Abstract
Methylene diphenyl diisocyanate (MDI), the most abundantly produced diisocyanate worldwide, is among the best recognized chemical causes of occupational asthma. The bulk of synthesized MDI, the 4,4’ isomer, has been the focus of most biochemical research to date. The biological reactivity of other MDI isomers (2,2’ and 2,4’), present at concentrations approaching 50% in some commercial products, remains less clear. We hypothesized 2,2’ and 2,4’ MDI react with glutathione (GSH), a major anti-oxidant of the lower airways, similarly to 4,4’ MDI, and that the products could be characterized using a combination of LC-UV-MS and MS/MS. Purified 2,2’ and 2,4’ MDI isomers were mixed with GSH in pH-buffered aqueous phase at 37°C and reaction products were analyzed at varying time points. Within minutes, S-linked bis(GSH)-MDI conjugates were detectable as the dominant [M+H]+ ion, with an 865.25 m/z and more intense [M+2H]2+ ions of the same nominal mass. Upon longer reaction, [M+H]+ ions with greater retention times and the 558.17 m/z expected for mono(GSH)-MDI reaction products were observed, and exhibited MS/MS collision-induced dissociation (CID)-fragmentation patterns consistent with cyclized structures. Compared with 4,4’ MDI, 2,2’ and 2,4’ isomers exhibit similar rapid reactivity with GSH and formation of bis(GSH)-MDI conjugates, but greater formation of cyclized mono(GSH) conjugates following extended reaction times (10 minutes to 2 hours). Further translational studies will be required to determine if the present in vitro findings extend to the complex lower airway microenvironment in vivo.
Keywords: Methylene Diphenyldiisocyanate (MDI), Isomer, Glutathione, Conjugate, Cyclized
Introduction
Methylene diphenyl diisocyanate (MDI), the most abundantly produced diisocyanate, is widely used in many different industries [1–3]. Inhalation of MDI into the lower airways has been reported to cause asthma in hypersensitized individuals and animal models, presumably due to chemical modification of “self” molecules in a manner that triggers inflammation [4–7]. Crude and technical grade preparations of MDI typically contain > 50% of the 4,4’ isomer, which been the focus of most biomedical research to date [3,6,8,9]. However, contemporary MDI formulations may also contain other (2,4’ and 2,2’) isomers (some with concentrations reaching nearly 50%) [3,10–12], whose biological reactivity remains unclear (Figure 1).
Figure 1.
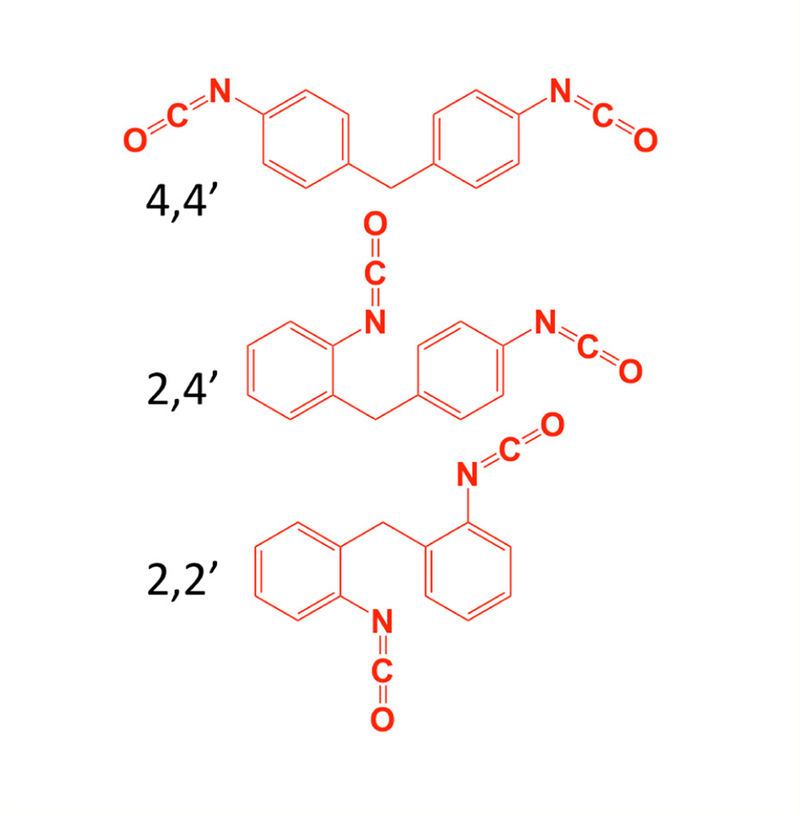
Three different isomers of MDI.
One self molecule susceptible to diisocyanate reactivity in vitro and in vivo is the unique tripeptide, glutathione (GSH), a major anti-oxidant of the lower airways [13–18]. The 4,4’ isomer of MDI exhibits preferential binding to free thiols (as present in GSH) vs. NH2 groups (present on proteins), and rapidly forms S-linked bis and mono(GSH)-MDI conjugates [16,19,20]. In vitro, MDI-GSH conjugates are cleaved into their corresponding (cys-gly) conjugates by human gamma glutamyl transpeptidase-1 [21], the primary step in metabolism along the mercapturic acid pathway [22]. GSH reacts with aliphatic hexamethylene diisocyanate vapors in vivo [18], and protects airway cells against exposure in vitro [23]. GSH also reacts with aliphatic (2-cyclohexyl-and 2-chloroethyl-) monoisocyanate metabolites of anti-cancer drugs (Lomustine and Carmustine) [24].
The biological reactivity of 2,2’ and 2,4’ isomers of MDI, remains relatively unknown, and may differ from that of 4,4’ MDI due to intrinsic differences between isomers. Under polyurethane manufacturing conditions (80°C, dry toluene, nitrogen blanket), N=C=O in MDI’s para vs. ortho is 3.8 −5.6 times more reactive with alcohols [25]; however, the influence of N=C=O’s position (para vs. ortho) in 2,2’ and 2,4’ MDI with biological molecules remains unknown. This investigation compared 2,2’ and 2,4’ vs. 4,4’ MDI isomers’ reactivity with GSH in aqueous phase at 37°C and neutral pH. A combination of LC-UV-MS and MS/MS techniques were employed to identify and compare the GSH reaction products with the different MDI isomers.
