Abstract
Loss of function mutations in COQ4 lead to fatal neonatal mitochondrial disorders with CoQ10 deficiency. In two siblings with childhood-onset, slowly progressive ataxia from a consanguineous mating from Turkey, whole exome sequencing identified homozygous missense mutations in COQ4 (Gly55Val). Blood levels of CoQ10 were either below or at the low end of the normal range. The more severely affected of the siblings was given a high dose of CoQ10 (2000 mg/day) for one month, following which significant improvement in neurological signs and symptoms was noted. Our report indicates that COQ4 mutations are a rare cause of ataxia and that CoQ10 supplementation is a personalized treatment for this form of ataxia.
Keywords: Ataxia, CoQ4 Mutation, CoQ10 Deficiency, Genetics, Therapy
Introduction
Ataxia is a clinical feature of hundreds of disorders, and even pure cerebellar ataxia without other symptoms can be caused by mutations in at least 40 dominant [1] and 45 recessive genes [2]. Whole exome and whole genome sequencing has recently allowed the identification of novel genes involved in numerous rare Mendelian disorders including ataxia, but many cases of isolated or recessive ataxia still remain unexplained [3,4]. When there are two or more affected individuals from a consanguineous mating, gene identification using homozygosity mapping is an efficient way to identify new genes for rare disorders [5,6]. In rare cases, gene identification can lead to personalized therapy, as exemplified here.
Patients and Methods
Patients
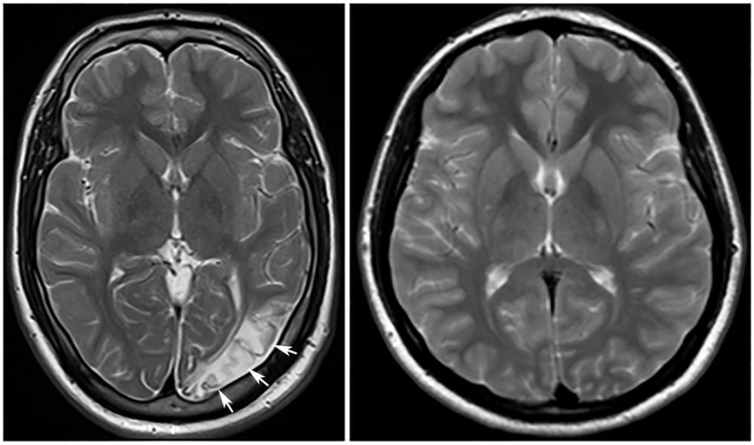
The index case is a 26 year-old male with slowly progressive ataxia and spasticity. His parents are first cousins. The proband had walking difficulty at the age of 8, followed by partial epilepsy four years later and lost the ability to ambulate by age 16. Neurological examination revealed cognitive deterioration, dysarthria, inability to walk even with support but able to stand with support, severe spasticity in the lower limbs, mild spasticity in the upper limbs, moderate weakness in proximal leg muscles, dysmetria and dysdiadochokinesia. His oculomotor movements and sphincter were normal with symmetrical deep tendon reflexes in both limbs. However, Babinski sign was positive bilaterally. A brain MRI of index case at time of evaluation showed cortical and subcortical T2 hyperintensity, not limited to a specific vascular territory, as described in a previous report describing COQ4 mutations [5] (Fig. 1). He was treated with carbamazepine (600 mg twice daily) and clonazepam (2 mg, twice daily).
Fig. 1: MRI of index case.
T2 weighted axial images of the patient (left) and age-matched control (right). Patient’s image shows cortical and subcortical signal changes in the left occipital region (arrow) and mild atrophy.
The proband’s 27 year-old sister’s symptoms also started when she was 8 years old but progressed more slowly than the proband. She developed partial epilepsy when she was 12 years old. Physical examination demonstrated dysarthria, spastic-ataxic gait, mild spasticity in all four extremities, although more prominently in the lower extremities. She could walk without support. Dysmetria and dysdiadokokinesis were present. Eye movements were normal. Her cognition was impaired. Babinski sign was positive bilaterally with no sphincter dysfunction.
Routine biochemistry, hemogram and metabolic tests were normal in both. Whole spine MRI did not reveal any pathology. However, brain magnetic resonance imaging (MRI) showed cerebral and cerebellar atrophy. She had been treated with levatiracetam (1000 mg, twice daily).
