Abstract
Background:
Optimal parameters of transcutaneous auricular vagus nerve stimulation (taVNS) are still undetermined. Given the vagus nerve’s role in regulating heart rate (HR), it is important to determine safety and HR effects of various taVNS parameters.
Objective:
We conducted two sequential trials to systematically test the effects of various taVNS parameters on HR.
Methods:
15 healthy individuals participated in the initial two-visit, crossover exploratory trial, receiving either tragus (active) or earlobe (control) stimulation each visit. Nine stimulation blocks of varying parameters (pulse width: 100 μs, 200 μs, 500 μs; frequency: 1 Hz, 10 Hz, 25 Hz) were administered each visit. HR was recorded and analyzed for stimulation-induced changes. Using similar methods and the two best parameters from trial 1 (500μs 10 Hz and 500μs 25 Hz), 20 healthy individuals then participated in a follow-up confirmatory study.
Results:
Trial 1- There was no overall effect of the nine conditions on HR during stimulation. However multivariate analysis revealed two parameters that significantly decreased HR during active stimulation compared to control (500μs 10 Hz and 500μs 25 Hz; p < 0.01). Additionally, active taVNS significantly attenuated overall sympathetic HR rebound (post-stimulation) compared to control (p < 0.001). Trial 2- For these two conditions, active taVNS significantly decreased HR compared to control (p = 0.02), with the strongest effects at 500μs 10 Hz (p = 0.032).
Conclusion:
These studies suggest that 60s blocks of tragus stimulation are safe, and some specific parameters modulate HR. Of the nine parameters studied, 500μs 10 Hz induced the greatest HR effects.
Keywords: Transcutaneous auricular vagus nerve, stimulation (taVNS), Ear stimulation, Autonomic nervous system, Heart rate, Vagus nerve stimulation
Introduction
The vagus nerve (CN X) is a mixed sensory and motor nerve that has efferent projections travelling throughout the abdomen, targeting nearly every major organ and is highly involved in the parasympathetic nervous system [1,2]. This parasympathetic response is centrally initiated in the periventricular nucleus (PVN) of the hypothalamus [3], sending efferent projections to the brainstem and heart via CN X, releasing acetylcholine (Ach) and slowing heart rate (HR). This HR effect was best demonstrated in the late 1800s by Otto Loewi who demonstrated bradycardia in a frog model via vagus nerve stimulation (VNS) of a frog [4] and since has been replicated in various animal studies [5,6]. Interestingly, closed-loop HR maintenance has also been described in a VNS pig model [7]. One can experience this vagal-mediated decrease in HR in humans through gentle rubbing on the vagus nerve via the carotid sinus, a technique known as carotid massage [8,9].
Clinically, VNS is FDA-approved for epilepsy, treatment-resistant depression, and morbid obesity. A major concern during the development of therapeutic cervical VNS was the earlier described, and potentially unsafe, parasympathetic and cardiac effects. Clinical trials monitored for adverse events throughout the implantation and treatment course, ultimately concluding VNS is a safe modality [10,11]. There have been several trials exploring the direct effect of VNS on HR in epileptic patients, revealing no unsafe stimulation-induced effects [12–16]. Contrary to the null findings of these early studies, a small-sample study by Frei and Osorio demonstrated HR decreases immediately upon initiation of VNS, followed by HR rebound spike upon termination [17]. Their findings also reveal variable HR effects between patients, but consistent within patients. Matheny et al. also described an induced cardiac arrest from non-therapeutic VNS during revascularization surgery [18]. These findings suggest that in-vivo electrical stimulation of the human vagus nerve may activate the parasympathetic nervous system and modulates HR.
Transcutaneous auricular vagus nerve stimulation (taVNS) is a relatively new form of noninvasive brain stimulation, which may replicate conventional VNS by stimulating the auricular branch of the vagus nerve (ABVN) [19]. If ABVN stimulation enters the main vagus bundle, evaluating safety is important, as the para sympathetic effects of the vagus nerve may cause inadvertent adverse events. There have been no prospective studies of the HR effects of taVNS, nor whether these effects are parameter specific.
In order to demonstrate the safety profile of taVNS, we conducted two trials exploring the HR effects of various stimulation parameters. The initial trial conducted was not an exhaustive exploration of parameters but rather a reasonable combination of high and low settings based on prior cervical VNS trials [20,21]. We hypothesized that parameters of higher energy density (larger pulse width, higher frequency) would be more effective in changing heart rate than would lower energy combinations, similar to prior cervically implanted VNS trials [22]. These energy-dense parameter combinations would also likely pose the highest safety risk. We then took the two most promising candidate parameters from trial 1 (in terms of immediate HR decrease) and conducted a controlled trial of the two optimal parameters against each other and against a control to definitively determine, of the nine parameters explored in trial 1, which taVNS parameter combination most effectively modulated the parasympathetic nervous system as measured by HR. Heart rate changes are thus a marker of vagus nerve engagement or activity.
Methods
Two sequential trials are included in this report. The first (trial 1) was a two-visit, controlled, crossover trial in which we tested the HR effects of nine combinations of taVNS parameters. The second (trial 2) was also a two-visit, controlled, crossover trial conducted in a separate, taVNS naive cohort of participants in which we tested the HR effects of two optimal parameters identified during trial 1. Both trials had similar methods, each consisting of two separate 1-h experimental visits (active or control on the first visit, opposite condition on second visit) Fig. 1a. Safety was reported by minor and major adverse event monitoring. The primary behavioral outcome measure was the immediate change in HR during stimulation.
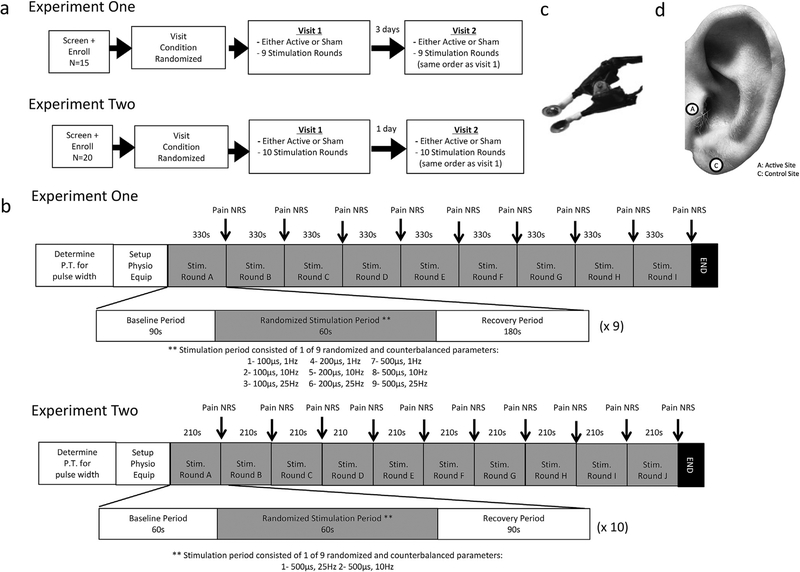
Fig. 1. Study design.
a) Participant flow diagram of study design. b) Timeline of experimental visit demonstrating stimulation periods and parameters. c) Image of custom ear stimulation electrodes used. d) Ear stimulation targets used; both trials utilized similar targets.
