Abstract
Ischemia–reperfusion injury (IRI) is a major, but potentially preventable contributor to the final tissue damage after an acute myocardial infarction. Many therapies have demonstrated successful reduction of IRI in preclinical settings, but none has shown improved outcomes in clinical studies. Part of the failure to translate new therapies to clinical settings can be attributed to the reliance on small animal models in preclinical studies. While animal models encapsulate the complexity of the systemic in vivo environment, they do not necessarily recapitulate human physiology. In this study, we utilized cardiac tissue engineering methods and cardiomyocytes derived from human induced pluripotent stem cells to establish a human tissue-engineered model of IRI. The resulting cardiac constructs were subjected to conditions that simulated ischemia–reperfusion. We demonstrated the presence of reperfusion injury and the ability to distinguish ischemic and reperfusion injury. We also demonstrated reductions in IRI following ischemic preconditioning, modification of reperfusion conditions, and addition of cardioprotective therapeutics. This study establishes the utility of a human tissue model for studying key aspects of IRI and the potential for improving translation of therapeutic strategies into the clinical setting.
Impact Statement
Reducing ischemia–reperfusion injury would significantly improve patient survival. Current preclinical models are inadequate because they rely on animals, which do not emulate human physiology and the clinical setting. We developed a human tissue platform that allowed us to assess the human cardiac response, and demonstrated the platform's utility by measuring injury during ischemia–reperfusion and the effects of cardioprotective strategies. The model provides a foundation for future studies on how patient-specific backgrounds may affect response to therapeutic strategies. These steps will be necessary to help translate therapies into the clinical setting.
Keywords: myocardium, disease modeling, bioreactor, human cardiac tissue, cardioprotection
Introduction
The most effective approach to improve clinical outcomes after myocardial infarction (MI) is reperfusion of the occluded artery.1 However, reperfusion itself leads to additional injury, termed ischemia–reperfusion injury (IRI).2 IRI involves the death of cells that were otherwise still viable at the end of the ischemic insult, and it is a significant contributor to the final infarct size. IRI is potentially preventable and limiting it will be a major step in managing cardiovascular disease.
Despite the importance of reducing IRI, there is currently no effective clinical therapy. Ischemic preconditioning—subjecting the heart to short periods of ischemia and reperfusion that cause no damage before the actual ischemic insult—has reduced the final infarct size in animals.3 However, ischemic preconditioning is not a clinically relevant therapy because it must occur before the MI. Instead, efforts to reduce IRI have focused on the modification of reperfusion conditions or addition of therapeutics during reperfusion.
Currently, the therapeutic development for IRI relies on animal studies. However, physiological differences between animals and humans can hinder the clinical predictability of these studies.4–6 Significantly, none of the therapeutics that have demonstrated decreased IRI in preclinical studies have improved outcomes in large clinical trials.7–10 This lack of success indicates that current preclinical models are inadequate,11 and that new approaches predictive of human pathophysiology of ischemia and reperfusion are needed. In particular, human tissue models are potentially powerful tools to facilitate translation into the clinical setting.12
In this study, we created a human tissue model of myocardial IRI. The model utilized human induced pluripotent stem cell-derived cardiomyocytes (hiPS-CMs) to study the effects of IRI on a human platform. However, hiPS-CMs are notoriously fetal in nature,13 so we promoted their maturation through culture in a tissue engineering bioreactor.14–19 The resulting cardiac constructs were subjected to simulated ischemia–reperfusion and tested for therapeutic strategies to reduce IRI. We saw increases in cell injury upon reperfusion compared to simulated ischemia only, and the reduction of IRI through ischemic preconditioning and modification of reperfusion conditions.
Materials and Methods
Detailed methods are provided in the Supplementary Data (Supplementary Data are available online at www.liebertpub.com/tea).
Cell culture
The hiPS cell line WTC11 was kindly provided by Dr. Bruce Conklin (through a Material Transfer Agreement with the Gladstone Institute of Cardiovascular Disease, UCSF). The hiPS cells were maintained in mTeSR 1 medium (STEMCELL Technologies) and differentiated into cardiomyocytes by following published methods routinely used in our laboratory.20 Briefly, differentiation was achieved through modification of the Wnt pathway using CHIR 99021 and Wnt-C59 (Tocris).
Bioreactor design
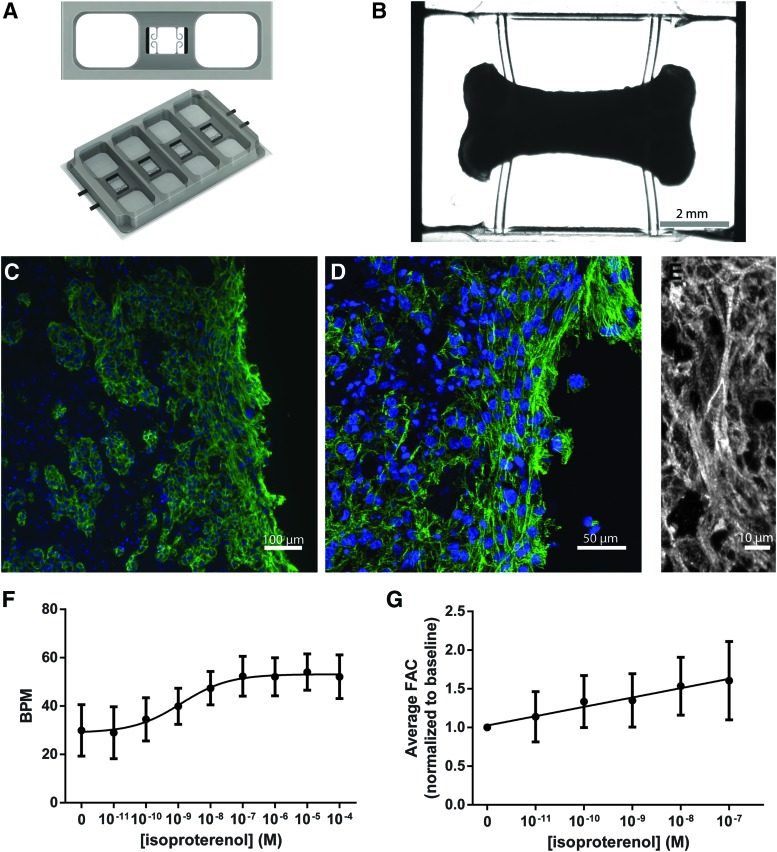
The bioreactors were formed from polydimethylsiloxane (PDMS) in a custom-designed mold. The bioreactor itself contained a small well (6 mm × 8 mm × 2 mm) for construct formation inside a larger well (41 mm × 12 mm × 6.5 mm), serving as a culture medium reservoir. The construct formed around two pairs of flexible PDMS pillars that were spaced 4.5 mm apart. Each bioreactor supported the formation of four independent constructs (Fig. 1A).
FIG. 1.
Bioreactor for the formation and maturation of human cardiac constructs. (A, B) Overview of the bioreactor. Constructs formed by seeding hiPS-CMs in a collagen–fibrinogen hydrogel around flexible pillars. Flexible pillars provided mechanical conditioning through passive tension and allowing for auxotonic contractions. (C–E) Immunofluorescence images demonstrating the presence of aligned and cross-striated hiPS-CMs along the edge of the construct. 4′,6-Diamidino-2-phenylindole (blue), cardiac troponin T (green/white). (F, G) Response of cardiac constructs to β-adrenergic stimulation using isoproterenol. (F) BPM for chronotropic response and (G) FAC as an indicator of force generation for ionotropic response. Data are presented as mean ± SD (n = 6). hiPS-CM, human induced pluripotent stem cell-derived cardiomyocyte; BPM, beats per minute; FAC, fractional area change; SD, standard deviation. Color images are available online.
Bioreactor cultivation of cardiac constructs
hiPS-CMs were generated in monolayers by staged differentiation over a period of 12–17 days. The resulting cells were early-stage cardiomyocytes that were used to form tissue constructs and matured during subsequent bioreactor culture similar to our previous studies.17 Approximately 25 × 106 hiPS-CMs/mL were encapsulated in a hydrogel containing 1.5 mg/mL of rat collagen type I (Corning) and 4 mg/mL bovine fibrinogen (Sigma). One hundred twenty microliter of cell–hydrogel solution was placed into each well of the bioreactor to form a cardiac construct around the pillars. The constructs were cultured in RPMI 1640 medium supplemented with 2% B27 (Thermofisher), 213 μg/mL ascorbic acid, 100 U/mL penicillin, and 100 μg/mL streptomycin. Aprotinin (33 μg/mL; Sigma) was added to the media for the first 7 days of culture. Constructs were analyzed and used for modeling of IRI either after 2 weeks of culture (relatively mature constructs) or 3 days of culture (immature constructs).
