Abstract
Background
Senescence is a natural barrier for the body to resist the malignant transformation of its own cells. This work investigated the senescence characteristics of cancer cells in vitro.
Material/Methods
Human cervical cancer HeLa cells were treated with different concentrations of doxorubicin for 3 days, with or without subsequent extended culture in drug-free medium for 6 days. Senescent cell ratios between these 2 culture schemes were calculated. Expression of 2 senescence-associated secretory factors, IL-6 and IL-8, were detected by RT-PCR and ELISA. Doxorubicin treatment induced epithelial-mesenchymal transition in cancer cells. The proportions of senescent cells in epithelial-like and mesenchymal-like sub-groups were calculated. Doxorubicin-treated HeLa cells were stained with Vimentin antibody and sorted by flow cytometry. Senescent cell marker p16INK4a and IL-8 expression in Vimentin-high and Vimentin-low cells were detected by Western blot.
Results
We found that less than 1% of HeLa cells showed senescence phenotype after treatment with doxorubicin for 3 days. However, the proportion of senescent cells was significantly increased when the doxorubicin-treated cells were subsequently cultured in drug-free medium for another 6d. RT-PCR and ELISA results showed that this prolonged culture method could further improve the expression of IL-6 and IL-8. We also found that the senescent cells were mainly epithelial-like type and few presented mesenchymal-like shape. p16INK4a and IL-8 expression were decreased in cell fraction with higher Vimentin expression.
Conclusions
Our results suggested the existence of time delay effect in doxorubicin-induced senescence of HeLa cells, and epithelial-mesenchymal transition may resist doxorubicin-induced cell senescence.
MeSH Keywords: Cell Aging, Doxorubicin, Epithelial-Mesenchymal Transition
Background
Cellular senescence refers to an irreversibly stable state after losing the proliferative ability [1]. The main features of senescence include [2]: 1) the cell stops dividing and enters a state similar to terminal differentiation; 2) expression of the characteristic senescence-associated β-galactosidase(SA-β-Gal); 3) chromatin condensation to form heterochromatin (senescence-associated heterochromatic foci), telomere shortening and hyper-methylation of histone H3K9; 4) expression of a large amount of matrix reconstruction proteins, chemokines and inflammatory factors such as PAI1, MMPs, CXCL1, IL-1, IL-6, and IL-8 [3–8], also known as senescence-associated secretory phenotype (SASP) [9].
Senescence is a natural barrier for the body to resist the malignant transformation of its own cells. Some key events in the process of carcinogenesis, such as DNA damage and hyper-activation of oncogenes, can induce cell senescence and thus inhibit the occurrence of cancer cells [10]. Moreover, many chemotherapy drugs can induce the senescence of cancer cells and lead to its extinction ultimately [11]. Therefore, broken of the senescence barrier may promote the occurrence of cancers. For example, CD11b+ gr-1+ myeloid-derived suppressor cells could enhance the senescence evading of tumor cells and promote their proliferation by secreting the antagonistic molecule IL1RA of IL1R [12].
Chemotherapy is one of the main methods to treat cancer. Chemotherapy drugs can not only induce apoptosis [13], but also promote the senescence of cancer cells [14]. In this study, we used doxorubicin-treated HeLa cells as a model of in vitro cellular senescence. We found the existence of time delay effect in drug-induced cancer cell senescence, and epithelial-mesenchymal transition could inhibit cell senescence.
Material and Methods
Cell culture
HeLa cells were obtained from the Institute of Biochemistry and Cell Biology, Shanghai Institute for Biological Science, Chinese Academy of Sciences. Cells were cultured in DMEM medium containing 10% fetal bovine serum, 100 U/ml penicillin and 100 μg/ml streptomycin. Doxorubicin was purchased from Meilun Biotechnology Co. LTD, Dalian, China.
MTT assay
The cells were seeded into 96-well plate at 5000/well and cultured overnight, then fresh medium containing doxorubicin was added and cultured for 3d. After that, 0.5 mg/ml MTT solution was added to each well and further incubated for 2 h. Subsequently, the supernatant was discarded and the remnant was fully dissolved in DMSO. The absorbance value of 570 nm was detected for each well.