Recruitment
The index case was referred to medical genetics with an initial diagnosis of ataxia. Genetic counseling was offered and all individuals, which included the two affected individuals, 2 unaffected siblings and parents, gave their voluntary informed written consent to participate. The local study protocol was approved by the Ethics Committee at Erciyes University, Kayseri, Turkey. The genetic study has been reviewed and approved by the University of Michigan institutional review board. Following identification of the mutation in CoQ10 biosynthesis, the treating neurologist attempted a trial with CoQ10 supplementation in the more severely affected individual. After this was successful, approval for CoQ10 treatment was granted from local health insurances, both patients were under long term treatment and were re-assessed after ~ 1 year.
CoQ10 levels
Plasma CoQ10 (Q10, 87853) was analyzed by the Mayo Clinic (Rochester, MN). Since samples were in transport more than 72 hours, only total (not reduced) plasma CoQ10 values were reliable and reported. CoQ10 levels of the sister, patient 2, although not treated with CoQ10, was measured at the same times as patient 1.
Next generation exome sequencing
DNA was isolated from whole blood using the Qiagen Gentra Puregene kit. Next generation exome sequencing was performed by the University of Michigan DNA core facility. Exome was captured by the SeqCap EZ Exome v3.0 kit (Roche, CA, USA), and paired ends were sequenced on HiSeq2000 to an average depth of 52 X. Variants of interest were validated and tested for segregation patterns, such as verification of heterozygosity, by Sanger sequencing.
Results
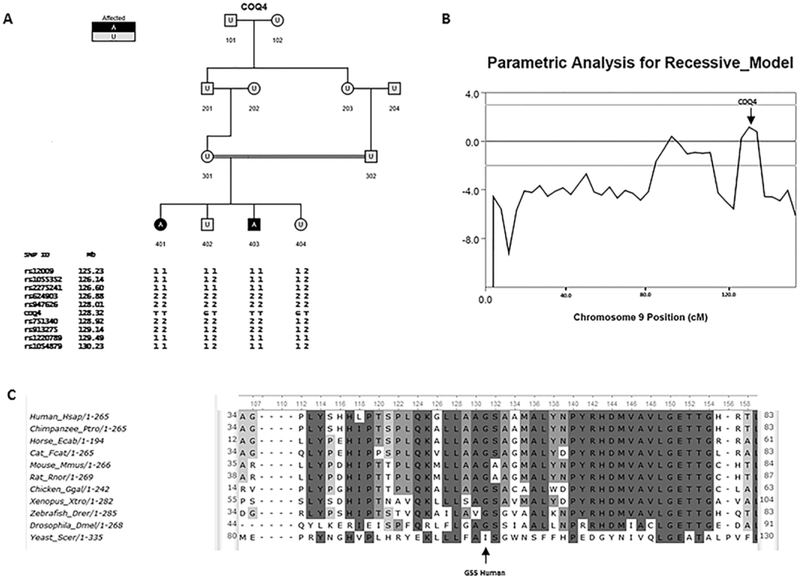
DNA samples from a sibling pair with childhood-onset ataxia were submitted for next generation exome sequencing. Common SNPs were used for linkage analysis using Merlin [7] with a pedigree loop included to account for the first cousin mating (Fig. 2A). Model parameters were for a fully penetrant rare recessive disease with a minor allele frequency of 0.0001. Only one region on distal chromosome 9 showed a LOD score above 1.0 under this model (Fig 2B). The region implicated contained 51 genes. We identified only one gene, COQ4, with a homozygous mutation that was predicted to be damaging, exon2:c.G164T :p.G55V. Both parents were heterozygous. This particular mutation has not been previously reported [8] and the glycine at this position is conserved in all vertebrates (Fig 2C). As C0Q4 mutations lead to CoQ10 deficiency [9], plasma CoQ10 was measured. CoQ10 levels were below or at the low end of normal (Table 1). CoQ10 treatment has been suggested [9,10] and has helped in humans and animal models with CoQ10 deficiency [11] and in some of the more prevalant causes of ataxia [12]. The treating neurologist therefore offered treatment with high dose (2000 mg/day) CoQ10 to the more severely affected patient. After one month on therapy the patient was re-evaluated, and another blood sample of the index patient was drawn. The Scale for the Assessment and Rating of Ataxia (SARA) [13], a validated rating scale for ataxia, was used to quantify severity of cerebellar dysfunction. Following 1 month of treatment with CoQ10, although serum CoQ10 values were not improved, clinical evaluation and subjective report showed a marked improvement. The total SARA score improved from 30 to 10, with the biggest improvement in gait and station, from being wheelchair bound, unable to walk even with support, or standing unaided, to now being able to walk with a walker and standing without support. After ~ 1 year of maintained therapy with CoQ10, the patient remained much improved from baseline, with a SARA score of 17. Subsequently the affected sister was also initiated on CoQ10 with an improvement in the SARA score at the initiation of treatment of 31 to 12 following long term treatment.
