Abstract
Purpose: To identify from the anterior segment the structural variables of the eyes that can be used to distinguish acute primary angle-closure (APAC) eyes or primary angle-closure suspect (PACS) eyes from normal eyes.
Patients and methods: We used a Pentacam scanner to measure participants’ anterior eye segments. We assessed each anterior segment structure variable on the basis of receiver operating characteristic curves using the area under the curve (AUC).
Results: AUCs for eyes in men with APAC were 1.000 for central anterior chamber depth (ACD), 0.982 for peripheral ACD, 0.916 for anterior chamber angle (ACA), and 0.992 for anterior chamber volume (ACV). AUCs for eyes in women with APAC were 0.997 for central ACD, 0.942 for peripheral ACD, 0.922 for ACA, and 0.946 for ACV. AUCs for eyes in men with PACS were 0.933 for central ACD, 0.930 for peripheral ACD, 0.887 for ACA, and 0.937 for ACV. AUCs for eyes in women with PACS were 0.960 for central ACD, 0.957 for peripheral ACD, 0.937 for ACA, and 0.937 for ACV. The negative predictive values (%) in men with APAC were 100 for all the four variables (central ACD, peripheral ACD, ACA, and ACV). The negative predictive values (%) in women with APAC were 100 for central ACD, 98.7 for peripheral ACD, 97.1 for ACA, and 97.9 for ACV. The negative predictive values (%) in men with PACS were 98.6 for central ACD, 100 for peripheral ACD, 98.5 for ACA, and 99.4 for ACV. The negative predictive values (%) in women with PACS were 100 for central ACD, 98.7 for peripheral ACD, 97.1 for ACA, and 97.9 for ACV.
Conclusions: The central ACD, peripheral ACD, ACA, and ACV measurements seem to be excellent markers to identify eyes without APAC or PACS.
Keywords: acute primary angle-closure, anterior segment, Pentacam, glaucoma
Introduction
Patients with primary angle-closure glaucoma (PACG) have narrow anterior-chamber angles and optic nerve damage. However, some patients with narrow angles lack optic nerve damage signs, a condition known as primary angle-closure (PAC). Untreated acute primary angle-closure (APAC) can lead to severe vision reduction and is a leading cause of blindness throughout the world.1
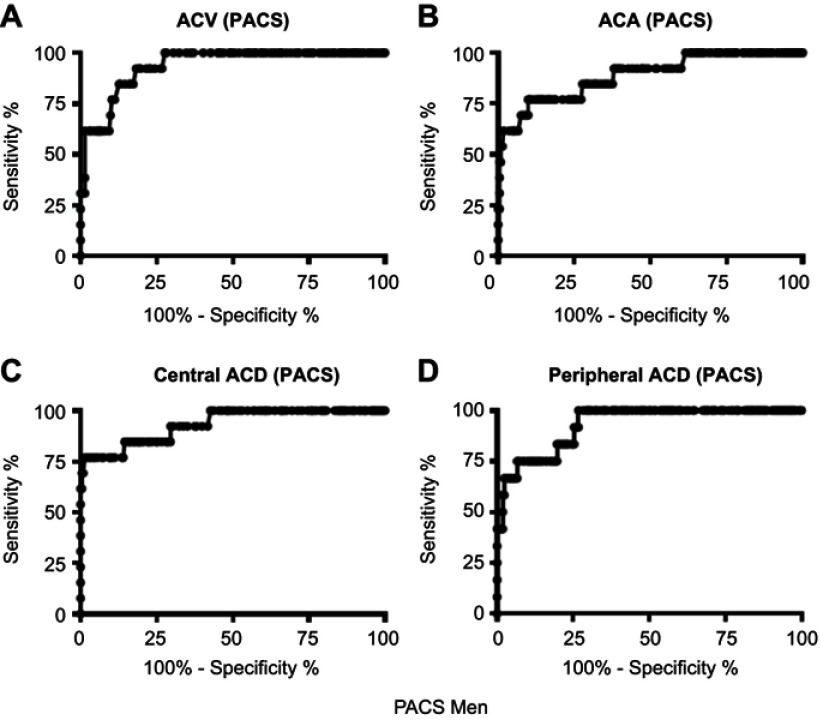
Figure 5.
Receiver operating characteristic curves for central ACD, peripheral ACD, ACA, and ACV to identify eyes with PACS in men.
Abbreviations: ACD, anterior chamber depth; ACA, anterior chamber angle; ACV, anterior chamber volume; PACS, primary angle-closure suspect.
In Japan, the population-based Tajimi study found that the incidence of PACG was 0.6%, and the Kumejima study found that it was 2.2%; these are intermediate or relatively high incidences among those in industrialized countries.2,3 Therefore, identifying patients with PAC and providing them with appropriate prophylactic treatments is desirable.
The methods used to identify patients with PAC by slit-lamp biomicroscopy include those reported by Schaffer,4 van Herick,5 and Smith.6 These tests have high sensitivity and specificity for identifying PAC if the examinations are performed by experienced ophthalmologists.7 However, the results are subjective and difficult to quantify.8,9 Therefore, new techniques to quantify anterior segment structural variables to identify eyes with PAC are needed.