Histology and immunostaining
Constructs were fixed in 4% paraformaldehyde, frozen, and cut into 10-μm thick sections that were stained with cardiac troponin T antibody (1:100, MS-295-P1; Thermofisher), and visualized with goat anti-mouse Alexa Fluor 488 (Thermofisher; A-11001). Nuclei were counterstained with 4′,6-diamidino-2-phenylindole.
Isoproterenol dose–response
To evaluate construct function, isoproterenol (Sigma; I6504) was used to measure chronotropic and ionotropic responses. Twenty-second long videos were taken at each concentration of isoproterenol and analyzed in MATLAB using a custom-written code, similar to our previous studies,17 to measure the beating frequency and the fractional area change (FAC). FAC was determined by masking the construct area and measuring the change in area between the relaxed and contracted state. Response was determined by comparing each construct to its baseline. Dose–response curves were analyzed in GraphPad Prism 6 software using built-in nonlinear regression tools.
Modeling of ischemia and ischemia–reperfusion
To model ischemia, the constructs were washed, and incubated in 120 μL of ischemic solution (119 mM NaCl, 12 mM KCl, 1.2 mM NaH2PO4, 1.3 MgSO4, 0.5 MgCl2, 0.9 CaCl2, 20 mM sodium lactate, and 5 mM HEPES, pH = 6.4) added to each bioreactor construct formation well. The small volume promoted metabolic waste accumulation and allowed constructs to modify their extracellular environment. These conditions were used to mimic the nutrient deprivation, hyperkalemia, high lactate concentration, and low extracellular pH seen during ischemia in the heart. Bioreactors were placed into a hypoxic chamber, perfused by anoxic gas (95% N2, 5% CO2), and cultured at 37°C for 6 h (Supplementary Fig. S1A).
To model reperfusion, constructs were returned to a mixture of air and 5% CO2 to restore oxygenation, and 2 mL media were added to the bioreactors to fully washout the accumulated waste, replenish nutrients, and reestablish extracellular pH and ionic balance (Supplementary Fig. S1B). The media consisted of RPMI 1640 supplemented with 1.4 mM calcium chloride (to establish physiological calcium concentration of 1.8 mM21) and 2% B27 without antioxidants (Thermofisher 10889038), at pH = 7.4. Bioreactors were placed into the incubator at 37°C for 3 h before endpoint analysis was performed.
Normoxic (control) constructs were cultured under the conditions used for reperfusion for the entire duration of the experiment (9 h) (Supplementary Fig. S1C).
Treatments to reduce IRI
For ischemic preconditioning, constructs were subjected to simulated ischemia for 30 min, followed by simulated reperfusion for 15 min. The constructs were then subjected to normal simulated ischemia–reperfusion; the exact conditions for both stages are detailed above
The reperfusion medium was modified to test cardioprotective strategies. To test reperfusion with acidic media, the reperfusion medium was titrated down to pH = 6.4 using 1 N HCl. To test the effects of drugs, either cyclosporine A (CsA, 1 μM; Sigma C1832) or N-acetyl-l-cysteine (NAC, 5 mM; Sigma A9165) was added to the reperfusion medium.
All treatment groups were compared to normoxic control and constructs subjected to normal simulated ischemia–reperfusion. All media used for simulated reperfusion were checked for pH and titrated to a pH = 7.4 using 1 N NaOH or 1 N HCl, except for acidic reperfusion (pH = 6.4) (Supplementary Fig. S1C).
Construct characterization after simulated ischemia–reperfusion
Cell death was assessed by measuring cell membrane permeability through lactate dehydrogenase (LDH, 88954; Thermofisher) and adenylate kinase (AK) release (LT07; Toxilight, Lonza) into the media. Cell viability was determined using RealTime-Glo (Promega; G9712). Mitochondrial membrane permeability (MMP) was assessed using JC-1 dye by measuring excitation at 485 nm and comparing emission at 528 and 590 nm. Reactive oxygen species (ROS) levels were measured using ROS-Glo (Promega; G8820). Construct intracellular pH was measured using pHrodo Green dye (ThermoFisher; P35373). All assays were used according to manufacturer's instructions.
Western blot
For Western blot analysis, 3–4 constructs per group were pooled together, flash-frozen, and homogenized. Protein concentrations were determined using bicinchoninic acid assay, and 30 μg of total protein was added to each lane. Membranes were probed using antibodies, as follows, and developed under chemiluminescence: all antibodies obtained from Cell Signaling Technology: cleaved caspase-3 (9664), total caspase-3 (9668), phospho-Akt (4060), Akt (pan) (2920), and β-tubulin (86298). Quantification was done in Licor Image Studio software.
Statistical analysis
Two-way unpaired t-test was used to compare two groups, while analysis of variance with post-hoc Tukey's HSD was used to compare three groups. p < 0.05 was used as a statistically significant difference. Statistical tests were performed using GraphPad Prism 6 software.
Results
Cardiac construct formation
Cardiac tissue constructs were formed by encapsulating hiPS-CMs in a collagen–fibrinogen hydrogel in the bioreactor. The construct formation well contained two pairs of flexible pillars for the cardiac constructs to form around and attach to, inside a larger well for medium addition (Fig. 1A, B). The flexible pillars provided passive tension to the construct to help align the cardiomyocytes, and they allowed for auxotonic contractions promoting mechanical conditioning and cardiomyocyte maturation.
After 2 weeks of bioreactor culture, the constructs were examined for their morphology. Histology revealed that the tissues were more compact at the periphery than at the construct center (Fig. 1C), likely due to the construct size limiting oxygen and nutrient availability to the center. The cardiomyocytes in the peripheral layers of the construct appeared more mature with aligned and cross-striated morphology (Fig. 1D, E).
Responses to β-adrenergic stimulation were measured using serial dilutions of isoproterenol. A positive chronotropic response was demonstrated, with an increase from 29.9 ± 11 beats per minute (BPM) at baseline to 54.0 ± 7.5 BPM at 10 μM isoproterenol, and an EC50 of 1.3 nM (Fig. 1F). The constructs were further analyzed for the FAC, a proxy for force generation. Increases in FAC from baseline to 100 nM were observed. Nonlinear regression of the dose–response data determined that the changes in FAC were significant and are indicative of a positive ionotropic response to isoproterenol (Fig. 1G and Supplementary Fig. S2). Taken together, the constructs demonstrated comprehensive responses to β-adrenergic stimulation.
Distinguishing ischemic and reperfusion injury
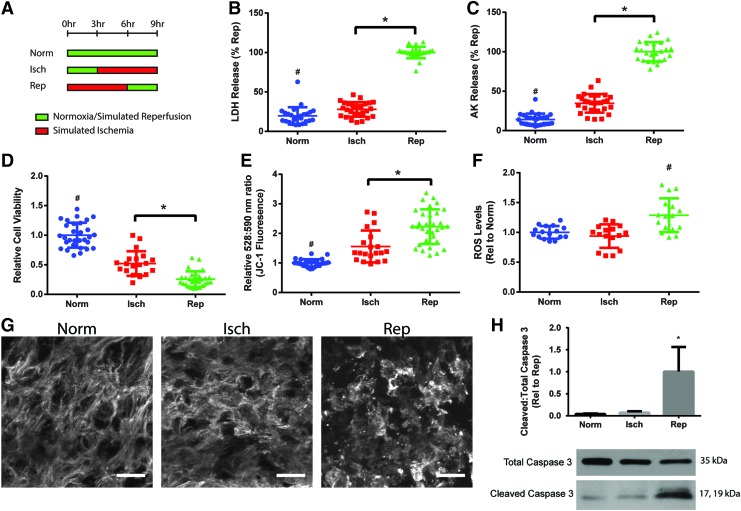
Because in vitro models examine IRI in non-physiological settings, they must first be validated to demonstrate that IRI can be observed. We specifically aimed to distinguish ischemic injury from reperfusion injury by comparing the construct responses to ischemia only (“Isch”) and ischemia followed by reperfusion (“Rep”). The Isch and Rep groups were further compared to control constructs cultured under normoxic culture conditions (“Norm”) (Fig. 2A).
FIG. 2.
Validating presence of ischemia–reperfusion injury in cardiac constructs. (A) Response of constructs to simulated ischemia (“Isch”) and simulated ischemia followed by reperfusion (“Rep”) compared to constructs cultured under normoxia (“Norm”). Constructs were analyzed for (B, C) cell death as measured by LDH and AK release, (D) cell viability as measured using RealTime-Glo assay, (E) mitochondrial membrane permeability as measured by JC-1 dye, where increased ratio of emission at 528 and 590 nm is correlated with higher permeability, and (F) ROS. Data in (B–E) (five independent experiments) and (F) (four independent experiments) and are shown as individual data points with mean ± SD. (G) Representative images of cardiac construct ultrastructure. Cardiac troponin T (white), scale bar = 20 μm. (H) Apoptosis determined by Western blot analysis of cleaved and total caspase 3. Data from three independent experiments are shown as mean ± SD. Statistical analysis done using ANOVA with post-hoc Tukey's HSD, #Indicates statistical significance compared to all other groups, *Indicates significant difference between groups, p < 0.05. LDH, lactate dehydrogenase; AK, adenylate kinase; ROS, reactive oxygen species, ANOVA, analysis of variance. Color images are available online.
