Short abstract
Background
Frontotemporal lobar degeneration (FTLD) is the third most common dementing neurodegenerative disease with nearly 80% having no known etiology.
Objective
Growing evidence that neurodegeneration can be linked to dysregulated metabolism prompted us to measure a panel of trophic factors, receptors, and molecules that modulate brain metabolic function in FTLD.
Methods
Postmortem frontal (Brodmann’s area [BA]8/9 and BA24) and temporal (BA38) lobe homogenates were used to measure immunoreactivity to Tau, phosphorylated tau (pTau), ubiquitin, 4-hydroxynonenal (HNE), transforming growth factor-beta 1 (TGF-β1) and its receptor (TGF-β1R), brain-derived neurotrophic factor (BDNF), nerve growth factor, neurotrophin-3, neurotrophin-4, tropomyosin receptor kinase, and insulin and insulin-like growth factor-1 (IGF-1) and insulin-like growth factor-2 (IGF-2) and their receptors by direct-binding enzyme-linked immunosorbent assay.
Results
FTLD brains had significantly elevated pTau, ubiquitin, TGF-β1, and HNE immunoreactivity relative to control. In addition, BDNF and neurotrophin-4 were respectively reduced in BA8/9 and BA38, while neurotrophin-3 and nerve growth factor were upregulated in BA38, and tropomyosin receptor kinase was elevated in BA24. Lastly, insulin and insulin receptor expressions were elevated in the frontal lobe, IGF-1 was increased in BA24, IGF-1R was upregulated in all three brain regions, and IGF-2 receptor was reduced in BA24 and BA38.
Conclusions
Aberrantly increased levels of pTau, ubiquitin, HNE, and TGF-β1, marking neurodegeneration, oxidative stress, and neuroinflammation, overlap with altered expression of insulin/IGF signaling ligand and receptors in frontal and temporal lobe regions targeted by FTLD. Dysregulation of insulin-IGF signaling networks could account for brain hypometabolism and several characteristic neuropathologic features that characterize FTLD but overlap with Alzheimer’s disease, Parkinson’s disease, and Dementia with Lewy Body Disease.
Keywords: neurodevelopmental disorders, neuropathology, neurodegenerative diseases
Introduction
Frontotemporal lobar degeneration (FTLD) is the third most common dementing neurodegenerative disease in the United States, surpassed only by Alzheimer’s disease (AD) and Dementia with Lewy Body Disease (DLBD; Erkkinen et al., 2018). The onset of FTLD-related symptoms begins between 45 and 65 years of age, and survival is just 3 to 5 years after diagnosis (Warren et al., 2013; Bennion-Callister and Pickering-Brown, 2014; Bang et al., 2015). In contrast, onset of nonfamilial AD or DLBD is 65 years or older, and survival is 7 to 10 years after diagnosis (Korczyn and Reichmann, 2006; Mitchell et al., 2009; Kua et al., 2014; Chene et al., 2015). Because only 20% of FTLD cases have a clear familial or genetic link, 80% have no known etiology (Rascovsky et al., 2011; Bang et al., 2015). Accompanying motor neuron disease with degeneration of brainstem motor nuclei and spinal anterior horn cells (Kertesz et al., 2005; Bennion-Callister and Pickering-Brown, 2014) accelerates the course and abbreviates survival. The neuropathology of FTLD is characterized by prominent atrophy of gray and white matter structures in the frontal lobe, insular cortex, and anterior temporal and cingulate regions (Kertesz et al., 2005; Rascovsky et al., 2011). Atrophy is associated with neuronal loss, gliosis, and accumulations of neuronal and glial cytoplasmic inclusions that contain misfolded and mutated proteins such as the microtubule-associated protein tau, TAR DNA-binding protein 43 kDa (TDP-43), fused-in-sarcoma, or ubiquitin (Hasegawa et al., 2008; Inukai et al., 2008; Kumar-Singh, 2011). Misfolded proteins continue to be identified in FTLD, but their causal or consequential roles remain uncertain.
The neurotrophins, brain-derived neurotrophic factor (BDNF), nerve growth factor (NGF), and neurotrophin-3 (NT3; Sortilin) and neurotrophin-4/5 interact with high-affinity neurotrophic tropomyosine receptor kinase (Trk), which are tyrosine kinases, or low-affinity p75 neurotrophin receptor (p75NTR; Chao and Lee, 2004; Zanardini et al., 2016). Trk signaling promotes neuronal survival, proliferation, growth, and plasticity, whereas p75 signaling supports remodeling of axons and dendrites (Huang and Reichardt, 2001; Gomez-Palacio-Schjetnan and Escobar, 2013; Zanardini et al., 2016). Despite evidence for neurotrophin signaling abnormalities in AD (Song et al., 2015), convincing data have not been generated with respect to FTLD. For example, BDNF protein expression in the frontal or temporal lobes is reduced in AD but not in FTLD (Ferrer et al., 2000). Similarly, cerebrospinal fluid levels of BDNF and NGF are reduced in AD but not in FTLD (Blasko et al., 2006). While serum levels of BDNF may be lower in FTLD relative to controls (Zanardini et al., 2016), the levels are not predictive of FTLD subtypes (Woolley et al., 2012).
Although most studies designed to examine roles for neurotrophin dysfunction in FTLD have been disappointingly negative, the overall number of relevant publications is small, perhaps due to difficulty in collecting well-characterized cases. Further research is justified due to the known interrelationships between neurotrophins and other molecules that can be mutated in FTLD, and clinical or experimental data showing that neurotrophin treatments can rescue some aspects of neurodegeneration (Arenas and Persson, 1994; Hefti, 1994; Allen et al., 2013; Shruthi et al., 2017). For example, progranulin, a secreted growth factor that regulates neuronal survival, synaptogenesis, and synaptic function, is normally cotransported with BDNF in axons and dendrites but mutated in FTLD (Petoukhov et al., 2013; Zanardini et al., 2016). Therefore, BDNF’s functions could be impaired by progranulin mutations. In addition, p75NTR and pro-NGF may have critical roles in the pathogenesis of FTLD-tau because upregulation of the p75NTR or increased signaling through high-affinity pro-NGF promotes tau hyperphosphorylation (Shen et al., 2018) and activation of prodeath/antisurvival signaling (Chen et al., 2008) via the Akt/GSK-3β pathway (Shen et al., 2018).
An additional factor contributing to FTLD pathogenesis is oxidative stress, possibly due to mutations in TDP-43, which upregulates the unfolded protein response (UPR) leading to accumulation of ubiquitin aggregates and Golgi fragments (Tong et al., 2012). Lipid peroxidation is another potential source of oxidative stress in neurodegenerative diseases (Shoeb et al., 2014). Elevated levels of 4-hydroxynonenal (HNE) adducts in FTLD frontal cortex have been associated with increased gliosis (Martinez et al., 2008), suggesting that the astrocytic responses contribute to neurodegeneration.
On the other hand, consideration should be given to the rapidly growing literature supporting roles for insulin and insulin-like growth factor (IGF)-linked metabolic derangements, stress, and neuroinflammatory responses as mediators of AD and DLBD/Parkinson’s disease (PD; Mattson et al., 1999; Blass et al., 2002; Rivera et al., 2005; Steen et al., 2005; Grunblatt et al., 2007; Craft, 2009; Giovannone et al., 2009; Tong et al., 2009; Craft et al., 2013; de la Monte, 2014; de la Monte and Tong, 2014). Evidence that brain metabolic abnormalities also occur in FTLD has been provided by 18F-fluorodeoxyglucose-positron emission tomography studies demonstrating frontal and temporal lobe glucose hypometabolism (Jeong et al., 2005b; Poljansky et al., 2011; Renard et al., 2011), corresponding with the major regions of neurodegeneration and distinct from the patterns in AD (Jeong et al., 2005a; Toney et al., 2011; Roman and Pascual, 2012). Impairments in brain glucose utilization compromise cell survival, energy metabolism, mitochondrial function, myelin integrity, and synaptic plasticity (Mattson et al., 1999; de la Monte and Wands, 2005; de la Monte, 2017). Furthermore, many neuropathologic features in FTLD, including tau hyperphosphorylation, impaired neuronal plasticity and neurotransmitter functions, white matter atrophy, and cerebral microvascular disease could be explained by insulin- and IGF-related metabolic dysfunctions (Mattson et al., 1999; de la Monte and Wands, 2005; de la Monte, 2017), as demonstrated in AD and DLBD (Grunblatt et al., 2007). The present work assesses the presence and nature of insulin/IGF metabolic, neurotrophin, and oxidative stress-related abnormalities in human postmortem brains with FTLD.