Both studies were conducted at the Medical University of South Carolina (MUSC) Brain Stimulation Lab, approved by MUSC Institutional Review Board (IRB), and registered on ClinicalTrials.gov (NCT02835885).
Participants and inclusion criteria
Participants were enrolled in either study after passing a taVNS screening form which listed the inclusion criteria as the following: Age 18–45, no personal or family history of seizure, mood, or cardiovascular disorders, no facial or ear pain, no recent ear trauma, no metal implants including pacemakers, not pregnant, no dependence on alcohol or recent illicit drug use, and not taking any pharmacological agents known to increase seizure risk (bupropion, neuroleptics, albuterol, theophylline, antidepressants, thyroid medications, or stimulants).
Trial 1
Fifteen healthy adults (7 female) were enrolled after meeting inclusion criteria. Participants attended two 1-h visits (randomized active, control) in which they lay supine with their neck and head elevated in a comfortable position. They were instructed to stay awake and maintain a still, comfortable position (no task was given, the only instruction was not to communicate with the research observer unless asked for verbal ratings) while receiving either active or control stimulation delivered to their left ear for 9 different stimulation rounds (one round per parameter). Each round consisted of a baseline period of 90s, stimulation period of 60s, and recovery period of 180s. The order of the different stimulation parameters was identical within both subject visits (active, control) for each individual, but were randomized across the group (Fig. 1b).
Trial 2
Twenty healthy adults (10 female) were enrolled after signing consent and meeting inclusion criteria. Participants attended two 1-h experimental visits (randomized active, control) with methods identical to trial 1, testing only two parameter combinations. Each round consisted of a baseline period (60s), stimulation period (60s), and recovery period (90s), 210s total (Fig. 1b).
taVNS stimulation system and electrodes
The stimulation system was custom developed at the MUSC Brain Stimulation Laboratory. It consists of a commercially avail able, FDA-cleared Digitimer DS7A constant current stimulator (Digitimer Ltd., USA) used with custom-built electrodes (built by BWB and AWB, Fig. 1c). Electrodes had a stimulation surface diameter of 1 cm, and Ten20 conductive paste (Weaver and Company, USA) was used to deliver stimulation to ear targets. Stimulation targets were cleaned with alcohol swabs (70% isopropyl alcohol) to ensure surface oils were removed and decrease skin resistance.
Ear stimulation targets
Both trials employed identical stimulation targets (See Fig. 1d). The active condition was direct electrical stimulation delivered to the inner side of the left tragus (anode in the ear canal, cathode on the surface of the tragus) as based on a review of several prior studies exploring the tragus nerve anatomy [19,23], tragus-evoked potentials [24–26], auricular acupuncture trials [27,28], and a concurrent taVNS/fMRI trial [29].
The left earlobe, used as control, has minimal ABVN innervation [19]. The control stimulation condition received identical stimulation parameters as the active condition. We included this condition in order to explore the hypothesized non-vagal effects of ear targets and the effect of simple sensory stimulation. Subjects were not informed which condition they were getting, or which position we thought might have greater vagal effects.
TaVNS parameters
Trial 1
Stimulation parameters varied by pulse width (100 μs, 200 μs, 500 μs) and frequency (1 Hz, 10 Hz, 25 Hz) creating nine different combinations of stimulation parameters. These parameters were chosen to cover a wide range (low to high) of both pulse width and frequency. The stimulation current was delivered at 200% of each participant’s individual perceptual threshold (PT) for each of the three pulse widths investigated in this trial (100 μs, 200 μs, 500 μs) and repeated for each stimulation condition (tragus and earlobe). PT procedure entailed delivering single pulses of stimulation to the target area and obtaining verbal confirmation of stimulation perception while modulating the current intensity via parametric estimation by sequential testing (PEST) method. This allows for determination of the minimum current intensity perceived by the participant at a specific pulse width.
Trial 2
These parameters (500 μs, 10 Hz or 500 μs, 25 Hz) were chosen from trial 1 as the most likely candidates to modulate heart rate. Parameters were randomized and counterbalanced over each of the ten rounds (five rounds of each parameter). Similar to trial 1, the stimulation current (mA) was delivered at 200% of each participant’s PT.
Safety and tolerability reporting
During both trials, participants were monitored for adverse events during each stimulation session regardless of condition – extreme decreases in HR to less than 35 beats per minute (BPM), respiration difficulty, and cardiac arrest, skin discomfort, irritation, headache, facial pain, and dizziness. No major or minor adverse events were reported during either trial.
Participants reported pain ratings of each stimulation parameter using a numerical rating scale (NRS) ranging from 0 to 10 after each of the nine stimulation parameters. “0” was used as the lowest rating for no sensation perceived; “10” was the highest pain rating for extreme, intolerable pain. Participants were able to use 0.5 increments with a rating of 1 representing the lowest rating where stimulation is felt with no pain.
HR analysis and statistics
Trial 1
HR was recorded using a Thought Technology system (Thought Technology Ltd), which measured HR using a blood volume pulse (BVP) sensor worn on the right index finger. BioGraph Infinity Software was used for both online safety monitoring as well as offline analysis. All HR was down sampled to 8 Hz and exported to be analyzed in IBM SPSS Statistics version 23 (IBM Corp, USA).
Trial 2
HR was recorded using a 2-channel Biopac ECG system (Biopac Systems Inc, USA), which measured HR using electrocardiogram electrodes attached to the subject’s chest. AcqKnowledge 4.1 soft ware was used for both online safety monitoring as well as offline analysis. All HR was consolidated into 5sec epoch bins and exported to be analyzed in IBM SPSS Statistics version 23 (IBM Corp, USA). Participant 20 of trial 2 had excessive artifact in stimulation round C of the control visit, which was excluded from analysis. The remaining 9 stimulation rounds for this participant were kept in the analysis.
Analysis for both trials was conducted in 5-s bins. There were 12 bins for stimulation period (totaling 60s); 12 bins for recovery (totaling 60s). The baseline period used was the final 5 s before stimulation started. Change scores for the stimulation period were calculated as the difference in HR in BPM during stimulation bin and baseline. Change scores for the recovery period were calculated from the final stimulation 5-s bin (bin 12).
Pre-processing of HR data was blindly conducted by an investigator who did not collect the primary data. This served as a quality control of the data and removal of any movement or electrical artifacts. Following processing, three 2-way repeated measures ANOVA statistical analysis were conducted to determine: 1) the overall effect of stimulation condition (active vs control); 2) the immediate change in HR during the stimulation period; 3) the change in HR during the recovery period. Partial eta-squared was used to determine effect sizes.
