Abstract
Aim:
The aim of present study is to investigate the effect of fatty acids on the outer membrane vesicles (OMVs) produced by Bacteroides spp.
Background:
Bacteroides spp. is the important member of Gut microbiota that employ OMVs production for interact with host. Besides, dietary fatty acids could influence on determination of gut microbiota composition and immune response. In this regard, we evaluated the effect of fatty acids on the growth and OMVs production of Bacteroides fragilis and Bacteroides thetaiotaomicron.
Methods:
B. fragilis and B. thetaiotaomicron were grown on BHI broth with and without palmitic and palmitoleic acids as saturated and unsaturated fatty acids, respectively. OMVs were extracted using multiple centrifugation and tris-ethylene diamine tetra acetic acid (EDTA)-Sodium deoxy cholate buffers. Physicochemical properties of OMVs were detected by electron microscopy (SEM), Bradford Coomassie brilliant blue assay and SDS-PAGE. Data were analyzed with One-way ANOVA using SPSS.
Results:
The growths of both Bacteroides were significantly increased by palmitic acid. Nevertheless, palmitoleic acid had no significant effect on them. Palmitic acid significantly decreased and increased the production of B. fragilis OMVs at low and high concentration, respectively. However, the production of B. thetaiotaomicron OMVs was not significantly affected by palmitic acid. Although palmitoleic acid had a significant decreasing effect on the production of B. fragilis OMVs, it significantly increased the production of B. thetaiotaomicron OMVs at low concentration.
Conclusion:
In conclusion we reported that palmitic acid had a stimulatory effect on the growth of B. fragilis and B. thetaiotaomicron and had a dose dependent effect on the production of B. fragilis OMVs. Also producing of B. thetaiotaomicron OMVs was affected by palmitoleic acid in a dose dependent manner.
Key Words: Bacteroides fragilis, Bacteroides thetaiotaomicron, Outer membrane vesicle, Palmitic acid, Palmitoleic acid
Introduction
A diverse and complex microbial community colonizes human host, especially in the gastrointestinal tract (GIT) that refers as the “gut microbiota”. This microbial community has co-evolved with host to create a mutually beneficial relationship (1). Gut microbiota constantly interacts with the host and has determining roles in its functions due to having various physiological effects including maintenance of intestinal epithelium integrity, energy harvest from diet, colonization resistance, regulation of glucose/lipid metabolism and immune system (2, 3, 4). The gut microbiota consists of bacteria, archaea, protozoa, fungi and viruses. The Bacteroidetes and Firmicutes are two dominant phyla in human gut microbiota (5).The composition of the gut microbiota is affected by genetic background and environmental factors including toxins, drugs, diet and pathogens (6). It has been found that unfavorable altered gut microbiota composition that is called “dysbiosis”, disrupts gut microbiota-host interactions which increases prone to diseases. Dysbiosis is correlated with intestinal and extra-intestinal disorders like irritable bowel syndrome (IBS), coeliac disease, inflammatory bowel disease (IBD), asthma, allergy, cardiovascular disease (CVD), metabolic syndrome and obesity (6-10). It has been suggested that diet is one of the most potent determinants of gut microbiota composition. It affects gut microbiota-host interactions through alternation of microbial metabolites, components and host metabolism. For example, dietary fatty acids have influential role on metabolic syndrome such as obesity, type 2 diabetes, hyper tension and rheumatoid arthritis (11-14). Dietary saturated fatty acids (SFAs) such as palmitic acid are able to activate inflammatory responses and promote metabolic syndrome. Conversely, polyunsaturated fatty acids (PUSAs) could suppress inflammatory responses (15).
