Abstract
Purpose
We compared the three-dimensional printed non-coplanar template (3DPNCT) plans with 3D-printed coplanar template (3DPCT) plans for radioactive seed implantation (RSI) in lung cancer and explored the differences between the two technologies.
Material and methods
33 patients with peripheral lung cancer that received 3DPCT-assisted RSI in our department between June 2017 and February 2018 were analyzed. A 3DPNCT plan was re-designed for all patients. The prescribed dose and seed activity in the new plan were the same as the 3DPCT plan. The data in the two plans were compared, including seed number, needle number, number of needles needed to cross the ribs, and dosimetry parameters. Dosimetry parameters included D90, Dmean, MPD (minimum peripheral dose), V100, V150, CI (conformity index), EI (external index), HI (homogeneity index) of target volume, D2cc of spinal cord and aorta, and V20 of affected side lung. We used a paired t-test and two groups of related non-parameters tests to examine statistical significance. A p value < 0.05 was considered statistically significant.
Results
We found no significant difference in dosimetry parameters (p > 0.05), except MPD. The mean MPD of the 3DPNCT plan was significantly higher than the 3DPCT plan (88.5 Gy and 81.8 Gy, respectively, p = 0.017). The number of needles used in the 3DPNCT plan and the number of needles needed to cross the ribs were significantly less compared with the 3DPCT plan (p = 0.000).
Conclusions
The dose distributions of the two 3DPCT plans were similar. 3DPNCT plan had a higher dose in target volume margin, with fewer needles and fewer breaks to the ribs.
Keywords: 3D printing template, seed implantation, dosimetry, peripheral lung cancer
Purpose
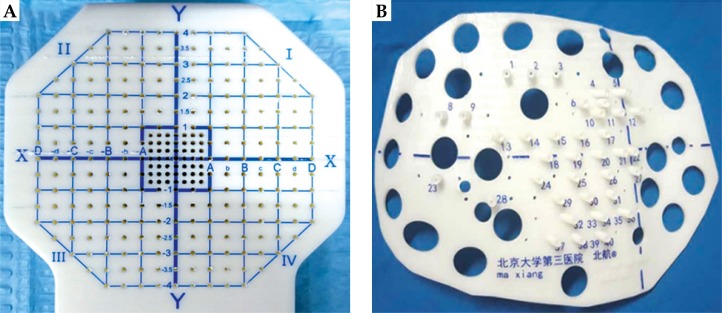
Radioactive seed implantation plays an important role in the treatment of local tumors [1]. The image-guided brachytherapy treatment planning system (B-TPS) has been widely used in the procedure of radioactive seed implantation, including preoperative plan design, intraoperative optimization, and post-operative evaluation. However, due to the error of insertion and complex anatomical structure, implantation highly depends on the experience of the clinician, if only guided by image alone, which might give rise to a significant deviation between the post-operative dose and preoperative planning. The appearance of the three-dimensional printing template (3DPT) technology has been deemed a milestone for radioactive seed implantation. Guided by the 3DPT, preoperative planning can be accurately realized in surgery [2]. Currently, 3DPT is divided into two types: 3D-printing non-coplanar template (3DPNCT), characterized by individualization, and non-coplanarity of the needle path and 3D-printing coplanar template (3DPCT), characterized by all needles path in the same direction [3] (Figure 1). In the present study, we compared the application of 3DPNCT and 3DPCT, which included designing the radioactive seed implantation plan for a thoracic tumor, and further analyzed the differences at the planning step to provide theoretical support for choosing a more reliable implantation technology and a more optimal strategy.
Fig. 1.
Three-dimensional printing template: A) 3D-printing coplanar template (3DPCT) (the distance between needles path is 5 mm); B) 3D-printing non-coplanar template (3DPNCT) (the little holes around the guide post on the template were pre-reserved needles path)
Material and methods
Clinical general information
We enrolled 33 patients with peripheral lung cancer who received computed tomography (CT)-guided radioactive iodine-125 (125I) seeds assisted by 3DPCT at our department. Our patients selection criteria were as follows: 1) failure to carry out surgery or external radiotherapy; 2) solitary tumor and tumor size ≤ 8 cm; 3) definitive pathological diagnosis; 4) suitable puncture access: bones and large blood vessels avoiding; 5) no bleeding tendency; 6) good general condition (Karnofsky performance status > 70) with expected survival > 3 months. All patients had complete radiological information and preoperative planning data. The general information, such as target lesions and preoperative planning are shown in Table 1.
