Dear Editor:
Infants born prematurely or who suffer a global hypoxic ischemic encephalopathy (HIE) are at high risk for motor problems, which manifest as feeding delays during their hospital admission. Oromotor dyscoordination is common in these infants, and feeding difficulty is the primary reason for delayed discharge [1]. Many infants who do not master this motor skill before term age (40–42 weeks gestation) will receive a gastrostomy tube (G-tube) for direct gastric feeding. Furthermore, feeding difficulties in infants are associated with later language delays, even in the absence of gross motor impairment [2].
Currently, the only treatment to improve oromotor skills during feeding consists of occupational therapy working with the infant to encourage safe feeding behavior. In other brain injuries, pairing brain stimulation with rehabilitative motor training has shown promise to stimulate activity-dependent neuroplasticity and remodeling of motor cortex [3–6]. In animal models of CNS injury and adults after stroke, vagus nerve stimulation via implanted cervical electrodes (VNS) improves function when paired with motor activity [3,6–8]. Recently, transcutaneous stimulation of the auricular branch of the vagus nerve (taVNS) has emerged as a non-invasive form of VNS with minimal side effects [3,6,9].
Our premise is that in babies at high risk for motor problems with feeding delay, brain stimulation via taVNS delivered simultaneously with active sucking from a bottle will enhance cortical plasticity involved in learning oromotor skills, leading to better oral feeding. As a first step, we sought to determine feasibility, and refine and optimize the protocol for delivering taVNS stimulation in neonates. We describe 5 patients who have successfully undergone taVNS paired feedings in a phase 0 study.
1. Study design
Institutional Review Board of the Medical University of South Carolina approved this phase 0 study. Written informed consent was obtained from parents prior to enrollment. An independent safety committee monitored adverse events. Inclusion criteria: Clinically stable infants, on minimal respiratory support (nasal cannula, or room air), who are either premature >33weeks gestational age (GA), or ≥35weeks with HIE, and working on oral feeding. Exclusion criteria: Infants who are unstable requiring respiratory support, or have major congenital anomalies or cardiomyopathy.
2. taVNS stimulation protocol
Participants received active taVNS via custom-made electrodes placed on the left tragus. Stimulation was delivered by a Digitimer DS7AH (Fig. 1). We determined the Perceptual Threshold (PT) at rest prior to sessions 1 & 6 starting with 0.1mA, frequency 25Hz, pulse width 500μs. We increased stimulation in 0.1mA increments until the PT was achieved by observation of the infant’s facial expression, fidgety movements, or Neonatal pain scale score (NIPS). We then decreased stimulation by 0.1mA below the PT and delivered taVNS while the infant was actively sucking from the bottle, to ensure coupling of motor activity and stimulation. We stopped stimulation when the infant stopped sucking, or at the end of a 2-minute train of successful sucking (due to concerns about skin damage from the stimulation). We conducted taVNS-paired feeding once a day, up to 30 minutes, for 10–22 days. Software recorded the actual amount and duration of stimulus during each session up to the maximum of 30-minute feeding session. Physiological data including heart rate (HR) was collected by routine cardiorespiratory monitoring. The NIPS score was recorded at start, middle and end of taVNS session.
Fig. 1. TaVNS electrode positioning on left tragus, and equipment setup.
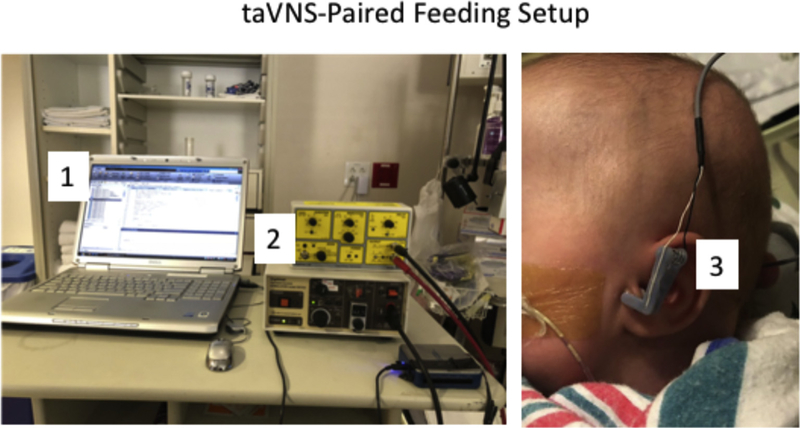
A computerized script (1) is used to communicate with a constant current stimulator (2). Stimulator delivers taVNS via custom ear electrodes (3) attached to the left ear of the neonate.