Isch constructs demonstrated significant increases in cell death over Norm constructs, as evidenced by measurements of LDH and AK release. Reperfusion led to further cell injury with a significant increase in cell death in Rep over Isch constructs (Fig. 2B, C). Furthermore, the Isch group demonstrated a significant decrease in cell viability compared to Norm controls, and reperfusion led to a further decrease in the Rep group (Fig. 2D). These results demonstrated that simulated ischemia caused cell injury in the cardiac constructs, and that reperfusion further exacerbated injury compared to ischemia alone.
JC-1 staining was used to further characterize IRI by assessing MMP. Mitochondrial permeability transition pore (MPTP) opening with subsequent mitochondria depolarization is known to be a critical step leading to cell death due to reperfusion injury.22 We observed a significant increase in MMP in the Isch constructs over the Norm group (Fig. 2E). Reperfusion led to an even larger increase in MMP, with a significant increase in Rep over Isch constructs, indicating opening of the MPTP during reperfusion.
Reperfusion and restoration of physiologic oxygen levels in the infarct region lead to rapid generation of ROS and oxidative stress that contributes to reperfusion injury.23 Rep constructs demonstrated higher levels of ROS compared to Isch and Norm constructs (Fig. 2F), indicating that oxidative stress after reperfusion could be contributing to IRI in the human tissue model.
Constructs were also evaluated for ultrastructural changes after ischemia and reperfusion. Normoxic constructs demonstrated aligned and striated staining for cardiac troponin T. This organization was disrupted to some extent in Isch constructs and even more so in Rep constructs compared to Norm constructs (Fig. 2G).
While ischemia leads to initial activation of apoptotic pathways to cause cell death, reperfusion leads to a further increase in apoptosis that contributes to injury.24–27 We assessed apoptosis in the constructs by examining the cleavage of caspase 3 into its active form through Western blot analysis. Isch constructs demonstrated a small increase in the ratio of cleaved and total caspase 3, while Rep constructs demonstrated a significantly higher increase in caspase 3 cleavage (Fig. 2H). Overall, these results demonstrated that reperfusion led to increased injury over ischemia alone in the constructs.
Effects of construct maturity
As a part of model validation, we established the importance of the bioreactor in providing a platform for maturation of hiPS-CMs by examining the responses of relatively immature cardiac constructs to simulated ischemia–reperfusion. Immature cardiac constructs were tested after 3 days of bioreactor culture, compared to the 2 weeks of culture for other studies. Three days was after they first started to demonstrate spontaneous contractions, but before they had time to structurally and functionally mature.
Immature constructs were subjected to ischemia–reperfusion and compared to immature normoxic (control) cardiac constructs. Reperfused constructs demonstrated no difference in LDH release compared to normoxic controls, but they did have significantly higher AK release (Supplementary Fig. S3A, B). The differences in cell death were much smaller than those seen in matured constructs. Cell viability and MMP measurements demonstrated no difference between the groups (Supplementary Fig. S3C, D). The immature cardiac constructs did not demonstrate the comprehensive response observed in their more mature counterparts (Fig. 2). We concluded that construct maturation in the bioreactor is a critical component for modeling IRI using the human tissue model.
Ischemic preconditioning
We next sought to demonstrate the utility of the system for examining various strategies to reduce IRI in the cardiac constructs. Ischemic preconditioning is the only treatment known to robustly decrease IRI in animal models,3 but this treatment has not been rigorously demonstrated to decrease IRI in humans. Importantly, preconditioning cannot be studied in acute MI patients as it must occur before the ischemic insult. A human tissue model would thus be useful to study ischemic preconditioning in a controllable setting.
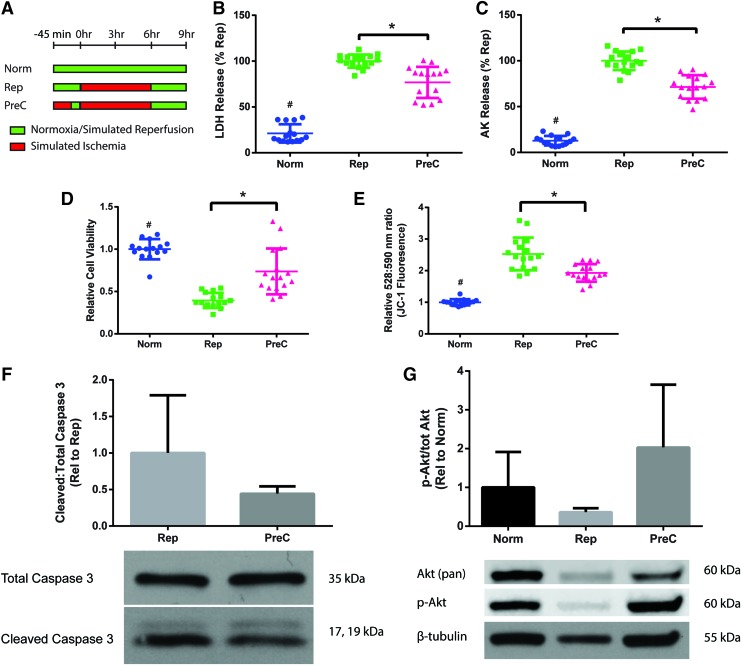
We simulated ischemic preconditioning by subjecting the constructs to 30 min of ischemia and 15 min of reperfusion before the simulated ischemia–reperfusion regimen (“PreC”) (Fig. 3A). Preconditioned constructs demonstrated a decrease in cell death, as measured by LDH and AK release (Fig. 3B, C), and an increase in cell viability compared to the Rep group (Fig. 3D). Furthermore, JC-1 staining demonstrated that preconditioning helped to partially prevent the increase in MMP seen in reperfused constructs (Fig. 3E). Western blot analysis also indicated that preconditioning resulted in a trend of decreased apoptosis and caspase 3 cleavage, although the differences were not statistically significant (Fig. 3F).
FIG. 3.
Ischemic preconditioning of cardiac constructs (“PreC”) to decrease ischemia–reperfusion injury. (A) Schematic of the experimental protocol with the comparison groups. Constructs were analyzed for (B, C) cell death as measured by LDH and AK release, (D) cell viability as measured using RealTime-Glo assay, and (E) mitochondrial membrane permeability as measured by JC-1 dye, where increased ratio of emission at 528 and 590 nm is correlated with higher permeability. Data are from four independent experiments and are shown as individual data points with mean ± SD. Statistical analysis done using ANOVA with post-hoc Tukey's HSD, #Indicates statistical significance compared to all other groups, *Indicates significant difference between groups, p < 0.05. Western blot analysis of (F) apoptosis by cleaved to total caspase 3 ratio and (G) prosurvival kinase activation by phosphorylated to total Akt ratio. Data are from three independent experiments, and are shown as mean ± SD. Data were not statistically significant by unpaired t-test and ANOVA, p < 0.05. Color images are available online.
Ischemic preconditioning is cardioprotective, at least in part, through the activation of prosurvival kinases.28,29 Western blot analysis of the cardiac constructs determined that there was increased phosphorylation and activation of the cell survival factor Akt in the preconditioned constructs (Fig. 3G). This difference was not statistically significant, but it does suggest that the activation of kinases in animals can be seen in vitro in human tissue models. Overall, these results support the use of engineered cardiac constructs as a human model of ischemic preconditioning.
Targeting rapid normalization of intracellular pH
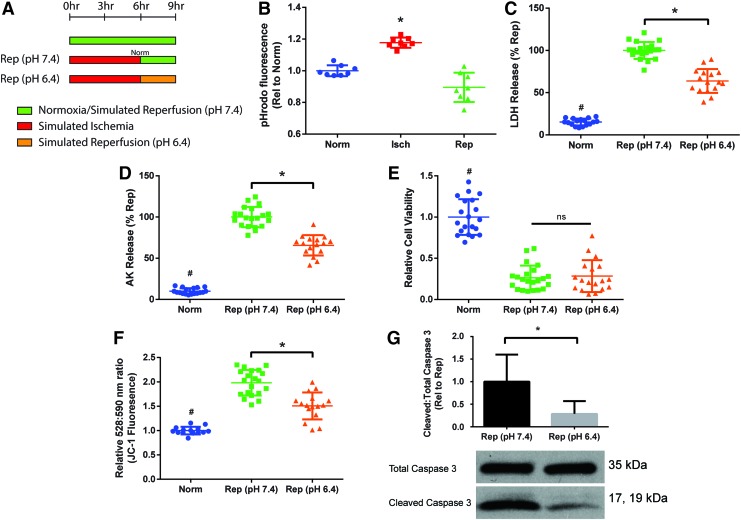
We next aimed to test therapeutic strategies that can be applied during reperfusion. Our first therapeutic target was the rapid normalization of intracellular pH seen during reperfusion. Ischemia leads to intracellular acidosis, while reperfusion causes a rapid restoration of intracellular pH, intracellular calcium overload, and eventually, cell death.30,31 We attempted to minimize the proton gradient during reperfusion to reduce IRI by reperfusion with a medium pH of 6.4 (“Rep [pH 6.4]”) and compared it to normal simulated reperfusion with a culture medium pH of 7.4 (“Rep [pH 7.4]”) (Fig. 4A).
