Short abstract
In the supraoptic nucleus (SON), the incidence of dye coupling among oxytocin (OT) neurons increases significantly in nursing mothers. However, the type(s) of connexin (Cx) involved is(are) unknown. In this study, we specifically investigated whether Cx36 plays a functional role in the coupling between OT neurons in the SON of lactating rats. In this brain region, Cx36 was mainly coimmunostained with vasopressin neurons in virgin female rats, whereas in lactating rats, Cx36 was primarily colocalized with OT neurons. In brain slices from lactating rats, application of quinine (0.1 mM), a selective blocker of Cx36, significantly reduced dye coupling among OT neurons as well as the discharge/firing frequency of spikes/action potentials and their amplitude, and transiently depolarized the membrane potential of OT neurons in whole-cell patch-clamp recordings. However, quinine significantly reduced the amplitude, but not frequency, of inhibitory postsynaptic currents in OT neurons; the duration of excitatory postsynaptic currents was reduced but not their frequency and amplitude. Furthermore, the excitatory effect of OT (1 pM) on OT neurons was significantly weakened and delayed by quinine, and burst firing was absent in the presence of this inhibitor. Lastly, Western blotting analysis revealed that the presence of combined, but not alone, quinine and OT significantly reduced the amount of Cx36 in the SON. Thus, Cx36-mediated junctional communication plays a crucial role in autoregulatory control of OT neuronal activity, likely by acting at the postsynaptic sites. The level of Cx36 is modulated by its own activity and the presence of OT.
Keywords: autoregulation, connexin 36, gap junction, oxytocin, supraoptic nucleus
Introduction
Gap junctions, composed of connexin (Cx), are the essential component for electrical synaptic transmission between adjacent neurons. Increased junctional communication is critical for coordinated neuronal activity, which is typically displayed by magnocellular neurons in the supraoptic nucleus (SON) of female mammals during lactation (Hatton et al., 1987). The incidence of dye coupling through gap junctions among oxytocin (OT) neurons in the SON increases significantly in nursing rats (Hatton & Yang, 2002). Functionally, this is consistent with the appearance of synchronized activation of OT neurons in burst firing during suckling (Wang et al., 1995, 1996b). Both Cx32 and Cx43 have been identified in the SON (Micevych et al., 1996). Cx32 mRNA levels in the SON presented two peaks of expression, first during late pregnancy and then again during lactation; however, increased dye coupling between magnocellular neurons appeared only during lactation but not late pregnancy (Micevych et al., 1996). It seems that the elevation of Cx32 mRNA levels has no causal association with the increased interneuronal communication. Moreover, no difference in astrocytic Cx43 levels was identified between lactating and virgin rats, and no heterocellular dye coupling was found between astrocytes and neurons in the SON (Hatton et al., 1987; Micevych et al., 1996; Hatton & Yang, 2002), although astrocytes showed higher structural and functional plasticity during lactation (Wang & Hatton, 2009; Wang et al., 2017) and Cx43 likely participated in heterocellular excitation during hyperosmotic stress (Yuan et al., 2010). Thus, it is unlikely that Cx32 and Cx43 are directly involved in intercellular junctional communication between OT neurons during lactation. It is possible that Cx36, an identified component of interneuronal gap junctions (Potolicchio et al., 2012), is involved in the junctional communication between OT neurons during lactation. However, current evidence indicative of Cx36 involvement in hypothalamic neuroendocrine function is based on only one report that Cx36 was present in the subset of paraventricular neurons containing corticotropin-releasing hormone (Westberg et al., 2009). As a whole, it remains a question whether Cx36 is present in OT neurons and involved in the junctional communication during lactation.
To address this question, we examined the expression of Cx36 in the SON of rats using immunohistochemistry and Western blotting. Then, whole-cell patch-clamp recordings were used to observe effects of quinine, a selective blocker of Cx36 (Gajda et al., 2005), on OT-evoked excitation of OT neurons during lactation (Wang et al., 2006; Wang & Hatton, 2006, 2007a, 2007b). The results support the involvement of Cx36 in autoregulatory effect of OT on OT neurons during lactation.
Materials and Methods
Animals
Experiments were performed using virgin (42- to 60-day-old) and lactating (30–34 days older than virgins) female Sprague-Dawley rats. All animal procedures were in strict accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved by Institutional Animal Care and Use Committees of the University of California, Riverside and Harbin Medical University.
Slice Preparation
Rats were decapitated, and the brains were quickly removed and immersed in ice-cold slicing solution that was oxygenated by bubbling with a compressed gas mixture of 95% O2/5% CO2. Coronal slices (200 μm thick) containing SONs were prepared as previously described (Wang & Hatton, 2009) and incubated at room temperature (21°C–23°C) for 1 hr in oxygenated regular artificial cerebrospinal fluid (aCSF) before drug treatment or application for protein analyses. The regular aCSF contained (in mM) 126 NaCl, 3 KCl, 1.3 MgSO4, 2.4 CaCl2, 1.3 NaH2PO4, 26 NaHCO3, 10 glucose, 0.2 ascorbic acid, pH 7.4, and 305 mOsm/kg, oxygenated with 95% O2/5% CO2. For slicing, one third of NaCl in the regular aCSF formulation was replaced by sucrose in the amount to keep osmolality preserved.
Immunohistochemistry and Confocal Microscopy
Immunostaining was performed based on our earlier reports (Wang & Hatton, 2009) with minor modifications. In brief, brains were fixed in 4% paraformaldehyde for 72 hr and then cut into 50-μm thick slices in cold 0.1 M phosphate-buffered solution (PBS). The slices were permeated with 0.3% Triton X-100 in PBS for 30 min, and nonspecific binding was blocked with 0.3% gelatin in PBS. The slices were then incubated overnight at 4°C with primary antibodies against mouse OT-neurophysin (NP, 1:400, H. Gainer, National Institute of Neurological Disorders and Stroke Cat# PS-38, RRID:AB_2315026, Bethesda, MD,USA), rabbit vasopressin (VP)-NP (1: 400, Abcam Cat# ab39363, RRID:AB_778778, Shanghai, China), and goat Cx36 (1: 300, Santa Cruz Biotechnology Cat# sc-14904, RRID:AB_2111311, Santa Cruz, CA, USA). After rinsing, slices were incubated with species-matched fluorescent donkey anti-goat IgG H&L Alexa Fluor® 647-conjugated antibody (Abcam Cat# ab150131, RRID:AB_2732857, Shanghai, China), donkey anti-mouse IgG H&L Alexa Fluor® 555-conjugated reabsorbed antibody (Abcam Cat# ab150110, RRID:AB_2783637, Shanghai, China), and donkey anti-rabbit IgG H&L Alexa Fluor® 488-conjugated reabsorbed antibody (Abcam Cat# ab150061, RRID:AB_2571722, Shanghai, China), respectively; each at dilution of 1:1000 for 1.5 hr at room temperature. Finally, Hoechst staining (4’,6-diamidino-2-phenylindole, 0.5 μg/ml, 15 min, Sigma, CAS#:28718-90-3, Shanghai, China) was used to label nuclei.
For each treatment, 12 slices from the middle part of the SON of 3 virgin females and 3 lactating rats were imaged, 10–20 μm from the surface using a laser scanning confocal microscope (Leica TCP SP2, Wetzlar, Germany or Zeiss LSM510, Oberkochen, Germany) equipped with a 63× objective. Multiple fluorophores were imaged sequentially, and distribution pattern and colocalization of different molecules were analyzed. We present data using optimized staining under conditions reported earlier. To obtain such conditions, serial dilutions of the primary antibody, staining with preabsorbed (immune-neutralization) primary antibody, no primary antibody, and no secondary antibody controls were applied. The number of Cx36-positive OT neurons and VP neurons were counted in the representative fields of five slices under a 40× objective. Cells were considered positive if their fluorescence intensities exceeded the fluorescence level + 3 standard deviations of the control with no primary antibody (Malarkey & Parpura, 2011).
