Abstract
Chronic inflammation of the salivary glands from pathologic conditions such as Sjögren’s syndrome can result in glandular destruction and hyposalivation. To understand which molecular factors may play a role in clinical cases of salivary gland hypofunction, we developed an aquaporin 5 (AQP5) Cre mouse line to produce genetic recombination predominantly within the acinar cells of the glands. We then bred these mice with the TNF-αglo transgenic line to develop a mouse model with salivary gland–specific overexpression of TNF-α; which replicates conditions seen in sialadenitis, an inflammation of the salivary glands resulting from infection or autoimmune disorders such as Sjögren’s syndrome. The resulting AQP5-Cre/TNF-αglo mice display severe inflammation in the salivary glands with acinar cell atrophy, fibrosis, and dilation of the ducts. AQP5 expression was reduced in the salivary glands, while tight junction integrity appeared to be disrupted. The immune dysregulation in the salivary gland of these mice led to hyposalivation and masticatory dysfunction.
Keywords: sialadenitis, aquaporin 5, xerostomia, transgenic mouse, acinar cells, tight junctions
Introduction
Saliva production aides in mastication while promoting proper oral hygiene (Tiwari 2011). Pathologic conditions that disrupt saliva flow, however, can lead to xerostomia accompanied by an associated increased incidence of dental caries. In general, hyposalivation can be a consequence of aging, medications, inflammation, or radiation therapy for head and neck cancer. Sialadenitis is a condition characterized by salivary gland inflammation that can be caused by infection, sialolithiasis (salivary stones), and autoimmune disorders such as Sjögren’s syndrome (SS; Seifert and Donath 1976). In some cases, hyposalivation from medications, aging, or disease can lead to bacterial colonization within the salivary duct, resulting in sialadenitis (Grisius and Fox 2003). TNF-α is an important mediator of inflammation in response to infection, and increased TNF-α expression has been often associated with inflammatory conditions that cause salivary gland hypofunction (Kang et al. 2011). For example, systemic inflammation by intraperitoneal injection of lipopolysaccharide in rats caused inhibition of saliva secretion as well as an upregulation of TNF-α in the salivary gland (Fernandez-Solari et al. 2010). In addition, human biopsies of patients with primary SS (Oxholm et al. 1992; Fox et al. 1994; Boumba et al. 1995; Kang et al. 2011) implicate TNF-α as having a major role in the pathophysiology of the disease. Besides the obvious immunopathologic effects on the cytoarchitecture of the salivary glands, increased TNF-α production can affect saliva production by inducing toxicity in sympathetic neurons (Soliven and Wang 1995), inhibiting the aquaporin 5 (AQP5) expression (Yamamura et al. 2012), disrupting tight junction formation (Baker et al. 2008), causing apoptosis (Sisto et al. 2009), and promoting basement membrane degradation (Azuma et al. 1997). Although increased TNF-α is highly associated with SS, TNF-α expression alone may not be the only inflammatory agent responsible for the pathophysiology of this autoimmune disorder, since the anti-TNF-α antibody infliximab does not appear to show any efficacy in treating SS (Mariette et al. 2004). The lack of efficacy of infliximab may be due to the fact that SS is a multifactorial disease (Oxholm et al. 1992) or because clinical neutralization of TNF-α may not have occurred early enough during the disease process (Zhou et al. 2017).
To better understand the genetic factors influencing saliva production, a Cre line was generated as a tool to conditionally knockout or overexpress a specific gene primarily within the salivary gland. We chose the AQP5 promoter to drive Cre expression because this water channel is expressed predominantly on the apical membrane of salivary gland acinar cells and is essential for saliva production (Sasaki et al. 2007). Cre-mediated recombination in this transgenic mouse line was primarily limited to only the salivary and the lacrimal glands. To determine if these mice could be used to generate a mouse model of salivary gland hypofunction, we bred the AQP5-Cre mice to the TNF-αglo transgenic line (Rozas et al. 2015; Hall et al. 2016) for conditional overexpression of this proinflammatory cytokine within the salivary gland, essentially to mimic the inflammatory conditions typically seen in cases of sialadenitis. The conditional overexpression of TNF-α in our AQP5/TNF-αglo mice led to recruitment of lymphocytes and macrophages into the salivary glands. AQP5 expression was reduced while the apical tight junctions in the acinar cells appeared to be disrupted. The immunopathology caused by the increased TNF-α expression subsequently led to acinar cell atrophy and hyposalivation in the mice. With the AQP5/TNF-αglo mice, we were able to demonstrate that the AQP5-Cre line can be used for targeted gene manipulation to specifically mimic pathologic conditions affecting the salivary glands, such as sialadenitis.
Materials and Methods
Human Samples
Human parotid salivary gland biopsies were obtained from patients (all female) at the School and Hospital of Stomatology, Wuhan University, China. These samples came from subjects presenting dry mouth and low saliva flow, but only 2 of the 5 patients had autoantibodies indicative of SS. Histoscore was calculated as previously described (Sun et al. 2012). The procedures were performed per the guidelines of the National Institutes of Health regarding the use of human tissues and with permission from the Institutional Ethical Board of the Wuhan University.