Table 1.
General clinical information
| Characteristics | |
|---|---|
| Sex (n) | |
| Male | 20 |
| Female | 13 |
| Age (years)* | 61 (17-84) |
| Lesion location (n) | |
| Superior lobe of left lung | 5 |
| Upper lobe of left lung | 7 |
| Superior lobe of right lung | 7 |
| Middle lobe of right lung | 9 |
| Upper lobe of right lung | 5 |
| Lesion length (cm)* | 4.5 (2.1-7.5) |
| Lesion volume (cc)* | 36.2 (3.2-204.5) |
| Prescribed dose (Gy)* | 160 (120-170) |
| Radioactivity (mCi)* | 0.68 (0.58-0.8) |
The data are shown as median value (range).
System planning
B-TPS (KLSIRPS-3D) was provided by the Beijing University of Aeronautics and Astronautics and Beijing Astro Technology Ltd., Co. Planning system source data originated from the official and latest manuscripts of the American Association of Physicists in Medicine (AAPM), and the 125I seeds model calculation algorithm was MCNP (Monte Carlo N Particle Transport Code) based on water phantom [4,5]. The seeds type was 6711_1985 (Shanghai GMS Pharmaceutical Co., Ltd.). The brachytherapy treatment planning system have been inputted the model to consider the inter-seed attenuation effect [6].
Preoperative planning
All procedures were conducted according to the international guideline [3]. Two days before the operation, patients obtained a CT-scan (Brilliance Bigbore CT, Philips Co., Ltd.) to locate the lesions, using a slice thickness of 5 mm. The patient’s position depended on the lesion location and included supine, prone, and lateral positions. We fixed the patients with a vacuum pad and marked position lines and template-guiding lines on their skin surface. CT imaging data were transmitted to B-TPS to design the preoperative plan, such as delineating gross tumor volume (GTV) and organs at risk (OARs) (including spinal cord, aorta, and affected lung), defining prescribed doses and radioactivity of seeds, determining needle pathway (distribution and orientation), calculating the number of radioactive seeds and their spatial distribution, and calculating the dosimetric distribution into GTV and OARs (spinal cord, aorta, and affected lung). We optimized the treatment plan (the physicist adjusted the distribution of needles and seeds manually) to ensure that doses of 90% GTV (D90 of GTV) matched the prescribed doses as closely as possible. As recommended in previously published literature, the prescribed dose was 120-170 Gy [3].
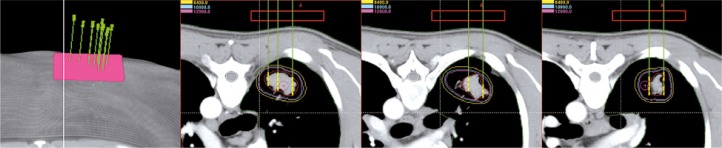
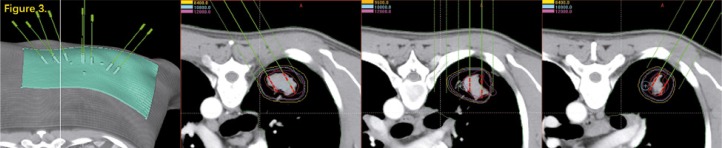
All patients have completed the treatment and the image data, target area, and treatment plan assisted by 3DPCT were available in B-TPS (Figure 2). We re-designed the treatment plan assisted by 3DPNCT for all patients in B-TPS (Figure 3). The prescribed doses and seeds radioactivity of the new plan (3DPNCT plan) was the same as the original plan (3DPCT plan).
Fig. 2.
The CT image following 3DPCT. The needle pathway was consistent in the different scanning layers
Fig. 3.