Western Blots and Coimmunoprecipitation
Methods for protein analysis were the same as previously reported (Wang et al., 2013). In comparing the levels of Cx36 in virgin and lactating female rats, the brains were collected after decapitation, and the SON was pouched out for extraction of proteins. In evaluating effects of OT (Sigma, CAS#:50-56-6, St. Louis, MO, USA) and quinine (quinine monohydrochloride dehydrate, Sigma, CAS#:6119-47-7), St. Louis, MO, USA treatments on Cx36 levels, 28 hypothalamic slices from 5 lactating rats were obtained as described earlier and then evenly allocated into four different incubation chambers (each condition contained 7 slices, 1–2 from each animal) for various drug treatments and controls, as shown in the Results section. SONs were then punched out and lysed. The lysates were centrifuged to remove insoluble components before protein levels were quantitated using a plate reader. Protein aliquots (60 μg) were loaded and separated on 10% sodium dodecyl sulphate-polyacrylamide gel electrophoresis gels and then transferred onto polyvinylidene difluoride membranes. After blocking with 1% gelatin for 1 hr at room temperature, membranes were incubated with goat anti-Cx36 (1: 500, see earlier) overnight at 4°C. To calibrate protein loads, mouse anti-actin (1: 500, Santa Cruz Biotechnology Cat# sc-47778, RRID:AB_626632, Santa Cruz, CA. USA) or rabbit anti-tubulin (1: 500, Santa Cruz Biotechnology Cat# sc-9104, RRID:AB_2241191, and Wanleibio, WL01931, Shanghai, China) antibodies were used (1 hr at room temperature) to probe membranes preincubated for 1 hr at room temperature in a blocking solution containing 5% dry milk. Bands were visualized using horseradish peroxidase-conjugated secondary antibodies (Santa Cruz Biotechnology Cat# sc-516102, RRID:AB_2687626; Cat# sc-2030, RRID:AB_631747; Cat# sc-2020, RRID:AB_631728, Shanghai, China) and an enhanced chemiluminescence system. In addition, a parallel labeling was performed using Fluorescein Irdye800 goat secondary antibody (Rockland Cat# 600-132-096, RRID:AB_217905, Philadelphia, PA, USA) and Alexa Fluor® 680-conjugated mouse secondary antibody (Thermo Fisher Scientific Cat# A-21057, RRID:AB_141436, Waltham, MA USA). The membranes were scanned with an Odyssey infrared imaging system.
The methods of detecting molecular association of Cx36 with OT-NP, using a coimmunoprecipitation technique, were utilized as previously described (Wang & Hatton, 2009), with minor modification. In brief, total lysates were precleared with protein A/G agarose (Millipore), and then 1.5 µg of immunoprecipitation antibody against Cx36 was added to the SON lysate containing 1.0 mg of protein to form immune complexes. After overnight incubation at 4°C, the immune complexes were captured by adding 50 µl of protein A/G agarose bead slurry through gently rocking for 2 hr at 4°C. The protein-loaded agarose beads were then collected and washed before being resuspended in sample buffer and boiled for 10 min. The beads were then removed by centrifugation and the supernatant was run on a 10% sodium dodecyl sulphate-polyacrylamide gel electrophoresis gel. Following electrophoresis, proteins were transferred to membranes, which were sequentially probed with antibodies against Cx36 or OT-NP (both at dilution of 1:300) for 4 hr at room temperature, each followed by secondary antibodies and enhanced chemiluminescence detection. Positive and negative controls consisted of total lysates and nonspecific IgG, respectively. All reagents were from GE Healthcare (Little Chalfont, Buckinghamshire, UK) or Tanon Science & Technology (Shanghai, China).
Patch-Clamp Recordings
Patch-clamp recording procedures for SON were similar to those described previously (Wang & Hatton, 2004). Briefly, slices were bathed in the regular aCSF for 1 hr before being transferred to a recording chamber. Whole-cell patch-clamp recordings were obtained from the somata of OT neurons using an Axopatch 200B amplifier or Multiclamp 700B amplifier (Molecular Devices, San Jose,CA, USA) under the visualization by a microscope (Eclipse FN1, Nikon, Shanghai, China). The pipette solution for recording from OT neurons in the SON contained (in mM) 145 K-gluconate, 10 KCl, 1MgCl2, 10 HEPES, 1 EGTA, 0.01 CaCl2, 2 Mg-ATP, 0.5 Na2-GTP, pH 7.3, adjusted with KOH. Signals were filtered, sampled at 5 kHz, and analyzed offline using Clampfit 10 software (Molecular Devices). The parameters for analysis within 10 min epochs included resting membrane potential (mV), membrane conductance (nS), frequency of action potentials (Hz), peak amplitude of action potentials (spike, mV), duration of action potentials expressed as the full width at half maximum (FWHM) of spike amplitude (ms), peak amplitude of after-hyperpolarization potential (AHP, mV), and the duration of AHP expressed as FWHM (ms). In addition, the frequency and peak amplitude of excitatory postsynaptic currents (EPSCs) and inhibitory postsynaptic currents (IPSCs) were also analyzed; rise and decay times of PSCs were calculated as time constants (tau) to reach 1-1/e and 1/e of the peak amplitude, respectively. OT neurons had the nonphasic firing and were post-electrophysiology identified using immunohistochemical labeling as previously described (Wang & Hatton, 2004).
Dye Coupling
For dye-coupling experiments, the brain slices were preincubated in external solution at 35°C for 30 min without (control) or with the presence of quinine (0.1 mM) and then subjected to whole-cell recording. Lucifer yellow (LY; 0.08% w/v; dipotassium salt, Sigma, CAS#:71206-95-6) was added to a regular pipette solution. After establishing a whole-cell recording, we allowed dye to diffuse for 10 min directly into the impaled cell in the current-clamp mode and indirectly, via gap junctional coupling, to adjacent cells(s); thus, treated cells were exposed to quinine in total of 40 min. Patch pipette was retrieved allowing cells to reseal and retain LY. Intracellular delivery and retention of LY were visualized under a laser scanning confocal microscope as stated earlier. The slices were fixed, and post hoc immunohistochemical staining was done to identify OT neurons. Imaging area, encompassing multiple images, covered ∼100 µm radius around the impaled cell. As was the case for OT-NP labeling (see earlier), cells were considered LY positive if their fluorescence intensity exceeded the fluorescence level + 3 standard deviations of the control condition prior to dye filling. The incidence of dye-labeled OT neurons is reported as the number of LY-stained cells. If only patch-camped OT neuron contained LY, that is, no dye transfer occurred, the cell was considered uncoupled, and the incidence was reported as 1.
Data Analysis
The number of subjects (cells, slices, or rats) required for comparison was estimated using power analysis (set at 80% and α = .05) and guided by our previously published work (Wang & Hatton, 2004, 2007a). Student’s t test, paired t test, χ2 test, and one-way analysis of variance (ANOVA) with Tukey’s post hoc test (electrophysiology) and Kruskal–Wallis H test (Western blots) were used for statistical analyses (using SigmaStat 12 program); p < .05 was considered significant. All measures were expressed as mean ± SEM in percentage of control values, or as otherwise noted.