Quantitative Real-time Polymerase Chain Reaction and Western Blot
Salivary glands were harvested from AQP5/TNF-αglo (Cre+) and control TNF-αglo (Cre-) mice at 20 mo of age. For quantitative real-time polymerase chain reaction (qPCR), tissues were homogenized in TRIZOL (Thermo Fisher Scientific), and quantitative real-time PCR was performed as previously described (Prochazkova et al. 2013) with TaqMan primers for murine TNF-α, IL-1β, IL-6, and GAPDH (Thermo Fisher Scientific). Statistical differences were assessed with an unpaired t test (Prism 6; GraphPad), with all data expressed as mean ± SEM and a significance level set at P ≤ 0.05. Western blot to examine active TNF-α signaling was conducted as previously described (Hall et al. 2016).
Salivary Gland Secretory Analysis
Mice were euthanized at either 5 wk or 20 wk (chronic inflammation) of age. After perfusion with 4% paraformaldehyde, tissues were collected from mice and fixed in 4% paraformaldehyde overnight. Paraffin sections (5 μm) were then used for histopathologic analysis with the following stains: hematoxylin and eosin, mucicarmine, and Masson’s trichrome (Histoserv). The focus score was calculated by counting the number of inflammatory cell infiltrates per 200 × 200–μm square for male and female mice (aged 5 or 20 wk). Immunohistochemistry was performed as described (Hall et al. 2016) with the following primary antibodies: TNF-α (IW-PA1079; IHC World), MAC-2 (MA1-940; Thermo Fisher Scientific), ZO-1 (zonula occludens 1; 40-2200, Thermo Fisher Scientific), CD-3 (A0452; Dako-Agilent), and AQP5 (AB15858; Sigma-Millipore). For apoptosis, TUNEL staining was performed with the TUNEL TACS-XL Basic Kit (4828-30-K; Trevigen). Saliva was induced with pilocarpine (5 mg/kg; Sigma-Aldrich) and measured as previously described (Hall et al. 2010). As hyposalivation and salivary gland inflammation can affect masticatory function, mice were also tested with a dolognawmeter (Dolan et al. 2010; Hall et al. 2016). Trials were conducted during the day, with at least 1 d of rest between runs. All statistical differences for pilocarpine-induced saliva flow and gnawing times were analyzed with an unpaired t test (Prism 6; GraphPad), with all data expressed as the mean ± SEM and a significance level set at P ≤ 0.05.
Results
Generation of AQP5-Cre Mice
Our laboratory has been involved in the genetic manipulation of cytokine signaling in the salivary glands of mice with a MMTV-Cre (mouse mammary tumor virus) line. However, the MMTV promoter is active in other tissues, particularly the mammary glands, resulting in other extra–salivary gland effects (Nandula et al. 2007; Hall et al. 2010). Here we sought to generate another Cre line with more restricted salivary gland expression with the AQP5 promoter, particularly since this water channel is highly expressed on the apical membrane of salivary gland acinar cells and is important in saliva production (Sasaki et al. 2007). A region of the AQP5 promoter from a mouse BAC clone that was shown to drive expression in salivary acinar cells was amplified by PCR (Zhou et al. 2008). This 5.6-kb AQP5 promoter fragment was subcloned with IRES-Cre-BGH-polyA (Appendix Fig. 1a). To test whether the AQP5 promoter would be functional in epithelial salivary gland cells, we cotransfected the A5 rat submandibular cell line with the AQP5-Cre vector and Cre stoplight, a Cre reporter plasmid (He et al. 1989; Yang and Hughes 2001). Without recombination, only red fluorescence is seen, but in the presence of active Cre, the plasmid is recombined to cause expression of EGFP in place of DsRed1. As seen in Appendix Figure 1b, green fluorescence was detected within cotransfected cells, suggesting that Cre is expressed by the AQP5 promoter and would be active in vivo within the salivary gland epithelial cells.
The AQP5-Cre transgene was then microinjected to generate AQP5-Cre transgenic mice. Mouse founder lines were identified by Southern blot (Appendix Fig. 1c), and line C4 was later expanded and bred to a double-fluorescent Cre reporter mouse (mT/mG) to follow the Cre expression pattern (Muzumdar et al. 2007). Similar to the Cre stoplight, the mT/mG mice display ubiquitous tdTomato red fluorescence unless Cre is present to recombine the transgene and produce green fluorescence. As seen in Appendix Figure 2a, recombination is seen in target tissues of the submandibular, parotid, and lacrimal glands. Green fluorescence was strongly observed in the serous acini of the parotid glands with less seen in the acini of submandibular glands, potentially because of the mixture of serous and mucous acini (Appendix Fig. 2a, b). Unlike the salivary glands, lacrimal glands showed a mixed recombination-mediated fluorescence pattern, possibly due to glandular differences in AQP5 localization (Sasaki et al. 2007). Minimal green fluorescence was detected in all other studied tissues, even the lungs, where AQP5 is also expressed (Appendix Fig. 2a; Flodby et al. 2010).
