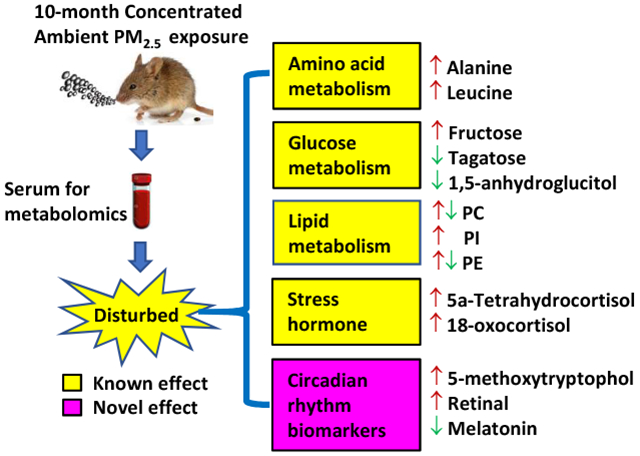
Abstract
Chronic ambient fine particulate matter (PM2.5) exposure correlates with various adverse health outcomes. Its impact on the circulating metabolome–a comprehensive functional readout of the interaction between an organism’s genome and environment–has not however been fully understood. This study thus performed metabolomics analyses using a chronic PM2.5 exposure mouse model. C57Bl/6J mice (female) were subjected to inhalational concentrated ambient PM2.5 (CAP) or filtered air (FA) exposure for 10 months. Their sera were then analyzed by liquid chromatography-mass spectrometry (LC-MS) and gas chromatography-mass spectrometry (GC-MS). These analyses identified 2570 metabolites in total, and 148 of them were significantly different between FA- and CAP-exposed mice. The orthogonal partial least-squares discriminant analysis (OPLS-DA) and heatmap analyses displayed evident clustering of FA- and CAP-exposed samples. Pathway analyses identified 6 perturbed metabolic pathways related to amino acid metabolism. In contrast, biological characterization revealed that 71 differential metabolites were related to lipid metabolism. Furthermore, our results showed that CAP exposure increased stress hormone metabolites, 18-oxocortisol and 5a-tetrahydrocortisol, and altered the levels of circadian rhythm biomarkers including melatonin, retinal and 5-methoxytryptophol.
Keywords: PM2.5, metabolomics analysis, the stress response, circadian rhythm disruption
Graphical Abstract
1. Introduction
Ambient fine particulate matter (PM2.5) pollution is a leading challenge for global public health (Cohen et al., 2017). It correlates with various adverse health effects from respiratory diseases to cardiometabolic abnormalities (Mukherjee and Agrawal, 2018). Its underlying biological mechanisms/action modes have yet not been well understood. PM2.5 inhalation has been shown to result in a pronounced pulmonary inflammation in humans and animal models, which has been long believed to subsequently cause systemic inflammation and various cardiometabolic effects and thus be central within the development of various adverse health effects caused by ambient PM2.5 exposure (Brook et al., 2010). Most recently, a rapidly increasing number of studies have indicated that ambient PM2.5 exposure also correlates with a variety of neural effects, implicating neural mechanisms in the toxic actions of PM2.5 (Li et al., 2017; Ying et al., 2014). This extension of putative mechanisms has merited further studies to thoroughly document the toxicity of PM2.5 using high throughput techniques, which will not only verify these putative mechanisms but also provide additional potential mechanisms.
As each metabolite in the biological fluids reflects the status of relevant metabolic pathway(s), profiling the whole collection of metabolites (the metabolome) using high throughput techniques including liquid chromatography-mass spectrometry (LC-MS) and gas chromatography-mass spectrometry (GC-MS) helps illustrate an individual’s comprehensive (patho)physiology. This is referred to as a metabolomics analysis. It has recently been exploited to comprehensively document the pathophysiological changes caused by intra-tracheal instillation of ambient PM2.5 in rats (Wang et al., 2017; Zhang et al., 2017). These studies have revealed marked effects of short-term intra-tracheal instillation of PM2.5 on the metabolome of urine, blood, and lung. Specifically, short-term intra-tracheal instillation of ambient PM2.5 was shown to mainly impact lipid and nucleotide metabolism in the lung, and alter metabolism of amino acids, glyoxylate and dicarboxylate, nitrogen, and methane in the blood and/or urine. These studies have advanced our understanding about ambient PM2.5 toxicity, also validated the application of metabolomics in PM2.5 toxicological study. However, cautions should still be taken when extrapolating these data, as intra-tracheal instillation is not the primary/main route of ambient PM2.5 exposure.
More recently, we employed the metabolomics strategy to ascertain whether short-term decrease in ambient PM2.5 concentration using air purifiers is sufficient to reduce adverse effects of inhalation exposure to PM2.5 in apparently healthy college students (Li et al., 2017). These metabolomics analyses demonstrated that acute PM2.5 inhalation was significantly associated with alterations in circulating glucose, amino acids, and lipids. Furthermore, examining the signature of circulating stress hormone metabolites strongly suggested that short-term inhalational ambient PM2.5 exposure elicits a marked stress response, adding the latter to the potential mechanisms for the progression of adverse health effects caused by ambient PM2.5 exposure. Notably, most toxic actions of ambient PM2.5 have been shown to be cumulative (EPA, Integrated Science Assessment for Particulate Matter), warranting further studies to assess the metabolomics effect of chronic exposure to PM2.5. As it is relatively difficult to determine the personal long-term PM2.5 exposure level, a mouse model using a versatile aerosol concentration enrichment system (VACES) was thus exploited in the present study. The metabolomics analyses revealed both previously identified and novel alterations in the circulating metabolome by chronic exposure to PM2.5, adding a comprehensive insight into the ambient PM2.5 toxicity.
2. Materials and Methods
2.1. Concentrated ambient PM2.5 (CAP) or filtered air (FA) exposure
All mouse-related procedures in this work were previously approved by the institutional animal care and use committee (IACUC) of Fudan University, and all the mice were treated humanely with regard for alleviation of sufferings. Specifically, C57Bl/6J female mice (3-week-old, 11/group and 22 in total) were purchased from the Animal Center of Fudan University (Shanghai, China) and acclimated in the animal facility for 2 weeks before exposure to FA or CAP. The group size of 11 was determined through the power analysis using the previously published effect of CAP exposure on the circulating IL-6 (Chen et al.,2018). As per the calculation with an online calculator (www.stat.ubc.ca/~rollin/stats/ssize/n2.html), the statistic power is 0.9. The monitoring and exposure of ambient aerosol and the exposure atmosphere were performed using a VACES as described previously (Geller et al., 2005; Maciejczyk et al., 2005). Briefly, 5-week-old mice were randomly grouped and exposed to FA or CAP from March 2016 to January 2017 for a total 10-month-exposure. The exposure was performed 5 days/week and 8 hours/day with no exposure during the weekends. Throughout the whole exposure period, the mice were housed in standard cages with relative humidity of 40 to 60% and temperature of 18 to 25°C under a 12-hour dark/12-hour light cycle.
2.2. Sampling and elemental composition analysis of PM2.5
The PM2.5 samples in CAP and FA chambers were collected every week using Teflon filters (Teflo, pore size of 2 μm, 37 mm, Pall Life Sciences, Ann Arbor, USA). The mass of PM2.5 was determined by the difference of the filter between pre- and post-exposure. To determine their elemental composition, the collected filters were immersed in nitric acid solution (1%) after wetting with ethanol, followed by 48-hour sonication in an ultrasonic bath and 2-week passive acid digest. A full suite of trace elements in the extracts were quantitated by inductively coupled plasma-mass spectrometry (ICP-MS) (ThermoFinnigan, ELEMENT2, San Jose, USA). With a sensitivity of over 2 Mcps/ng·g−1 for a mid-mass element and off peak noise of < 0.2 cps irrespective of mass, the machine can reliably measure sub pg·g−1 concentrations in any semiconductor process chemical (https://assets.thermofisher.com/TFS-Assets/CMD/Application-Notes/AN-30105-HR-ICP-MS-Trace-Metals-Sulfuric-Acid-AN30105-EN.pdf).