Materials and Methods
Reactivity of different MDI isomers with GSH
Purified 2,2’ MDI (CAS# 2536–05-02/Desmodur 22M), 2,4’ MDI (CAS# 5873–54-1/Desmodur 24MI) and 4,4’ MDI (CAS# 101–68-8/ Desmodur 44M) were obtained from the International Isocyanate Institute (Boonton, New Jersey). Purity was certified by GC-MS (98.54%, 99%, and 98.6% for 2,2’, 2,4’ and 4,4’ MDI respectively). Each isomer of MDI was initially diluted in extra dry 99.8% acetone (≤ 0.005% water) manufactured by Acros Organics (Morris Plains, NJ) to achieve a 10% weight/volume (w/v) stock solustion. MDI stock solutions were prepared within minutes of use for each experiment, and further diluted 100-fold in HPLC grade water buffered to pH 7.4 with 200 mM sodium phosphate (JT Baker; Center Valley, PA) containing 20 mM reduced glutathione (GSH) from Sigma-Aldrich (St. Louis, MO). Reaction conditions were based on prior published studies of GSH reactivity with 4,4’-MDI, containing a slight (2.5:1) molar excess of GSH’s reactive SH to MDI’s N=C=O groups to drive the reaction forward [19,26].
Reaction solutions were immediately vortexed and then incubated with end-over-end rotation (15 rotations/minute) for varying time periods ranging from 1 minute to 2 hours. GSH solutions were pre-equilibrated to 37°C and all experiments were performed in a 37°C temperature regulated room. Following varying durations of reactivity, samples were immediately filtered, mixed 1:10 with water/0.1% formic acid (to stabilize thiocarbamate linkages and prepare for LC-MS), stored at 4°C and analyzed within 2 hours, or stored at −80°C. Prior to analysis all samples were microfuged (16,000g) before transfer to LC-MS vials. All experiments were repeated on three different days with fresh reagents and included control reactions without GSH or MDI [26].
LC-UV-MS and MS/MS analysis of MDI-GSH reaction products
Reaction products of different MDI isomers with GSH were assessed through LC-MS/MS using a C18 LC column and electrospray ionization (ESI) in positive mode, with an increasing gradient of acetonitrile for elution. Samples were analyzed on an Agilent G6550A QTOF system coupled to an Agilent 1290 Infinity LC system, using a rapid resolution HT Zorbax Eclipse Plus C18 column (2.1 × 50 mm, 1.8 μm) from Agilent Technologies (Santa Clara, CA).
Samples (5 μL) were loaded and eluted over a 5 minutes period starting at time 0 with a 98:2 ratio of water:acetonitrile, increasing to 85:15 between 0 and 1 minute, 60:40 between 1 and 3 minutes, 5:95 between 3 and 4 minutes, up to 2:98 by 4.5 minutes and held till 5 minutes. All water and acetonitrile solutions contained 0.1% formic acid. Positive electrospray ionization (ESI+) was performed using the following parameters: gas temperature-280°C, gas flow-11 l/min, nebulizer-40 psig, sheath gas temp-350°C, sheath gas flow-11, Vcap-4000V, nozzle voltage-2000 V, fragmentor voltage-175V, skimmer voltage 65V, octopole RF peak voltage 750 V. The m/z values of all ions present in the mass spectra were corrected against two reference ions (purine, [M +H]+ m/z 112.9856 and 1H, 1H, 3H tetra(fluoropropoxy) phosphazene, [M+H]+ m/z 922.0097). The data acquisition range for LC-MS was from 110 to 1700 m/z. UV light absorbance (210, 254 nm) coupled to LC-MS was captured by diode array detection.
For MS/MS analyses, the collision energy was automatically set using Agilent MassHunter Acquisition software according to the formula, slope x (m/z)/100 + offset; with the slope of 5 and offset of 2.5. MS/MS data were obtained for the 5 most intense ions, in some experiments with preference given to species of interest with m/z’s of 865.25, 558.17, 532.12, 199.12, or 106.06 ± 100 ppm. Data were acquired and analyzed using MassHunter Workstation software from Agilent.
Data Analysis
New reaction products were identified by comparison of experimental samples’ total ion chromatograms (TICs), base peak chromatograms (BPCs), and chromatograms of ultraviolet (UV) light absorbance (at 210, 254 nm) versus control reactions performed without GSH or MDI. Extracted ion chromatograms (EICs) for [M+H]+ ions with defined m/z values and peak identification were accomplished with MassHunter Software. ChemDraw Professional 16.0 (PerkinElmer; Branford, CT) was used for chemical structure modelling, based on the exact mass of newly formed products (e.g. [M+H]+ ions), and their MS/MS fragmentation pattern upon CID.