3. Demographics
Four patients were born prematurely (25–27 weeks GA, 665–990 grams), and one was born at 35 weeks GA and suffered HIE. Infants were >42 weeks GA when enrolled (mean: 47.2 weeks GA; sd: 5.86; range: 42–53 weeks GA), and were candidates for G-tube placement. Full oral feeds were defined as ≥ 130 cc/kg/day, with adequate weight gain for discharge.
4. HR reduction with taVNS
taVNS produced a mean decrease of 20 ± 9 bpm or 13 ± 5% drop in HR within 20 ± 10 seconds after starting stimulation with feeding (as also seen in healthy adult volunteers [9]). The effect of taVNS on heart rate was transient, within the normal limits of neonatal heart rate fluctuations, and not of clinically significant magnitude, but was reproducible in the 5 enrolled infants. The heart rate change with onset of stimulation was so reproducible that we adjusted positioning of the electrodes when the heart rate decrease was not observed, to ensure target attainment after burping or repositioning the infant. Our early experience in these neonates suggests that heart rate changes may be used to monitor taVNS stimulation and ensures CNS target engagement in terms of earlobe position and contact, and the individual dose [9,10].
5. Outcome
The infants had attempted p.o. feeds for 30–101 days prior to taVNS. Daily feeding volumes at enrollment were 35–64% of total feeds for the 7 days prior to treatment. Patients received 10–25 taVNS sessions. One infant received 25 treatments at parents’ request. In these infants who had feeding difficulty due to delayed initiation of feeds from illness or prematurity, 4 of the 5 infants were able to achieve full oral feedings and weight gain adequate for discharge. These 4 attained full oral feeds within 7–23 days from the start of taVNS-paired feeding. Three infants avoided G-tubes. One attained full oral feedings for 4 days but received a G-tube during another surgery for hernia repair, per mother’s request, to be used in case feeding deteriorated in the post-operative period.
6. Safety
We stimulated a mean of 14±6min during active sucking. Using subthreshold taVNS (mean 0.84 ± 0.17mA), there were no adverse events of bradycardia (HR < 80 bpm), worsening of swallowing, hoarseness, earlobe skin irritation or burns, or elevation ofneonatal infant pain scale scores.
7. Conclusions
taVNS paired with feeding in newborns is feasible with promising preliminary results. Further refinements and formal doubleblind testing are needed to determine if our approach enhances learning and mastery of this important motor task. To speculate this early is not wise, however, preliminary data suggests that treatment efficacy may be based on the infant’s baseline number of feeds by mouth and may not necessarily be related to postnatal age. If taVNS paired-feeding improves oromotor skills, it may also be useful in other forms of rehabilitation in newborns, infants, and children.
Acknowledgments
Research reported in this publication was supported by funding from the National Institutes of Health National Center of Neuromodulation for Rehabilitation, NIH/NICHD Grant Number P2CHD086844 which was awarded to the Medical University of South Carolina. The contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH or NICHD.
Footnotes
8. Conflicts of interest
The Medical University of South Carolina (MUSC) has filed a provisional patent, in the name of some of the authors (BWB, DDJ, MSG, WHD, PMS, MD), for an automated system for delivering the taVNS described in this letter. We further confirm the order of authors listed in this manuscript has been approved by all of us and that the work described in this manuscript is not currently under consideration for publication in another journal.
Contributor Information
Bashar W. Badran, Department of Psychiatry, Brain Stimulation Laboratory, Medical University of South Carolina, Charleston, SC, USA; Department of Biomedical Engineering, City College of New York, NewYork, NY, USA; US Army Research Laboratory, Aberdeen Proving Ground, MD, USA.