Material and Methods
Materials
MaxiSorp Nunc 96-well plates used for enzyme-linked immunosorbent assays (ELISAs) were from Thermo Fisher Scientific (Waltham, MA, USA). Superblock-TBS and horseradish peroxidase-conjugated secondary antibodies were from Pierce Chemical Co (Rockford, IL, USA). Primary monoclonal antibodies and other immunodetection reagents were purchased from Abcam (Cambridge, MA, USA), EMD Millipore (now Millipore/Sigma, Billerica, MA, USA), Santa Cruz Biotechnology (Dallas, TX, USA), Vector Laboratories (Burlingame, CA, USA), Invitrogen (Carlsbad, CA, USA), or Life Technologies/Thermo Fisher, Inc. (Waltham, MA, USA). Fine chemicals were purchased from CalBiochem (Carlsbad, CA, USA) or Sigma-Aldrich (St. Louis, MO, USA).
Human Brain Tissue
Postmortem frontal and temporal lobe tissue was obtained from the Taub Brain Bank at Columbia University in New York (Keller et al., 2008; Vonsattel et al., 2008). Postmortem diagnoses of FTLD were made by systematic evaluation and scoring of atrophy, histopathological lesions, and immunohistochemical staining for phosphorylated tau (pTau), ubiquitin, and TDP-43 using standard criteria (Rascovsky et al., 2011; Coyle-Gilchrist et al., 2016). Aged normal controls were also processed and banked using a standardized protocol (Keller et al., 2008; Vonsattel et al., 2008). Permission to use deidentified postmortem human brain tissue for this research was granted by the Lifespan Hospitals institutional review board.
Direct-Binding ELISAs
Frontal (Brodmann’s areas [BA]8/9 and BA24) and temporal (BA38) lobe cortical tissue homogenates were prepared in 5 volumes of lysis buffer containing 50 mM Tris (pH 7.5), 150 mM NaCl, 5 mM EDTA (pH 8.0), 50 mM NaF, 0.1% Triton X-100, and protease and phosphatase inhibitors (Moroz et al., 2008). Clarified supernatants obtained by centrifuging the samples at 14,000 × g for 15 min were used in direct-binding ELISAs to measure immunoreactivity to insulin, insulin receptor (insulin R), insulin-like growth factor-1 (IGF-1), IGF-1R, insulin-like growth factor-2 (IGF-2), IGF-2R, NGF, BDNF, NT3, neurotrophin-4 (NT4), Trk, tau, pTau, transforming growth factor-beta 1 (TGF-β1), TGF- β1R, and HNE (Tong et al., 2009). The functions of each factor are summarized in Table 1.
Table 1.
Trophic and Stress Factors and Their Functions.
| Gene | Protein | CNS cell expression | Functions | Disease correlation |
|---|---|---|---|---|
| Nerve growth factor (NGF) | Nerve growth factor (NGF) | Neuron | • Produced in the cortex and hippocampus• Involved in the survival, growth, and maintenance of sensory and sympathetic fibers (i.e., trigeminal dorsal root neurons)• Required for the maturation of cholinergic-hippocampal interaction | Decreased cholinergic activity in AD basal forebrain |
| Brain-derived neurotrophic factor (BDNF) | Brain-derived neurotrophic factor (BDNF) | Neuron | • Important in the survival and maintenance of sensory neurons, retinal ganglion, some cholinergic neurons, spinal motor neurons, and some dopaminergic neurons• Involved in synaptic plasticity (long-term potentiation) | Decreased in AD, PD, HD, ALS |
| Sortilin 1 (SORT1) | Neurotrophin-3 (NT3) | Neuron, oligodendrocyte | • Required for the normal development of oligodendrocytes• Involved in synaptic plasticity• Promotes the survival of visceral and proprioceptive sensory neurons | |
| Neurotrophin-4 (NTF4) | Neurotrophin-4 (NT4) | Neuron | • Required for the survival of peripheral sensory neurons but not sympathetic or motor neurons | |
| Neurotrophic receptor tyrosine kinase 1 (NTRK1) | Tropomyosin receptor kinase (Trk) | Neuron | • 3 types: TrkA, TrkB, TrkC• TrkA binds NGF• TrkB binds BDNF and NT4• TrkC binds NT3• TrkA found in the cholinergic basal forebrain, striatum, and hippocampus• TrkB and TrkC found in the cholinergic basal forebrain but more widely distributed• Can associate with the pan neurotrophin receptor, p75NTR | Decreased TrkB in AD |
| 4-hydroxynonenal (HNE) | • End product of lipid peroxidation• Increased expression during oxidative stress• Secondary signaling molecule that regulates cell death, growth, and differentiation | |||
| INS | Insulin | • Peptide hormone secreted by the beta islets cells of the pancreas in response to a rise in blood glucose• Promotes the uptake of glucose into muscle and adipose tissue for anabolic purposes (glycolysis and glycogen synthesis) | Insulin resistance in AD | |
| Insulin-like growth factor-1 (IGF-1) | Insulin-like growth factor-1 (IGF-1) | • Protein released by the liver in response to growth hormone stimulation• Regulates cell growth and differentiation | Increased in PD |
Note. AD = Alzheimer’s disease; ALS = amyotrophic lateral sclerosis; CNS = central nervous system; HD = Huntington’s disease; PD = Parkinson’s disease.
Four replicate protein samples (100 ng/50 µl) were adsorbed to the well bottoms (96-well Nunc MaxiSorp plates) by overnight incubation at 4°C and then blocked for 3 hr with Superblock (TBS). After washing, the samples were incubated with primary antibody (0.1–0.4 µg/ml) for 1 hr at 37°C. Immunoreactivity was detected with horseradish peroxidase-conjugated secondary antibody and Amplex UltraRed soluble fluorophore. Fluorescence intensity was measured (Ex 565 nm/Em 595 nm) in a SpectraMax M5 microplate reader (Molecular Devices, Sunnyvale, CA, USA). Binding specificity was determined from parallel control reactions with primary or secondary antibodies omitted. Immunoreactivity was normalized to protein content in parallel wells as determined with the NanoOrange Protein Quantification Kit. Protein quantification checks were performed to adjust for any variability in loading that may have occurred with robotic dilution of the stock sample homogenates.
Quality Assurance
The studies and data analyses were performed under code and repeated twice to demonstrate reproducibility of results. The animal source, nature, commercial source, and RRID number for all immunodetection reagents are provided in Supplementary Table 1. Antibody specificity was further assessed by measuring immunoreactivity in control studies in which the primary antibodies were omitted, or serially diluted to demonstrate progressive binding extinction. The election to use ELISAs rather than Western blotting to measure immunoreactivity in postmortem human tissues was based on the impracticality of simultaneously analyzing large numbers of cases on a single membrane, together with concerns about variable postmortem artefacts and disease states associated with oxidative stress leading to artificial declines in the expression of full-length proteins, despite preservation of epitopes.
Statistical Analysis
Data depicted in box plots reflect group medians (horizontal bar), 95% confidence interval limits (upper and lower box limits), and range (whiskers). Data were analyzed using GraphPad Prism 7 (GraphPad Inc., San Diego, CA). Intergroup comparisons were made by analysis of variance (ANOVA) with post hoc repeated measures Tukey tests and Student t tests. Significant p values are shown within the graph panels.
Results
Human Subject Groups
The study included 13 controls and 11 patients with FTLD. Diagnoses were confirmed in all cases by postmortem examination of the brain. Although the controls were somewhat older, the mean ages of the control and FTLD groups did not differ significantly (Table 2). Ten of the 11 FTLD cases were male compared with 8 of 13 in the control group. Mean postmortem intervals and brain pH were similar between the FTLD and control cases. None of the FTLD cases had identified genetic mutations linked to familial forms of the disease.
Table 2.
Subject Characteristics.
| Group | No. of cases | Age | Sex | PMI (hr) | pH | Brain wt (g) |
|---|---|---|---|---|---|---|
| FTLD | 11 | 69.6 ± 11.2 | 10 M, 1 F | 5.3 ± 3.6 | 6.54 ± 0.28 | 1,194.3 ± 125.1 |
| Control | 13 | 77.7 ± 12.1 | 8 M, 5 F | 5.2 ± 4.6 | 6.66 ± 0.2 | 1,251.7 ± 141.3 |
Note. FTLD = frontotemporal lobar degeneration; PMI = postmortem interval.