SA-β-Gal staining
The SA-β-Gal staining kit was purchased from Beyotime Biotechnology Co., LTD, Haimen, China. SA-β-Gal working solution was prepared according to the manufacturer’s instructions. The cells were fixed with 4% paraformaldehyde for 5 min, followed by washing 3 times with PBS, then incubated in the working solution at 37 degrees overnight. The stained cells were observed under microscope.
RNA Extraction and RT-PCR
Total RNA was isolated using Trizol reagent from the cells cultured in 6-cm dish followed by concentration determination. RNA (2 μg) was reverse transcribed to cDNA with the RevertAid reverse transcription kit (Thermo Scientific, Illinois, USA). The primers used in this study were as follows: IL-6: CCC CTG ACC CAA CCA CAA AT (forward), GCC CAG TGG ACA GGT TTC TG (reverse); IL-8: GGT GCA GTT TTG CCA AGG AG (forward), TTC CTT GGG GTC CAG ACA GA (reverse); PAI1: CAG ACC AAG AGC CTC TCC AC (forward), GGT TCC ATC ACT TGG CCC AT (reverse); GAPDH: CTC TGC TCC TGT TCG AC (forward), GCG CCC AAT ACG ACC AAA TC (reverse). The PCR products were analyzed by 1.5% agarose gel electrophoresis.
ELISA
Medium from cells treated with or without doxorubicin were collected. 50 μL samples were analyzed in IL-6 and IL-8 ELISA plates (Thermo Scientific, Cat # BMS213HS and KHC0081) and quantified according to standard curves.
Flow cytometry
Cells cultured in 10-cm dish were digested with trypsin and washed with PBS followed by fixing with Cytofix/Cytoperm kit (BD Biosciences, CA, USA), then stained with FITC-conjugated Vimentin antibody (Abcam Co., MA, USA). Flow cytometric analysis was carried out using a FACSCalibur (BD Biosciences, CA, USA).
Cell morphology analysis
After 0.1μg/ml doxorubicin treatment for 3 d and a prolonged culture of 6d, HeLa cell morphology showed heterogeneity. Cells were divided into epithelial (E)-like or mesenchymal (M)-like groups according to their appearance by 3 pathologists. If the length of a cell (longest axis) is more than 3 times of its width (vertical to the longest axis), this cell was tagged as M-like group. Otherwise it was ascribed to E-like group.
Western blot
Cells collected by FACS were washed by PBS and lysed in RIPA buffer. Protein content was determined by the Bradford assay (Beyotime Institute of Biotechnology, Haimen, China). 50μg proteins were separated in a 15% SDS-polyacrylamide gel electrophoresis and transferred to PVDF membrane. The membranes were first blocked with 5% (w/v) nonfat dry milk in TBST and then probed with the indicated primary antibodies with gentle shaking at 4°C overnight. After washing 3 times, the membranes were incubated with the HRP-conjugated secondary antibodies for 1 h. The signals were detected using an enhanced chemiluminescence detection kit (Thermo Scientific, Illinois, USA). The anti-E-Cadherin (1: 500 dilution), ani-Vimentin (1: 1000 dilution), anti-IL-8(1: 200 dilution), anti-p16INK4a (1: 500 dilution) and anti-GAPDH (1: 5000 dilution) antibodies were purchased from Abcam Co., MA, USA (Cat # ab323410, ab8069, ab18672, ab108349 and ab8245).
Statistics
Statistical analyses were performed using GraphPad Prism software. Each experiment was repeated least 3 times independently. All values and error bars were represented as mean ±SD. Two-tailed unpaired t tests were used to determine statistical significance between 2 experimental groups.