Results
Primary reaction product of 2,2’ and 2,4’ MDI isomers with GSH
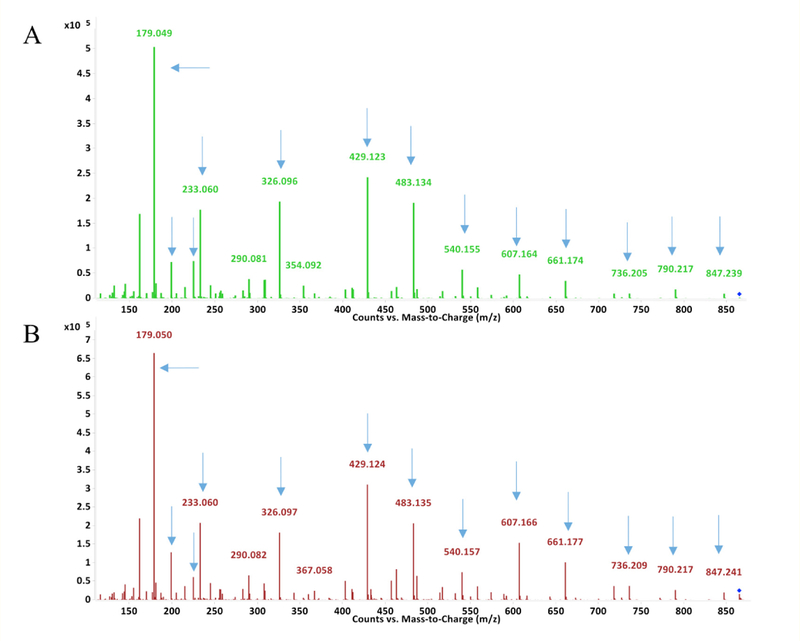
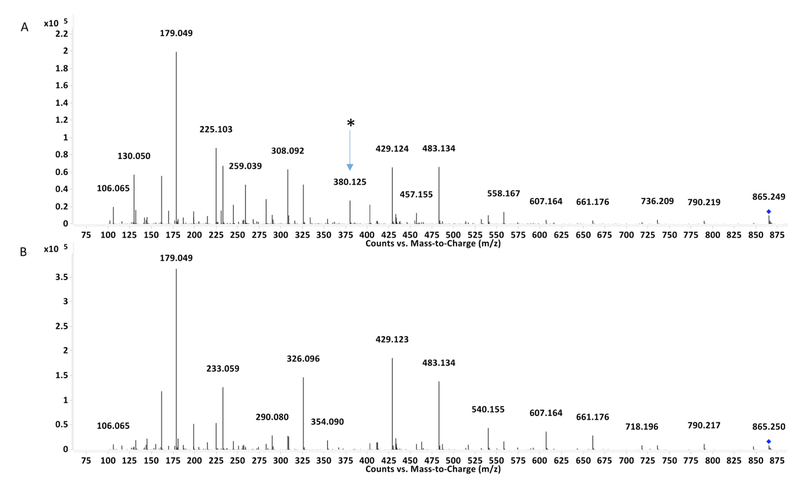
When purified 2,2’ and 2,4’ MDI isomers were incubated with GSH under at 37°C in aqueous phase at pH 7.4, a prominent new product was readily identified by comparing TICs of experimental vs. control reactions performed without MDI. Within 1 minute (Figure 2), this primary reaction product was distinguishable as an [M+H]+ ion with an 865.25 m/z and a more intense [M+2H]2+ ion (433.12 m/z), consistent with that previously described for bis(GSH)-MDI products with 4,4’ MDI [21]. The different isomers’ bis(GSH)-MDI reaction products had slightly different retention times on the C18 LC column; those comprised of 2,2’ MDI < 2,4’ MDI < 4,4’ MDI under the reverse phase chromatography conditions employed.
Figure 2.
Primary reaction product of glutathione with different isomers of MDI. Left: LC-MS TIC’s of reactions of glutathione without MDI (A), or with 2,2’ (B), 2,4’ (C) or 4,4’ (D) MDI for 1 minute. Y-axis represents ion intensity and X-axis represents LC retention time in minutes. Right: MS of products (Y-axis = ion intensity and X-axis depicts m/z) with LC retention times shown by the arrows in Panels B, C, and D are shown in Panels E, F, and G respectively for GSH with 2,2’, 2,4’ and 4,4’ MDI. GSH and GSSG represent glutathione in its reduced and oxidized states respectively, while cys-gly, a high intensity ion, is a minor contaminant of GSH.
MS/MS CID produced fragments of the 865 m/z [M+H]+ ion derived from reaction of 2,2’ and 2,4’ MDI with GSH, are shown in figure 3, and support the structures proposed in figure 4. Daughter ions of the 865.25 m/z [M+H]+ parent ion include a 607 m/z [M+H]+ ion characteristic of S,S’-linked bis(GSH)-MDI (See supplemental materials, figure 15). The 847.24 m/z daughter ion likely results from loss of water (~18 amu) from the 865.25 m/z [M+H]+ parent ion, while the 790.22 m/z [M+H]+ daughter ion likely results from loss of the glycine residue. The 736 m/z daughter ion is consistent with loss of a single γ-glu from bis(GSH)-MDI, and the 661.18 m/z daughter ion with fur-ther loss of gly. The 540.16 m/z [M+H]+ daughter ion likely results from loss of water and one glutathione moiety from the parent 865 m/z [M+H]+ ion. The 429.12 and 483.13 m/z daughter ions are consistent with fragmentation of the S-cys linkage of one GSH and subsequent loss of γ-glu or gly from the 2nd GSH group S-linked to MDI. The prominent 326.097 m/z daughter ion is consistent with a fragment con-taining the cysteine group of GSH linked to MDI cleaved between the N-C bond of MDI’s other N=C=O group. The dominant 179 m/z and the 233.06 daughter ions are expected for the cys-gly and γ-glu-cys fragments from GSH. Daughter ions expected from fragmented MDI, of 225.10 and 199.12 m/z, were also observed.
Figure 3.
MS/MS analysis of new 865 m/z [M+H]+ product of different MDI isomers with GSH. The major new [M+H]+ ion with an 865 m/z was subjected to LC-MS/MS. Fragmented daughter ions are shown from the major new 865 m/z [M+H]+ parent ion observed in reactions of GSH with 2,2’ MDI (Panel A) or 2,4’ MDI (Panel B). The Y-axis reflects ion intensity and the X-axis depicts m/z. Daughter ions detailed in the text are highlighted by arrows. Comparison with 4,4’ MDI reactions are shown in Fig. 6.
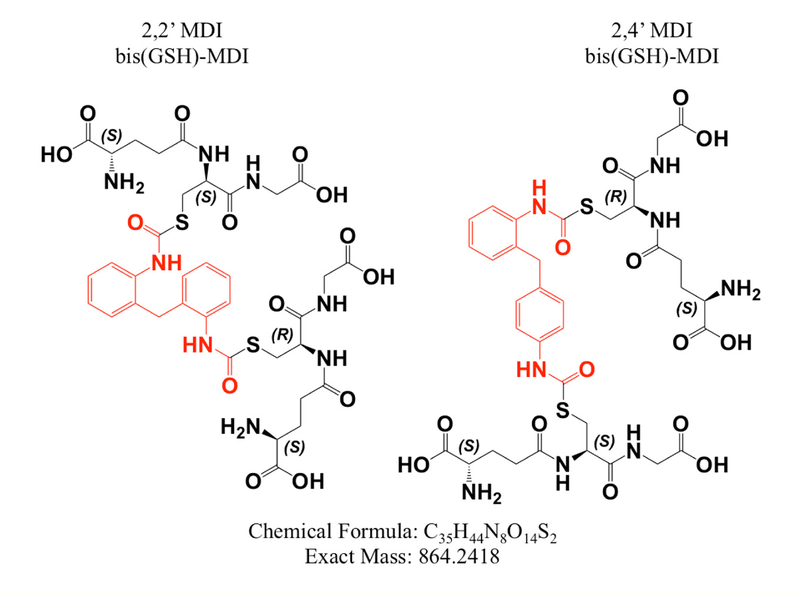
Figure 4.