Results
Cx36 Is Expressed in OT Neurons in the SON
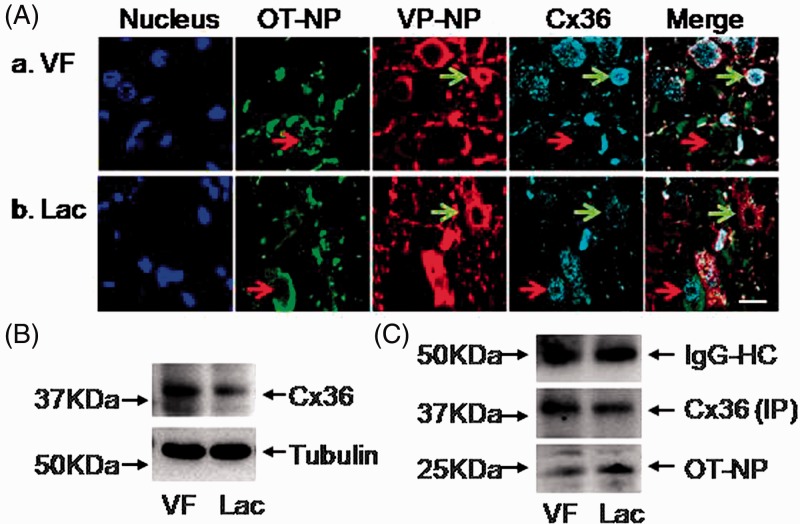
We first examined the expression of Cx36 in the SON using immunohistochemistry and Western blots in five pairs of virgin and lactating females. As shown in Figure 1(A), Cx36 was present in the SON of both virgin and lactating females. In the virgin females, Cx36 was mainly localized in VP neurons as it coimmunostained with VP-NP (91.7 ± 5.3% of VP neurons and 63.0 ± 10.9% of OT neurons; n = 5 slices, p < .05 between the two types of cells by Student’s t test). In lactating rats, Cx36 was mostly localized in OT neurons, as OT-NP neurons coimmunostained with Cx36 (68.3 ± 9.3% of OT neurons; n = 5 slices), while staining of VP neuronal somata for Cx36 was significantly reduced (55.0 ± 7.3% of VP neurons; n = 5 slices p < .01 compared with that in the virgin females by Student’s t test). Cx36 protein was also identified in Western blotting in eight pairs of virgin and lactating females (Figure 1(B)). The level of Cx36 in SONs of lactating rats was reduced to 51.2 ± 16.0% of the amount found in SONs of virgin rats (n = 8 in each group, p = .028; Student’s t test), perhaps due to the above reduction of Cx36 allocation in the population of VP neurons. To assess the association of Cx36 with OT neurons in virgin and lactating females, coimmunoprecipitation of Cx36 with OT-NP (Figure 1(C)) was performed. The results showed that Cx36-pulled down OT-NP in lactating rats was 1.61 ± 0.1 fold higher than that in virgin rats (each group n = 5, p < .05 by Student’s t test). These findings indicate that Cx36 has the overall reduced level in the SON of lactating rats. However, due to differential cell type-specific expression, there seems to be preferential preservation of Cx36 levels in OT neurons, where this protein could be a component of OT neuronal gap junctions.
Figure 1.
Cx36 expression in rat supraoptic nucleus (SON). (A) Representative confocal microscopic images taken from the SON of VF (a) and Lac (b) female rats. From left to the right: Hoechst staining on cell nuclei, and immunostaining of OT-NP, VP-NP, Cx36, and their merges; the red and green arrows point to OT and VP neurons, respectively. Scale bar, 20 μm. (B) Western blots showing the expression of Cx36 proteins in the SON of VF and Lac rats, respectively. Tubulin immunoreactivity was used as loading control. (C) Western blotting bands showing Cx36 (IP)-pulled down OT-NP in VF and Lac female rats. There was a 1.61 ± 0.1 fold higher association of Cx36 with OT in the SON of lactating rats relative (normalized to 1.0) to that in virgin rats (n = 5 in each group; p < .05 by Student’s t test). Cx36 was used as a control for loading.
Cx = connexin; VF = virgin female; Lac = lactating; IgG-HC = heavy chain of a nonspecific immunoglobulin; IP = immunoprecipitated protein; OT-NP = oxytocin-neurophysin; VP-NP = vasopressin-neurophysin.
Effects of Quinine on the Electrical Activity of OT Neurons in the SON
The expression of Cx36 in OT neurons suggests its functional association with OT neuronal activity. To test this possibility, we observed effects of quinine on the firing activity of OT neurons in brain slices from lactating rats using whole-cell patch-clamp recordings; positive identification of OT neurons was obtained by post hoc immunohistochemistry. The results from nine neurons showed that application of quinine (0.1 mM, 10 min) significantly reduced action potential/spike amplitude when compared with the control period prior to the application of quinine (–54.8 ± 3.7 mV in control and –50.1± 4.4 mV in quinine, p = .032, one-way ANOVA followed by Tukey’s post hoc test). In addition, quinine depolarized the membrane potential (–53.3 ± 1.1 mV in control and –51.9 ± 1.1 mV in quinine, p = .048, one-way ANOVA followed by Tukey’s post hoc test) and increased the width of AHP (expressed as the FWHM; 23.0 ± 2.1 ms in control and 27.7 ± 3.3 ms in quinine, p = .046, one-way ANOVA followed by Tukey’s post hoc test; Figure 2(A); Table 1). These effects are similar to quinine effects on dopaminergic neurons of the substantia nigra from mice brain slices (Zou et al., 2018). However, there was no significant change in the membrane conductance (2.1 ± 0.3 nS in control and 2.2 ± 0.3 nS, p = .329), spike width (expressed as FWHM; 1.8 ± 0.2ms in control and 2.4 ± 0.5 ms, p = .143), firing rate/frequency (3.0 ± 1.5 Hz in control and 1.0 ± 0.9 Hz, p = .217), and AHP peak amplitude (11.8 ± 0.6 in control and 11.5 ± 0.7, p = .979; see Part A of Table 1). Noteworthy is that among the 9 OT neurons, 5 cells showed significant reduction in the firing rate (2.1–652.5 fold), 3 cells with low basal firing rate had no change (<20% change), and 1 previously almost silent cell randomly exhibited several spikes (change of firing rate from 0.003 Hz to 0.61 Hz), suggesting inhibition of actively firing neurons.
Figure 2.
Effects of quinine on the firing activity of OT neurons and dye coupling in the SON of brain slices from lactating rats. (A) Firing activity: (Aa) an entire current-clamp recording of the firing activity of an OT neuron; (Ab) expanded episodes within the recording, during, and after treatment with quinine; (Ac) features of spikes at different time points within the expanded episodes. The dashed horizontal lines indicate the peak of the first spike (top line), the level of resting membrane potential (mid line), and the first spike AHP valley (bottom line). The vertical dual arrow indicates the spike amplitude. The horizontal dashed dual arrows (open heads) indicate the full width of AHP at half maximum amplitude (FWHM). Two horizontal arrows mark the FWHM of the action potential duration. (B) Dye coupling: representative images showing magnocellular neurons filled (directly or by coupling) with LY (left) and their immunohistochemical identification as OT neurons in control slices (a) and in slices treated with quinine (0.1 mM, 30 min; b). Merged LY and OT-NP images are shown on the right sides. Scale bar, 20 µm. The preincubation of slices with quinine significantly reduced the incidence of dye coupling between OT neurons when compared with control (n = 7 in each group; 3.43 ± 0.75 OT neurons in control, while 1.43 ± 0.43 when treated with quinine; p = .039 by Student’s t test). Other annotations refer to Figure 1.
OT-NP = oxytocin-neurophysin; LY = Lucifer yellow.
Table 1.
Effects of Quinine (Qu) Without and With OT on the Firing Activity of OT Neurons in the SON of Brain Slices From Lactating Rats.
| Groups | AP amp (mV) | AP duration,FWHM (ms) | AP freq (Hz) | AHP duration,FWHM (ms) | RMP (-mV) | Cond (nS) |
|---|---|---|---|---|---|---|
| A. Control | 54.8±3.7 | 1.8±0.2 | 3.0±1.5 | 23.0±2.1 | 53.3±1.1 | 2.1±0.3 |
| Qu-10min | 50.1±4.4* | 2.4±0.5 | 1.0±0.9 | 27.7±3.3* | 51.9±1.1* | 2.2±0.3 |
| Qu-20min | 62 | 1.4 | 0.0016±0.04* | 28.7 | 53.9±9.9 | 2.1±0.5 |
| B. Control | 61.3±2.4 | 1.7±0.1 | 0.1±0.03 | 27.0±3.8 | 62.3±1.1 | 2.6±0.4 |
| OT-10min | 53.6±2.6* | 2.0±0.2* | 0.8±0.5* | 23.6±3.1 | 53.4±2.3* | 2.5±0.4 |
| OT-20min | 51.8±2.0* | 2.0±0.2* | 0.1±0.01* | 25.4±3.6 | 54.2±2.0* | 2.6±0.4 |
| C. Control (Qu-20min) | 62 | 2.6 | 0.002±0.002 | 28.7 | 53.9±1.7 | 2.1±0.2 |
| Qu-OT-10min | 64.8±3.2 | 2.3±0.0 | 0.7±0.4 | 32.3±1.3 | 52.9±1.9 | 2.3±0.2 |
| Qu-OT-20min | 50.5±6.2 | 1.6±1.0 | 0.6±0.6 | 28.2±3.0 | 49.6±1.5 | 2.5±0.2 |
Note. (A) Recordings were taken from 9, 9, and 5 cells for control, Qu for 10 min, and for 20 min, respectively. (B) Recordings were based on 8 cells for all the groups. (C) Recordings were taken from 5 cells; a subset of cells, 1, 2, or 5 cells showing spike(s) in the control (preincubation with Qu for 20 min), Qu-OT 10 min, and Qu-OT-20 min, respectively; *p < .05 compared with the control, one-way ANOVA, and Tukey’s post hoc test, as well as χ2 test (see text for details). Note that due to low number of samples in Qu-20 min (A), statistical analysis was not possible. All measures were expressed as mean ± SEM with the exception when reporting on measurements of AP Amp, AP FWHM, AHP Amp, and AHP FWHM from single neurons. OT = oxytocin; SON = supraoptic nucleus; AP = action potential; amp = amplitude; freq = frequency of action potential discharges; AHP = after-hyperpolarization potential; FWHM = the full with at half maximum; RMP = resting membrane potential; Cond = conductance.