FIG. 4.
Reperfusion with acidic media (“Rep [pH 6.4]”) to prevent rapid intracellular pH normalization and reduce ischemia–reperfusion injury. (A) Schematic of the experimental protocol for the comparison groups. (B) Measurement of intracellular pH using pHrodo dye. Higher fluorescence signal indicates a lower intracellular pH. Data are from two independent experiments. Constructs were analyzed for (C, D) cell death as measured by LDH and AK release, (E) cell viability as measured using RealTime-Glo assay, and (F) mitochondrial membrane permeability as measured by JC-1 dye, where increased ratio of emission at 528 and 590 nm is correlated with higher permeability. Data are from four independent experiments and are shown as individual data points with mean ± SD. Statistical analysis was done using ANOVA with post-hoc Tukey's HSD, #Indicates statistical significance compared to all other groups, *Indicates significant difference between groups, p < 0.05. (G) Western blot analysis of apoptosis by cleaved to total caspase 3 ratio. Data are from three independent experiments, mean ± SD. *Indicates significant difference between groups as determined by unpaired t-test, p < 0.05. ns, not significant. Color images are available online.
pHrodo staining confirmed a lower intracellular pH in the cardiac constructs after simulated ischemia (Fig. 4B). Reperfusion with acidic media demonstrated a decrease in cell death, as assessed by LDH and AK release (Fig. 4C, D). However, the rep (pH 6.4) group did not demonstrate improvements in cell viability as measured by cell activity (Fig. 4E), presumably due to acidic media inhibiting normal cell activity. The rep (pH 6.4) group did have a significant decrease in MMP compared to the rep (pH 7.4) group, although the levels of MMP were still significantly higher than in normoxic constructs (Fig. 4F). Western blot also confirmed that reperfusion with acidic media led to a significant decrease in the caspase 3 cleavage compared to Rep constructs (Fig. 4G). Reperfusion with acidic media demonstrated that in our constructs, IRI could be reduced by modifying reperfusion conditions.
Targeting opening of the MPTP
We next sought to reduce IRI in the constructs by preventing reperfusion-induced opening of the MPTP with subsequent mitochondria depolarization and cell death.22,32 Cyclophilin D (CypD) is a regulator of the MPTP, and it has been found that knockout of CypD in animals is cardioprotective.33 CsA can regulate CypD and decrease IRI by inhibiting opening of the MPTP.34
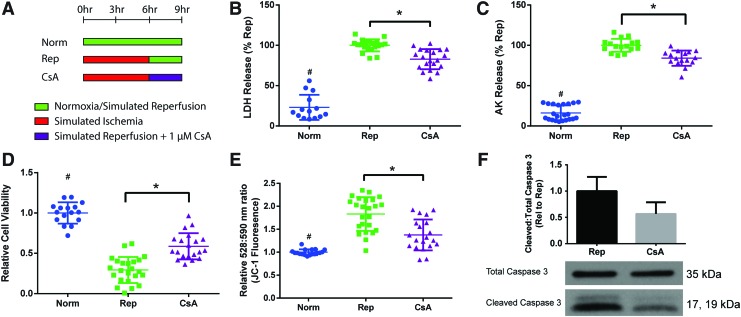
We assessed the efficacy of CsA to reduce IRI in the engineered cardiac constructs. The addition of CsA (1 μM) during reperfusion (“CsA”) (Fig. 5A) reduced cell death as shown by LDH and AK release, and led to increased cell viability (Fig. 5B–D). CsA-treated constructs also displayed decreased MMP compared to reperfused constructs (Fig. 5E). CsA treatment led to a nonsignificant decrease in caspase 3 cleavage (Fig. 5F). These results indicate that CsA can decrease IRI in the human tissue model through inhibition of MPTP opening.
FIG. 5.
CsA addition to prevent opening the mitochondrial permeability transition pore during reperfusion. (A) 1 μM CsA was added to the reperfusion media (“CsA”). Constructs were analyzed for (B, C) cell death as measured by LDH and AK release, (D) cell viability as measured using RealTime-Glo assay, and (E) mitochondrial membrane permeability as measured by JC-1 dye, where increased ratio of emission at 528 and 590 nm is correlated with higher permeability. Data are from four independent experiments and are shown as individual data points with mean ± SD. Statistical analysis was done using ANOVA with post-hoc Tukey's HSD, #Indicates statistical significance compared to all other groups, *Indicates significant difference between groups, p < 0.05. (F) Western blot analysis of apoptosis by cleaved to total caspase 3 ratio. Data are from three independent experiments, mean ± SD. Not significant by unpaired t-test, p < 0.05. CsA, cyclosporine A. Color images are available online.
Targeting oxidative stress
Reperfusion leads to rapid increases in ROS generation due to reactivation of the electron transport chain.35 The large amount of ROS overwhelms endogenous antioxidants, damages cellular membranes and proteins, and induces opening of the MPTP to contribute to IRI.23,36 We explored the use of antioxidants to target oxidative stress and help reduce IRI.
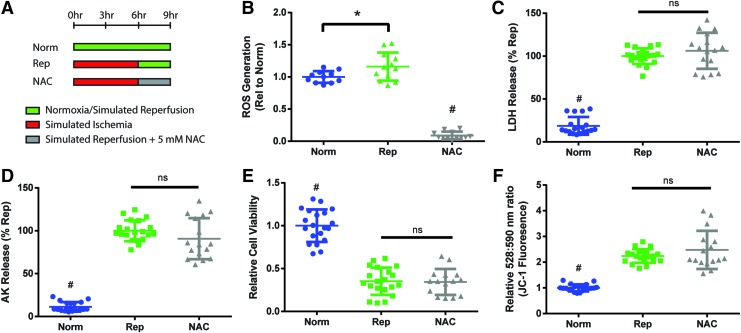
The antioxidant, NAC (5 mM), was added during reperfusion to test if it could reduce IRI (“NAC”) (Fig. 6A). In the tissue-engineered cardiac constructs, the administration of NAC on reperfusion significantly decreased ROS levels compared to normoxic and reperfused constructs, which confirmed its antioxidant properties (Fig. 6B). However, NAC-treated constructs demonstrated no difference in cell death (Fig. 6C, D), cell viability (Fig. 6E), or MMP levels (Fig. 6F) compared to reperfused constructs. These results indicated that NAC could reduce ROS levels in the constructs, but it remained ineffective as a treatment for IRI in this in vitro model.
FIG. 6.
Addition of NAC to reduce reperfusion-induced oxidative stress. (A) 5 mM NAC was added to the reperfusion media (“NAC”). Constructs were analyzed for (B) levels of ROS, (C, D) cell death as measured by LDH and AK release, (E) cell viability as measured using RealTime-Glo assay, and (F) mitochondrial membrane permeability as measured by JC-1 dye, where increased ratio of emission at 528 and 590 nm is correlated with higher permeability. Data aggregated from (B) three and (C–F) four independent experiments. Individual data points with mean ± SD. Statistical analysis was done using ANOVA with post-hoc Tukey's HSD, #Indicates statistical significance compared to all other groups, *Indicates significant difference between groups, p < 0.05. NAC, N-acetyl-l-cysteine. Color images are available online.
Discussion
We proposed the first human tissue-engineered model of myocardial IRI as an improved platform for preclinical studies of IRI. Currently, studies of IRI and cardioprotective therapies rely on the use of animals and animal-derived cardiomyocytes.37 However, animal use, particularly rodents, can be problematic because animal hearts have physiological differences that can influence their response to IRI.4–6
Instead, we sought to build a human tissue model by utilizing hiPS-CMs to form engineered cardiac muscle. hiPS cells are a robust and patient-specific source of cardiomyocytes for in vitro models of this kind. Notably, isolated and immature cardiomyocytes are more resistant to ischemic injury38; therefore, hiPS-CMs have a limited utility for modeling IRI because of their fetal-like phenotype. Cardiac tissue engineering can promote hiPS-CM maturation14–19 and help develop a more predictive human tissue model of IRI.