2.3. Serum sample collection
Following the 10-month exposure to FA or CAP, all the mice were euthanized, and their blood was harvested from the orbital venous plexus. All blood samples were set at room temperature allowing to clot for 30 minutes. Serum samples were then obtained through centrifugation at 4°C for 15 min in the speed of 3000 rpm, and stored at −80°C before LC-MS or GC-MS tests.
2.4. Serum sample preparation for GC-MS and LC-MS
80 μL sera from each mouse were thoroughly mixed with 10 μL of internal standard buffer (2-chlorophenylalanine in methyl alcohol, 0.3 mg/mL) and 240 μL of cold methanol-acetonitrile (v/v, 2:1) via vortexing and sonication. The mixture was then incubated at −20°C for 20 minutes, and centrifuged at 4 °C for 10 minutes with a speed of 14000 rpm. The supernatants were then harvested for LC-MS or dried under vacuum before derivatization for GC-MS.
2.5. Gas chromatography-mass spectrometry (GC-MS)
Derivatization of GC-MS samples was conducted following a previous report (Peng et al., 2015) with minor modifications. In brief, each sample was added with 80μL of methoxyamine (15 mg/mL, in pyridine). The mixture was then vortexed for 2 minutes and incubated for 90 minutes at 37 °C. 20 μL of n-hexane and 80 μL of bis(trimethylsilyl) trifluoroacetamide (BSTFA) (with 1% of trimethylchlorosilane) were then added. The solution was vortexed for 2 minutes, then reacted for 60 minutes at 70 °C, and finally incubated at room temperature for 30 minutes before GC-MS.
1 μL derivatized solution was then injected into the GC-MS system (Agilent 7890A-5975C, USA) using splitless mode. A non-polar DB-5 capillary column (J&W Scientific, 30 m × 250 μm I.D.) was used to perform the separation with a constant flow rate of 1.0 mL/min with carrier gas of high purity helium. The programmed GC temperatures were as follows: 15°C/min, 50°C-125°C; 5°C/min, 125°C-210°C; 10°C/min, 210°C-270°C; 20°C/min, 270°C-305°C with a final maintenance for 5 minutes at 305°C. Full scan mode (mass-to-charge ratio range of 50 to 600) with the acquisition rate set at 20 spectrum/second was used. The filament bias was set as −70 V and the electron impact (EI) ion source was held at 230°C.
2.6. Liquid chromatography-mass spectrometry (LC-MS)
The LC-MS test was carried out on a Waters UPLC I-class system with a sample manager and a binary solvent delivery manager, coupling with a Waters Q-TOF Mass Spectrometer equipped with an electrospray interface (Waters Corporation, Milford, USA). LC was performed using an Acquity BEH C18 column (100 mm × 2.1 mm i.d., 1.7 μm; Waters, Milford, USA). 3.00μL sample was first injected into the whole system with (A) H2O containing 0.1% of formic acid and (B) acetonitrile containing 0.1% of formic acid. The separation was obtained with programmed gradients as follows: 5%-25% (B) over 0-1.5 min, 25%-100%(B) over 1.5-10.0 min, 100% (B) over 10.0-13.0 min, 100%-5% (B) over 13.0-13.5 min, and holding for 1 min with a flow rate of 0.40 mL/min at 5% (B). The desolvation and source temperatures were set at 500°C and 120°C respectively with a desolvation gas flow of 900 L/h. The column temperature was set at 45.0 °C. Centroid data was collected at 0.1s scan time and 0.02s interscan delay time with m/z range between 50 to 1000.
2.7. Quality control (QC) samples
Quality control samples were prepared by mixing a small volume of all the samples in both groups. All the QC samples were spaced evenly with one in every ten samples to assess the repeatability of the tests.
2.8. Metabolomics data processing
ChromaTOF(V4.34, LECO) was used to align raw GC-MS data. The obtained data matrix provides information about mass-to-charge ratio (m/z), sample information, peak intensity and retention time. The peaks from internal standards, derivatization procedure, noise or column bleed were then removed, and peaks of one metabolite were combined and normalized using the total peak intensity of each sample. XCMS and the Aglient Mass Hunter Quanlitative were used to process the raw LC-MS data for peak disconvolution, alignment, integration and normalization, producing a matrix with information on m/z, peak intensity and retention time.
The normalized data sets were subjected to multivariate statistical analysis using SIMCA-P software (Version 14.0, Umetrics, Umeå, Sweden), including the principle component analysis (PCA) and the orthogonal partial least-squares discriminant analysis (OPLS-DA), which are developed specifically for the analysis of omics datasets (Madsen et al., 2010). Specifically, the outliers were identified using the principle component analysis (PCA) with mean-center scaling. The orthogonal partial least-squares discriminant analysis (OPLS-DA) with unit variance (UV) scaling was then carried out to extract the differential metabolites between FA and CAP groups. The OPLS-DA evaluates variations in frame areas between groups: variations in the measured data are partitioned into two blocks with one containing those that correlate with the class identifier and the other including those orthogonal to the first block and thus do not contribute to the discrimination between groups (Madsen et al., 2010). The OPLS-DA model was cross-validated by withholding one-seventh of the samples in seven successive simulation to guard against over fitting (Cloarec et al., 2005) and the maximum number of iterations was fixed at 200 to ensure convergence of the OPLS algorithm (Westerhuis et al., 2010). Variable contribution of the OPLS-DA model was ranked by the variable importance in the projection (VIP), and VIP > 1.0 was considered as relevant to group discrimination. Matlab was used to transform the loadings from the OPLS-DA models and plot the color-coded correlation coefficients of all variables. NIST 11 standard mass spectral database and Fiehn database was referred to annotate the ion peaks from GC-MS test, and Metlin database (https://metlin.scripps.edu/), human metabolite HMBD database (http://www.hmdb.ca/)and the Lipidomics Gateway database (http://www.lipidmaps.org/) was referred to annotated the ion peak from LC-MS test.
2.9. KEGG pathway analysis
To identify the perturbed biological pathways, the clustering analysis on the differential metabolite data was performed using the Kyoto Encyclopedia of Genes and Genomes (http://www.kegg.jp, KEGG) and the clusterProfiler package in R that calculates the enrichment of KEGG terms using the hypergeometric distribution (Yu et al., 2012). To address the statistical issue due to multiple comparisons, the false discovery rate (FDR) controlling procedure was performed as previously described (Storey, 2002), and the calculated q-value (also known as adjusted p-value) was used to identify the perturbed KEGG pathways. All the pathways with adjusted p<0.05 were considered as the biological pathways perturbed by chronic exposure to CAP.
2.10. Statistics
Unless otherwise noted, all data were presented as mean ± SEM, and subjected to multivariate statistical method test and student t test. Statistical analyses were done using GraphPad Prism (Version 6, La Jolla, CA, USA) and SIMCA-P software (Version 14.0, Umetrics, Umeå, Sweden). p < 0.05 and VIP > 1.0 was considered significant.
3. Results
3.1. Characterization of CAP exposure
During the exposure period, the average concentration of ambient PM2.5 was 41.7 ± 25.7 μg/m3, and the average PM2.5 concentrations of the CAP and FA chambers were 236.9 ± 158.9 and 12.1 ± 4.7 μg/m3, respectively. As the exposure was performed 5 days/week and 8 hours/day (thus 5 days/7 days × 8 hours/24 hours=5/21 of total time was exposed to FA or CAP), the average PM2.5 exposure levels in this study (ConcentrationAmbient × 16/21+ ConcentrationChamber × 5/21) were 34.7 and 88.2 μg/m3 for FA and CAP-exposed mice respectively. This PM2.5 exposure level in CAP-exposed group was remarkably higher than the Chinese national ambient air quality standard (35 μg/m3), but was quite common in heavily polluted areas such as Beijing, China (Zhang and Cao, 2015). Supplemental Table 1 demonstrates the elemental compositions of PM2.5 in FA and CAP chambers. The relatively high abundance of Ca, Si, Al and Fe in PM2.5 is an indicative of its origination from construction and building emissions (Tan et al., 2016). This character of PM2.5 in this study was consistent with the undergoing major construction on Fenlin campus of Fudan University.
