Abstract
Introduction:
Matrix metalloproteinase-9 (MMP-9) plays an important role in the pathophysiology of sepsis. A single-nucleotide polymorphism (SNP) at position -1562 (C/T) in the MMP-9 gene has been associated with differential MMP-9 expression, being higher when the -1562 T allele is present. We evaluated the association of the SNP MMP9 -1562 C/T with severity and mortality in patients with sepsis to establish whether the prognosis of the disease is affected.
Materials and Methods:
A case-control study exploratory was carried out in a cohort of infected patients. 540 individuals were selected in total, 270 patients with sepsis and 270 controls (infected but non-septic), classified according to the 2016 consensus (Sepsis-3). The presence of the single-nucleotide polymorphism (SNP; allele T and/or allele C) was determined through analyses of restriction fragment length polymorphism and plasma levels of MMP-9 were determined through enzyme-linked immunosorbent assay immunoassay.
Results:
SNP MMP-9 -1562 has two known alleles (T and C), with predominance of the C over the T allele; in the group of patients with sepsis, T allele was found in 7.2% of cases, while C allele in the rest (92.8%); in comparison, in the group of infected but non-septic patients, frequencies were 9.4% for T allele and 90.6% for the C allele (P = .33). Also, the presence of the polymorphic T allele was not related to the levels of MMP-9 in patients with sepsis in comparison with infected but non-septic patients 780 (397-1375) ng/mL vs 646 (172-1249) ng/mL (P = .64). There was also no association between the SNP and sepsis mortality (P = .78).
Conclusions:
We concluded that there was no association between the SNP MMP9 -1562 C/T and sepsis or between the SNP MMP9 -1562 C/T and sepsis mortality in the Northeastern Colombian septic patient cohort. Further research is needed to clarify the correlation among sepsis, genetic factors with allele T and MMP-9 plasma concentration.
Keywords: matrix metalloproteinase 9, prognosis, sepsis, single-nucleotide polymorphism
Introduction
Sepsis is considered a public health problem and one of the leading causes of death worldwide.1 The last consensus of sepsis-3 defined it as a “life-threatening organ dysfunction caused by a dysregulated host response to infection.”2 In Colombia, Ortíz et al3 reported a monthly incidence rate of sepsis of 3.6 per 100 admissions per hospital and Leon et al reported a mortality of 18.5% and 45.6% in patients with severe sepsis and septic shock at 28 days, respectively.4
Several biomarkers have been described in the pathophysiology of sepsis, including matrix metalloproteinase-9 (MMP-9), which may represent a promising tool for the prognosis of patients with sepsis. MMP-9 is a proteinase that participates in the proteolytic regulation of intra- and extracellular matrix. MMP-9 is inhibited by TIMP15 and is involved in several processes such as the immune exacerbation,6 chemotaxis,7 and angiogenesis.8 In sepsis, MMP-9 has been linked to an increase in vascular permeability due to the degradation of collagen present in the vascular basement membrane, facilitating the migration of inflammatory cells and modulating the inflammatory response.9 Also, it is involved in vascular hyporeactivity, in animal models and cardiovascular disease,10 lung injury,11 in the coagulation/fibrinolysis system and in platelet function.12
Sepsis is a multifactorial and heterogeneous process that involves humoral and cellular immunological reactions. the stratification of the disease, the prognosis and the response to therapeutic management is difficult in these patients. For that reason, the International Sepsis Definitions Conference, in 2001, proposed a new scale of “predisposition, infection, response and organ dysfunction” (PIRO scale) which includes the study of genetic predisposition as a possible promising biomarker in the course of sepsis.13 Some of these genetic variabilities have been reported by Villar et al.14 This group and other authors reviewed single-nucleotide polymorphisms (SNPs) in CD14, TLR2, and TNF genes as factors that modulate clinical presentation, responses to medical treatment and susceptibility to sepsis or infection.15,16 Similar studies have reported MMP-9 geners 3918242 playing an important role in the pathophysiology of sepsis. A single-nucleotide polymorphism at position -1562 in its gene has been associated with different MMP-9 levels of expression.