Phosphorylated Tau
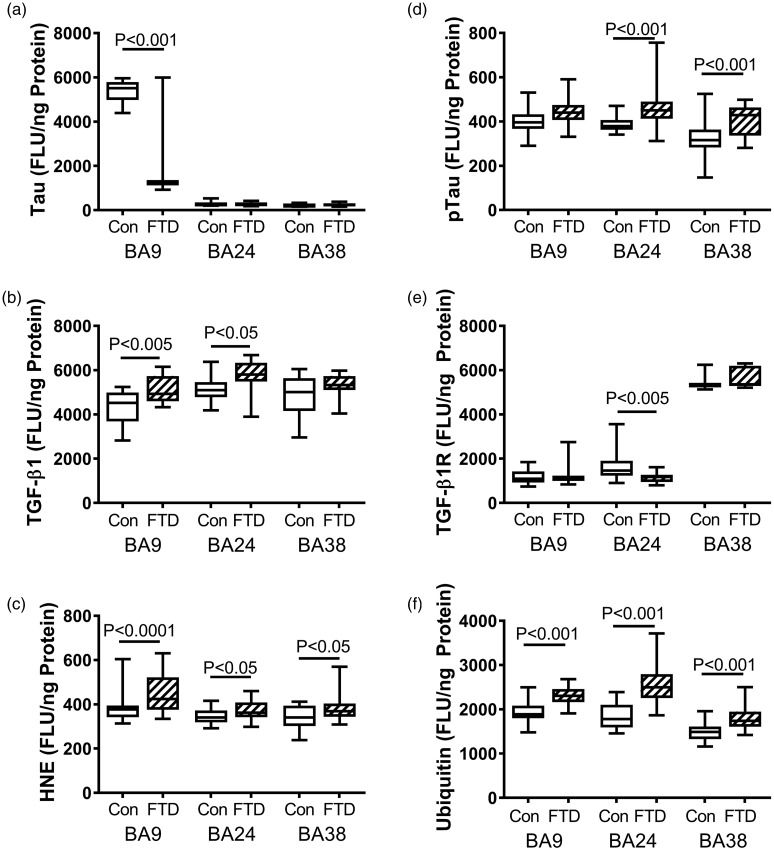
Tau is a microtubule-associated protein that stabilizes the cytoskeleton and helps mediate dynamic cellular processes such as transport and cell division via physiological cycles of phosphorylation and dephosphorylation. However, aberrant phosphorylation of tau results in aggregation of the fibrils, ubiquitination of the protein, and activation of the UPR which triggers oxidative stress, neuronal dysfunction, and death. Elevated levels of pTau and protein ubiquitination are consistent neuropathologic features of FTLD. The ELISA studies demonstrated significantly reduced mean levels of Tau in the BA8/9 region of FTLD brains, but levels similar to control in the other two brain regions examined (Figure 1(a)). The mean levels of pTau were significantly higher in the BA24 and BA38 but not BA8/9 regions of FTLD brains relative to control (Figure 1(d)). Correspondingly, the mean levels of ubiquitin immunoreactivity were significantly higher in all three regions of FTLD brains relative to control (Figure 1(f)).
Figure 1.
FTD-associated changes in markers of oxidative stress. Fresh-frozen postmortem microdissected control (n = 13) and FTD (n = 11) human brain samples from BA 8/9 (prefrontal), BA24 (anterior cingulate), and BA38 (temporal pole) were used to measure (a) Tau, (b) TGF-β1, (c) HNE, (d) pTau, (e) TGF-β1 receptor (TGF-β1R), and (f) ubiquitin by direct-binding ELISAs with results normalized to protein concentration. Levels of immunoreactivity were measured in arbitrary FLU. Box plots depict mean (horizontal bar), 95% confidence interval limits (upper and lower limits of the boxes), and range (stems). Data were analyzed by two-way ANOVA with the Tukey post hoc significance test. Significant between-group differences are indicated within the panels.
BA = Brodmann’s area; FLU = fluorescence light unit; FTD = frontotemporal lobar degeneration; HNE = 4-hydroxynonenal; pTau = phosphorylated Tau; TGF-β1 = transforming growth factor-beta 1.
Indices of Neuroinflammation and Oxidative Stress
TGF-β1 is a proinflammatory cytokine and known mediator of neuroinflammation in various neurodegenerative diseases, including AD (Masliah et al., 2001). TGF-β1 receptor is a membrane-bound serine/threonine protein kinase receptor that interacts with TGF superfamily signaling ligands, transducing growth and cytokine functions (Saarma and Sariola, 1999). The studies demonstrated elevated mean levels of TGF-β1 in all three regions of FTLD brains, although the intergroup differences were significant only for BA8/9 and BA24 (Figure 2(b)). The mean level of TGF-β1 receptor expression was modestly but significantly lower in the BA24 region of FTLD brains, whereas in BA8/9 and BA38, the expression levels were similar in the FTLD and control groups.
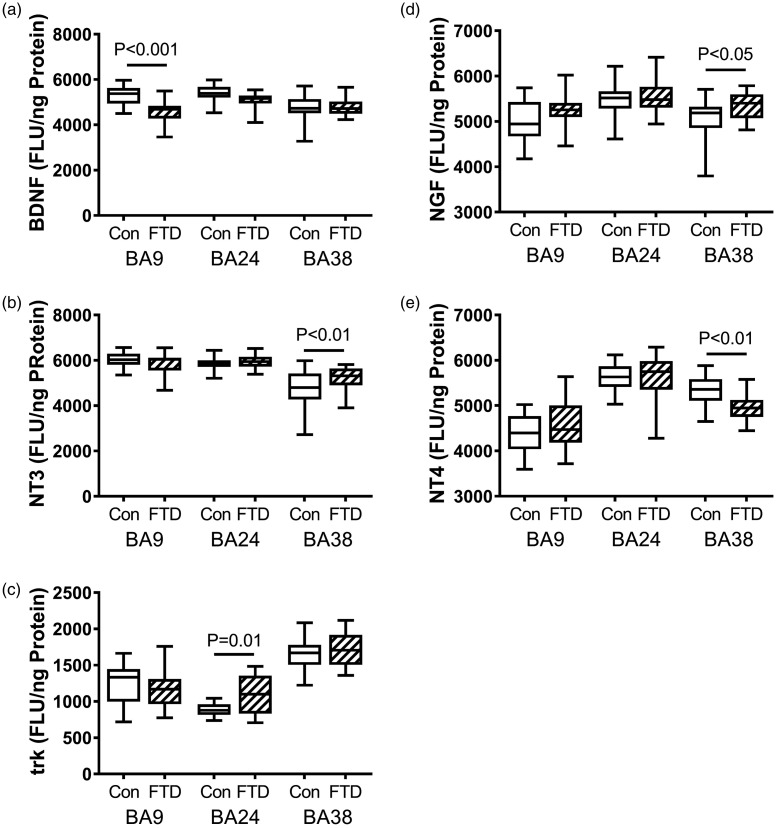
Figure 2.
FTD-associated regional alterations in neurotrophin and neurotrophin receptor immunoreactivity. Fresh-frozen postmortem microdissected control (n = 13) and FTD (n = 11) human brain samples from BA 8/9 (prefrontal), BA24 (anterior cingulate), and BA38 (temporal pole) were used to measure (a) BDNF, (b) NT3, (c) Trk, (d) NGF, and (e) NT4 by direct-binding ELISAs with results normalized to protein concentration. Levels of immunoreactivity were measured in arbitrary FLU. Box plots depict mean (horizontal bar), 95% confidence interval limits (upper and lower limits of the box), and range (stems). Data were analyzed by two-way ANOVA with the Tukey post hoc significance test. Significant between-group differences are indicated within the panels.
BA = Brodmann’s area; BDNF = brain-derived neurotrophic factor; FLU = fluorescence light unit; FTD = frontotemporal lobar degeneration; NGF = nerve growth factor; NT3 = neurotrophin-3; NT4 = neurotrophin-4; Trk = tropomyosin receptor kinase.
Neuroinflammation and oxidative stress disrupt membrane integrity leading to lipid peroxidation. Elevated levels of HNE mark lipid peroxidation in various neurodegenerative diseases, including AD and DLBD (Tong et al., 2009; Shoeb et al., 2014). In FTLD, the mean levels of HNE immunoreactivity were significantly elevated relative to control in all three regions (Figure 3(f)).