In five cells, the presence of quinine was extended to 20 min, four cells fell into silence, that is, they did not discharge action potentials. Statistically, the firing rate (0.0016 ± 0.039 Hz) in cells exposed to quinine for 20 min was significantly lower when compared with the activity of the same cells in control period, that is, prior to the treatment (control) and to the first 10 min of the treatment with quinine (n = 5, p = .041 and p = .028, respectively, one-way ANOVA followed by Tukey’s test). The membrane conductance remained stable throughout the experiment (2.1 ± 0.5 nS in quinine for 20 min; p = .477 vs. the control, one-way ANOVA followed by Tukey’s test). However, membrane potential recovered to the control levels (–53.8 ± 9.9 mV at 20 min of quinine; n = 5, p = .411 vs. control, one-way ANOVA followed by Tukey’s test). These results are consistent with the general effect of Cx36 in maintaining the excitability of neurons.
To assess the extent to which quinine affected the gap junctional coupling between OT neurons, we analyzed the incidence of dye coupling. We recorded from magnocellular neurons in current-camp mode while allowing for intercellular diffusion of LY dissolved in pipette solution. The slices were then fixed, and OT neuron identity was established by a post hoc immunohistochemistry. In control/untreated slices, LY spread from the impaled OT neuron (n = 7) to adjacent neurons giving the incidence of dye coupling of 3.43 ± 0.75 OT neurons (an uncoupled neuron would give the incidence of dye coupling of 1.0; Figure 2(Ba)). The treatment of slices with quinine (0.1 mM; 40 min) significantly reduced the incidence of dye coupling between OT neurons (n = 7, 1.43 ± 0.43; p = .039 by Student’s t test; Figure 2(Bb)). Of note, quinine failed to increase action potential duration (Table 1), which is expected if quinine acted as a potassium channel blocker (Lin et al., 1998; Imai et al., 1999). Taken together, these finding indicate that quinine in our experimental conditions acts as a specific blocker of Cx36-associated gap junctions.
Effects of Quinine on Postsynaptic Currents of OT Neurons in the SON
The firing activity of OT neurons is closely associated with the activity of synaptic inputs (Wang & Hatton, 2004, 2007a). Thus, we further examined effects of quinine on EPSCs and IPSCs, as OT neurons receive both glutamatergic and GABAergic inputs (Wang & Hatton, 2004). As shown in Figure 3 and Table 2, quinine (n = 5; 0.1 mM, 20 min) did not significantly influence the EPSC amplitude (15.7 ± 1.3 pA in control and 16.5 ± 1.7 pA in quinine, p = .332, one-way ANOVA followed by Tukey’s test) or the frequency of EPSCs (5.3 ± 1.7 Hz in control and 6.0 ± 1.3 Hz in quinine, n = 6, p = .692, one-way ANOVA followed by Tukey’s test); shorter 10 min application of quinine (n=6) had a similar lack of effect on amplitude and frequency of EPSCs, but it significantly reduced the EPSC duration. By contrast, quinine treatment (0.1 mM, 20 min) significantly reduced the amplitude (28.0 ± 4.3 pA in control and 25.3 ± 3.8 pA in quinine, p = .030, one-way ANOVA followed by Tukey’s test) but not the frequency (4.3 ± 0.4 Hz in control and 4.2 ± 0.8 Hz, n = 6, p = 0.653, one-way ANOVA followed by Tukey’s test) of IPSCs. In three cells, longer presence of quinine (20 min) further reduced the IPSC amplitude (20.0 ± 5.8 pA and its frequency (3.6 ± 1.0 Hz) although the small sample size did not have enough post-hoc power to test for a statistically significant difference. These results are consistent with the view that Cx36 plays a role in OT synaptic activity, likely at the postsynaptic sites.
Figure 3.
Effects of quinine on the synaptic activity of OT neurons in the SON of brain slices from lactating rats. (A) Voltage-clamp recordings of EPSCs at a holding potential of –70 mV. (B) Voltage-clamp recordings of IPSCs at a holding potential of –20 mV. The three panels show the full recordings (1), expanded episodes (2), and representative single events (3), respectively.
Table 2.
Effects of Quinine (Qu) on Spontaneous EPSCs and IPSCs of OT Neurons.
| Groups | Amp (pA) | Duration, FWHM (ms) | Rise time, tau (ms) | Decay time, tau (ms) | Freq. (Hz) |
|---|---|---|---|---|---|
| EPSCs | |||||
| Control (n = 6) | 15.7 ± 1.3 | 3.5 ± 0.3 | 6.5± 1.0 | 34.0 ± 18.9 | 5.4 ± 1.7 |
| Qu-10 min (n = 6) | 16.5 ± 1.7 | 3.1 ± 0.3* | 10.2 ± 3.8 | 19.5 ± 7.5 | 6.0 ± 1.3 |
| Qu-20 min (n = 5) | 14.1 ± 2.1 | 3.3 ± 0.4 | 6.9 ± 2.2 | 21.1 ± 6.7 | 2.7 ± 0.7 |
| IPSCs | |||||
| Control (n = 6) | 28.0 ± 4.3 | 6.8 ± 0.3 | 12.3 ± 6.1 | 29.1 ± 12.41 | 4.3 ± 0.4 |
| Qu-10 min (n = 6) | 25.3 ± 3.8* | 6.5 ± 0.3 | 26.6 ± 21.1 | 28.1 ± 8.41 | 4.2 ± 0.8 |
| Qu-20 min (n = 3) | 20.0 ± 5.8 | 6.1 ± 0.7 | 5.9 ± 0.9 | 39.1 ± 11.07 | 3.6 ± 1.0 |
Note. Rise and decay times are given as time constants (tau) to reach 1-1/e and 1/e of the peak amplitude, respectively. *p < .05 compared with control, one-way ANOVA with Tukey’s post hoc test, as well as χ2 test (see text for details). EPSC = excitatory postsynaptic potential; IPSP = inhibitory postsynaptic potential; OT = oxytocin; Amp = amplitude; FWHM = the full with at half maximum; Freq = frequency of events.
Effects of Quinine on OT-Evoked Increase in Firing Activity of OT Neurons
OT has excitatory effects on OT neuronal activity (Figure 4), which has been extensively studied elsewhere (Wang et al., 2006; Wang & Hatton, 2006, 2007a, 2007b). The major effects include increased firing rate, depolarization of membrane potential, reduction of spike amplitude, and increase in spike width (Wang et al., 2006; Figure 4 and Table 1).
Figure 4.
Effects of OT on the firing activity of OT neurons in the acute SON slice preparations from lactating rats. (A) Full current-clamp recording of the firing activity of an OT neuron; (B) clustered firing episodes (a burst is shown in the middle panel); (C) features of spikes. Other annotations refer to Figure 2.
OT = oxytocin.