The gut microbiota has significant roles on human physiology and metabolism. It produces essential metabolites from diet such as short-chain fatty acids (SCFAs) which act as source of energy for colonocytes, signaling molecules and epigenetic factor for modulation host functions (16, 17). Also immune system and host’s defenses are associated with composition and function of microbiota (16-18). One way to interact between gut microbiota and the host is to produce outer membrane vesicles (OMVs). OMVs are nano sized particles, 20 to 250 nm, which produced by gram negative bacteria (19, 20). The component of OMVs includes proteins, hydrolytic enzymes, toxin or lipopolysaccharide (LPS), DNA and RNA (21, 22). Recent studies demonstrated that Bacteroides spp. derived OMVs have significant role in maintenance of homeostasis and regulation of immune system. For example, OMVs containing capsular polysaccharide A (PSA) from B. fragilis modulate the immune system and tolerance to intestinal commensal bacteria. B. thetaiotaomicron OMVs modulate intestinal macrophages in a sulphatase dependent manner. Also hydrolytic enzymes that are packaged into Bacteroides spp. derived OMVs, contribute to maintenance of homeostasis (19-27). As mention above, the gut microbiota composition is affected by many factor especially diet, dietary SFAs and PUSAs. According to importance of dietary fatty acids and Bacteroides spp. and their OMVs, we evaluated the effect of palmitic and palmitoleic acids, as saturated and unsaturated fatty acids, on the growth and the production of OMVs from B. fragilis and B. thetaiotaomicron.
Methods
Bacterial strains and growth condition
B. fragilis ATCC 23745 and B. thetaiotaomicron ATCC 10774 were grown in brain heart infusion (BHI) broth supplemented with hemin (5µg/ml) and menadione (1µg/ml). These media incubated at 37 °C under anaerobic conditions provided 80% N2, 10% Co2 and 10% H2.
Fatty acids preparation and bacterial inoculation
Palmitic acid (PO500) and palmitoleic acid SIGMA-ALDRICH, GERMANY (P9417) solutions were prepared at 20 mg/ml concentration in absolute ethanol. Fatty acid solutions were sterilized using a 0.22µm-pore-size polyvinylidene difluoride filter (Millipore, Billerica, MA). Palmitic acid and palmitoleic acid solutions were added to BHI broth media at different concentration, 0, 125, 250 and 500 µg/L. B. fragilis and B. thetaiotaomicron were inoculated at 1.5×108 CFU ml-1 to BHI broth enriched with palmitic and palmitoleic acid and incubated under anaerobic conditions for an overnight. Finally, the optical density (OD) was measured by ELISA reader (Epoch Biotech ELX50).
OMVs purification
After an overnight incubation under anaerobic conditions as described previously, OMVs were isolated (28). Briefly, five hundred milliliters of bacterial cultures were centrifuged at 5000g at 4 °C. The cell pellets were washed twice by phosphate buffer solution (PBS). Then cell pellet was re-suspend in sodium chloride 9% solution. The cell suspension was homogenized and concentrated by centrifugation for 1 hour at 6500g at 4 °C. The OMVs were isolated using Tris-ethylene diamine tetra acetic acid (EDTA)-sodium deoxycholate (Sigma-Aldrich, USA) buffers and centrifugation for 90 minutes at 20000g, 4 °C. The OMVs concentrated were re-suspended in 3% sucrose solution.
Scanning electron microscopy (SEM)
To determine morphology and size of OMVs, these vesicles were fixed using 2.5% glutaraldehyde and 2% paraformaldehyde in PBS. Gold coated samples were prepared by sputter coater (KYKY Technology, China) and examined using SEM (KYKY Technology, China).
Quantitative and qualitative determination of protein
After the extraction, the protein concentration of OMVs was measured by Bradford protein assay. Determination of OMVs proteins by Bradford Coomassie brilliant blue assay was confirmed by measuring absorbance at 590 nm (29). To determine protein profile electrophoresis of OMV was performed by slab gel containing 12% SDS-PAGE. Then separated proteins were stained with Coomassie brilliant blue and were de-stained by acid acetic 1 %( 30).
Statistical Analyses
Data were analyzed with One-Way ANOVA based on LSD test using SPSS version 24.0 (SPSS Inc., Chicago, IL, USA). All results demonstrate as mean± Standard deviation (SD). In all experiments, P<0.05 was considered statistically significant.