3.2. Alterations of serum metabolome by chronic CAP exposure
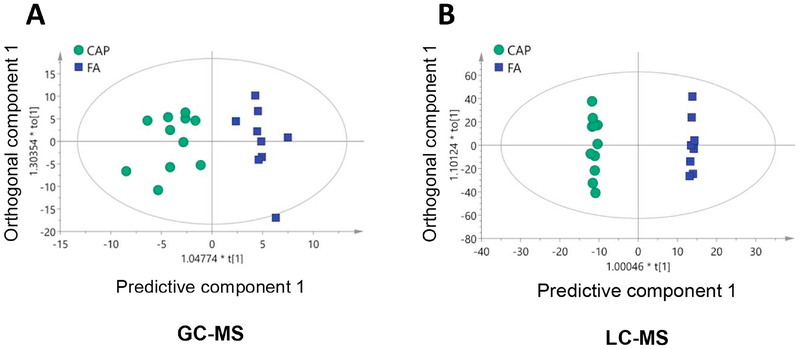
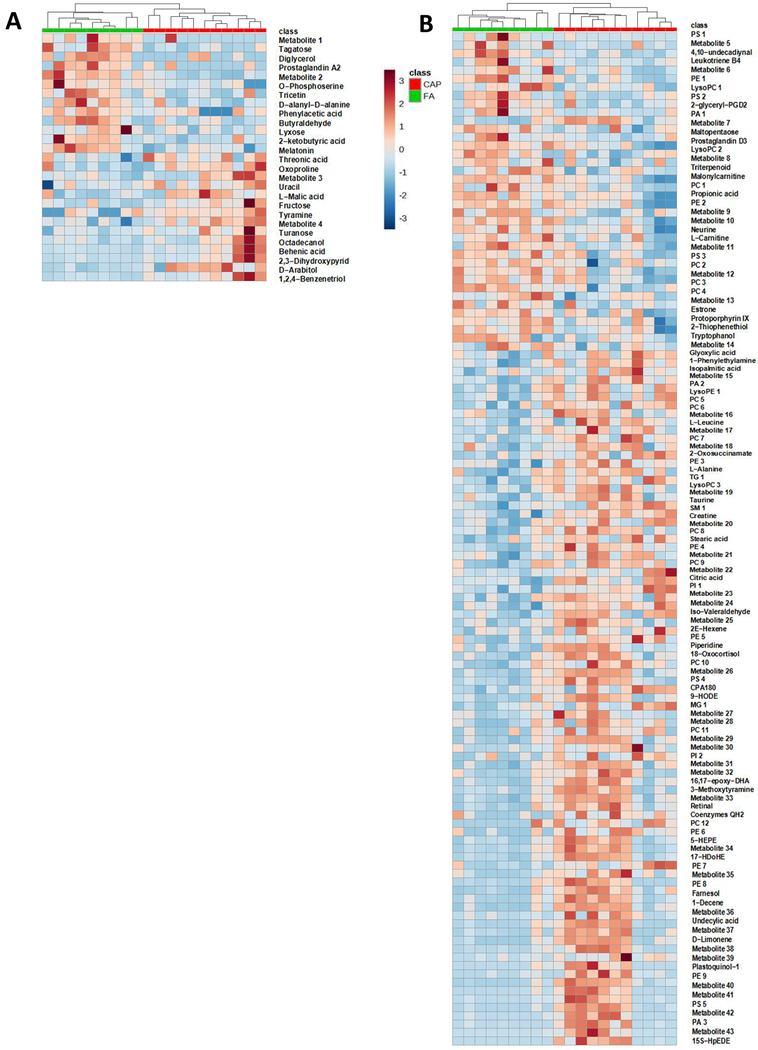
To thoroughly document the metabolic effects of CAP exposure, sera of these FA- or CAP-exposed mice were harvested and analyzed by LC-MS and GC-MS. These metabolomics analyses identified 2570 metabolites in total. Of them, 148 metabolites were significantly different among the FA and CAP groups. As shown in Supplemental Tables 2 and 3, CAP-exposed mice versus FA-exposed controls had 97 significantly increased metabolites and 51 significantly decreased metabolites. To overview the metabolic effect of chronic exposure to CAP in this murine model, OPLS-DA score was calculated for each sample and the plots (Figures 1A and B) reveal that despite marked individual variations, a clustering of FA- and CAP-exposed samples was evident for both GC-MS (R2X=0.335, R2Y=0.861, Q2=0.642, Figure 1A) and LC-MS (R2X=0.437, R2Y=0.998, Q2=0.548, Figure 1B). To further illustrate CAP exposure-induced alterations in the circulating metabolome, heatmap analyses were performed. Consistent with the OPLS-DA score plotting, Figures 2A and B demonstrate evident clustering of FA- and CAP-exposed samples.
Figure 1. Chronic CAP exposure alters the circulating metabolome.
Mice were exposed to FA or CAP for 10 months. Their sera were collected and subjected to LC-MS and GC-MS analyses. OPLS-DA score of each sample was calculated and plotted using GC-MS (A) or LC-MS (B) results.
Figure 2. Heatmaps of differential metabolites.
Mice were exposed to FA or CAP for 10 months. Their sera were collected and subjected to LC-MS and GC-MS analyses. The identified differential metabolites were used to perform heatmap analyses. The color represents the metabolite concentration of each sample calculated by peak area normalization method.
3.3. Metabolic pathways impacted by chronic exposure to CAP
To determine which metabolic pathway(s) is impacted by chronic exposure to CAP, KEGG metabolic pathway analyses were performed. Table 1 shows that chronic exposure to CAP significantly impacted 6 metabolic pathways, including protein digestion and absorption, glycine, serine and threonine metabolism, D-Alanine metabolism, carbon metabolism, ATP-binding cassette (ABC) transporters, and biosynthesis of amino acids. Notably, all these metabolism pathways are related to amino acid metabolism. However, as shown in Table 1, the proteinogenic amino acids impacted by chronic exposure to CAP were L-alanine and L-leucine. Both of them were increased in CAP-exposed mice versus FA-exposed controls.
Table 1.
Metabolic pathways perturbed by chronic exposure to CAP.
| Perturbed Pathway | Adjusted p value | Differential Metabolites | CAP/FA ratio |
|---|---|---|---|
| Protein digestion and absorption | 1.76e-03 | Tyramine | 1.64 |
| L-Alanine | 1.28 | ||
| Propionic acid | 0.69 | ||
| Piperidine | 2.11 | ||
| L-Leucine | 1.17 | ||
| Glycine, serine and threonine metabolism | 1.67e-02 | 2-ketobutyric acid | 0.73 |
| Creatine | 1.43 | ||
| Glyoxylic acid | 0.93 | ||
| D-Alanine metabolism | 1.67e-02 | D-Alanyl-D-alanine | 0.62 |
| L-Alanine | 1.28 | ||
| Carbon metabolism | 2.14e-02 | L-Malic acid | 1.44 |
| Erythrose 4-phosphate | 0.23 | ||
| Citric acid | 1.67 | ||
| L-Alanine | 1.28 | ||
| Glyoxylic acid | 0.93 | ||
| ABC transporters | 3.14e-02 | Fructose | 1.49 |
| L-Alanine | 1.28 | ||
| Taurine | 1.38 | ||
| L-Leucine | 1.17 | ||
| Biosynthesis of amino acids | 3.14e-02 | 2-ketobutyric acid | 0.73 |
| Erythrose 4-phosphate | 0.23 | ||
| Citric acid | 1.67 | ||
| L-Alanine | 1.28 | ||
| L-Leucine | 1.17 |
3.4. Effects of chronic exposure to CAP on lipid metabolisms
Ambient PM2.5 exposure has been shown to impact lipid metabolism in apparently healthy college students and rodent models (Li et al., 2017; Wang et al., 2017; Zhang et al., 2017). Consistent with these studies, Tables 2 and 3 show that of those 148 differential metabolites, 71 were related to lipid metabolism. Table 2 reveals that 33 differential metabolites were related to metabolism of glycerophospholipid, encompassing the leukotriene precursor-providers phosphatidylethanolamine (PE), phosphatidylcholine (PC) and phosphatidylinositides (PI). Chronic exposure to CAP significantly increased circulating metabolites of sphingolipids including SM(d16:1/18:1), GlcCer(d18:1/14:0), and psychosine sulfate (Table 2). In addition, our metabolomics analyses showed that chronic CAP exposure significantly impacted the metabolism of other lipids including sterols, prenols, glycerolipids, and lysophospholipids (Table 2).