Results
Trial 1
Participants, perceptual thresholds (PT), and stimulation current
15 (7 female) healthy, right-handed individuals (mean age 26.5 ± 4.99 SD) were included in this study. All participants completed both visits without any dropout. Perceptual thresholds (PT) varied by stimulation site and pulse width (Table 1). The cur rent at which taVNS was delivered was a scale multiplier of the PT (200%). Mean stimulation currents were as follow (mean ± SD mA): 100 μs (tragus- 9.28 ± 2.56; earlobe 6.57 ± 1.83) 200 μs (tragus-5.32 ± 1.60; earlobe 3.64 ± 1.26) 500 μs (tragus- 3.0 ± 0.93; earlobe 1.97 ± 0.70). It was also determined that tragus stimulation cur rents were higher than earlobe perceptual thresholds for each pulse width (paired t-test; 100 μs: t (14) = 3.864, p = 0.002; 200 μs: t (14) = 3.506, p = 0.003; 500 μs: t (14) = 3.797, p = 0.002).
Table 1.
Trial 1 perceptual threshold and stimulation current.
| Pulse Width | Perceptual Threshold ± SD (mA) | Stim Current ± SD (mA) | Significant? (p value) | |||
|---|---|---|---|---|---|---|
| Tragus (Active) | Earlobe (Control) | Tragus (Active) | Earlobe (Control) | |||
| 100μs | 4.64 ± 1.28 | 3.28 ± 0.91 | 9.28 ± 2.56 | 6.57 ± 1.83 | Y (p = 0.002) | |
| 200μs | 2.66±0.80 | 1.82±0.63 | 5.32±1.60 | 3.64±1.26 | Y (p = 0.003) | |
| 500μs | 1.5±0.47 | 0.99 ± 0.35 | 3 ± 0.93 | 1.97±0.71 | Y (p = 0.002) | |
Adverse events and pain ratings
There were no minor or major adverse events during the experimental sessions nor spontaneously reported following exit of the trial. Minor redness was seen at the sight of stimulation that disappeared within 5 min of stimulation completion.
For the nine various parameters, as the pulse width and frequency increased, so did the pain rating numeric rating scale (NRS), although the highest mean tragus pain rating was 2.133, SD 1.34 (500 μs, 25 Hz) and the highest mean earlobe pain rating was 1.23, SD 0.42 (100 μs, 10 Hz). Although some of these NRS scale ratings show statistical significance between stimulation conditions (paired t-test; 500μs 10 Hz: t (14) = 3.44, p = 0.004; 25 Hz: t (14) = 3.237, p = 0.006), and did not have a large outcome on the results as the NRS requires a pain difference of more than 2 points to be clinically relevant [30]. Parametric NRS scores and statistics are described in Table 2.
Table 2.
Trial 1 NRS pain ratings
| Parameter | Mean NRS Pain Rating ± SD | Significant? (p value) | ||
|---|---|---|---|---|
| Tragus (Active) |
Earlobe (Control) |
|||
| 100μs | 1 Hz | 1.27 ± 0.59 | 1.0 ± 0.0 | N |
| 10Hz | 1.67±0.82 | 1.23±0.42 | N | |
| 25 Hz | 1.57±0.82 | 1.13±0.35 | N | |
| 200μs | 1 Hz | 1.27±0.46 | 1.0±0.0 | N |
| 10Hz | 1.43 ± 0.82 | 1.03±0.13 | N | |
| 25 Hz | 2.1 ±1.36 | 1.33±1.05 | N | |
| 500μs | 1 Hz | 1.2±0.56 | 1.07±0.26 | N |
| 10Hz | 1.77±0.86 | 1.0±0.0 | Y (0.004) | |
| 25 Hz | 2.13±1.34 | 1.17±0.36 | Y (0.006) | |
Effect of stimulation condition on HR
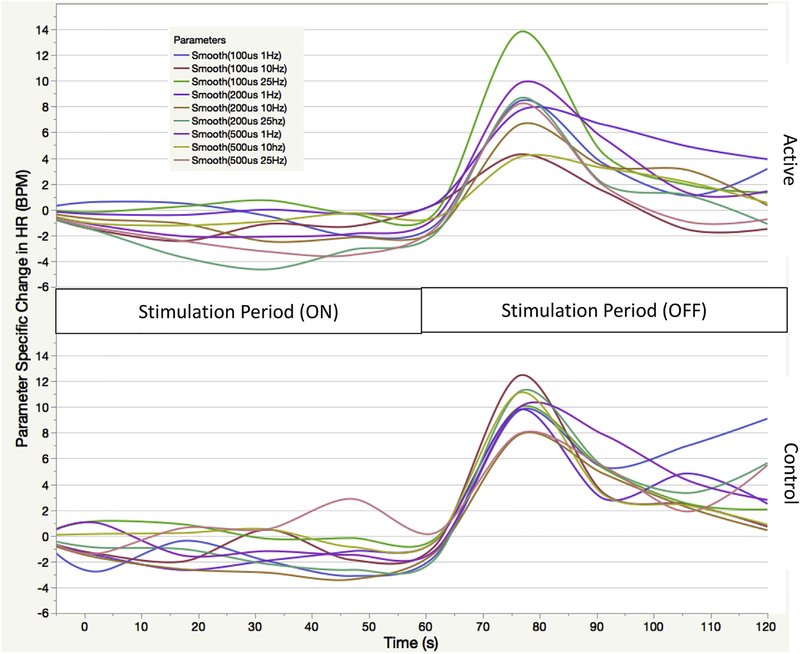
Note the overall pattern of effect on HR over time (Fig. 2). taVNS has a recognizable physiologic signature: when stimulation begins (stimulation period), HR decreases immediately and is sustained at this lower level. Upon termination of stimulation (recovery period), there is an immediate reorientation spike in HR that elevates past baseline for nearly 30 s, followed by a regression back to the mean resting HR. The nine different stimulation parameters each have a varied effect on HR, with some inducing larger decreases than other parameters.
Fig. 2. Stimulation response by parameter over time.
Active and control traces of all nine parameters tested in trial 1 demonstrating the physiological signature of taVNS. Time periods identified as 60s during stimulation and 60s post stimulation (120s total)
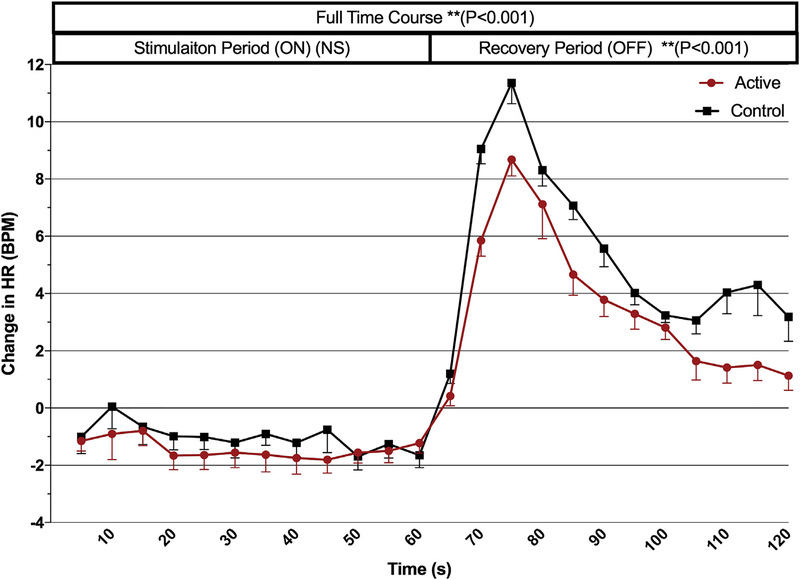
Mean changes from baseline in the stimulation and recovery periods of all active vs. all control stimulation rounds are shown in Fig. 3. To determine the overall time course effect of taVNS on HR, we grouped all parameters by condition (active vs control, 5s bins for 24 epochs total over 120s) and conducted a repeated measures ANOVA demonstrating an overall effect of stimulation condition for the entire time course (stimulation and recovery periods combined; F (1,718) = 7.825, p < 0.001). When all parameters are grouped together, both active and control stimulation have a nonsignificant slowing effect on HR during the stimulation period (60s), with a mean active HR decrease from baseline of 1.43 BPM, SEM 0.20, and control mean HR decrease of 1.02, SEM 0.20.