The literature reports show contradictory findings in behavior of polymorphism SNP MMP-9 -1562 C/T and its expression in sepsis. This study is the first to describe the possible genetic association of SNP MMP-9 -1562 C/T, in the largest cohort of a Colombian population, with sepsis. To assess the prognostic value of SNP MMP-9 -1562 C/T in sepsis we sought to (1) study the association of the SNP with presence and absence of sepsis; (2) stratify an analysis between the presence of the SNP with biomarkers for organic dysfunction, focusing on cardiovascular dysfunction, since the heart is the major affected organ to increase mortality; and (3) determine the relationship of the SNP with plasma levels of MMP-9 and (4) the association of the SNP with mortality in sepsis. This study was supported by the reporting of Genetic Association Studies (STREGA)—An Extension of the STROBE Statement.17
Materials and Methods
Type of study
Informed consent was obtained from all patients, or from their legal representative if the patient was unable to consent. The present study was approved by the Ethics Committee of the Autonomous University of Bucaramanga (0056/2009 and 0066/2015), Universidad Industrial de Santander UIS (0012/2014) (0029/2017), and the various hospitals that participated of the study. Design was defined as a cases and controls nested no match 1: 1 in a cohort, performed in the metropolitan area of Bucaramanga from 2009 (January) to 2013 (December).
Study design
The participants were followed up for 28 days to check your survival or severity, and the data were obtained by reviewing the clinical history and interviewing the patient or close family member.
Inclusion criteria ware as follows: (1) patients over 18 years old, admitted to the emergency department or intensive care unit; (2) patients were included if they fulfilled the sepsis criteria published in 2001 for sepsis, severe sepsis, or septic shock; then all the variables were subsequently reclassified using the 2016 sepsis. Exclusion criteria were as follows: (1) pregnancy; (2) patients with known chronic inflammatory diseases (cancer and autoimmune disease); (3) immunosuppression; (4) heart failure prior to the infectious process; and (5) diagnosis of sepsis or septic shock for longer than 72 h.
Clinical variables
We assessed severity and type of infection using severity scales (SOFA, APACHE II). We also used the CHARLSON co-morbidity index. Enrolled patients were followed up to 28 days after being enrolled in the study to evaluate mortality.
Clinical and biomarker data
We obtained clinical data, such as mean arterial pressure, platelet count, bilirubin, creatinine, blood urea nitrogen (to determine SOFA scale and organic dysfunction), troponin I, brain natriuretic peptide (NT-proBNP) (to identify cardiovascular dysfunction), plasma level MMP-9, metalloproteinase inhibitor TIMP-1, and determination SNP MMP-9 -1562 C/T (rs 3918242).
Peripheral blood collection was performed as soon as the patient was enrolled in the study, it means within the first 72 h of sepsis diagnosis. Tubes were centrifuged at 1000g for 15 min to get plasma or serum, respectively (with or without anticoagulant). Supernatants were aliquoted and stored at −80°C until analysis. We quantified the following biomarkers in serum: troponin I, NT-proBNP, BUN, creatinine, and bilirubin, by means of electrochemiluminescence using the COBAS 6000 analyzer (Roche, Bogota, Colombia). We measured MMP-9 and TIMP-1 levels in plasma by enzyme-linked immunosorbent assay (ELISA) using Quantikine kits (R&D Systems, Minneapolis, USA).
DNA extraction
We extracted DNA from 200 μL of peripheral blood from buffy coat using the QIAamp DNA blood kit, following the manufacturer’s recommendations (QIAGEN, Germantown, USA). DNA quality was visually verified by electrophoresis using 1% agarose gels and stored at −80 in freezer.
Detection of the SNP MMP-9 -1562 C/T
Amplification of the promoter region that contained the SNP was performed by PCR, using the forward primer 5′ GCC-TGG-TGG-CAC-ATA-GTA-GGC-CC-3′ and the reverse primer 5′ CTT-CCT-AGC-CAG-CCG-GCA-TC-3′ (Bionner, Alameda, USA) at a concentration of 0.2 μM in a final volume of 25 μL of master mix (Thermo Scientific, Waltham, USA). PCR reactions were run in a MJ Research PTC-100 thermocycler (Marshall Scientific, Hampton, USA), using the following cycles: 1 min of initial denaturation at 95°C, followed by 30 cycles of 94°C for 30 s, 58°C for 30 s, and 72°C for 30 s, with a final extension step of 10 min at 72°C. Amplification was verified by electrophoresis in 2% agarose gels by the presence of a band of 435 bp. For detecting the presence of the SNP, the amplicons were digested with 2 units of the restriction endonuclease SphI (Thermo Scientific, Waltham, USA) in 10 μL final volume for 17 h at 37°C. The resulting fragments were run by electrophoresis in 3% agarose gels and examined using the Gel Doc XR Gel Documentation System (Bio-Rad, Hercules, USA), and the bands were analyzed with ImagenLab software 15.2.1 (Bio-Rad, Hercules, USA) (Figure 1). The procedure was carried out in the facilities of the Laboratory of Genetic and Molecular Biology in Autonomous University of Bucaramanga, UNAB.
Figure 1.
