Abstract
Objective: Meropenem is a parenteral carbapenem antibiotic which has a broad spectrum of activity against aerobes and anaerobes. Meropenem’s bactericidal activity is determined by the time during which meropenem concentration remains above the minimal inhibition concentration (MIC) during the dosing interval. Thus, prolonged infusion is the optimal way to maximize the time-dependant activity. However, studies to date have shown that carbapenems and in particular, meropenem, are relatively unstable in solution. The aims of this study were therefore (1) to establish the effects of temperature on the concentration of a generic brand reconstituted meropenem solution and (2) to determine whether 24-hour continuous infusion is possible without concentrations dropping below the recommended 90%. Method: Preliminary examination was carried out by the means of nuclear magnetic resonance (NMR) spectroscopy. Meropenem was subsequently assayed using high-performance liquid chromatography (HPLC). The method was developed and validated in compliance with International Council for Harmonisation (ICH) guidelines. Meropenem’s stability was examined at two temperatures 22°C and 33°C to mimic average and high temperature in hospital wards. Solutions were prepared aseptically at the clinically relevant concentration. Results: NMR results obtained showed an increase in open ring methyl groups peak intensity, indicating that meropenem begins to degrade upon dissolution (d=1.05 and 1.25). Results obtained from quantitative HPLC confirm that meropenem concentrations dropped to 90% of initial concentration at 7.4 hours and 5.7 hours at 22°C and 33°C, respectively. Conclusion: Although results obtained indicate that meropenem should not be continuously infused over 24 hours, it is possible that meropenem could be continuously infused for at least 7 hours if temperature does not exceed 22°C and for 5 hours if temperature does not exceed 33°C.
Keywords: meropenem, stability, continuous infusions, temperature, IV bags, normal saline
Introduction
Antibiotic resistance is increasing to dangerously high levels worldwide threatening the effective prevention and treatment of an ever-increasing range of infections. The increasing occurrence of infection caused by multidrug-resistant, gram-negative bacterial pathogens challenges clinicians to adopt new administration strategies with the intent of optimizing bactericidal activity of currently available beta-lactams.1-3
Meropenem is a parenteral carbapenem that is structurally related to beta-lactam antibiotics such as penicillin and cephalosporin. It has an excellent bactericidal activity against a wide range of clinically significant gram-negative and gram-positive aerobic and anaerobic bacteria, thus known as a broad spectrum antibiotic.4-7 Meropenem is a time-dependant antibiotic; hence, bactericidal effects are closely correlated to the time at which concentrations remain above the MIC.8 Periods at which concentrations are above the MIC (T > MIC) is a major parameter determining efficacy. When concentrations drop lower than the MIC (T < MIC), bacterial growth resumes immediately because meropenem has no significant postantibiotic effect.9,10
Meropenem is currently administered via intermittent bolus infusion (over ≈ 15-30 minutes) which has numerous advantages, including better utilization of intravenous access, less concern about drug degradation over time, and fewer compatibility concerns. This mode of administration, however, results in concentrations falling below the MIC.11 Although administration by continuous infusion maximizes the pharmacodynamic properties, carbapenems, in particular meropenem, are known to be quite unstable, hence a major parameter that needs to be studied.12 The stability of meropenem should be maintained throughout the infusion time in order for the patient to receive abundant amounts of active drug needed to achieve clinical cure while avoiding exposure to harmful degradation products.13
Various studies have demonstrated that continuous infusion of meropenem is beneficial in terms of efficacy based on pharmacokinetics and pharmacodynamics.14-18 However, to comply with the European and US Pharmacopeia’s, beta-lactam antibiotics should always contain at least 90% to 110% of the initial concentration.13
One of the major limitations of continuous infusion meropenem is that it would demand frequent drug preparation and administration due to its short stability.16,17 This presents a challenge in practice as it requires pharmacy staff to produce multiple preparations and nursing staff to coordinate the multiple administrations throughout the day. Moreover, the problem is compounded in hospital wards where temperatures are not controlled, therefore exposing the infusion to elevated temperatures that can compromise stability.14 Therefore, the aims of this study were (1) to establish the effects of temperature on the decomposition of reconstituted meropenem solutions and (2) to determine whether 24-hour continuous infusion is possible without concentrations dropping below the recommended 90% of the initial concentration.