Fig. 3. Time course analysis for trial 1.
All 9 stimulation parameters’ effect on mean heart rate (grouped by condition, active/control) are represented in this time course analysis. An overall effect of condition is revealed (120s time course, p < 0.001), as well as the blunting of HR spike by active stimulation during the recovery period (p < 0.001). (Error bars = SEM).
Exploring the change in HR during the post-stimulation period (from the final 5s bin of stimulation), the sympathetic rebound that occurs upon termination of taVNS was blunted by active stimulation compared to control, demonstrating a condition (active, con trol) effect of rebound spike (F (1,358) = 7.843, p = 0.005). The peak HR rebound was achieved during the third 5-s bin (15 s post-taVNS) with a max rebound in HR for active taVNS of 8.153 BPM and control stimulation of 11.361 BPM. The sympathetic spike time- course analysis revealed a significant condition-time interaction (F (6.675,2389.737) = 6.224, p < 0.001), as the effects of active stimulation return to baseline more quickly than do the effects of control stimulation.
Multivariate analysis of HR to determine parametric effects
We conducted a multivariate analysis to determine parameter- specific effects. Several parameters were determined to have an effect of condition on the decrease in HR during stimulation (Table 3). Two parameters that had the largest, significant effects of condition on HR, in which active stimulation decreased HR more than control stimulation: 500 μs, 25 Hz (active HR −3.13 BPM, control 0.799, t (1) = 26.874, p < 0.001); 500 μs, 10 Hz (active HR −0.929 BPM, control 0.290, t (1) = 6.936, p = 0.01). A multivariate analysis was performed on the sympathetic reorientation spike, showing several parameters that suppressed the sympathetic spike during the recovery period (Table 4). The optimal parameters for suppression of the parasympathetic rebound based on magnitude difference of peak spike suppression were 100 μs, 10 Hz and 500 μs, 10 Hz.
Table 3.
Trial 1 mean change in HR during stimulation.
| Parameter | Mean Change in HR from Baseline ± SEM (Average over 60s Stimulation Period) |
Partial Eta Sq. | Condition Effect? (p value) | ||
|---|---|---|---|---|---|
| Tragus (Active) |
Earlobe (Control) |
||||
| 100μs | 1Hz | −0.744 ± .781 | −2.40 ± .781 | 0.007 | N |
| 10 Hz | −1.17±.0.461 | −0.891 ±0.461 | 0.001 | N | |
| 25 Hz | 0.24±0.362 | 0.524±0.362 | 0.001 | N | |
| 200μs | 1 Hz | 0.07 ± 0.631 | −2.01 ±0.631 | 0.016 | Y (0.018) |
| 10 Hz | −1.54±0.353 | −2.86±0.353 | 0.021 | Y (0.005) | |
| 25 Hz | −3.57±0.436 | −1.81 ±0.436 | 0.024 | Y (0.006) | |
| 500μs | 1Hz | −2.17±0.337 | −0.857±0.337 | 0.022 | Y (0.006) |
| 10 Hz | −0.93±0.335 | 0.290±0.335 | 0.019 | Y (0.01) | |
| 25 Hz | −3.13±0.545 | 0.799±0.545 | 0.072 | Y (<0.001) | |
Table 4.
Trial 1 mean change in recovery HR.
| Parameter | Mean Change in Recovery HR ± SD (Average over 60s Recovery Period) |
Partial Eta Sq. | Condition Effect? (p value) | ||
|---|---|---|---|---|---|
| Tragus (Active) |
Earlobe (Control) |
||||
| 100μs | 1Hz | 3.859 ± 0.998 | 7.296 ± 0.998 | 0.017 | Y (0.015) |
| 10 Hz | 1.244 ± 0.547 | 4.077 ±0.547 | 0.038 | Y (<0.001) | |
| 25 Hz | 4.348±0.581 | 5.2±0.581 | 0.003 | N | |
| 200μs | 1 Hz | 5.436±0.672 | 5.76±0.672 | 0.000 | N |
| 10 Hz | 3.591 ±0.476 | 4.59±0.476 | 0.007 | N | |
| 25 Hz | 2.995±0.600 | 5.792 ±0.600 | 0.031 | Y (0.001) | |
| 500μs | 1 Hz | 4.512±00.647 | 5.755 ±00.647 | 0.005 | N |
| 10 Hz | 2.703±0.539 | 4.926±0.539 | 0.025 | Y (0.004) | |
| 25 Hz | 2.644±0.601 | 5.474±0.601 | 0.032 | Y (0.006) | |
Trial 2
Participants, perceptual thresholds (PT), and stimulation current
Twenty (10 female) healthy, right-handed individuals (mean age 25.65 ± 5.53 SD) were included in this study. All participants completed both visits without any dropout. Mean stimulation currents were as follows (mean ± SD mA): 500 μs (tragus-2.09 ± 0.97; earlobe 2.04 ± 0.82). Using a paired 2-tailed t-test, there was no difference in perceptual threshold between the two stimulation sites (Table 5).
Table 5.
Trial 2 mean perceptual threshold, stimulation current, and pain ratings.
| Tragus (Active) |
Earlobe (Control) | Significant? (p value) | |
|---|---|---|---|
| PT ± SD (mA) | 1.045 ± 0.48 | 1.02 ± 0.41 | N |
| Stim Current ± SD (mA) | 2.09 ± 0.97 | 2.04 ± 0.82 | |
| Mean 500 μs 10 Hz NRS Pain Rating ± SD | 1.35 ± 0.68 | 2.24 ± 1.28 | Y (P = 0.009) |
| Mean 500 μs 25 Hz NRS Pain Rating ± SD | 1.32 ± 0.57 | 2.10 ± 1.17 | Y (P = 0.01) |
Adverse events and pain ratings
Similar to trial 1, there were no minor or major adverse events during trial 2. The mean NRS scores for pain are described in Table 5, and although there was a significant difference in pain between parameter-specific stimulation targets when analyzed (10 Hz: paired t-test; t (19) = 2.746, p = 0.092; 25 Hz: paired t-test t(19) = 2.68, p = 0.01), these are not reflective of the minimal pain reflected in the ratings (mean pain difference between conditions less than one rating point). These pain ratings are considered relatively painless for both conditions [30].