Results
Time delay effect in doxorubicin-induced cell senescence
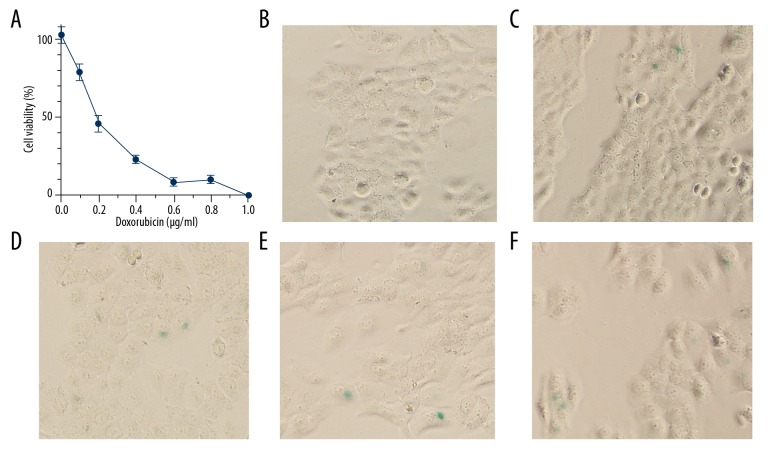
First, we used MTT assay to determine the dose-survival curve of HeLa cells treated with various concentrations of doxorubicin for 3 days. As shown in Figure 1A, the half lethal concentration of doxorubicin was 0.21 μg/ml. At 0.1 μg/ml of doxorubicin, the inhibition rate was nearly 20%. Next, we used SA-β-Gal staining to detect doxorubicin-induced cell senescence. As shown in Figure 1B, there were almost no senescent cells in the control group, and very few senescent cells were detected after treatment with 0.05–0.4 μg/ml doxorubicin for 3 days (Figure 1C–1F). The inhibition rate at 0.4 μg/ml doxorubicin was close to 80%, indicating that higher concentrations of doxorubicin kill most cells and are unlikely to increase the proportion of senescent cells.
Figure 1.
Acute treatment with doxorubicin induced HeLa cells senescence at a low efficiency. (A) Dose-survival curve of HeLa cells to doxorubicin treatment for 3 days. (B–F) SA-β-Gal staining of HeLa cells treated by doxorubicin for 3 days at 0, 0.05, 0.1, 0.2, or 0.4 μg/ml, respectively.
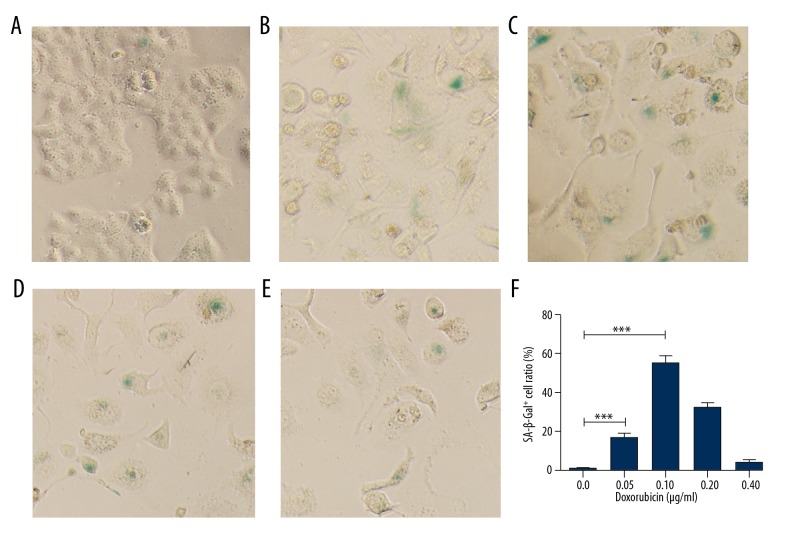
We subsequently tried another method to promote doxorubicin-induced cell senescence. We cultured HeLa cells in various concentrations of doxorubicin for 3 days, followed by growing in drug-free medium for another 6 days. SA-β-Gal staining revealed that this prolonged culture protocol significantly improved the senescent cell proportion at various concentrations of doxorubicin (Figure 2A–2E). The senescent cell proportion was approximately 60% after treatment with 0.1 μg/ml doxorubicin followed by prolonged culture (Figure 2F). These results indicate the time delay effect of doxorubicin-induced cell senescence of HeLa cells.
Figure 2.
Time delay effect of doxorubicin-induced senescence in HeLa cells. (A–E) HeLa cells were treated by doxorubicin for 3 days at 0, 0.05, 0.1, 0.2, or 0.4 μg/ml, respectively, and cultured in drug-free medium for 6 days followed by SA-β-Gal staining. (F) SA-β-Gal-positive cell proportions corresponding to A–E. *** p<0.001.
