Abstract
Objectives: Calotropin (CTP), a natural product isolated from Calotropis gigantea, has been identified as a potential anticancer agent. In this study, we aimed to investigate the effect CTP on colorectal cancer and the role of Yes-associated protein (YAP) in CTP-inhibited cell proliferation.
Methods: Cell viability and cell proliferation were detected by MTT and BrdU assay. Western blotting and immunofluorescence were performed to determine CTP-induced YAP dephosphorylation and nuclear localization. Western blotting, siRNA transfection and RT-PCR analysis were carried out to investigate the mechanisms of CTP-mediated YAP activation. The anti-tumor activities of CTP were observed in mice tumor models.
Results: We demonstrated that CTP inhibits the proliferation of colorectal cancer cells both in vitro and in vivo. Moreover, we showed that CTP activates YAP in colorectal cancer cells. Mechanistically, CTP promotes LATS1 degradation via the ubiquitination/proteasome pathway, resulting in YAP dephosphorylation and nuclear localization, leading to induce YAP target genes expression in colorectal cancer cells. Inhibition of YAP activity enhances CTP-mediated inhibition of cell proliferation, suggesting that YAP plays a protective role in CTP-induced antiproliferative effect.
Conclusion: Our results demonstrate that CTP markedly inhibits tumor growth and activates a protective role of YAP in colorectal cancer cells, indicating that combination of CTP and YAP targeting drugs may be a promising strategy for colorectal cancer treatment.
Keywords: colorectal cancer; calotropin, CTP; cell proliferation; YAP; LATS1
Introduction
Calotropin (CTP) is one of the cardenolides isolated from Calotropis gigantea that exhibits significant cytotoxicity against several cancer cells. CTP induces cell cycle arrest through decreasing the expression levels of CDK1 and CDK2 and promotes apoptosis by regulating the cytotoxic T lymphocyte-associated antigen - mediated TGFβ/ERK signaling pathway in lung cancer cells.1,2 CTP also promotes apoptosis via downregulating the expression levels of antiapoptotic proteins in K562 cells.3 In colon cancer cells, CTP inhibits cell growth through inhibition of Wnt signaling pathway.4 Although this promising anticancer activities, the mechanisms responsible for the CTP-induced tumor growth inhibition remain unknown.
In recent years, the Hippo pathway has gained great interest as being a central player in regulating many aspects of tumor biology.5,6 Core components of the mammalian Hippo pathway contain a kinase cascade of MST1/2 (mammalian STE20-like kinase 1 and 2) and LATS1/2 (large tumor suppressor 1 and 2).7 When the Hippo pathway is activated by upstream signals, MST1/2 phosphorylate and activate LATS1/2. Activated LATS1/2 subsequently phosphorylates and inhibits Yes-associated protein (YAP) and TAZ (WW domain-containing transcription regulator 1) translocation into the nucleus, interaction with the TEAD family of transcription factors and activation of downstream genes (eg CTGF, CYR61).8,9 AS a major downstream effector of the Hippo pathway, YAP is important for cell proliferation and survival. As dysregulation of YAP is associated with several types of cancer such as breast, liver and colon cancer, YAP has been considered as a promising and important therapeutic target.10,11
CTP has been identified as a potential anticancer agent. However, the molecular mechanisms involved remain poorly understood.
Materials and methods
Cell culture, agents and antibodies
Human colorectal cancer cell lines HT-29 and HCT116 cells were purchased from the American Type Culture Collection. All cell lines were cultured in RPMI-1640 supplemented with 10% FBS (Gibco), 100 U/mL penicillin (Sigma) and 100 μg/mL streptomycin (Sigma) at 37°C in an atmosphere containing 5% CO2. CTP was gifted kindly from Hao-Fu Dai professor. The purity of CTP was proved to be ≥95% by chromatographic analysis. CTP was dissolved in DMSO and stored at −20°C for experimental use in this study. MG132, cycloheximide and verteporfin were obtained from MedChemExpress. The following antibodies were used in this study: LATS1 was purchased from Cell Signaling Technology; phosphorylated and total forms of YAP, and Goat Anti-Rabbit IgG H&L (Alexa Fluor ® 488) were purchased from Abcam.