Methods
Instrumentation
Nuclear magnetic resonance (NMR) spectroscopy was performed on 600 MHz base frequency Bruker Avance III two-channel FT-NMR spectrometer. Quantitative analysis was performed by the means of reverse-phase high-performance liquid chromatography (HPLC) using an Agilent 1260 HPLC system with single wavelength ultraviolet (UV) detection and ChemStation software.
Materials
Meropenem reference standard, Methanol (HPLC grade), acetonitrile (HPLC grade), ammonium acetate, glacial acetic acid, and paracetamol were purchased from Sigma Aldrich; 0.9% saline was bought from Baxter, and 100-mL 0.9% saline infusion bags were obtained from St George’s hospital, London, UK. All of the above materials were used without further purification. Pharmaceutical dosages from meropenem infusion vials (1000 mg) were prepared in a fume hood using an aseptic, nontouch technique. Generic brand, AstraZeneca, intravenous (IV) meropenem was reconstituted with 20 mL water for injection and further diluted with 50 mL of 0.9% saline.
Preliminary Investigation
Preliminary examination of meropenem was carried out by the means of NMR spectroscopy to provide an indication of how fast meropenem degraded and inform development of the HPLC method. Meropenem solution at the clinically relevant concentration [(1000 mg / 70 mL) × 1000 = 14 285 ppm] was placed in an NMR tube and instrument was programmed to periodically analyze throughout the day. The remainder of reconstituted solution was left at room temperature for 2 weeks and then analyzed by HPLC to understand the characteristics of meropenem degradation product/s.
Method Development
The aim was to develop a HPLC method capable of separating meropenem from its degradation products and measuring its concentration in infusion solutions, precisely and rapidly. Parameters investigated were mobile phase, column type, choice of internal standard, injection volume, flow rate, column temperature, linear range, and detection wavelength.
Method Validation
The developed method was tested for linearity, range, precision, accuracy, specificity, sensitivity, and robustness in compliance with ICH validation guidelines. The method was tested over the period of 5 days by running 3 replicates of a standard set of samples once a day for 5 days.
Reconstituting 1 g of meropenem powder for infusion with 20 mL water for injection and mixing with 50 mL of saline (as in clinical practice) gives an infusion concentration of 14 286 ppm meropenem. This solution will be diluted 1 in 50 for analysis, which reduces the amount of sample needed, reduces matrix effects, and gives a nominal concentration of 286 ppm meropenem, in the usual analytical range for quantitative HPLC with UV detection.
Analytical range was tested by injecting variable volumes (2, 4, 6, 8 10, 15, and 20 µL) of a 1000 ppm standard solution (equivalent to injecting 10 µL of a set of standards, concentrations from 200 to 2000 ppm). Precision was considered at 3 levels: repeatability, intermediate precision, and reproducibility. System and method precision were also considered.
Accuracy was determined by measurement of recovery and evaluated by using QC samples: (1) low (75 ppm), (2) medium (250 ppm), and (3) high (450 ppm).
Specificity/selectivity was assessed by running diluent blanks. Preparation of diluent blanks involved spiking 750 of saline, water for injection and mobile phase with 250 of internal standard.
Robustness parameters examined include changes in column temperature (±5°C), changes in flow rate (±0.2 mL/min), changes in mobile phase pH (±0.2 units), and changes in the mobile phase composition (95:5: v/v; ammonium acetate: acetonitrile).
Sample Preparation
Two 1-g meropenem pharmaceutical dose vials were emptied into a 250-mL beaker and reconstituted with 40 mL water for injection, and 100 mL 0.9% saline was added and the solution was stirred. Six 100-mL 0.9% saline polyvinyl chloride IV bags were emptied completely, dried, and injected using a 20-mL syringe with same amount (20 mL) of reconstituted bulk solution (14 285 ppm) and incubated at the relevant temperature, 22°C and 33°C. The concentration of meropenem was measured every hour for 21 hours after reconstitution and testing was performed in duplicate. The infusion solution was considered stable while the percentage of intact molecule remained above 90%.
Physical Observation
Two responses were measured: physical compatibility and color change. The solutions stored in IV bags were examined at every sampling interval for cloudiness, crystallization/precipitation, color change, and signs of interactions.