Results
The effect of palmitic and palmitoleic acids on B. fragilis and B. thetaiotaomicron growth
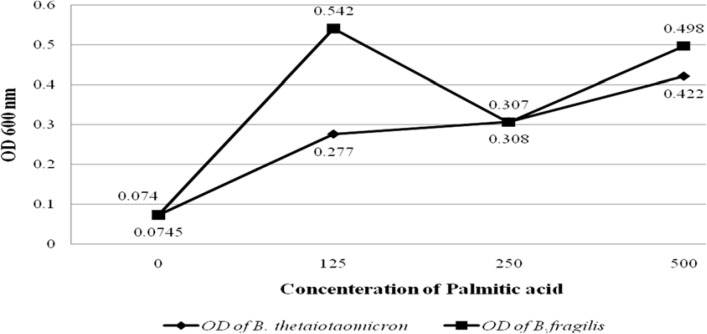
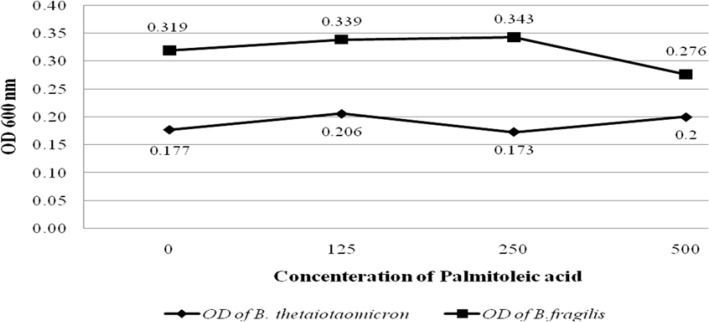
To evaluate the effect of palmitic and palmitoleic acid on the growth of the B. fragilis and B. thetaiotaomicron, different concentration of both fatty acids was added to BHI broth. The results showed that palmitic acid significantly increased the growth of B. fragilis (P value < 0.05) and B. thetaiotaomicron (P value < 0.05). Palmitic acid had most stimulatory effect on these bacteria at the high concentration, 500µg/L. Interestingly, the growth of B. fragilis was more stimulated compared with B. thetaiotaomicron by palmitic acid (figure 1). In contrast, palmitoleic acid had no significant stimulatory effect on the growth of the B. fragilis and B. thetaiotaomicron (figure 2).
Figure 1.
Graph shows the OD values of B. fragilis ATCC23745 and B. taiotaomicron ATCC 10774 growths in BHI broth treated with palmitic acid. The horizontal and vertical axes show the concentration of Palmitoleic acid and the OD values of bacterial growth, respectively
Figure 2.
Graph shows the OD values of B. fragilis ATCC23745 and B. thetaiotaomicron ATCC 10774 growths in BHI broth treated with Palmitoleic acid. The horizontal and vertical axes show the concentration of Palmitoleic acid and the OD values of bacterial growth, respectively
The effect of palmitic acid and palmitoleic acid on the production of B. fragilis and B. thetaiotaomicron derived-OMVs
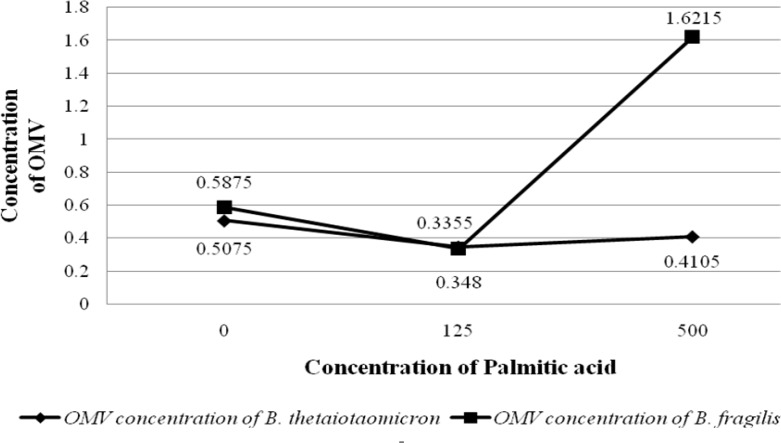
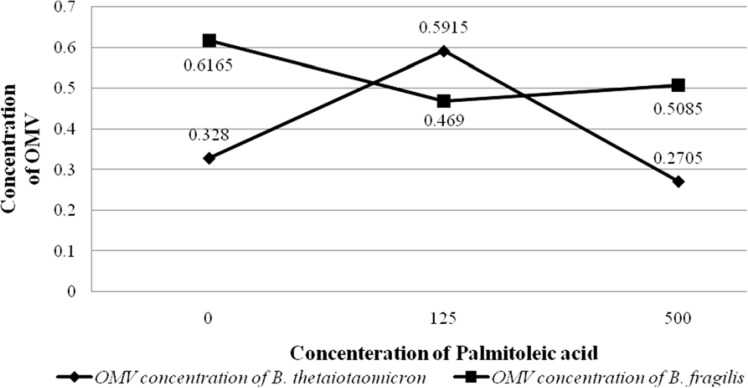
To evaluate the effect of palmitic acid and palmitoleic acid on the OMVs production of B. fragilis and B. thetaiotaomicron, these particles were extracted in BHI broth supplemented with different concentrations of fatty acids examined by SDS and Bradford methods that results of the experiments are shown in the figures (figure 3 and 4). Although palmitic acid significantly decreased the OMVs production of