The CT image following 3DPNCT. The needle orientation was different in different scanning layers. The dotted line was pre-reserved for the virtual access
Comparison and evaluation of planning
We compared several parameters of the 3DPNCT and 3DPCT treatment plans and analyzed the differences. The parameters included seed numbers, needle numbers, number of ribs passed through by the needles, and other related dosimetric parameters. The dosimetric parameters included D90, mean doses of GTV (Dmean), volume percent of GTV receiving 100% prescribed dose (V100), volume percent of GTV receiving 150% prescribed dose (V150), volume percent of GTV receiving 200% prescribed dose (V200), and minimum peripheral dose of GTV (MPD). The conformity index (CI) was used to evaluate the conformity of dose distribution [7]: CI = (VT, ref/VT) × (VT, ref/Vref), where VT, VT, ref, and Vref were the volume of GTV, the volume of GTV with the prescribed dose, and the total volume covered by prescribed dose (cm3), respectively. The ideal CI is 1, showing that the prescribed dose just covers the GTV, and the dose of outside GTV less than the prescribed dose. The greater the CI, the greater the volume of GTV with the prescribed dose and the smaller the volume outside GTV with the prescribed dose. The external index (EI) was used to describe the percentage of the volume outside GTV, exceeding the prescribed dose to the volume of GTV [8]: EI = (Vref – VT,ref)/VT × 100%. The most ideal EI is 0, showing that the dose of all volumes outside GTV are less than the prescribed dose. The greater the EI, the greater the volume of outside GTV with the prescribed dose. The homogeneity index (HI) was used to describe the homogeneity of dose distribution [8]: HI = (VT,ref – VT,1.5ref)/VT,ref × 100%, where VT,1.5ref was the volume of GTV with 150% prescribed dose (cm3). The most ideal HI is 100%, the greater the HI, the more homogenous the dose distribution of GTV. In terms of normal tissues, D2cc was used for the dose evaluation of the spinal cord and thoracic aorta (the dose received by normal tissues in the range of 2 cm3), and V20 was used for the lung on the affected side (the percentage of lung volume received 20 Gy dose).
Statistical methods
The Shapiro-Wilk test was used to verify whether the planning data of the two groups (3DPNCT and 3DPCT) conformed to the normal distribution. For data that conformed to the normal distribution, a paired sample t-test was used for the comparison. Non-parametric correlation sample rank sum test (Wilcoxon) was used for data that did not conform to the normal distribution. P < 0.05 was considered statistically significant. SPSS 20.0 software was used for the statistical analysis.
Results
The results of each parameter were compared between 3DPNCT and 3DPCT (Table 2). D90, Dmean, MPD, and V150 conformed to a normal distribution using a paired t-test (Table 2). A non-parametric rank sum test was used for the remaining parameters. There were no significant differences among the dosimetric parameters (p > 0.05), except for MPD. MPD of 3DPNCT was significantly higher than 3DPCT (88.5 Gy vs. 81.8 Gy, p = 0.017). The number of radioactive seeds used by both groups (3DPNCT and 3DPCT) was similar (mean, 61 and 60, respectively), with no statistical difference. However, the needle number and the number of needles passing through the ribs following 3DPNCT were both significantly less compared with 3DPCT (p = 0.000).
Table 2.
Parameter results compared between 3DPNCT and 3DPCT
| Parameters | Non-coplanar | Coplanar | Normal distribution test (Shapiro-Wilk) | p (Paired t/Wilcoxon tests) | ||||
|---|---|---|---|---|---|---|---|---|
| Interval | Median | Mean | Interval | Median | Mean | |||
| Number of seeds | 12-175 | 45.0 | 61.7 ±42.00 | 14-166 | 49.0 | 60.5 ±41.35 | Inconformity | 0.137 |
| Number of needles | 4-34 | 11.0 | 13.4 ±8.07 | 4-39 | 13.0 | 15.2 ±9.63 | Inconformity | 0.000 |
| Number of through rib needles | 0-15 | 1.0 | 2.8 ±3.89 | 0-28 | 5.0 | 7.0 ±6.01 | Inconformity | 0.000 |
| D90(Gy) | 142.3-176.5 | 163.2 | 163.8 ±8.25 | 129.6-195.4 | 162.6 | 162.8 ±11.62 | Conformity | 0.494 |
| Dmean(Gy) | 261.0-409.4 | 332.8 | 336.5 ±33.79 | 256.8-463.7 | 321.7 | 326.0 ±41.92 | Conformity | 0.136 |
| mPD (Gy) | 46.1-133.2 | 92.1 | 88.5 ±21.71 | 38.2-124.6 | 82.2 | 81.8 ±24.75 | Conformity | 0.017 |
| V100(%) | 90.0-98.3 | 91.0 | 91.5 ±1.60 | 89.3-100.0 | 90.9 | 91.6 ±2.39 | Inconformity | 0.662 |
| V150(%) | 40.6-77.0 | 62.3 | 61.4 ±7.46 | 36.0-87.1 | 60.0 | 59.3 ±10.20 | Conformity | 0.285 |