HPLC Assay
The samples were diluted 50-fold with mobile phase (700 μL), and paracetamol (250 μL; concentration 1000 ppm) was used as an internal standard (meropenem concentration = 286 ppm). The diluted samples were injected (injection volume 10 μL) via an auto sampler onto a Synergi 4 μm, POLAR-RP-80R, 250 × 4.6-mm column. The mobile phase was composed of 10-mM ammonium acetate buffer adjusted to pH 5.4 with acetic acid and acetonitrile (90:10: vol/vol), isocratic elution at a flow rate of 2.8 mL/min. The UV detector wavelength was set to 290 nm and the column temperature was set at 50±1°C.
Results
Method Validation
The internal standard corrected calibration curve displayed good linearity over the concentration range of 0 to 500 µL/mL. The representative linear equation was y = 0.9943x − 0.0566, with a correlation coefficient (R2) of 0.9998 (Figure 1). The regression line (R2 = .9996) in Figure 2 demonstrates linearity in the range of 0 to 2000 ppm.
Figure 1.
Showing average 7 concentration calibration (internal standard corrected) to assess linearity.
Figure 2.
Showing linearity in the range of 0 to 2000 ppm.
Good system precision was obtained where intrasample precision ranged between Percentage Relative Standard Deviation (%RSD) 0.01% and 0.37%. Intraday precision attained ranged between %RSD 0.25% and 1.90% and interday precision obtained ranged between %RSD 1.79% and 5.64%.
The percentage error determined from all standard QC samples was between 0.29 and 0.68%; therefore, percent recovery values obtained were at least 99.32%. For all blanks, there was no significant response. Meropenem reference standard solution was spiked with internal standard to ensure that peaks are well resolved.
Increasing the column temperature decreased the pressure and retention time. Slight changes in mobile phase pH displayed no significant difference in peak area or peak shape. The robustness study demonstrated that the method can optimally perform reproducibly when parameters change slightly.
The limit of detection was 5.55 ppm and the limit of quantitation was 16.82 ppm.
Preliminary Results
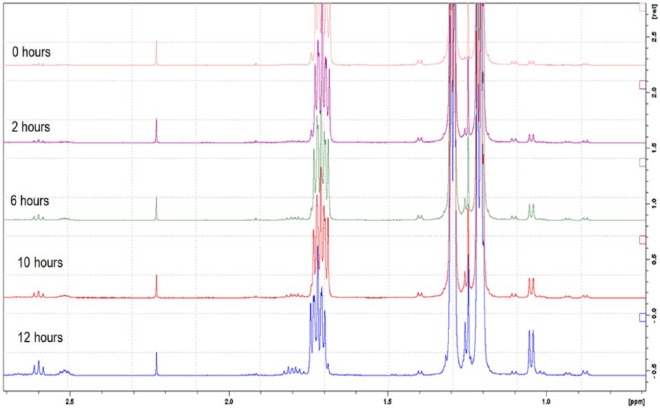
The results of the preliminary NMR investigation are shown in Figure 3. The solution was analyzed periodically by proton NMR with water suppression. The increase in peak intensity in the spectra, most prominently at chemical shifts of 1.05, 1.8, 2.5, and 2.6 ppm, suggests that meropenem begins to degrade upon dissolution. NMR was used as a qualitative method for characterization rather than a quantitative technique as it would have been difficult to quantify due peak overlap. NMR provided an estimation of how fast meropenem degraded; however, the instrument did not give an indication of concentration, so further testing by HPLC was needed.
Figure 3.
Preliminary nuclear magnetic resonance spectra of reconstituted meropenem.
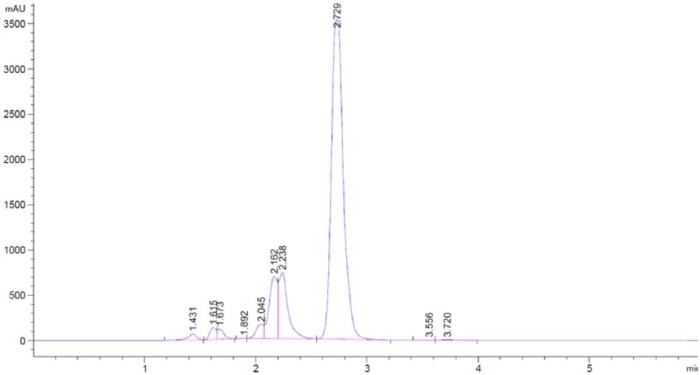
Preliminary investigation by the means of HPLC was carried out to give an indication of how many degradants meropenem has and to obtain the retention time of both meropenem and the degradation product/s. Results displayed in Figure 4 show the retention time of both meropenem and its degradation product/s. The spectra displays good resolution of meropenem and it degradant/s as no coelution was observed and the compounds are not distinguished from one another.