The presence of APAC and the anterior-chamber depth have been found to be associated.10,11 Advanced imaging technologies have enabled clinicians to assess structures of the anterior eye segment quantitatively.12 New imaging modalities such as the rotating Scheimpflug imaging device (Pentacam-Scheimpflug) and anterior segment optical coherence tomography (AS-OCT) are now commercially available. The Pentacam is made up of a rotating Scheimpflug camera that can record a three-dimensional virtual model of the anterior eye segment. The embedded software evaluates and quantifies biometric variables of the anterior segment using cross-sectional photographs from 0 to 360°.
The question then arises as to whether the Pentacam device can be used to detect changes in the anterior segments of eyes with APAC. The onset of APAC has been reported to be associated with the depth and volume of the anterior chamber as measured by the Pentacam device.8,9,13–15
Thus, our purpose was to determine the diagnostic capabilities of the biometric anterior segment variable values obtained with a Pentacam device to identify eyes with APAC. To accomplish this, we examined the anterior eye chambers of patients diagnosed with APAC, or PAC suspects (PACS), and compared these findings with those obtained from 458 normal eyes.
Material and methods
Patients
We recruited patients newly diagnosed with APAC at the Dokkyo Medical University Saitama Medical Center between January 2010 and July 2017. We defined APAC by the presence of ocular or periocular pain, nausea or vomiting, blurred vision with haloes, and an intraocular pressure (IOP) >30 mmHg. In addition, patients with APAC had at least three of the following signs: conjunctival injection, corneal epithelial edema, a mid-dilated unreactive pupil, or a shallow anterior chamber.16 We treated patients promptly with an intravenous drip of hyperosmotic agents following diagnoses. In case with severe corneal edema due to high IOPs, the Pentacam cannot take measurements. Thus, we only included patients whose anterior chamber structures could be examined after the treatment. We excluded patients who received surgical treatments, such as laser iridotomy, peripheral iridectomy, or cataract surgery, and also cases with bilateral APAC. Seven of the patients recruited had been dilated for ophthalmological examinations within 24 hrs of the onset of APAC symptoms.
We included eyes with appositional contacts between the peripheral iris and the posterior trabecular meshwork higher than 270° into the PACS group.17 We considered eyes with occludable angles and features of peripheral iris trabecular obstruction as having PAC. Such features included elevated IOP, the presence of peripheral anterior synechiae, iris whorling (distortion of radially orientated iris fibers), “‘glaukomflecken’” lens opacities, and excessive pigment deposition on the trabecular surface, but in the absence of a glaucomatous optic disc or visual field changes.17 We considered eyes with PAC showing glaucomatous optic disc changes (neuroretinal rim thinning, disc excavation, and/or optic disc hemorrhage attributable to glaucoma) or a glaucomatous visual field change (pattern standard deviation <5% and values outside the normal limits in the glaucoma hemifield test) as having PACG.17 For our analysis, we only used reliable visual field test results (ie, results with false-positive errors <15%, false-negative errors <15%, and fixation loss <20%). Finally, we classified 40 eyes from 40 patients (11 eyes of 11 men, and 29 eyes of 29 women) into the APAC group, and 48 eyes from 48 patients (13 eyes of 13 men, and 35 eyes of 35 women) into the PACS group. The age ranges of patients in the APAC group were 57–85 years with a mean of 73.6±8.7 years for men and 61–86 years with a mean of 74.1±7.4 years for women. The age range of patients in the PACS group were 57–85 years with a mean of 73.7±8.2 years for men and 61–86 years with a mean of 74.3±7.6 years for women.
We randomly selected normal eyes from age-matched individuals who underwent glaucoma screenings and were deemed to have normal eyes at our hospital during the same period (normal group). The eyes of the normal group did not have clinically significant cataracts that may affect visual acuity. In total, we studied 458 eyes of 213 men and 245 women. The population’s mean age were 74.2±6.8 years (ranging from 65 to 91 years) in men and 73.0±7.0 years (ranging from 55 to 93 years) in women (normal group). We found no significant differences in terms of age when comparing between the normal and APAC groups or between the normal and PACS groups.
All individuals underwent comprehensive ophthalmologic examinations including standardized refraction, measurement of best-corrected visual acuity using a Landolt ring, slit-lamp biomicroscopy, gonioscopy, and funduscopy. We performed gonioscopies in a dimly lit room with a four-mirror mini gonio lens (Ocular Instruments, Bellevue, WA, USA). We used the program central 24-2 threshold test of the Humphrey Field Analyzer (HFA) III 850(Carl Zeiss Meditec, Dublin, CA)An expert fundus examiner (TM) evaluated all glaucomatous nerve heads.
This study was a single-center, prospective comparative study. The Institutional Review Board of the Dokkyo Medical University Saitama Medical Center approved and the study protocol, and we conducted it in accordance with the tenets of the Declaration of Helsinki. The certificate approval number was 1635. We obtained written informed consent forms from all individuals after a full explanation of the nature of the experiments.
