Abstract
Aim:
We assessed effect of Akkermansia muciniphila and its extracellular vesicles on toll-like receptors and tight junction expression in human epithelial colorectal adenocarcinoma cells (Caco-2).
Background:
The intestinal microbiota plays an important role in the intestinal homeostasis through its metabolites and derivatives. Interacting with immune cells and intestinal epithelial pattern recognition receptors (PRRs), intestinal microbiota regulates the function of the digestive barrier and inflammation caused by the metabolic diseases.
Methods:
A. muciniphila was cultured on a mucin-containing medium and its EVs was extracted by ultracentrifugation. This bacterium was treated in the MOI=10 and its EVs at the concentrations of 0.1, 0.5 and 5 µg on Caco-2 cells. After 24 hours, the expression of tight junction and toll-like receptor genes were investigated by quantitative real time PCR method.
Results:
A. muciniphila increased the expression of tlr2 and tlr4. However, EVs at all of the concentrations showed a decrease in tlr4 expression. EVs at the concentrations of 0.1 and 0.5 µg/ml decreased the expression of tlr2. A. muciniphila significantly increased the expression of ocldn and cldn4. Both this bacterium and EVs increased the expression of zo2 in the cell line. Furthermore, this data show that A. muciniphila derived EVs have a dose-independent effect on Caco-2 cells.
Conclusion:
This preliminary research shows A. muciniphila and its EVs both may increase the integrity of the intestinal barrier. A. muciniphila derived EVs also reduces the inflammation so that EVs of this bacterium can be used as an appropriate target for the treatment of metabolic syndrome and inflammatory bowel diseases.
Key Words: Gut microbiota, Akkermansia muciniphila, Toll-like receptor, Tight junction protein, Extracellular vesicle
Introduction
The intestinal microbiota is the largest human microbiota reservoir that plays a significant role in human health and is involved in regulating many host physiological pathways such as the regulation of the digestive system stimulation, permeability of the digestive barrier, fat distribution, and energy homeostasis (1, 2). Intestinal microbiota reacts through MAMPs in the outer membrane surface, producing metabolites, enzymes, and secretion of vesicles with toll-like receptors (TLRs), resulting in tolerance and maintenance of intestinal immune homeostasis. Gut microbiota also interacts with the intestinal barrier, preserves the integrity of the intestinal barrier and provides protection against pathogenic bacteria (3, 4).
Gut microbiota dysbiosis can occur due to a variety of reasons, reduces the expression of proteins involved in the formation of tight junctions (TJ) such as zonula occludens and occludin and TLRs, altering the permeability of the gastrointestinal epithelial barrier and creating inflammatory cytokines (5, 6). The reduction of the integrity of the intestinal barrier and the formation of inflammation is associated with the onset and development of many diseases, including inflammatory intestinal diseases and metabolic diseases. Manipulation of the intestinal microbiota through prebiotics and probiotics can increase the beneficial bacteria, reduce inflammation and improve the function of the intestinal barrier (7).
One of the normal flora bacteria in the gastrointestinal tract, which has been recently introduced as the next-generation probiotics candidate, is the Akkermansia muciniphila (A.muciniphila) bacterium. A. muciniphila is an anaerobic Gram-negative bacterium belonging to the verrucomicrobia phylum that is located in the gastrointestinal mucosal layer and 1-4% of the fecal microbiota communities in the gut. This bacterium has been identified as a mucin-degrading bacterium and is considered as the closest bacterium to epithelial cells (8, 9). A. muciniphila also increases the number of goblet cells and mucus secretion, restoring the thickness of the intestinal mucus caused by High Fat Diet (HFD), thereby increasing the epithelial barrier integrity (2, 10). This bacterium plays an important role in the relationship between the gastrointestinal tract microbiota and the host, controlling the function of the digestive barrier and other physiological and hemostatic functions during obesity and type 2 diabetes (2, 11). Due to its role in stabilizing the thickness of the intestinal mucosa and maintaining the integrity of the digestive barrier, it increases the colonization of beneficial bacteria and reduces pathogenic bacteria (12).