Fig 2. Family with Linkage Analysis and mutation in COQ4.
A: Pedigree of consanguineous family, with genotypes of closely linked SNPs from linkage analysis shown next to COQ4 status (based on Sanger sequencing). B: Linkage analysis of chromosome 9, the only chromosome with LOD > 1, C: Cross species comparison of amino acid sequence alignment shows glycine at position 55 is conserved in all vertebrates.
Table 1:
Summary of clinical, laboratory and imaging findings of the reported patients
| Patient 1 | Patient 2 | ||||
|---|---|---|---|---|---|
| Before treatment |
After 1 month COQ10 (2000mg per day) |
After ~1 year of CoQ10 (2000mg per day) |
Before treatment |
After ~1 year of CoQ10 (2000mg per day) |
|
| Consanguinity | Product of first cousin marriages | ||||
| Sex | Male | Female | |||
| Age at onset (years) | 8 | ||||
| Age at evaluation (years) | 25 | 27 | 26 | 28 | |
| SARA score at evaluation | 30 | 10 | 17 | 17 | 12 |
| Gait | Unable to walk, even supported | Clearly abnormal, > 10 steps not possible | Marked staggering, intermittent support of the wall required | Gait difficulty | Gait difficulty |
| Station | Able to stand >10 sec with constant support | Able to stand >10 sec without support | Able to stand >10s in natural position only with intermittent support | Normal | Normal |
| Nose finger test | Tremor > 5 cm | Tremor <2 cm | Tremor with an amplitude <2cm | Tremor <2 cm | Tremor <2 cm |
| Speech | Dysarthria | Dysarthria | Dysarthria | Dysarthria | Dysarthria |
| COQ10 in blood mcg/L (433–1532 is normal range) | 500 | 461 | NA | 428 and 308 | NA |
| MRI | Cerebral (especially parietal-occipital region) and cerebellar atrophy | NA | Cerebral and cerebellar atrophy | NA | |
| Other features | Partial epilepsy, Cognitive deterioration | None | Partial epilepsy, Cognitive deterioration | None | |
Discussion
Our results suggest that certain mutations in COQ4 lead primarily to ataxia, in contrast to more damaging mutations that lead to lethal neonatal mitochondrial encephalomyopathy [9,10] with variable presentations, including one case with mainly neurological involvement [9] similar to our cases. Our results suggest that COQ4 deficiency can lead to ataxia that is responsive to CoQ10 treatment. Although only a single case, our results are consistent with animal models and prior evidence in patients with other CoQ10 deficiencies that demonstrate benefit from high dose CoQ10 therapy [14]. This case represents an early report of CoQ10 treatment for COQ4-deficiency ataxia. The SARA score improvement from 30 to 10 may be exaggerated by inexperience of the physician with the English form, and psychologically by the subjective clear improvement observed by all, the patient, his parents and the physician, The later scores (from 32 to 17 and 31 to 12) may be a more objective indicative of treatment response. We should also note that while blood CoQ10 levels were borderline low in both, it was not a good biomarker, as already initially, the level in the less severely affected sister was lower than in the more affected brother, and levels did not show improvement during treatment. Since blood was drawn in Turkey several days before analysis at the Mayo clinic, we can’t rule out technical problems with measurements in these delayed samples. The question of value of blood CoQ10 as a biomarker in such cases of ataxia clearly warrants further studies under optimal conditions and larger sample sizes.
Of relevance, recently, a case of multiple system atrophy, a severe form of ataxia with autonomic system failure, with mutations in COQ2 was reported who was treated with high-dose ubiquinol, a more bioavailable form of CoQ10. While plasma levels of CoQ10 improved in that case, phenotype, and SARA score of 40, in contrast to our cases reported here, did not improve [15]. More research is needed to determine whether similarities and differences are due to timing of the administration in relation to disease stage, different mutations, or other still unknown factors.
Conclusion
In Turkey, as in the US, CoQ10 is considered a supportive supplement rather than a prescription medication. This can limit access due to out-of-pocket costs associated with supplementation with high dose CoQ10. In these and similar cases such as vitamin deficiencies with demonstrated low blood levels, high dose supplementation should be considered a precise pharmacotherapy, and coverage should be available through prescription. The benefit of CoQ10 treatment may be suggested by the molecular etiology as well as blood levels, and clinical improvement with CoQ10 can significantly improve health and quality of life.