Biometric variables
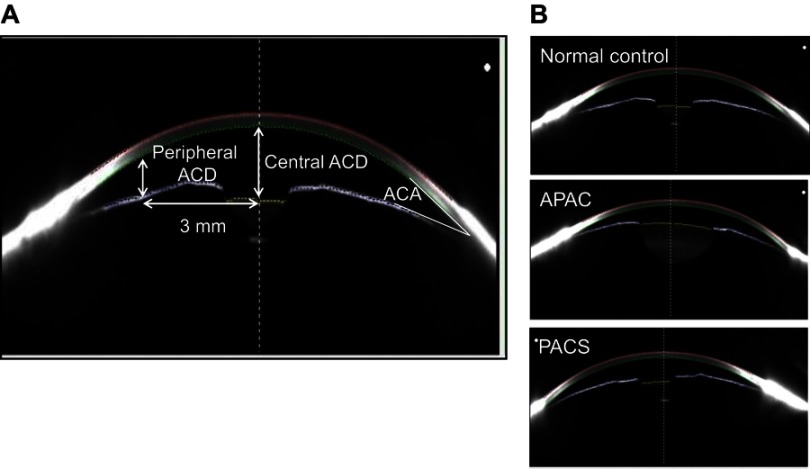
We took images all anterior eye segments with a Pentacam device (Oculus, Wetzlar, Germany) and calculated the anterior segment variable values, including central and peripheral anterior chamber depth (ACD), anterior chamber angle (ACA), and anterior chamber volume (ACV) using the software embedded in the Pentacam (Figure 1A). We defined ACD as the distance from the corneal endothelium to the anterior surface of the lens. The peripheral ACD was defined as the mean score of the distance from the posterior surface of the corneal endothelium to the anterior iris surface 3 mm away from the corneal apex in the superior, inferior, nasal, and temporal quadrants. We calculated ACA from the reconstructed anterior segment images, using the angle intersection between the posterior corneal surface and the iris. We defined ACV as solids bounded by the posterior surface of the cornea (12.0 mm around the corneal vertex) and by the iris and the lens.
Figure 1.
Biometric variables, including central anterior chamber depth (ACD), peripheral ACD, anterior chamber angle (ACA), and anterior chamber volume (ACV), were measured with a Pentacam (A). Representative images for a normal eye, an eye with APAC, and an eye with PACS (B).
Abbreviations: APAC, acute primary angle- closure; PACS, primary angle-closure suspect.
Statistical analyses
We calculated receiver operating characteristic (ROC) curves to determine optimal cutoff values that yielded the highest Youden index. This value corresponded to the point on the ROC curve that was farthest from the 45° diagonal line. We used area under the curve (AUC) analysis to compare ROC curves. We then calculated the positive predictive values (the true-positive/true- positive + false-positive values) and the negative predictive values (the true -negative/true-negative + false-negative values). We used the Prism Version 7.0 d software (GraphPad) to conduct our ROC analysis. We compared data between the groups using the StatMate version V software for Macintosh (ATMS, Tokyo, Japan) using unpaired t tests. We considered all P<0.05 as statistically significant.
Results
Representative cases
Of the 40 eyes in the APAC group, 10 were diagnosed with PACG because of the presence of a glaucomatous optic disc or a visual field change. Figure 1 shows representative images of the eyes of a normal woman, a woman with APAC, and a woman with PACS (Figure 1B). These figures show that the central and peripheral ACDs were shallower and the ACAs were narrower in eyes with APAC and PACS than in normal eyes. During Pentacam measurements in eyes with APAC, the mean IOPs were reduced to 35.6±16.3 mmHg (mean ± standard deviation) in men and 25.7±14.8 mmHg in women owing to treatment with intravenous drips of hyperosmotic agents before the procedure. The initial mean IOPs at presentation were 48.3±12.0 mmHg in men and 43.7±11.0 mmHg in women in the eyes with APAC. Table 1 shows the demographic and clinical characteristics of the studied patients.
Table 1.
Demographic and clinical characteristics of the studied patients
| Group | Variable | IOP (mmHg) | AL (mm) | PD (mm) |
|---|---|---|---|---|
| Age (years) | ||||
| APAC men | 73.6±8.7 | 35.6±16.3 | 22.88±0.99 | 3.81±0.90 |
| APAC women | 74.1±7.4 | 25.7±14.8 | 22.24±0.78 | 3.75±1.34 |
| PACS men | 73.7±8.2 | 12.6±3.9 | 22.51±0.73 | 2.48±0.66 |
| PACS women | 74.3±7.6 | 13.4±4.2 | 22.24±0.85 | 2.48±0.71 |
| Control men | 74.2±6.8 | 13.1±3.4 | 24.14±1.44 | 2.41±0.52 |
| Control women | 73.0±7.0 | 12.8±2.9 | 23.71±1.62 | 2.58±0.50 |
Abbreviations: APAC, acute primary angle-closure; PACS, primary angle-closure suspect; IOP, intraocular pressure; AL, axial length; PD, pupil diameter.