We sought to mature hiPS-CMs by encapsulating them in a collagen–fibrinogen hydrogel around flexible pillars for three-dimensional culture. The hydrogel would shrink around the pillars to condense the construct, and the pillars would provide passive tension to help align the cardiomyocytes and allow for auxotonic contractions for mechanical conditioning. The constructs were allowed to culture in the platform for 2 weeks to promote mechanical conditioning and maturation of the cardiomyocytes. While studies have shown enhanced maturation with longer bioreactor culture times,15,17 we decided to utilize 2 weeks to allow for increased experimental throughput.
The resulting cardiac constructs demonstrated aligned and striated cardiomyocytes along the periphery, but not at the construct center. This indicates that further improvements to the bioreactor design are needed to achieve a spatially homogenous, dense, and fully mature tissue. Our constructs are still relatively immature compared to adult heart tissue.
Construct function was tested and showed an increase in beat frequency and FAC to β-adrenergic stimulation. Studies have demonstrated that contractile force can be directly measured by optically determining changes in construct length if the elastic modulus of the PDMS pillar is known.39,40 We were unable to reliably manufacture PDMS with the same modulus to directly determine construct contractile force, but by measuring changes in construct area (FAC), we could still indirectly assess changes in contractile force and ionotropic response to isoproterenol. Our constructs demonstrated functional maturation with both a positive chronotropic and ionotropic response to β-adrenergic stimulation, where positive ionotropic responses are not seen in immature cardiomyocyte monolayers.41 It is the presence of aligned and cross-striated cardiomyocytes at the construct periphery and the complete response to β-adrenergic stimulation that allowed us to utilize the platform to study IRI.
Ischemia is both a hypoxic and substrate-depleted environment due to occlusion of the upstream blood vessel that causes changes to the intracellular and extracellular environment. We simulated these aspects in vitro by placing our constructs in a hypoxic environment and in a solution that was devoid of metabolic substrates and recapitulated the expected extracellular ischemic changes, such as acidic pH, hyperkalemia, and lactate accumulation that inhibits glycolysis.
An ischemic time of 0.5–1 h is typically utilized in animal studies to assess injury.3 However, our model is still relatively immature compared to adult heart tissue, and a longer ischemic time was needed to see changes in cell injury. We utilized 6 h of simulated ischemia, similar to another study that assessed ischemic changes in an engineered heart construct utilizing rat cardiomyocytes.42 The clinical trials, CIRCUS and CYCLE, to assess CsA efficacy in reducing IRI included patients up to 6–12 h after MI symptom onset, which indicates that clinicians are interested in this ischemic time frame.8,9
Our bioreactor was specifically designed for study of IRI. It featured two spaces for medium addition that allowed for rapid changes between ischemia and reperfusion conditions. A small amount of ischemic solution was added to the construct well during simulated ischemia, which allowed for the accumulation of metabolic wastes around the cardiac constructs and for them to further modify their extracellular environment. To reperfuse, media were added to the large space surrounding the construct well, to rapidly restore nutrients and extracellular ion and pH balance with minimal manipulation.
To validate the model, we demonstrated critical aspects of IRI in constructs exposed to ischemia only and ischemia followed by reperfusion. Cell injury was assessed by at least two methodologically different assays as recommended by Nomenclature Committee on Cell Death guidelines.43 We determined changes in cell injury by measuring cell membrane permeability using LDH and AK release, cell activity and viability using RealTime-Glo assay, and MMP using JC-1 dye. We saw that our ischemia-only constructs demonstrated significant differences in all of our cell injury assays compared to normoxic control constructs, which established that simulated ischemia led to ischemic injury in our constructs. Furthermore, we saw that the increases in cell injury, as measured by our assays, were significantly magnified by reperfusion, which demonstrated that reperfusion led to increased injury over ischemia alone. Critically, we observed significant increases in MMP after reperfusion, which correlates with opening of the MPTP to cause cell death.22 Many efforts to treat IRI have focused on the opening of the MPTP as a critical therapeutic target.8,9
In addition to our primary assays to assess cell injury, we wanted to further characterize changes due to IRI. Apoptosis in IRI is thought to be driven by reperfusion,24–27 and as expected, we saw little caspase 3 activation in our ischemic constructs, but significantly increased apoptotic activity in our reperfused constructs. Caspase inhibition during reperfusion has helped decrease IRI,44 but it may ultimately have limited efficacy due to the significant amount of necrotic injury that is seen during reperfusion.45 We also saw disorganized cardiac ultrastructure after simulated ischemia–reperfusion, which may be the result of reperfusion-induced intracellular calcium overload leading to activation of intracellular proteases.46,47 Overall, these results support the utility of the proposed model in recapitulating IRI.
We also examined the responses of relatively immature constructs to ischemia–reperfusion in our model. The fetal heart is relatively resistant to hypoxia,48 and we observed that the immature cardiac constructs (after 3 days of culture, when macroscopic contractions are first observed) were also resistant to ischemic injury. These immature constructs did not demonstrate significant differences between ischemia–reperfusion and normoxia, and the lack of response helped to establish the importance of mechanical conditioning and maturation in developing a model of IRI.
While ischemic preconditioning has demonstrated robust and strong cardioprotection in animal models,3 study of it in humans has been limited due to lack of a robust platform.49 Evidence for cardioprotection by ischemic preconditioning in humans has been found through better outcomes in patients with preinfarction angina, patients undergoing coronary artery bypass surgeries, as well as in models of quiescent primary human ventricular cardiomyocytes and isolated atrial trabeculae.50–54 An engineered human tissue model offers a more physiological and robust platform to study ischemic preconditioning and evaluate its potential benefits. In this study, we demonstrated that ischemic preconditioning helped decrease IRI in a human tissue model.
Similar to what has been shown in animals, the preconditioned constructs displayed increased activation of prosurvival kinases. Further identification of the specific proteins that drive cardioprotection will be important because they may differ between humans and animals. For example, STAT3 activation and STAT5 attenuation were cardioprotective in mouse models,55 but the opposite was found in humans.56 Furthermore, cardioprotection through other more clinically relevant ischemic conditioning regimens, such as postconditioning and remote ischemic conditioning, has been found to function through similar activation of prosurvival pathways.57,58 Thus, the use of the human tissue model to study ischemic conditioning would help glean insights into modalities to reduce IRI.
We decided to only utilize one cycle of ischemic preconditioning in our studies to limit manipulation of the constructs. Studies have suggested that there is a minimum preconditioning stimulus that must be achieved through either number of ischemia–reperfusion cycles or duration of ischemia to provide cardioprotection.59–62 We utilized a longer 30-min ischemic time in our preconditioning regimen to provide a stronger single stimulus compared to the multiple cycles of 4–5 min used in animal studies.3 In our future studies, we wish to assess the molecular changes that are characteristic of crossing the preconditioning threshold to better understand its cardioprotective effects.
We also sought to reduce IRI after the ischemic insult by modifying reperfusion conditions. Ischemia leads to a switch from aerobic respiration to anaerobic glycolysis, a decrease in ATP turnover, and a decrease in intracellular pH.63 Reperfusion causes rapid normalization of intracellular pH by creating a large proton gradient between the intracellular and extracellular space, which drives intracellular calcium overload and cell death.30 However, pharmacological inhibition of the sodium-hydrogen exchanger to prevent rapid pH normalization has had mixed success.64–67
We instead leveraged the increased control offered in an in vitro model and sought to prevent rapid pH normalization by reperfusion with acidic media to decrease the pH gradient. We saw a decrease in cell death, MPTP opening, and apoptosis. Reperfusion with acidic media has previously demonstrated a reduction in IRI in rat cardiomyocytes and isolated hearts,68,69 and we have shown that it is also cardioprotective in a human tissue model. We decreased IRI by modifying the cellular environment during reperfusion, and the results also indicate that inhibition of intracellular pH normalization remains a viable therapeutic target.
The proposed model of IRI was also used for pharmacological screening of therapeutics that could be added during reperfusion to decrease IRI. Many drugs have demonstrated the ability to decrease the infarct size in animals, but none has shown benefits in large clinical trials.8–10 We sought to decrease IRI by targeting some of the putative mechanisms of damage—the use of CsA to prevent MPTP opening and the use of NAC to reduce oxidative stress.
CsA addition during reperfusion demonstrated a decrease in IRI, and critically, it also decreased MMP. This finding indicates that CsA was able to reduce reperfusion injury by preventing opening of the MPTP. Although CsA usage in the CYCLE and CIRCUS clinical trials did not demonstrate improved clinical outcomes,8,9 the trials have also been criticized for utilizing a different CsA formulation compared to an earlier trial,34 and for the increased use of cardioprotective platelet inhibitors in patients, which might mask any benefit of CsA administration.70 The encouraging results of CsA in the human tissue model indicate that the design of the clinical studies may have confounded potential cardioprotective effects.