Time delay effect in doxorubicin-induced SASP
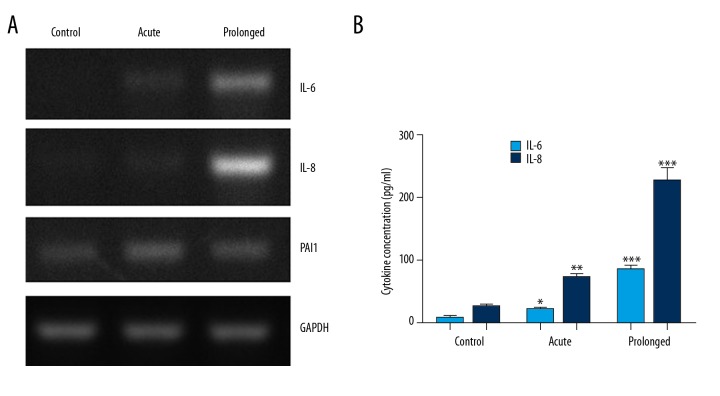
SASP is a hallmark of cell senescence. The expression of members of SASP, including IL-6, IL-8, and PAI1, were detected by RT-PCR in HeLa cells treated with 0.1 μg/ml doxorubicin for 3 days with or without prolonged culture (Acute or Prolonged). Figure 3A shows that the transcriptions of IL-6, IL-8, and PAI1 were induced by acute doxorubicin treatment. However, the IL-6 and IL-8 mRNA levels were further increased after prolonged culturing. Next, we determined the secreted IL-6 and IL-8 protein levels by ELISA. As shown in Figure 3B, after acute treatment with doxorubicin, the IL-6 and IL-8 levels in cell culture supernatant were significantly increased when compared to the control group (p<0.05 and p<0.01, respectively). In cell supernatant after prolonged culture, the IL-6 and IL-8 protein levels increased more (p<0.001 for both cytokines). These results confirmed the time delay effect of doxorubicin-induced senescence of HeLa cells.
Figure 3.
Time delay effect of doxorubicin-induced SASP. (A) 0.1 μg/ml doxorubicin treatment for 3 days (acute) induced the expressions of SASP markers IL-6, IL-8, and PAI1 in HeLa cells. When the cells were allowed to continuously grow in drug-free medium for another 6 days (prolonged), further upregulation of IL-6 and IL-8 was observed. (B) The secreted IL-6 and IL-8 levels in cell culture supernatant in prolonged, acute, or control group were determined by ELISA. * p<0.05, ** p<0.01 *** p<0.001.
EMT inhibited cell senescence
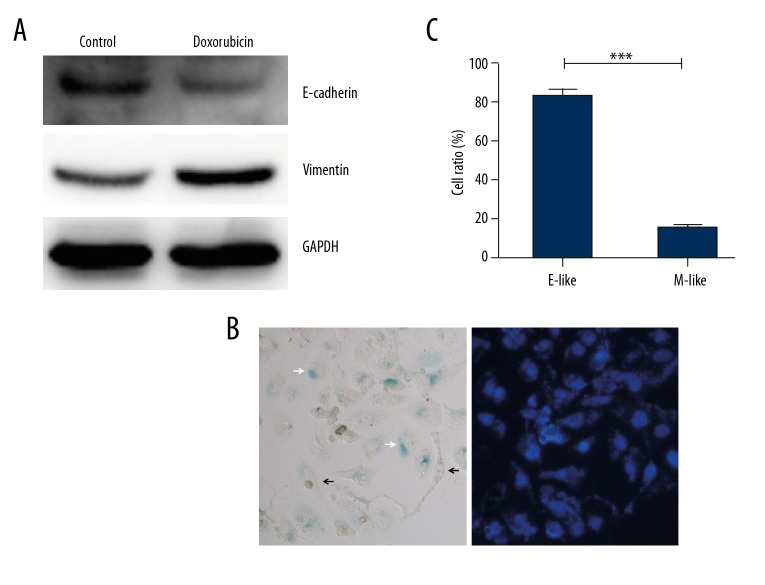
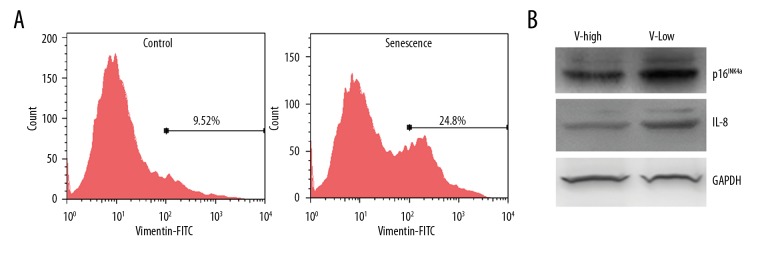
HeLa cells showed cobblestone-like epithelial cell morphology under normal culture conditions. Cancer cells can elicit the epithelial-mesenchymal transition (EMT) when treated with doxorubicin [15,16]. We also found that after 0.1 μg/ml doxorubicin treatment for 3 days and a prolonged culture for 6 days, HeLa cell morphology showed heterogeneity (Figure 2C). Some cells swelled but still had a nearly round shape, while others presented a spindle-like shape with multiple branches that resembled the EMT. We performed Western blot analysis to determine the expression of EMT markers E-Cadherin and Vimentin. Figure 4A shows that after doxorubicin treatment, E-Cadherin expression was decreased while Vimentin expression was increased, indicating that doxorubicin induces EMT in HeLa cells. These doxorubicin-treated cells can be divided into epithelial (E)-like or mesenchymal (M)-like groups according to their appearance (Figure 4B). After SA-β-Gal staining, we found that the proportion of SA-β-Gal-positive cells in the E-like group was significantly higher than in the M-like group (Figure 4C). We next stained the prolonged cultured HeLa cells with anti-Vimentin antibody and performed FACS to collect Vimentinhigh and Vimentinlow sub-groups (Figure 5A). Western blot results showed that IL-8 and p16INK4a expression in the Vimentinhigh sub-group was significantly lower than that in the Vimentinlow sub-group (Figure 5B). Together, these results indicate that EMT inhibits cell senescence.