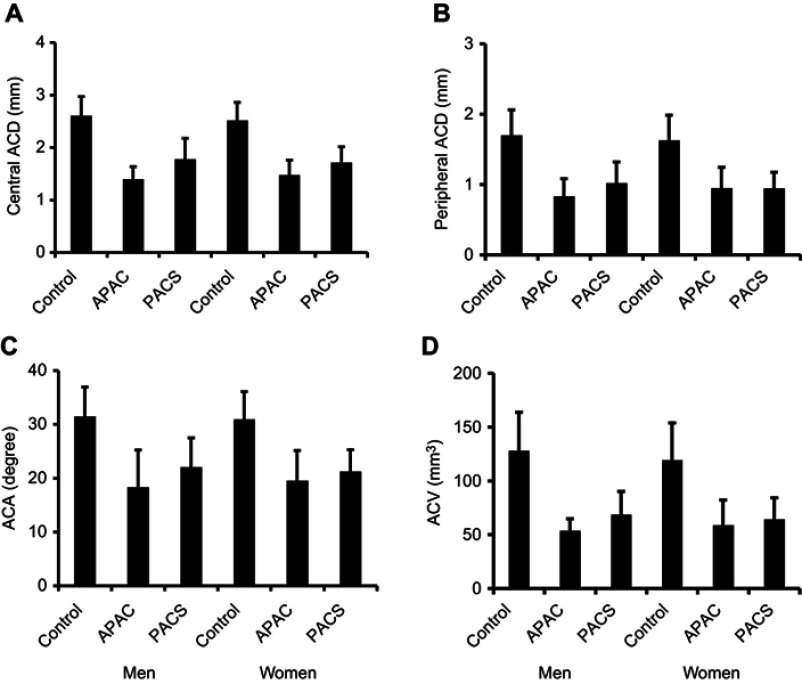
Mean values of anterior segment biometric variables
Figure 2A–D shows the mean values for central ACD, peripheral ACD, ACA, and ACV for individuals in the normal control. APAC and PACS groups for both genders. The values for the APAC and PACS groups were significantly lower than those in the control group (P<0.001–0.05, Figure 2A–D). However, we found no significant differences in these values when comparing between the APAC and PACS groups, except for the value of the central ACD in women (P<0.05, Figure 2A).
Figure 2.
Bar graphs showing central ACD (A), peripheral ACD (B), ACA (C) and ACV (D). Bars represent means of values with 95% confidence intervals.
Abbreviations: ACD, anterior chamber depth; ACA, anterior chamber angle; ACV, anterior chamber volume; APAC, acute primary angle-closure; PACS, primary angle-closure suspect.
AUC and optimal cutoff values to distinguish between eyes with APAC or PACS as determined by ROC curves
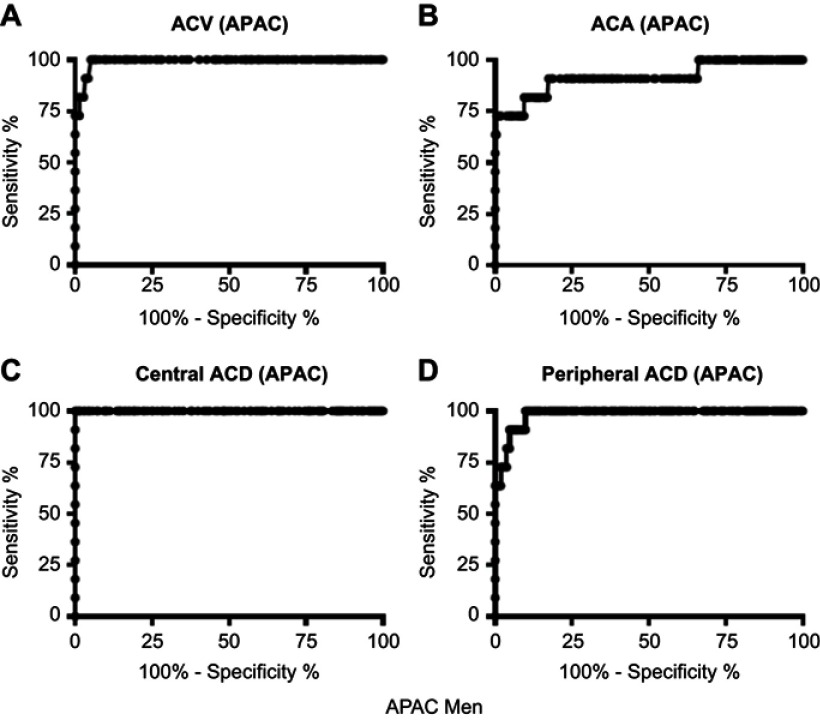
The ROC curve for the central ACD values to identify eyes with APAC was farthest from the diagonal line compared with curves for the other biometric variables; in both men and women (Figures 3 and 4). The AUCs of the anterior segment structures biometric variables determined by the ROC curves ranged from 0.916 to 1.000 (Table 2). The highest AUC was obtained for the central ACD with significant differences from the other biometric variables in women (P<0.05, Table 2).
Figure 3.
Receiver operating characteristic curves for central ACD, peripheral ACD, ACA, and ACV to identify eyes with APAC in men.
Abbreviations: ACD, anterior chamber depth; ACA, anterior chamber angle; ACV, anterior chamber volume; APAC, acute primary angle-closure.
Figure 4.
Receiver operating characteristic curves for central ACD, peripheral ACD, ACA, and ACV to identify eyes with APAC in women.
Abbreviations: ACD, anterior chamber depth; ACA, anterior chamber angle; ACV, anterior chamber volume; APAC, acute primary angle-closure.
Table 2.