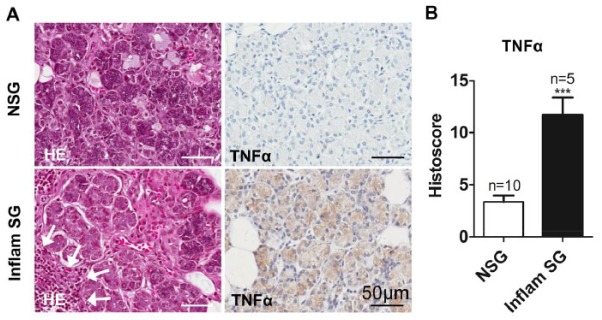
The AQP5-Cre mice were next employed to genetically manipulate the expression of a gene strongly associated with salivary gland dysfunction. Because of the known proinflammatory nature of TNF-α, we wanted to determine if overexpression of this cytokine alone would cause sialadenitis. Before the genetic manipulation of TNF-α levels in mice, the upregulation of TNF-α expression was confirmed in clinical cases of hyposalivation. Specifically, parotid gland biopsies from human subjects presenting xerostomia were examined to see if there was any correlation between inflammation and TNF-α expression. In contrast to the controls, lymphocytic infiltrates were observed in the parotid gland, and there was an associated increase in TNF-α staining from patients experiencing dry mouth, as seen with the histoscore (Fig. 1a, b). This suggests a link between TNF-α expression and inflammatory-mediated hyposalivation.
Figure 1.
TNF-α expression correlates with salivary gland hypofunction and inflammation. (A) Parotid gland biopsies were obtained from patients (all female) experiencing low saliva flow. Physical examination of these patients indicated inflammation of the parotid glands, but only 2 of 5 had a confirmed diagnosis of primary SS (positive for autoantibodies indicative of SS). Inflammatory infiltrates are seen histologically in patients with dry mouth (arrows). Immunohistochemistry shows increased TNF-α expression in association with inflammation and dry mouth. (B) The histoscore (total cell intensity divided by total cell number) confirms significantly increased TNF-α expression within the inflamed parotid glands. ***P ≤ 0.001. Values are presented as mean ± SEM. HE, hematoxylin and eosin; NSG, normal salivary gland; SG, salivary gland; SS, Sjögren’s syndrome.
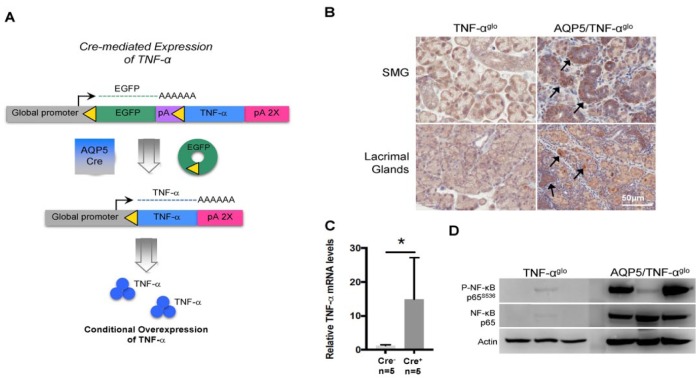
Following confirmation that elevated TNF-α expression is present in clinical cases of salivary gland hypofunction, we next wanted to study its role in the pathogenesis of salivary gland inflammation in a mouse model. We chose to breed the AQP5-Cre mice with the TNF-αglo transgenic line (Hall et al. 2016) for overexpression of TNF-α in the acinar cells of the salivary gland (Fig. 2a). Transgenic expression of TNF-α, while exceeding levels typical in cases of sialadenitis, will still allow us to determine if overexpression alone causes inflammation and to understand its immunologic impact. AQP5/TNF-αglo mice were born at the expected genotypic ratio of 25%. Although AQP5/TNF-αglo mice exhibited no signs of wasting, they were consistently 5 g smaller than their littermate controls. Nonetheless, there was no statistical difference in serum TNF-α levels (data not shown). Staining for TNF-α confirmed localized overexpression within the salivary and lacrimal glands of the AQP5/TNF-αglo (Fig. 2b), which was further demonstrated by real-time PCR (Fig. 2c). Active downstream TNF-α signaling was demonstrated with increased phosphorylated levels of NF-κB in the submandibular glands of the AQP5/TNF-αglo mice, albeit at variable levels (Fig. 2d).
Figure 2.