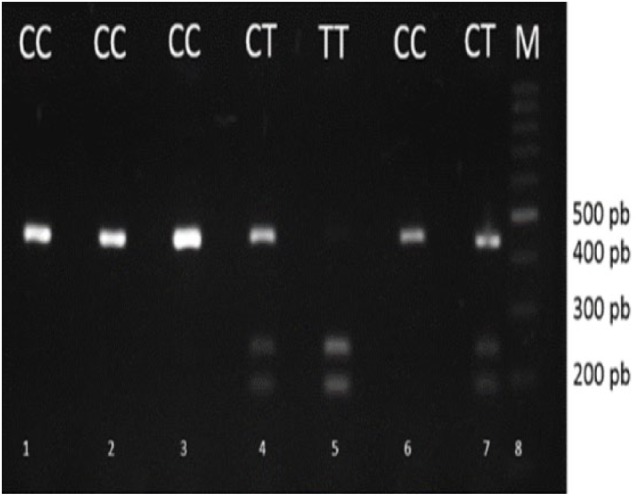
SNP MMP-9 -1562 C/T, is detected by RFLP. RFLP indicates restriction fragment length polymorphism.
The 435-bp product obtained from PCR was digested by the restriction enzyme SphI, fragments generated by RFLP were separated by electrophoresis in 2% agarose gels. Lanes 1, 2, 3 and 6: homozygous CC; Lanes 4 and 7: heterozygous CT; Lane 5: homozygous TT; M: molecular weight marker (100 bp).
Sample size
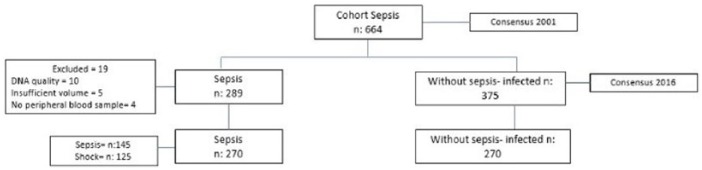
The original cohort was composed of 664 patients, classified according to the consensus of Sepsis 2001.18 From this cohort, participants were subsequently reclassified according to the new Sepsis-3 criteria, yielding a total of 540 eligible participants, 270 of which had sepsis (cases) and 270 who, albeit infected, were determined as non-septic patients (controls).
Sample selection was non-probabilistic, using the largest available sample (Figure 2); however, sample size in the present project corresponds to a pilot study. Due to participant availability, sample size calculation was, therefore, done based on the largest available sample for matched populations. It is worth mentioning that the number of patients contemplated was based on the consultation estimates for this pathology in our institution and in published studies. This study will lay the necessary clinical and statistical bases for further studies on a larger scale, estimating the role of SNPs in a multifactorial disease such as sepsis.
Figure 2.
Population of study and classification according to consensus 2016.
Statistical analyses
We used STATA 14v software (StataCorp, Texas, USA) for the statistical analyses. Only bivariate analyzes were performed. First, we studied the distribution of quantitative variables by graphical representation analysis and found that these did not have a normal distribution. Hence, a descriptive univariate analysis was performed, and quantitative variables were reported as medians and interquartile ranges. Categorical variables were reported as frequencies and percentages. Comparisons of quantitative variables between groups were carried out using the Wilcoxon-Mann-Whitney test. Comparisons between groups on categorical variables were carried out with the chi-square test. SNP correlation with the different biomarkers was assessed using Kruskal-Wallis test. Also, the genotypic and allelic frequencies were calculated to determine the Hardy-Weinberg equilibrium using R Studio 3.3.3 software (RStudioInc, Boston, USA) We use in stratified logistic regression, between the presence of the polymorphic allele T (CT-TT vs CC), dominant inheritance model. To check whether a polymorphic allele copy is enough to generate the interest event, through the SNPstats platform (Institut Catalá dOncologia,Cataluña, España).
Results
540 adult patients were enrolled, 270 classified as patients with sepsis and 270 as infected but non-septic patients were used as controls. In septic group, 53% of the patients had sepsis and 47% had septic shock; 50% were women and 254 (94.1%) were mestizos; 76 (23%) of the septic group died within the 30 days of follow-up, and none of the control group died within this period. The most frequent diagnoses were urinary tract infection (34%), low respiratory tract infection (24%), soft tissue infection (26%), intraabdominal infection (8%), and others (7.5%). We found that the MMP-9 levels in the septic group were significantly higher than the controls (589 ng/mL vs 196 ng/mL, respectively, P < .001); on the contrary, TIMP-1 levels were lower in the patients with sepsis than in the controls (306 ng/mL vs 847 ng/mL, respectively, P < .001). Demographical and clinical data of the participants are detailed in Table 1.
Table 1.
