Abstract
Introduction:
Lichen planus pigmentosus (LPP) is a distinct clinical entity commonly encountered in the Indian population.
Aim:
To study the clinicoetiological profile of LPP at a tertiary care hospital.
Methods:
A total of 100 patients with clinically and histopathologically confirmed diagnosis of LPP were included. Demographic details including the age of onset, duration of disease, symptoms, and family history were obtained. History regarding any precipitating factors, cosmetics, drug intake, and associated cutaneous or systemic diseases was taken. Clinical examination of the skin, oral cavity, hair, and nails was carried out.
Results:
Of the total 100 patients, 56 (56%) were females and 44 (44%) males with age ranging from 18 to 54 years (mean age - 31.23 years). The duration of disease ranged from 2 to 60 months with a mean of 19.31 months. Cosmetic disfigurement (68%) was the commonest complaint, followed by itching (41%) while, 30% of the patients were asymptomatic. History of topical mustard oil and hair dye application was present in 62% and 48% of the cases each. Other topicals included perfumes (24%), aftershave lotion (36%), and cosmetics (20%). Face (54%) and neck (48%) were the commonest sites affected, followed by upper back (36%), upper limbs, and chest (each 32%). A total of 11 patients showed only flexural involvement. The commonest pattern of pigmentation was diffuse (56%) followed by reticular in 16%. The color of the pigmentation varied from slate grey to brownish-black in varying proportions. A positive association was found between hypothyroidism with diffuse LPP where the P value was <0.001.
Conclusion:
LPP is a distinct clinical entity caused by diverse etiological factors and shows varied clinical patterns. All the patients should be advised to stop using mustard oil/henna/hair dye/after shave lotions and cosmetics. Hypothyroidism can be considered to be a disease associated with LPP and all the patients should be investigated for the same.
Keywords: Lichen planus, lichen planus pigmentosus, mustard oil, pigmentation
Introduction
The term lichen plans pigmentosus (LPP) was first used by Shima in 1956 for a condition considered to be a variant of lichen planus.[1] It is characterized by insidious onset of blue–brown macules over the sun-exposed areas and the flexures. Rarely, involvement can be generalized. Palms, soles, and nails are not affected, and involvement of the oral mucosa is infrequent. In contrast to the classic lesions of lichen planus, the lesions of LPP usually are asymptomatic, although some lesions may be associated with pruritus or a burning sensation. Although LPP is a frequently encountered disorder in Indians, very few studies have been done to study its clinical profile. No definite etiological factors have been identified. Hepatitis C virus, mustard oil, amla oil, and paraphenylenediamine in hair dye have been implicated as precipitating factors in predisposed individuals.[2] However, no temporal correlation has been found between the development or aggravation of pigmentation and the use of oils, hair cosmetics or toiletries as of yet. Hence, we conducted this study to determine the clinicoetiological profile of patients with LPP.
Materials and Methods
It was a hospital-based prospective observational study and 100 patients (who fulfilled the inclusion criteria and gave consent) were recruited based on convenient sampling. The study period was 1 year, and it was conducted in a single hospital. The aim was to study the clinicoetiological profile of patients with LPP. The inclusion criteria included: either sex, age >= 18 years, clinical criteria: slate grey to violaceous to brown pigmentation, histopathological criteria:
Basal cell degeneration, civatte bodies +/−, band-like inflammatory infiltrate in upper dermis, dermal melanophages, perivascular lymphohistiocytic infiltrate. The exclusion criteria included: age <18 years, those who took any treatment in the past 3 months, pregnant and lactating women, conditions mimicking LPP like dermal melanosis, Nevus of Ota, Ashy dermatoses (AD), macular amyloidosis, and those who do not give informed written consent. Some authors consider AD, LPP, and EDP as the same condition, whereas others consider them to be different.[3] The major difference between LPP and EDP/AD is the presence of an erythematous border in the early active phase of EDP.[3] As there is significant clinicohistological overlap between these, only the patients who showed lichenoid reaction on histology and fulfilled other histological criteria were recruited. Known cases of LP with post inflammatory hyperpigmentation were not included. Demographic details of all patients who fulfilled the inclusion criteria were taken. Information was noted regarding any precipitating factors, use of cosmetics, and associated cutaneous or systemic diseases. A detailed cutaneous examination was done.
Results
The characteristics of the patients are given in Table 1.
Table 1.