Targeted overexpression of TNF-α in the salivary glands through AQP5-Cre-expressing mice. (A) A schematic demonstrating targeted conditional overexpression of TNF-α in the salivary glands that is generated by breeding AQP5-Cre mice with the TNF-αglo transgenic line. (B) The resulting AQP5/TNF-αglo mice display targeted overexpression of TNF-α (arrows) in the submandibular and lacrimal glands. (C) Elevated TNF-α mRNA levels demonstrated by quantitative real time PCR. (D) Elevated phosphorylation of NF-κB reveals active TNF-α signaling as a result of transgene recombination. *P < 0.05. Values are presented as mean ± SEM. AQP5, aquaporin 5; SMG, submandibular gland.
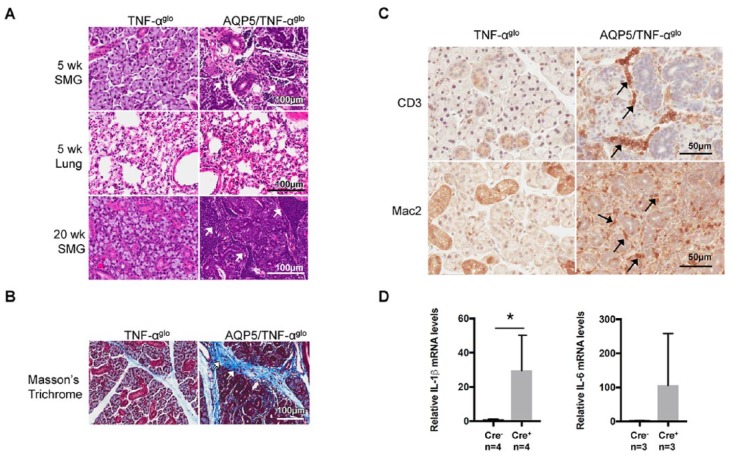
Conditional overexpression of TNF-α disrupted normal immune homeostasis and caused glandular destruction, where, at 5 wk of age, lymphocytic infiltrates were apparent within the salivary glands of the AQP5/TNF-αglo mice (Fig. 3a). No inflammatory infiltrates were observed in other tissues, including the lungs. By 20 wk of age, chronic TNF-α signaling caused widespread destruction and severe inflammation throughout the salivary gland (Fig. 3a). The gender of the mice did not correlate with the degree of inflammation (Appendix Fig. 2c). Because inflammation can lead to glandular fibrosis among patients with SS (Koski et al. 2001), we next stained the salivary glands of the AQP5/TNF-αglo mice with Masson’s trichome and saw abnormal fibrotic collagen deposition, suggestive of inflammatory-mediated interstitial fibrosis (Fig. 3b). We characterized the lymphocytic infiltrates seen within the salivary glands by immunohistochemistry, particularly since T-helper type 1 T cells have been implicated in the development of autoimmune disorders such as SS (Ishimaru and Hayashi 2009). CD3 staining of the AQP5/TNF-αglo mice showed T-cell infiltrates within the salivary glands with an accompanying recruitment of macrophages (as seen with Mac2 staining; Fig. 3c). Interestingly, SSA and SSB autoantibodies, specific serological markers of SS, were not observed in our mouse model (data not shown). The recruitment of T cells and macrophages could be a result of the chemotactic nature of TNF-α (Ming et al. 1987) or could result from the induction of other inflammatory cytokines. Indeed, real-time PCR showed upregulation of other proinflammatory cytokines, including a significant induction of IL-1β and a trend toward increased IL-6 (Fig. 3d).
Figure 3.
Inflammation in the submandibular gland resulting from conditional overexpression of TNF-α. (A) Inflammatory infiltrates are seen in the submandibular glands of the AQP5/TNF-αglo mice at 5 wk of age (arrows), while other tissues, including the lungs, show no histologic signs of inflammation. A focus score of 90 ± 31 inflammatory infiltrates was counted per 200 × 200–μm square for male and female AQP5/TNF-αglo mice (total of 10 squares counted), while no inflammation was seen in the Cre- controls. By wk 20, severe inflammation is present in the submandibular glands, leading to glandular destruction (inflammatory foci were too numerous to count). (B) Chronic inflammation results in interstitial fibrosis, as seen by the abnormal collagen deposition shown by Masson trichrome staining. (C) CD3 staining shows recruitment of T cells in the submandibular glands of the AQP5/TNF-αglo mice. Mac2 staining shows an accompanying infiltration of macrophages into the submandibular glands around the blood vessels and ducts. (D) Additional proinflammatory cytokines are induced by overexpression of TNF-α in the submandibular gland, including a significant increase in IL-1β (*P ≤ 0.05, n = 4) and a trend toward increased IL-6 (n = 4). Values are presented as mean ± SEM. AQP5, aquaporin 5; SMG, submandibular gland.