AUC and optimal anterior segment biometric variable cutoff values determined by ROC curves to identify eyes with APAC
| Variable | AUC (95%CI) | P-value | Optimal cutoff value |
|---|---|---|---|
| Men (n=11) | |||
| Central ACD | 1.000 (1.000–1.000) | 1.82 mm | |
| Peripheral ACD | 0.982 (0.961–1.002) | 0.37 | 1.27 mm |
| ACA | 0.916 (0.803–1.028) | 0.14 | 26.5 degrees |
| ACV | 0.992 (0.981–1.003) | 0.49 | 71.5 mm3 |
| Women (n=29) | |||
| Central ACD | 0.997 (0.993–1.001) | 1.97 mm | |
| Peripheral ACD | 0.942 (0.888–0.996) | 0.029 | 1.18 mm |
| ACA | 0.922 (0.867–0.996) | 0.021 | 23.9 degrees |
| ACV | 0.946 (0.901–0.992) | 0.027 | 73.5 mm3 |
Abbreviations: AUC, area under the curve; ROC, receiver operating characteristic curve; APAC, acute primary angle-closure; CI, confidence interval; ACD, anterior chamber depth; ACA, anterior chamber angle; ACV, anterior chamber volume; P, values obtained by comparison with AUC of the central ACD.
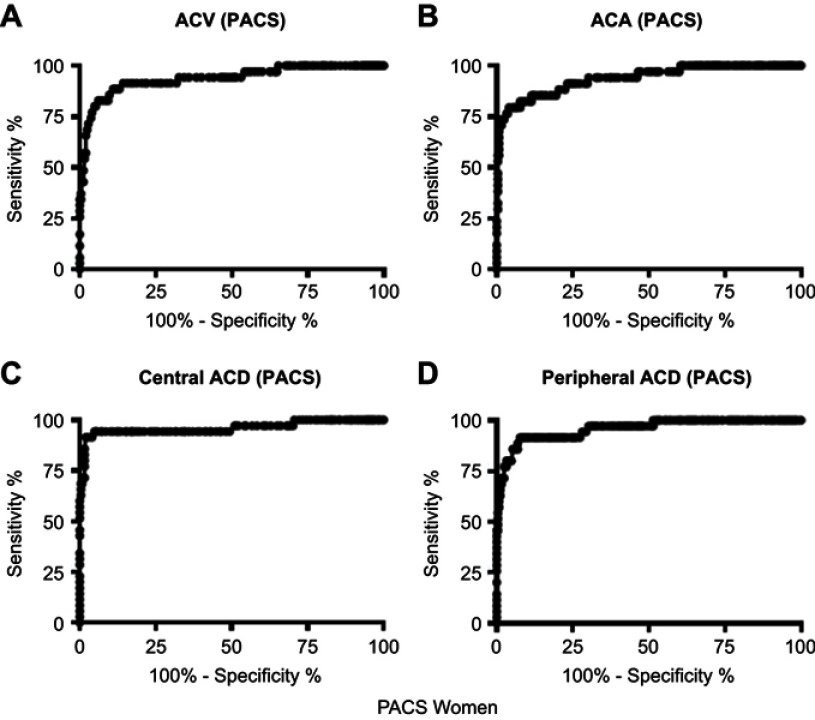
The ROC curves for each biometric variable to identify eyes with PACS in both genders were shifted leftward and downward compared with the curves for the APAC group (Figures 3–6). The AUCs ranged from 0.887 to 0.960 (Table 3). This indicates that these structural variables are less useful for identifying eyes with PACS than eyes with APAC. The optimal cutoff values were generally lower for the APAC group than for the PACS group in men (except in the case of the ACA values); we found no trends in women.
Figure 6.
Receiver operating characteristic curves for central ACD, peripheral ACD, ACA, and ACV to identify eyes with PACS in women.
Abbreviations: ACD, anterior chamber depth; ACA, anterior chamber angle; ACV, anterior chamber volume; PACS, primary angle-closure suspect.
Table 3.
AUC and optimal anterior segment biometric variable cutoff values determined by ROC curves to identify eyes with PACS
| Variable | AUC (95%CI) | P-value | Optimal cutoff value |
|---|---|---|---|
| Men (n=13) | |||
| Central ACD | 0.933 (0.859–1.006) | 1.97 mm | |
| Peripheral ACD | 0.930 (0.870–0.989) | 0.91 | 1.47 mm |
| ACA | 0.887 (0.786–0.989) | 0.61 | 24.9 degrees |
| ACV | 0.937 (0.888–0.986) | 0.89 | 96.5 mm3 |
| Women (n=35) | |||
| Central ACD | 0.960 (0.913–1.008) | 1.97 mm | |
| Peripheral ACD | 0.957 (0.921–0.993) | 0.84 | 1.18 mm |
| ACA | 0.937 (0.889–0.984) | 0.14 | 23.5 degrees |
| ACV | 0.937 (0.886–0.986) | 0.12 | 79.5 mm3 |
Abbreviations: AUC, area under the curve; ROC, receiver operating characteristic curve; PACS, primary angle-closure suspect; CI, confidence interval; ACD, anterior chamber depth; ACA, anterior chamber angle; ACV, anterior chamber volume; P, values obtained in comparison with the AUC of the central ACD.