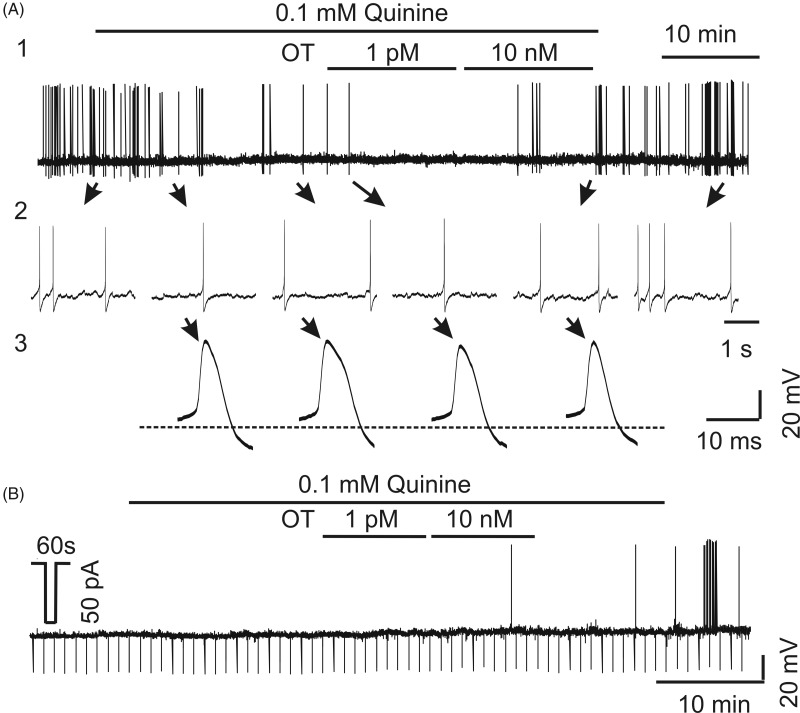
Because Cx36 is involved in postsynaptic currents of OT neurons, blocking its function might also interfere with OT-evoked increased firing activity of OT neurons. To test this hypothesis, we observed the influence of quinine on the effect of OT. As shown in Figure 5, in the presence of quinine (20 min), adding 1 pM OT for 10 min only excited 2 out of 5 neurons, while further addition of 10 nM OT for 10 min made all the 5 neurons to show increased depolarization of membrane potential (p = .025 vs. control period, that is, 20 min in quinine prior to adding OT; χ2 test). However, the firing rate (0.6±0.6 Hz) was not significantly changed from that in control period (0.002 + 0.002 Hz; p = .186, χ2 test) due to one cell that showed a dramatic increase in the firing rate (from 0.008 Hz to 3.2 Hz). Noteworthy is that compared with the relative high incidence of OT-evoked burst firing (Wang & Hatton, 2007a; Wang et al., 2016), no neurons showed burst firing in response to OT in the presence of quinine. In the 5 cells tested, 4 of them fired at a low frequency (1–2 spikes/min; see Part C of Table 1), which basically excluded the possibility to discharge in burst. These findings indicate that OT modulation of OT neuronal activity, besides previously reported multiple underlying mechanisms (Hou et al., 2016), also involves Cx36 activity, perhaps via fast, gap junctional interneuronal communication.
Figure 5.
Effects of OT on the firing activity of OT neurons in the acute SON slice preparations from lactating rats in the presence of quinine. (A) Current-clamp recordings of the firing activity—A1: full recording; A2: expanded firing episodes; A3: features of spikes; horizontal dashed line indicates the resting membrane potential. (B) Membrane conductance assay. The downward voltage shifts indicate the voltage changes in response to hyperpolarizing currents (50 pA, 60 s).
OT = oxytocin.
Effects of OT on the Amount of Cx36 in the Presence of Quinine
Results presented earlier indicate functional association between Cx36 amount and OT neuronal activity. Using Western blots, we further examined if OT and quinine would affect the level of Cx36 in the SON from 5 lactating female rats. As shown in Figure 6, 10-min treatments of the SON slices with either OT (1pM, 10 min) or quinine (0.1 M, 10 min) did not significantly affect the amount of Cx36 when compared with untreated controls. However, application of OT to cells pretreated for 10 min with quinine (which was kept throughout the experiment so that the accumulative exposure to quinine was 20 min) significantly reduced levels of detected Cx36 in the SON (63.7 ± 12.1% of the control, n = 7, p = .024 by with Kruskal–Wallis H test). Due to the acute nature (10 min) of the treatments, this effect likely represents enhanced degradation of Cx36 (Arumugam et al., 2005) or a decrease in translation of Cx36 mRNA. This finding is in agreement with the electrophysiological effects of OT in the presence of quinine on the firing activity of OT neurons (Figure 5 and Part C of Table 1).
Figure 6.
Effects of OT on the levels of Cx36 in the SON of lactating female rats in acute slice preparations. (A) Western blots showing the levels of Cx36 proteins in control (CTR), OT (1 pM, 10 min), quinine (0.1 mM, 10 min) and OT (1 pM, 10 min) in presence of quinine (Qu-OT); slices were preincubated for 10 min with quinine (so, total of 20 min for Qu; Qu-OT), respectively. (B) Bar graph summarizing the effect of OT, Qu, or OT-Qu on Cx36 levels relative (normalized to 100%) to the control. Each data point represents an average of measurements obtained from seven brain SON samples from five rats; p < .05 between control and Qu-OT with Kruskal–Wallis H test. Actin immunoreactivity was used as loading control.
CTR = control; OT = oxytocin; Qu = quinine; Qu-OT = oxytocin in presence of quinine.
Discussion
In the present study, we first verified the expression of Cx36 at protein level in the SON in both OT and VP neurons. We then showed the shift in Cx36 expression in lactating female rats toward OT neurons. Furthermore, observing effects of quinine, a Cx36-specific blocker, revealed Cx36 function in maintenance of basal firing rate as well as mediating OT-elicited excitation of OT neurons. Finally, OT in the presence of quinine reduced the level of Cx36. Thus, this study indicates the presence of Cx36 in the SON and its functional role in autoregulatory roles of OT on OT neuronal activity.
Cx36 in the SON
Gap junctions are channel-forming structures in the plasma membranes that allow for direct metabolic and electrical communication between cells in the mammalian brain. Among 20 Cx genes identified in mice and 21 in humans, Cx36, Cx45, or Cx57 proteins are thought to form homocellular, that is, neuron–neuron, gap junctions in murine models (Sohl et al., 2005). Gap junctions play essential roles in interneuronal communication, which is particularly meaningful in synchronized neuronal activity (Gajda et al., 2005). Gap junctions are heavily utilized by magnocellular, both VP and OT, neurons under certain physiological conditions. Namely, lactation significantly increased interneuronal dye couplings between OT neurons (Hatton et al., 1987), while hyperosmotic stress increases VP neuron activity in a gap junction-dependent manner (Yuan et al., 2010). The histological basis of gap junctional involvement in OT/VP neuronal activity is the function of different types of Cx channels. Previous studies have revealed the presence of Cx32 in SON neurons and Cx43 in SON astrocytes (Micevych et al., 1996). VP synthesis and release induced by hyperosmotic stimulation depend on increased expression of Cx43 and its function in the SON (Jiang et al., 2014). In this study, we provide the evidence of Cx36 presence in SON neurons based on studies using immunohistochemistry, Western blotting, and electrophysiology, thereby allowing a new venue to explore the biological functions of gap junctions. Of note, quinine by acting intracellularly can block Cx36 and Cx50 junctional currents in a reversible and concentration-dependent manner; however, it did not substantially block astrocytic gap junction channels formed by Cx26, Cx30, and Cx43 (Nagy et al., 2003), and only moderately affected Cx45 junctions binding site (Srinivas et al., 2001). Because Cx50 has not been implicated in the homocellular coupling (Srinivas et al., 2001), quinine represents a specific blocker for neuronal Cx36-forming channels. Consistent with this notion, quinine reduced dye coupling between OT neurons (Figure 2) without exhibiting blocking effects on potassium currents underlying the repolarization phase of action potential, as action potential duration was unaffected (Table 1). A reduction in action potential amplitude recorded from OT neurons treated with quinine (Table 1) could represent its action on voltage-gated sodium channels, as seen in isolated spiral ganglion neurons in culture (Imai et al., 1999). However, this is a pharmacologically highly unlikely scenario because of a lack of blocking action on potassium channels, which are more sensitive to quinine than voltage-gated sodium channels (Lin et al., 1998). Thus, the influence of quinine on OT neuronal activity provides functional evidence for the presence of Cx36 in the SON. Cx36 is an essential component of inter-OT neuronal gap junctions. Of course, it is possible that some novel yet unidentified type(s) of Cx is/are sensitive to quinine is present in the SON or that quinine has some side effects that would affect interpretation of our data.