B. fragilis at low concentration (P value < 0.01) but significantly increased it at high concentration (P value < 0.002). Based on statistical analyzes; it was showed that palmitic acid effect on the production of OMVs from B. fragilis was dose dependent due to the presence of a significant increasing effect at high concentration (P value < 0.001). The production of B. thetaiotaomicron OMVs was not significantly affected by palmitic acid at both concentrations (figure 3 and 4). On other hand, palmitoleic acid had a decreasing effect on the production of B. fragilis OMVs and the low concentration was statistically significant (P value < 0.03). We reported that the palmitoleic acid had a significant increasing effect on the production of B. thetaiotaomicron OMVs at low concentration (P value < 0.03) (Figure 5 and 6). Also, we identified a dose dependent effect of palmitoleic acid on the B. thetaiotaomicron OMVs production because of the high concentration compared with low concentration had a significant reducing effect (P value < 0.01) in this regard.
Figure 3.
Graph shows the OD values of OMVs production by B. fragilis ATCC23745 and B. thetaiotaomicron ATCC 10774 in BHI broth supplemented with Palmitic acid. The horizontal and vertical axes show the concentration of Palmitoleic acid and the OD values of bacterial growth, respectively
Figure 4.
Graph shows the OD values of OMVs production by B. fragilis ATCC23745 and B. thetaiotaomicron ATCC 10774 in BHI broth supplemented with Palmitoleic acid. The horizontal and vertical axes show the concentration of Palmitoleic acid and the OD values of bacterial growth, respectively
Figure 5.
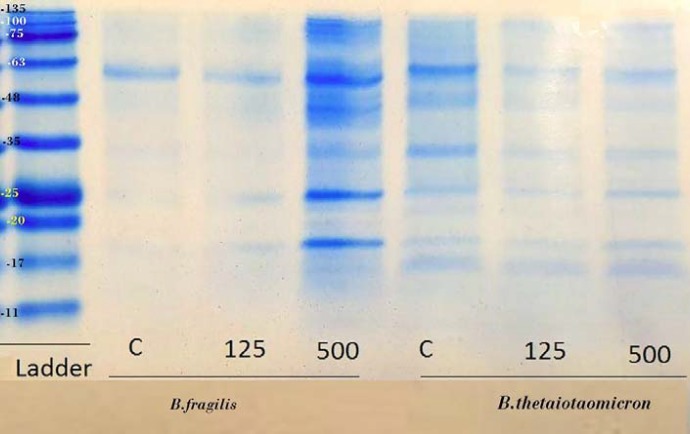
The protein profile of B. fragilis ATCC23745 and B. thetaiotaomicron ATCC 10774 derived OMVs which are produced in BHI broth supplemented with high and low concentration of palmitic acid. The protein profiles of OMVs from B. fragilis and B. thetaiotaomicron were compared using SDS-PAGE according to Claassen et al. (1996)
Figure 6.