Cell viability assay
Cells were cultured in 96-well plates overnight and then exposed to CTP (0–10 µM) for 24 hrs. The cell viabilities were determined by MTT assay. Briefly, 10 μL of MTT was added to each well. After 4 hrs of incubation, the medium was removed and 200 μL of dimethylsulfoxide was added to dissolve the crystal formazan dye. The absorbance was measured at 570 nm on an enzyme-linked immunosorbent assay reader.
BrdU assay
BrdU signaling was assayed using a BrdU Cell Proliferation Assay Kit (Abcam, ab126556). Briefly, cells were cultured in 96-well plates and exposed to CTP (0.1 and 0.2 µM) for 24 hrs. Subsequently, 10 μM BrdU was added to each well, and samples were incubated for 12 h at 37°C. BrdU signaling was determined by measuring the absorbance at 450 nm.
Western blot
Cells were harvested and lysed with radioimmunoprecipitation assay buffer. Protein concentrations were quantified by using a BCA protein assay kit (Thermo, 23227). Proteins were size fractionated by 10% SDS-PAGE and transferred to polyvinylidene fluoride membranes. After blocking, the membranes were incubated with primary antibodies at 4°C overnight and then incubated with secondary antibodies at room temperature for 2 hrs. Target proteins were examined using enhanced chemiluminescence reagents (WBKLS0100; Merck Millipore).
RT-PCR analysis
Total RNA was isolated using TRIzol reagent (Thermo Fisher Scientific). cDNA was prepared from 1 mg of total RNA, using reverse transcriptase and random hexamers from RevertAid First Strand cDNA Synthesis Kit (Thermo Fisher Scientific). Quantitative real-time PCR was performed by mixing cDNA, gene-specific primers and IQ SYRB Green Supermix and detected by Mx3005P QPCR System (Agilent). The 2-pCt method was used to quantitate fold changes by normalizing to GAPDH. Primers for RT-qPCR were as follows: Human CTGF: 5‘-AAAAGTGCATCCGTACTCCCA-3‘ and 5‘-CCGTCGGTACATACTCCACAG-3‘; Human CYR61: 5‘-AGCCTCGCATCCTATACAACC-3‘ and 5‘-TTCTTTCAC-AAGGCGGCACTC-3‘; Human LATS1: 5‘-ACCGCTTC-AAATGTGACTGTGATGCCACCT-3‘ and 5‘-CTTCCTTG-GGCAAGCTTGGCTGATCCTCT-3‘; Human GAPDH: 5‘-GAGCGAGATCCCTCCAAAAT-3‘ and 5‘-GGCTGTTGT-CATACTTCTCATGG-3‘.
Immunofluorescence
Cells were fixed with 4% paraformaldehyde at room temperature for 30 mins, washed 3 times with PBS and then incubated with 0.1% Triton X-100 for permeabilization; non-specific receptors on cells were blocked for 30 mins with 5% BSA. Cells were stained with anti-YAP antibody (Abcam, ab52771) overnight at 4°C and then incubated with Alexa Fluor 488–conjugated goat anti-rabbit IgG (Abcam, ab150077) at room temperature for 1 hr. Nuclei were stained with DAPI. Images were captured using confocal laser scanning microscopy (Olympus, FV1000).
Animal models
All animal experiments complied with the National Institutes of Health (USA)’s Guide for the Care and Use of Laboratory Animals (NIH Publication No 8023, revised 1978), and all studies were approved by the Institutional Animal Care and Treatment Committee of Hainan Medical College. HT-29 tumor models were established in BALB/c immunodeficient nude mice at 6–8 weeks of age. When the tumor volumes reached 100 mm3, mice were randomly divided into 2 groups and each tumor model was injected with CTP (50 mg/kg) or vehicle (DMSO), respectively, through the tail vein once every 2 days. Animals were sacrificed after 3 weeks. Tumor tissues were isolated and frozen in liquid nitrogen or fixed in 10% formalin immediately.
Statistical analysis
Statistical analysis was performed using GraphPad Prism 6.0 (GraphPad Software). Comparisons between the two groups were performed by the Student’s t-test. Statistical significance was defined as *P<0.05, **P<0.01 and ***P<0.001.