Table 2.
Chronic CAP exposure impacts lipid metabolism.
| Lipid | Metabolites | CAP/FA ratio | p value |
|---|---|---|---|
| Sphingolipid | SM(d16:1/18:1) | 1.39 | 0.0059 |
| GlcCer(d18:1/14:0) | 3.79 | 0.0111 | |
| Psychosine sulfate | 31.39 | 0.0274 | |
| Sterol lipid | 1alpha-hydroxy-23-[3-(1-hydroxy-1-methylethyl) phenyl]-22,22,23,23-tetradehydro-24,25,26,27-tetranorvitamin D3 /1alpha-hydroxy-23-[3-(1-hydroxy-1-methylethyl) phenyl]-22,22,23,23-tetradehydro-24,25,26,27-tetranorcholecalciferol | 1.58 | 0.0052 |
| Taurochenodeoxycholic acid 7-sulfate | 0.72 | 0.0226 | |
| Prenol lipid | D-Limonene | 4.92 | 0.0069 |
| Coenzymes QH2 | 3.04 | 0.0214 | |
| (−)-Fusicoplagin A | 0.66 | 0.0314 | |
| Plastoquinol-1 | 5.66 | 0.0458 | |
| Glycerolipid | TG(14:1(9Z)/18:1(11Z)/14:1(9Z)) | 1.29 | 0.0249 |
| MG(0:0/22:6(4Z,7Z,10Z,13Z,16Z,19Z)/0:0) | 2.35 | 0.0342 | |
| Lysophospholipid | LysoPC(20:5(5Z,8Z,11Z,14Z,17Z)) | 0.66 | 0.0048 |
| LysoPC(18:0) | 0.48 | 0.0190 | |
| LysoPC( 18:3(6Z,9Z, 12Z)) | 1.35 | 0.0112 | |
| LysoPE(18:0/0:0) | 1.12 | 0.0123 | |
| Glycerophospholipid | PA(P-16:0/15:1(9Z)) | 12.59 | 0.0025 |
| PA(P-16:0/20:5(5Z,8Z,11Z,14Z,17Z)) | 0.53 | 0.0312 | |
| PA(18:1(9Z)/22:2(13Z,16Z)) | 1.1 | 0.0337 | |
| PC(13:0/0:0) | 1.2 | 0.0031 | |
| PC(10:0/4:0) | 0.69 | 0.0032 | |
| PC(16:0/22:5(4Z,7Z,10Z,13Z,16Z)) | 0.81 | 0.0113 | |
| PC(15:0/18:1(11Z)) | 1.67 | 0.0140 | |
| PC(16:0/P-18:0) | 2.17 | 0.0159 | |
| PC(14:0/20:1(11Z)) | 1.13 | 0.0192 | |
| PC(P-17:0/0:0) | 1.43 | 0.0350 | |
| PC(18:2(9Z,12Z)/P-18:1(11Z)) | 2.43 | 0.0403 | |
| PC(15:0/22:4(7Z,10Z,13Z,16Z)) | 1.6 | 0.0414 | |
| PC(22:5(7Z,10Z,13Z,16Z,19Z)/16:0) | 0.81 | 0.0445 | |
| PC(18:1(11Z)/22:6(4Z,7Z,10Z,13Z,16Z,19Z)) | 0.79 | 0.0479 | |
| PC(20:3(5Z,8Z,11Z)/P-18:1(11Z)) | 3.07 | 0.0499 | |
| PE(P-16:0/0:0) | 1.53 | 0.0025 | |
| PE(22:4(7Z,10Z,13Z,16Z)/P-18:1(11Z)) | 0.45 | 0.0026 | |
| PE(16:0/22:6(4Z,7Z,10Z,13Z,16Z,19Z)) | 3.74 | 0.0060 | |
| PE(22:6(4Z,7Z,10Z,13Z,16Z,19Z)/0:0) | 1.27 | 0.0075 | |
| PE(18:3(6Z,9Z,12Z)/P-18:1(11Z)) | 3.24 | 0.0112 | |
| PE(O-18:1(1Z)/20:4(5Z,8Z,11Z,14Z)) | 2.11 | 0.0138 | |
| PE(18:0/20:4(5Z,8Z,10E,14Z)(12OH[S])) | 0.71 | 0.0149 | |
| PE(20:5(5Z,8Z,11Z,14Z,17Z)/0:0) | 6.91 | 0.0308 | |
| PE(22:4(7Z,10Z,13Z,16Z)/22:6(4Z,7Z,10Z,13Z,16Z,19Z)) | 4.02 | 0.0325 | |
| PI(P-16:0/12:0) | 1.75 | 0.0016 | |
| PI (O-18:0/22:6(4Z,7Z,10Z,13Z,16Z,19Z)) | 2.58 | 0.0415 | |
| PS(19:1(9Z)/22:4(7Z,10Z,13Z,16Z)) | 0.78 | 0.0037 | |
| PS(16:0/22:2(13Z,16Z)) | ∞* | 0.0056 | |
| PS(17:1(9Z)/0:0) | 2.27 | 0.0101 | |
| PS(19:0/0:0) | 10.89 | 0.0126 | |
| PS(O-18:0/22:6(4Z,7Z,10Z,13Z,16Z,19Z)) | 0.1 | 0.0390 | |
| PS(17:1(9Z)/22:2(13Z,16Z)) | 0.48 | 0.0398 | |
| CPA(18:0) | 2.27 | 0.0203 |
PS(16:0/22:2(13Z,16Z)) was not detected in FA exposed samples.
Table 3.
Chronic CAP exposure impacts free fatty acid metabolism.
| Metabolites | CAP/FA ratio | p value | |
|---|---|---|---|
| Saturated fatty acid | Stearic acid | 1.44 | 0.0079 |
| Isopalmitic acid | 1.06 | 0.0479 | |
| Undecylic acid | 4.76 | 0.0294 | |
| Behenic acid | 2.41 | 0.0208 | |
| Threonic acid | 1.22 | 0.0338 | |
| 2-ketobutyric acid | 0.73 | 0.0242 | |
| 2R-aminoheptanoic acid | 2.39 | 0.0492 | |
| 2-hydroxy-nonadecanoic acid | 2.02 | 0.0339 | |
| 16-fluoro-7Z-hexadecenoic acid | 3.44 | 0.014 | |
| 2-chloro-acetic acid | 1.14 | 0.0166 | |
| Propionic acid | 0.69 | 0.0175 | |
| 3,3-Dibromo-2-n-hexylacrylic acid | 0.42 | 0.0174 | |
| N-Acetylaminooctanoic acid | 0.8 | 0.0178 | |
| Unsaturated fatty acid | 2E,4E-undecadienoic acid | 4.54 | 0.0007 |
| 17-HDoHE | 3.49 | 0.0035 | |
| 12-oxo-5E,8E,10Z-dodecatrienoic acid | 5.30 | 0.0011 | |
| 5,6-Epoxy-8,11,14-eicosatrienoic acid | 2.18 | 0.0047 | |
| Eicosapentaenoic acid | 0.6 | 0.0022 | |
| 2E,4E-undecadienoic acid | 4.54 | 0.0007 | |
| Fatty acid metabolism (Acylcarnitine) | 3-hydroxypentadecanoyl carnitine | 2.41 | 0.0223 |
| Malonylcarnitine | 0.66 | 0.0462 | |
| L-Palmitoylcarnitine | 1.64 | 0.0484 | |
| Carnitine metabolism | Carnitine | 0.75 | 0.0211 |
Fatty acids are the major components of lipids (Mohammad, 2015). Table 3 demonstrates that chronic CAP exposure significantly changed levels of 13 saturated fatty acids and 6 unsaturated fatty acids. The carnitine shuttle is essential for transportation of fatty acids and their subsequent β-oxidation in mitochondrial matrixes. Notably, chronic exposure to CAP not only significantly decreased the circulating carnitine level, but also significantly changed the levels of intermediates of the carnitine shuttle including 3-hydroxypentadecanoyl carnitine, malonylcarnitine, and l-palmitoylcarnitine (Table 3).
