Abstract
A 35-year-old man presented to his optician with sudden onset diplopia and a 1-week history of headaches. He was noted to have sixth nerve palsy. The following day he was admitted to hospital with confusion and expressive dysphasia. He had been due to travel to Ghana on business and had received yellow fever (YF) vaccination 18 days prior to onset of headaches. His initial cerebrospinal fluid (CSF) revealed elevated protein, increased white cell count but was PCR negative for standard viral pathogens. Herpes simplex virus (HSV)-1 was detected by PCR in CSF at a very low level from a second lumbar puncture performed 6 days later, and the patient was treated for HSV meningoencephalitis. However, retrospective investigation for yellow fever vaccine-associated neurological disease revealed increasing titres of YF IgG in three serial CSF samples, and no evidence of HSV antibodies in CSF or plasma, ruling out HSV encephalitis.
Keywords: infection (neurology), meningitis, vaccination/immunisation
Background
Yellow fever (YF) virus is an arbovirus endemic in many countries in Africa and South America. The mortality rate of YF during epidemics can rise to 50%1; however, there has been an effective live-attenuated vaccine available since the 1930s. YF is the only disease for which countries may require a certificate of vaccination as a condition of entry,2 and as such over 125 000 doses of the YF vaccine are administered in the UK each year.3 4 The vaccine is not recommended in infants younger than 6 months of age due to the risk of postinfectious encephalitis and a risk assessment is advised prior to vaccinating patients over the age of 60 years.5 However, more recently, a new pattern of neurological adverse events have been recognised, termed yellow fever vaccine-associated neurological disease (YEL-AND) and occurring in vaccine recipients of any age. YEL-AND presents within 30 days of vaccination, symptoms include fever and headache, and can progress to one or more of the following: focal neurological dysfunction, confusion, seizures, Guillain-Barré syndrome, encephalomyelitis and coma.5 6 This case aims to increase awareness of YEL-AND, especially in those age groups not classically associated with adverse events, and to also highlight the relative difficulty in diagnosing this disease.
Case presentation
A 35-year-old British man presented to his optician with sudden onset horizontal diplopia and reported frontal headaches for the previous 7 days. He was referred to the emergency ophthalmology department, who diagnosed a right-sided sixth cranial nerve palsy and arranged an outpatient MRI for the following week. However, the next day he presented to the emergency department with confusion, word finding difficulty and fever. The patient had no significant previous medical history other than depression for which he was taking fluoxetine. His young daughter had been hospitalised with bronchiolitis and pneumonia the week prior to his admission and Streptococcus pyogenes had been isolated in her throat swab. The patient had been due to travel to Ghana for business and had received several travel vaccines; hepatitis A, typhoid, combined diphtheria, tetanus and polio, and YF vaccine were administered 18 days prior to onset of his headaches. In addition, he had received the meningitis ACWY vaccine 7 days prior to symptom onset. He had also taken two doses of atovaquone/proguanil for malaria prophylaxis. The patient did not report any recent travel, and had never travelled outside of Europe. He was admitted to hospital with suspected meningoencephalitis and commenced on empirical intravenous aciclovir and ceftriaxone. After 3 days without improvement of symptoms, the patient was transferred to the specialist Infectious Diseases Unit at the Queen Elizabeth University Hospital, Glasgow.
Investigations
The patient had a CT of the head, chest X-ray and electroencephalography (EEG) on admission, all of which were normal. He subsequently had an MRI of the head, which was also unremarkable with no features suggestive of encephalitis. Liver function tests were normal, and all other biochemistry and haematology results were within range, except for a mild lymphopenia (1.0×109/L). Autoimmune encephalitis was investigated and antibodies to a ganglioside panel, LGI1, CASPR2, dsDNA and the N-methyl-D-aspartate (NMDA) receptor were all negative. The antinuclear antibodies (ANA) screen was weakly positive (titre 1:160, homogenous pattern). Blood cultures were negative, serology tests including hepatitis A, B, C and E, HIV-1/2, syphilis, Lyme disease and Q fever were negative, Cryptococcus neoformans antigen test was negative and the interferon-gamma release assay for tuberculosis was also negative. A respiratory sample was positive for respiratory syncytial virus (RSV) by PCR (influenza A/B, rhinovirus, adenovirus, parainfluenza 1–4, human metapneumovirus, coronavirus, Mycoplasma pneumoniae, Bordetella pertussis and mumps in respiratory samples were all negative). A lumbar puncture (LP) was performed 2 days after admission and the cerebrospinal fluid (CSF) demonstrated a raised protein (0.8 g/L) and a high white cell count (WCC) (231 cells/µL) consisting of 100% lymphocytes, glucose was normal. Bacterial culture was negative and viral PCR was negative for herpes simplex virus (HSV) 1 and 2, varicella zoster virus (VZV), enterovirus, parechovirus and RSV. A second CSF was collected 6 days later and this was again sent to the local biochemistry, microbiology and virology laboratory, in addition CSF and plasma were sent to the Rare and Imported Pathogens Laboratory (RIPL), Porton, for YF antibody and RNA detection. In this second sample, the protein was again raised (1.05 g/L), WCC had reduced to 120 cells/µL, glucose was normal, culture was negative; however, HSV-1 DNA was detected by PCR at a very low level (cycle threshold (Ct) 38.49). The virology laboratory advised that this may be a false positive result, as the initial CSF, collected 9 days after symptom onset, was HSV-1 negative and the second sample was only very weakly positive. However, due to the patient’s neurological symptoms, CSF pleocytosis and the potential severity of untreated HSV encephalitis, the patient was treated with 21 days intravenous aciclovir for possible HSV-1 meningoencephalitis. After completion of aciclovir treatment, a third LP was performed, the protein was mildly raised (0.59 g/L), the WCC was 26 cells/µL and viral PCR was negative. The patient was discharged the following day after 22 days on the ward.