Like other bacteria, gastrointestinal tract commensal bacteria produce extracellular vesicles (EVs). EVs have a spherical shape of 20 to 250 nm in diameter that is produced by many Gram positive and negative bacteria. These molecules are released and secreted at all stages of bacterial growth and in many culture media and different stages of bacterial life during pathogenicity and as a normal flora (13). EVs of gastrointestinal commensal bacteria play a role in adjusting the immune system and regulating the intestinal hemostasis and the epithelial intestinal permeability (14). In a recent study, it has been found that A. muciniphila derived EVs (A.mEV) has a better effect on the regulation of the immune system and intestinal hemostasis and protection of colitis in mice than the bacterium itself (15). Given that the intestine is a place for interaction between microbiota and host and Caco-2 cell line represents intestinal cells and is the best model for testing TJ proteins (16, 17), we have decided to investigate the effect of the bacterium and its EVs on this cell line. Our aim has been to compare the effects of A. muciniphila and its EVs on inflammation and TJ in vitro.
Methods
Culture of A. muciniphila
A. muciniphila MucT (ATCC BAA-835) was purchased from DSMZ institute. It was cultured anaerobically in a basal mucin-based medium under the anaerobic conditions (80% N2, 10% H2 and 10% CO2) at 37 °C for 3-7 days (9). After growth, A. muciniphila was inoculated to brain heart infusion broth (BHI) (Quelab) supplemented with 0.5% mucin (Sigma-Aldrich) under the above conditions for 24 hours. Cultures were centrifuged and washed with an anaerobic PBS and used for EVs extraction.
EVs Extraction
Bacterial cultures were pelleted at 10000 g for 20 min. The EVs were extracted with ultracentrifuge at 150000g for 2 hours at 4 °C as previously described (18). The pellets were re-suspended in 3% sucrose and stored at − 80 °C. The morphology of EVs was monitored by scanning electron microscope (SEM), then total protein concentration measured by the Bradford Protein Assay.
Cell culture Treatment
The Caco-2 (ATCC® HTB-37) as human epithelial colorectal adenocarcinoma cell line were cultured at 37°C in 5% CO2 in Dulbecco's Modified Eagle's Medium (DMEM)(Gibco), supplemented with 10% heat inactivated fetal bovine serum (FBS)(Gibco) and 1% penicillin-streptomycin (Gibco) in six well plates (Sorfa). Afterward, Caco2 were infected with A. muciniphila at Multiplicity of infection (MOI) 10 ratios (i.e. 10 bacteria per cell). Besides, different concentrations of EVs (0.1, 0.5 and 5 µg) were used for treatment of Caco-2 cell culture. For each experiment, equal volumes of PBS or sucrose were used as control separately. All of the experiments were performed in triplicate.
Trypan blue exclusion assay
The effects of A. muciniphila and its EVs on the viability of cells were determined using trypan blue exclusion assay as described. Briefly, cells were treated with MOI 10 A. muciniphila and variable concentrations of EVs for 24 h at 37 °C. Treated cells incubated and then trypsinized. Equal volume of trypan blue mixed with cell suspension and calculated using a hemocytometer. Live and dead cells were counted with confocal microscopy. The viability percentage was determined using the following formula:
% Viability= (Live Cell Count/Total Cell Count) ×100
RNA extraction and cDNA synthesis
After 24 h post infection, total RNA was extracted from Caco-2 cells using RNeasy Plus Mini kit (Qiagen). cDNA was synthesis from 500 ng total RNA using PrimeScript RT Reagent Kit (Takara) according to the manufacture's instruction.
Quantitative Real-Time PCR Experiments
Real-time PCR was performed by 2X SYBR Premix Ex Taq II (Takara) with Roche LightCycler 96 instrument. The mRNA expression levels of tlr2, tlr4, ocldn, zo1, zo2, zo3 and cldn4 were evaluated. gapdh was used as a reference gene. Finally, raw data were analyzed according to the ΔΔCT method and gapdh was used as an internal control to normalize gene expression. Sequence of primers used in this study was prepared from the Primer Bank (Table 1). All of the experiments were performed in triplicate.