On the other hand, the antioxidant NAC failed to reduce IRI in the cardiac constructs, despite the known importance of oxidative stress in causing reperfusion injury.23,36 NAC decreased the overall ROS levels in the construct, but despite this reduction, NAC did not seem to inhibit cell death, improve cell viability, or affect MMP. Antioxidants have shown mixed results in preclinical studies and small clinical trials,71–73 and more recently, the specific targeting of mitochondrial ROS generation has become of interest.74 Early results have indicated that these therapies can reduce IRI and need to be further explored.75 We are continuing to use the human tissue model as a platform for pharmacologic testing of therapeutics against IRI.
In our studies, we chose to focus on the cardiomyocyte responses to IRI and only incorporated hiPS-CMs into the model. Cardiomyocytes only account for one-third of the cell population in the heart,76 and the other cardiac cell types have important roles in IRI. Cardiac endothelial cells and fibroblasts can help mitigate cardiomyocyte injury through paracrine signaling77–79; however, they can also propagate IRI through microvascular obstruction and inflammation.80,81 The inclusion of endothelial cells and fibroblasts in future models will be important for developing a more physiological platform. There are many studies that have incorporated endothelial cells and a microvasculature into their cardiac constructs,82–84 and we can now utilize these methods to enhance our model of IRI.
It is also recognized that our model does not represent the entire heart. In our studies, the entire cardiac construct is subjected to the same conditions and ischemic time, but in an MI, different areas of the heart are subjected to different degrees of injury. Our constructs did not readily regain contractile function after IRI and are more representative of the core infarct area. To assess the infarct border zone and compare its response to the core area, it will be necessary for us to alter simulated ischemia–reperfusion conditions. Furthermore, we would like to assess how different regions of the infarct affect each other and the remote noninfarcted heart. This will allow us to better understand how treatments can salvage the entire area at risk and the heart response as a whole.
Despite encouraging results, it is difficult to determine the clinical predictivity of the cardiac constructs for pharmacological screening. CsA helped decrease IRI in the cardiac construct and in other preclinical studies, but it has not been effective in patients.8,9 This difficulty is further compounded by the fact that no therapeutic has robustly demonstrated improved clinical outcomes in patients.
Part of these differences in the results between preclinical and clinical studies has been attributed to the differences between patients themselves. Patients have many different comorbidities and take multiple medications that can affect their susceptibility to IRI and response to ischemic conditioning.85–87 The patient background needs to be accounted for in preclinical studies. Thus, the human tissue-engineered model has significant advantages over animal models for clinical translation. Recent studies have illustrated that hiPS-CMs can capture patient-specific disease backgrounds and cardiac tissue engineering can help recapitulate pathological phenotypes, such as hypertrophy.88–91 The use of hiPS-CMs offers the opportunity to study IRI on different genetic backgrounds.
Conclusion
The human tissue-engineered model developed in this study demonstrated key aspects of IRI, enabled assessment of the differential responses to ischemia and reperfusion, and saw the reduction of IRI through various therapeutic strategies. We anticipate that the use of patient-specific hiPSCs will allow for recapitulation of patient comorbidities, and further development of the cardiac tissue engineering system will lead to more accurate simulation of the in vivo setting. Additional study on the human tissue model will help in developing therapies to reduce IRI and facilitate their translation into the clinical setting.
Supplementary Material
Acknowledgments
The authors gratefully acknowledge funding of this work provided by NIH (grants 2R01 HL076485 and EB002520) and NSF (NSF CELL-MET Engineering Research Center, EEC-1647837).
Disclosure Statement
No competing financial interests exist.
References
- 1. Van de Werf F., Bax J., Betriu A., et al. Management of acute myocardial infarction in patients presenting with persistent ST-segment elevation: the Task Force on the Management of ST-Segment Elevation Acute Myocardial Infarction of the European Society of Cardiology. Eur Heart J 29, 2909, 2008 [DOI] [PubMed] [Google Scholar]
- 2. Yellon D.M., and Hausenloy D.J. Myocardial reperfusion injury. N Engl J Med 357, 1121, 2007 [DOI] [PubMed] [Google Scholar]
- 3. Jones S.P., Tang X.L., Guo Y., et al. The NHLBI-Sponsored Consortium for preclinicAl assESsment of cARdioprotective Therapies (CAESAR): a new paradigm for rigorous, accurate, and reproducible evaluation of putative infarct-sparing interventions in mice, rabbits, and pigs. Circ Res 116, 572, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hamlin R.L., and Altschuld R.A. Extrapolation from mouse to man. Circ Cardiovasc Imaging 4, 2, 2011 [DOI] [PubMed] [Google Scholar]
- 5. O'Hara T., and Rudy Y. Quantitative comparison of cardiac ventricular myocyte electrophysiology and response to drugs in human and nonhuman species. Am J Physiol Heart Circ Physiol 302, H1023, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Gibbs C.L. Cardiac energetics: sense and nonsense. Clin Exp Pharmacol Physiol 30, 598, 2003 [DOI] [PubMed] [Google Scholar]
- 7. Limalanathan S., Andersen G.Ø., Kløw N.E., Abdelnoor M., Hoffmann P., and Eritsland J. Effect of ischemic postconditioning on infarct size in patients with ST-elevation myocardial infarction treated by primary PCI results of the POSTEMI (POstconditioning in ST-Elevation Myocardial Infarction) randomized trial. J Am Heart Assoc 3, e000679, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ottani F., Latini R., Staszewsky L., et al. Cyclosporine A in reperfused myocardial infarction the multicenter, controlled, open-label CYCLE trial. J Am Coll Cardiol 67, 365, 2016 [DOI] [PubMed] [Google Scholar]
- 9. Cung T.T., Morel O., Cayla G., et al. Cyclosporine before PCI in patients with acute myocardial infarction. N Engl J Med 373, 1021, 2015 [DOI] [PubMed] [Google Scholar]
- 10. Roolvink V., Ibáñez B., Ottervanger J.P., et al. Early intravenous beta-blockers in patients with ST-segment elevation myocardial infarction before primary percutaneous coronary intervention. J Am Coll Cardiol 67, 2705, 2016 [DOI] [PubMed] [Google Scholar]
- 11. Rossello X., and Yellon D.M. Cardioprotection: the disconnect between bench and bedside. Circulation 134, 574, 2016 [DOI] [PubMed] [Google Scholar]
- 12. Ribas J., Sadeghi H., Manbachi A., et al. Cardiovascular organ-on-a-chip platforms for drug discovery and development. Appl In Vitro Toxicol 2, 82, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Guo L., Abrams R.M.C., Babiarz J.E., et al. Estimating the risk of drug-induced proarrhythmia using human induced pluripotent stem cell-derived cardiomyocytes. Toxicol Sci 123, 281, 2011 [DOI] [PubMed] [Google Scholar]
- 14. Nunes S.S., Miklas J.W., Liu J., et al. Biowire: a platform for maturation of human pluripotent stem cell-derived cardiomyocytes. Nat Methods 10, 781, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Tiburcy M., Hudson J.E., Balfanz P., et al. Defined engineered human myocardium with advanced maturation for applications in heart failure modeling and repair. Circulation 135, 1832, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Shadrin I.Y., Allen B.W., Qian Y., et al. Cardiopatch platform enables maturation and scale-up of human pluripotent stem cell-derived engineered heart tissues. Nat Commun 8, 1825, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ronaldson-Bouchard K., Ma S.P., Yeager K., et al. Advanced maturation of human cardiac tissue grown from pluripotent stem cells. Nature 556, 239, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Li R.A., Keung W., Cashman T.J., et al. Bioengineering an electro-mechanically functional miniature ventricular heart chamber from human pluripotent stem cells. Biomaterials 163, 116, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Agarwal A., Goss J.A., Cho A., McCain M.L., and Parker K.K. Microfluidic heart on a chip for higher throughput pharmacological studies. Lab Chip 13, 3599, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Burridge P.W., Matsa E., Shukla P., et al. Chemically defined generation of human cardiomyocytes. Nat Methods 11, 855, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Larsson L., and Ohman S. Serum ionized calcium and corrected total calcium in borderline hyperparathyroidism. Clin Chem 24, 1962, 1978 [PubMed] [Google Scholar]