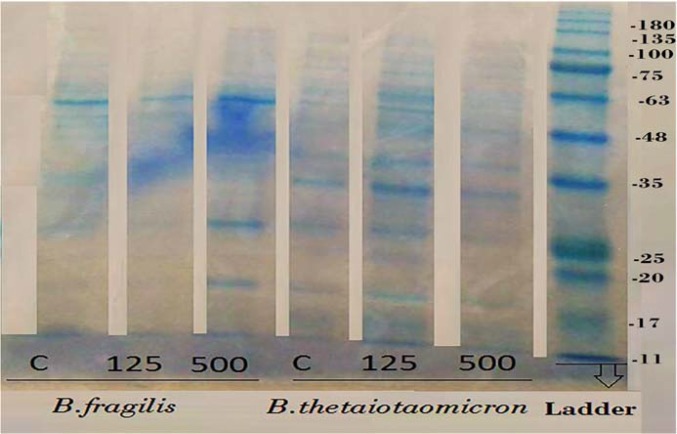
The protein profile of B. fragilis ATCC23745 and B. thetaiotaomicron ATCC 10774 derived OMVs which are produced in BHI broth supplemented with high and low concentration of palmitoleic acid. The protein profiles of OMVs from B. fragilis and B. thetaiotaomicron were compared using SDS-PAGE according to Claassen et al. (1996)
Discussion
Bacteroides spp. have significant roles in the gut microbiota-host interactions through various mechanisms including OMVs production. These particles influence the regulation of immune system and homeostasis (31, 32). On the ther hand, diet-derived saturated and unsaturated fatty acids have inflammatory and anti-inflammatory properties, respectively (33, 34). Here, we aimed to evaluate the effects of palmitic and palmitoleic acids (as saturated and unsaturated fatty acids) on the growth and the production of OMVs from B. fragilis and B. thetaiotaomicron.
It has been shown that diet has substantial role on the gut microbiota composition (35). In this regard, some studies focused on the role of high fat diet as an important factor on the pattern of gut microbiota (36, 37). Also, the effect of dietary fatty acids on the intestinal bacterial growth was assessed (38). For example, fatty acids derived from human and cow milk inhibits the growth of Lactobacillus bifidus (39). Furthermore, the growth of Bacteroides ruminicola, one species of rumen bacteria, was decreased in the presence of palmitic acid (40). Some researches indicated that unsaturated long-chain fatty acids (C18) have stimulation effect on the growth of microorganisms in low concentration (41). In present study, palmitic acid acts as a significant stimulant for the growth of both B. fragilis and B. thetaiotaomicron, while palmitoleic acid has no significant effect on the growth of these bacteria. The stimulatory effect of unsaturated fatty acids on the growth of bacteria decreases along with the increase in the length of the fatty acid chain. Also, fatty acids which have induction effect on the bacterial growth might have opposite effect at other concentration. Therefore, fatty acids can be considered as a stimulator or inhibitor agent on the bacterial growth depending on their structure and concentration (41).
B. fragilis and B. thetaiotaomicron are two important members of gut microbiota due to having important potentials such as regulating of immune responses and homeostasis (42, 43). It has been demonstrated that, B. fragilis and B. thetaiotaomicron employ OMVs production to exert their effects in gut microbiota-host interactions (44, 45). Considering the important roles of Bacteroides spp. derived OMVs, the effects of palmitic acid and palmitoleic acid which are components of diet, on the production of these vesicles were investigated for the first time. Our results illustrate that palmitic acid has a significant stimulatory effect on the production of B. fragilis derived OMVs at the high concentration but it significantly decreased production of B. fragilis OMVs at low concentration. However, palmitic acid had no significant effect on the production of B. thetaiotaomicron OMVs. Palmitoleic acid, as an unsaturated fatty acid, significantly decreased the OMVs production from B. fragilis and significantly increased the production of B. thetaiotaomicron OMVs at low concentration. It can be concluded that the effect of palmitic acid on the production of OMVs from B. fragilis and also the effect of palmitoleic acid on the production of OMVs from B. thetaiotaomicron was dose dependent. Therefore, fatty acids can act as a stimulator or inhibitor agent on the production of OMVs depending on their concentration. However, further molecular investigations and animal models are required to illustrate in details the effect of palmitic and palmitoleic acid on the growth of Bacteroides spp. and OMVs production.