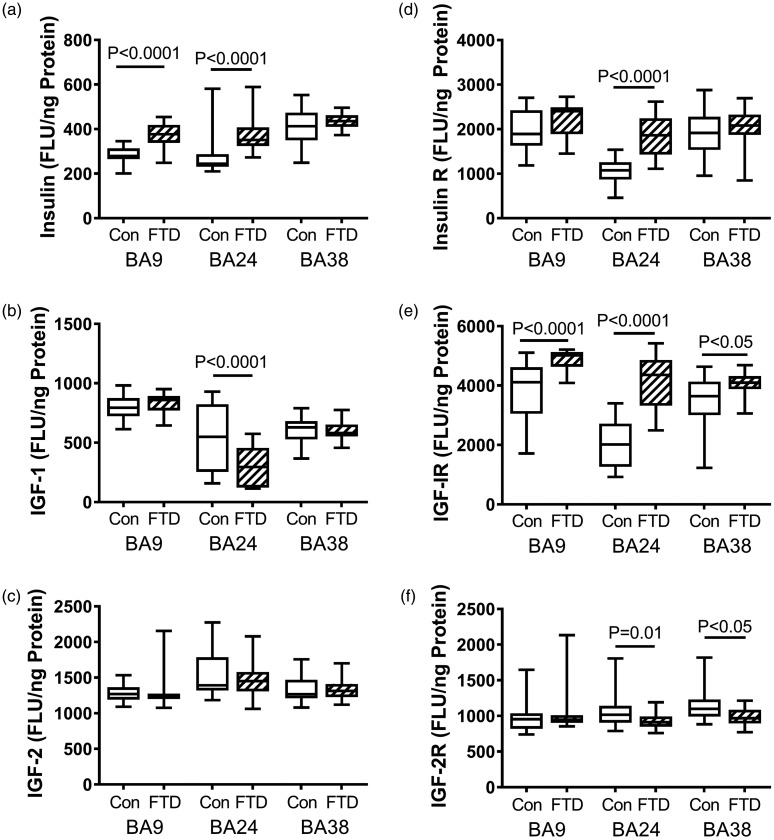
Figure 3.
FTD-associated regional changes in insulin/IGF trophic factors and receptors. Fresh-frozen postmortem microdissected control (n = 13) and FTD (n = 11) human brain samples from BA 8/9 (prefrontal), BA24 (anterior cingulate), and BA38 (temporal pole) were used to measure (a) insulin, (b) IGF-1, (c) IGF-2, (d) insulin receptor, (e) IGF-1 receptor, and (f) IGF-2 receptor by direct-binding ELISAs with results normalized to protein concentration. Levels of immunoreactivity were measured in arbitrary FLU. Box plots depict mean (horizontal bar), 95% confidence interval limits (upper and lower limits of the boxes), and range (stems). Data were analyzed by two-way ANOVA with the Tukey post hoc significance test. Significant between-group differences are indicated within the panels.
BA = Brodmann’s area; FLU = fluorescence light unit; FTD = frontotemporal lobar degeneration; IGF-1 = insulin-like growth factor-1; IGF-2 = insulin-like growth factor-2.
Neurotrophins and Receptor
We measured brain levels of four neurotrophins, including BDNF (Figure 2(a)), NGF (Figure 2(d)), NT3 (Figure 2(b)), and NT4 (Figure 2(e)), and the neurotrophin receptor, Trk (Figure 2(c)). Neurotrophins promote growth and survival of neurons and glia, and neurotrophin receptors transmit intracellular signals that drive related responses to trophic factor stimulation. In FTLD, BDNF expression was significantly reduced in BA8/9, and NT4 was significantly reduced in BA38, whereas NGF and NT3 were significantly increased in BA38. Otherwise, the mean levels of neurotrophin expression were similar in control and FTLD samples. Trk expression was modestly but significantly elevated in BA24 of FTLD brains but similar to control in BA8/9 and BA38 (Figure 2(e)).
Insulin, IGFs, and Receptors
Insulin, IGF-1 and IGF-2 are regulators of cell growth and metabolism with distinct mechanisms of action. Insulin works to decrease blood glucose by increasing the cell’s permeability to sugars, while IGFs regulate the cell cycle in response to trophic factor stimulation. Insulin deficiency in the brain is a feature of AD (Rivera et al., 2005; Steen et al., 2005; de la Monte, 2017), whereas systemic insulin deficiency is fundamental to Type 1 diabetes mellitus. In contrast, elevated levels of insulin mark states of insulin resistance, as occurs in Type 2 diabetes mellitus. In brains with FTLD, the mean levels of insulin immunoreactivity were significantly elevated in the anterior frontal (BA8/9) and cingulate (BA24) regions but not significantly changed relative to control in the temporal pole (BA38; Figure 3(a)). In contrast, IGF-1 immunoreactivity was significantly reduced in BA24 of FTLD brains (Figure 3(b)). Otherwise, the regional levels of IGF-1 and IGF-2 expression were similar in FTLD and control samples (Figure 3(b) and (c)).
Insulin receptor expression was significantly elevated relative to control in BA24 but not BA8/9 or BA38 (Figure 3(d)). The mean levels of IGF-1 receptor expression were significantly higher in BA8/9, BA24, and BA38 of FTLD relative to control samples (Figure 3(e)). In contrast, IGF-2 receptor expression was modestly but significantly reduced in the BA24 and BA38 regions of FTLD brains, whereas the mean levels of IGF-2 receptor immunoreactivity were similar in BA8/9 of control and FTLD samples (Figure 3(f)).
Discussion
The primary goal of this work was to determine if FTLD is associated with significant alterations in the expression of insulin, IGFs, and their corresponding receptors. The working hypothesis is that in FTLD, like AD (Rivera et al., 2005; Craft, 2009; de la Monte et al., 2009; Talbot et al., 2012; de la Monte, 2014; Talbot, 2014) and Parkinson Disease/DLBD (Clarkson et al., 2001; Giovannone et al., 2009; Tong et al., 2009; Godau et al., 2010; Picillo et al., 2013; Athauda and Foltynie, 2016; Ayadi et al., 2016), impairments in brain insulin and IGF signaling mechanisms mediate brain metabolic dysfunction, together with increased phosphorylation of Tau, neuroinflammation, oxidative stress, neuronal loss, and impaired neuronal plasticity (de la Monte and Wands, 2005). Furthermore, all these abnormalities have been linked to insulin/IGF deficiencies or resistances in humans and experimental models with neurodegeneration (de la Monte and Wands, 2005; Craft, 2009; de la Monte, 2012; Craft et al., 2013; de la Monte, 2014; Talbot, 2014). A potential limitation of this study is that the sample sizes were relatively small due to limited availability of well-characterized cases in the brain bank. The calculated power for alpha = .05 was .72, which is somewhat below the standard power desired of .80 and therefore could account for differences in gene expression that were graphically evident but did not reach statistical significance.
The study evaluated three brain regions targeted by FTLD including BA8/9 in the prefrontal region, BA24 in the anterior cingulate region of the frontal lobe, and BA38 within the temporal pole. BA8/9 is important for executive functions, attention, planning of motor activity, and working memory. BA24 modulates executive functions, basic instinct, motivation, and reward pathways via limbic circuitry. BA38 has functional roles in language usage, including semantic processing, speech comprehension, and naming. Therefore, the clinical features/deficits in FTLD are associated with neurodegeneration within these brain regions. Correspondingly, 18F-fluorodeoxyglucose-positron emission tomography studies revealed pronounced brain hypometabolism overlapping with atrophy of these same brain regions in FTLD (Jeong et al., 2005a, 2005b; Poljansky et al., 2011; Renard et al., 2011), helping to distinguish FTLD from AD and DLBD by noninvasive methods (Brown et al., 2014).
In the present study, the brains with FTLD had significantly elevated mean levels of pTau, ubiquitin, TGF-β1, and HNE, reflecting known neuropathologic processes including aberrantly increased Tau phosphorylation, protein ubiquitination, neuroinflammation, and oxidative stress with lipid peroxidation product accumulations (Martinez et al., 2008). Although the degree of increased pTau in BA8/9 did not reach statistical significance, in that same region, the mean level of total Tau was strikingly reduced. Therefore, the relative levels of Tau phosphorylation were likely elevated and probably to greater extents in BA8/9 than in BA24 and BA38. Reduced levels of Tau expression have been reported in association with late stages of human neurodegenerative diseases (Lowe, 1998) and could reflect underlying severe atrophy with fiber loss and synaptic disconnection.
The higher levels of ubiquitin were anticipated because all forms of FTLD exhibit increased ubiquitin immunoreactivity (Attems and Jellinger, 2013). Mechanistically, ubiquitin accumulation in FTLD is mediated by protein misfolding and aggregation, UPR activation of ubiquitin ligase, and attendant ubiquitin tagging of misfolded proteins such as pTau (Martinez et al., 2008; Attems and Jellinger, 2013). Alternatively, mutations in the ubiquitin gene can lead to misfolding and accumulation of ubiquitin (Tong et al., 2012). Because none of the cases were familial in etiology, the increased levels of ubiquitin immunoreactivity were most likely due to an accumulation of aberrantly pTau or other unknown proteins. Activation of the ubiquitin pathway leads to increased oxidative stress and neuroinflammation. Correspondingly, HNE immunoreactivity was significantly increased in all three brain regions studied. In addition, TGF-β1, which can function as a proinflammatory cytokine (Zheng et al., 2016), was upregulated in BA8/9 and BA24. Although this response provides evidence of chronic neuroinflammation in FTLD, the full spectrum of proinflammatory responses was not evaluated. Previous reports have demonstrated that neurodegeneration in FTLD is associated with activation of several other proinflammatory cytokines and chemokines (Blasko et al., 2006). However, differential regional expression/activation of specific cytokines and chemokines could account for the nonsignificant elevation of TGF-β1 in BA38, despite the presence of neuroinflammation. The fact that TGF-β1 receptor expression was not elevated in FTLD indicates that the neuroinflammatory responses to TGF-β1 as well as other ligands within the superfamily are likely mediated by cytokine activation rather than increased signal transduction via upregulated expression of the receptor.