Reproductive Stages and Cx Functions
A dramatic feature of junctional communication/connectivity is its function-associated expression. In response to environmental demands, the connectivity between adjacent neurons in the SON, as assessed by dye coupling, is increased during the periods of lactation and hyperosmotic stimulation (Hatton et al., 1984). Cx43 has been extensively identified as the major astrocytic gap junctional protein that does not function in direct interneuronal communication, although it could be involved in astrocyte–neuron signaling by operating as a hemichannel to influence VP neuronal activity (Yuan et al., 2010). By contrast, Cx32 is generally considered as the major component of the gap junctions among SON neurons ever since the early identification in the Hatton laboratory (Micevych et al., 1996). Increased expression of Cx32 during lactation suggests its involvement in the increased neuronal connectivity, as per dye coupling, which facilitates synchronization of burst firing (Hatton, 1990). However, the lack of Cx-specific gap junctional blockers makes it difficult to confirm the function of Cx32 in this process, albeit Cx32 knockout mice could be used (Iacobas et al., 2007). Similarly, Cx36 knockout animals (Iacobas et al., 2007) should be used to confirm our present findings, albeit to our knowledge a Cx36 knockout rat is not available at present. Albeit not reported in the literature, it is tempting to speculate that such Cx36 knockout animals would show a lactating phenotype. Meanwhile, based on not only the histology and protein identification but also the usage of a Cx36-specific blocker in the SON, the present study provides evidence for the participation of Cx36 in OT-evoked excitation of OT neurons, a functional basis of the synchronized action of OT neurons (Wang et al., 1995, 1996a, 1996b). In addition, Cx36 was predominately expressed in VP neurons in virgin rats, while in lactating rats, Cx36 expression was preserved in OT neurons and downregulated in VP neurons (Figures 1 and 7). Whether other Cxs could participate in OT-evoked activation of OT neurons during lactation remains to be examined.
Figure 7.
Diagram of the Cx36 expression in the SON and OT modulation of Cx36 expression in OT neurons. (A) Expression of Cx36 in OT- (top row) and VP- (bottom row) neurons in virgin female rats (column a) and lactating rats (column b). Note the retrieval of astrocytic processes in lactation allowing for the gap junctional coupling between OT neurons. (B) Hypothetical mechanisms underlying time-dependent OT modulation of Cx36 expression and functions: (Ba) short-time OT action (5–10 min); (Bb) longer time OT action (> 10 min). The “x” by OTR shows its putative downregulation during prolonged presence of OT.
Cx = connexin; OT = oxytocin; VP = vasopressin; SON = supraoptic nucleus; cAMP = cyclic AMP; CREB = cAMP response element binding protein; F-actin = filamentous actin; G-J = gap junction; pERK 1/2 = phosphorylated extracellular signal-regulated protein kinase 1/2; OTR = OT receptors; PGs = prostaglandins; for other annotations refer to Figure 1. See text for details.
Cx36 and OT Neuronal Activity
Neuronal gap junction is essential for the synchronization of electrical activity of junctionally coupled neurons. For instance, Cx36-associated gap junctions contribute to network synchronization in CA3 pyramidal cells in neonatal hippocampus (Molchanova et al., 2016) and blockade of Cx36 with quinine attenuates seizure severity in pentylenetetrazole-kindled rats (Faridkia et al., 2016). This gap junctional coupling is applicable for OT neurons during lactation. In lactating rats, suckling-evoked synchronization of burst firing depends on an increase in OT levels in the SON (Moos et al., 1989); blocking OT receptors (OTR) also blocks the burst discharges (Moos & Richard, 1988; Wang & Hatton, 2007a). The excitatory effect of OT on OT neurons is associated with synaptic inputs on OT neurons and astrocytic plasticity as previously reviewed (Hatton & Wang, 2008; Hou et al., 2016). It is likely that suckling- and OT-evoked retraction of astrocytic processes (Wang & Hatton, 2009), otherwise hindering plasma membrane to plasma membrane interactions, that is, coupling by gap junctions, of neighboring OT neurons, allows for the formation of functional Cx36 containing gap junctions (Figure 7(A)), thereby promoting the synchronization of burst discharges among adjacent OT neurons.
This synchronizing function of Cx36 is based on its regulation of OT neuronal activity and its responses to OT stimulation (Figure 7(B)). Clearly, blocking Cx36 with quinine reduced the firing rate and spike amplitude along with causing a transient depolarization of the membrane potential in OT neurons. These effects were correlated with the elongation of the recovery of the membrane potential from the spike AHP, which is a rate-limiting factor of firing rate and the burst discharges. The transient depolarization of membrane potential could be due to the reduced IPSC amplitude that is an indicator of cellular electrical inhibition. However, whether quinine-sensitive Cx36 is solely responsible for the synchronized burst of OT neurons or the occurrence of milk ejections remains to be investigated.
Possible Mechanism Underlying Modulation of Cx36 Expression in OT Neurons
The observation in the present study revealed that Cx36 is not only a critical component of OT-evoked firing activity but also a target of OT actions (Figure 7(B)). During suckling stimulation, OT levels around OT neurons increase gradually (Moos et al., 1989), which can largely account for the increased rate of tonic firing activity before burst discharges (Wang et al., 1996b; Wang & Hatton, 2004, 2005). The occurrence of the burst (Wang & Hatton, 2004, 2005) or elongated OT action (Wang et al., 2006) can reversely decrease the firing rate. In response to OT stimulation, the activation of OTR can increase mobilization of G protein βγ subunit (Wang & Hatton, 2007a) along with downstream phosphorylated extracellular signal-regulated protein kinase 1/2 (pERK 1/2; Wang & Hatton, 2007b), prostaglandin synthesis (Wang et al., 2017), and actin cytoskeletal reorganization (Wang & Hatton, 2007b). In addition, via Gαq/11-phospholipase C-inositol trisphosphate receptors pathway, activation of OTR can increase cytosolic Ca2+ by recruitment of this ion from the endoplasmic reticulum store (Arthur et al., 2007), which also leads to increase prostaglandin synthesis (Soloff et al., 2000). The increased pERK 1/2 (Cushing et al., 2005), which has inhibitory mutual interaction with protein kinase A (Zhong et al., 2003), and actin remodeling (Liu et al., 2014) have been associated with increased Cx36 expression. Thus, along with OT-evoked formation of filamentous actin at the membrane subcortical areas in OT neurons (Wang & Hatton, 2007b) and retraction of astrocytic processes (Wang & Hatton, 2009), the expression of Cx36 and its formation of gap junctions should also be increased, thereby promoting the burst generation and their synchrony. Further study to examine the expression of Cx36 following short OT treatment is warranted to verify this possibility.
By contrast, following the burst (Wang et al., 1996b) or during prolonged OT treatment, that is, > 10 min (Wang et al., 2006), the firing activity of OT neurons reduces dramatically. The postburst inhibition during suckling can be explained by burst-evoked increase in extracellular potassium (Leng et al., 1988) and high potassium-evoked expansion of astrocytic processes in the presence of high level of OT (Wang & Hatton, 2009). Because activation of potassium channels is negatively correlated with the expression of Cx36 (Medina-Ceja & Ventura-Mejia, 2010; Lebreton et al., 2015; Zhou et al., 2017), it is reasonable to propose that the burst firing dampens the activity of Cx36 and that results in the inhibition. During extended time of OT treatment, the expression of protein kinase A is increased (Wang et al., 2017) as result of prostaglandin activation of EP receptor (Shibuya et al., 2000) and reduced pERK1/2 inhibition of protein kinase A expression (Zhong et al., 2003), while filamentous actin network is disrupted (Wang & Hatton, 2007b). Protein kinase A activity could reduce Cx36 expression through the activation of cyclic AMP response element binding protein (CREB; Arumugam et al., 2005). CREB is an important downstream signal of OT functions in the brain (Tomizawa et al., 2003; Guzman et al., 2013); the activation of CREB could be a direct result of protein kinase A activation (Wang et al., 2017) as well as a delayed response to OT-increased pERK 1/2 in OT neurons (Wang & Hatton, 2007b). In addition, one might expect desensitization of OTRs or their removal from the plasma membrane in the prolong presence of OT, which would result in a reduced mobilization of Ca2+ from the intracellular stores, and also reduced pERK1/2 formation and PG synthesis. Direct evidence supporting these regulatory processes (Figure 7) remains to be collected.