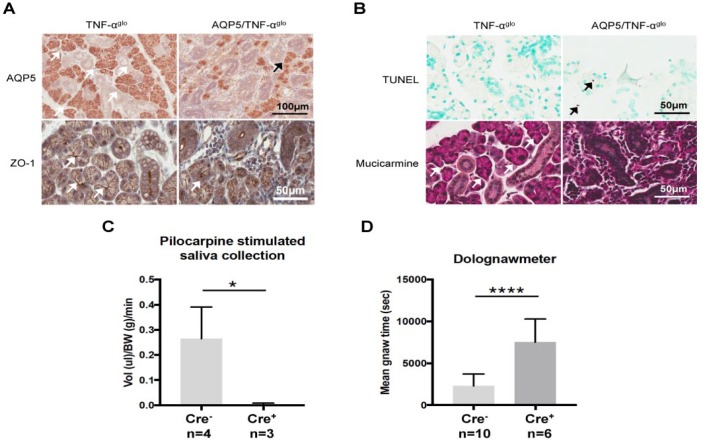
Besides the immunologic features seen in the AQP5/TNF-αglo mice, acinar cell function was assessed. Since the localization and expression of AQP5 is altered in SS (Steinfeld et al. 2001; Lai et al. 2016), AQP5 was examined in the mice by immunohistochemistry. As shown in Figure 4a, reduced levels of AQP5 staining were seen in the salivary glands of the AQP5/TNF-αglo mice. We additionally stained for the tight junction protein ZO-1 in the AQP5/TNF-αglo mice, as TNF-α treatment affects tight junction function (Baker et al. 2008), and its expression is decreased in the labial salivary glands of patients with SS (Ewert et al. 2010). As seen in Figure 4a, the acinar cells in the AQP5/TNF-αglo mice show reduced and abnormal distribution of ZO-1, suggesting that TNF-α affects the proper formation of the apical tight junctions. With reduced AQP5 staining and tight junction defects, we next wanted to determine if these defects were caused by apoptosis within the salivary glands of the AQP5/TNF-αglo mice. Increased apoptosis with nuclear fragmentation (Fig. 4b) was seen in the salivary glands of the AQP5/TNF-αglo mice, possibly resulting in the observed reduced AQP5 staining. We also examined whether the mucous acini were affected by overexpression of TNF-α as well, so we evaluated mucin production with mucicarmine and saw a loss of mucous acini with a replacement of the glandular tissue by inflammatory infiltrates (Fig. 4b).
Figure 4.
Overexpression of TNF-α affects salivary function causing apoptosis, hyposalivation, and masticatory dysfunction. (A) The submandibular glands of the AQP5/TNF-αglo mice show reduced expression of the water channel AQP5 and the tight junction protein ZO-1 (arrows), suggesting possible secretory dysfunction. (B) Apoptotic nuclear fragmentation (arrows) seen in the submandibular glands of the AQP5/TNF-αglo mice. Acinar cell atrophy was confirmed with mucin stain mucicarmine (white arrows in the Cre- TNF-αglo mice), where the AQP5/TNF-αglo mice show a loss of mucous acini. (C) Pilocarpine-induced saliva flow is reduced in the Cre+ AQP5/TNF-αglo mice as compared with the Cre- TNF-αglo mice, suggesting severe salivary gland hypofunction (**P ≤ 0.05; 20-wk-old mice). (D) Dolognawmeter testing to measure masticatory dysfunction reveals longer gnawing times for Cre+ AQP5/TNF-αglo mice versus Cre- littermate controls (****P ≤ 0.001; 20-wk old mice—the final 3 of 10 trials are recorded and averaged). Values are presented as mean ± SEM. AQP5, aquaporin 5; ZO-1, zonula occludens 1.
With these histologic findings of inflammation and associated glandular immunopathology, we next examined if there was actual salivary gland hypofunction in these mice. We injected mice with the muscarinic agonist pilocarpine to study saliva flow and saw severe hyposalivation in the AQP5/TNF-αglo mice (Fig. 4c). Clinical cases of sialadenitis are often accompanied by painful swelling and tenderness to the salivary gland, so, to determine if this could affect masticatory function, we tested the AQP5/TNF-αglo mice in a dolognawmeter, which records the time needed for a mouse to gnaw through a plastic dowel (Dolan et al. 2010). The AQP5-Cre/TNF-αglo mice exhibited longer gnawing times as compared with the controls, possibly as a result of glandular inflammation (Fig. 4d). Saliva, however, is known to aide in swallowing by lubricating food for consumption, so, although the plastic dowel is not consumed by the mice, we cannot rule out hyposalivation as a contributor to longer gnawing times for the AQP5-Cre/TNF-αglo mice.
Discussion
Our laboratory previously demonstrated that alterations in TGF-β signaling can have deleterious effects on salivary gland homeostasis (Nandula et al. 2007; Hall et al. 2010). To continue our investigations examining the effects of manipulating cytokine signaling in the salivary gland, we next wanted to conditionally overexpress TNF-α. However, a better approach to restricting Cre recombination specifically within the salivary gland was needed (Hall et al. 2010), so we developed the AQP5-Cre line. Analysis of AQP5 promoter activity with the mT/mG Cre reporter mouse line showed that recombination was limited to the salivary and lacrimal glands. To determine if the AQP5-Cre line could be used to mimic pathologic hyposalivation disorders, we then bred the AQP5-Cre mice with the TNF-αglo transgenic line to overexpress TNF-α within the salivary glands, a feature common to many salivary gland disorders.