Sensitivity and specificity for identifying eyes with APAC and PACS
The sensitivities for identifying eyes with APAC ranged from 80.0% to 100%, with the specificities ranging from 82.6% to 100% (Table 4). The sensitivities and specificities were almost the same for eyes with PACS when comparing the values between genders.
Table 4.
Sensitivity, specificity, and positive and negative predictive values of anterior segment biometric variables to identify eyes with APAC
| Variable | Sensitivity % (95%CI) | Specificity % (95%CI) | Positive predictive value % (95%CI) | Negative predictive value % (95%CI) |
|---|---|---|---|---|
| Men (n=11) | ||||
| Central ACD | 100 (71.5–100) | 100 (98.3–100) | 100 (97.3–100) | 100 (98.2–100) |
| Peripheral ACD | 100 (71.5–100) | 90.1 (85.3–93.7) | 33.3 (15.4–48.1) | 100 (97.9–100) |
| ACA | 90.9 (58.7–99.7) | 82.6 (76.9–87.5) | 26.8 (12.8–41.2) | 100 (97.8–100) |
| ACV | 100 (66.4–100) | 93.9 (89.8–96.7) | 50.0 (35.1–66.5) | 100 (98.2–100) |
| Women (n=29) | ||||
| Central ACD | 100 (86.3–100) | 90.3 (86.0–93.6) | 84.8 (68.4–97.5) | 100 (98.9–100) |
| Peripheral ACD | 88.0 (68.8–97.5) | 88.7 (84.2–92.3) | 59.1 (44.3–73.7) | 98.7 (96.4–99.9) |
| ACA | 80.0 (59.3–93.2) | 89.9 (85.5–93.3) | 62.9 (48.4–79.1) | 97.1 (94.6–99.1) |
| ACV | 84.0 (63.9–95.5) | 91.4 (87.3–94.6) | 66.7 (51.3–82.5) | 97.9 (95.6–99.3) |
Abbreviations: APAC, acute primary angle-closure; CI, confidence interval; ACD, anterior chamber depth; ACA, anterior chamber angle; ACV, anterior chamber volume.
The positive predictive values were generally low to moderate, except for that in the central ACD to identify eyes with APAC or PACS (Tables 4 and 5). This indicates that these structural variables can identify eyes with APAC and PACS to some extent but are sometimes incomplete. Conversely, the negative predictive values were almost 100%, which indicates that these optimal cutoff values are excellent indicators for excluding eyes without APAC or PACS.
Table 5.
Sensitivity, specificity, and positive and negative predictive values of anterior segment biometric variables to identify eyes with PACS
| Variable | Sensitivity % (95%CI) | Specificity % (95%CI) | Positive predictive value % (95%CI) | Negative predictive value % (95%CI) |
|---|---|---|---|---|
| Men (n=13) | ||||
| Central ACD | 76.9 (46.2–95.0) | 98.6 (95.9–99.7) | 76.9 (43.1–99.7) | 98.6 (95.9–99.7) |
| Peripheral ACD | 100 (73.5–100) | 73.2 (66.8–79.1) | 19.4 (12.4–29.1) | 100 (97.7–100) |
| ACA | 76.9 (46.2–94.9) | 90.1 (85.3–93.8) | 32.3 (20.8–50.1) | 98.5 (95.5–99.7) |
| ACV | 92.3 (64.0–99.8) | 81.7 (75.8–86.6) | 24.5 (17.1–34.3) | 99.4 (97.1–99.8) |
| Women (n=35) | ||||
| Central ACD | 91.4 (76.9–98.2) | 98.0 (95.3–99.3) | 86.5 (69.5–99.7) | 98.8 (96.3–99.9) |
| Peripheral ACD | 91.4 (76.9–98.2) | 92.2 (88.2–95.2) | 64.7 (49.2–76.8) | 99.1 (96.7–99.8) |
| ACA | 79.4 (62.1–91.3) | 95.9 (92.6–98.0) | 67.4 (51.2–82.8) | 97.5 (95.4–99.3) |
| ACV | 88.6 (73.3–96.8) | 89.0 (84.4–92.6) | 56.1 (41.7–72.1) | 98.2 (95.5–99.6) |
Abbreviations: PACS, primary angle-closure suspect; CI, confidence interval; ACD, anterior chamber depth; ACA, anterior chamber angle; ACV, anterior chamber volume.
Discussion
The results of this study indicate that the values for the anterior segment structural variables in eyes with APAC or PACS were significantly different from those in the eyes of the control group. However, the differences between the APAC and PACS groups were not significant, except in the case of the ACD values in women. The optimal cutoff values for each variable, as determined by ROC curves, showed high sensitivity and specificity for distinguishing eyes with APAC from normal eyes, and this was the case for both genders. Although the negative predictive values for these structural variables were almost 100% when using optimal cutoff values, the positive predictive values were generally low to moderate. Therefore, the results of the anterior chamber measurements using the Pentacam device can be used to rule out APAC and PACS. Moreover, the positive predictive values in the case of the central ACD values were relatively high in the APAC and PACS groups. The central ACD may represent a marker for detecting APAC and PACS but only to some extent, because its positive predictive value is not 100%.