Effect of stimulation condition on HR
Trial 2 replicated a physiological response to taVNS. As stimulation starts, there is an immediate bradycardia, which persists throughout the entire stimulation period. Upon termination of stimulation, a HR spike occurs, lasting approximately 30 s. The overall effect on HR during the entire time course (120s period) is not significantly different by condition. Fig. 4 shows the mean changes from baseline in the stimulation and recovery periods of all active vs. all control stimulation rounds.
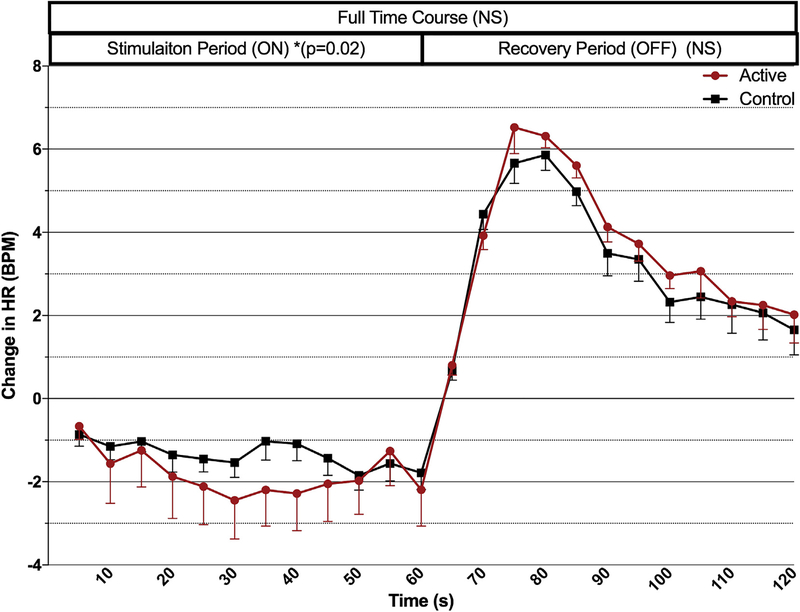
Fig. 4. Time course analysis for trial 2.
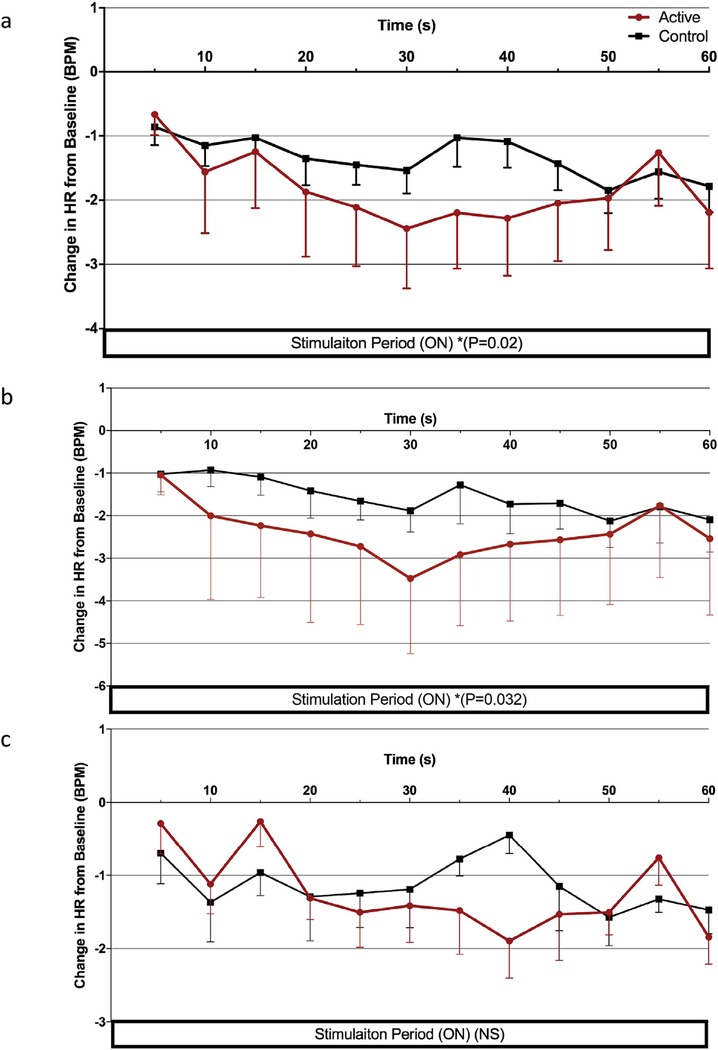
Both stimulation parameters’ effects on mean heart rate (grouped by condition, active/control) are represented in this time course analysis. Active taVNS significantly decreases HR during the stimulation period (p = 0.02). (Error bars = SEM).
Exploring the effect of condition on the stimulation period alone (first 60s), when both parameters are grouped by condition, active taVNS has a significant decrease in HR compared to control stimulation (repeated measures ANOVA, F (1,466) = 5.365, P < 0.02) (Fig. 5a). During the 60-s post stimulation period (from the final 5s bin of stimulation), the sympathetic rebound that occurs upon termination of taVNS was not significantly different between conditions.
Fig. 5. Stimulation effects on HR of trail 2 parameters.
a) Stimulation period effects of taVNS reveal significant decrease in HR during active stimulation compared to control (p =0.02) b) Effects of 500μs 10 Hz parameter on HR; active stimulation significantly decreased HR compared to control (p = 0.032) d) Effects of 500μs 25 Hz. (Error bars = SEM).
Parameter-specific effects on HR
We split into parameter specific changes by condition (500 μs, 10 Hz and 500 μs, 25 Hz; active vs control) and con ducted two specific time period analyses based on parameter and condition (60s stimulation period, 60s recovery period). In the stimulation period of the 500 μs, 10 Hz parameter, active stimulation induces a significant decrease in HR compared to control (repeated measures ANOVA, f (1,478) = 4.79, p = 0.032) (Fig. 5b). Mean decrease for the active condition was −2.40 BPM ±0.275 vs. control, which produced a −1.56 BPM ±0.275 change from baseline (Table 6). The 500 μs, 25 Hz parameter showed non-significant effect of condition on HR throughout the stimulation period (Fig. 5c) and neither control condition induced significant reduction in HR compared to the 5s initial baseline.
Table 6.
Trial 2 mean change in HR from baseline.
| Parameter | Mean Change in HR from Baseline ± SEM (Average over 60s Stimulation Period) |
Partial Eta Sq. | Condition Effect? (p value) |
||
|---|---|---|---|---|---|
| Tragus (Active) |
Earlobe (Control) |
||||
| 500μs | 10 Hz | −2.40 ± 0.275. | −1.56 ± 0.275 | 0.01 | Y (P = 0.032) |
| 25 Hz | −1.244±.180 | −1.036±1.84 | 0.001 | N | |
Discussion
taVNS in healthy adults delivered for 60s periods is feasible and safe, demonstrating parameter-specific effects on HR decrease and on sympathetic rebound. In trial 1 we found the optimal taVNS parameters which modulate heart rate are those which are most energy dense, specifically 500μs 10 Hz and 500μs 25 Hz parameters. We then tested these two parameters for replication in trial 2 and demonstrated the 500μs 10 Hz parameter as the most effective at decreasing HR in healthy individuals. To our knowledge, this is the first prospective trial to systematically test the safety and physiological effects of various taVNS parameters.