Table 1.
Sequence of primers used in qPCR
| Primer name | Forward primer | Reverse primer | Product size (bp) |
|---|---|---|---|
| gapdh | GGAGCGAGATCCCTCCAAAAT | GGCTGTTGTCATACTTCTCATGG | 197 |
| tlr 2 | TTATCCAGCACACGAATACACAG | AGGCATCTGGTAGAGTCATCAA | 160 |
| tlr 4 | AGACCTGTCCCTGAACCCTAT | CGATGGACTTCTAAACCAGCCA | 147 |
| zo1 | CAACATACAGTGACGCTTCACA | CACTATTGACGTTTCCCCACTC | 105 |
| zo2 | ATGGAAGAGCTGATATGGGAACA | TGCTGAACTGCAAACGAATGAA | 242 |
| zo3 | CAGACAGGCGACCACATCG | GGTGCAGGTCTTGAGTATCTGA | 90 |
| ocldn | AAGAGTTGACAGTCCCATGGCATAC | ATCCACAGGCGAAGTTAATGGAAG | 133 |
| cldn4 | GGGGCAAGTGTACCAACTG | GACACCGGCACTATCACCA | 109 |
Statistical analysis
Analysis of relative gene expression data was performed by GraphPad Prism 6 (GraphPad, La Jolla, CA). Data comparisons was performed with unpaired t-test. Results with P<0.05 were considered to be statistically significant.
Results
Morphology and characterization of EVs
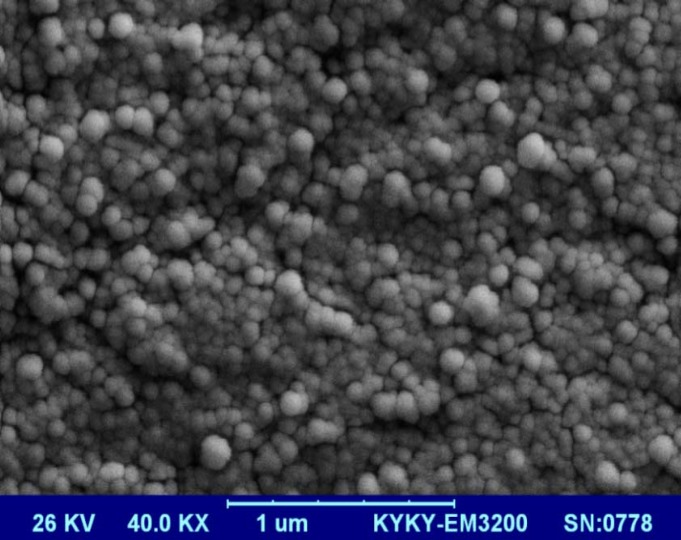
Scanning Electron Microscopy (SEM) analysis of A.mEV showed a spherical shape and a range of 40 to 150 nm in size (Figure 1).
Figure 1.
A Scanning electron microscopy of A.mEVs (magnification: ×40K)
Effects of A. muciniphila and its EVs on TLRs
After 24 hours, no difference in cell viability was observed in both control and treatment groups. It is widely approved that, the gut flora influences the intestinal immune system and have a crucial role in the immune system homeostasis by interaction with TLRs. Thus, to determine the effect of A. muciniphila and A.mEV in Caco2 Cell line, this bacterium and its EVs concentrations treated overnight and then gene expressions were measured by qPCR.