Figure 4.
Preliminary high-performance liquid chromatography of aged meropenem sample to indicated degradation product/s retention time.
Physical Observation Results
Upon preparation, the solution was clear and colorless, but as the samples began to age, the color of the solution became tinged yellow. The older the sample, the more distinct the color change became. It was also observed that the change in color was noticeable for solutions incubated at 33°C (7 hours) before solutions stored 22°C (12 hours).
Quantitative Results
Figure 5 shows the influence of time on meropenem concentrations at 22°C and 33°C, indicating the time at which meropenem concentration drops 10%. All data were drift-corrected and corrected with the internal standard. The slopes of the regression lines shown are statistically significant at the 99% level of confidence, indicating that meropenem concentrations decrease with time.
Figure 5.
Showing the gradual decrease in meropenem concentration at both 22°C and 33°C.
Results show that meropenem maintained 90% intact molecule for 7.4 hours at 22°C and within 20 hours, 71.2% of the initial concentration remained. Meropenem maintained 90% intact molecule for 5.7 hours at 33°C and within 20 hours, 61.4% of initial concentration was maintained.
Discussion
In recent years, stability studies have been given significant attention due to their importance in development and quality control of pharmaceutical products.19 Meropenem has no significant postantibiotic effect as bactericidal activity is determined by the time at which the drug concentration remains 4 or 5 times above the MIC. The interval between intermittent doses may result in meropenem concentrations falling below the MIC as high peak concentrations cannot enhance the bactericidal activity of meropenem.20
Determining meropenem stability could give an insight as to whether continuous infusions are feasible, thus increasing patient response to therapy which can theoretically delay development of resistance. Numerous recent studies have assessed the clinical benefits of continuous/extended infusion compared with current intermittent dosing to optimize the bacterial activity of parenteral antibiotics for treating infections.12,15,21-25
Fehér et al studied groups of patients receiving meropenem to determine whether the administration of meropenem via a 4-hour extended infusion leads to a more favorable clinical outcome. From this observational study, it was concluded that treatment of febrile neutropenia was more successful with extended 4-hour infusions than 30-minute bolus infusion, with 68.4% and 40.9% of patients clinically cured, respectively (P < .001).21 Dulhunty et al observed the clinical and pharmacokinetic differences between continuous and intermittent dosing in critically ill patients with severe sepsis. Results obtained from this prospective controlled trial demonstrated that continuous infusion achieved higher antibiotic plasma concentration and also achieved higher rates of clinical cure (70% vs 43%; P = .0037).23
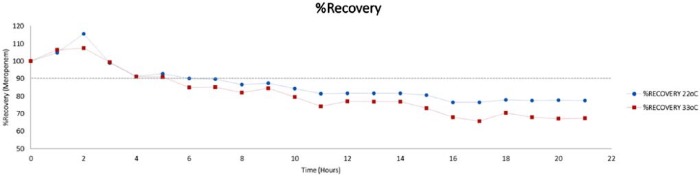
Continuously infusing meropenem will reduce the workload related to multiple administrations; however, the stability of meropenem is an important factor that needed consideration. Our findings confirmed that the stability of meropenem in solution was influenced by the storage temperature. Drug concentrations dropped to 90% of initial concentration after 7.4 hours at 22°C and 5.7 hours at 33°C (Figure 6) (Table 1). The visual color change observed in meropenem solution is not reported in meropenem stability studies; however, it is likely due to the degradation process and the formation of degradation product. Change in color usually indicates that the beta-lactam ring has been hydrolyzed. It is recommended that solutions that have changed color should not be administered to patients as changes in physical compatibility occur with loss of potency and an increase in toxicity.19
Figure 6.
Showing meropenem percent recovery at all sampling intervals.
Table.1.
Showing the Percentage of Concentration Remaining at Each Sampling Interval for 22°C and 33°C.
| Time (h) | 0 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | 19 | 20 | 21 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| %R 22°C | 100 | 105 | 115 | 99 | 91 | 93 | 90 | 90 | 87 | 87 | 84 | 81 | 82 | 82 | 82 | 81 | 76 | 76 | 78 | 78 | 78 | 78 |
| %R 33°C | 100 | 106 | 107 | 99 | 91 | 91 | 85 | 85 | 82 | 84 | 79 | 74 | 77 | 77 | 77 | 73 | 68 | 66 | 70 | 68 | 67 | 67 |
Note. %R= %Recovery.