No minor or major adverse effects were observed, suggesting 60s periods of taVNS at 200% of perceptual threshold is safe and tolerable [21,31]. As pulse width increases, less current is needed for the sensation to be perceived, confirming the strength-duration property of electrical stimulation. The earlobe appears to require more stimulation current than the tragus as determined by the sensory threshold testing. The mean overall pain score for active taVNS ratings were 1.6 (trial 1) and 2.17 (trial 2), versus control stimulation ratings of 1.1 (trial 1) and 1.35 (trial 2). These are considered perceivable but not painful, making taVNS a relatively painless method. This is both an advantage and a limitation for this trial. By titrating to each location, we were able to deliver currents at that location relative to pain threshold there. We thus eliminated any difference between active and control in terms of pain. Un fortunately, the tradeoff for matching on painfulness, as the PT differed between the active and control sites, was that we delivered more dose (absolute current) to the tragus, introducing a potential dose confound. We do not believe this explains our differences, as a retrospective review of individuals receiving higher dosage did not demonstrate larger effects. This may be due to the narrow range of stimulation current at each pulse width, and we cannot determine whether large increases in dose would result in greater effects.
The multivariate analyses conducted in trial 1 demonstrated significant, parameter-specific effects on HR during both stimulation and post-stimulation periods. Conditions with more energy- dense parameters had noticeably larger effects. Notably, the parameters with highest HR slowing effects were those with higher pulse width and frequency (500 μs, 10 Hz and 500 μs, 25 Hz). The highest suppressors of the sympathetic rebound in HR post stimulation were those with 10 Hz frequency (500 μs, 10 Hz and 100 μs, 10 Hz).
Trial 2 tested for, and replicated, several aspects of trial 1, con firming that the energy-dense parameters determined to be the best modulators of HR in the first trial induced an overall significant effect of bradycardia during the stimulation period. When split by parameter, the optimal parameter at inducing decreases in heart rate was 500 μs, 10 Hz.
The taVNS parameter space is vast, and systematic parametric optimization trials are needed to determine optimal stimulation parameters. Our trial suggests that specific taVNS parameters may be better than others at modulating HR, which we suspect is mediated through the activation of the main vagus nerve bundle. taVNS at 500μs 10 Hz decreases heart rate during stimulation, and is suspected to be a parasympathetic response modulated by the ABVN, which innervates the tragus [32], however these effects are substantially smaller than bradycardia induced by direct cervical VNS [17].
Blunting of sympathetic rebound was an unexpected finding in trial 1. It has been demonstrated in prior studies that this sympathetic spike in HR occurs [33,34], and is thought to be a reorienting phenomenon. It is also seen pharmacologically, in the reciprocal effect of noradrenergic blockade via beta-blockers [35]. The reciprocal mechanisms are thought to maintain physiological homeostasis, and rapid activation/deactivation of stimulation of either system has a strong reciprocal action that subsequently occurs. One prior taVNS trial suggests that sympathetic nervous activity is reduced upon stimulation [36], although the findings did not replicate in trial 2, as the blunting may not be seen due to greater parasympathetic activation in the stimulation period, causing larger reciprocal spikes in the active taVNS condition. The vagus has been implicated in the reciprocal of this phenomena (sympathetic activation to parasympathetic rebound) [37] suggesting there may be a homeostatic mechanism which becomes activated when either one of the independent functioning autonomic responses is activated.
There have been several studies exploring the behavioral or therapeutic effects of taVNS, many of which are positive [38–41]. The extant literature suggests that tragus stimulation directly modulates the vagus network via the ABVN. We and others have demonstrated that stimulation of the ABVN has a direct effect on the afferent projection of the vagus nerve [29], with similarities in blood oxygen level dependent (BOLD) signal to cervically implanted VNS [42,43]. These fMRI findings suggest one possible mechanistic hypothesis for the immediate, sustained decrease in HR: stimulation is entering the afferent vagus system toward the central nervous system, activating the parasympathetic efferent cholinergic pathway and targeting the viscera, including the heart. Pulse width and frequency are likely important in order to activate this system. Higher pulse widths and frequencies of ABVN stimulation clearly induce larger parasympathetic responses than does taVNS stimulation using lower energy parameters.
This field is still in its infancy, and there is a lack of consensus on the optimal parameters for taVNS. There is an infinite combination of current, frequency, pulse width, and duty cycle [24–28,38,42,43] that would be impossible to exhaustively test, however likely candidates emerge which are comparable to implantable VNS.
Limitations
It is impossible to truly say these effects are a direct cause of stimulating the ABVN, as the only way to definitively say that would require dissection and direct nerve recording. However, based on prior literature and cadaver trials [19], tragus stimulation is likely directly stimulating the ABVN, whereas the earlobe has little to no ABVN innervation, and therefore less parasympathetic-derived effect. Although nearly all controlled taVNS trials explore the lobule as a control or sham region in behavioral studies, perhaps it may be a biologically active region of stimulation in terms of physiological response. We suspect this may be due to anatomical variance and distal projections of the ABVN that inadvertently receive stimulation. Future studies should explore a third, off-ear stimulation site to avoid this confound.