Figure 4.
Morphological changes of senescence cells induced by doxorubicin. HeLa cells were treated with 0.1 μg/ml doxorubicin for 3 days followed by prolonged culture in drug-free medium for 6 days. (A) Expression of E-Cadherin and Vimentin were determined by Western blot. (B) After staining with SA-β-Gal kit and DAPI, most of the senescent cells showed an epithelial-like shape (white arrow), while mesenchymal-like cells were mainly SA-β-Gal-negative (black arrow). (C) Proportions of SA-β-Gal positive cells in epithelial(E)-like or mesenchymal(M)-like cells. *** p<0.001.
Figure 5.
EMT inhibited doxorubicin-induced cell senescence. HeLa cells were treated with 0.1 μg/ml doxorubicin for 3 days followed by prolonged culture in drug-free medium for 6 days. The cells were collected and stained with FITC-conjugated anti-Vimentin antibody (A). After sorting with flow cytometry, Vimentinhigh (V-high) and Vimentinlow (V-low) sub-group cells were used to detect the expression of p16INK4a and IL-8 by Western blot (B).
Discussion
There are many causes of cell senescence, such as continuous cell replication, oncogene hyper-activation, radiation, and chemicals treatment [17]. Several reports had shown that cancer cell senescence can be detected immediately after treatment of doxorubicin for 2–3 days [18–20]. In this study, we found that acute doxorubicin treatment induced HeLa cell senescence at an extremely low efficiency. If the acute doxorubicin-treated cells were allowed to grow in drug-free medium for another 6 days, the proportion of senescent cells significantly increases. Furthermore, in these prolongedly cultured cells, the transcription and secreted protein levels of IL-6 and IL-8, 2 members of SASP, were further significantly enhanced when compared to acute doxorubicin treatment. These results suggest that doxorubicin-induced HeLa cell senescence has a time delay effect.
Doxorubicin is a commonly used chemotherapy drug that can induce DNA cross-linking and lead to cell apoptosis, autophagy, and/or senescence [21–23]. Unlike apoptosis, senescence is a relatively stable state with active metabolism. Doxorubicin can induce cell apoptosis in 24 h in a dose-dependent manner [24]. The time-delayed effect of doxorubicin induced cell senescence, indicating that the cell fate (apoptosis or senescence) was determined by a different mechanism.