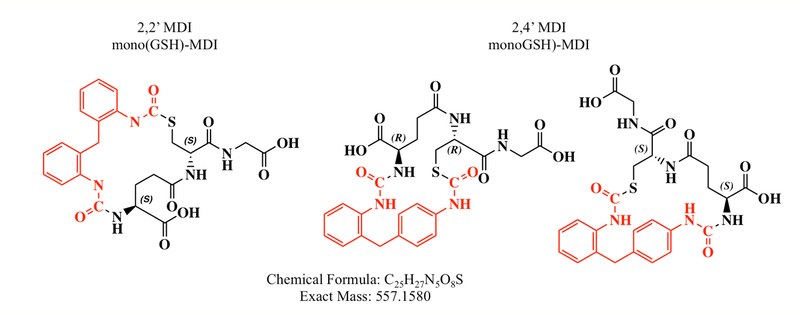
Proposed chemical structures for bis(GSH)-MDI reaction products formed with 2,2’ or 2,4’ MDI. The primary reaction products 2,2’ and 2,4’ MDI form with GSH are modeled based on the 865.25 m/z of the parent molecule and its CID fragmentation pattern during LC-MS/MS shown in figure 3. Theoretical chemical formulas and exact mass provided beneath the structures are the same for both compounds.
The MS/MS fragmentation pattern of 2,2’ and 2,4’ bis(GSH)-MDI are nearly identical to that of bis(GSH)-MDI generated from 4,4’ MDI in prior reports [19] and in head-to-head experiments in this study (See Figure 6). Thus, 2,2’ and 2,4’ MDI react rapidly with GSH at 37°C in pH buffered solution. Their primary reaction products are S-linked bis(GSH)-MDI conjugates, similar to those previously described with 4,4’ MDI [16].
Figure 6.
MS/MS analysis of 865 m/z [M+H]+ product of GSH with different MDI isomers: 2,2’ (A), 2,4’ (B), 4,4’ (C).
Bis(GSH)-MDI formation with different MDI isomers followed by UV light absorbance
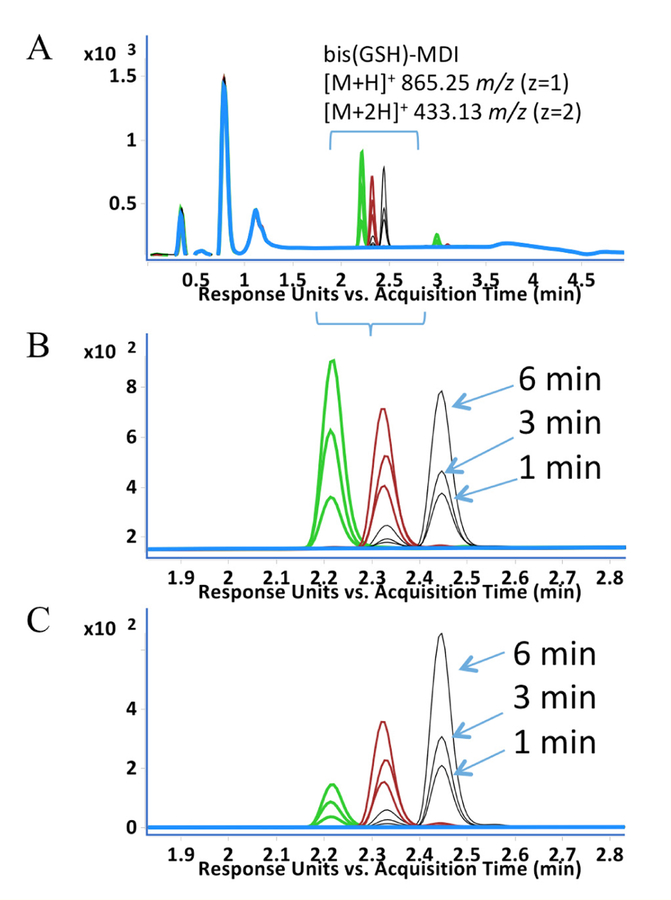
The amount of bis(GSH)-MDI reaction products formed over time, with 2,2’, 2,4’ and 4,4’ MDI isomers, were measured based on UV light absorbance at two different wavelengths; 210 nm (generally reflective of peptide bonds) and 254 nm (generally reflective of MDI’s ring structures) [25,27,28]. The data demonstrate qualitatively similar (increasing) formation of bis(GSH)-MDI conjugates with each of the different MDI isomers within the first 6 minutes of reaction (Figure 7). Quantitation of reaction rates, however, was not possible as extinction coefficients for the different MDI isomers’ bis(GSH) conjugates are unknown and likely differ substantially at different UV light wavelengths, based on studies by Nagy., et al. of alcoholic derivatives of 2,4’ vs. 4,4’ MDI [25].
Figure 7.
LC-UV light chromatograms of GSH reaction products with different MDI isomers following 1, 3, and 6 minute reactions. A. Entire LC-UV (A210) chromatogram, B. Limited region of the LC-UV (A210) chromatogram, and C. Limited region of the LC-UV (A254) chromatogram highlighting time frame (~1.9 to 2.8 min) when bis(GSH)-MDI elutes. Samples were analyzed following GSH reactivity without MDI (blue), or with 2,2’ (green), 2,4’ (red), or 4,4’ (black) MDI. Y-axis represents UV light absorbance and X-axis represents LC retention time in minutes. Samples following 1, 3 and 6 minutes are shown as labeled.