Alterations in neurotrophin or neurotrophin receptor expression were mainly detected in BA38. The significant reduction in BDNF expression in BA8/9 overlaps with the presence of severely reduced Tau expression, together with increased ubiquitin, TGF-β1, and HNE immunoreactivities. BDNF is a neuroprotective and neurotrophic peptide essential for learning, memory, and metabolism and guards against neurobehavioral deficits, impairments in synaptic integrity, and neuronal loss (Corse et al., 1999; Almli et al., 2000; Lynch et al., 2007; Marosi and Mattson, 2014). Its significant inhibition in AD correlates with neurofibrillary tangle accumulation and amyloid-beta neurotoxicity (Jiao et al., 2016). Although experimental data suggest that Tau overexpression inhibits BDNF (Rosa et al., 2016), in FTLD, this phenomenon could not account for the significant reductions in BDNF because Tau expression was not elevated. However, Tau pathology can disrupt BDNF signaling through Trk and attendant activity-dependent secretion of BDNF (Mazzaro et al., 2016). Although our finding of reduced BDNF in BA8/9 is discordant with results in a previous report (Ferrer et al., 2000), the authors’ use of Western blotting was less sensitive than the ELISAs employed in the present study.
The other three neurotrophins measured, NGF, NT3, and NT4, were significantly modulated in BA38 and not in the BA8/9 or BA24 frontal lobe regions. While NGF and NT3 expression were significantly increased, NT4, also known as neurotrophin-5 or neurotrophin-4/5, was significantly reduced. NT3, NGF, and BDNF mainly signal through TrkA, whereas NT4 signals through TrkB (Huang and Reichardt, 2001; Chen et al., 2008). NT3 also signals neuroprotective effects through TrkC (Pinon et al., 1996; Yamauchi et al., 2005). The TrkA receptor is high affinity, whereas the TrkB receptor is low affinity. Because there is no apparent overlap between NT4 and other neurotrophins and TrkA and TrkB are distinct, the reduced levels of NT4 would not likely have been compensated for by upregulation of NT3 or NGF.
Although the modest but significant increases in NT3 and NGF and attendant activation of TrkA may have compensated for the reduced levels of BDNF, the latter was observed in BA8/9 and not in BA38 where NT3, NT4, and NGF were significantly modulated. Moreover, the impact of reduced NT4 expression in FTLD is uncertain because genetic depletion of the corresponding gene produces subtle cellular abnormalities and a normal adult phenotype (Pollock et al., 2003). The increased mean level of NT3 may represent a compensatory response because previous studies demonstrated upregulation of NT3 following ischemic injury to the brain (Chung et al., 2017). NGF, which regulates growth, proliferation, survival, and homeostasis of neurons (Allen et al., 2013), is upregulated and probably dysregulated in AD (Hefti, 1994; Scott et al., 1995; Allen et al., 2013). Higher cortical and subcortical levels of NGF have been attributed to impaired retrograde transport to various regions with attendant deprivation of NGF-stimulated blood flow and episodic memory (Scott et al., 1995). The significantly elevated NGF expression in BA38 may have similar consequences in FTLD.
The finding of higher mean levels of insulin expression, with or without upregulation of the insulin receptor in BA8/9 and BA24, provides new evidence for brain (frontal lobe) insulin resistance in FTLD and illustrates that brain metabolic dysfunction linked to impaired insulin signaling represents a feature shared in common with AD and DLBD (Tong et al., 2009; Athauda and Foltynie, 2016; de la Monte, 2017). However, the absence of altered insulin or insulin receptor expression in the FTLD temporal lobe (BA38) helps distinguish the nature of underlying metabolic dysfunctions in FTLD from AD. Because insulin signaling plays vital roles in synaptic plasticity needed for learning and memory, neuroprotection, cellular homeostasis, and energy metabolism, many of the clinical and neuropathologic frontal lobe-related abnormalities in FTLD may be attributable to insulin resistance. Furthermore, increased oxidative stress with activation of the UPR leading to neuroinflammation, aberrant tau phosphorylation, and protein ubiquitination in FTLD may also be mediated by impaired downstream signaling through PI3K-Akt pathways that regulate metabolism and enable activation of GSK-3β as occurs in AD (Duarte et al., 2012).
FTLD brains also exhibited reduced levels of IGF-1 expression in BA24, indicating IGF-1 deficiency in the anterior cingulate. Furthermore, the increased expression of IGF-1 receptor in BA8/9, BA24, and BA38 in the setting of normal IGF-1 expression reflects IGF-1 resistance in both the frontal and temporal lobes. In the brain, insulin and IGF-1 activate their own receptors but signal through highly related pathways beginning with the insulin receptor substrate docking protein that transmits signals downstream through Erk-MAPK and PI3K-Akt pathways (de la Monte and Wands, 2005). Like insulin, IGF-1 has neurotrophic and neuroprotective effects; however, IGF-1 has a more dominant role in promoting growth (de la Monte and Wands, 2005). In postmitotic neurons, IGF-1 stimulates growth of neuritic processes and cell fibers needed to establish and maintain connections and mediate plasticity (de la Monte and Wands, 2005). Another key role of IGF-1 is to support myelin maintenance/integrity, survival, and function of oligodendrocytes (de la Monte, 2012). Inhibition of IGF-1 signaling due to receptor resistance may be an important factor contributing to the severe white matter atrophy with myelin and axonal loss in FTLD.
Finally, IGF-2 expression was unchanged in FTLD, but IGF-2 receptor expression was significantly reduced in BA24 and BA38, which are both interconnected with the limbic circuit. Reduced levels of IGF-2 receptor also occur in AD, PD, and DLBD, particularly in the late stages of disease (Rivera et al., 2005; Steen et al., 2005; Tong et al., 2009; de la Monte, 2017). IGF-2 receptor, like insulin and IGF-1, can activate PI3K-Akt pathways (Hale et al., 2013; Mu et al., 2017). Therefore, reduced expression of IGF-2 receptors could limit activation of those pathways. The IGF-2R, also called, “cation independent mannose-6-phosphate receptor,” is multifunctional and binds to IGF-2 at the cell surface, and mannose-6-phosphate-tagged proteins in the trans-Golgi network (Brown et al., 2009). IGF-2R depletion leads to motor and speech deficits and language delays. Therefore, language and motor impairments associated with FTLD, including variants with motor neuron disease, could be mediated by inhibition of IGF-2R expression (Peter et al., 2017). Included among IGF-2R’s roles are its ability to (a) sequester IGF-2 and regulate growth, particularly during development (Brown et al., 2009); (b) activate TGF-β1 (Leksa et al., 2005); (c) be imprinted by genomic methylation and regulate expression or silencing of the maternal or paternal allele (Delaval and Feil, 2004); and (d) respond to stimulation by insulin or IGF-1 (Heidenreich, 1993; Dore et al., 2000). Among these features, the effects of imprinting and late-life deimprinting may be highly relevant to neurodegeneration because methylation/demethylation and attendant alterations in gene expression and function are influenced by aging and environmental exposures (Zampieri et al., 2015), that is, factors that have the potential to drive occurrences of sporadic forms of neurodegeneration, including FTLD.
Altogether, the findings herein support the hypothesis that in FTLD, like AD, PD, and DLBD, dysregulated insulin/IGF signaling in brain regions targeted by neurodegeneration mediate brain metabolic dysfunction and contribute to disease pathogenesis. One exciting aspect of these observations and conclusions is that like AD and PD, it may be possible to repurpose antidiabetes agents such as insulin and analogues of the glucagon-like peptide 1 incretin (Holscher, 2014a, 2014b; Biessels and Reagan, 2015; de la Monte et al., 2017) to treat FTLD, including its varied cytopathologies linked to metabolic dysfunction, similar to therapeutic concepts currently emerging for AD (de la Monte, 2017; de la Monte et al., 2017; Clarke et al., 2018).