Sociodemographic characteristics of the study population.
| Variable | Sepsis N = 270 | Infected but non-septic N = 270 | P value |
|---|---|---|---|
| Age (years) | 61 (48-75) | 57 (43-71) | .01 |
| Male | 135 | 141 | .6 |
| Female | 135 | 129 | .6 |
| Creatinine (mg/dL) | 1 (0.75-2.01) | 0.78 (0.64-0.96) | <.001 |
| BUN (mg/dL) | 24.9 (16.3-44.5) | 13.7 (10.42-19) | <.001 |
| Bilirubin total (mg/dL) | 0.4 (0.22-0.8) | 0.2 (0.2-0.4) | <.001 |
| NT-proBNP (pg/mL) | 2099 (590-8937) | 329 (153-867) | <.001 |
| Troponin I (ng/mL) | 0.1 (0.1-0.4) | 0.1 (0.1-0.1) | .2 |
| MAP (mmHg) | 68 (62-79) | 70 (68-75) | <.001 |
| Platelets (mm3) | 232 (168-303) | 248 (194-307) | .020 |
| SOFA | 2 (1-5) | 0 (0-1) | <.001 |
| APACHE | 12.5 (8-18) | 7 (5-10) | <.001 |
| CHARLSON | 1 (1-3) | 1 (0-2) | .1 |
| MMP-9 (ng/mL) | 589 (229-1098) | 196 (120-300) | <.001 |
| TIMP-1 (ng/mL) | 306 (170.5-579.2) | 847 (615.8-1169) | <.001 |
Data presented as median (25th to 75th percentile).
Abbreviations: APACHE, Acute Physiology and Chronic Health Evaluation; BUN, blood urea nitrogen; CHARLSON, Charlson Comorbidity Index; MAP, mean arterial pressure; MMP-9, matrix metalloproteinase 9; NT-proBNP, cerebral natriuretic peptide; SOFA, Sequential Organ Failure Assessment; TIMP-1, tissue inhibitor of metalloproteinase 1.
No significant differences were observed between the proportions of the three possible genotypes (CC, CT, and TT) and the septic and control groups. Genotype distribution for the SNP MMP9-1562 C/T is summarized in Table 2.
Table 2.
Genotype and allele frequency distribution.
| Variable | Sepsis | Infected but non-septic | P value | OR | CI 95% |
|---|---|---|---|---|---|
| SNP MMP-9 -1562 | |||||
| CC, n (%) | 231 (85.87) | 223 (82.66) | .30 | 1 | |
| CT, n (%) | 38 (13.75) | 43 (15.87) | 1.21 | 0.75-1.95 | |
| TT, n (%) | 1 (0.37) | 4 (1.48) | 0.50 | 0.46-3.3 | |
| SNP MMP-9 -1562 allele | |||||
| C, n (%) | 501 (92.8) | 489 (90.6) | .33 | 0.32 | 0.23-1.32 |
| T, n (%) | 39 (7.2) | 51 (9.4) | 0.33 | 0.45 | 0.53-1.34 |
CT and TT, polymorphic genotype; T, polymorphic allele; MMP-9, matrix metalloproteinase 9.
We did not identify an allelic association with the plasma levels of MMP-9 and other biomarkers of organic dysfunction in patients with sepsis and none of these variables are confounding factors and infected individuals’ group, significant differences were found between the presence of the T allele in TIMP-1, APACHE, and CHARLSON (Table 3). Similarly, no significant differences were observed between the presence of the polymorphic allele with survival at 30 days of follow-up and cardiovascular dysfunction (Tables 4 and 5).
Table 3.
Correlation of SNP MMP-9 -1562 genotype with plasma levels of MMP-9 and biomarkers of organ dysfunction in sepsis and controls.