Qualitative differences between MDI isomers reaction products with GSH over extended reaction times
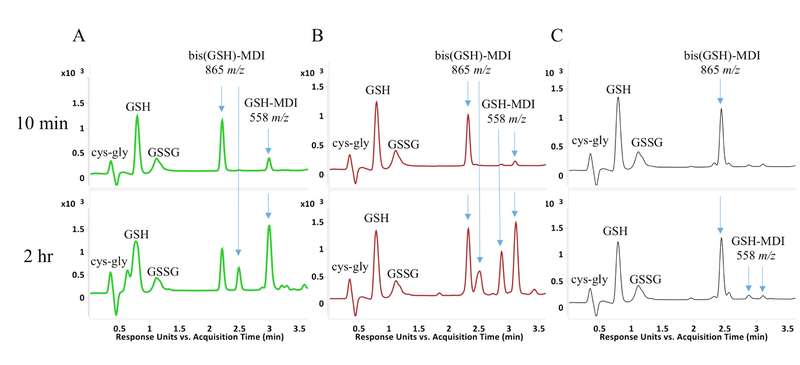
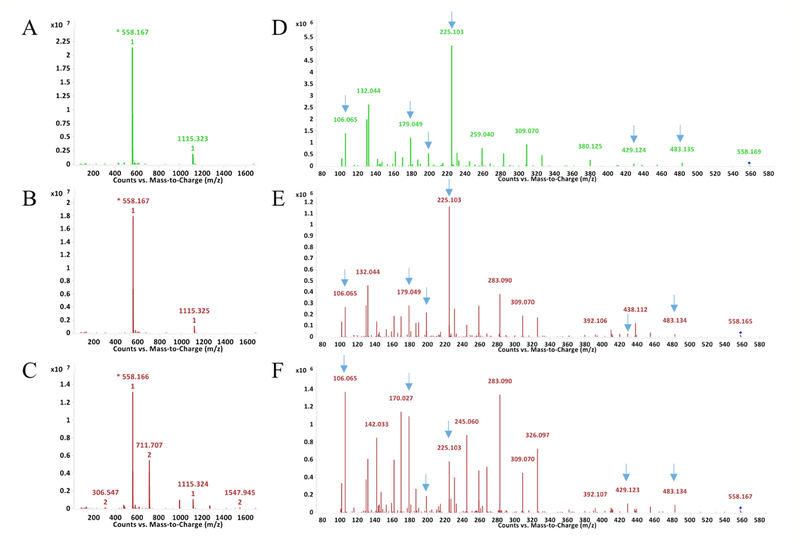
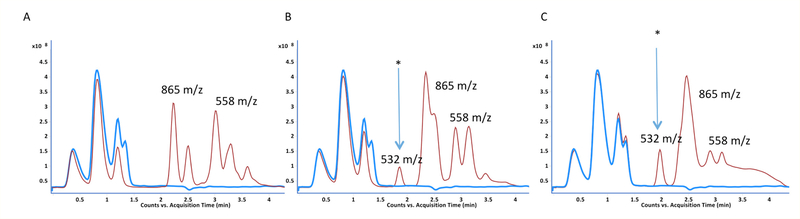
LC-UV(A210 nm)-MS analysis of GSH reaction products with MDI following extended reaction times (10 minutes to 2 hours) revealed unexpected qualitative differences between isomers (Figure 8). Reactions with the 2,4’ and 2,2’ vs. the 4,4’ isomer of MDI resulted in greater amounts of 558.17 m/z [M+H]⁺ ions than bis(GSH)-MDI.
Figure 8.
Differences between MDI isomers following extended reaction times with GSH. The LC-UV (A210) chromatograms are shown for reactions of GSH with 2,2’ (A), 2,4’ (B) or 4,4’ (C) MDI for 10 minutes (top row) or 2 hr (bottom row). Peaks with [M+H]+ ions that possess m/z’s corresponding to bis (865 m/z) or mono(GSH)-MDI (558 m/z) reaction products are highlighted. *Note 1 peak for mono(GSH)-MDI from 2,2’ MDI, two peaks for mono(GSH)-MDI from 2,4’ MDI, and much lower level dual peaks for mono(GSH)-MDI with 4,4’ MDI.
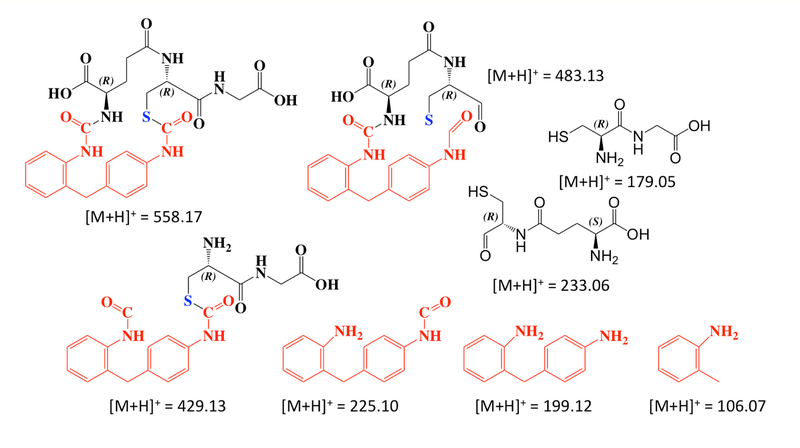
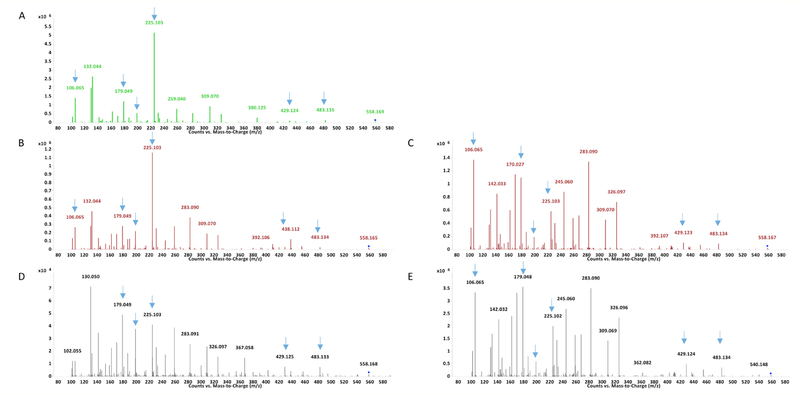
Notably 2,2’ MDI resulted in one, while 2,4’ MDI reactions resulted in two, major new peaks of 558.17 m/z [M+H]+ ions following 2 hr reactions. MS/MS analyses of the 558.17 m/z [M+H]+ ions (Figure 9 and Figure 10) are consistent with cyclized mono(GSH)-MDI (Figure 11), in which the free thiol (cys side chain) and amino terminus (γ-glu) of GSH bind a single MDI, as previously described [19]. MS/MS CID fragments of the 558.17 m/z [M+H]+ ion include 483.13 m/z and 429.12 m/z daughter ions consistent with loss of gly or γ-glu, as well as 225.10, 199.12, and 106.07 m/z daughter ions consistent with fragmentation of MDI, and the 179 m/z cys-gly fragment of GSH. The fragmentation pattern of the 558 m/z [M+H]+ ions (from 2,4’ MDI-GSH) with shorter retention times displayed qualitative differences compared to those with longer retention times and those derived from 2,2’ MDI (Figure 9). Further comparison of MS/MS CID fragmentation spectra for 558 m/z [M+H]+ ions generated upon reaction of GSH with 4,4’ vs 2,2’ and 2,4’ MDI are provided in (Figure 12).