Characteristics of 100 patients with lichen planus pigmentosus
| Parameter | Number of patients (percent) Total=100 | |
|---|---|---|
| Total patients (100) | Males | 44% |
| Females | 56% | |
| Mean age (years) Range : 18 to 54 years | Males | 28±1.6 |
| Females | 31±1.4 | |
| Mean duration of disease (months) Range -2-60 months | 19.31 | |
| Initial site of presentation | Neck | 28% |
| Face | 24% | |
| ARms | 18% | |
| Back | 11% | |
| Flexures (Neck/inframammary areas) | 10% | |
| Most commonly involved sites | Face | 54% |
| Neck | 48% | |
| Upper back | 36% | |
| Upper limbs | 32% | |
| Flexures (axilla/infra mammary areas) | 13% | |
| Symptoms | Cosmetic disfigurement | 68% |
| Itching | 41% | |
| Burning | 15% | |
| Asymptomatic | 30% | |
| Etiological agents | Mustard oil/Almond oil | 62% |
| Hair dye | 48% | |
| Perfumes/deodorants | 24% | |
| Aftershave lotions | 36% | |
| Cosmetic creams (fair and lovely) | 20% | |
| Pattern of pigmentation | Diffuse | 56% |
| Reticular | 16% | |
| Inversus | 13% | |
| Blotchy | 12% | |
| Follicular | 5% | |
| Zosteriform/segmental | 3% | |
| Oral mucosal involvement | 8% | |
| Nail involvement | 14% | |
| Associated diseases | Vitiligo | 1% |
| Hypothyroidism | 22% | |
| Hepatitis C | 4% | |
| Chronic urticaria | 12% | |
| Tuberculosis | 5% | |
| Canities | 24% | |
| Concomitant Lichen planus | 0% | |
A total of 100 patients were enrolled in the study. Of 100 patients (age range, 18–54 years), 56 were females (mean age, 31 ± 1.4 years), and 44 were males (mean age, 28 ± 1.6 years). Maximum number of patients (42%) were in the age group 18–27 years, 30% belonged to 28-37 years, while the rest belonged to the age group 38 to 58 years. Only seven patients belonged to age above 50 years. The duration of disease ranged from 2 to 60 months with a mean of 19.31 months. Majority of the patients (54%) had the disease for 2 months to 12 months, while the rest had the disease since more than 1 year. In the drug history, 18 patients were on thyroxine, 9 on cetirizine and 1 was on antitubercular therapy. The initial site of presentation was the neck in 28% of the patients and face in 24%. A total of 9% had simultaneous involvement in multiple sites. Maximum number of patients had bilateral symmetrical involvement (60%). Face (54%) and neck (48%) were the commonest sites affected. In the face, the common areas involved were periocular area, nasal bridge, preauricular areas, and the temples.
The various clinical patterns of LPP are enumerated in Table 1. The color of LPP varied from slate grey to bluish to brownish–black. Multiple shades were noted in each patient. At places like the face, it was usually more of light to dark brown to slate -grey, while over the neck, flexures, back it was more bluish–brown in color.
Oral mucosal involvement was seen in eight patients in the form of violaceous pigmentation over bilateral buccal mucosae; however, they were asymptomatic. Nails were involved in 14% of the patients in the form of longitudinal ridging in 12 patients and longitudinal melanonychia in four patients. Palms, soles, and scalp were not involved in any of the patients in our study.
Discussion
The term LPP was first coined by Shima in 1956 for a condition considered to be a variant of lichen planus. However, the detailed description of the disease was given by Bhutani et al.[4] It is commonly seen in Indian, Asian and middle east population. The exact prevalence of the disease is unknown. According to the Global consensus forum, acquired macules of pigmentation of uncertain etiology involving predominantly the head and neck region or the trunk/flexures, with or without present or past evidence of lichen planus elsewhere, predominantly occurring in the people of South Asian origin (e.g., India, Pakistan, Sri Lanka) and the African Continent, may be termed LPP.[3]
In our study, females outnumbered males with a male to female ratio of 1:1.3, similar to Bhat et al. (56.70% females and 43.30% males) and Sindhura et al. (1:2.2).[5,6] Maximum number (42%) of our patients belonged to age group 18 to 27 years. This may be attributed to the increased outdoor activity leading to excess sun exposure and using various cosmetic products in this young age group. LPP is usually an asymptomatic disease and the only bothersome complaint is the cosmetic disfigurement. However, 41% of our patients complained of itching while 15% complained of burning. Bhat et al.[5] reported itching in 50% of their patients. Itching is considered by few to be a marker of activity and progression.[7] The underlying cause of lichen planus pigmentosus is currently unknown. However, studies suggest that the condition may be triggered by a number of agents like viral infections, UV light, or the application of certain oils to the hair or skin (i.e. mustard oil, amla oil, or hair dyes, sindoor). It is considered to be a type IV hypersensitivity reaction to unknown antigen by few. These oils act as potent photosensitizers on exposure to ultraviolet radiation triggering a lichenoid reaction at the dermoepidermal junction. In our study, the most common triggering agents encountered were topical mustard oil and hair dye application in 62% and 48% of the cases. Mustard oil contains allyl isothycynate, while hair dye contain PPD, both being potent photosensitizers. Other topicals included perfumes (24%), aftershave lotion (fitkari) (36%) and cosmetics (20%). These substances contain photosensitizers, such as musk ambrette, sandalwood oil, and bergamot oil, used in high-end perfumes, as well as rosemary and lavender oil. As for men, some colognes and aftershaves contain lime. There is already evidence in literature according to which these cosmetic products are known to cause or aggravate LPP as proved by patch-testing by several studies.[8,9] However, we could not perform the patch testing.