| V200(%) | 15.3-52.9 | 28.8 | 30.1 ±8.57 | 11.8-60.8 | 26.5 | 28.8 ±12.34 | Inconformity | 0.561 |
| CI | 0.52-0.84 | 0.73 | 0.71 ±0.10 | 0.47-0.83 | 0.74 | 0.71 ±0.10 | Inconformity | 0.514 |
| EI (%) | 6.5-69.1 | 23.1 | 29.2 ±18.84 | 9.2-110.9 | 20.0 | 29.7 ±23.72 | Inconformity | 0.330 |
| HI (%) | 17.7-55.6 | 31.6 | 32.9 ±7.74 | 12.5-73.8 | 33.4 | 36.6 ±12.03 | Conformity | 0.090 |
| D2cc (Gy) of spinal cord | 0-69.1 | 3.5 | 8.8 ±14.30 | 0-75.4 | 3.4 | 8.7 ±15.00 | Inconformity | 0.117 |
| D2cc (Gy) of aorta | 0-146.2 | 1.0 | 16.2 ±32.44 | 0-142.6 | 0.92 | 12.8 ±28.09 | Inconformity | 0.434 |
| V20 (%) of affected lung | 1.7-64.2 | 15.4 | 21.1 ±17.49 | 1.8-70.0 | 14.0 | 20.3 ±17.45 | Inconformity | 0.160 |
Discussion
3D printing template have solved the shortcomings of free-handed puncture, such as heavy dependence on personal experience of operators, uneven implantation quality, lack of effective quality control methods, etc. It greatly improves the accuracy, safety, and efficiency of seed implantation operation [2,3,9]. Some evidence has indicated that both 3DPNCT and 3DPCT could guarantee the guidance of the insertion needle and the consistency between the post-operative dose and the preoperative plan [10,11]. However, for thoracic tumors, the choice of planning templates to design the treatment remains unclear, due to the motion of the tumor and the position of ribs as an obstacle. The present study fully compared the difference between the 3DPNCT and 3DPCT plans. Furthermore, our study was instructional for constructing a feasible choice of guiding-template and understanding the standard process of radioactive seed implantation. Because of the high-risk of seed implantation for central lung cancer patients and the quality of implantation that cannot be guaranteed, our study refined the treatment site of peripheral lung cancer lesions to ensure the feasibility of the plan.
In the present study, most dosimetric parameters showed no significant difference between the target area and normal tissues, suggesting that both 3DPNCT and 3DPCT were able to meet the dosimetrics needed, which was consistent with previous studies [2,9,10,11,12]. MPD is another expression for D100, which means the minimum dose that can cover the target area [3]. MPD following 3DPNCT was significantly higher than 3DPCT, suggesting that the lesion margin could receive higher doses with 3DPNCT planning. In Ma et al. study, V100 and D90 were 5% and 8%, and the 2-year overall survival and local control rates improved to 18.9% and 23.3%, respectively [13]. Therefore, 3DPNCT planning demonstrated a potential advantage to increase local control rate and to reduce recurrence.
In terms of the number of needle punctures, the treatment planning based on 3DPNCT was significantly better than 3DPCT (p < 0.05). These results indicated that 3DPNCT could significantly reduce the total number of needle punctures and avoid the needles passing through the ribs, which obviously shortens the operation time, and reduces the risk of side effects and suffering of the patients. It is more flexible to design treatment planning under 3DPNCT (puncture by coplanar and non-coplanar are both available), which corresponds to the concept of individualized treatment. Although we need to optimize the intra-operative planning and pre-reserve needle pathways due to tumor motion and operation error, we could perform the surgery well assisted by 3DPNCT [11]. The shortcoming of 3DPNCT compared with 3DPCT is that the pre-reserved needle path is only a hole and not controlled by the guide post. When using pre-reserved needle path for puncture during the actual procedure, the quality of puncture is greatly affected by personal experience. It may be necessary to verify the needle position and adjust the direction of needle repeatedly, which may affect the efficiency and increase the risk of complications of the operation [14]. In addition, both plan designing and template manufacturing of 3DPNCT are quite complex and expensive, which impair its popularization.
Conclusions
The major dosimetric parameters of 3DPNCT planning are similar to 3DPCT plans. Both are able to meet the clinical treatment needs. However, the margin dose is higher and the total number of needles and needles through the ribs are reduced using 3DPNCT planning compared with 3DPCT, which indicates that 3DPNCT has potential advantages to improve local control, reduce operation risk, and benefit more patients.