3.5. Effects of chronic exposure to CAP on circulating saccharides
Ambient PM2.5 exposure correlates with abnormalities on homeostatic regulation of glucose metabolism and thus type 2 diabetes mellitus (Baja et al., 2010; Balti et al., 2014; Hansen et al., 2016). Table 4 shows that although chronic CAP exposure did not remarkedly alter serum glucose level, it significantly upregulated the level of circulating fructose, which is believed to be particularly harmful for cardiometabolic homeostasis (Hannou et al., 2018). Furthermore, CAP exposure significantly decreased the levels of circulating 1,5-anhydroglucitol and tagatose, both of which are inversely correlated with risk for type 2 diabetes mellitus (Ensor et al., 2014; Hashimoto and Koga, 2015). Additionally, chronic exposure to CAP significantly decreased erythrose 4-phosphate, which is an intermediate in Calvin cycle and the pentose phosphate pathway (Loureiro et al., 2017).
Table 4.
Chronic CAP exposure impacts saccharide metabolism.
| Metabolites | CAP/FA ratio | p value | |
|---|---|---|---|
| Monosaccharide | 1,5-Anhydroglucitol | 0.35 | 0.00003 |
| Tagatose | 0.29 | 0.0012 | |
| Fructose | 1.49 | 0.0385 | |
| Monosaccharide Phosphate | Erythrose 4-phosphate | 0.23 | 0.0376 |
| Disaccharide | Turanose | 1.85 | 0.0266 |
| Oligosaccharide | Maltopentaose | 0.62 | 0.0347 |
3.6. Chronic exposure to CAP increases the levels of stress hormone metabolites
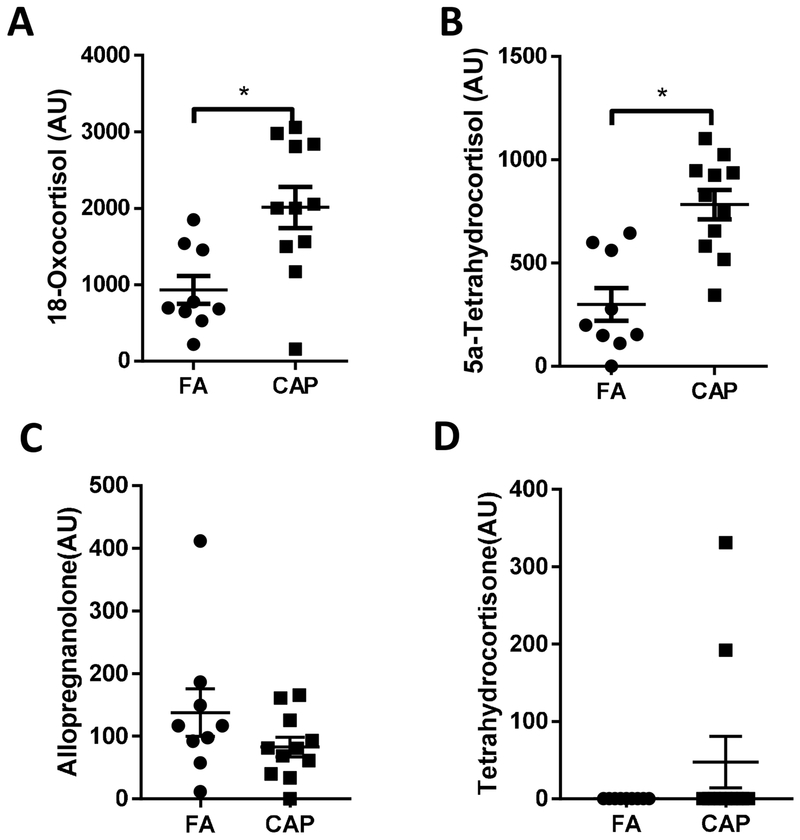
Short-term ambient PM2.5 exposure has been shown to increase circulating stress hormones, which has been implicated in the progression of various adverse health effects caused by ambient PM2.5 exposure (Li et al., 2017). Consistent with these studies, Figure 3 reveals that chronic exposure to CAP significantly increased circulating 18-oxocortisol and 5a-tetrahydrocortisol, two metabolites of cortisol. In addition, the level of allopregnanolone, a barbiturate-like modulator of central gamma-aminobutyric acid receptor that modifies behaviors including the stress response, was slightly decreased and level of tetrahydrocortisone, a metabolite of cortisone, was slightly increased (Figures 3C and D).
Figure 3. Chronic CAP exposure increases stress hormone metabolites.
Mice were exposed to FA or CAP for 10 months. Their sera were collected and subjected to LC-MS and GC-MS analyses. Two stress hormone metabolites, 18-oxocortisol (A) and 5a-tetrahydrocortisol (B), were identified as differential metabolites. Allopregnanolone (C) and tetrahydrocortisone (D) were also detected but not identified as differential metabolites. *p< 0.05 and VIP >1.0, multivariate statistical method and student t test.
3.7. Chronic exposure to CAP changes the levels of circadian rhythm-related biomarkers
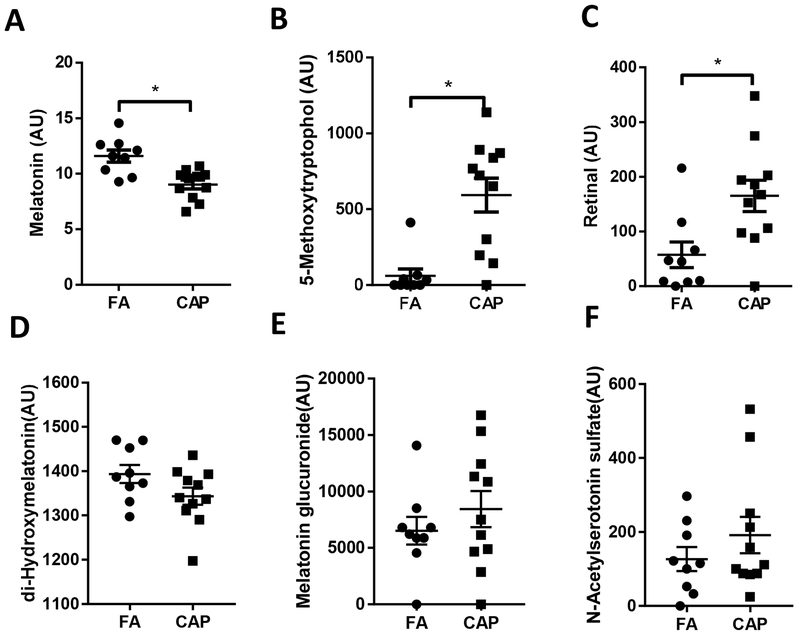
Circadian rhythm plays a vital role in maintenance of cardiometabolic homeostasis. Figure 4 indicates that chronic CAP exposure altered the levels of circulating circadian rhythm biomarkers including melatonin, 5-methoxytryptophol, retinal, di-Hydroxymelatonin, melatonin glucuronide and N-Acetylserotonin sulfate. Specifically, CAP-exposed mice versus controls had significantly decreased melatonin (Figure 4A) and significantly increased 5-methoxytryptophol and retinal (Figures 4B and 4C). Among the identified three melatonin metabolites, di-Hydroxymelatonin level was slightly decreased and the levels of melatonin glucuronide and N-Acetylserotonin sulfate were slightly increased (Figures 4D–F).
Figure 4. Chronic CAP exposure changes circulating circadian rhythm-related biomarkers.
Mice were exposed to FA or CAP for 10 months. Their sera were collected and subjected to LC-MS and GC-MS analyses. Three circadian rhythm-related biomarkers, melatonin (A), 5-methoxytryptophol (B), retinal (C), were identified as differential metabolites. Other three melatonin metabolites, di-Hydroxymelatonin (D), melatonin glucuronide (E) and N-acetylserotonin (F) were also detected but not identified as differential metabolites. *p< 0.05 and VIP >1.0, multivariate statistical method and student t test.