Upregulation of TNF-α levels is considered a factor in the pathogenesis of SS (Oxholm et al. 1992; Fox et al. 1994; Boumba et al. 1995; Kang et al. 2011). TNF-α is a pleiotropic cytokine that can produce a range of immunomodulatory effects by binding onto the TNFR1 or TNFR2 receptors that are expressed on almost all cells (Sisto et al. 2009). While TNF-α is predominantly expressed by macrophages and lymphocytes, enrichment of salivary gland epithelial cells from patients with SS showed that these nonmyeloid cells can also express TNF-α (Fox et al. 1994; Sisto et al. 2009). By secreting TNF-α, acinar cells may participate in the pathophysiology of SS rather than just act as targets of autoimmunity (Fox et al. 1994). Increased secretion of TNF-α can, in turn, affect acinar cell function by decreasing AQP5 expression (Yamamura et al. 2012) and reducing transepithelial resistance (Baker et al. 2008), both of which will result in secretory dysfunction. Blocking TNF-α signaling alone, however, does not appear to prevent SS-related sialadenitis, as anti-TNF-α therapy showed no efficacy in treating SS (Mariette et al. 2004; Moutsopoulos et al. 2008). Neutralization of TNF-α may need to occur at an earlier time point. In the NOD model of SS, for example, lymphocytic infiltrates appear at 10 wk of age, but TNF-α is increased at 4 wk (Zhou et al. 2017). So, TNF-α may act as an instigator of inflammation in many salivary gland disorders, even if it is not the sole player.
In summary, an AQP5-Cre line was used to generate a mouse model of targeted overexpression of TNF-α that mimics some aspects of salivary gland disorders. Overexpression of TNF-α caused inflammation accompanied by acinar cell atrophy. TNF-α overexpression and resulting inflammation were restricted to the salivary gland through the AQP5-Cre. There was no inflammation in other tissues, including the lung, where AQP5 is known to be expressed. Rather, inflammation was predominantly localized to only the salivary and lacrimal glands through the AQP5-Cre (Zhou et al. 2008; Flodby et al. 2010). The AQP5-Cre mice therefore provide a valuable means of genetically examining other factors that might contribute to salivary pathophysiology.
Author Contributions
A. Limaye, Z.J. Sun, A.B. Kulkarni, contributed to conception, design, data analysis, drafted and critically revised the manuscript; B.E. Hall, contributed to conception, design, and data analysis, drafted the manuscript; L. Zhang, A. Cho, M. Prochazkova, C. Zheng, M. Walker, contributed to data analysis, drafted the manuscript; F. Adewusi, P.D. Burbelo, contributed to analysis, drafted and critically revised the manuscript; I.S. Ambudkar, J.C. Dolan, B.L. Schmidt, contributed to data analysis, critically revised the manuscript. All authors gave final approval and agree to be accountable for all aspects of the work.
Supplemental Material
Supplemental material, DS_10.1177_0022034519837240 for Targeted TNF-α Overexpression Drives Salivary Gland Inflammation by A. Limaye, B.E. Hall, L. Zhang, A. Cho, M. Prochazkova, C. Zheng, M. Walker, F. Adewusi, P.D. Burbelo, Z.J. Sun, I.S. Ambudkar, J.C. Dolan, B.L. Schmidt and A.B. Kulkarni in Journal of Dental Research
Acknowledgments
We thank Dr. Kenneth Yamada for critical reading of the manuscript, Niklas Malmstrom for technical assistance, and the Veterinary Resource Core, the Gene Transfer Core, and the Combined Technical Research Core of the Division of Intramural Research for providing expert support.
Footnotes
This work was supported by the Division of Intramural Research of the National Institute of Dental and Craniofacial Research, National Institutes of Health.
The authors declare no potential conflicts of interest with respect to the authorship and/or publication of this article.
A supplemental appendix to this article is available online.