Supplemental Material
Supplemental Material for Altered Brain Expression of Insulin and Insulin-Like Growth Factors in Frontotemporal Lobar Degeneration: Another Degenerative Disease Linked to Dysregulation of Insulin Metabolic Pathways by Connie J. Liou, Ming Tong, Jean P. Vonsattel and Suzanne M. de la Monte in ASN Neuro
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by AA-11431, NS-096525, and HD-057100 from the National Institute on Alcohol Abuse and Alcoholism of the National Institutes of Health.
References
- Allen S. J., Watson J. J., Shoemark D. K., Barua N. U., Patel N. K. (2013). GDNF, NGF and BDNF as therapeutic options for neurodegeneration. Pharmacol Ther, 138, 155–175. [DOI] [PubMed] [Google Scholar]
- Almli C. R., Levy T. J., Han B. H., Shah A. R., Gidday J. M., Holtzman D. M. (2000). BDNF protects against spatial memory deficits following neonatal hypoxia-ischemia. Exp Neurol, 166, 99–114. [DOI] [PubMed] [Google Scholar]
- Arenas E., Persson H. (1994). Neurotrophin-3 prevents the death of adult central noradrenergic neurons in vivo. Nature, 367, 368–371. [DOI] [PubMed] [Google Scholar]
- Athauda D., Foltynie T. (2016). Insulin resistance and Parkinson’ s disease: A new target for disease modification? Prog Neurobiol, 145–146, 98–120. [DOI] [PubMed] [Google Scholar]
- Attems J., Jellinger K. (2013). Neuropathological correlates of cerebral multimorbidity. Curr Alzheimer Res, 10, 569–577. [DOI] [PubMed] [Google Scholar]
- Ayadi A. E., Zigmond M. J., Smith A. D. (2016). IGF-1 protects dopamine neurons against oxidative stress: Association with changes in phosphokinases. Exp Brain Res, 234, 1863–1873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bang J., Spina S., Miller B. L. (2015). Frontotemporal dementia. Lancet, 386, 1672–1682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennion-Callister J., Pickering-Brown S. M. (2014). Pathogenesis/genetics of frontotemporal dementia and how it relates to ALS. Exp Neurol, 262(Pt B), 84–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biessels G. J., Reagan L. P. (2015). Hippocampal insulin resistance and cognitive dysfunction. Nat Rev Neurosci, 16, 660–671. [DOI] [PubMed] [Google Scholar]
- Blasko I., Lederer W., Oberbauer H., Walch T., Kemmler G., Hinterhuber H., Marksteiner J., Humpel C. (2006). Measurement of thirteen biological markers in CSF of patients with Alzheimer’s disease and other dementias. Dement Geriatr Cogn Disord, 21, 9–15. [DOI] [PubMed] [Google Scholar]
- Blass J. P., Gibson G. E., Hoyer S. (2002). The role of the metabolic lesion in Alzheimer’s disease. J Alzheimers Dis, 4, 225–232. [DOI] [PubMed] [Google Scholar]
- Brown J., Jones E. Y., Forbes B. E. (2009). Interactions of IGF-II with the IGF2R/cation-independent mannose-6-phosphate receptor mechanism and biological outcomes. Vitam Horm, 80, 699–719. [DOI] [PubMed] [Google Scholar]
- Brown R. K., Bohnen N. I., Wong K. K., Minoshima S., Frey K. A. (2014). Brain PET in suspected dementia: Patterns of altered FDG metabolism. Radiographics, 34, 684–701. [DOI] [PubMed] [Google Scholar]
- Chao M. V., Lee F. S. (2004). Neurotrophin survival signaling mechanisms. J Alzheimers Dis, 6, S7–S11. [DOI] [PubMed] [Google Scholar]
- Chen L. W., Yung K. K., Chan Y. S., Shum D. K., Bolam J. P. (2008). The proNGF-p75NTR-sortilin signalling complex as new target for the therapeutic treatment of Parkinson’s disease. CNS Neurol Disord Drug Targets, 7, 512–523. [DOI] [PubMed] [Google Scholar]
- Chene G., Beiser A., Au R., Preis S. R., Wolf P. A., Dufouil C., Seshadri S. (2015). Gender and incidence of dementia in the Framingham Heart Study from mid-adult life. Alzheimers Dement, 11, 310–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung J. Y., Kim M. W., Im W., Hwang I. K., Bang M. S., Kim M. (2017). Expression of neurotrophin-3 and trkC following focal cerebral ischemia in adult rat brain with treadmill exercise. Biomed Res Int, 2017, 9248542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke J. R., Ribeiro F. C., Frozza R. L., De Felice F. G., Lourenco M. V. (2018). Metabolic dysfunction in Alzheimer’ s disease: From basic neurobiology to clinical approaches. J Alzheimers Dis, 64, S405–S426. [DOI] [PubMed] [Google Scholar]
- Clarkson E. D., Zawada W. M., Bell K. P., Esplen J. E., Choi P. K., Heidenreich K. A., Freed C. R. (2001). IGF-I and bFGF improve dopamine neuron survival and behavioral outcome in parkinsonian rats receiving cultured human fetal tissue strands. Exp Neurol, 168, 183–191. [DOI] [PubMed] [Google Scholar]
- Corse A. M., Bilak M. M., Bilak S. R., Lehar M., Rothstein J. D., Kuncl R. W. (1999). Preclinical testing of neuroprotective neurotrophic factors in a model of chronic motor neuron degeneration. Neurobiol Dis, 6, 335–346. [DOI] [PubMed] [Google Scholar]
- Coyle-Gilchrist I. T., Dick K. M., Patterson K., Vazquez Rodriquez P., Wehmann E., Wilcox A., Lansdall C. J., Dawson K. E., Wiggins J., Mead S., Brayne C., Rowe J. B. (2016). Prevalence, characteristics, and survival of frontotemporal lobar degeneration syndromes. Neurology, 86, 1736–1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craft S. (2009). The role of metabolic disorders in Alzheimer disease and vascular dementia: Two roads converged. Arch Neurol, 66, 300–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craft S., Cholerton B., Baker L. D. (2013). Insulin and Alzheimer’s disease: Untangling the web. J Alzheimers Dis, 33(Suppl 1), S263–S275. [DOI] [PubMed] [Google Scholar]
- de la Monte S. M. (2012). Triangulated mal-signaling in Alzheimer’ s disease: Roles of neurotoxic ceramides, ER stress, and insulin resistance reviewed. J Alzheimers Dis, 30(Suppl 2), S231–S249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de la Monte S. M. (2014). Type 3 diabetes is sporadic Alzheimer’s disease: Mini-review. Eur Neuropsychopharmacol, 24, 1954–1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de la Monte S. M. (2017). Insulin resistance and neurodegeneration: Progress towards the development of new therapeutics for Alzheimer’s disease. Drugs, 77, 47–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de la Monte S. M., Longato L., Tong M., Wands J. R. (2009). Insulin resistance and neurodegeneration: Roles of obesity, type 2 diabetes mellitus and non-alcoholic steatohepatitis. Curr Opin Investig Drugs, 10, 1049–1060. [PMC free article] [PubMed] [Google Scholar]
- de la Monte S. M., Tong M. (2014). Brain metabolic dysfunction at the core of Alzheimer’s disease. Biochem Pharmacol, 88, 548–559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de la Monte S. M., Tong M., Schiano I., Didsbury J. (2017). Improved brain insulin/IGF signaling and reduced neuroinflammation with T3D-959 in an experimental model of sporadic Alzheimer’s disease. J Alzheimers Dis, 55, 849–864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de la Monte S. M., Wands J. R. (2005). Review of insulin and insulin-like growth factor expression, signaling, and malfunction in the central nervous system: Relevance to Alzheimer’s disease. J Alzheimers Dis, 7, 45–61. [DOI] [PubMed] [Google Scholar]
- Delaval K., Feil R. (2004). Epigenetic regulation of mammalian genomic imprinting. Curr Opin Genet Dev, 14, 188–195. [DOI] [PubMed] [Google Scholar]
- Dore S., Kar S., Zheng W. H., Quirion R. (2000). Rediscovering good old friend IGF-I in the new millenium: Possible usefulness in Alzheimer’ s disease and stroke. Pharm Acta Helv, 74, 273–280. [DOI] [PubMed] [Google Scholar]