Outcome and follow-up
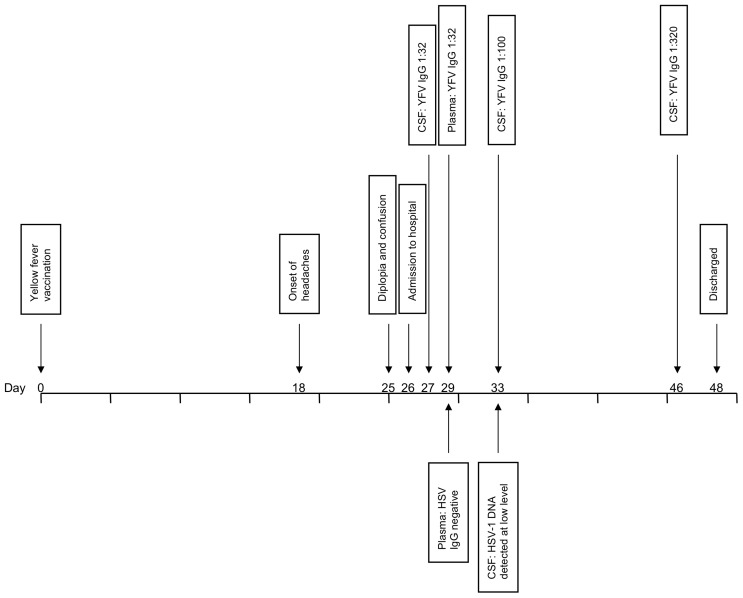
The patient’s sixth nerve palsy resolved within a few days and no double vision was documented after day 7 of his admission. On discharge, the patient was still experiencing headaches, and reported fatigue and myalgia. The day prior to his discharge the reference laboratory reported that YF IgG was detected in the patient’s plasma at a titre of 1:32 and at a titre of 1:100 in the CSF, suggesting local IgG production within the central nervous system (CNS). YF virus RNA was negative in both plasma and CSF (figure 1). On receipt of these results, further investigation into a potential YEL-AND diagnosis was then performed. All three CSF samples collected during the patient’s admission were retrospectively tested in parallel at RIPL; titres of YF IgG increased from 1:32 to 1:100 to 1:320 (figure 1). The CSF was also tested for measles, rubella, VZV and HSV IgG, with no intrathecal antibody production detected for any of these viruses, casting further doubt on the diagnosis of an HSV meningoencephalitis and increasing suspicion that the patient’s symptoms were due to YEL-AND. The patient returned to an outpatient clinic 1 month after discharge, at which time he still reported severe headaches. At this point, the patient was finding it difficult to concentrate for long periods of time and his depression symptoms had worsened. The patient attended a final outpatient appointment 3 months later, by which time the headaches had subsided, and he was now returning to work in a phased manner. At this appointment (4 months after symptom onset), blood was tested for HSV type specific antibodies; no IgG was detected to either HSV-1 or HSV-2, ruling out HSV-1 as the cause of the patient’s neurological symptoms and hospital admission.
Figure 1.
Clinical course timeline. CSF, cerebrospinal fluid; HSV, herpes simplex virus; YFV, yellow fever vaccine.
Discussion
This case met the definition of ‘suspect’ YEL-AND as defined by McMahon et al in 20076; a history of fever, mental status change, focal neurological dysfunction, pleocytosis and elevated protein in the CSF. Furthermore, presenting within 30 days of YF vaccination and with no evidence of an alternative diagnosis. There was an initial delay in investigating YEL-AND as a differential diagnosis for a number of reasons. First, although YEL-AND had been mentioned as a potential diagnosis at admission, it was disregarded due to the patients young age. Second, after a low level HSV-1 PCR positive result on his second CSF sample, the patient was treated for an HSV meningoencephalitis. This proved to be a misdiagnosis as follow-up testing confirmed that the patient was negative for HSV antibodies 4 months after discharge. The results from the reference laboratory demonstrated intrathecal YF antibody production, with titres of YF IgG in the CSF increasing in serial samples, and titres in the CSF greater than that of the plasma.