| Variables | Sepsis N = 270 | Infected but not septic N = 270 | ||||
|---|---|---|---|---|---|---|
| CC (n = 231) | CT + TT (n = 39) | P value | CC | CT + TT | P value | |
| MMP-9 (ng/mL) | 577 (218.1-1083) | 780 (397-1375) | .14 | 884 (535-1474) | 646 (172-1249) | .64 |
| TIMP-1 (ng/mL) | 305 (169-555) | 304 (186-640) | .63 | 222 (139-355) | 66 (34-162) | .05 |
| MAP (mmHg) | 68 (61-80) | 69 (67-72) | .39 | 70 (68-75) | 70 (68-73) | .72 |
| Platelets (mm3) | 230 (170-304) | 237 (168-300) | .93 | 245 (193-309) | 253 (204-299) | .59 |
| Creatinine (mg/dL) | 1.09 (0.74-1.95) | 0.98 (0.78-2.74) | .84 | 0.8 (0.6-0.9) | 0.6 (0.6-0.7) | .50 |
| BUN (mg/dL) | 25 (16.33-45.27) | 24 (16.3-36.9) | .40 | 14.5 (11.8-21.2) | 10.1 (9.4-11.7) | .1 |
| Bilirubin total (mg/dL) | 0.15 (0.07-0.23) | 0.12 (0.07-0.19) | .59 | 0.3 (0.2-0.5) | 0.2 (0.2-0.2) | .25 |
| NT-proBNP (pg/mL) | 2102 (613-8776) | 1656 (413-9554) | .79 | 323.6 (161.2-866.5) | 373 (138.2-876.2) | .83 |
| Troponin I (ng/mL) | 0.1 (0.1-0.19) | 0.1 (0.1-0.18) | .90 | 0.1 (0.1-0.1) | 0.1 (0.1-0.1) | .70 |
| SOFA | 2 (1-5) | 2 (1-4) | .36 | 1 (1-1) | 0 (0-0) | .1 |
| APACHE | 13.8 (8-19) | 12.65 (9-15) | .46 | 8 (6-10.5) | 4 (3.7-4) | .02 |
| CHARLSON | 2 (1-3) | 1 (1-4) | .78 | 1 (1-2) | 0 (0-0) | .03 |
Data presented as median (25th to 75th percentile).
Abbreviations: APACHE, Acute Physiology and Chronic Health Evaluation; BUN, blood urea nitrogen; CHARLSON, Charlson Comorbidity Index; MAP, mean arterial pressure; MMP-9, matrix metalloproteinase 9; proBNP, cerebral natriuretic peptide; SOFA, Sequential Organ Failure Assessment; TIMP-1, tissue inhibitor of metalloproteinase 1.
Table 4.
SNP MMP-9 -1562 C/T and survival in sepsis.
| Allele | Survivor, n (%) | Non-survivor, n (%) | OR (CI 95%) | P value |
|---|---|---|---|---|
| CC (n = 232) | 168 (86.60) | 64 (85.33) | 1 | .78 |
| CT + TT (n = 38) | 26 (13.40) | 12 (14.67) | 1.47 (0.68-3.17 |
Abbreviations: CI, confidence interval; OR, odds ratio.
The population is grouped with the presence or absence of the polymorphic allele T and its association with survival in sepsis.
Table 5.
SNP MMP-9 -1562 C/T with echocardiographic variables (diastolic dysfunction).
| Allele | DDVI altered, n (%) | DDVI not altered, n (%) | P value |
|---|---|---|---|
| CC (n = 226) | 65 (80.2) | 161 (85.2) | .47 |
| CT + TT (n = 44) | 16 (19.8) | 28 (14.8) |
Abbreviation: DDVI, Left Ventricular Diastolic Dysfuntion.
CT and TT, polymorphic genotype; T, polymorphic allele.
Discussion
MMP-9 is secreted by cells of innate immunity such as neutrophils; therefore, this molecule is involved in the immune exacerbation in sepsis. However, literature presents conflicting reports regarding the possible role of plasma MMP-9 in sepsis. A study conducted by Lorente et al19 found that the levels of MMP-9 were elevated in patients who had severe sepsis that survived and recently a study developed by Niño et al20 found no association between MMP-9 levels and mortality. On the other hand, Hoffmann et al21 demonstrated that plasma levels MMP-9 in patients with severe sepsis were significantly higher compared with healthy individuals. Also, interestingly, the inhibition of MMP-9 significantly reduces the bacterial load and the resulting immunopathology in a co-infection model. This highlights the inhibition of MMP-9 as a possible therapeutic agent.22
The SNP MMP-9 -1562 C/T polymorphism has been associated with the development of diabetic microvascular complication and coronary artery disease.23–25 However, the study of polymorphism and its relationship with sepsis is not clear. On one hand, Martin et al26 found that there was no association between the presence of MMP-9 -1562 C/T polymorphism and the development of sepsis. In contrast, the study done by Collazos et al27 reported that the presence of the polymorphic allele T is related to a higher concentration of MMP-9 in plasma in severe sepsis.