- 22. Halestrap A.P., and Richardson A.P. The mitochondrial permeability transition: a current perspective on its identity and role in ischaemia/reperfusion injury. J Mol Cell Cardiol 78, 129, 2015 [DOI] [PubMed] [Google Scholar]
- 23. Kim J.S., Jin Y., and Lemasters J.J. Reactive oxygen species, but not Ca2+ overloading, trigger pH- and mitochondrial permeability transition-dependent death of adult rat myocytes after ischemia-reperfusion. Am J Physiol Heart Circ Physiol 290, H2024, 2006 [DOI] [PubMed] [Google Scholar]
- 24. Gao E., Boucher M., Chuprun J.K., Zhou R.H., Eckhart A.D., and Koch W.J. Darbepoetin alfa, a long-acting erythropoietin analog, offers novel and delayed cardioprotection for the ischemic heart. Am J Physiol Heart Circ Physiol 293, H60, 2007 [DOI] [PubMed] [Google Scholar]
- 25. Gottlieb R.A., Burleson K.O., Kloner R.A., Babior B.M., and Engler R.L. Reperfusion injury induces apoptosis in rabbit cardiomyocytes. J Clin Invest 94, 1621, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Zhao Z.Q., Nakamura M., Wang N.P., et al. Reperfusion induces myocardial apoptotic cell death. Cardiovasc Res 45, 651, 2000 [DOI] [PubMed] [Google Scholar]
- 27. Dumont E.A.W.J., Hofstra L., van Heerde W.L., et al. Cardiomyocyte death induced by myocardial ischemia and reperfusion : measurement with recombinant human annexin-V in a mouse model. Circulation 102, 1564, 2000 [DOI] [PubMed] [Google Scholar]
- 28. Schulman D., Latchman D.S., and Yellon D.M. Urocortin protects the heart from reperfusion injury via upregulation of p42/p44 MAPK signaling pathway. Am J Physiol Heart Circ Physiol 283, H1481, 2002 [DOI] [PubMed] [Google Scholar]
- 29. Uchiyama T., Engelman R.M., Maulik N., and Das D.K. Role of Akt signaling in mitochondrial survival pathway triggered by hypoxic preconditioning. Circulation 109, 3042, 2004 [DOI] [PubMed] [Google Scholar]
- 30. Kalogeris T., Baines C.P., Krenz M., and Korthuis R.J. Cell biology of ischemia/reperfusion injury. Int Rev Cell Mol Biol 298, 229, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Qian T., Nieminen A.L., Herman B., and Lemasters J.J. Mitochondrial permeability transition in pH-dependent reperfusion injury to rat hepatocytes. Am J Physiol 273, C1783, 1997 [DOI] [PubMed] [Google Scholar]
- 32. Seidlmayer L.K., Juettner V.V., Kettlewell S., Pavlov E.V., Blatter L.A., and Dedkova E.N. Distinct mPTP activation mechanisms in ischaemia-reperfusion: contributions of Ca2+, ROS, pH, and inorganic polyphosphate. Cardiovasc Res 106, 237, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Baines C.P., Kaiser R.A., Purcell N.H., et al. Loss of cyclophilin D reveals a critical role for mitochondrial permeability transition in cell death. Nature 434, 658, 2005 [DOI] [PubMed] [Google Scholar]
- 34. Piot C., Croisille P., Staat P., et al. Effect of cyclosporine on reperfusion injury in acute myocardial infarction. N Engl J Med 359, 473, 2008 [DOI] [PubMed] [Google Scholar]
- 35. Zweier J.L., Flaherty J.T., and Weisfeldt M.L. Direct measurement of free radical generation following reperfusion of ischemic myocardium. Proc Natl Acad Sci U S A 84, 1404, 1987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Toyokuni S. Reactive oxygen species-induced molecular damage and its application in pathology. Pathol Int 49, 91, 1999 [DOI] [PubMed] [Google Scholar]
- 37. Lecour S., Bøtker H.E., Condorelli G., et al. ESC working group cellular biology of the heart: position paper: improving the preclinical assessment of novel cardioprotective therapies. Cardiovasc Res 104, 399, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Ostadalova I., Ostadal B., Kolár F., Parratt J.R., and Wilson S. Tolerance to ischaemia and ischaemic preconditioning in neonatal rat heart. J Mol Cell Cardiol 30, 857, 1998 [DOI] [PubMed] [Google Scholar]
- 39. Vandenburgh H., Shansky J., Benesch-Lee F., et al. Drug-screening platform based on the contractility of tissue-engineered muscle. Muscle Nerve 37, 438, 2008 [DOI] [PubMed] [Google Scholar]
- 40. Hansen A., Eder A., Bönstrup M., et al. Development of a drug screening platform based on engineered heart tissue. Circ Res 107, 35, 2010 [DOI] [PubMed] [Google Scholar]
- 41. Pillekamp F., Haustein M., Khalil M., et al. Contractile properties of early human embryonic stem cell-derived cardiomyocytes: beta-adrenergic stimulation induces positive chronotropy and lusitropy but not inotropy. Stem Cells Dev 21, 2111, 2012 [DOI] [PubMed] [Google Scholar]
- 42. Katare R.G., Ando M., Kakinuma Y., and Sato T. Engineered heart tissue: a novel tool to study the ischemic changes of the heart in vitro. PLoS One 5, e9275, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Galluzzi L., Aaronson S.A., Abrams J., et al. Guidelines for the use and interpretation of assays for monitoring cell death in higher eukaryotes. Cell Death Differ 16, 1093, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Mocanu M.M., Baxter G.F., and Yellon D.M. Caspase inhibition and limitation of myocardial infarct size: protection against lethal reperfusion injury. Br J Pharmacol 130, 197, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. McCully J.D., Wakiyama H., Hsieh Y.J., Jones M., and Levitsky S. Differential contribution of necrosis and apoptosis in myocardial ischemia-reperfusion injury. Am J Physiol Heart Circ Physiol 286, H1923, 2004 [DOI] [PubMed] [Google Scholar]
- 46. Chen M., Won D.J., Krajewski S., and Gottlieb R.A. Calpain and mitochondria in ischemia/reperfusion injury. J Biol Chem 277, 29181, 2002 [DOI] [PubMed] [Google Scholar]
- 47. Hernando V., Inserte J., Sartório C.L., Parra V.M., Poncelas-Nozal M., and Garcia-Dorado D. Calpain translocation and activation as pharmacological targets during myocardial ischemia/reperfusion. J Mol Cell Cardiol 49, 271, 2010 [DOI] [PubMed] [Google Scholar]
- 48. Sedmera D., Kucera P., and Raddatz E. Developmental changes in cardiac recovery from anoxia-reoxygenation. Am J Physiol Regul Integr Comp Physiol 283, R379, 2002 [DOI] [PubMed] [Google Scholar]
- 49. Ludman A.J., Yellon D.M., and Hausenloy D.J. Cardiac preconditioning for ischaemia: lost in translation. Dis Model Mech 3, 35, 2010 [DOI] [PubMed] [Google Scholar]
- 50. Yellon D.M., Alkhulaifi A.M., and Pugsley W.B. Preconditioning the human myocardium. Lancet 342, 276, 1993 [DOI] [PubMed] [Google Scholar]
- 51. Walsh S.R., Tang T.Y., Kullar P., Jenkins D.P., Dutka D.P., and Gaunt M.E. Ischaemic preconditioning during cardiac surgery: systematic review and meta-analysis of perioperative outcomes in randomised clinical trials. Eur J Cardiothorac Surg 34, 985, 2008 [DOI] [PubMed] [Google Scholar]
- 52. Masci P.G., Andreini D., Francone M., et al. Prodromal angina is associated with myocardial salvage in acute ST-segment elevation myocardial infarction. Eur Heart J Cardiovasc Imaging 14, 1041, 2013 [DOI] [PubMed] [Google Scholar]
- 53. Ikonomidis J.S., Tumiati L.C., Weisel R.D., Mickle D.A., and Li R.K. Preconditioning human ventricular cardiomyocytes with brief periods of simulated ischaemia. Cardiovasc Res 28, 1285, 1994 [DOI] [PubMed] [Google Scholar]
- 54. Walker D.M., Walker J.M., Pugsley W.B., Pattison C.W., and Yellon D.M. Preconditioning in isolated superfused human muscle. J Mol Cell Cardiol 27, 1349, 1995 [DOI] [PubMed] [Google Scholar]
- 55. Aleshin A., Ananthakrishnan R., Li Q., et al. RAGE modulates myocardial injury consequent to LAD infarction via impact on JNK and STAT signaling in a murine model. Am J Physiol Heart Circ Physiol 294, H1823, 2008 [DOI] [PubMed] [Google Scholar]
- 56. Heusch G., Musiolik J., Kottenberg E., Peters J., Jakob H., and Thielmann M. STAT5 activation and cardioprotection by remote ischemic preconditioning in humans: short communication. Circ Res 110, 111, 2012 [DOI] [PubMed] [Google Scholar]