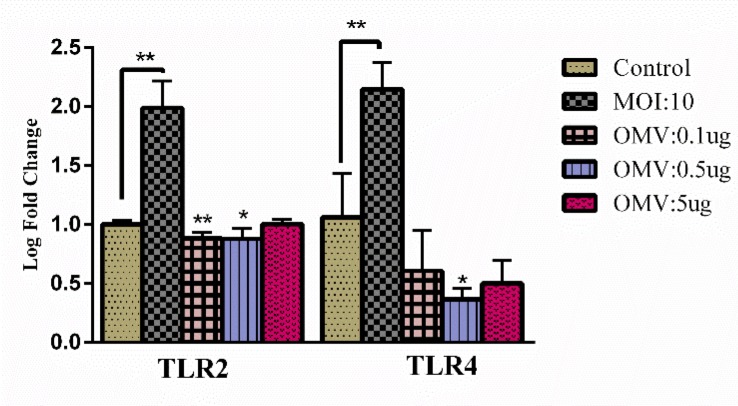
A. muciniphila in the Caco2 cell line has significantly increased tlr2 and tlr4 gene expression. In response to EVs 0.1 and 0.5 µg/ml, expression of tlr2 were decreased but not in 5 µg/ml. Interestingly, the expression of tlr4 was significantly decreased in response to 0.5 µg/ml of EVs concentrations (Figure 2).
Figure 2.
Effects of A. muciniphila and its EVs on TLRs. The Caco-2 cells were treated with A. muciniphila at MOI 10 and different concentrations of A.mEV (0.1, 0.5 and 5 µg); *, **'P<0.05 and P<0.01 were considered statistically significant, respectively. gapdh was used as an internal control
Effects of A. muciniphila and its EVs on tight junctions
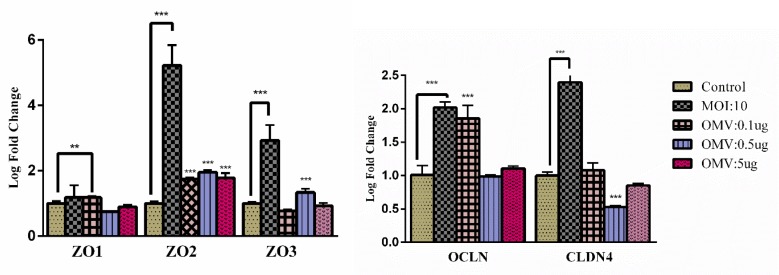
Gut microbiota and their derivatives increases TJ proteins in the intestinal barrier, improves intestinal homeostasis and thereby create healthy conditions in the gut. Significantly A. muciniphila increased TJ gene expressions such as ocldn, cldn4, zo2 and zo3 but a slightly increased expression of zo1.
A.mEV increased expression of ocldn (Figure 3A) and zo1 at the mRNA level in response to 0.1 µg/ml concentration but decreased zo3 expression (not significant). At the EVs 0.5 µg/ml expression of zo3 was increased. In response to EVs 0.5 µg/ml expression of cldn4 was decreased. All of the EVs concentrations significantly increased zo2 expression. (Figure 3B). This result initially shows that the effect of A.mEV on studied genes may be dose-independent.
Figure 3.
Effects of A. muciniphila and its EV on tight junctions: The Caco-2 cells were treated with A. muciniphila at MOI 10 and different concentrations of A.mEV (0.1, 0.5 and 5 µg); **, ***; P<0.01 and P<0.001 were considered statistically significant, respectively. gapdh was used as an internal control
Discussion
Increasing intestinal barrier permeability and inflammatory cytokines production play significant role in developing intestinal and metabolic diseases, and gut microbiota have critical role in regulating the host immune system by various signaling pathways such as TLRs (19, 20). In present study, it has been shown that A. muciniphila contributes to an increase in the expression of tlr2 and tlr4. In the same vein, in the Ottman’s study who investigated the effect of A. muciniphila on HEK-Blue cells, an increase in the expression of tlr2 and tlr4 has been shown (20). In the another study shown that A. muciniphila LPS is different from E.coli LPS and is not considered as a strong agonist for TLR4 (21). Additionally, it has been shown that A. muciniphila can increase the expression of tlr2 through the Amuc-1100 protein not LPS in mice (22). Since the LPS of commensal and pathogenic bacteria is different, it leads the host immune system to tolerance, and these stimulations are probably as a result of intestinal immune tolerance to A. muciniphila. Most of commensal bacteria cause immunomodulatory by producing OMVs, furthermore participate in immune system regulation and intestine homeostasis. In present study, A.mEV decreased tlr4 and tlr2 expression. In this regard, research investigating the effect of OMV of E.coli C25, a commensal bacterium, on tlr4 expression in the Caco-2 cell line revealed that its OMV also has decreased tlr4 expression (23). In a study by Shen on OMV of Bacteroides fragilis, it has been shown that PSA in its OMV reacting with TLR2 and triggering the secretion of anti-inflammatory cytokines (24).