To our knowledge, this is one of a few studies that examined the impact of elevated temperatures (experienced on hospital ward) on meropenem’s stability. Results obtained in this study suggest that in clinical settings with ambient temperatures below 33°C, extended infusion could be considered. Results from previous studies support our findings as they also suggested that the stability of meropenem reconstituted in solution is influenced by the storage temperature. At lower ambient temperatures, meropenem is stable for a longer time than when stored at elevated temperatures.26 Drug concentration is significantly reduced at high storage temperatures and should not be administered in tropical countries.14 Smith et al looked at the stability of meropenem in different infusion devices at low temperatures and confirmed that meropenem was stable for 5 days at 5°C.25 However, Kuti et al suggested that meropenem is stable for only ~4 to 6 hours at room temperature, and its pharmacokinetic properties, when administered by continuous infusion, are largely unknown.27
Conclusion
Although results obtained determine that meropenem should not be continuously infused over 24 hours, it is suggested that patients can receive continuously infused meropenem for at least 7 hours if temperature does not exceed 22°C and for 5 hours if temperature does not exceed 33°C.
Acknowledgments
We would like to acknowledge support from Antimicrobials department in St Georges Hospitals and also the technical support provided by Dr Jean-Marie Peron at Kingston University on NMR data acquisition and analysis.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The authors are grateful to Kingston University for the provision of laboratory space and technical support for S. Fawaz. This research did not receive any specific grant from funding agencies in the public, commercial, or for not-for-profit sectors.
ORCID iD: Stephen Barton  https://orcid.org/0000-0002-9661-8416
https://orcid.org/0000-0002-9661-8416
References
- 1. Neu HC. The crisis in antibiotic resistance. Science. 1992;257(5073):1064-1074. [DOI] [PubMed] [Google Scholar]
- 2. Kumarasamy KK, Toleman MA, Walsh TR, et al. Emergence of a new antibiotic resistance mechanism in India, Pakistan, and the UK: a molecular, biological, and epidemiological study. Lancet Infect Dis. 2010;10(9):597-602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lambert PA. Mechanisms of antibiotic resistance in Pseudomonas aeruginosa. J R Soc Med. 2002;95(suppl 41):22-26. [PMC free article] [PubMed] [Google Scholar]
- 4. Zhanel GG, Wiebe R, Dilay L, et al. Comparative review of the carbapenems. Drugs. 2007;67(7):1027-1052. [DOI] [PubMed] [Google Scholar]
- 5. Bedikian A, Okamoto MP, Nakahiro RK, et al. Pharmacokinetics of meropenem in patients with intra-abdominal infections. Antimicrob Agents Chemother. 1994;38(1):151-154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Aryal S. Differences Between Gram Positive and Gram Negative Bacteria. Date unknown. http://www.microbiologyinfo.com/differences-between-gram-positive-and-gram-negative-bacteria/. Accessed August 31, 2015.