Pakravan et al examined the fellow eyes of patients with a previous APAC attacks and reported that eyes with ACV ≤100 μL, ACD ≤2.1 mm, and ACA ≤26° may be at risk of developing APAC.8 They also reported high sensitivity and specificity for distinguishing eyes at high risk of APAC, as with our test for PACS. However, these authors did not examine the positive and negative predictive values to determine the rates of false positives and false negatives, respectively. In addition, they studied only 18 normal eyes in the control group, which may have led to an overestimation of the diagnostic abilities of these biometric variables. In contrast, we had 458 normal eyes in our control group. The fellow eyes of APAC cases are not always PACS positive.17
The AUC values, along with their sensitivity and specificity, as determined by our ROC curves were excellent for identifying eyes with APAC in both genders (particularly those of the central ACD values). This suggests that these structural variables of the ocular anterior segment are good indicators of the presence of APAC in eyes. However, the positive predictive values for these structural variables were generally low to moderate when applying optimal cutoff values, whereas the negative predictive values were almost 100%. These findings indicate that the false-positive rate was relatively high, and the false-negative rate was essentially zero.
Using these measurements, patients with values larger than the optimal cutoff values can be ruled out as having no APAC or PACS. In contrast, if their measurements have lower values than the optimal cutoff values, approximately one-half of the patients will have APAC or PACS because the positive predictive values range from 19.4% to 100%. This indicates that the structural variables should not be used solely for determining which eyes are affected by APAC or PACS. Additional clinical examinations, such as conventional slit-lamp biomicroscopy, gonioscopy, and the provocation test, are also necessary to identify eyes with APAC or PACS. We recommend that clinicians use the optimal cutoff values of these structural variables, in combination with the results of conventional slit-lamp biomicroscopy, gonioscopy, and the provocation test, to select eyes that require prophylactic treatments to prevent APAC.
Distinguishing eyes with significant APAC risks from normal eyes is important to prescribe prophylactic treatments. Without treatment, 22% of eyes with PACS progress to PAC over a period of 5 years.18 Therefore, we also analyzed the anterior segment structures of the patients with PACS to verify whether the measurements differed from those of normal eyes.
According to a report, the use of central ACD cutoff values ≤2.1 mm to identify eyes at high risk of developing APAC8 would yield a number of false-positive cases, but the optimal cutoff values in that report were higher than ours. From our results, 17 eyes out of 213 in men and 20 eyes out of 245 in women in the control group had ACD values between 2.1 and 1.97 mm. This indicates that even when using the recommended published cutoff values, identifying eyes that have a true risk of developing APAC is difficult.
Usually, eyes with acute phase PAC have not undergone surgical interventions. In most studies, the anterior segment structural variables have been examined by AS-OCT or a Pentacam in eyes with a history of APAC treated surgically.19 Sng et al reported on the results of pretreatments based on anterior segment images obtained by AS-OCT during the acute phase of APAC and identified a shallower ACD and steeper iris curvature in eyes with APAC than in fellow eyes.20 Although we did not examine the iris curvature of eyes with APAC in our study, we did not measure the central ACDs and found that the measurement may represent a marker for detecting APAC and PACS.
The results of the Kumejima study showed that APAC affects more women than men.3 In addition, an Asian study reported that women have narrower anterior segment biometric values than men as determined by AS-OCT values.21 Because the AS-OCT light sources are different from a Pentacam, we could not compare our ACA values with those. However, the anterior segment biometric variables differ between men and women; therefore, we analyzed the results by dividing our indivduals into men and women.
While using the central ACD to diagnose APAC and PACS, it is difficult to distinguish eyes with plateau iris syndrome from normal eyes. The ACA should be a better structural variable for differentiating eyes with APAC or PACS associated with plateau iris syndrome.
Conclusion
In conclusion, the anterior segment structural variables quantified by a Pentacam device may be used to identify eyes with APAC and PACS with high sensitivity and specificity. When applying the optimal cutoff values determined by ROC curves, we found that the positive predictive values were not good enough to distinguish eyes with APAC from those with PACS. However, given the high negative predictive values for these structural variables (almost 100%) when using the optimal cutoff values, these structural variables should be useful to identify eyes without APAC or PACS.