- Duarte A. I., Moreira P. I., Oliveira C. R. (2012). Insulin in central nervous system: More than just a peripheral hormone. J Aging Res, 2012, 384017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erkkinen M. G., Kim M. O., Geschwind M. D. (2018). Clinical neurology and epidemiology of the major neurodegenerative diseases. Cold Spring Harb Perspect Biol, 10, pii: a033118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrer I., Marin C., Rey M. J., Ribalta T. (2000). Brain-derived neurotrophic factor in patients with frontotemporal dementia. Neurosci Lett, 279, 33–36. [DOI] [PubMed] [Google Scholar]
- Giovannone B., Tsiaras W. G., de la Monte S., Klysik J., Lautier C., Karashchuk G., Goldwurm S., Smith R. J. (2009). GIGYF2 gene disruption in mice results in neurodegeneration and altered insulin-like growth factor signaling. Hum Mol Genet, 18, 4629–4639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godau J., Herfurth M., Kattner B., Gasser T., Berg D. (2010). Increased serum insulin-like growth factor 1 in early idiopathic Parkinson’ s disease. J Neurol Neurosurg Psychiatry, 81, 536–538. [DOI] [PubMed] [Google Scholar]
- Gomez-Palacio-Schjetnan A., Escobar M. L. (2013). Neurotrophins and synaptic plasticity. Curr Top Behav Neurosci, 15, 117–136. [DOI] [PubMed] [Google Scholar]
- Grunblatt E., Salkovic-Petrisic M., Osmanovic J., Riederer P., Hoyer S. (2007). Brain insulin system dysfunction in streptozotocin intracerebroventricularly treated rats generates hyperphosphorylated tau protein. J Neurochem, 101, 757–770. [DOI] [PubMed] [Google Scholar]
- Hale L. J., Welsh G. I., Perks C. M., Hurcombe J. A., Moore S., Hers I., Saleem M. A., Mathieson P. W., Murphy A. J., Jeansson M., Holly J. M., Hardouin S. N., Coward R. J. (2013). Insulin-like growth factor-II is produced by, signals to and is an important survival factor for the mature podocyte in man and mouse. J Pathol, 230, 95–106. [DOI] [PubMed] [Google Scholar]
- Hasegawa M., Arai T., Nonaka T., Kametani F., Yoshida M., Hashizume Y., Beach T. G., Buratti E., Baralle F., Morita M., Nakano I., Oda T., Tsuchiya K., Akiyama H. (2008). Phosphorylated TDP-43 in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Ann Neurol, 64, 60–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hefti F. (1994). Neurotrophic factor therapy for nervous system degenerative diseases. J Neurobiol, 25, 1418–1435. [DOI] [PubMed] [Google Scholar]
- Heidenreich K. A. (1993). Insulin and IGF-I receptor signaling in cultured neurons. Ann N Y Acad Sci, 692, 72–88. [DOI] [PubMed] [Google Scholar]
- Holscher C. (2014. a). Central effects of GLP-1: New opportunities for treatments of neurodegenerative diseases. J Endocrinol, 221, T31–T41. [DOI] [PubMed] [Google Scholar]
- Holscher C. (2014. b). Insulin, incretins and other growth factors as potential novel treatments for Alzheimer’ s and Parkinson’ s diseases. Biochem Soc Trans, 42, 593–599. [Google Scholar]
- Huang E. J., Reichardt L. F. (2001). Neurotrophins: Roles in neuronal development and function. Annu Rev Neurosci, 24, 677–736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inukai Y., Nonaka T., Arai T., Yoshida M., Hashizume Y., Beach T. G., Buratti E., Baralle F. E., Akiyama H., Hisanaga S., Hasegawa M. (2008). Abnormal phosphorylation of Ser409/410 of TDP-43 in FTLD-U and ALS. FEBS Lett, 582, 2899–2904. [DOI] [PubMed] [Google Scholar]
- Jeong Y., Cho S. S., Park J. M., Kang S. J., Lee J. S., Kang E., Na D. L., Kim S. E. (2005. a). 18F-FDG PET findings in frontotemporal dementia: An SPM analysis of 29 patients. J Nucl Med, 46, 233–239. [PubMed] [Google Scholar]
- Jeong Y., Park K. C., Cho S. S., Kim E. J., Kang S. J., Kim S. E., Kang E., Na D. L. (2005. b). Pattern of glucose hypometabolism in frontotemporal dementia with motor neuron disease. Neurology, 64, 734–736. [DOI] [PubMed] [Google Scholar]
- Jiao S. S., Shen L. L., Zhu C., Bu X. L., Liu Y. H., Liu C. H., Yao X. Q., Zhang L. L., Zhou H. D., Walker D. G., Tan J., Gotz J., Zhou X. F., Wang Y. J. (2016). Brain-derived neurotrophic factor protects against tau-related neurodegeneration of Alzheimer’ s disease. Transl Psychiatry, 6, e907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller C. E., Amaya Mdel P., Cortes E. P., Mancevska K., Vonsattel J. P. (2008). Electronic tracking of human brain samples for research. Cell Tissue Bank, 9, 217–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kertesz A., McMonagle P., Blair M., Davidson W., Munoz D. G. (2005). The evolution and pathology of frontotemporal dementia. Brain, 128, 1996–2005. [DOI] [PubMed] [Google Scholar]
- Korczyn A. D., Reichmann H. (2006). Dementia with Lewy bodies. J Neurol Sci, 248, 3–8. [DOI] [PubMed] [Google Scholar]
- Kua E. H., Ho E., Tan H. H., Tsoi C., Thng C., Mahendran R. (2014). The natural history of dementia. Psychogeriatrics, 14, 196–201. [DOI] [PubMed] [Google Scholar]
- Kumar-Singh S. (2011). Progranulin and TDP-43: Mechanistic links and future directions. J Mol Neurosci, 45, 561–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leksa V., Godar S., Schiller H. B., Fuertbauer E., Muhammad A., Slezakova K., Horejsi V., Steinlein P., Weidle U. H., Binder B. R., Stockinger H. (2005). TGF-beta-induced apoptosis in endothelial cells mediated by M6P/IGFII-R and mini-plasminogen. J Cell Sci, 118, 4577–4586. [DOI] [PubMed] [Google Scholar]
- Lowe J. (1998). Establishing a pathological diagnosis in degenerative dementias. Brain Pathol, 8, 403–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch G., Kramar E. A., Rex C. S., Jia Y., Chappas D., Gall C. M., Simmons D. A. (2007). Brain-derived neurotrophic factor restores synaptic plasticity in a knock-in mouse model of Huntington’ s disease. J Neurosci, 27, 4424–4434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marosi K., Mattson M. P. (2014). BDNF mediates adaptive brain and body responses to energetic challenges. Trends Endocrinol Metab, 25, 89–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez A., Carmona M., Portero-Otin M., Naudi A., Pamplona R., Ferrer I. (2008). Type-dependent oxidative damage in frontotemporal lobar degeneration: Cortical astrocytes are targets of oxidative damage. J Neuropathol Exp Neurol, 67, 1122–1136. [DOI] [PubMed] [Google Scholar]
- Masliah E., Ho G., Wyss-Coray T. (2001). Functional role of TGF beta in Alzheimer’s disease microvascular injury: Lessons from transgenic mice. Neurochem Int, 39, 393–400. [DOI] [PubMed] [Google Scholar]
- Mattson M. P., Pedersen W. A., Duan W., Culmsee C., Camandola S. (1999). Cellular and molecular mechanisms underlying perturbed energy metabolism and neuronal degeneration in Alzheimer’s and Parkinson’s diseases. Ann N Y Acad Sci, 893, 154–175. [DOI] [PubMed] [Google Scholar]
- Mazzaro N., Barini E., Spillantini M. G., Goedert M., Medini P., Gasparini L. (2016). Tau-driven neuronal and neurotrophic dysfunction in a mouse model of early tauopathy. J Neurosci, 36, 2086–2100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell S. L., Teno J. M., Kiely D. K., Shaffer M. L., Jones R. N., Prigerson H. G., Volicer L., Givens J. L., Hamel M. B. (2009). The clinical course of advanced dementia. N Engl J Med, 361, 1529–1538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moroz N., Tong M., Longato L., Xu H., de la Monte S. M. (2008). Limited Alzheimer-type neurodegeneration in experimental obesity and type 2 diabetes mellitus. J Alzheimers Dis, 15, 29–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mu X., Qi W., Liu Y., Zhou J., Li Y., Rong X., Lu L. (2017). IGF-II-mediated downregulation of peroxisome proliferator-activated receptor-gamma coactivator-1alpha in myoblast cells involves PI3K/Akt/FoxO1 signaling pathway. Mol Cell Biochem, 432, 199–208. [DOI] [PubMed] [Google Scholar]
- Peter B., Lancaster H., Vose C., Fares A., Schrauwen I., Huentelman M. (2017). Two unrelated children with overlapping 6q25.3 deletions, motor speech disorders, and language delays. Am J Med Genet A, 173, 2659–2669. [DOI] [PubMed] [Google Scholar]