Results
Calotropin inhibits the growth of colorectal cancer cells both in vitro and in vivo
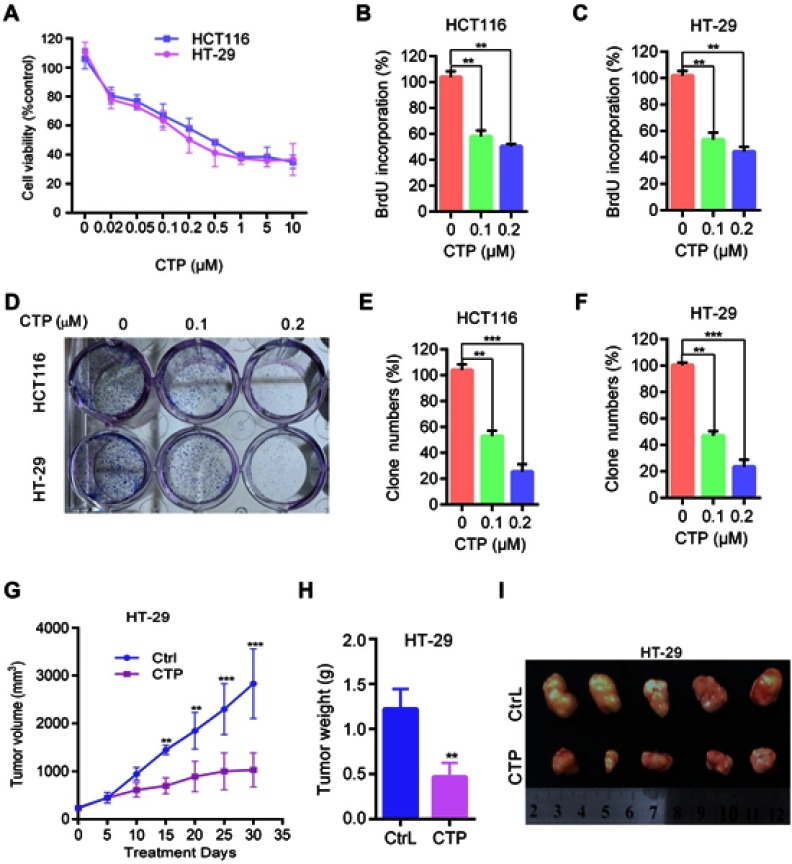
To examine the effect of CTP on colorectal cancer cells, human colorectal cancer cell lines HT-29 and HCT116 were treated with various concentrations of CTP for 24 hrs, followed by MTT assay. As shown in Figure 1A, CTP markedly suppressed colorectal cancer cell proliferation in a dose-dependent manner in both cell lines. Consistently, BrdU assays showed that there were a significantly lower percentage of BrdU-positive cells in CTP-treated cells in a dose-dependent manner (Figure 1B and C). In addition, CTP significantly suppressed cell proliferation in colorectal cancer cells, as shown by reduced colony formation (Figure 1D–F). Collectively, these results demonstrate that CTP inhibits the proliferation of colorectal cancer cells in vitro. To evaluate the effect of CTP on colorectal cancer growth in vivo, BALB/c nude mice were inoculated with HT-29 cells and treated with CTP or vehicle. As shown in Figure 1G, CTP treatment significantly decreased the rate of tumor growth compared to that in the control group. Consistently, tumor weight was reduced in CTP-treated mice compared with those treated with control treatment (Figure 2H). In addition, the size of CTP-treated tumors was much smaller than that of the control group (Figure 1I). Taken together, these data suggest that CTP inhibits the growth of colorectal cancer both in vitro and in vivo.
Figure 1.
Calotropin inhibits cell proliferation in colorectal cancer cells. (A) Cell viability was measured using an MTT assay in HT-29 and HCT116 cells treated with the indicated concentrations of CTP for 24 hrs. (B and C) Cells were treated with the indicated concentrations of CTP for 24 hrs, and the degree to which CTP inhibited cell proliferation was measured using BrdU labeling. (D–F) Cell proliferation was measured by colony formation assay in HT-29 and HCT116 cells treated with the indicated concentrations of CTP and grown for 7 days. (G–I) BALB/c nude mice were inoculated with HT-29 cells and treated with CTP or vehicle. Tumor volumes were measured at indicated time points (G). Tumor weights at time of sacrifice (H). Images of isolated tumors derived from CTP-or vehicle-treated mice (I). **P<0.01, ***P<0.001.