In the intestinal medium, intestinal microbiota interacts with TJ proteins, causing the protection of the integrity, the improvement of intestinal barrier function, and the protection against pathogenic bacteria and metabolites resulting from them. In the present study, we showed that A. muciniphila increased TJs such as ocldn, cldn4, zo1, zo2 and zo3, and in addition AmEV increased the expression of ocldn and zo2. These results indicate the significant role of A. muciniphila and AmEV in increasing the expression of TJ proteins which are involved in the regulation of the assembly of TJ proteins and the stability of the intestinal barrier (25). Additionally, E. coli strain Nissle 1917 (EcN) has been shown that increases TJ proteins such as cldn14 and zo1 expression (26, 27). The effects of A.mEV on mice fed HFD have been investigated in a study by Chelakkot et al. and it has been found that EVs of this bacterium increases the expression of TJ proteins, reduces body weight, and improves metabolic function (28). A recent study showed that A. muciniphila regulates TJ proteins such as Ocldn and Cldn3 and increases intestinal barrier integrity by affecting on TLR2(22). In recent studies, it has been shown that the oral gavage of A. muciniphila can increase the thickness of the mucus and decrease the permeability of the intestinal epithelial barrier. Therefore, it can be concluded that as the thickness of the intestinal epithelial mucus decreases and the permeability of the epithelial barrier increases in the obesity, A. muciniphila is directly effective in controlling and reducing obesity (2). Additionally, in a study conducted on OMV of EcN bacterium in the intestinal cell line, it has been shown that it improves the up-regulation of TJ proteins such as cldn14 and zo1 and decreases the expression of cldn2. Thus, it reduces the permeability of the intestinal barrier (25). There have been many studies on the safety of probiotics. The leaky gut hypothesis (the increase in the permeability of the intestinal barrier) occurs in metabolic and inflammatory bowel diseases, leading to the passage of bacteria, metabolites and their derivatives outside the intestine and the stimulation of the immune system. Considering the results obtained in this study on human intestine cell line and the significant role of A. muciniphila and A.mEV in reducing permeability and inflammation, it can be suggested that EVs of this bacterium is a more suitable treatment target than the live bacteria and can be used in the treatment of many inflammatory diseases to regulate the integrity of the intestinal barrier and reduce inflammation. Further investigations are pivotal to accredit these conclusions.
Acknowledgment
This project was supported by Iran Biotech Fund grant No. 94/10243, Pasteur Institute of Iran and National Institute for Medical Research Development grant No. 942995.We would like to thank the personnel of Mycobacteriology and Pulmonary Research Department of Pasteur Institute of Iran for their cooperation.
Conflict of interests
The authors declare that they have no conflict of interest.