Disclosure
Authors report no conflict of interest.
References
- 1.Wang J, Chai S, Zheng G, et al. Expert consensus statement on computed tomography-guided (125)I radioactive seeds permanent interstitial brachytherapy. J Cancer Res Ther. 2018;14:12–17. doi: 10.4103/jcrt.JCRT_888_17. [DOI] [PubMed] [Google Scholar]
- 2.Ji Z, Jiang Y, Guo F, et al. Dosimetry verification of radioactive seed implantation for malignant tumors assisted by 3D printing individual templates and CT guidance. Appl Radiat Isot. 2017;124:68–74. doi: 10.1016/j.apradiso.2016.12.009. [DOI] [PubMed] [Google Scholar]
- 3.Wang J, Zhang F, Guo J, et al. Expert consensus workshop report: Guideline for three-dimensional printing template-assisted computed tomography-guided (125)I seeds interstitial implantation brachytherapy. J Cancer Res Ther. 2017;13:607–612. doi: 10.4103/jcrt.JCRT_412_17. [DOI] [PubMed] [Google Scholar]
- 4.Nath R, Anderson LL, Luxton G, et al. Dosimetry of interstitial brachytherapy sources: recommendations of the AAPM Radiation Therapy Committee Task Group No. 43. American Association of Physicists in Medicine. Med Phys. 1995;22:209–234. doi: 10.1118/1.597458. [DOI] [PubMed] [Google Scholar]
- 5.Rivard MJ, Coursey BM, DeWerd LA, et al. Update of AAPM Task Group No. 43 Report: A revised AAPM protocol for brachytherapy dose calculations. Med Phys. 2004;31:633–674. doi: 10.1118/1.1646040. [DOI] [PubMed] [Google Scholar]
- 6.Safigholi H, Sardari D, Karimi Jashni S, et al. An analytical model to determine interseed attenuation effect in low-dose-rate brachytherapy. J Appl Clin Med Phys. 2013;14:4226. doi: 10.1120/jacmp.v14i3.4226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Van’t Riet A, Mak AC, Moerland MA, et al. A conformation number to quantify the degree of conformality in brachytherapy and external beam irradiation: application to the prostate. Int J Radiat Oncol Biol Phys. 1997;37:731–736. doi: 10.1016/s0360-3016(96)00601-3. [DOI] [PubMed] [Google Scholar]
- 8.Saw CB, Suntharalingam N. Quantitative assessment of interstitial implants. Int J Radiat Oncol Biol Phys. 1991;20:135–139. doi: 10.1016/0360-3016(91)90149-x. [DOI] [PubMed] [Google Scholar]
- 9.Ji Z, Jiang Y, Su L, et al. Dosimetry verification of 125I seeds implantation with three-dimensional printing noncoplanar templates and CT guidance for paravertebral/retroperitoneal malignant tumors. Technol Cancer Res Treat. 2017:1533034617723221. doi: 10.1177/1533034617723221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhe J, Yuliang J, Fuxin G, et al. Dosimetry evaluation for CT guided 125I seeds implantation assisted by three-dimensional-printing coplanar coordinate template in chest malignant tumor. Chin J Nucl Med Mol Imaging. 2018;38:4–8. [Google Scholar]
- 11.Zhe J, Yuliang J, Fuxin G, et al. Dosimetric assessment of CT-guided radioactive seed implantation assisted by 3D printing non-coplanar template in treatment of chest malignant tumor. Chin J Radiat Oncol. 2017;26:754–758. [Google Scholar]
- 12.Ran P, Yuliang J, Zhe J, et al. Dosimetric analysis of 3D-printed coplanar template-assisted and CT-guided 125I seed implantation for the treatment of malignant tumors. Chin J Radiat Oncol. 2017;26:1062–1066. [Google Scholar]
- 13.Ma X, Yang Z, Jiang S, et al. Effectiveness and safety of a robot-assisted 3D personalized template in 125I seed brachytherapy of thoracoabdominal tumors. J Contemp Brachytherapy. 2018;10:368–379. doi: 10.5114/jcb.2018.77957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yang XH, Huang XY. Complications of CT-guided percutaneous lung puncture biopsy: an analysis of influencing factors. J Interv Radiol. 2013;22:658–662. [Google Scholar]