4. Discussion
Both randomized controlled trial and toxicological animal studies have showed that short-term ambient PM2.5 exposure causes remarkable alteration in the circulating metabolome, offering a deep insight into the biological mechanism whereby acute PM2.5 exposure causes adverse health effects (Li et al., 2017; Wang et al., 2017; Zhang et al., 2017). However, despite those numerous epidemiological studies showing that chronic exposure to PM2.5 correlates with various cardiometabolic abnormalities (EPA, Integrated Science Assessment for Particulate Matter), how it impacts the circulating metabolome has not been fully understood yet. In this study, we thoroughly documented the effect of chronic exposure to CAP on the circulating metabolome using a well-studied mouse model. The major findings include that 1) as evidenced by the evident clustering of FA- and CAP-exposed samples, chronic exposure to CAP remarkably altered the circulating metabolome; 2) almost all perturbed metabolic pathways identified by KEGG pathway analyses were related to metabolism of amino acids, specifically L-alanine and L-leucine; 3) biological characterization of the differential metabolites revealed that the metabolism of lipids, particularly glycerophospholipid, was most frequently targeted by chronic exposure to CAP; 4) chronic CAP exposure increased the level of fructose, and decreased the levels of 1,5-anhydroglucitol and tagatose; 5) chronic CAP exposure significantly altered the levels of circulating circadian rhythm biomarkers including melatonin, 5-methoxytryptophol, and retinal.
One of the most important findings in the present study is the evident clustering of FA- and CAP-exposed samples in both OPLS-DA score plotting and heatmap analysis. These clusterings strongly suggest that chronic CAP exposure markedly altered the circulating metabolome. They are consistent with numerous previous studies showing that chronic ambient PM2.5 exposure correlates with various systemic and/or extra-pulmonary effects (Chen et al., 2017; Chen et al., 2018; Gorr et al., 2014; Hu et al., 2017; Sancini et al., 2014; Zhang et al., 2017). Notably, in spite of those numerous studies investigating the toxicity of chronic ambient PM2.5 exposure, its biomarker(s) has not yet been established. These evident clusterings of FA- and CAP-exposed samples in the present study, even in the presence of a marked individual variation, strongly suggests that the toxicity of PM2.5 may be well reflected by a signature of circulating metabolites.
In the present study, our KEGG pathway analyses have identified six metabolic pathways that were significantly impacted by chronic exposure to CAP. It is noteworthy that all of these impacted pathways were related to metabolism of amino acids. These data suggest that amino acid metabolism may be one of the most important targets by chronic ambient PM2.5 exposure. As shown in Table 1, the proteinogenic amino acids impacted by chronic exposure to CAP include alanine and leucine. Chronic exposure to CAP significantly increased their circulating levels. Interestingly, although alanine and leucine belong to different classes of amino acids and have different biological functions, both have been shown to correlate with susceptibility to type 2 diabetes (Newgard et al., 2009; Sattar et al., 2004). They are believed to be even better predictors for the development of diabetes in the setting of obesity than lipids do (Melnik, 2012; Newgard et al., 2009). Therefore, these impacts on amino acid metabolism by chronic exposure to CAP may also reflect impairment of glucose homeostasis. This is consistent with the rapidly increasing studies showing that ambient PM2.5 exposure results in various abnormalities on glucose metabolism (Esposito et al., 2016). However, further studies are still needed to determine whether these increases in circulating alanine and leucine are indicative of impaired glucose homeostasis in the context of PM2.5 pollution, and whether they reflect a novel mechanism whereby exposure to ambient PM2.5 impairs glucose homeostasis.
Consistent with the impairment of glucose homeostasisas suggested by the increased circulating alanine and leucine, the present metabolomics analyses revealed significant effects of chronic exposure to CAP on metabolism of saccharides that are relevant to glucose homeostasis. It is a consensus that increased fructose consumption is one of the primary culprits for the present global epidemic of diabetes (Bidwell, 2017; Hannou et al., 2018). In this study, we observed that chronic CAP exposure significantly upregulated the level of circulating fructose. To our best knowledge, this is the first study showing that exposure to PM2.5 may perturb fructose metabolism. Fructose has been shown to increase inflammation and insulin resistance (Bidwell, 2017; Hannou et al., 2018), two major components shared by type 2 diabetes and the pathophysiology due to exposure to PM2.5. As such, further studies are warranted to determine whether this perturbation of fructose metabolism contributes to the development of diabetes related with ambient PM2.5 exposure. In addition, the present metabolomics analyses revealed that chronic CAP exposure significantly decreased circulating 1,5-anhydroglucitol and tagatose. In contrast to fructose, both of them are negatively correlated with type 2 diabetes (Espinosa and Fogelfeld, 2010). Along with the results of KEGG pathway analyses, these effects of chronic exposure to CAP on circulating saccharides strongly suggest that even though it does not alter the fasting glucose level, chronic exposure to CAP in female mice markedly impairs glucose homeostasis and thus likely contributes to the development of type 2 diabetes.
In addition to effects on metabolism of amino acids and saccharides, this study showed that chronic CAP exposure remarkably impacts metabolism of lipids. As shown in Tables 2 and 3, 71 or 48% differential metabolites were related to lipid metabolism. This is consistent with several previous metabolomics analyses on short-term PM2.5 exposure (Li et al., 2017; Wang et al., 2017; Zhang et al., 2017). Furthermore, these results show that metabolism of glycerophospholipids is most frequently targeted by chronic exposure to CAP, as per the biological classification of differential metabolites on Table 2. Glycerophospholipids are not only a crucial structural component of various biological membranes, but also the primary source for the precursors of prostanglandins and other leukotrienes, two crucial classes of mediators for inflammatory responses (Aoki and Narumiya, 2012). In addition, chronic exposure to CAP significantly increased circulating metabolites of sphingolipids including SM(d16:1/18:1), GlcCer(d18:1/14:0), and psychosine sulfate (Table 2). Sphingolipids are another class of membrane lipids that play a role in the signaling cascades involved in inflammation (Chiurchiu et al., 2018; Grosch et al., 2018). Alterations in glycerophospholipid and sphingolipid metabolism have been shown not only reflect inflammation but also play a crucial role in the pathogenesis of inflammatory diseases like psoriasis (Zeng et al., 2017). Notably, the inflammatory response is also widely believed to be central in the pathophysiology due to ambient PM2.5 exposure (Brook et al., 2010). Therefore, although verification is still needed, this demonstration of alterations in glycerophospholipid metabolism by our metabolomics analyses may reflect marked inflammatory response to CAP inhalation, which has been repeatedly shown by our studies and others’ (Chen et al., 2018; Fiordelisi et al., 2017).
Most recently, we demonstrated that reduction of ambient PM2.5 using air purifiers markedly decreased circulating stress hormones (Li et al., 2017), strongly suggesting that exposure to PM2.5 may induce the stress response. The present metabolomics analyses have identified four metabolites related to the stress response. Of them, two metabolites, 18-oxocortisol and 5a-tetrahydrocortisol (Figure 3), were significantly different between the FA and CAP groups. Furthermore, both of them were increased in CAP-exposed mice. These data have collectively corroborated that exposure to PM2.5 results in the stress response. Given the well-known cardiometabolic effects of the stress response, these results have merited further studies to delineate the role of the stress response in the progression of adverse health effects by PM2.5 exposure.
Disruption of circadian rhythm has been linked to numerous adverse health effects such as weight gain, inflammation, and even cancer (Van Dycke et al., 2015). Melatonin is a well-known hormone which is secreted by the pineal gland playing a critical role in the circadian rhythm regulation. Noticeably, in the present metabolomics analyses, it came out to be one of the differential metabolites with the lowest p value (Supplemental Table 2). The present results additionally show that the levels of circulating 5-methoxytryptophol and retinal, two other well-known circadian rhythm biomarkers, were also significantly impacted by chronic exposure to CAP. In addition to these three differential circadian rhythm-related metabolites, the present metabolomics analysis also detected three other circadian rhythm-related metabolites that are comparable between the FA and CAP groups (Figures 4D–F). These data collectively suggest that ambient PM2.5 exposure may disrupt circadian rhythm. This is somehow consistent with one previous study demonstrating that particulate matter increase may blunt daytime urinary sodium excretion and nocturnal blood pressure dipping (Tsai et al., 2012). Along with this previous study, our demonstration of alterations in circulating melatonin, 5-methoxytryptophol, and retinal further suggests that the disruption of circadian rhythm may even mediate the progression of various adverse health effects by ambient PM2.5 exposure, warranting further studies to verify this novel toxicity of PM2.5.