Given the discrepancies between the studies of plasma levels of MMP-9 in sepsis, this study aims to clarify the impact of the polymorphism 1562 C/T in the promoter region of the MMP-9 gene with sepsis. The epigenetic studies carried out found that region 1562 of the MMP-9 gene is a binding site for transcription factors that increase gene expression. Furthermore, in this position, the presence of the SNP rs3918242, which is generated by replacing a cytosine nucleotide (C) with one of thymine (T),28 has been indicated. When the C allele is present, the rate of transcription decreases because the binding of the transcription factors is inhibited; therefore, the presence of the polymorphic T allele generates a higher level of MMP-9 in blood.28
In the present study, we evaluated the association of MMP-9 -1562 C/T gene polymorphism with development of sepsis and for the first time, the polymorphism was evaluated as a potential tool for prognostic and clinical outcomes in patients with sepsis according to the new classification of sepsis-3. We found that the MMP-9 -1562 C/T gene polymorphism was not associated with development of sepsis and there was no significant association between the polymorphism of MMP-9 with sepsis mortality, or with cardiovascular dysfunction or changes in plasma levels of MMP-9 in patients with sepsis.
On the other hand, from the allele frequencies, we found that the studied population shows Hardy-Weinberg equilibrium conditions. Likewise, our results suggest that the allele frequencies behave according to chance pairing rather than genetic drift, panmixia, or any other selective force that would affect random distribution of the alleles. Our results are also in line with those of León et al,29 showing that the population of Bucaramanga does not present a homogeneic structure (Fst = 0.0038), as there are no subpopulations in the six polymorphisms studied and the association results are not seen with the studied outcomes.29
In our results, the presence of the -1562 C/T polymorphism of MMP-9 was not associated with mortality in patients with sepsis. Like our study, a multicenter study developed by Martin et al26 in 90 ICU patients with severe sepsis and 91 uninfected controls patients with trauma and brain stroke found that there was no association between the presence of the polymorphism in non-survivors in comparison with the survivors in patients with sepsis (P = .1). Undoubtedly, we have a larger sample and a higher mortality rate in patients with sepsis, which increases the power of our results. Consequently, it is very likely that there is no real association between this polymorphism and the outcomes studied in our population. On the other hand, the control group of our study uses, infected non septic patients unlike Martin et al who examined patients without infection, which highlights the diagnostic value of sepsis and not merely infection.
The association of the MMP-9 -1562 C/T gene polymorphism and development of sepsis is poorly understood due to the low number of studies in patients with sepsis. We found that the polymorphism of MMP-9 did not have a significant association with the development of sepsis compared with non-septic infected patients. As we did, Martin et al26 found that there was no association between the MMP-9 -1562 C/T gene polymorphism and the presence of sepsis (P = .3).
Several studies have found that high levels of blood MMP-9 are associated with sepsis. We found that patients with sepsis with CT + TT showed a greater median of MMP-9 plasma concentration; however, this difference was not significant (P = .14) (Table 3). In contrast, a study carried out by Collazos et al27 in 90 patients with severe sepsis, 102 with HIV monoinfection, 111 with HIV-hepatitis C infection, and 86 non-infected controls (45 stroke and 41 trauma patients) demonstrated that the presence of the T allele is associated with a higher plasma concentration of MMP-9 in patients with sepsis (P = .042), due to the size of the sample and the dispersion of the data.
Although the association of elevated levels of MMP-9 with cardiac dysfunction and loss of vascular tone has been demonstrated in animal models of sepsis,18 the role of MMP-9 in sepsis with cardiovascular disease in humans remains understudied. We could not show an association between the SNP of MMP-9 and markers of cardiac dysfunction (serum troponin I, NT-proBNP, mean arterial pressure and DDVI) of individual alleles of polymorphism in patients with sepsis.
In addition, the allelic frequencies of the SNP MMP-9 -1562 C/T of the present investigation were compared with the 1000 Genomes database, finding the same percentage in the present study of the polymorphic T allele (15.52% vs 7.3%), for which it is presented that the wild allele C in the studied population is fixed. Due to the contradictions in the levels of MMP-9 in sepsis between this and other studies, we believe that it is likely there is a polymorphism or mutation in the MMP-9 gene that may play a synergistic role with other causing elements of mortality and severity in sepsis, leaving it as a poor predictor on its own.
However, the literature on this topic is quite limited, preventing us to firmly conclude the above. Still, there are other plausible explanations for the observed absence of association: (1) the interaction of the MMP-9 gene with other genes involved in the mechanisms of the sepsis, (2) the presence of other relevant influencing haplotypes of SNPs in the MMP-9 gene or, (3) epigenetic and other genetic mechanisms of control mediated by the existing microenvironment, including inflammatory mediators and their byproducts. All these hypotheses fall beyond the scope of this study, which emphasizes the need to design future studies to address and identify them, and thus clarify the role of SNP MMP-9 -1562 C/T in sepsis.