- Petoukhov E., Fernando S., Mills F., Shivji F., Hunter D., Krieger C., Silverman M. A., Bamji S. X. (2013). Activity-dependent secretion of progranulin from synapses. J Cell Sci, 126, 5412–5421. [DOI] [PubMed] [Google Scholar]
- Picillo M., Erro R., Santangelo G., Pivonello R., Longo K., Pivonello C., Vitale C., Amboni M., Moccia M., Colao A., Barone P., Pellecchia M. T. (2013). Insulin-like growth factor-1 and progression of motor symptoms in early, drug-naive Parkinson’s disease. J Neurol, 260, 1724–1730. [DOI] [PubMed] [Google Scholar]
- Pinon L. G., Minichiello L., Klein R., Davies A. M. (1996). Timing of neuronal death in trkA, trkB and trkC mutant embryos reveals developmental changes in sensory neuron dependence on Trk signalling. Development, 122, 3255–3261. [DOI] [PubMed] [Google Scholar]
- Poljansky S., Ibach B., Hirschberger B., Manner P., Klunemann H., Hajak G., Marienhagen J. (2011). A visual [18F]FDG-PET rating scale for the differential diagnosis of frontotemporal lobar degeneration. Eur Arch Psychiatry Clin Neurosci, 261, 433–446. [DOI] [PubMed] [Google Scholar]
- Pollock G. S., Robichon R., Boyd K. A., Kerkel K. A., Kramer M., Lyles J., Ambalavanar R., Khan A., Kaplan D. R., Williams R. W., Frost D. O. (2003). TrkB receptor signaling regulates developmental death dynamics, but not final number, of retinal ganglion cells. J Neurosci, 23, 10137–10145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rascovsky K., et al. (2011). Sensitivity of revised diagnostic criteria for the behavioural variant of frontotemporal dementia. Brain, 134, 2456–2477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renard D., Collombier L., Castelnovo G., Fourcade G., Kotzki P. O., LaBauge P. (2011). Brain FDG-PET changes in ALS and ALS-FTD. Acta Neurol Belg, 111, 306–309. [PubMed] [Google Scholar]
- Rivera E. J., Goldin A., Fulmer N., Tavares R., Wands J. R., de la Monte S. M. (2005). Insulin and insulin-like growth factor expression and function deteriorate with progression of Alzheimer’s disease: Link to brain reductions in acetylcholine. J Alzheimers Dis, 8, 247–268. [DOI] [PubMed] [Google Scholar]
- Roman G., Pascual B. (2012). Contribution of neuroimaging to the diagnosis of Alzheimer’s disease and vascular dementia. Arch Med Res, 43, 671–676. [DOI] [PubMed] [Google Scholar]
- Rosa E., Mahendram S., Ke Y. D., Ittner L. M., Ginsberg S. D., Fahnestock M. (2016). Tau downregulates BDNF expression in animal and cellular models of Alzheimer’ s disease. Neurobiol Aging, 48, 135–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saarma M., Sariola H. (1999). Other neurotrophic factors: Glial cell line-derived neurotrophic factor (GDNF). Microsc Res Tech, 45, 292–302. [DOI] [PubMed] [Google Scholar]
- Scott S. A., Mufson E. J., Weingartner J. A., Skau K. A., Crutcher K. A. (1995). Nerve growth factor in Alzheimer’ s disease: Increased levels throughout the brain coupled with declines in nucleus basalis. J Neurosci, 15, 6213–6221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen L. L., Manucat-Tan N. B., Gao S. H., Li W. W., Zeng F., Zhu C., Wang J., Bu X. L., Liu Y. H., Gao C. Y., Xu Z. Q., Bobrovskaya L., Lei P., Yu J. T., Song W., Zhou H. D., Yao X. Q., Zhou X. F., Wang Y. J. (2018). The ProNGF/p75NTR pathway induces tau pathology and is a therapeutic target for FTLD-tau. Mol Psychiatry, 23, 1813–1824. [DOI] [PubMed] [Google Scholar]
- Shoeb M., Ansari N. H., Srivastava S. K., Ramana K.V. (2014). 4-Hydroxynonenal in the pathogenesis and progression of human diseases. Curr Med Chem, 21, 230–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shruthi S., Sumitha R., Varghese A. M., Ashok S., Chandrasekhar Sagar B. K., Sathyaprabha T. N., Nalini A., Kramer B. W., Raju T. R., Vijayalakshmi K., Alladi P. A. (2017). Brain-derived neurotrophic factor facilitates functional recovery from ALS-cerebral spinal fluid-induced neurodegenerative changes in the NSC-34 motor neuron cell line. Neurodegener Dis, 17, 44–58. [DOI] [PubMed] [Google Scholar]
- Song J. H., Yu J. T., Tan L. (2015). Brain-derived neurotrophic factor in Alzheimer’s disease: Risk, mechanisms, and therapy. Mol Neurobiol, 52, 1477–1493. [DOI] [PubMed] [Google Scholar]
- Steen E., Terry B. M., Rivera E. J., Cannon J. L., Neely T. R., Tavares R., Xu X. J., Wands J. R., de la Monte S. M. (2005). Impaired insulin and insulin-like growth factor expression and signaling mechanisms in Alzheimer’s disease – Is this type 3 diabetes? J Alzheimers Dis, 7, 63–80. [DOI] [PubMed] [Google Scholar]
- Talbot K. (2014). Brain insulin resistance in Alzheimer’s disease and its potential treatment with GLP-1 analogs. Neurodegener Dis Manag, 4, 31–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talbot K., Wang H. Y., Kazi H., Han L. Y., Bakshi K. P., Stucky A., Fuino R. L., Kawaguchi K. R., Samoyedny A. J., Wilson R. S., Arvanitakis Z., Schneider J. A., Wolf B. A., Bennett D. A., Trojanowski J. Q., Arnold S. E. (2012). Demonstrated brain insulin resistance in Alzheimer’ s disease patients is associated with IGF-1 resistance, IRS-1 dysregulation, and cognitive decline. J Clin Invest, 122, 1316–1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toney L. K., McCue T. J., Minoshima S., Lewis D. H. (2011). Nuclear medicine imaging in dementia: A practical overview for hospitalists. Hosp Pract (1995), 39, 149–160. [DOI] [PubMed] [Google Scholar]
- Tong J., Huang C., Bi F., Wu Q., Huang B., Zhou H. (2012). XBP1 depletion precedes ubiquitin aggregation and Golgi fragmentation in TDP-43 transgenic rats. J Neurochem, 123, 406–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong M., Dong M., de la Monte S. M. (2009). Brain insulin-like growth factor and neurotrophin resistance in Parkinson’s disease and dementia with Lewy bodies: Potential role of manganese neurotoxicity. J Alzheimers Dis, 16, 585–599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vonsattel J. P., Del Amaya M. P., Keller C. E. (2008). Twenty-first century brain banking. Processing brains for research: The Columbia University methods. Acta Neuropathol, 115, 509–532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren J. D., Rohrer J. D., Rossor M. N. (2013). Clinical review. Frontotemporal dementia. BMJ, 347, f4827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolley J. D., Strobl E. V., Shelly W. B., Karydas A. M., Robin Ketelle R. N., Wolkowitz O. M., Miller B. L., Rankin K. P. (2012). BDNF serum concentrations show no relationship with diagnostic group or medication status in neurodegenerative disease. Curr Alzheimer Res, 9, 815–821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamauchi J., Chan J. R., Miyamoto Y., Tsujimoto G., Shooter E. M. (2005). The neurotrophin-3 receptor TrkC directly phosphorylates and activates the nucleotide exchange factor Dbs to enhance Schwann cell migration. Proc Natl Acad Sci U S A, 102, 5198–5203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zampieri M., Ciccarone F., Calabrese R., Franceschi C., Burkle A., Caiafa P. (2015). Reconfiguration of DNA methylation in aging. Mech Ageing Dev, 151, 60–70. [DOI] [PubMed] [Google Scholar]
- Zanardini R., Ciani M., Benussi L., Ghidoni R. (2016). Molecular pathways bridging frontotemporal lobar degeneration and psychiatric disorders. Front Aging Neurosci, 8, 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng C., Zhou X. W., Wang J. Z. (2016). The dual roles of cytokines in Alzheimer’ s disease: Update on interleukins, TNF-alpha, TGF-beta and IFN-gamma. Transl Neurodegener, 5, 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Material for Altered Brain Expression of Insulin and Insulin-Like Growth Factors in Frontotemporal Lobar Degeneration: Another Degenerative Disease Linked to Dysregulation of Insulin Metabolic Pathways by Connie J. Liou, Ming Tong, Jean P. Vonsattel and Suzanne M. de la Monte in ASN Neuro