- 7. Prince BT, McMahon BJ, Jain M, Peters AT. Meropenem tolerance in a patient with probable fulminant piperacillin-induced immune hemolytic anemia. J Allergy Clin Immunol Pract. 2015;3(3):452-453. [DOI] [PubMed] [Google Scholar]
- 8. Harrison MP, Moss SR, Featherstone A, Fowkes AG, Sanders AM, Case DE. The disposition and metabolism of meropenem in laboratory animals and man. J Antimicrob Chemother. 1989;24(suppl A):265-277. [DOI] [PubMed] [Google Scholar]
- 9. Drusano GL, Hutchison M. The pharmacokinetics of meropenem. Scand J Infect Dis. 1995;96(suppl):11-6. [PubMed] [Google Scholar]
- 10. Thyrum PT, Yeh C, Birmingham B, Lasseter K. Pharmacokinetics of meropenem in patients with liver disease. Clin Infect Dis. 1997;24(suppl2):S184-S190. [DOI] [PubMed] [Google Scholar]
- 11. Mollá-Cantavella S, Ferriols-Lisart R, Torrecilla-Junyent T, Alós-Almiñana M. Intravenous meropenem stability in physiological saline at room temperature. Eur J Hosp Pharm. 2014;21(4):202-207. [Google Scholar]
- 12. Denooz R, Charlier C. Simultaneous determination of five beta-lactam antibiotics (cefepim, ceftazidim, cefuroxim, meropenem and piperacillin) in human plasma by high-performance liquid chromatography with ultraviolet detection. J Chromatogr B Analyt Technol Biomed Life Sci. 2008;864(1-2):161-167. [DOI] [PubMed] [Google Scholar]
- 13. Manning L, Wright C, Ingram PR, et al. Continuous infusions of meropenem in ambulatory care: clinical efficacy, safety and stability. PloS One. 2014;9(7):e102023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Jaruratanasirikul S, Sriwiriyajan S. Stability of meropenem in normal saline solution after storage at room temperature. Southeast Asian J Trop Med Public Health. 2003;34(3):627-629. [PubMed] [Google Scholar]
- 15. Thalhammer F, Traunmuller F, Menyawi IE, et al. Continuous infusion versus intermittent administration of meropenem in critically ill patients. J Antimicrob Chemother. 1999;43(4):523-527. [DOI] [PubMed] [Google Scholar]
- 16. de Cordova PB, Lucero RJ, Hyun S, Quinlan P, Price K, Stone PW. Using the nursing interventions classification as a potential measure of nurse workload. J Nurs Care Qual. 2010;25(1):39-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Beswick S, Hill PD, Anderson MA. Comparison of nurse workload approaches. J Nurs Manag. 2010;18(5):592-598. [DOI] [PubMed] [Google Scholar]
- 18. Jenkins A, Hills T, Santillo M, Gilchrist M. Drug Stability Working Group of the BSAC UK OPAT Initiative. Extended stability of antimicrobial agents in administration devices. J Antimicrob Chemother. 2017;72(4):1217-1220. [DOI] [PubMed] [Google Scholar]
- 19. NHS Pharmaceutical Quality Assurance Committee. Standard Protocol for Deriving and Assessment of Stability Part 1—Aseptic Preparations (Small Molecule). 2nd ed. England: 2011. https://www.sps.nhs.uk/wp-content/uploads/2017/06/Stability-part-1-small-molecules-v4-April-17.pdf [Google Scholar]
- 20. Novelli A, Fallani S, Cassetta MI, Conti S, Mazzei T. Postantibiotic leukocyte enhancement of meropenem against Gram-positive and Gram-negative strains. Antimicrob Agents Chemother. 2000;44(11):3174-3176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Fehér C, Rovira M, Soriano A, et al. Effect of meropenem administration in extended infusion on the clinical outcome of febrile neutropenia: a retrospective observational study. J Antimicrob Chemother. 2014;69(9):2556-2562. [DOI] [PubMed] [Google Scholar]
- 22. Zobell JT, Ferdinand C, Young DC. Continuous infusion meropenem and ticarcillin-clavulanate in pediatric cystic fibrosis patients. Pediatr Pulmonol. 2014;49(3):302-306. [DOI] [PubMed] [Google Scholar]
- 23. Dulhunty JM, Roberts JA, Davis JS, et al. Continuous infusion of beta-lactam antibiotics in severe sepsis: a multicenter double-blind, randomized controlled trial. Clin Infect Dis. 2013;56(2):236-244. [DOI] [PubMed] [Google Scholar]
- 24. Wang D. Experience with extended-infusion meropenem in the management of ventilator-associated pneumonia due to multidrug-resistant Acinetobacter baumannii. Int J Antimicrob Agents. 2009;33(3):290-291. [DOI] [PubMed] [Google Scholar]
- 25. Smith DL, Bauer SM, Nicolau DP. Stability of meropenem in polyvinyl chloride bags and an elastomeric infusion device. Am J Health Syst Pharm. 2004;61(16):1682-1685. [DOI] [PubMed] [Google Scholar]
- 26. Patel PR, Cook SE. Stability of meropenem in intravenous solutions. Am J Health Syst Pharm. 1997;54(4):412-421. [DOI] [PubMed] [Google Scholar]
- 27. Kuti JL, Nightingale CH, Knauft RF, Nicolau DP. Pharmacokinetic properties and stability of continuous-infusion meropenem in adults with cystic fibrosis. Clin Ther. 2004;26(4):493-501. [DOI] [PubMed] [Google Scholar]