Doxorubicin can induce epithelial-mesenchymal transition that endows cancer cells with increased mobility and drug resistance [25]. We found that the appearance of most of the SA-β-Gal-positive cells after doxorubicin treatment showed an epithelial-like round shape. Expression of the mesenchymal marker Vimentin was inversely related with p16INK4a and IL-8, further indicating that the process of epithelial-mesenchymal transition can inhibit cell senescence. In accordance with our findings, Al-Khalaf et al. reported that p16INK4a and its downstream miRNA targets can inhibit EMT through suppressing the EMT inducer ZEB1 in primary human fibroblasts and breast cancer cells [26]. Ansieau et al. also showed that Twist1 and Twist2 induction was sufficient to override oncogene-induced senescence and lead to complete EMT simultaneously in human cells [27]. Although the details of the linkage between EMT and cell senescence are still unclear, our results might explain why evading cell senescence can promote carcinogenesis.
Conclusions
Our results suggest the existence of a time delay effect in doxorubicin-induced senescence of HeLa cells, and epithelial-mesenchymal transition may resist doxorubicin-induced cell senescence.
Footnotes
Source of support: This study was supported by the Shanghai Natural Science Foundation (16ZR1403600)
Conflicts of interest
None.
References
- 1.Storer M, Mas A, Robert-Moreno A, et al. Senescence is a developmental mechanism that contributes to embryonic growth and patterning. Cell. 2013;155:1119–30. doi: 10.1016/j.cell.2013.10.041. [DOI] [PubMed] [Google Scholar]
- 2.Salama R, Sadaie M, Hoare M, Narita M. Cellular senescence and its effector programs. Genes Dev. 2014;28:99–114. doi: 10.1101/gad.235184.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Coppe JP, Patil CK, Rodier F, et al. A human-like senescence-associated secretory phenotype is conserved in mouse cells dependent on physiological oxygen. PLoS One. 2010;5:e9188. doi: 10.1371/journal.pone.0009188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Benelli R, Stigliani S, Minghelli S, et al. Impact of CXCL1 overexpression on growth and invasion of prostate cancer cell. Prostate. 2013;73:941–51. doi: 10.1002/pros.22640. [DOI] [PubMed] [Google Scholar]
- 5.McCarthy DA, Clark RR, Bartling TR, et al. Redox control of the senescence regulator interleukin-1alpha and the secretory phenotype. J Biol Chem. 2013;288:32149–59. doi: 10.1074/jbc.M113.493841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang P, Han L, Shen H, et al. Protein kinase D1 is essential for Ras-induced senescence and tumor suppression by regulating senescence-associated inflammation. Proc Natl Acad Sci USA. 2014;111:7683–88. doi: 10.1073/pnas.1310972111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Medina RJ, O’Neill CL, O’Doherty TM, et al. Ex vivo expansion of human outgrowth endothelial cells leads to IL-8-mediated replicative senescence and impaired vasoreparative function. Stem Cells. 2013;31:1657–68. doi: 10.1002/stem.1414. [DOI] [PubMed] [Google Scholar]
- 8.Eren M, Boe AE, Murphy SB, et al. PAI-1-regulated extracellular proteolysis governs senescence and survival in Klotho mice. Proc Natl Acad Sci USA. 2014;111:7090–95. doi: 10.1073/pnas.1321942111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kuilman T, Peeper DS. Senescence-messaging secretome: SMS-ing cellular stress. Nat Rev Cancer. 2009;9:81–94. doi: 10.1038/nrc2560. [DOI] [PubMed] [Google Scholar]
- 10.Campisi J. Aging, cellular senescence, and cancer. Annu Rev Physiol. 2013;75:685–705. doi: 10.1146/annurev-physiol-030212-183653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ewald JA, Desotelle JA, Wilding G, Jarrard DF. Therapy-induced senescence in cancer. J Natl Cancer Inst. 2010;102:1536–46. doi: 10.1093/jnci/djq364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Di Mitri D, Toso A, Chen JJ, et al. Tumour-infiltrating Gr-1+ myeloid cells antagonize senescence in cancer. Nature. 2014;515:134–37. doi: 10.1038/nature13638. [DOI] [PubMed] [Google Scholar]