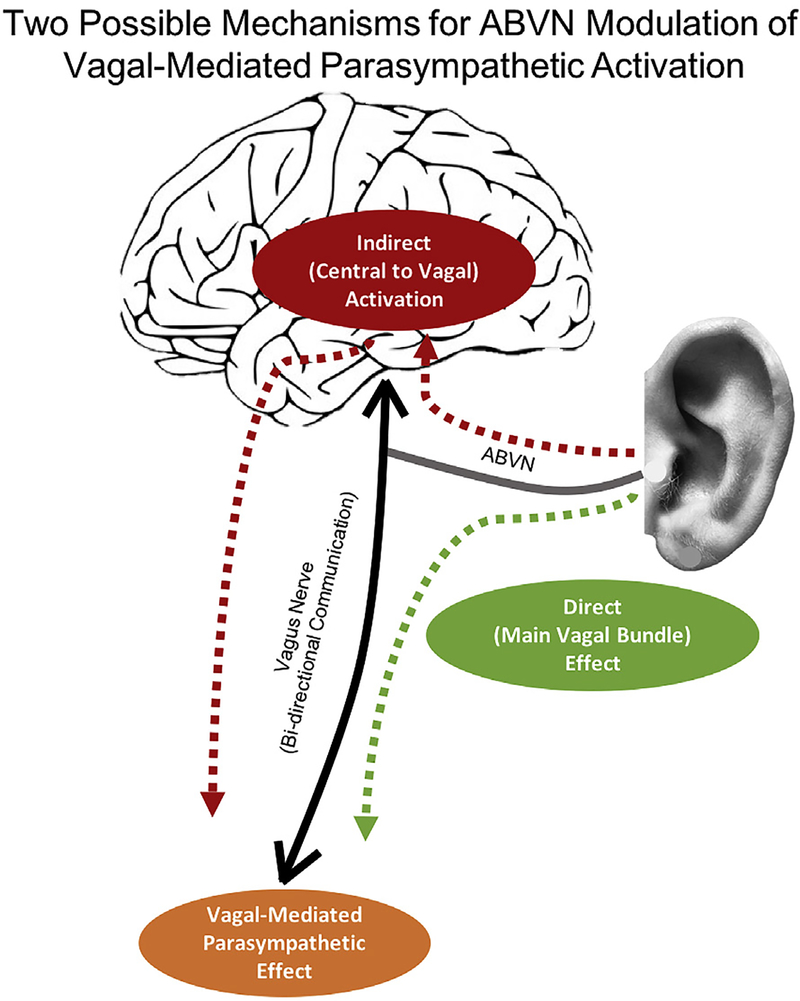
It is also unclear whether the effects seen are through the hypothesized afferent central targets of the vagus system (ear-brain- vagus-body), or whether they are modulating parasympathetic response via direct efferent projections from the ear to the periphery (ear-vagus-heart). Fig. 6 proposes two possible mechanisms for parasympathetic activation via stimulation of the ABVN. This is difficult to determine without systematic, parametric testing using combined taVNS and neuroimaging paradigms, or with in- vivo microelectrode recording of nerve dissections in animal models, or in animal models where the ABVN is lesioned. It is curious that our parameter findings are in the same range as implanted therapeutic VNS parameters, it is still unclear as to why cardiac effects are seen compared to the general lack of cardiac findings when applied clinically. Perhaps a head-to-head intra operative study prospectively looking at physiological response to both implanted and non-invasive is warranted.
Fig. 6.
Proposed theoretical mechanisms of taVNS-mediated parasympathetic activation.
Conclusion
taVNS administered for 1 min at 200% perceptual threshold is safe and has parameter-specific effects on heart rate. Upon systematic exploration, the optimal parameter at modulating HR is 500 μs, 10 Hz. These parameters and electrode location may be considered the most biologically active and in future studies could, this change in HR could be used as an individual dosing mechanism for the subsequent use of taVNS as a therapeutic device for neuropsychiatric disorders. Further neuroimaging trials should explore these parameters to determine their central effects.
Funding
Financial support from: NIH R21/R33 (5R21MH106775–02), National Center of Neuromodulation for Rehabilitation (NC NM4R) (5P2CHD086844–03), COBRE Brain Stimulation Core (5P20GM109040–04).
References
- [1].Monkhouse S Cranial nerves: functional anatomy. Cambridge University Press; 2005. [Google Scholar]
- [2].Tracey KJ. Physiology and immunology of the cholinergic antiinflammatory pathway. J Clin Invest 2007;117(2):289–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Buijs RM, Chun SJ, Niijima A, Romijn HJ, Nagai K. Parasympathetic and sym pathetic control of the pancreas: a role for the suprachiasmatic nucleus and other hypothalamic centers that are involved in the regulation of food intake. J Comp Neurol 2001;431(4):405–23. [DOI] [PubMed] [Google Scholar]
- [4].Loewi O Über humorale übertragbarkeit der herznervenwirkung. Pflugers Arch für Gesamte Physiol Menschen Tiere 1921;189(1):239–42. [Google Scholar]
- [5].McEwen LM. The effect on the isolated rabbit heart ofvagal stimulation and its modification by cocaine, hexamethonium and ouabain. J Physiol 1956;131(3): 678–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Fouad FM, Tarazi RC, Ferrario CM, Fighaly S, Alicandri C. Assessment of parasympathetic control of heart rate by a noninvasive method. Am J Physiol 1984;246(6 Pt 2):H838–42. [DOI] [PubMed] [Google Scholar]
- [7].Tosato M, Yoshida K, Toft E, Nekrasas V, Struijk JJ. Closed-loop control of the heart rate by electrical stimulation of the vagus nerve. Med Biol Eng Comput 2006;44(3):161–9. [DOI] [PubMed] [Google Scholar]
- [8].Lim S, Anantharaman V, Teo W, Goh P, Tan A. Comparison of treatment of supraventricular tachycardia by Valsalva maneuver and carotid sinus mas sage. Ann Emerg Med 1998;31(1):30–5. [PubMed] [Google Scholar]
- [9].Schweitzer P, Teichholz LE. Carotid sinus massage. Its diagnostic and therapeutic value in arrhythmias. Am J Med 1985;78(4):645–54. [DOI] [PubMed] [Google Scholar]
- [10].Ben-Menachem E, Manon-Espaillat R, Ristanovic R, Wilder B, Stefan H, Mirza W, et al. Vagus nerve stimulation for treatment of partial seizures: 1. A controlled study of effect on seizures. Epilepsia 1994;35(3):616–26. [DOI] [PubMed] [Google Scholar]
- [11].Premchand RK, Sharma K, Mittal S, Monteiro R, Dixit S, Libbus I, et al. Autonomic regulation therapy via left or right cervical vagus nerve stimulation in patients with chronic heart failure: results of the ANTHEM-HF trial. J Card Fail 2014;20(11):808–16. [DOI] [PubMed] [Google Scholar]
- [12].Premchand RK, Bhaskar Rao B, Partani K. A rare case of acquired aortopulmonary fistula with bicuspid aortic valve: report of successful surgical repair. BMJ Case Rep 2014:2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Holder LK, Wernicke JF, Tarver WB. Treatment of refractory partial seizures: preliminary results of a controlled study. Pacing Clin Electrophysiol 1992;15(10 Pt 2):1557–71. [DOI] [PubMed] [Google Scholar]
- [14].Uthman B, Wilder B, Penry J, Dean C, Ramsay R, Reid S, et al. Treatment of epilepsy by stimulation of the vagus nerve. Neurology 1993;43(7):1338-. [DOI] [PubMed] [Google Scholar]
- [15].Ramsay RE, Uthman BM, Augustinsson LE, Upton AR, Naritoku D, Willis J, et al. Vagus nerve stimulation for treatment of partial seizures: 2. Safety, side effects, and tolerability. First International Vagus Nerve Stimulation Study Group. Epilepsia 1994;35(3):627–36. [DOI] [PubMed] [Google Scholar]
- [16].Setty AB, Vaughn BV, Quint SR, Robertson KR, Messenheimer JA. Heart period variability during vagal nerve stimulation. Seizure 1998;7(3):213–7. [DOI] [PubMed] [Google Scholar]
- [17].Frei MG, Osorio I. Left vagus nerve stimulation with the neurocybernetic prosthesis has complex effects on heart rate and on its variability in humans. Epilepsia 2001;42(8):1007–16. [DOI] [PubMed] [Google Scholar]
- [18].Matheny RG, Shaar CJ. Vagus nerve stimulation as a method to temporarily slow or arrest the heart. Ann Thorac Surg 1997;63(6):S28–9. [DOI] [PubMed] [Google Scholar]
- [19].Peuker ET, Filler TJ. The nerve supply of the human auricle. Clin Anat 2002;15(1):35–7. [DOI] [PubMed] [Google Scholar]