References
- 1.Cani PD, Osto M, Geurts L, Everard A. Involvement of gut microbiota in the development of low-grade inflammation and type 2 diabetes associated with obesity. Gut Micro. 2012;3:279–88. doi: 10.4161/gmic.19625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Everard A, Belzer C, Geurts L, Ouwerkerk JP, Druart C, Bindels LB, et al. Cross-talk between Akkermansia muciniphila and intestinal epithelium controls diet-induced obesity. Proc Natl Acad Sci USA. 2013;110:9066–71. doi: 10.1073/pnas.1219451110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Spiljar M, Merkler D, Trajkovski M. The immune system bridges the gut microbiota with systemic energy homeostasis: focus on TLRs, mucosal barrier, and SCFAs. Front Immunol. 2017;8:1353. doi: 10.3389/fimmu.2017.01353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Valentini M, Piermattei A, Di Sante G, Migliara G, Delogu G, Ria F. Immunomodulation by gut microbiota: role of Toll-like receptor expressed by T cells. J Immunol Res . 2014;2014:586939. doi: 10.1155/2014/586939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Teixeira TF, Collado MC, Ferreira CL, Bressan J, Maria do Carmo GP. Potential mechanisms for the emerging link between obesity and increased intestinal permeability. Nut Res. 2012;32:637–47. doi: 10.1016/j.nutres.2012.07.003. [DOI] [PubMed] [Google Scholar]
- 6.Lin L, Zhang J. Role of intestinal microbiota and metabolites on gut homeostasis and human diseases. BMC Immuno. 2017;18:2. doi: 10.1186/s12865-016-0187-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lopetuso LR, Scaldaferri F, Bruno G, Petito V, Franceschi F, Gasbarrini A. The therapeutic management of gut barrier leaking: the emerging role for mucosal barrier protectors. Eur Rev Med Pharmacol Sci. 2015;19:1068–76. [PubMed] [Google Scholar]
- 8.Derrien M, Van Baarlen P, Hooiveld G, Norin E, Müller M, De Vos WM. Modulation of Mucosal Immune Response, Tolerance, and Proliferation in Mice Colonized by the Mucin-Degrader Akkermansia muciniphila. Front Microbiol. 2011;2:166. doi: 10.3389/fmicb.2011.00166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Derrien M, Vaughan EE, Plugge CM, De Vos WM. Akkermansia muciniphila gen nov sp no a human intestinal mucin-degrading bacterium. Int J Syst Evol Microbiol. 2004;54:1469–76. doi: 10.1099/ijs.0.02873-0. [DOI] [PubMed] [Google Scholar]
- 10.Shin NR, Lee JC, Lee HY, Kim MS, Whon TW, Lee MS, et al. An increase in the Akkermansia spp population induced by metformin treatment improves glucose homeostasis in diet-induced obese mice. Gut. 2014;63:727–35. doi: 10.1136/gutjnl-2012-303839. [DOI] [PubMed] [Google Scholar]
- 11.Rokhsefat S, Lin A, Comelli EM. Mucin–microbiota interaction during postnatal maturation of the intestinal ecosystem: clinical implications. Dig Dis Sci. 2016;61:1473–86. doi: 10.1007/s10620-016-4032-6. [DOI] [PubMed] [Google Scholar]
- 12.Derrien M, Belzer C, De Vos WM. Akkermansia muciniphila and its role in regulating host functions. Microb Pathog. 2017;106:171–81. doi: 10.1016/j.micpath.2016.02.005. [DOI] [PubMed] [Google Scholar]
- 13.Lee EY, Bang JY, Park GW, Choi DS, Kang JS, Kim HJ, et al. Global proteomic profiling of native outer membrane vesicles derived from Escherichia coli. Proteomics. 2007;7:3143–53. doi: 10.1002/pmic.200700196. [DOI] [PubMed] [Google Scholar]