The present study provides a deep insight into the metabolic effect of chronic exposure to ambient PM2.5 through the metabolomics analysis of the plasma in a chronic PM2.5 exposure mouse model. However, several limitations should be noted. Firstly, the present study performed the metabolomics analysis of plasma from the female only. Further metabolomics analysis of plasma from the male is required to determine whether there is a gender difference in the metabolic response to exposure to ambient PM2.5. Secondly, the present study did not investigate the development of the metabolic effects due to exposure to ambient PM2.5, which requires the metabolomics analysis of plasma at a series of timepoints. Thirdly, the present study did not determine the role of these metabolic alterations in the pathogenesis due to exposure to ambient PM2.5. Fourthly, the present study did not determine the components of ambient PM2.5 responsible for these metabolic effects.
5. Conclusions
This study using metabolomics analyses demonstrates marked alterations in the circulating metabolome by chronic exposure to CAP, which not only reflect well-known adverse health effects of PM2.5 inhalation such as inflammation and impairment of glucose homeostasis, but also provide novel potential mechanisms for the toxicity of PM2.5, including activation of the stress response and disruption of the circadian rhythm. These findings reaffirm the importance of using the metabolomics strategy to advance our understanding of the toxicity of a complex pollutant like ambient PM2.5, and also add a deep mechanistic insight into the toxic actions of ambient PM2.5.
Supplementary Material
Highlights.
Chronic exposure to CAP disturbs 6 amino acid-related metabolic pathways in mice
Chronic CAP exposure significantly perturbs lipid metabolism in mice
Chronic exposure to CAP changes the levels of circulating saccharides in mice
Chronic exposure to CAP increases the levels of stress hormone metabolites in mice
Chronic CAP exposure changes levels of circadian rhythm-related biomarkers in mice
Metabolomics analyses identified both known and unknown alterations in circulating biomarkers by chronic PM2.5 exposure adding an integral mechanistic insight into the ambient PM2.5 toxicity.
Acknowledgments
Funding
This work was supported by the National Institutes of Health (R01ES024516 to ZY), the American Heart Association (13SDG17070131 to ZY), the National Natural Science Foundation of China (Grant No. 81770805 to ZY and 81500216 to MC) and Shanghai Pujiang Program (17PJ1401300 to YX).
List of relevant abbreviations and definitions:
- FA
filtered air
- PM2.5
ambient fine particulate matter
- CAP
concentrated ambient PM2.5
- LC-MS
liquid chromatography-mass spectrometry
- GC-MS
gas chromatography-mass spectrometry
- VACES
versatile aerosol concentration enrichment system
- ICP-MS
inductively coupled plasma-mass spectrometry
- PCA
principle component analysis
- OPLS-DA
the orthogonal partial least-squares discriminant analysis
- KEGG
the Kyoto Encyclopedia of Genes and Genomes database
- BSTFA
bis(trimethylsilyl) trifluoroacetamide
- TMCS
trimethylchlorosilane
- QC
quality control
- m/z
mass-to-charge ratio
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Ethics approval and consent to participate
All procedures of this study were approved by the Institutional Animal Care and Use Committee (IACUC) at Fudan University, and all the animals were treated humanely and with regard for alleviation of suffering.
Availability of data and material
All datasets in the present study available from the corresponding author on reasonable request.
Competing interests
The authors declare no conflict of interests in this study.
References
- Aoki T, Narumiya S, 2012. Prostaglandins and chronic inflammation. Trends Pharmacol Sci 33, 304–311. [DOI] [PubMed] [Google Scholar]
- Baja ES, Schwartz JD, Wellenius GA, Coull BA, Zanobetti A, Vokonas PS, Suh HH, 2010. Traffic-related air pollution and QT interval: modification by diabetes, obesity, and oxidative stress gene polymorphisms in the normative aging study. Environ Health Perspect 118, 840–846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balti EV, Echouffo-Tcheugui JB, Yako YY, Kengne AP, 2014. Air pollution and risk of type 2 diabetes mellitus: a systematic review and meta-analysis. Diabetes Res Clin Pract 106, 161–172. [DOI] [PubMed] [Google Scholar]
- Bidwell AJ, 2017. Chronic Fructose Ingestion as a Major Health Concern: Is a Sedentary Lifestyle Making It Worse? A Review. Nutrients 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brook RD, Rajagopalan S, Pope CA 3rd, Brook JR, Bhatnagar A, Diez-Roux AV, Holguin F, Hong Y, Luepker RV, Mittleman MA, Peters A, Siscovick D, Smith SC Jr., Whitsel L, Kaufman JD, 2010. Particulate matter air pollution and cardiovascular disease: An update to the scientific statement from the American Heart Association. Circulation 121, 2331–2378. [DOI] [PubMed] [Google Scholar]
- Chen M, Liang S, Zhou H, Xu Y, Qin X, Hu Z, Wang X, Qiu L, Wang W, Zhang Y, Ying Z, 2017. Prenatal and postnatal mothering by diesel exhaust PM2.5-exposed dams differentially program mouse energy metabolism. Part Fibre Toxicol 14, 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen M, Qin X, Qiu L, Chen S, Zhou H, Xu Y, Hu Z, Zhang Y, Cao Q, Ying Z, 2018. Concentrated Ambient PM2.5-Induced Inflammation and Endothelial Dysfunction in a Murine Model of Neural IKK2 Deficiency. Environ Health Perspect 126, 027003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiurchiu V, Leuti A, Maccarrone M, 2018. Bioactive Lipids and Chronic Inflammation: Managing the Fire Within. Front Immunol 9, 38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cloarec O, Dumas ME, Trygg J, Craig A, Barton RH, Lindon JC, Nicholson JK, Holmes E, 2005. Evaluation of the orthogonal projection on latent structure model limitations caused by chemical shift variability and improved visualization of biomarker changes in H-1 NMR spectroscopic metabonomic studies. Anal Chem 77, 517–526. [DOI] [PubMed] [Google Scholar]
- Cohen AJ, Brauer M, Burnett R, Anderson HR, Frostad J, Estep K, Balakrishnan K, Brunekreef B, Dandona L, Dandona R, Feigin V, Freedman G, Hubbell B, Jobling A, Kan H, Knibbs L, Liu Y, Martin R, Morawska L, Pope CA 3rd, Shin H, Straif K, Shaddick G, Thomas M, van Dingenen R, van Donkelaar A, Vos T, Murray CJL, Forouzanfar MH, 2017. Estimates and 25-year trends of the global burden of disease attributable to ambient air pollution: an analysis of data from the Global Burden of Diseases Study 2015. Lancet 389, 1907–1918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ensor M, Williams J, Smith R, Banfield A, Lodder RA, 2014. Effects of Three Low-Doses of D-Tagatose on Glycemic Control Over Six Months in Subjects with Mild Type 2 Diabetes Mellitus Under Control with Diet and Exercise. J Endocrinol Diabetes Obes 2, 1057. [PMC free article] [PubMed] [Google Scholar]
- Espinosa I, Fogelfeld L, 2010. Tagatose: from a sweetener to a new diabetic medication? Expert Opin Investig Drugs 19, 285–294. [DOI] [PubMed] [Google Scholar]
- Esposito K, Petrizzo M, Maiorino MI, Bellastella G, Giugliano D, 2016. Particulate matter pollutants and risk of type 2 diabetes: a time for concern? Endocrine 51, 32–37. [DOI] [PubMed] [Google Scholar]