Figure 9.
LC-MS and MS/MS analysis of new 558 m/z [M+H]+ product formed when GSH reacts with 2,2’ and 2,4’ MDI. LC-MS analyses of the major new [M+H]+ ions in A210 chromatograms are shown on the left for reactions of GSH with 2,2’ MDI (A) and for two different peaks formed with 2,4’ MDI (B longer retention time and C shorter retention time). During LC-MS/MS, the major reaction products with the 558 m/z [M+H]+ (e.g. parent ions) from Panels A - C yielded the corresponding daughter fragments shown in Panels D though F respectively, and detailed further in Figure 10. Comparison of the mono(GSH)-MDI 558 m/z [M+H]+ ions from 2,2’ and 2,4’ vs. 4,4’ MDI (in head-to-head experiments) are shown in Figure 12.
Figure 10.
Predicted MS/MS CID fragments of mono(GSH)-MDI.
Figure 11.
Proposed chemical structures for mono(GSH)-MDI reaction products with a 558 m/z [M+H]+. The major GSH reaction products that form following extended reaction times (10 minutes to 2 hours) with 2,2’ and 2,4’ MDI are modeled based on LC-MS/MS CID fragmentation patterns shown in Figure 9. Note 2 chemically distinct reaction products are proposed for 2,4’ MDI depending upon which N=C=O group (ortho vs. para position) is bound to GSH’s NH2 vs. SH moiety. Theoretical chemical formula and exact mass provided beneath structures are the same for each compound.
Figure 12.
MS/MS analysis of 558 m/z [M+H]+ product of different MDI isomers with GSH. During LC-MS/MS, upon CID@30.5eV the 558 m/z parent ions yield the following spectra if generated from 2,2’ MDI (Panel A), 2,4’ MDI, Panel B major peak (longer retention), Panel C minor peak (shorter retention time), and 4,4’ MDI (Panels D and E, later and earlier eluting peaks respectively).
The rationale for bis(GSH)-MDI and cyclized mono(GSH)-MDI products with multiple retention times remains unclear, and may represent different conformers of the same molecule, or structural differences. Evidence for the presence of S,N’-as well as S,S’-linked bis(GSH)-MDI following 2 hr reactions of GSH with 2,2’ MDI is supported by the presence of a 380.12 m/z daughter ion (consistent with that expected γ-glu-MDI) upon MS/MS analysis of a peak eluting with a longer retention time (Figure 13). Reciprocal conjugation of GSH’s NH2 and SH moieties to 2,4’ MDI’s ortho vs. para N=C=O groups explain the presence of 558.17 m/z [M+H]+ ions with two distinct retention times most evident following 2 hr reactions (Figure 8 and 11).
Figure 13.
MS/MS comparison of peaks with different retention times but identical 865.25 m/z in reactions of 2,4’ MDI with GSH. Fol-lowing 2 hr reaction of 2,4’ MDI with GSH, two prominent peaks in the A210 chromatogram were observed with the same (865.25) m/z. MS/MS analysis of the 865 m/z [M+H]+ ion with the longer retention time, appearing only at the later time points is shown in Panel A and contrasted the CID of the 865 m/z [M+H]+ ion with the shorter retention time appearing at the earliest time points. Note the 380.125 m/z daughter ion that would arise as a γ-glu-MDI fragment from an N-linked MDI conjugate, as well as the 607 m/z fragment indicative of S,S’-linked bis(GSH)-MDI, suggesting the possibility of mixed reaction products eluting with the same retention time, or potential on column rearrangement.
Discussion
This study utilized LC-UV-MS and MS/MS approaches to provide new information on biological reactivity of different MDI isomers, which may be influenced by proximity of their N=C=O groups to the molecule’s methylene bridge. The present investigation focused on the reactivity of 2,2’ and 2,4’ MDI with GSH, a major anti-oxidant of the lower airways, and found marked similarities with that previously described for 4,4’ MDI [16,19,26]. Under the present experimental conditions (aqueous phase, pH 7.4, 37°C) 2,2’, 2,4’ and 4,4’ MDI form primarily bis(GSH)-MDI conjugates within minutes. However, following longer reaction times (≥ 10 minutes), 2,2’ MDI and 2,4’ MDI form greater amounts of mono(GSH)-MDI, in a form likely stabilized by cyclization [19]. LC-UV-MS and MS/MS approaches facilitated characterization of the 2,2’ and 2,4’ MDI isomers’ reaction products with GSH and comparison with those of 4,4’ MDI.
The initially formed bis(GSH)-MDI conjugates with 2,2’ and 2,4’ MDI have longer retention times than GSH (on a C18 column under reverse phase conditions) and are readily distinguishable as a dominant new peak in LC-UV chromatograms at 210 and 254 nm. These bis(GSH)-MDI reaction products are also readily apparent in corresponding LC-MS TICs as [M+H]+ ions with an 865.25 m/z and as higher intensity [M+2H]2+ ions of the same nominal mass. Upon MS/MS, the different isomers’ primary bis(GSH)-MDI products exhibited similar CID-fragmentation patterns, including a daughter ion (607 m/z) that differentiates S,S’ vs. potential S,N’-or N,N’-linked conjugates. The primary bis(GSH)-MDI products of 2,4’ MDI have a slightly longer retention time than those for 2,2’ MDI, but a slightly shorter retention time than those of 4,4’ MDI under reverse phase chromatography conditions on a C18 column. Bis(GSH)-MDI reaction products that form following longer reaction times (2 hr) exhibit multiple retention times, which may reflect different conformations of the same molecule or intramolecular (S-N) rearrangements (as mentioned in Section 3C and below).
Cyclized mono(GSH)-MDI conjugates (Figure 8) are possible if GSH’s amino terminus (γ-glu) and its free thiol (side chain of cysteine) both react with a single MDI. Molecules with these properties are distinguishable in LC-MS TICs and BPCs as [M+H]+ ions possessing a 558.17 m/z and distinct CID fragmentation pattern upon MS/MS, as previously described [19]. These conjugates form to varying degrees with 2,2’, 2,4’ and 4,4’ MDI, have longer retention times than bis(GSH)-MDI, and may elute at multiple retention times under the described reverse phase LC-MS conditions. The biologic relevance of cyclized mono(GSH)-MDI vs. bis(GSH)-MDI is unknown. However, if cyclization provides stability, as shown for some biologically active peptides [29], it could reduce transcarbamylating potential and thus, lower antigenicity and toxicity.