Our 10-min treatments with OT or quinine alone did not significantly affect the level of Cx36, while combined OT and Qu treatment did. A 10-min treatment certainly seems too short to reduce Cx36 levels via gene expression. As Cx36 protein have a relatively short half-life ∼ 3.1 hr (Wang et al., 2015), it is possible that noticeable degradation would occur within the time frame of our experiments, as we have similarly previously seen in the case of glial fibrillary acidic protein (GFAP) (Wang & Hatton, 2009) following the activation of OTR signaling in the SON (Wang et al., 2017). Furthermore, reduced Cx36 levels might be the result of a decrease in the translation of the Cx36 mRNA. Future experiments to discern underlying mechanism(s) for the observed phenomenon could include mRNA analysis along with longer lasting treatments. It is tempting to speculate that the observed effect of combined OT and Qu treatment might be a consequence of some additive effect of each agent, perhaps through pERK 1/2 in OT neurons. Namely, pERK 1/2 reduces the expression of plasmalemmal sodium chloride cotransporter in mouse distal convoluted tubule (Zhou et al., 2012), and it is possible that it could have a similar effect on Cx36. While it is known that OT can increase pERK1/2 levels in OT neurons, it is unknown whether quinine could cause an additive effect. Future experiment will be necessary to test this possible scenario.
Taken together, Cx36 is an essential component of gap junctions in magnocellular neurosecretory OT neurons in the SON. Its expression in OT neurons during lactation is, in part, responsible for the synchronized burst firing and likely the ensuing bolus release of OT into the bloodstream.
Acknowledgments
We thank the late Dr. Glenn I. Hatton for supporting this study initially.
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the National Natural Science Foundation of China (grant no. 31471113, Y.-F. W.), the higher education talents funds of Heilongjiang province (grant no. 002000154, Y.-F. W.), and the Civitan International McNulty Award (V. P.).
References
- Arthur P., Taggart M. J., Mitchell B. F. (2007). Oxytocin and parturition: A role for increased myometrial calcium and calcium sensitization? Front Biosci, 12, 619–633. [DOI] [PubMed] [Google Scholar]
- Arumugam H., Liu X., Colombo P. J., Corriveau R. A., Belousov A. B. (2005). NMDA receptors regulate developmental gap junction uncoupling via CREB signaling. Nat Neurosci, 8, 1720–1726. [DOI] [PubMed] [Google Scholar]
- Cushing P., Bhalla R., Johnson A. M., Rushlow W. J., Meakin S. O., Belliveau D. J. (2005). Nerve growth factor increases connexin43 phosphorylation and gap junctional intercellular communication. J Neurosci Res, 82, 788–801. [DOI] [PubMed] [Google Scholar]
- Faridkia Z., Yaghmaei P., Nassiri-Asl M. (2016). Protective effect of quinine on chemical kindling and passive avoidance test in rats. Iran Red Crescent Med J, 18, e25490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gajda Z., Szupera Z., Blazso G., Szente M. (2005). Quinine, a blocker of neuronal cx36 channels, suppresses seizure activity in rat neocortex in vivo. Epilepsia, 46, 1581–1591. [DOI] [PubMed] [Google Scholar]
- Guzman Y. F., Tronson N. C., Jovasevic V., Sato K., Guedea A. L., Mizukami H., Nishimori K., Radulovic J. (2013). Fear-enhancing effects of septal oxytocin receptors. Nat Neurosci, 16, 1185–1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatton G. I. (1990). Emerging concepts of structure-function dynamics in adult brain: The hypothalamo-neurohypophysial system. Prog Neurobiol, 34, 437–504. [DOI] [PubMed] [Google Scholar]
- Hatton G. I., Perlmutter L. S., Salm A. K., Tweedle C. D. (1984). Dynamic neuronal-glial interactions in hypothalamus and pituitary: Implications for control of hormone synthesis and release. Peptides, 5(Suppl 1), 121–138. [DOI] [PubMed] [Google Scholar]
- Hatton G. I., Wang Y. F. (2008). Neural mechanisms underlying the milk ejection burst and reflex. Prog Brain Res, 170, 155–166. [DOI] [PubMed] [Google Scholar]
- Hatton G. I., Yang Q. Z. (2002). Peripartum interneuronal coupling in the supraoptic nucleus. Brain Res, 932, 120–123. [DOI] [PubMed] [Google Scholar]
- Hatton G. I., Yang Q. Z., Cobbett P. (1987). Dye coupling among immunocytochemically identified neurons in the supraoptic nucleus: Increased incidence in lactating rats. Neuroscience, 21, 923–930. [DOI] [PubMed] [Google Scholar]
- Hou D., Jin F., Li J., Lian J., Liu M., Liu X., Xu Y., Zhang C., Zhao C., Jia S., Jiao R., Liu X. Y., Wang X., Zhang Y., Wang Y.-F. (2016). Model roles of the hypothalamo-neurohypophysial system in neuroscience study. Biochem Pharmacol (Los Angel), 5, 3: e211. [Google Scholar]
- Iacobas D. A., Iacobas S., Spray D. C. (2007). Connexin-dependent transcellular transcriptomic networks in mouse brain. Prog Biophys Mol Biol, 94, 169–185. [DOI] [PubMed] [Google Scholar]
- Imai S., Suzuki T., Sato K., Tokimasa T. (1999). Effects of quinine on three different types of potassium currents in bullfrog sympathetic neurons. Neurosci Lett, 275, 121–124. [DOI] [PubMed] [Google Scholar]
- Jiang S., Wang Y. Q., Xu C. F., Li Y. N., Guo R., Li L. (2014). Involvement of connexin43 in the acute hyperosmotic stimulus induced synthesis and release of vasopressin in the supraoptic nucleus of rats. Mol Med Rep, 10, 2165–2171. [DOI] [PubMed] [Google Scholar]
- Lebreton F., Pirog A., Belouah I., Bosco D., Berney T., Meda P., Bornat Y., Catargi B., Renaud S., Raoux M., Lang J. (2015). Slow potentials encode intercellular coupling and insulin demand in pancreatic beta cells. Diabetologia, 58, 1291–1299. [DOI] [PubMed] [Google Scholar]
- Leng G., Shibuki K., Way S. A. (1988). Effects of raised extracellular potassium on the excitability of, and hormone release from, the isolated rat neurohypophysis. J Physiol, 399, 591–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin X., Chen S., Tee D. (1998). Effects of quinine on the excitability and voltage-dependent currents of isolated spiral ganglion neurons in culture. J Neurophysiol, 79, 2503–2512. [DOI] [PubMed] [Google Scholar]
- Liu X., Yan F., Yao H., Chang M., Qin J., Li Y., Wang Y., Pei X. (2014). Involvement of RhoA/ROCK in insulin secretion of pancreatic beta-cells in 3D culture. Cell Tissue Res, 358, 359–369. [DOI] [PubMed] [Google Scholar]
- Malarkey E. B., Parpura V. (2011). Temporal characteristics of vesicular fusion in astrocytes: Examination of synaptobrevin 2-laden vesicles at single vesicle resolution. J Physiol, 589, 4271–4300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medina-Ceja L., Ventura-Mejia C. (2010). Differential effects of trimethylamine and quinine on seizures induced by 4-aminopyridine administration in the entorhinal cortex of vigilant rats. Seizure, 19, 507–513. [DOI] [PubMed] [Google Scholar]
- Micevych P. E., Popper P., Hatton G. I. (1996). Connexin 32 mRNA levels in the rat supraoptic nucleus: Up-regulation prior to parturition and during lactation. Neuroendocrinology, 63, 39–45. [DOI] [PubMed] [Google Scholar]