Acknowledgements
We thank Linda Gates for excellent technical assistance, and NS078560 for funding.
Footnotes
Authors declare that they don’t have any conflicts of interest
References
- 1.Sun YM, Lu C, Wu ZY. Spinocerebellar ataxia: relationship between phenotype and genotype - a review Clin Genet. 2016;90(4):305–14. [DOI] [PubMed] [Google Scholar]
- 2.Beaudin M, Klein CJ, Rouleau GA, Dupre N. Systematic review of autosomal recessive ataxias and proposal for a classification. Cerebellum Ataxias. 2017;4:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fogel BL, Lee H, Deignan JL, Strom SP, Kantarci S, Wang X, et al. Exome sequencing in the clinical diagnosis of sporadic or familial cerebellar ataxia. JAMA Neurol. 2014;71(10):1237–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fogel BL, Lee H, Strom SP, Deignan JL, Nelson SF. Clinical exome sequencing in neurogenetic and neuropsychiatric disorders. Ann N Y Acad Sci. 2016;1366(1):49–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lander ES, Botstein D. Homozygosity mapping: a way to map human recessive traits with the DNA of inbred children. Science. 1987;236(4808):1567–70. [DOI] [PubMed] [Google Scholar]
- 6.Scott EM, Halees A, Itan Y, Spencer EG, He Y, Azab MA, et al. Characterization of Greater Middle Eastern genetic variation for enhanced disease gene discovery. Nat Genet. 2016;48(9):1071–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Abecasis GR, Cherny SS, Cookson WO, Cardon LR. Merlin--rapid analysis of dense genetic maps using sparse gene flow trees. Nat Genet. 2002;30(1):97–101. [DOI] [PubMed] [Google Scholar]
- 8.Lek M, Karczewski KJ, Minikel EV, Samocha KE, Banks E, Fennell T, et al. Analysis of protein-coding genetic variation in 60,706 humans. Nature. 2016;536(7616):285–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brea-Calvo G, Haack TB, Karall D, Ohtake A, Invernizzi F, Carrozzo R, et al. COQ4 mutations cause a broad spectrum of mitochondrial disorders associated with CoQ10 deficiency. Am J Hum Genet. 2015;96(2):309–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chung WK, Martin K, Jalas C, Braddock SR, Juusola J, Monaghan KG, et al. Mutations in COQ4, an essential component of coenzyme Q biosynthesis, cause lethal neonatal mitochondrial encephalomyopathy. J Med Genet. 2015;52(9):627–35. [DOI] [PubMed] [Google Scholar]
- 11.Salviati L, Trevisson E, Rodriguez Hernandez MA, Casarin A, Pertegato V, Doimo M, et al. Haploinsufficiency of COQ4 causes coenzyme Q10 deficiency. J Med Genet. 2012;49(3):187–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lo RY, Figueroa KP, Pulst SM, Lin CY, Perlman S, Wilmot G, et al. Coenzyme Q10 and spinocerebellar ataxias. Mov Disord. 2015;30(2):214–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schmitz-Hübsch T, du Montcel ST, Baliko L, Berciano J, Boesch S, Depondt C, Giunti P, Globas C, Infante J, Kang JS, Kremer B, Mariotti C, Melegh B, Pandolfo M, Rakowicz M, Ribai P, Rola R, Schöls L, Szymanski S, van de Warrenburg BP, Dürr A, Klockgether T, Fancellu R. Scale for the assessment and rating of ataxia: development of a new clinical scale. Neurology. 2006;66(11):1717–20. [DOI] [PubMed] [Google Scholar]
- 14.Desbats MA, Lunardi G, Doimo M, Trevisson E Salviati L. Genetic bases and clinical manifestations of coenzyme Q10 (CoQ 10) deficiency. J Inherit Metab Dis. 2015; 38(1):145–56. [DOI] [PubMed] [Google Scholar]
- 15.Mitsui J, Koguchi K, Momose T, Takahashi M, Matsukawa T, Yasuda T, Tokushige SI, Ishiura H, Goto J, Nakazaki S, Kondo T, Ito H, Yamamoto Y, Tsuji S. Three-Year Follow-Up of High-Dose Ubiquinol Supplementation in a Case of Familial Multiple System Atrophy with Compound Heterozygous COQ2 Mutations. Cerebellum. 2017; 16(3):664–672. [DOI] [PMC free article] [PubMed] [Google Scholar]