Abbreviations: CTP, calotropin; CrtL, control.
Figure 2.
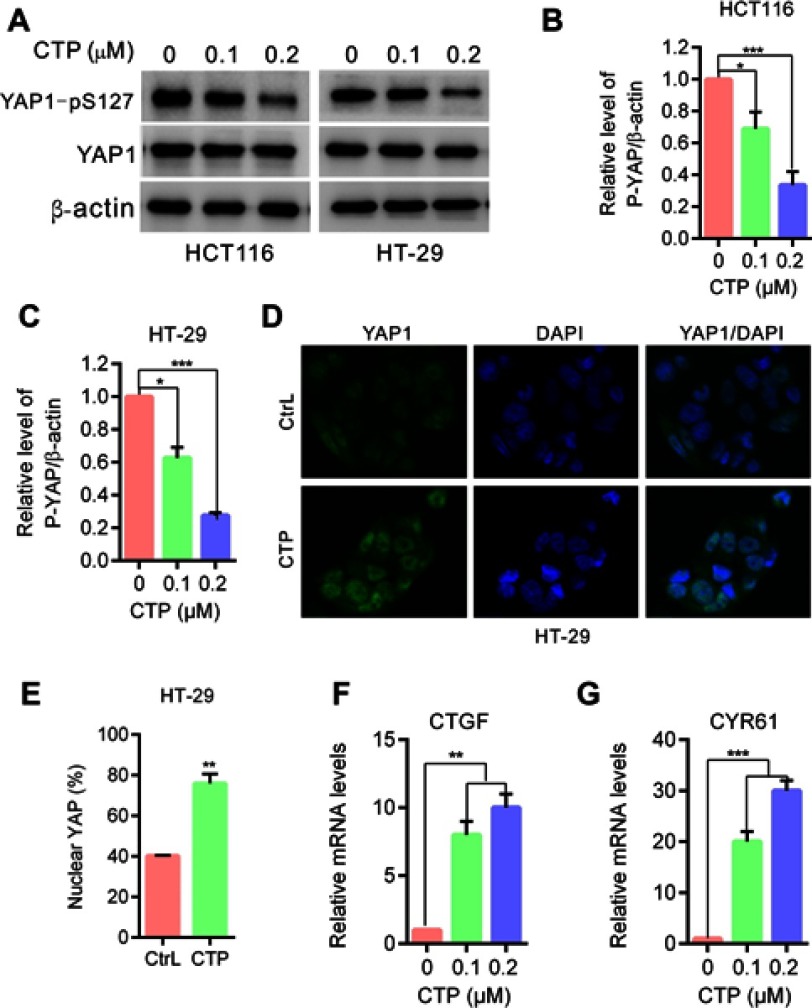
YAP is activated in calotropin-treated colorectal cancer cells. (A) Immunoblot analysis of phosphorylation of YAP (S127) in HT-29 and HCT116 cells treated with the indicated concentrations of CTP for 24 hrs. Total YAP expression served as internal controls. (B and C) ImageJ densitometric analysis of the p-YAP/β-actin ratios from immunoblots. (D) HT-29 cells treated with CTP for 24 hrs,and YAP subcellular localization was determined by immunofluorescence staining for endogenous YAP (green) along with DAPI for DNA (blue). (E) Quantitative analysis of YAP nuclear translocation. (F and G) CTGF and CYR61 mRNA expression in cells treated with the indicated concentrations of CTP for 24 hrs were evaluated by RT-qPCR. *P<0.05, **P<0.01, ***P<0.001.
Abbreviations: CTP, calotropin; CrtL, control.
YAP is activated in calotropin-treated colorectal cancer cells
To address whether the Hippo pathway is involved in CTP-inhibited cell proliferation in colorectal cancer cells, we first detected the phosphorylation status of YAP, a major downstream effector of the Hippo pathway. As shown in Figure 2A–C, CTP treatment promoted YAP dephosphorylation at S127 in colorectal cancer cells. As expected, CTP treatment induced YAP nuclear localization in colorectal cancer cells (Figure 2D and E). After translocation into the nucleus, YAP interacts with the TEAD family of transcription factors and activates of downstream genes (eg CTGF, CRY61).9,12 Consistent with this observation, CTP treatment strongly induced the expression of CTGF and CRY61, as measured by quantitative RT-PCR (Figure 2F and G). Collectively, these data demonstrated that CTP enhances YAP dephosphorylation and nuclear localization and promotes YAP target genes expression in colorectal cancer cells.