Dorothea D. Jenkins, Department of Pediatrics, Medical University of South Carolina, Charleston SC, USA.
William H. DeVries, Department of Psychiatry, Brain Stimulation Laboratory, Medical University of South Carolina, Charleston, SC, USA
Morgan Dancy, Department of Psychiatry, Brain Stimulation Laboratory, Medical University of South Carolina, Charleston, SC, USA.
Philipp M. Summers, Department of Psychiatry, Brain Stimulation Laboratory, Medical University of South Carolina, Charleston, SC, USA
Georgia M. Mappin, Department of Psychiatry, Brain Stimulation Laboratory, Medical University of South Carolina, Charleston, SC, USA
Henry Bernstein, Department of Biomedical Engineering, City College of New York, New York, NY, USA.
Marom Bikson, Department of Biomedical Engineering, City College of New York, New York, NY, USA.
Patricia Coker-Bolt, Division of Occupational Therapy, College of Health Professions, Medical University of South Carolina, Charleston, SC, USA.
Mark S. George, Brain Stimulation Laboratory, Department of Psychiatry, Medical University of South Carolina, Charleston, SC, USA Department of Neurology, Medical University of South Carolina, Charleston, SC, USA; Department of Neurology, Medical University of South Carolina, Charleston, SC, USA.
References
- [1].Adamkin DH. Feeding problems in the late preterm infant. Clin Perinatol 2006;33:831–7. abstract ix. [DOI] [PubMed] [Google Scholar]
- [2].Adams-Chapman I, Bann CM, Vaucher YE, Stoll BJ. Eunice Kennedy Shriver National Institute of child H and human development neonatal research N. Association between feeding difficulties and language delay in preterm infants using bayley scales of infant development-third edition. J Pediatr 2013;163(680–5):e1–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Dawson J, Pierce D, Dixit A, et al. Safety, feasibility, and efficacy of vagus nerve stimulation paired with upper-limb rehabilitation after ischemic stroke. Stroke 2016;47:143–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Pruitt DT, Schmid AN, Kim LJ, et al. Vagus nerve stimulation delivered with motor training enhances recovery of function after traumatic brain injury. J Neurotrauma 2016;33:871–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Porter BA, Khodaparast N, Fayyaz T, et al. Repeatedly pairing vagus nerve stimulation with a movement reorganizes primary motor cortex. Cerebr Cortex 2012;22:2365–74. [DOI] [PubMed] [Google Scholar]
- [6].Redgrave JN, Moore L, Oyekunle T, et al. Transcutaneous auricular vagus nerve stimulation with concurrent upper limb repetitive task practice for poststroke motorrecovery: a pilot study.JStroke Cerebrovasc Dis 2018. (in press) , 10.1016/j.jstrokecerebrovasdis.2018.02.056. [DOI] [PubMed] [Google Scholar]
- [7].Khodaparast N, Hays SA, Sloan AM, et al. Vagus nerve stimulation delivered during motor rehabilitation improves recovery in a rat model of stroke. Neurorehabilitation Neural Repair 2014;28:698–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Khodaparast N, Kilgard MP, Casavant R, et al. Vagus nerve stimulation during rehabilitative training improves forelimb recovery after chronic ischemic stroke in rats. Neurorehabilitation Neural Repair 2016;30:676–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Badran BW, Mithoefer OJ, Summer CE, et al. Short trains of transcutaneous auricular vagus nerve stimulation (taVNS) have parameter-specific effects on heart rate. Brain Stimul 2018. 1935–861X, (in press), 10.1016/j.brs.2018.04.004 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Badran BW, Dowdle LT, Mithoefer OJ, et al. Neurophysiologic effects of trans-cutaneous auricular vagus nerve stimulation (taVNS) via electrical stimulation of the tragus: a concurrent taVNS/fMRl study and review. Brain Stimul 2018;11:492–500. [DOI] [PMC free article] [PubMed] [Google Scholar]