In our study, the commonest pattern of pigmentation was diffuse (56%) [Figure 1] followed by reticular in 16% of the patients, followed by blotchy pigmentation in 12%. A total of 32% patients had multiple patterns of pigmentation. Follicular LPP was also noted in three patients. Flexural LPP also known as LPP inversus was found in 13 patients [Figure 2]. It involves sites like axillae, groins, inframammary areas, and other sun-protected sites. It is more common in postmenopausal women. It can begin as violaceous papules and immediately transit to flat macules which follow the lines of cleavage.[10] LPP at these flexural sites can be explained by the repeated friction in these areas leading to koebnerization, tight-fitted clothing, and also by the concentrated accumulation of topicals like mustard oil and perfumes/deodorants.[11] Kanwar et al. reported that the pigmentation was diffuse in 77%, reticular in 10%, blotchy in 7%, and perifollicular in 6% of patients.[2] Four of our patients had segmental/zosteriform pattern [Figure 3]. This pattern may be explained by mosaicism determined during embryogenesis which determine cell populations with different immunological and antigenic properties.[12]
Figure 1.
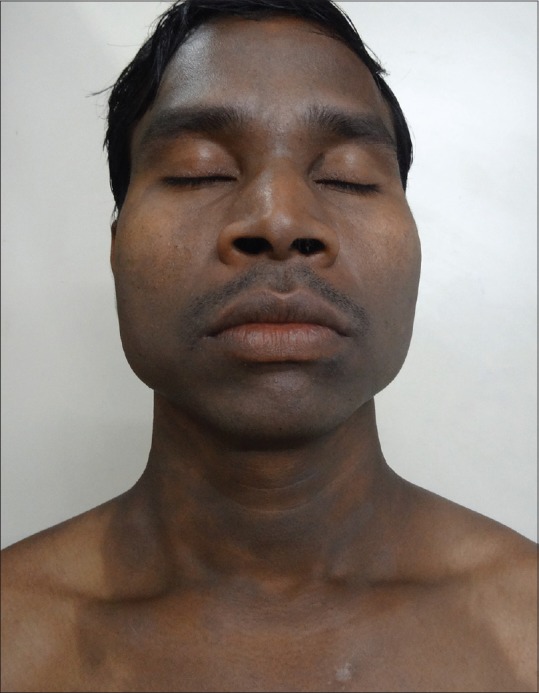
Diffuse LPP over face and neck
Figure 2.
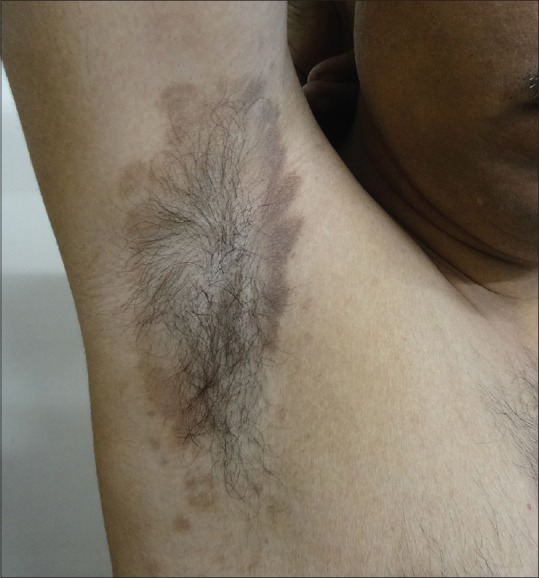
LPP inversus
Figure 3.