Acknowledgment
We thank all the personnel of Mycobacteriology and Pulmonary Research Department, Pasteur Institute of Iran for their assistance in this project. This project was supported by Iran Biotech Fund grant No. 94/10243 and National Institute for Medical Research Development grant No. 942995.
Conflict of interests
The authors declare that they have no conflict of interest.
References
- 1.Turnbaugh PJ, Ley RE, Hamady M, Fraser-Liggett CM, Knight R, Gordon JI. The human microbiome project. Nat. 2007;449 doi: 10.1038/nature06244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brown EM, Sadarangani M, Finlay BB. The role of the immune system in governing host-microbe interactions in the intestine. Nat immunol. 2013;14:660. doi: 10.1038/ni.2611. [DOI] [PubMed] [Google Scholar]
- 3.Geurts L, Neyrinck AM, Delzenne NM, Knauf C, Cani PD. Gut microbiota controls adipose tissue expansion, gut barrier and glucose metabolism: novel insights into molecular targets and interventions using prebiotics. Benef Microbes. 2014;5:3–17. doi: 10.3920/BM2012.0065. [DOI] [PubMed] [Google Scholar]
- 4.Turnbaugh PJ, Ley RE, Mahowald MA, Magrini V, Mardis ER, Gordon JI. An obesity-associated gut microbiome with increased capacity for energy harvest. Nat. 2006;444:1027. doi: 10.1038/nature05414. [DOI] [PubMed] [Google Scholar]
- 5.Marchesi JR. Prokaryotic and eukaryotic diversity of the human gut. Advanc App Microbiol. 2010:43–62. doi: 10.1016/S0065-2164(10)72002-5. [DOI] [PubMed] [Google Scholar]
- 6.Tanoue T, Umesaki Y, Honda K. Immune responses to gut microbiota-commensals and pathogens. Gut Microbes. 2010;1:224–33. doi: 10.4161/gmic.1.4.12613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ley RE, Turnbaugh PJ, Klein S, Gordon JI. Microbial ecology: human gut microbes associated with obesity. Nat. 2006;444:1022. doi: 10.1038/4441022a. [DOI] [PubMed] [Google Scholar]
- 8.Ley RE, Bäckhed F, Turnbaugh P, Lozupone CA, Knight RD, Gordon JI. Obesity alters gut microbial ecology. Proc Natl Acad Sci U S A. 2005;102:11070–5. doi: 10.1073/pnas.0504978102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang H, DiBaise JK, Zuccolo A, Kudrna D, Braidotti M, Yu Y, et al. Human gut microbiota in obesity and after gastric bypass. Proc Natl Acad Sci U S A. 2009;106:2365–70. doi: 10.1073/pnas.0812600106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Turnbaugh PJ, Hamady M, Yatsunenko T, Cantarel BL, Duncan A, Ley RE, et al. A core gut microbiome in obese and lean twins. Nat. 2009;457 doi: 10.1038/nature07540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lorente-Cebrián S, Costa AG, Navas-Carretero S, Zabala M, Martínez JA, Moreno-Aliaga MJ. Role of omega-3 fatty acids in obesity, metabolic syndrome, and cardiovascular diseases: a review of the evidence. J Physiol Biochem. 2013;69:633–51. doi: 10.1007/s13105-013-0265-4. [DOI] [PubMed] [Google Scholar]
- 12.Riediger ND, Othman RA, Suh M, Moghadasian MH. A systemic review of the roles of n-3 fatty acids in health and disease. J Am Dietetic Assoc. 2009;109:668–79. doi: 10.1016/j.jada.2008.12.022. [DOI] [PubMed] [Google Scholar]
- 13.Claesson MJ, Jeffery IB, Conde S, Power SE, O’connor EM, Cusack S, et al. Gut microbiota composition correlates with diet and health in the elderly. Nat. 2012;488 doi: 10.1038/nature11319. [DOI] [PubMed] [Google Scholar]
- 14.Nicholson JK, Holmes E, Kinross J, Burcelin R, Gibson G, Jia W, et al. Host-gut microbiota metabolic interactions. Sci. 2012;336:1262–7. doi: 10.1126/science.1223813. [DOI] [PubMed] [Google Scholar]