The present report focuses on soluble MDI-GSH reaction products that were quantitatively most abundant, based on UV-light absorbance, however additional products were also present. A precipitate was observed in longer (2 hour) reactions of MDI with GSH solutions, possibly polyureas, as previously described for MDI in aqueous phase [30]. The precipitate was insoluble in water or organic solvents and thus, could not be analysed by LC-MS, but may be characterized in future studies using solid state analytical methods (FTIR, NMR). MDI-GSH reactions also contained low levels of GSH-conjugated to partially hydrolyzed MDI (GSH-MDI*NH2) not detectable based on UV-light absorbance, but readily apparent in LC-MS TICs, exclusively with 2,4’ and 4,4’, but not 2,2’ MDI (see figure 14). In contrast, complete hydrolysis products of 2,2’, 2,4’ and 4,4’ MDI were below the limit of detection (0.03 μM; e.g. ≤ 0.001% of starting material) defined using purified methylene diamine isomer standards (see supplemental materials, figure 15 and Supplemental Methods).
Figure 14.
LC-MS TIC of GSH reaction products following extended (2hr) reaction time with different MDI isomers. (A) Reactions performed with 2,2’ MDI, (B) Reactions performed with 2,4’ MDI, (C) Reactions performed with 4,4’ MDI. Solid thick blue line represents samples without MDI (control), while thin red line depicts signal from samples with different MDI isomer. Note the [M+H]+ ion with the 532 m/z consistent with previously described mono(GSH)-MDI*NH2, a conjugate of GSH with partially hydrolyzed MDI (amine terminated), as previously described with 4,4’ MDI [19, 21], is present in reactions with 4,4’ and 2,4’ MDI but not 2,2’ MDI.
The potential derivation of mono(GSH)-MDI (in cyclized form, or containing partially hydrolysed MDI) via intra-or inter-molecular rearrangement of bis(GSH)-MDI (vs. direct reactivity of GSH with MDI) is a provocative hypothesis suggested by the order of product formation in this study (e.g. mono(GSH)-MDI*NH2 observed only at the latest time points, and cyclized forms increasing over time). The finding are consistent with prior studies on 4,4’ MDI by Reisser., et al. [16], which suggest dynamic formation of mono(GSH)-MDI secondary to bis(GSH)-MDI formation. Further studies with purified bis(GSH)-MDI prepared with different MDI isomers should yield more definitive insight into the temporal order of bis vs. mono GSH-MDI reaction product formation.
The present data should be interpreted with recognition of the studies strengths and weakness. The strengths of the study include the precise methodology for separation of reaction products and determination of their mass, availability of purified MDI isomers, and the controlled in vitro study design. Conversely, our reductionist in vitro system possesses recognizable limitations towards understanding complex interactions that may occur in vivo. While we replicated normal body pH, temperature and relative ratios of reactants and organic solvent, our in vitro reactions didn’t include a multitude of other factors in the airway microenvironment (e.g. surfactant, proteins, GSH-dependent enzymes) that may influence MDI reactivity in vivo. Nonetheless, together with prior reports on other diisocyanates [17,18,20,31,32], the present data support the concept that GSH in airway fluid represents a likely target for 2,2’ and 2,4’, as well as the 4,4’ isomer of MDI.
Conclusion
In summary, we demonstrate similarities and differences in the reactivity of different isomers of MDI with GSH, a major anti-oxidant of the lower airways. The three MDI isomers studied (2,2’, 2,4’ and 4,4’) all react rapidly with GSH, forming bis(GSH)-MDI conjugates within minutes. Following longer reaction times (10 minutes to 2 hours) 2,2’ and 2,4’ MDI form greater amounts of cyclized mono(GSH)-MDI compared to 4,4’-MDI. Further studies will be required to determine if similar GSH reactivity with different MDI isomers occurs in the lower airways of exposed workers, and if so, the implications with respect to MDI toxicity and allergenicity.
Supplementary Material
Figure 5.
Predicted MS/MS CID fragments of bis(GSH)-MDI.
Acknowledgements
We would like to acknowledge the technical support of Drs. Terence Wu and Mousumi Ghosh from the Yale West Campus Analytical Core and Center for Molecular Innovation.
Funding
The work was supported by a grant from the Centers for Disease Control’s National Institute for Occupational Safety and Health (OH010941) and a gift from the International Isocyanate Institute. Any conclusions are those of the author and not of the funding Institutes.
Abbreviations
- AUC
Area Under the Curve
- BPC
Base Peak Current
- CID
Collision Induced Dissociation
- EIC
Extracted Ion Chromatogram
- GSH
Glutathione in Reduced State
- GSSG
Glutathione in Oxidized State
- MDI
Methylene Diphenyl-Diisocyanate
- TIC
Total Ion Chromatogram
Footnotes
Declarations of Interest
None.
Bibliography
- 1.Global Insulation staff. http://www.globalinsulation.com/news/item/1359-basf-tobuild-new-methylene-diphenyl-diisocyana-teunit-in-louisiana. Last accessed 02/15/2019.
- 2.Statista https://www.statista.com/statistics/750809/mdi-demand-worldwide/. Last accessed 02/15/2019.
- 3.Allport DC, et al. “MDI and TDI : a safety, health and the environment: a source book and practical guide” Wiley, New York: (2003). [Google Scholar]
- 4.Mapp CE., et al. “Combined asthma and alveolitis due to diphenylmethane diisocyanate (MDI) with demonstration of no crossed respiratory reactivity to toluene diisocyanate (TDI)”. Annals of Allergy 545 (1985): 424–429. [PubMed] [Google Scholar]
- 5.Sabbioni G., et al. “Comparison of biological effects with albumin adducts of 4,4’-methylenediphenyl diisocyanate in workers”. Archives of Toxicology 914 (2017): 1809–1814. [DOI] [PubMed] [Google Scholar]
- 6.Pauluhn J. “Brown Norway rat asthma model of diphenylmethane-4,4’-diisocyanate (MDI): analysis of the elicitation dose-response relationship”. Toxicological Sciences 1042 (2008): 320–331. [DOI] [PubMed] [Google Scholar]
- 7.Wisnewski AV., et al. “Antigenic changes in human albumin caused by reactivity with the occupational allergen diphenylmethane diisocyanate”. Analytical Biochemistry 4002 (2010): 251–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Luna LG., et al. “Quantitation of 4,4’-methylene diphenyl diisocyanate human serum albumin adducts”. Toxicology Reports 1 (2014): 743–751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sabbioni G., et al. “Determination of a new biomarker in subjects exposed to 4,4’-methylenediphenyl diisocyanate”. Biomarkers 156 (2010): 508–515. [DOI] [PubMed] [Google Scholar]
- 10.http://www.huntsman.com/polyurethanes/Media%20Library/a_MC1CD1F5AB7BB1738E040EBCD2B6B01F1/Products_MC1CD1F5AB8081738E040EBCD2B6B01F1/Adhesives%20%20Coatings_MD362C55B00D70D0EE040EBCD2C6B681C/files/ACE%20brochure_final_June_4_2013.pdf. Last accessed 02/15/2019.