More recent diagnostic criteria for YEL-AND have been produced by the Centre for Disease Control and Prevention (CDC).1 To meet the criteria for a case to be considered ‘probable’ or ‘definite’ requires one of the following: isolation of vaccine virus strain in serum or CSF, virus quantitation in serum, YF-specific IgM in CSF or amplification of vaccine virus strain in the CSF (box 1). Of these tests, only amplification of YF by reverse transcriptase PCR is available in the UK. Detection of YF RNA in the CSF is often negative in published cases of YEL-AND,7–9 most likely due to the period between vaccination and presentation, hence confirming a diagnosis of YEL-AND in the UK is problematic. YF IgM in CSF is considered diagnostic of a definitive case of YEL-AND, as IgM is not believed to cross the blood–brain barrier and as such is assumed to result from local virus replication.1 As CSF IgM testing is not available in the UK, investigation of intrathecal IgG antibody production was performed in this case. Plasma and CSF were tested for HSV, measles, VZV, rubella and YF IgG. Plasma was positive for measles, VZV, rubella and YF IgG and the CSF was positive for YF IgG only, suggesting that the YF IgG present in the CSF was due to local antibody production. Demonstrating intrathecal production of pathogen-specific IgG is an established method of proving viral invasion of the CNS, widely accepted in the field of neurovirology,10 with such methods being considered diagnostic in cases of HSV and VZV CNS infections.11 Unfortunately, as only semiquantitative analysis of YF IgG titres were available, we were unable to analyse the results as a specific antibody index, as is standard practice for intrathecal antibody production. However, we would consider such a substantial rise in CSF titres over a 19-day period, and the low levels of YF-specific IgG in plasma to be considered highly suggestive of intrathecal antibody production. According to the CDC diagnostic criteria, this patient would not have met the criteria for a suspect case, as although he had clinical signs of encephalitis he did not have neuroimaging consistent with inflammation or an EEG consistent with an encephalopathy (box 1). We still consider this case to represent a suspect case of YEL-AND, as there are case reports of patients classified as definite YEL-AND who do not have neuroimaging consistent with inflammation,6 12 therefore the absence of abnormal imaging results does not preclude the diagnosis.
Box 1. CDC’s Yellow Fever Vaccine Safety Working Group case definition for yellow fever vaccine-associated neurological disease. (Box adapted from Staples et al 1).
‘Suspect’ neurotropic disease
-
Neurological disease presenting with one or more of the following:
Fever, focal neurological dysfunction, mental status change, seizures, cerebrospinal fluid (CSF) pleocytosis, elevated CSF protein.
-
One or more of the following:
Neuroimaging consistent with inflammation.
Electroencephalogram finding consistent with encephalopathy.
Symptoms occur within 1 and 30 days of vaccination with yellow fever (YF) vaccine, either given alone or in combination with other vaccinations.
No evidence of other diagnoses.
‘Probable’ neurotropic disease
Fulfils criteria for suspect neurotropic disease.
-
One or more of the following:
YF vaccine strain isolated from blood (>7 days postvaccination).
YF vaccine virus concentration in serum >1000 pfu/mL (on any day).
‘Definite’ neurotropic disease
Fulfils criteria for suspect neurotropic disease.
-
One or more of the following:
YF vaccine strain isolated from CSF.
YF-specific IgM detected in CSF.
Amplification of YF vaccine strain from CSF.