Calotropin promotes degradation of LATS1
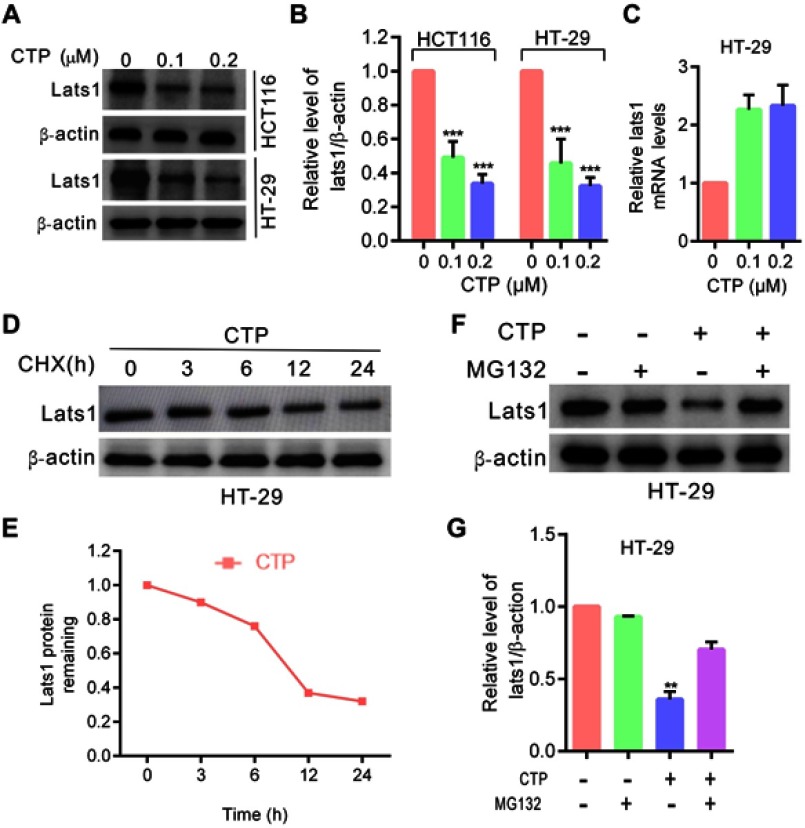
Although YAP transcriptional activity is regulated by several mechanisms, as a key downstream effector of the Hippo pathway, YAP is regulated directly by LATS1/2 kinases. Phosphorylation by LATS1/2 at S127 sequesters YAP in the cytoplasm by promoting its binding to 14–3-3 proteins.7,13,14 Therefore, we investigated whether LATS1 kinase is involved in CTP-induced YAP activation in colorectal cancer cells. As shown in Figure 3A and B, LATS1 expression was significantly decreased upon CTP treatment. To determine the mechanism underlying the regulation of LATS1 by CTP, we performed quantitative RT-PCR analysis after 24 hrs of CTP treatment. The results showed that there were no significant changes in mRNA levels of LATS1, suggesting that transcription regulation might not account for the decreased LATS1 expression upon CTP treatment (Figure 3C). Thus, we next examined whether LATS1 was degraded through the ubiquitination/proteasome pathway. As shown in Figure 3D and E,CTP treatment shortened the half-life of endogenous LATS1 protein. Moreover, we demonstrated that downregulation of LATS1 protein caused by CTP was reversed by treating cells with the proteasome inhibitor MG132 (Figure 3F and G). Collectively, these results indicated that CTP promoted LATS1 degradation through the ubiquitination/proteasome pathway.
Figure 3.