- 11.New Advances in Polymeric MDI Variants (2018). http://www.monomers.basf.com/cm/internet/en/function/conversions:/publish/content/Produkte/Isocyanates/Lupranat_27_06_2017/Lupranat_MI.pdf. Last accessed 02/15/2019.
- 12.Lupranat -Product overview (2018). http://www.monomers.basf.com/cm/internet/en/content/Produkte/Isocyanates/lupranat#. Last accessed 02/15/2019.
- 13.Cantin AM., et al. “Normal alveolar epithelial lining fluid contains high levels of glutathione”. Journal of Applied Physiology 631 (1987): 152–157. [DOI] [PubMed] [Google Scholar]
- 14.Cross CE., et al. “Oxidants, antioxidants, and respiratory tract lining fluids”. Environmental Health Perspectives 10210 (1994): 185–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.van der Vliet A., et al. “Determination of low-molecular-mass antioxidant concentrations in human respiratory tract lining fluids”. American Journal of Physiology 276 (1999): L289–L296. [DOI] [PubMed] [Google Scholar]
- 16.Reisser M., et al. “Synthesis, characterization, and solvolysis of mono-and bis-S-(glutathionyl) adducts of methylene-bis-(phenylisocyanate) (MDI)”. Chemical Research in Toxicology 1510 (2002): 1235–1241. [DOI] [PubMed] [Google Scholar]
- 17.Wisnewski AV., et al. “Toluene diisocyanate reactivity with glutathione across a vapor/liquid interface and subsequent transcarbamoylation of human albumin”. Chemical Research in Toxicology 2410 (2011): 1686–1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wisnewski AV., et al. “Reaction products of hexamethylene diisocyanate vapors with “self” molecules in the airways of rabbits exposed via tracheostomy”. Xenobiotica 485 (2018): 488–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wisnewski AV., et al. “Connecting glutathione with immune responses to occupational methylene diphenyl diisocyanate exposure”. Chemico-Biological Interactions 2051 (2013): 38–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lalko JF., et al. “The selective peptide reactivity of chemical respiratory allergens under competitive and non-competitive conditions”. Journal of Immunotoxicology 103 (2013): 292–301. [DOI] [PubMed] [Google Scholar]
- 21.Wisnewski AV., et al. “In vitro cleavage of diisocyanate-glutathione conjugates by human gamma-glutamyl transpeptidase-1”. Xenobiotica 468 (2016): 726–732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bakke JE., et al. “Metabolism of 2,4’,5-trichlorobiphenyl by the mercapturic acid pathway”. Science 2174560 (1982): 645–647. [DOI] [PubMed] [Google Scholar]
- 23.Wisnewski AV., et al. “Glutathione protects human airway proteins and epithelial cells from isocyanates”. Clinical and Experimental Allergy 353 (2005): 352–357. [DOI] [PubMed] [Google Scholar]
- 24.Brajtburg J., et al. “Lysis of human promyelocytic HL-60 cells by amphotericin B in combination with 2-chloroethyl-1-nitrosoureas: role of the carbamoylating activity of nitrosoureas”. Cancer Research 5011 (1990): 3274–3278. [PubMed] [Google Scholar]
- 25.Nagy L., et al. “Kinetics of Uncatalyzed Reactions of 2,4′-and 4,4′-Diphenylmethane-Diisocyanate with Primary and Secondary Alcohols”. International Journal of Chemical Kinetics 499 (2017): 643–655. [Google Scholar]
- 26.Wisnewski AV., et al. “Glutathione reaction products with a chemical allergen, methylene-diphenyl diisocyanate, stimulate alternative macrophage activation and eosinophilic airway inflammation”. Chemical Research in Toxicology 28 (2015): 729–737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cantor CR and Schimmel PR. “Biophysical chemistry / Part 2, Techniques for the study of biological structure and function” W.H. Freeman., San Francisco: (1980). [Google Scholar]
- 28.Tse KS., et al. “A study of serum antibody activity in workers with occupational exposure to diphenylmethane diisocyanate”. Allergy 405 (1985): 314–320. [DOI] [PubMed] [Google Scholar]
- 29.Hruby VJ. Conformational restrictions of biologically active peptides via amino acid side chain groups”. Life Science 313 (1982): 189–199. [DOI] [PubMed] [Google Scholar]
- 30.Sekizawa J and Greenberg MM. W.H. Organization, U.N.E. Programme, IPoC. Safety, I.-O.P.f.t.S.M.o. Chemicals, Diphenylmethane Diisocyanate (MDI), World Health Organization; (2001). [Google Scholar]
- 31.Wisnewski AV., et al. “Hexamethylene diisocyanate (HDI) vapor reactivity with glutathione and subsequent transfer to human albumin”. Toxicology In Vitro 272 (2013): 662–671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pauluhn J., et al. “Analysis of biomarkers in rats and dogs exposed to polymeric methylenediphenyl diisocyanate (pMDI) and its glutathione adduct”. Toxicology 2223 (2006): 202–212. [DOI] [PubMed] [Google Scholar]
- 33.MacDougall D., et al. “Guidelines for data acquisition and data quality evaluation in environmental chemistry”. Analytical Chemistry 5214 (1980): 2242–2249. [Google Scholar]
- 34.Forsythe JG., et al. “ Structural characterization of methylenedianiline regioisomers by ion mobility-mass spectrometry, tandem mass spectrometry, and computational strategies: I. Electrospray spectra of 2-ring isomers”. Analytical Chemistry 869 (2014): 4362–4370. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.