- 57. Tsang A., Hausenloy D.J., Mocanu M.M., and Yellon D.M. Postconditioning: a form of ‘modified reperfusion’ protects the myocardium by activating the phosphatidylinositol 3-kinase-Akt pathway. Circ Res 95, 230, 2004 [DOI] [PubMed] [Google Scholar]
- 58. Schmidt M.R., Redington A., and Bøtker H.E. Remote conditioning the heart overview: translatability and mechanism. Br J Pharmacol 172, 1947, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Iliodromitis E.K., Kremastinos D.T., Katritsis D.G., Papadopoulos C.C., and Hearse D.J. Multiple cycles of preconditioning cause loss of protection in open-chest rabbits. J Mol Cell Cardiol 29, 915, 1997 [DOI] [PubMed] [Google Scholar]
- 60. Schulz R., Post H., Vahlhaus C., and Heusch G. Ischemic preconditioning in pigs: a graded phenomenon: its relation to adenosine and bradykinin. Circulation 98, 1022, 1998 [DOI] [PubMed] [Google Scholar]
- 61. Yamasaki K., Fujiwara H., Tanaka M., et al. Preconditioning with 15-minute ischemia extends myocardial infarct size after subsequent 30-minute ischemia in rabbits. Jpn Circ J 61, 344, 1997 [DOI] [PubMed] [Google Scholar]
- 62. Johnsen J., Pryds K., Salman R., Løfgren B., Kristiansen S.B., and Bøtker H.E. The remote ischemic preconditioning algorithm: effect of number of cycles, cycle duration and effector organ mass on efficacy of protection. Basic Res Cardiol 111, 10, 2016 [DOI] [PubMed] [Google Scholar]
- 63. Robergs R.A., Ghiasvand F., and Parker D. Biochemistry of exercise-induced metabolic acidosis. Am J Physiol Regul Integr Comp Physiol 287, R502, 2004 [DOI] [PubMed] [Google Scholar]
- 64. Gumina R.J., Mizumura T., Beier N., Schelling P., Schultz J.J., and Gross G.J. A new sodium/hydrogen exchange inhibitor, EMD 85131, limits infarct size in dogs when administered before or after coronary artery occlusion. J Pharmacol Exp Ther 286, 175, 1998 [PubMed] [Google Scholar]
- 65. Javadov S., Choi A., Rajapurohitam V., Zeidan A., Basnakian A.G., and Karmazyn M. NHE-1 inhibition-induced cardioprotection against ischaemia/reperfusion is associated with attenuation of the mitochondrial permeability transition. Cardiovasc Res 77, 416, 2008 [DOI] [PubMed] [Google Scholar]
- 66. Théroux P., Chaitman B.R.R., Danchin N., et al. Inhibition of the sodium–hydrogen exchanger with cariporide to prevent myocardial infarction in high-risk ischemic situations. Main results of the GUARDIAN trial. Guard during ischemia against necrosis (GUARDIAN) investigators. Circulation 102, 3032, 2000 [DOI] [PubMed] [Google Scholar]
- 67. Zeymer U., Suryapranata H., Monassier J.P., et al. The Na(+)/H(+) exchange inhibitor eniporide as an adjunct to early reperfusion therapy for acute myocardial infarction. Results of the evaluation of the safety and cardioprotective effects of eniporide in acute myocardial infarction (ESCAMI) trial. J Am Coll Cardiol 38, 1644, 2001 [DOI] [PubMed] [Google Scholar]
- 68. Bond J.M., Herman B., and Lemasters J.J. Protection by acidotic pH against anoxia/reoxygenation injury to rat neonatal cardiac myocytes. Biochem Biophys Res Commun 179, 798, 1991 [DOI] [PubMed] [Google Scholar]
- 69. Inserte J., Barba I., Hernando V., et al. Effect of acidic reperfusion on prolongation of intracellular acidosis and myocardial salvage. Cardiovasc Res 77, 782, 2008 [DOI] [PubMed] [Google Scholar]
- 70. Heusch G. CIRCUS: a kiss of death for cardioprotection? Cardiovasc Res 108, 215, 2015 [DOI] [PubMed] [Google Scholar]
- 71. Effect of 48-h intravenous trimetazidine on short- and long-term outcomes of patients with acute myocardial infarction, with and without thrombolytic therapy; A double-blind, placebo-controlled, randomized trial. The EMIP-FR Group. European Myocardial Infarction Project–Free Radicals. Eur Heart J 21, 1537, 2000 [DOI] [PubMed] [Google Scholar]
- 72. Flaherty J.T., Pitt B., Gruber J.W., et al. Recombinant human superoxide dismutase (h-SOD) fails to improve recovery of ventricular function in patients undergoing coronary angioplasty for acute myocardial infarction. Circulation 89, 1982, 1994 [DOI] [PubMed] [Google Scholar]
- 73. Sochman J., Vrbská J., Musilová B. and Rocek, M. Infarct size limitation: acute N-acetylcysteine defense (ISLAND trial): preliminary analysis and report after the first 30 patients. Clin Cardiol 19, 94, 1996 [DOI] [PubMed] [Google Scholar]
- 74. Chouchani E.T., Pell V.R., Gaude E., et al. Ischaemic accumulation of succinate controls reperfusion injury through mitochondrial ROS. Nature 515, 431, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Valls-Lacalle L., Barba I., Miró-Casas E., et al. Succinate dehydrogenase inhibition with malonate during reperfusion reduces infarct size by preventing mitochondrial permeability transition. Cardiovasc Res 109, 374, 2016 [DOI] [PubMed] [Google Scholar]
- 76. Pinto A.R., Ilinykh A., Ivey M.J., et al. Revisiting cardiac cellular composition. Circ Res 118, 400, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Leucker T.M., Ge Z.D., Procknow J., et al. Impairment of endothelial-myocardial interaction increases the susceptibility of cardiomyocytes to ischemia/reperfusion injury. PLoS One 8, e70088, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Pabla R., and Curtis M.J. Endogenous protection against reperfusion-induced ventricular fibrillation: role of neuronal versus non-neuronal sources of nitric oxide and species dependence in the rat versus rabbit isolated heart. J Mol Cell Cardiol 28, 2097, 1996 [DOI] [PubMed] [Google Scholar]
- 79. Abrial M., Da Silva C.C., Pillot B., et al. Cardiac fibroblasts protect cardiomyocytes against lethal ischemia-reperfusion injury. J Mol Cell Cardiol 68, 56, 2014 [DOI] [PubMed] [Google Scholar]
- 80. Kawaguchi M., Takahashi M., Hata T., et al. Inflammasome activation of cardiac fibroblasts is essential for myocardial ischemia/reperfusion injury. Circulation 123, 594, 2011 [DOI] [PubMed] [Google Scholar]
- 81. Niccoli G., Burzotta F., Galiuto L., and Crea F. Myocardial no-reflow in humans. J Am Coll Cardiol 54, 281, 2009 [DOI] [PubMed] [Google Scholar]
- 82. Zhang B., Montgomery M., Chamberlain M.D., et al. Biodegradable scaffold with built-in vasculature for organ-on-a-chip engineering and direct surgical anastomosis. Nat Mater 15, 669, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Chiu L.L.Y., Montgomery M., Liang Y., Liu H., and Radisic M. Perfusable branching microvessel bed for vascularization of engineered tissues. Proc Natl Acad Sci U S A 109, E3414, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Sekine H., Shimizu T., Sakaguchi K., et al. In vitro fabrication of functional three-dimensional tissues with perfusable blood vessels. Nat Commun 4, 1399, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Sivaraman V., Hausenloy D.J., Wynne A.M., and Yellon D.M. Preconditioning the diabetic human myocardium. J Cell Mol Med 14, 1740, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Ferdinandy P., Hausenloy D.J., Heusch G., Baxter G.F., and Schulz R. Interaction of risk factors, comorbidities, and comedications with ischemia/reperfusion injury and cardioprotection by preconditioning, postconditioning, and remote conditioning. Pharmacol Rev 66, 1142, 2014 [DOI] [PubMed] [Google Scholar]
- 87. Fan Y., Yang S., Cao Y., and Huang Y. Effects of acute and chronic atorvastatin on cardioprotection of ischemic postconditioning in isolated rat hearts. Cardiovasc Ther 31, 187, 2013 [DOI] [PubMed] [Google Scholar]
- 88. Yazawa M., Hsueh B., Jia X., et al. Using induced pluripotent stem cells to investigate cardiac phenotypes in Timothy syndrome. Nature 471, 230, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Song H., Zandstra P.W., and Radisic M. Engineered heart tissue model of diabetic myocardium. Tissue Eng Part A 17, 1869, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Wang G., McCain M.L., Yang L., et al. Modeling the mitochondrial cardiomyopathy of Barth syndrome with induced pluripotent stem cell and heart-on-chip technologies. Nat Med 20, 616, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Hirt M.N., Sörensen N.A., Bartholdt L.M., et al. Increased afterload induces pathological cardiac hypertrophy: a new in vitro model. Basic Res Cardiol 107, 307, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.