- [20].Zabara J Inhibition of experimental seizures in canines by repetitive vagal stimulation. Epilepsia 1992;33(6):1005–12. [DOI] [PubMed] [Google Scholar]
- [21].Penry JK, Dean JC. Prevention of intractable partial seizures by intermittent vagal stimulation in humans: preliminary results. Epilepsia 1990;31(s2): S40–3. [DOI] [PubMed] [Google Scholar]
- [22].DeGiorgio C, Schachter S, Handforth A, Salinsky M, Thompson J, Uthman B, et al. Prospective long-term study of vagus nerve stimulation for the treat ment of refractory seizures. Epilepsia 2000;41(9):1195–200. [DOI] [PubMed] [Google Scholar]
- [23].Lang J Auris externa–außenohr Klinische Anatomie des Ohres. Springer; 1992. p. 1–30. [Google Scholar]
- [24].Fallgatter A, Neuhauser B, Herrmann M, Ehlis A-C, Wagener A, Scheuerpflug P, et al. Far field potentials from the brain stem after transcutaneous vagus nerve stimulation. J Neural Transm 2003;110(12):1437–43. [DOI] [PubMed] [Google Scholar]
- [25].Fallgatter AJ, Ehlis A-C, Ringel TM, Herrmann MJ. Age effect on far field potentials from the brain stem after transcutaneous vagus nerve stimulation. Int J Psychophysiol 2005;56(1):37–43. [DOI] [PubMed] [Google Scholar]
- [26].Polak T, Markulin F, Ehlis A-C, Langer JB, Ringel TM, Fallgatter AJ. Far field potentials from brain stem after transcutaneous vagus nerve stimulation: optimization of stimulation and recording parameters. J Neural Transm 2009;116(10):1237–42. [DOI] [PubMed] [Google Scholar]
- [27].Wang S-M, Peloquin C, Kain ZN. The use of auricular acupuncture to reduce preoperative anxiety. Anesth Analg 2001;93(5):1178–80. [DOI] [PubMed] [Google Scholar]
- [28].Greif R, Laciny S, Mokhtarani M, Doufas AG, Bakhshandeh M, Dorfer L, et al. Transcutaneous electrical stimulation of an auricular acupuncture point de creases anesthetic requirement. The Journal of the American Society of Anesthesiologists 2002;96(2):306–12. [DOI] [PubMed] [Google Scholar]
- [29].Badran BW, Dowdle LT, Mithoefer OJ, LaBate NT, Coatsworth J, Brown JC, et al. Neurophysiologic effects of transcutaneous auricular vagus nerve stimulation (taVNS) via electrical stimulation of the tragus: a concurrent taVNS/fMRI study and review. Brain Stimul 2018;11(3):492–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Hawker GA, Mian S, Kendzerska T, French M. Measures of adult pain: visual analog scale for pain (VAS pain), numeric rating scale for pain (NRS pain), McGill pain questionnaire (MPQ), short-form McGill pain questionnaire (SF- MPQ), chronic pain grade scale (CPGS), short Form-36 bodily pain scale (SF-36 BPS), and measure of intermittent and constant osteoarthritis pain (ICOAP). Arthritis Care Res 2011;63(Suppl 11):S240–52. [DOI] [PubMed] [Google Scholar]
- [31].Sackeim HA, Rush AJ, George MS, Marangell LB, Husain MM, Nahas Z, et al. Vagus nerve stimulation (VNS™) for treatment-resistant depression: efficacy, side effects, and predictors of outcome. Neuro psychopharmacology 2001;25(5):713–28. [DOI] [PubMed] [Google Scholar]
- [32].Benarroch EE, editor. The central autonomic network: functional organization, dysfunction, and perspective. Mayo Clinic Proceedings; Elsevier; 1993. [DOI] [PubMed] [Google Scholar]
- [33].Cobb JL, Santer RM. Electrophysiology of cardiac function in teleosts: cholinergically mediated inhibition and rebound excitation. J Physiol 1973;230(3): 561–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Koizumi K, Kollai M. Control of reciprocal and non-reciprocal action of vagal and sympathetic efferents: study of centrally induced reactions. J Auton Nerv Syst 1981;3(2–4):483–501. [DOI] [PubMed] [Google Scholar]
- [35].Paton JF, Boscan P, Pickering AE, Nalivaiko E. The yin and yang of cardiac autonomic control: vago-sympathetic interactions revisited. Brain Res Brain Res Rev. 2005;49(3):555–65. [DOI] [PubMed] [Google Scholar]
- [36].Clancy JA, Mary DA, Witte KK, Greenwood JP, Deuchars SA, Deuchars J. Non- invasive vagus nerve stimulation in healthy humans reduces sympathetic nerve activity. Brain stimulation 2014;7(6):871–7. [DOI] [PubMed] [Google Scholar]
- [37].Mezzacappa ES, Kelsey RM, Katkin ES, Sloan RP. Vagal rebound and recovery from psychological stress. Psychosom Med 2001;63(4):650–7. [DOI] [PubMed] [Google Scholar]
- [38].Bauer S, Baier H, Baumgartner C, Bohlmann K, Fauser S, Graf W, et al. Transcutaneous vagus nerve stimulation (tVNS) for treatment of drug- resistant epilepsy: a randomized, double-blind clinical trial (cMPsE02). Brain stimulation 2016;9(3):356–63. [DOI] [PubMed] [Google Scholar]
- [39].Capone F, Assenza G, Di Pino G, Musumeci G, Ranieri F, Florio L, et al. The effect of transcutaneous vagus nerve stimulation on cortical excitability. J Neural Transm 2015;122(5):679–85. [DOI] [PubMed] [Google Scholar]
- [40].Laqua R, Lotze M, Leutzow B, Usichenko T. fMRI evidence for a reduction in affective processing of thermal pain in responders of transcutaneous vagal nerve stimulation (TVNS). Clin Neurophysiol 2016;127(3):e9. [Google Scholar]
- [41].Magis D, Gérard P, Schoenen J. Transcutaneous Vagus Nerve Stimulation (tVNS) for headache prophylaxis: initial experience. J Headache Pain 2013;14(S1):P198. [Google Scholar]
- [42].Kraus T, Hösl K, Kiess O, Schanze A, Kornhuber J, Forster C. BOLD fMRI deactivation of limbic and temporal brain structures and mood enhancing effect by transcutaneous vagus nerve stimulation. J Neural Transm 2007;114(11):1485–93. [DOI] [PubMed] [Google Scholar]
- [43].Kraus T, Kiess O, Hösl K, Terekhin P, Kornhuber J, Forster C. CNS BOLD fMRI effects of sham-controlled transcutaneous electrical nerve stimulation in the left outer auditory canal–a pilot study. Brain stimulation 2013;6(5):798–804. [DOI] [PubMed] [Google Scholar]