- Fiordelisi A, Piscitelli P, Trimarco B, Coscioni E, Iaccarino G, Sorriento D, 2017. The mechanisms of air pollution and particulate matter in cardiovascular diseases. Heart Fail Rev 22, 337–347. [DOI] [PubMed] [Google Scholar]
- Geller MD, Biswas S, Fine PA, Sioutas C, 2005. A new compact aerosol concentrator for use in conjunction with low flow-rate continuous aerosol instrumentation. Journal of Aerosol Science 36, 1006–1022. [Google Scholar]
- Gorr MW, Velten M, Nelin TD, Youtz DJ, Sun Q, Wold LE, 2014. Early life exposure to air pollution induces adult cardiac dysfunction. Am J Physiol Heart Circ Physiol 307, H1353–1360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grosch S, Alessenko AV, Albi E, 2018. The Many Facets of Sphingolipids in the Specific Phases of Acute Inflammatory Response. Mediators Inflamm 2018, 5378284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannou SA, Haslam DE, McKeown NM, Herman MA, 2018. Fructose metabolism and metabolic disease. J Clin Invest 128, 545–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen AB, Ravnskjaer L, Loft S, Andersen KK, Brauner EV, Baastrup R, Yao C, Ketzel M, Becker T, Brandt J, Hertel O, Andersen ZJ, 2016. Long-term exposure to fine particulate matter and incidence of diabetes in the Danish Nurse Cohort. Environ Int 91, 243–250. [DOI] [PubMed] [Google Scholar]
- Hashimoto K, Koga M, 2015. Indicators of glycemic control in patients with gestational diabetes mellitus and pregnant women with diabetes mellitus. World J Diabetes 6, 1045–1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Z, Chen M, Zhou H, Tharakan A, Wang X, Qiu L, Liang S, Qin X, Zhang Y, Wang W, Xu Y, Ying Z, 2017. Inactivation of TNF/LT locus alters mouse metabolic response to concentrated ambient PM2.5. Toxicology 390, 100–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Cai J, Chen R, Zhao Z, Ying Z, Wang L, Chen J, Hao K, Kinney PL, Chen H, Kan H, 2017. Particulate Matter Exposure and Stress Hormone Levels: A Randomized, Double-Blind, Crossover Trial of Air Purification. Circulation 136, 618–627. [DOI] [PubMed] [Google Scholar]
- Loureiro I, Faria J, Santarem N, Smith TK, Tavares J, Cordeiro-da-Silva A, 2017. Potential drug targets in the pentose phosphate pathway of trypanosomatids. Curr Med Chem. [DOI] [PubMed] [Google Scholar]
- Maciejczyk P, Zhong MH, Li Q, Xiong J, Nadziejko C, Chen LC, 2005. Effects of subchronic exposures to concentrated ambient particles (CAPs) in mice: II. The design of a CAPs exposure system for biometric telemetry monitoring. Inhal Toxicol 17, 189–197. [DOI] [PubMed] [Google Scholar]
- Madsen R, Lundstedt T, Trygg J, 2010. Chemometrics in metabolomics-A review in human disease diagnosis. Analytica Chimica Acta 659, 23–33. [DOI] [PubMed] [Google Scholar]
- Melnik BC, 2012. Leucine signaling in the pathogenesis of type 2 diabetes and obesity. World J Diabetes 3, 38–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohammad S, 2015. Role of Free Fatty Acid Receptor 2 (FFAR2) in the Regulation of Metabolic Homeostasis. Curr Drug Targets 16, 771–775. [DOI] [PubMed] [Google Scholar]
- Mukherjee A, Agrawal M, 2018. A Global Perspective of Fine Particulate Matter Pollution and Its Health Effects. Rev Environ Contam Toxicol 244, 5–51. [DOI] [PubMed] [Google Scholar]
- Newgard CB, An J, Bain JR, Muehlbauer MJ, Stevens RD, Lien LF, Haqq AM, Shah SH, Arlotto M, Slentz CA, Rochon J, Gallup D, Ilkayeva O, Wenner BR, Yancy WS Jr., Eisenson H, Musante G, Surwit RS, Millington DS, Butler MD, Svetkey LP, 2009. A branched-chain amino acid-related metabolic signature that differentiates obese and lean humans and contributes to insulin resistance. Cell Metab 9, 311–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng ZX, Wang Y, Gu X, Xue Y, Wu Q, Zhou JY, Yan C, 2015. Metabolic transformation of breast cancer in a MCF-7 xenograft mouse model and inhibitory effect of volatile oil from Saussurea lappa Decne treatment. Metabolomics 11, 636–656. [Google Scholar]
- Sancini G, Farina F, Battaglia C, Cifola I, Mangano E, Mantecca P, Camatini M, Palestini P, 2014. Health risk assessment for air pollutants: alterations in lung and cardiac gene expression in mice exposed to Milano winter fine particulate matter (PM2.5). Plos One 9, e109685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sattar N, Scherbakova O, Ford I, O’Reilly DS, Stanley A, Forrest E, Macfarlane PW, Packard CJ, Cobbe SM, Shepherd J, 2004. Elevated alanine aminotransferase predicts new-onset type 2 diabetes independently of classical risk factors, metabolic syndrome, and C-reactive protein in the west of Scotland coronary prevention study. Diabetes 53, 2855–2860. [DOI] [PubMed] [Google Scholar]
- Storey JD, 2002. A direct approach to false discovery rates. Journal of the Royal Statistical Society Series B-Statistical Methodology 64, 479–498. [Google Scholar]
- Tan JH, Duan JC, Ma YL, He KB, Cheng Y, Deng SX, Huang YL, Si-Tu SP, 2016. Long-term trends of chemical characteristics and sources of fine particle in Foshan City, Pearl River Delta: 2008-2014. Science of the Total Environment 565, 519–528. [DOI] [PubMed] [Google Scholar]
- Tsai DH, Riediker M, Wuerzner G, Maillard M, Marques-Vidal P, Paccaud F, Vollenweider P, Burnier M, Bochud M, 2012. Short-term increase in particulate matter blunts nocturnal blood pressure dipping and daytime urinary sodium excretion. Hypertension 60, 1061–1069. [DOI] [PubMed] [Google Scholar]
- Van Dycke KC, Rodenburg W, van Oostrom CT, van Kerkhof LW, Pennings JL, Roenneberg T, van Steeg H, van der Horst GT, 2015. Chronically Alternating Light Cycles Increase Breast Cancer Risk in Mice. Curr Biol 25, 1932–1937. [DOI] [PubMed] [Google Scholar]
- Wang X, Jiang S, Liu Y, Du X, Zhang W, Zhang J, Shen H, 2017. Comprehensive pulmonary metabolome responses to intratracheal instillation of airborne fine particulate matter in rats. Sci Total Environ 592, 41–50. [DOI] [PubMed] [Google Scholar]
- Westerhuis JA, van Velzen EJJ, Hoefsloot HCJ, Smilde AK, 2010. Multivariate paired data analysis: multilevel PLSDA versus OPLSDA. Metabolomics 6, 119–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ying Z, Xu X, Bai Y, Zhong J, Chen M, Liang Y, Zhao J, Liu D, Morishita M, Sun Q, Spino C, Brook RD, Harkema JR, Rajagopalan S, 2014. Long-term exposure to concentrated ambient PM2.5 increases mouse blood pressure through abnormal activation of the sympathetic nervous system: a role for hypothalamic inflammation. Environ Health Perspect 122, 79–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu GC, Wang LG, Han YY, He QY, 2012. clusterProfiler: an R Package for Comparing Biological Themes Among Gene Clusters. Omics-a Journal of Integrative Biology 16, 284–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng C, Wen B, Hou G, Lei L, Mei Z, Jia X, Chen X, Zhu W, Li J, Kuang Y, Zeng W, Su J, Liu S, Peng C, 2017. Lipidomics profiling reveals the role of glycerophospholipid metabolism in psoriasis. Gigascience 6, 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Hu H, Shi Y, Yang X, Cao L, Wu J, Asweto CO, Feng L, Duan J, Sun Z, 2017. (1)H NMR-based metabolomics study on repeat dose toxicity of fine particulate matter in rats after intratracheal instillation. Science of the Total Environment 589, 212–221. [DOI] [PubMed] [Google Scholar]
- Zhang YL, Cao F, 2015. Fine particulate matter (PM 2.5) in China at a city level. Sci Rep 5, 14884. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.