- 14.Ahmadi Badi S, Moshiri A, Fateh A, Rahimi Jamnani F, Sarshar M, Vaziri F, et al. Microbiota-Derived Extracellular Vesicles as New Systemic Regulators. Front Microbiol. 2017;8:1610. doi: 10.3389/fmicb.2017.01610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kang CS, Ban M, Choi EJ, Moon HG, Jeon JS, Kim DK, et al. Extracellular vesicles derived from gut microbiota, especially Akkermansia muciniphila, protect the progression of dextran sulfate sodium-induced colitis. Plos One. 2013;8:e76520. doi: 10.1371/journal.pone.0076520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gao Y, Li S, Wang J, Luo C, Zhao S, Zheng N. Modulation of Intestinal Epithelial Permeability in Differentiated Caco-2 Cells Exposed to Aflatoxin M1 and Ochratoxin A Individually or Collectively. Toxins. 2017;10:13. doi: 10.3390/toxins10010013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Larregieu CA, Benet LZ. Drug discovery and regulatory considerations for improving in silico and in vitro predictions that use Caco-2 as a surrogate for human intestinal permeability measurements. AAPS J. 2013;15:483–97. doi: 10.1208/s12248-013-9456-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Siadat SD, Vaziri F, Eftekhary M, Karbasian M, Moshiri A, Aghasadeghi MR, et al. Preparation and evaluation of a new lipopolysaccharide-based conjugate as a vaccine candidate for brucellosis. Osong Public Health Res Perspect. 2015;6:9–13. doi: 10.1016/j.phrp.2014.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kamada N, Seo SU, Chen GY, Núñez G. Role of the gut microbiota in immunity and inflammatory disease. Nat Rev Immunol. 2013;13:321. doi: 10.1038/nri3430. [DOI] [PubMed] [Google Scholar]
- 20.Ottman N, Reunanen J, Meijerink M, Pietilä TE, Kainulainen V, Klievink J, et al. Pili-like proteins of Akkermansia muciniphila modulate host immune responses and gut barrier function. Plos One. 2017;12:e0173004. doi: 10.1371/journal.pone.0173004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Reunanen J, Kainulainen V, Huuskonen L, Ottman N, Belzer C, Huhtinen H, et al. Akkermansia muciniphila adheres to enterocytes and strengthens the integrity of the epithelial cell layer. Appl Environ Microbiol. 2015;81:3655–62. doi: 10.1128/AEM.04050-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Plovier H, Everard A, Druart C, Depommier C, Van Hul M, Geurts L, et al. A purified membrane protein from Akkermansia muciniphila or the pasteurized bacterium improves metabolism in obese and diabetic mice. Nat Med. 2017;23:107. doi: 10.1038/nm.4236. [DOI] [PubMed] [Google Scholar]
- 23.Patten DA, Hussein E, Davies SP, Humphreys PN, Collett A. Commensal-derived OMVs elicit a mild proinflammatory response in intestinal epithelial cells. Microbiol. 2017;163:702–11. doi: 10.1099/mic.0.000468. [DOI] [PubMed] [Google Scholar]
- 24.Shen Y, Giardino Torchia ML, Lawson GW, Karp CL, Ashwell JD, Mazmanian SK. Outer membrane vesicles of a human commensal mediate immune regulation and disease protection. Cell Host Microbe. 2012;12:509–20. doi: 10.1016/j.chom.2012.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Alvarez CS, Badia J, Bosch M, Giménez R, Baldomà L. Outer membrane vesicles and soluble factors released by probiotic escherichia coli nissle 1917 and commensal ecor63 enhance barrier function by regulating expression of tight junction proteins in intestinal epithelial cells. Front Microbiol. 2016;7:1981. doi: 10.3389/fmicb.2016.01981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hering N, Richter J, Fromm A, Wieser A, Hartmann S, Günzel D, et al. TcpC protein from E coli Nissle improves epithelial barrier function involving PKCζ and ERK1/2 signaling in HT-29/B6 cells. Mucosal Immunol. 2014;7:369. doi: 10.1038/mi.2013.55. [DOI] [PubMed] [Google Scholar]
- 27.Ukena SN, Singh A, Dringenberg U, Engelhardt R, Seidler U, Hansen W, et al. Probiotic Escherichia coli Nissle 1917 inhibits leaky gut by enhancing mucosal integrity. Plos One. 2007;2:e1308. doi: 10.1371/journal.pone.0001308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chelakkot C, Choi Y, Kim DK, Park HT, Ghim J, Kwon Y, et al. Akkermansia muciniphila-derived extracellular vesicles influence gut permeability through the regulation of tight junctions. Exp Mol Med. 2018;50:e450. doi: 10.1038/emm.2017.282. [DOI] [PMC free article] [PubMed] [Google Scholar]