ORCID iD: L. Zhang  https://orcid.org/0000-0001-9049-8807
https://orcid.org/0000-0001-9049-8807
References
- Azuma M, Motegi K, Aota K, Hayashi Y, Sato M. 1997. Role of cytokines in the destruction of acinar structure in Sjögren’s syndrome salivary glands. Lab Invest. 77(3):269–280. [PubMed] [Google Scholar]
- Baker OJ, Camden JM, Redman RS, Jones JE, Seye CI, Erb L, Weisman GA. 2008. Proinflammatory cytokines tumor necrosis factor-alpha and interferon-gamma alter tight junction structure and function in the rat parotid gland Par-C10 cell line. Am J Physiol Cell Physiol. 295(5):C1191–C1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boumba D, Skopouli FN, Moutsopoulos HM. 1995. Cytokine mRNA expression in the labial salivary gland tissues from patients with primary Sjögren’s syndrome. Br J Rheumatol. 34(4):326–333. [DOI] [PubMed] [Google Scholar]
- Dolan JC, Lam DK, Achdjian SH, Schmidt BL. 2010. The dolognawmeter: a novel instrument and assay to quantify nociception in rodent models of orofacial pain. J Neurosci Methods. 187(2):207–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ewert P, Aguilera S, Alliende C, Kwon YJ, Albornoz A, Molina C, Urzúa U, Quest AF, Olea N, Pérez P, et al. 2010. Disruption of tight junction structure in salivary glands from Sjögren’s syndrome patients is linked to proinflammatory cytokine exposure. Arthritis Rheum. 62(5):1280–1289. [DOI] [PubMed] [Google Scholar]
- Fernandez-Solari J, Prestifilippo JP, Ossola CA, Rettori V, Elverdin JC. 2010. Participation of the endocannabinoid system in lipopolysaccharide-induced inhibition of salivary secretion. Arch Oral Biol. 55(8):583–590. [DOI] [PubMed] [Google Scholar]
- Flodby P, Borok Z, Banfalvi A, Zhou B, Gao D, Minoo P, Ann DK, Morrisey EE, Crandall ED. 2010. Directed expression of Cre in alveolar epithelial type 1 cells. Am J Respir Cell Mol Biol. 43(2):173–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox RI, Kang HI, Ando D, Abrams J, Pisa E. 1994. Cytokine mRNA expression in salivary gland biopsies of Sjögren’s syndrome. J Immunol. 152(11):5532–5539. [PubMed] [Google Scholar]
- Grisius MM, Fox PC. 2003. Salivary gland diseases. In: Burkett’s oral medicine diagnosis and treatment. 10th ed. London (UK): BC Decker Inc; p. 235–265. [Google Scholar]
- Hall BE, Zhang L, Sun ZJ, Utreras E, Prochazkova M, Cho A, Terse A, Arany P, Dolan JC, Schmidt BL, et al. 2016. Conditional TNF-α overexpression in the tooth and alveolar bone results in painful pulpitis and osteitis. J Dent Res. 95(2):188–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall BE, Zheng C, Swaim WD, Cho A, Nagineni CN, Eckhaus MA, Flanders KC, Ambudkar IS, Baum BJ, Kulkarni AB. 2010. Conditional overexpression of TGF-beta1 disrupts mouse salivary gland development and function. Lab Invest. 90(4):543–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He XJ, Ship J, Wu XZ, Brown AM, Wellner RB. 1989. Beta-adrenergic control of cell volume and chloride transport in an established rat submandibular cell line. J Cell Physiol. 138(3):527–535. [DOI] [PubMed] [Google Scholar]
- Ishimaru N, Hayashi Y. 2009. Regulation of T cell activation in Sjögren’s syndrome. Jpn Dent Sci Rev. 45(1):41–45. [Google Scholar]
- Kang EH, Lee YJ, Hyon JY, Yun PY, Song YW. 2011. Salivary cytokine profiles in primary Sjögren’s syndrome differ from those in non-Sjögren sicca in terms of TNF-α levels and Th-1/Th-2 ratios. Clin Exp Rheumatol. 29(6):970–976. [PubMed] [Google Scholar]
- Koski H, Janin A, Humphreys-Beher MG, Sorsa T, Malmström M, Konttinen YT. 2001. Tumor necrosis factor-alpha and receptors for it in labial salivary glands in Sjögren’s syndrome. Clin Exp Rheumatol. 19(2):131–137. [PubMed] [Google Scholar]
- Lai Z, Yin H, Cabrera-Pérez J, Guimaro MC, Afione S, Michael DG, Glenton P, Patel A, Swaim WD, Zheng C, et al. 2016. Aquaporin gene therapy corrects Sjögren’s syndrome phenotype in mice. Proc Natl Acad Sci U S A. 113(20):5694–5699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mariette X, Ravaud P, Steinfeld S, Baron G, Goetz J, Hachulla E, Combe B, Puéchal X, Pennec Y, Sauvezie B, et al. 2004. Inefficacy of infliximab in primary Sjögren’s syndrome: results of the randomized, controlled Trial of Remicade in Primary Sjögren’s Syndrome (TRIPSS). Arthritis Rheum. 50(4):1270–1276. [DOI] [PubMed] [Google Scholar]
- Ming WJ, Bersani L, Mantovani A. 1987. Tumor necrosis factor is chemotactic for monocytes and polymorphonuclear leukocytes. J Immunol. 138(5):1469–1474. [PubMed] [Google Scholar]