- Molchanova S. M., Huupponen J., Lauri S. E., Taira T. (2016). Gap junctions between CA3 pyramidal cells contribute to network synchronization in neonatal hippocampus. Neuropharmacology, 107, 9–17. [DOI] [PubMed] [Google Scholar]
- Moos F., Poulain D. A., Rodriguez F., Guerne Y., Vincent J. D., Richard P. (1989). Release of oxytocin within the supraoptic nucleus during the milk ejection reflex in rats. Exp Brain Res, 76, 593–602. [DOI] [PubMed] [Google Scholar]
- Moos F., Richard P. (1988). Characteristics of early- and late-recruited oxytocin bursting cells at the beginning of suckling in rats. J Physiol, 399, 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagy J. I., Ionescu A. V., Lynn B. D., Rash J. E. (2003). Coupling of astrocyte connexins Cx26, Cx30, Cx43 to oligodendrocyte Cx29, Cx32, Cx47: Implications from normal and connexin32 knockout mice. Glia, 44, 205–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potolicchio I., Cigliola V., Velazquez-Garcia S., Klee P., Valjevac A., Kapic D., Cosovic E., Lepara O., Hadzovic-Dzuvo A., Mornjacovic Z., Meda P. (2012). Connexin-dependent signaling in neuro-hormonal systems. Biochim Biophys Acta, 1818, 1919–1936. [DOI] [PubMed] [Google Scholar]
- Shibuya I., Kabashima N., Ibrahim N., Setiadji S. V., Ueta Y., Yamashita H. (2000). Pre- and postsynaptic modulation of the electrical activity of rat supraoptic neurones. Exp Physiol, 85(Spec No), 145S–151S. [DOI] [PubMed] [Google Scholar]
- Sohl G., Maxeiner S., Willecke K. (2005). Expression and functions of neuronal gap junctions. Nat Rev Neurosci, 6, 191–200. [DOI] [PubMed] [Google Scholar]
- Soloff M. S., Jeng Y. J., Copland J. A., Strakova Z., Hoare S. (2000). Signal pathways mediating oxytocin stimulation of prostaglandin synthesis in select target cells. Exp Physiol, 85(Spec No), 51S–58S. [DOI] [PubMed] [Google Scholar]
- Srinivas M., Hopperstad M. G., Spray D. C. (2001). Quinine blocks specific gap junction channel subtypes. Proc Natl Acad Sci U S A, 98, 10942–10947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomizawa K., Iga N., Lu Y. F., Moriwaki A., Matsushita M., Li S. T., Miyamoto O., Itano T., Matsui H. (2003). Oxytocin improves long-lasting spatial memory during motherhood through MAP kinase cascade. Nat Neurosci, 6, 384–390. [DOI] [PubMed] [Google Scholar]
- Wang H. Y., Lin Y. P., Mitchell C. K., Ram S., O'Brien J. (2015). Two-color fluorescent analysis of connexin 36 turnover: Relationship to functional plasticity. J Cell Sci, 128, 3888–3897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang P., Qin D., Wang Y. F. (2017). Oxytocin rapidly changes astrocytic GFAP plasticity by differentially modulating the expressions of pERK 1/2 and protein kinase A. Front Mol Neurosci, 10, 262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S. C., Zhang F., Zhu H., Lv C., Liu X., Wang Y.-F., Qin D., Currie S. N. (2016). Suckling-induced burst discharges of supraoptic oxytocin neurons in rats: Prostaglandin mediation of oxytocin actions. Int J Adv Res, 4, 1111–1122. [Google Scholar]
- Wang Y. F., Hatton G. I. (2004). Milk ejection burst-like electrical activity evoked in supraoptic oxytocin neurons in slices from lactating rats. J Neurophysiol, 91, 2312–2321. [DOI] [PubMed] [Google Scholar]
- Wang Y. F., Hatton G. I. (2005). Burst firing of oxytocin neurons in male rat hypothalamic slices. Brain Res, 1032, 36–43. [DOI] [PubMed] [Google Scholar]
- Wang Y. F., Hatton G. I. (2006). Mechanisms underlying oxytocin-induced excitation of supraoptic neurons: Prostaglandin mediation of actin polymerization. J Neurophysiol, 95, 3933–3947. [DOI] [PubMed] [Google Scholar]
- Wang Y. F., Hatton G. I. (2007. a). Dominant role of betagamma subunits of G-proteins in oxytocin-evoked burst firing. J Neurosci, 27, 1902–1912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y. F., Hatton G. I. (2007. b). Interaction of extracellular signal-regulated protein kinase 1/2 with actin cytoskeleton in supraoptic oxytocin neurons and astrocytes: Role in burst firing. J Neurosci, 27, 13822–13834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y. F., Hatton G. I. (2009). Astrocytic plasticity and patterned oxytocin neuronal activity: Dynamic interactions. J Neurosci, 29, 1743–1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y. F., Negoro H., Honda K. (1995). Effects of hemitransection of the midbrain on milk-ejection burst of oxytocin neurones in lactating rat. J Endocrinol, 144, 463–470. [DOI] [PubMed] [Google Scholar]
- Wang Y. F., Negoro H., Honda K. (1996. a). Amplitudes and time courses of milk ejection bursts of oxytocin neurons in the midbrain-hemitransected lactating rat. Brain Res, 719, 203–206. [DOI] [PubMed] [Google Scholar]
- Wang Y. F., Negoro H., Honda K. (1996. b). Milk ejection bursts of supraoptic oxytocin neurones during bilateral and unilateral suckling in the rat. J Neuroendocrinol, 8, 427–431. [DOI] [PubMed] [Google Scholar]
- Wang Y. F., Ponzio T. A., Hatton G. I. (2006). Autofeedback effects of progressively rising oxytocin concentrations on supraoptic oxytocin neuronal activity in slices from lactating rats. Am J Physiol Regul Integr Comp Physiol, 290, R1191–R1198. [DOI] [PubMed] [Google Scholar]
- Wang Y. F., Sun M. Y., Hou Q., Parpura V. (2013). Hyposmolality differentially and spatiotemporally modulates levels of glutamine synthetase and serine racemase in rat supraoptic nucleus. Glia, 61, 529–538. [DOI] [PubMed] [Google Scholar]
- Westberg L., Sawa E., Wang A. Y., Gunaydin L. A., Ribeiro A. C., Pfaff D. W. (2009). Colocalization of connexin 36 and corticotropin-releasing hormone in the mouse brain. BMC Neurosci, 10, 41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan H., Gao B., Duan L., Jiang S., Cao R., Xiong Y. F., Rao Z. R. (2010). Acute hyperosmotic stimulus-induced Fos expression in neurons depends on activation of astrocytes in the supraoptic nucleus of rats. J Neurosci Res, 88, 1364–1373. [DOI] [PubMed] [Google Scholar]
- Zhong M., Yang M., Sanborn B. M. (2003). Extracellular signal-regulated kinase 1/2 activation by myometrial oxytocin receptor involves Galpha(q)Gbetagamma and epidermal growth factor receptor tyrosine kinase activation. Endocrinology, 144, 2947–2956. [DOI] [PubMed] [Google Scholar]
- Zhou B., Wang D., Feng X., Zhang Y., Wang Y., Zhuang J., Zhang X., Chen G., Delpire E., Gu D., Cai H. (2012). WNK4 inhibits NCC protein expression through MAPK ERK1/2 signaling pathway. Am J Physiol Renal Physiol, 302, F533–F539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou J., Chen H., Yang C., Zhong J., He W., Xiong Q. (2017). Reversal of TRESK downregulation alleviates neuropathic pain by inhibiting activation of gliocytes in the spinal cord. Neurochem Res, 42, 1288–1298. [DOI] [PubMed] [Google Scholar]
- Zou L., Xue Y., Jones M., Heinbockel T., Ying M., Zhan X. (2018). The effects of quinine on neurophysiological properties of dopaminergic neurons. Neurotox Res, 34, 62–73. [DOI] [PubMed] [Google Scholar]