Abbrevation list
ACA, Anterior chamber angle; ACD, Anterior chamber depth; ACV, Anterior chamber volume; APAC, Acute primary angle-closure; AUC, Area under the curve; IOP, Intraocular pressure; PAC, Primary angle-closure; PACG, Primary angle-closure glaucoma; PACS, Primary angle-closure suspect; PD, Pupil diameter; ROC, Receiver operating characteristic.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Quigley HA, Broman AT. The number of people with glaucoma worldwide in 2010 and 2020. Br J Ophthalmol. 2006;90(3):262–267. doi: 10.1136/bjo.2005.081224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yamamoto T, Iwase A, Araie M, et al; Tajimi Study Group, Japan Glaucoma Society. The Tajimi Study report 2: prevalence of primary angle closure and secondary glaucoma in a Japanese population. Ophthalmology. 2005;112(10):1661–1669. [DOI] [PubMed] [Google Scholar]
- 3.Sawaguchi S, Sakai H, Iwase A, et al. Prevalence of primary angle closure and primary angle-closure glaucoma in a southwestern rural population of Japan: the Kumejima Study. Ophthalmology. 2012;119(6):1134–1142. doi: 10.1016/j.ophtha.2011.12.038 [DOI] [PubMed] [Google Scholar]
- 4.Shaffer RN. Primary glaucomas. Gonioscopy, ophthalmoscopy and perimetry. Trans Am Acad Ophthalmol Otolaryngol. 1960;64:112–127. [PubMed] [Google Scholar]
- 5.Van Herick W, Shaffer RN, Schwartz A. Estimation of width of angle of anterior chamber. Incidence and significance of the narrow angle. Am J Ophthalmol. 1969;68:626–629. doi: 10.1016/0002-9394(69)91241-0 [DOI] [PubMed] [Google Scholar]
- 6.Smith RJ. A new method of estimating the depth of the anterior chamber. Br J Ophthalmol. 1979;63:215–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dabasia PL, Edgar DF, Murdoch IE, Lawrenson JG. Noncontact screening methods for the detection of narrow anterior chamber angles. Invest Ophthalmol Vis Sci. 2015;56(6):3929–3935. doi: 10.1167/iovs.15-16727 [DOI] [PubMed] [Google Scholar]
- 8.Pakravan M, Sharifipour F, Yazdani S, Koohestani N, Yaseri M. Scheimpflug imaging criteria for identifying eyes at high risk of acute angle closure. J Ophthalmic Vis Res. 2012;7(2):111–117. [PMC free article] [PubMed] [Google Scholar]
- 9.Li X, Wang Z, Cao Q, Hu L, Tian F, Dai H. Pentacam could be a useful tool for evaluating and qualifying the anterior chamber morphology. Int J Clin Exp Med. 2014;7(7):1878–1882. [PMC free article] [PubMed] [Google Scholar]
- 10.Tornquist R. Shallow anterior chamber in acute glaucoma; a clinical and genetic study. Acta Ophthalmol Suppl. 1953;39:1–74. [PubMed] [Google Scholar]
- 11.Alsbirk PH. Anterior chamber depth and primary angle-closure glaucoma. I. An epidemiologic study in Greenland Eskimos. Acta Ophthalmol (Copenh). 1975;53:89–104. doi: 10.1111/j.1755-3768.1975.tb01142.x [DOI] [PubMed] [Google Scholar]
- 12.Konstantopoulos A, Hossain P, Anderson DF. Recent advances in ophthalmic anterior segment imaging: a new era for ophthalmic diagnosis? Br J Ophthalmol. 2007;91(4):551–557. doi: 10.1136/bjo.2006.103408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Oka N, Otori Y, Okada M, Miki A, Maeda N, Tano Y. Clinical study of anterior ocular segment topography in angle-closure glaucoma using the three-dimensional anterior segment analyzer pentacam. Nihon Ganka Gakkai Zasshi. 2006;110(5):398–403. [PubMed] [Google Scholar]
- 14.Rabsilber TM, Khoramnia R, Auffarth GU. Anterior chamber measurements using pentacam rotating scheimpflug camera. J Cataract Refract Surg. 2006;32(3):456–459. [DOI] [PubMed] [Google Scholar]
- 15.Rossi GC, Scudeller L, Delfino A, et al. Pentacam sensitivity and specificity in detecting occludable angles. Eur J Ophthalmol. 2012;22(5):701–708. doi: 10.5301/ejo.5000108 [DOI] [PubMed] [Google Scholar]
- 16.Lee JR, Sung KR, Han S. Comparison of anterior segment parameters between the acute primary angle closure eye and the fellow eye. Invest Ophthalmol Vis Sci. 2014;55(6):3646–3650. doi: 10.1167/iovs.13-13009 [DOI] [PubMed] [Google Scholar]
- 17.Foster PJ, Buhrmann R, Quigley HA, Johnson GJ. The definition and classification of glaucoma in prevalence surveys. Br J Ophthalmol. 2002;86(2):238–242. doi: 10.1136/bjo.86.2.238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Thomas R, George R, Parikh R, Muliyil J, Jacob A. Five year risk of progression of primary angle closure suspects to primary angle closure: a population based study. Br J Ophthalmol. 2003;87(4):450–454. doi: 10.1136/bjo.87.4.450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ang GS, Wells AP. Changes in Caucasian eyes after laser peripheral iridotomy: an anterior segment optical coherence tomography study. Clin Exp Ophthalmol. 2010;38(8):778–785. doi: 10.1111/j.1442-9071.2010.02360.x [DOI] [PubMed] [Google Scholar]
- 20.Sng CCA, Aquino MCD, Liao J, et al. Pretreatment anterior segment imaging during acute primary angle closure. Insights into angle closure mechanisms in acute phase. Ophthalmology. 2014;121(1):119–125. doi: 10.1016/j.ophtha.2013.08.004 [DOI] [PubMed] [Google Scholar]
- 21.Huang W, Gao X, Li X, et al. Anterior and posterior ocular biometry in healthy Chinese subjects: data based on AS-OCT and SS-OCT. PLoS One. 2015;10(3):e0121740. doi: 10.1371/journal.pone.0121740 [DOI] [PMC free article] [PubMed] [Google Scholar]