- Moutsopoulos NM, Katsifis GE, Angelov N, Leakan RA, Sankar V, Pillemer S, Wahl SM. 2008. Lack of efficacy of etanercept in Sjögren syndrome correlates with failed suppression of tumour necrosis factor alpha and systemic immune activation. Ann Rheum Dis. 67(10):1437–1443. [DOI] [PubMed] [Google Scholar]
- Muzumdar MD, Tasic B, Miyamichi K, Li L, Luo L. 2007. A global double-fluorescent Cre reporter mouse. Genesis. 45(9):593–605. [DOI] [PubMed] [Google Scholar]
- Nandula SR, Amarnath S, Molinolo A, Bandyopadhyay BC, Hall B, Goldsmith CM, Zheng C, Larsson J, Sreenath T, Chen W, et al. 2007. Female mice are more susceptible to developing inflammatory disorders due to impaired transforming growth factor beta signaling in salivary glands. Arthritis Rheum. 56(6):1798–1805. [DOI] [PubMed] [Google Scholar]
- Oxholm P, Daniels TE, Bendtzen K. 1992. Cytokine expression in labial salivary glands from patients with primary Sjögren’s syndrome. Autoimmunity. 12(3):185–191. [DOI] [PubMed] [Google Scholar]
- Prochazkova M, Terse A, Amin ND, Hall B, Utreras E, Pant HC, Kulkarni AB. 2013. Activation of cyclin-dependent kinase 5 mediates orofacial mechanical hyperalgesia. Mol Pain. 9:66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozas P, Lazcano P, Piña R, Cho A, Madrid R, Gonzalez-Billault C, Kulkarni AB, Utreras E. 2015. Targeted overexpression of tumor necrosis factor-α (TNF-α) in nociceptive tissues increased cyclin-dependent kinase 5 (Cdk5) activity with subsequent increased of Ca+2 influx on trigeminal ganglia neurons. Pain. 157(6):1346–1362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki Y, Tsubota K, Kawedia JD, Menon AG, Yasui M. 2007. The difference of aquaporin 5 distribution in acinar and ductal cells in lacrimal and parotid glands. Curr Eye Res. 32(11):923–929. [DOI] [PubMed] [Google Scholar]
- Seifert G, Donath K. 1976. Classification of the pathohistology of diseases of the salivary glands—review of 2,600 cases in the Salivary Gland Register. Beitr Pathol. 159(1):1–32. [DOI] [PubMed] [Google Scholar]
- Sisto M, D’Amore M, Caprio S, Mitolo V, Scagliusi P, Lisi S. 2009. Tumor necrosis factor inhibitors block apoptosis of human epithelial cells of the salivary glands. Ann N Y Acad Sci. 1171:407–414. [DOI] [PubMed] [Google Scholar]
- Soliven B, Wang N. 1995. Tumor necrosis factor-alpha regulates nicotinic responses in mixed cultures of sympathetic neurons and nonneuronal cells. J Neurochem. 64(2):883–894. [DOI] [PubMed] [Google Scholar]
- Steinfeld S, Cogan E, King LS, Agre P, Kiss R, Delporte C. 2001. Abnormal distribution of aquaporin-5 water channel protein in salivary glands from Sjögren’s syndrome patients. Lab Invest. 81(2):143–148. [DOI] [PubMed] [Google Scholar]
- Sun ZJ, Zhang L, Hall B, Bian Y, Gutkind JS, Kulkarni AB. 2012. Chemopreventive and chemotherapeutic actions of mTOR inhibitor in genetically defined head and neck squamous cell carcinoma mouse model. Clin Cancer Res. 18(19):5304–5313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiwari M. 2011. Science behind human saliva. J Nat Sc Biol Med. 2(1):53–58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamura Y, Motegi K, Kani K, Takano H, Momota Y, Aota K, Yamanoi T, Azuma M. 2012. TNF-α inhibits aquaporin 5 expression in human salivary gland acinar cells via suppression of histone H4 acetylation. J Cell Mol Med. 16(8):1766–1775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang YS, Hughes TE. 2001. Cre stoplight: a red/green fluorescent reporter of Cre recombinase expression in living cells. Biotechniques. 31(5):1036, 1038, 1040–1041. [DOI] [PubMed] [Google Scholar]
- Zhou B, Ann DK, Flodby P, Minoo P, Liebler JM, Crandall ED, Borok Z. 2008. Rat aquaporin-5 4.3-kb 5′-flanking region differentially regulates expression in salivary gland and lung in vivo. Am J Physiol Cell Physiol. 295(1):C111–C120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou J, Kawai T, Yu Q. 2017. Pathogenic role of endogenous TNF-α in the development of Sjögren’s-like sialadenitis and secretory dysfunction in non-obese diabetic mice. Lab Invest. 97(4):458–467. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, DS_10.1177_0022034519837240 for Targeted TNF-α Overexpression Drives Salivary Gland Inflammation by A. Limaye, B.E. Hall, L. Zhang, A. Cho, M. Prochazkova, C. Zheng, M. Walker, F. Adewusi, P.D. Burbelo, Z.J. Sun, I.S. Ambudkar, J.C. Dolan, B.L. Schmidt and A.B. Kulkarni in Journal of Dental Research