- 15.Lee JY, Plakidas A, Lee WH, Heikkinen A, Chanmugam P, Bray G, et al. Differential modulation of Toll-like receptors by fatty acids preferential inhibition by n-3 polyunsaturated fatty acids. J lipid Res. 2003;44:479–86. doi: 10.1194/jlr.M200361-JLR200. [DOI] [PubMed] [Google Scholar]
- 16.Bäckhed F, Ley RE, Sonnenburg JL, Peterson DA, Gordon JI. Host-bacterial mutualism in the human intestine. Sci. 2005;307:1915–20. doi: 10.1126/science.1104816. [DOI] [PubMed] [Google Scholar]
- 17.Hooper LV, Midtvedt T, Gordon JI. How host-microbial interactions shape the nutrient environment of the mammalian intestine. Ann Rev Nut. 2002;22:283–307. doi: 10.1146/annurev.nutr.22.011602.092259. [DOI] [PubMed] [Google Scholar]
- 18.Kamada N, Seo SU, Chen GY, Núñez G. Role of the gut microbiota in immunity and inflammatory disease. Nat Rev Immunol. 2013;13:321. doi: 10.1038/nri3430. [DOI] [PubMed] [Google Scholar]
- 19.Shen Y, Torchia MLG, Lawson GW, Karp CL, Ashwell JD, Mazmanian SK. Outer membrane vesicles of a human commensal mediate immune regulation and disease protection. Cell Host Microb. 2012;12:509–20. doi: 10.1016/j.chom.2012.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bonnington KE, Kuehn MJ. Protein selection and export via outer membrane vesicles. Biochim Biophys Acta. 2014;1843:1612–9. doi: 10.1016/j.bbamcr.2013.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schwechheimer C, Kuehn MJ. Outer-membrane vesicles from Gram-negative bacteria: biogenesis and functions. Nat Rev Microbiol. 2015;13:605. doi: 10.1038/nrmicro3525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Unal CM, Schaar V, Riesbeck K. Bacterial outer membrane vesicles in disease and preventive medicine. Semin Immunopathol. 2011;33:395–408. doi: 10.1007/s00281-010-0231-y. [DOI] [PubMed] [Google Scholar]
- 23.MacDonald IA, Kuehn MJ. Offense and defense: microbial membrane vesicles play both ways. Res Microbiol. 2012;163:607–18. doi: 10.1016/j.resmic.2012.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee EY, Choi DS, Kim KP, Gho YS. Proteomics in gram-negative bacterial outer membrane vesicles. Mass Spectrom Rev. 2008;27:535–55. doi: 10.1002/mas.20175. [DOI] [PubMed] [Google Scholar]
- 25.Kulp A, Kuehn MJ. Biological functions and biogenesis of secreted bacterial outer membrane vesicles. Annu Rev Microbiol. 2010;64:163–84. doi: 10.1146/annurev.micro.091208.073413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kulkarni HM, Jagannadham MV. Biogenesis and multifaceted roles of outer membrane vesicles from Gram-negative bacteria. Microbiol. 2014;160:2109–21. doi: 10.1099/mic.0.079400-0. [DOI] [PubMed] [Google Scholar]
- 27.Ellis TN, Kuehn MJ. Virulence and immunomodulatory roles of bacterial outer membrane vesicles. Microbiol Mol Biol Rev. 2010;74:81–94. doi: 10.1128/MMBR.00031-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Daliri SS, Kafil HS, Aghazadeh M, Fateh A, Yousefi M, Siadat SD. Extraction and Biological Evaluation of the Membrane Vesicles of Mycobacterium tuberculosis (CRBIP7 11) as Adjuvant and Vaccine Candidate. Jundishapur J Microbiol. 2017;10 [Google Scholar]
- 29.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–54. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 30.Claassen I, Meylis J, Van Der Ley P, Peeters C, Brons H, Robert J, et al. Production, characterization and control of a Neisseria meningitidis hexavalent class 1 outer membrane protein containing vesicle vaccine. Vaccine. 1996;14:1001–8. doi: 10.1016/0264-410x(96)00020-5. [DOI] [PubMed] [Google Scholar]