Calotropin promotes degradation of LATS1. (A) Immunoblot analysis of LATS1 in HT-29 and HCT1160 cells treated with CTP for 24 hrs. (B) ImageJ densitometric analysis of the LATS1/β-actin ratios from immunoblots. (C) LATS1 mRNA expression in cells treated with the indicated concentrations of CTP for 24 hrs was evaluated by RT-qPCR. (D) Immunoblot analysis of LATS1 in cells treated with CTP for 24 hrs in the presence of cycloheximide (CHX). (E) At each time point, the intensity of LATS1 was normalized to the intensity of β-actin first and then to the value at the 0-hr time point. (F) Immunoblot analysis of LATS1 in cells treated with CTP for 24 hrs in the presence and absence of MG132. (G) ImageJ densitometric analysis of the LATS1/β-actin ratios from immunoblots. **P<0.01, ***P<0.001.
Abbreviation: CTP, calotropin.
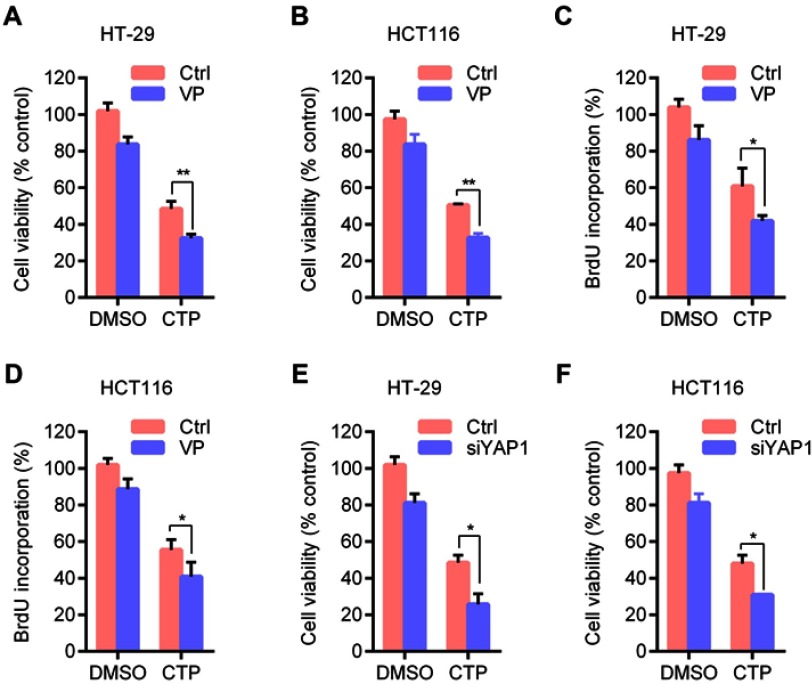
Inhibition of YAP activity enhances calotropin-inhibited cell proliferation in colorectal cancer cells
To determine whether YAP is involved in CTP-mediated inhibition of cell proliferation, we performed MTT and BrdU assays to detect cell viability and cell proliferation upon treatment with YAP inhibitor. As shown in Figure 4A and B, inhibition of YAP by Verteporfin (VP), a YAP inhibitor, resulted in significant lower cell viability than CTP treatment alone. Consistently, similar results were observed by BrdU assay (Figure 4C and D). Furthermore, knockdown of YAP by using siRNAs enhanced TCP-inhibited cell proliferation (Figure 4E and F). These findings suggested that CTP-induced YAP activation plays a protective role against CTP-induced inhibition of cell proliferation in colorectal cancer.
Figure 4.
Inhibition of YAP activity enhances calotropin-induced anti-proliferative effect. (A and B) Cell viability was measured by MTT assay in cells treated with DMSO or CTP for 24 hrs in the presence and absence of Verteporfin (VP). (C and D) Cell proliferation was measured using BrdU labeling in cells treated with DMSO or CTP for 24 hrs in the presence and absence of VP. (E and F) Cells were transfected with siYAP or control for 48 hrs and then treated with CTP for 24 hrs, and cell viability was measured by MTT assay. *P<0.05, **P<0.01.
Abbreviations: CTP, calotropin; DMSO, dimethyl sulfoxide.
Discussion
Natural products have been the major resource of compounds of value to medicine. CTP is one of the natural products isolated from Calotropis gigantea that exhibits potential anti-cancer activities. In this study, we demonstrated that CTP markedly suppresses cell proliferation in colorectal cancer cells. We also showed that CTP induces YAP activation through downregulation of LATS1, and Inhibition of YAP activity enhances CTP-inhibited cell proliferation in colorectal cancer cells, suggesting that YAP plays a protective role in suppressing CTP-inhibited cell proliferation.