- 13.Seah KS, Loh JY, Nguyen TTT, et al. SAHA and cisplatin sensitize gastric cancer cells to doxorubicin by induction of DNA damage, apoptosis and perturbation of AMPK-mTOR signalling. Exp Cell Res. 2018;370(2):283–91. doi: 10.1016/j.yexcr.2018.06.029. [DOI] [PubMed] [Google Scholar]
- 14.He Q, Au B, Kulkarni M, et al. Chromosomal instability-induced senescence potentiates cell non-autonomous tumourigenic effects. Oncogenesis. 2018;7:62. doi: 10.1038/s41389-018-0072-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pan JX, Chen G, Li JJ, et al. Isocorydine suppresses doxorubicin-induced epithelial-mesenchymal transition via inhibition of ERK signaling pathways in hepatocellular carcinoma. Am J Cancer Res. 2018;8:154–64. [PMC free article] [PubMed] [Google Scholar]
- 16.Han R, Xiong J, Xiao R, et al. Activation of beta-catenin signaling is critical for doxorubicin-induced epithelial-mesenchymal transition in BGC-823 gastric cancer cell line. Tumour Biol. 2013;34:277–84. doi: 10.1007/s13277-012-0548-3. [DOI] [PubMed] [Google Scholar]
- 17.Collado M, Serrano M. Senescence in tumours: Evidence from mice and humans. Nat Rev Cancer. 2010;10:51–57. doi: 10.1038/nrc2772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tong Y, Zhao W, Zhou C, et al. PTTG1 attenuates drug-induced cellular senescence. PLoS One. 2011;6:e23754. doi: 10.1371/journal.pone.0023754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rouibah H, Kebsa W, Lahouel M, et al. Algerian propolis potentiates doxorubicin mediated anticancer effect against human pancreatic PANC-1 cancer cell line through cell cycle arrest, apoptosis induction and P-glycoprotein inhibition. Anticancer Agents Med Chem. 2018;18:375–87. doi: 10.2174/1871520618666180110143239. [DOI] [PubMed] [Google Scholar]
- 20.Srdic-Rajic T, Santibanez JF, Kanjer K, et al. Iscador Qu inhibits doxorubicin-induced senescence of MCF7 cells. Sci Rep. 2017;7:3763. doi: 10.1038/s41598-017-03898-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Taatjes DJ, Koch TH. Nuclear targeting and retention of anthracycline antitumor drugs in sensitive and resistant tumor cells. Curr Med Chem. 2001;8:15–29. doi: 10.2174/0929867013374029. [DOI] [PubMed] [Google Scholar]
- 22.Bilardi RA, Kimura KI, Phillips DR, Cutts SM. Processing of anthracycline-DNA adducts via DNA replication and interstrand crosslink repair pathways. Biochem Pharmacol. 2012;83:1241–50. doi: 10.1016/j.bcp.2012.01.029. [DOI] [PubMed] [Google Scholar]
- 23.Rogalska A, Gajek A, Lukawska M, et al. Novel oxazolinoanthracyclines as tumor cell growth inhibitors-Contribution of autophagy and apoptosis in solid tumor cells death. PLoS One. 2018;13:e0201296. doi: 10.1371/journal.pone.0201296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Huang Z, Lin S, Long C, et al. Notch signaling pathway mediates Doxorubicin-driven apoptosis in cancers. Cancer Manag Res. 2018;10:1439–48. doi: 10.2147/CMAR.S160315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang Y, Weinberg RA. Epithelial-to-mesenchymal transition in cancer: Complexity and opportunities. Front Med. 2018;12(4):361–73. doi: 10.1007/s11684-018-0656-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Al-Khalaf HH, Aboussekhra A. p16(INK4A) induces senescence and inhibits EMT through microRNA-141/microRNA-146b-5p-dependent repression of AUF1. Mol Carcinog. 2017;56:985–99. doi: 10.1002/mc.22564. [DOI] [PubMed] [Google Scholar]
- 27.Ansieau S, Bastid J, Doreau A, et al. Induction of EMT by twist proteins as a collateral effect of tumor-promoting inactivation of premature senescence. Cancer Cell. 2008;14:79–89. doi: 10.1016/j.ccr.2008.06.005. [DOI] [PubMed] [Google Scholar]