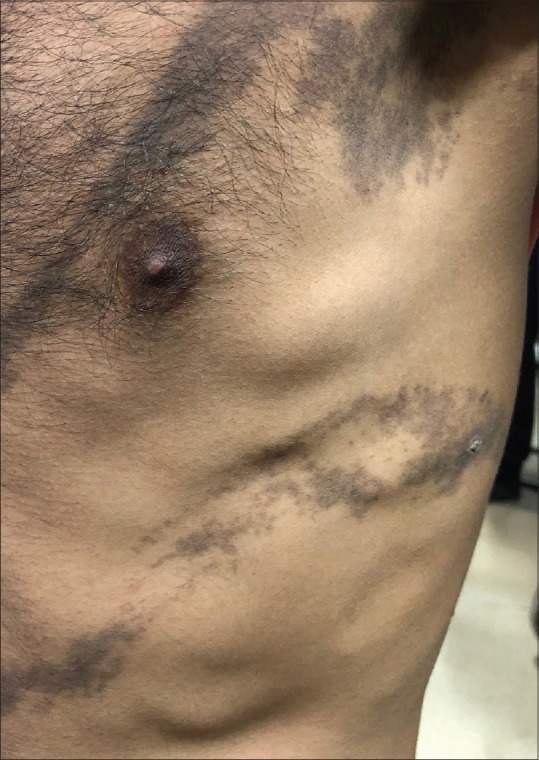
Zosteriform LPP
Only four of our patients were positive for hepatitis C. The prevalence of positive serology for hepatitis C virus is 60.6% in one study.[13] The prevalence of hypothyroidism in India is 11%. Hypothyroidism was found in 22% of our patients. And 18 patients had preceding hypothyroidism (were already on thyroxine), while 4 were diagnosed during the work up for LPP. In a study by Karnet al. on LPP, 31.7% of the patients were hypothyroid.[14] A positive association was found between hypothyroidism with diffuse LPP where the P value was <0.001 [Table 2]. LPP has also been linked to autoimmune diseases such as vitiligo and lupus erythematosus. One of our patient had diffuse LPP, sparing the vitiligo lesions.[15] Two of our patients had positive antinuclear antibody (ANA); however, they did not have any signs/symptoms of autoimmune disease. A total of 12 patients had a history of chronic urticaria, 24% had associated canities, two had a history of tubercular lymphadenitis, while three had pulmonary tuberculosis.
Table 2.
Association of hypothyroidism with type of pigmentation
| Type of pigmentation | Hypothyroidism absent (n=67) | Hypothyroidism present (n=33) | P | ||
|---|---|---|---|---|---|
| No. | % | No | % | ||
| Diffuse | 21 | 31.3 | 28 | 84.8 | <0.001 |
| Blotchy | 21 | 31.3 | 11 | 33.3 | 0.84 |
| Inversus | 16 | 23.9 | 9 | 27.3 | 0.71 |
In our study, oral mucosa was involved in 8% of our patients and nail in 14%, while in a study by Bhat et al., oral involvement was seen in 16.7% and nail changes in 23%.[5] Involvement of palms and soles has been reported previously; however, none of our patients had palmoplantar involvement.[16]
Conclusion
LPP is now becoming a common disease in Indian population, and the cosmetic disfigurement that it leads to is an area of concern. It has a chronic course. Treatment can lead to stabilization of the disease, but the pigmentation is resistant and takes many months to years to fade off. All the patients should be advised to stop using mustard oil/henna/hair dye/after shave lotions and cosmetics. Hypothyroidism could be considered to be a disease associated with LPP; however, more studies with large population are needed to confirm the same.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form the patient(s) has/have given his/her/their consent for his/her/their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Shima T. Lichen planus pigmentosus. Nippon Hifuka Gakkai Zaashi. 1956;66:346–53. [Google Scholar]
- 2.Kanwar AJ, Dogra S, Handa S, Parsad D, Radotra BD. A study of 124 Indian patients with lichen planus pigmentosus. Clin Exp Dermatol. 2003;28:481–5. doi: 10.1046/j.1365-2230.2003.01367.x. [DOI] [PubMed] [Google Scholar]
- 3.Kumarasinghe SPW, Pandya A, Chandran V, Rodrigues M, Dlova NC, Kang HY, et al. A global consensus statement on ashy dermatosis, erythema dyschromicum perstans, lichen planus pigmentosus, idiopathic eruptive macular pigmentation, and Riehl's melanosis. Int J Dermatol. 2018 doi: 10.1111/ijd.14189. doi: 10.1111/ijd. 14189. [DOI] [PubMed] [Google Scholar]