Dysregulation of Hippo pathway componentsis often correlated with aberrant cell growth and tumor formation of human cancers.15,16 As a downstream effector, YAP plays a key role in Hippo pathway to control cell proliferation. Elevated expression and nuclear localization of YAP have been frequently observed in human cancers such as lung cancers, colon cancers and breast cancers,6,10 where it promotes cell proliferation and tumor growth, acting as a powerful tumor promoter. But in some circumstances, YAP restricts cell expansion and play a possible suppressor function.17 In this study, we demonstrated that CTP activates YAP, and inhibition of YAP activity enhances CTP-inhibited cell proliferation in colorectal cancer cells, suggesting that YAP promotes cell proliferation against CTP agent pressure.
Although YAP transcriptional activity is regulated by several mechanisms, in most tumor cell types, the activity of YAP is mainly regulated by the Hippo pathway. In the Hippo pathway, LATS1/2 directly phosphorylates YAP for its cytoplasmic retention and proteasomal degradation, and depletion of LATS1/2 promotes YAP dephosphorylation and nuclear localization.7,8 Consistent with these observations, we demonstrated that CTP induces YAP activation by promoting LATS1 degradation via the ubiquitination/proteasome pathway. Downregulation of LATS1 enhances YAP dephosphorylation and nuclear localization and stimulates YAP target genes expression in colorectal cancer cells.
Post-translational modifications of LATS kinases are involved in several mechanisms. Differential phosphorylation of LATS1/2 affects their stability. LATS1 phosphorylation by NUAK1 reduces its protein levels, whereas KIBRA stabilizes LATS2 by augmenting its phosphorylation.18,19 Moreover, both LATS kinases are clients of the molecular chaperone HSP90 and inhibition of HSP90 reduces LATS1/2 stabilization.20 Otherwise, LATS protein stability is regulated also through ubiquitination by a number of E3 ligases.21,22 But in this study, detailed mechanism on CTP causing LATS1 destabilization needs further study.
Conclusion
We demonstrated that CTP inhibits the proliferation of colorectal cancer cells both in vitro and in vivo. We showed that CTP induces YAP activation through downregulation of LATS1, and inhibition of YAP activity enhances CTP-inhibited cell proliferation in colorectal cancer cells. Our findings suggest that CTP is a potential anticancer agent against colorectal cancer, and the combined use of YAP inhibitors may be a promising strategy for CTP-mediated anticancer therapy.
Acknowledgments
This work was funded by the National Natural Science Foundation of China (81860429) and Natural Science Foundation of Hainan Province (817145).
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Mo EP, Zhang RR, Xu J, et al. Calotropin from Asclepias curasavica induces cell cycle arrest and apoptosis in cisplatin-resistant lung cancer cells. Biochem Biophys Res Commun. 2016;478(2):710–715. doi: 10.1016/j.bbrc.2016.08.011 [DOI] [PubMed] [Google Scholar]
- 2.Tian L, Xie XH, Zhu ZH. Calotropin regulates the apoptosis of nonsmall cell cancer by regulating the cytotoxic T lymphocyte associated antigen 4mediated TGFbeta/ERK signaling pathway. Mol Med Rep. 2018;17(6):7683–7691. doi: 10.3892/mmr.2018.8853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang SC, Lu MC, Chen HL, et al. Cytotoxicity of calotropin is through caspase activation and downregulation of anti-apoptotic proteins in K562 cells. Cell Biol Int. 2009;33(12):1230–1236. doi: 10.1016/j.cellbi.2009.08.013 [DOI] [PubMed] [Google Scholar]
- 4.Park HY, Toume K, Arai MA, Sadhu SK, Ahmed F, Ishibashi M. Calotropin: a cardenolide from calotropis gigantea that inhibits Wnt signaling by increasing casein kinase 1alpha in colon cancer cells. Chembiochem. 2014;15(6):872–878. doi: 10.1002/cbic.201300786 [DOI] [PubMed] [Google Scholar]