Limitations of study: (1) classification under the new Sepsis-3 criteria affected the number of participants in each group; (2) given the stratification by survival, and the subgroups in genotypes, statistical power is limited; (3) using a single SNP as a biomarker can constitute a disadvantage; however, the role of such a polymorphism with a larger sample than presented in previous studies was a stronghold; (4) the study clarifies the non-association of SNP and encourages investigators to study other polymorphisms to generate predictive models accompanied by clinical variables; (5) it is important to mention that the number of enrolled patients was based on the consultation estimates for this pathology, both within our institution and in previous published studies; and (6) exploratory analyzes were made for the formulation of hypotheses, but not to discard them or confirm the association of the SNP with the event studied.
This is the first study of which we are aware on the genotypic frequency distribution of the SNP MMP-9 -1562 C/T assessing the relationship between it and sepsis and its prognostic role in patients with sepsis in Latin America and, until now, the one with the largest sample size. Also, statistical, and computational studies are required to better comprehend the prognostic value of MMP-9 SNPs in the outcome of sepsis, both on its own and in combination with other markers.
Conclusions
The present study is the first one to genotype the MMP-9 polymorphism -1562 C/T in a Latin American population, finding the possible no association between the SNP and sepsis in infected patients and between the SNP and mortality of patients with sepsis. Also, this variant did not correlate with MMP-9 plasma levels, or with of various biomarkers of cardiovascular disease.
Acknowledgments
The authors would like to thank the Administrative Department of Science, Technology and Innovation (COLCIENCIAS), Bank of the Republic, and the Autonomous University of Bucaramanga for administrative support.
Footnotes
Funding:The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Bank of the Republic -Foundation for the Promotion of Research and Technology (Project No. 3.467 ) and the Administrative Department of Science, Technology and Innovation (COLCIENCIAS) - contracts FIDUPREVISORA S.A No. FP44842-033-2015. The Autonomous University of Bucaramanga, and the Canadian Institutes of Health Research (FDN 143299). Funding agencies had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Declaration of Conflicting Interests:The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Author Contributions: Conceptualization: CB-M, SB-B, CMP, CIV, MEC, IB, DT-D
Data curation: CB-M, SB-B, CMP, SES
Formal analysis: CB-M, SES, CIV
Methodology: CB-M, SB-B, CMP, MEC, IB
Writing ± original draft: CB-M, SB-B, MCP
Writing ± review & editing: MFT-C, MEC, RM-V, RS, RI, APF.
ORCID iDs: Diego Torres-Dueñas  https://orcid.org/0000-0002-8006-7461
https://orcid.org/0000-0002-8006-7461
Richard Schulz  https://orcid.org/0000-0002-5045-3193
https://orcid.org/0000-0002-5045-3193
References
- 1. Mayr FB, Yende S, Angus DC. Epidemiology of severe sepsis. Virulence. 2014;5:4–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Singer M, Deutschman CS, Seymour C, et al. The third international consensus definitions for sepsis and septic shock (sepsis-3). JAMA. 2016;315:801–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ortíz G, Dueñas C, Rodríguez F, et al. Epidemiology of sepsis in Colombian intensive care units. Biomedica. 2014;34:40–47. [DOI] [PubMed] [Google Scholar]
- 4. Leon AL, Hoyos NA, Barrera LI, et al. Clinical course of sepsis, severe sepsis, and septic shock in a cohort of infected patients from ten Colombian hospitals. BMC Infect Dis. 2013;13:345–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Page-McCaw A, Ewald AJ, Werb Z. Matrix metalloproteinases and the regulation of tissue remodelling. Nat Rev Mol Cell Biol. 2007;8:221–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Khokha R, Murthy A, Weiss A. Metalloproteinases and their natural inhibitors in inflammation and immunity. Nat Rev Immunol. 2013;13:649–665. [DOI] [PubMed] [Google Scholar]
- 7. Song J, Wu C, Korpos E, et al. Focal MMP-2 and MMP-9 activity at the blood-brain barrier promotes chemokine-induced leukocyte migration. Cell Rep. 2015;10:1040–1054. [DOI] [PubMed] [Google Scholar]
- 8. Deryugina EI, Quigley JP. Pleiotropic roles of matrix metalloproteinases in tumor angiogenesis: contrasting, overlapping and compensatory functions. Biochim Biophys Acta. 2010;1803:103–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. de Souza P, Schulz R, da Silva-Santos JE. Matrix metalloproteinase inhibitors prevent sepsis-induced refractoriness to vasoconstrictors in the cecal ligation and puncture model in rats. Eur J Pharmacol. 2015;765:164–170. [DOI] [PubMed] [Google Scholar]