- 4.Bhutani LK, Bedi TR, Pandhi RK, Nayak NC. Lichen planus pigmentosus. Dermatologica. 1974;149:43–50. doi: 10.1159/000251470. [DOI] [PubMed] [Google Scholar]
- 5.Bhat RM, Mathanda TR, Jayaprakash CS, Dandakeri S. Clinical, histopathological characteristics and immunohistochemical findings in Lichen Planus Pigmentosus. Indian J Dermatol. 2017;62:612–17. doi: 10.4103/ijd.IJD_148_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sindhura KB, Vinay K, Kumaran MS, Saikia UN, Parsad D. Lichen planus pigmentosus: A retrospective clinico-epidemiologic study with emphasis on the rare follicular variant. J Eur Acad Dermatol Venereol. 2016;30:e142–4. doi: 10.1111/jdv.13454. [DOI] [PubMed] [Google Scholar]
- 7.Muthu SK, Narang T, Saikia UN, Kanwar AJ, Parsad D, Dogra S. Low-dose oral isotretinoin therapy in lichen planus pigmentosus: An open-label non-randomized prospective pilot study. Int J Dermatol. 2016;55:1048–54. doi: 10.1111/ijd.13293. [DOI] [PubMed] [Google Scholar]
- 8.Robles-Mendez JC, Rizo-Frias P, Herz-Ruelas ME, Pandya AG, Ocampo Candiani J. Lichen planus pigmentosus and its variants: Review and update. Int J Dermatol. 2018;57:505–14. doi: 10.1111/ijd.13806. [DOI] [PubMed] [Google Scholar]
- 9.Tienthavorn T, Tresukosol P, Sudtikoonaseth P. Patch testing and histopathology in Thai patients with hyperpigmentation due to Erythema dyschromicum perstans, Lichen planus pigmentosus, and pigmented contact dermatitis. Asian Pac J Allergy Immunol. 2014;32:185–92. doi: 10.12932/AP0376.32.2.2013. [DOI] [PubMed] [Google Scholar]
- 10.Sharma VK, Gupta V, Pahadiya P, Vedi KK, Arava S, Ramam M. Dermoscopy and patch testing in patients with lichen planus pigmentosus on face: A cross-sectional observational study in fifty Indian patients. Indian J Dermatol Venereol Leprol. 2017;83:656–62. doi: 10.4103/ijdvl.IJDVL_469_16. [DOI] [PubMed] [Google Scholar]
- 11.Chen S, Sun W, Zhou G, Chen S, Lu X. Lichen planus pigmentosus inversus: Report of three Chinese cases and review of the published work. J Dermatol. 2015;42:77–80. doi: 10.1111/1346-8138.12693. [DOI] [PubMed] [Google Scholar]
- 12.Vineet R, Sumit S, K GV, Nita K. Lichen planus pigmentosus in linear and zosteriform pattern along the lines of Blaschko. Dermatol Online J. 2015;21 [PubMed] [Google Scholar]
- 13.Al-Mutairi N, El-Khalawany M. Clinicopathological characteristics of lichen planus pigmentosus and its response to tacrolimus ointment: An open label, non-randomized, prospective study. J Eur Acad Dermatol Venereol. 2010;24:535–40. doi: 10.1111/j.1468-3083.2009.03460.x. [DOI] [PubMed] [Google Scholar]
- 14.Karn D, KC S, Timalsina M. Lichen Planus Pigmentosus: A study for association of thyroid dysfunction. Kathmandu Univ Med J. 2016;53:36–40. [PubMed] [Google Scholar]
- 15.Seidel A. Lichen planus pigmentosus (LPP) and lichen planus pigmentosus: 35 cases in Armenia, Colombia. J Am Acad Dermatol. 2015;72:AB114. [Google Scholar]
- 16.Dabas G, Vinay K, Parsad D, Chatterjee D, Kumaran MS. A retrospective study of lichen planus pigmentosus with focus on palmoplantar involvement. Clin Exp Dermatol. 2018 doi: 10.1111/ced.13696. doi: 10.1111/ced.13696. [DOI] [PubMed] [Google Scholar]