- 31.Fábrega MJ, Aguilera L, Giménez R, Varela E, Alexandra Cañas M, Antolín M, et al. Activation of immune and defense responses in the intestinal mucosa by outer membrane vesicles of commensal and probiotic Escherichia coli strains. Front Microbiol. 2016;7:705. doi: 10.3389/fmicb.2016.00705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lin L, Zhang J. Role of intestinal microbiota and metabolites on gut homeostasis and human diseases. BMC Immunol. 2017;18 doi: 10.1186/s12865-016-0187-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nicholls SJ, Lundman P, Harmer JA, Cutri B, Griffiths KA, Rye K, et al. Consumption of saturated fat impairs the anti-inflammatory properties of high-density lipoproteins and endothelial function. J Am College Cardio. 2006;48:715–20. doi: 10.1016/j.jacc.2006.04.080. [DOI] [PubMed] [Google Scholar]
- 34.Calder PC, Grimble RF. Polyunsaturated fatty acids, inflammation and immunity. Europe J Clin Nutr. 2002;56:S14. doi: 10.1038/sj.ejcn.1601478. [DOI] [PubMed] [Google Scholar]
- 35.Maslowski KM, Mackay CR. Diet, gut microbiota and immune responses. Nat Immunol. 2010;12 doi: 10.1038/ni0111-5. [DOI] [PubMed] [Google Scholar]
- 36.Zen Besten G, Van Eunen K, Groen AK, Venema K, Reijngoud DJ, Bakker BM. The role of short-chain fatty acids in the interplay between diet, gut microbiota, and host energy metabolism. J lipid Res. 2013;54:2325–40. doi: 10.1194/jlr.R036012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hildebrandt MA, Hoffmann C, Sherrill–Mix SA, Keilbaugh SA, Hamady M, Chen YY, et al. High-fat diet determines the composition of the murine gut microbiome independently of obesity. Gastroenterol. 2009;137:1716–24. doi: 10.1053/j.gastro.2009.08.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kau AL, Ahern PP, Griffin NW, Goodman AL, Gordon JI. Human nutrition, the gut microbiome and the immune system. Nat. 2011;474 doi: 10.1038/nature10213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tomarelli RM, Norris R, Rose C, Gyorgy P. The effect of fatty acids on the growth of strains of Lactobacillus bifidus. J Biol Chem. 1950;187:197–204. [PubMed] [Google Scholar]
- 40.Maczulak A, Dehority B, Palmquist D. Effects of long-chain fatty acids on growth of rumen bacteria. App Environ Microbiol. 1981;42:856–62. doi: 10.1128/aem.42.5.856-862.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nieman C. Influence of trace amounts of fatty acids on the growth of microorganisms. Bacteriol Rev. 1954;18:147. doi: 10.1128/br.18.2.147-163.1954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Round JL, Mazmanian SK. The gut microbiota shapes intestinal immune responses during health and disease. Nat Rev Immunol. 2009;9 doi: 10.1038/nri2515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ivanov II, Littman DR. Modulation of immune homeostasis by commensal bacteria. Curr Opin Microbiol. 2011;14:106–14. doi: 10.1016/j.mib.2010.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ahmadi Badi S, Moshiri A, Fateh A, Rahimi Jamnani F, Sarshar M, Vaziri F, et al. Microbiota-Derived extracellular vesicles as new systemic regulators. Front Microbiol. 2017;8:1610. doi: 10.3389/fmicb.2017.01610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ahmadi Badi S, Khatami SH, Irani SH, Siadat SD. Induction Effects of Bacteroides fragilis Derived Outer Membrane Vesicles on Toll Like Receptor 2, Toll Like Receptor 4 Genes Expression and Cytokines Concentration in Human Intestinal Epithelial Cells. Cell J. 2019;21:57–61. doi: 10.22074/cellj.2019.5750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ahmadi Badi S, Khatami SH, Irani SH, Siadat SD. Induction Effects of Bacteroides fragilis Derived Outer Membrane Vesicles on Toll Like Receptor 2, Toll Like Receptor 4 Genes Expression and Cytokines Concentration in Human Intestinal Epithelial Cells. Cell J. 2019;21:57–61. doi: 10.22074/cellj.2019.5750. [DOI] [PMC free article] [PubMed] [Google Scholar]