- 10. Gutierrez FRS, Lalu MM, Mariano FS, et al. Increased activities of cardiac matrix metalloproteinases matrix metalloproteinase (MMP)-2 and MMP-9 are associated with mortality during the acute phase of experimental trypanosoma cruzi infection. J Infect Dis. 2008;197:1468–1476. [DOI] [PubMed] [Google Scholar]
- 11. Pardo A, Cabrera S, Maldonado M, Selman M. Role of matrix metalloproteinases in the pathogenesis of idiopathic pulmonary fibrosis. Respir Res. 2016;17:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gresele P, Falcinelli E, Sebastiano M, Momi S. Matrix metalloproteinases and platelet function. Prog Mol Biol Transl Sci 2017;147:133–165. [DOI] [PubMed] [Google Scholar]
- 13. Opal SM. Concept of PIRO as a new conceptual framework to understand sepsis. Pediatr Crit Care Med. 2005;6:S55–S60. [DOI] [PubMed] [Google Scholar]
- 14. Villar J, Maca-Meyer N, Perez-Mendez L, Flores C. Bench-to-bedside review: understanding genetic predisposition to sepsis. Crit Care. 2004;8:180–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Heesen M, Bloemeke B, Schade U, et al. The -260 C->T promoter polymorphism of the lipopolysaccharide receptor CD14 and severe sepsis in trauma patients. Intensive Care Med. 2002;28:1161–1163. [DOI] [PubMed] [Google Scholar]
- 16. Koch A, Melbye M, Sorensen P, et al. Acute respiratory tract infections and mannose-binding lectin insufficiency during early childhood. JAMA. 2001;285:1316–1321. [DOI] [PubMed] [Google Scholar]
- 17. Little J, Higgins JPT, Ioannidis JPA, et al. STrengthening the REporting of genetic association studies (STREGA)—an extension of the STROBE statement. Genet Epidemiol. 2009;33:581–598. [DOI] [PubMed] [Google Scholar]
- 18. Levy MM, Fink P, Marshall JC, et al. SIS International Sepsis Definitions Conference. Crit Care Med. 2003;31:1250–1256. [DOI] [PubMed] [Google Scholar]
- 19. Lorente L, Martin MM, Sole-Violan J, et al. Association of sepsis-related mortality with early increase of TIMP-1/MMP-9 ratio. PLoS ONE. 2014;9:e94318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Niño ME, Serrano SE, Niño DC, et al. TIMP1 and MMP9 are predictors of mortality in septic patients in the emergency department and intensive care unit unlike MMP9/TIMP1 ratio: multivariate model. PLoS ONE. 2017;12:e0171191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hoffmann U, Brueckmann M, Borggrefe M. Matrix metalloproteinases and their inhibitors: promising novel biomarkers in severe sepsis. Crit Care. 2009;13:1006–1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Weiss G, Lai C, Fife ME, et al. Reversal of TREM-1 ectodomain shedding and improved bacterial clearance by intranasal metalloproteinase inhibitors. Mucosal Immunol. 2017;10:1021–1030. [DOI] [PubMed] [Google Scholar]
- 23. Zhang B, Ye S, Herrmann S-M, et al. Functional polymorphism in the regulatory region of gelatinase B gene in relation to severity of coronary atherosclerosis. Circulation. 1999;99:1784–1794. [DOI] [PubMed] [Google Scholar]
- 24. Opstad TB, Arnesen H, Pettersen A, Seljeflot I. The MMP-9 -1562 C/T polymorphism in the presence of metabolic syndrome increases the risk of clinical events in patients with coronary artery disease. PLoS ONE. 2014;9:e0106816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Zhang Z, Wu X, Cai T, et al. Matrix metalloproteinase 9 gene promoter (Rs 3918242) mutation reduces the risk of diabetic microvascular complications. Int J Environ Res Public Health. 2015;12:8023–8033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Martin G, Asensi V, Montes AH, et al. Role of plasma matrix-metalloproteases (MMPs) and their polymorphisms (SNPs) in sepsis development and outcome in ICU patients. Sci Rep. 2014;4:5002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Collazos J, Asensi V, Martin G, Montes AH, Suarez-Zarracina T, Valle-Garay E. The effect of gender and genetic polymorphisms on matrix metalloprotease (MMP) and tissue inhibitor (TIMP) plasma levels in different infectious and non-infectious conditions. Clin Exp Immunol. 2015;182:213–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Yin H, Zhao L, Zhang Y, Qin L. Polymorphism in matrix metalloproteinase-9 1562 C/T contributes to the risk of coronary artery disease. Int J Clin Exp Pathol 2016;9:2277–2282. [Google Scholar]
- 29. León FJ, Rondón F, Vargas CI, et al. Analysis of population genetic structure from Bucaramanga (Colombia) based on gene polymorphisms associated with regulation of blood pressure. Colomb Med. 2012;43:154–161. [PMC free article] [PubMed] [Google Scholar]