- 5.Yu FX, Zhao B, Guan KL. Hippo pathway in organ size control, tissue homeostasis, and cancer. Cell. 2015;163(4):811–828. doi: 10.1016/j.cell.2015.10.044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zanconato F, Cordenonsi M, Piccolo S. YAP/TAZ at the roots of cancer. Cancer Cell. 2016;29(6):783–803. doi: 10.1016/j.ccell.2016.05.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Meng Z, Moroishi T, Guan KL. Mechanisms of Hippo pathway regulation. Genes Dev. 2016;30(1):1–17. doi: 10.1101/gad.274027.115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hao Y, Chun A, Cheung K, Rashidi B, Yang X. Tumor suppressor LATS1 is a negative regulator of oncogene YAP. J Biol Chem. 2008;283(9):5496–5509. doi: 10.1074/jbc.M709037200 [DOI] [PubMed] [Google Scholar]
- 9.Zhao B, Ye X, Yu J, et al. TEAD mediates YAP-dependent gene induction and growth control. Genes Dev. 2008;22(14):1962–1971. doi: 10.1101/gad.1664408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Avruch J, Zhou D, Bardeesy N. YAP oncogene overexpression supercharges colon cancer proliferation. Cell Cycle. 2012;11(6):1090–1096. doi: 10.4161/cc.11.6.19453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cottini F, Hideshima T, Xu C, et al. Rescue of Hippo coactivator YAP1 triggers DNA damage-induced apoptosis in hematological cancers. Nat Med. 2014;20(6):599–606. doi: 10.1038/nm.3562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhao B, Li L, Lei Q, Guan KL. The Hippo-YAP pathway in organ size control and tumorigenesis: an updated version. Genes Dev. 2010;24(9):862–874. doi: 10.1101/gad.1909210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Moon S, Kim W, Kim S, et al. Phosphorylation by NLK inhibits YAP-14-3-3-interactions and induces its nuclear localization. EMBO Rep. 2017;18(1):61–71. doi: 10.15252/embr.201642683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dupont S, Morsut L, Aragona M, et al. Role of YAP/TAZ in mechanotransduction. Nature. 2011;474(7350):179–183. doi: 10.1038/nature10137 [DOI] [PubMed] [Google Scholar]
- 15.Harvey KF, Zhang X, Thomas DM. The Hippo pathway and human cancer. Nat Rev Cancer. 2013;13(4):246–257. doi: 10.1038/nrc3458 [DOI] [PubMed] [Google Scholar]
- 16.Yu FX, Luo J, Mo JS, et al. Mutant Gq/11 promote uveal melanoma tumorigenesis by activating YAP. Cancer Cell. 2014;25(6):822–830. doi: 10.1016/j.ccr.2014.04.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Barry ER, Morikawa T, Butler BL, et al. Restriction of intestinal stem cell expansion and the regenerative response by YAP. Nature. 2013;493(7430):106–110. doi: 10.1038/nature11693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Humbert N, Navaratnam N, Augert A, et al. Regulation of ploidy and senescence by the AMPK-related kinase NUAK1. Embo J. 2010;29(2):376–386. doi: 10.1038/emboj.2009.342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Xiao L, Chen Y, Ji M, Dong J. KIBRA regulates Hippo signaling activity via interactions with large tumor suppressor kinases. J Biol Chem. 2011;286(10):7788–7796. doi:10.1074/jbc.M110.173468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Huntoon CJ, Nye MD, Geng L, et al. Heat shock protein 90 inhibition depletes LATS1 and LATS2, two regulators of the mammalian hippo tumor suppressor pathway. Cancer Res. 2010;70(21):8642–8650. doi: 10.1158/0008-5472.CAN-10-1345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Furth N, Aylon Y. The LATS1 and LATS2 tumor suppressors: beyond the Hippo pathway. Cell Death Differ. 2017;24(9):1488–1501. doi: 10.1038/cdd.2017.99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Salah Z, Melino G, Aqeilan RI. Negative regulation of the Hippo pathway by E3 ubiquitin ligase ITCH is sufficient to promote tumorigenicity. Cancer Res. 2011;71(5):2010–2020. doi: 10.1158/0008-5472.CAN-10-3516 [DOI] [PubMed] [Google Scholar]