The YF vaccine contains the 17D strain of YF, this strain was selected for use in the vaccine after demonstration of the loss of neurotropism in animal models.13 However, adverse reactions to the vaccine, including neurological disease, are well known to occur at the extremes of age, and as such the vaccine is contraindicated in those under 6 months of age, and a risk assessment is advised prior to vaccinating patients over the age of 60 years.5 However, since 2001, there have been increasing reports of serious adverse reactions to the YF vaccine including neurological and viscerotropic disease, which can occur in vaccinees of any age.14 15 YF vaccine-associated viscerotropic disease mimics natural YF infection, in which the vaccine virus proliferates in multiple organs, causing multiple organ failure and has a case fatality rate of over 60%.1 Clinical syndromes of YEL-AND, which include meningoencephalitis, acute disseminated encephalomyelitis and Guillain-Barré syndrome, are seldom fatal,1 and have almost exclusively been observed following primary vaccination.5 The prevalence of YEL-AND has been estimated to be between 0.25 and 0.8 per 100 000 doses9 14 16; however, the prevalence of serious adverse neurological events may be much higher if acute meningitis is included as a distinct clinical syndrome.7 Accurate estimates of YEL-AND are difficult to ascertain as it may not be considered as a differential diagnosis in patients who do not fit into the age groups classically associated with such adverse events to YF vaccination. The majority of patients with YEL-AND make a full recovery, as did our patient; however, he still reported headaches, attention deficit and mood swings 2 months after onset of symptoms. Other cases of YEL-AND have documented similar symptoms continuing after hospital discharge6 7 17; hence, the neurological sequelae of YEL-AND should not be underestimated. With increasing outbreaks of YF in recent years,18 vaccination remains the best protection against disease in travellers. Severe adverse reactions to YF vaccination, including YEL-AND, are rare, and this case is intended to highlight the importance of vigilance in patients with a history of recent YF vaccination, regardless of age. Furthermore, this case demonstrates that ascertaining a probable or definite diagnosis of YEL-AND in the UK is challenging due to a lack of appropriate diagnostic tests, therefore the reporting rate of YEL-AND from the UK may be lower than that observed in other countries.
Patient’s perspective.
For several weeks leading up to my series of travel vaccinations, I had been under a significant level of work-related stress over a sustained period of time. As I was in very good physical condition at the time (having recently trained for and participated in a 5-a-side football tournament), I declared myself to be in good health when asked by the nurse during my initial appointment at the travel clinic, in spite of feeling mentally exhausted at that point.
In the weeks following these injections, I experienced a sustained period of headaches of an intensity and persistence like never before; however, I treated the symptoms with painkillers and assumed that these were merely a side-effect of the stress I was working under. Around 5 or 6 days prior to my hospital admission, I was becoming drowsy and found it difficult to stay awake while visiting my daughter, who had been hospitalised with a combination of pneumonia, tonsillitis and bronchitis (incidentally this was the reason why I did not go on the business trip to Africa). In the 48 hours or so prior to my own admission to hospital, the headaches became very severe, with the pain focused just behind the centre of my forehead. I recall piling several pillows on my head to induce drowsiness in order to sleep and escape the pain; I remember vividly a feeling of resignation and of not having the will to get out of bed.
In respect of my postdischarge experience, my mental health has worsened since the encephalitis. Two years on my pre-existing depression has deepened, with my medication dosage having since been doubled.
Learning points.
Yellow fever vaccine-associated neurological disease (YEL-AND) can occur at any age.
Diagnosing YEL-AND in the UK is difficult due to the lack of available tests required for a definitive diagnosis.
Low level positive PCR results can lead to misdiagnosis.
Footnotes
Contributors: EJG performed retrospective analysis of the patient’s case and prepared the manuscript. DJB was the consultant in charge during the patient’s hospital admission and initiated initial investigations into a YEL-AND diagnosis. RNG provided advice regarding laboratory diagnosis and interpretation of results. All authors proofread and edited the manuscript.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Patient consent for publication: Obtained.
References
- 1. Staples J, Gershman M, Fischer M. Centres for Disease Control and Prevention. Yellow fever vaccine: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep 2010;59:1–27. [PubMed] [Google Scholar]
- 2.World Health Organisation. International Health Regulations (2005). 3rd edn Geneva, 2016. [Google Scholar]
- 3. National Travel Health Network and Centre. Yellow fever vaccine usage by yellow fever vaccination centres in England, Wales and Northern Ireland 2017. London 2018. [Google Scholar]
- 4. Scotland HP. Summary of yellow fever vaccination centres 2015-2017. Glasgow, 2018. [Google Scholar]
- 5.Department of Health. Immunisation against infectious disease (Green Book). Public Health England 2013. [Google Scholar]
- 6. McMahon AW, Eidex RB, Marfin AA, et al. Neurologic disease associated with 17D-204 yellow fever vaccination: a report of 15 cases. Vaccine 2007;25:1727–34. 10.1016/j.vaccine.2006.11.027 [DOI] [PubMed] [Google Scholar]
- 7. Guimard T, Minjolle S, Polard E, et al. Short report: Incidence of yellow fever vaccine-associated neurotropic disease. Am J Trop Med Hyg 2009;81:1141–3. 10.4269/ajtmh.2009.09-0295 [DOI] [PubMed] [Google Scholar]
- 8. Pires-Marczeski FC, Martinez VP, Nemirovsky C, et al. Intrathecal antibody production in two cases of yellow fever vaccine associated neurotropic disease in Argentina. J Med Virol 2011;83:2208–12. 10.1002/jmv.22236 [DOI] [PubMed] [Google Scholar]
- 9. Lindsey NP, Rabe IB, Miller ER, et al. Adverse event reports following yellow fever vaccination, 2007–13. J Travel Med 2016;23:taw045 10.1093/jtm/taw045 [DOI] [PubMed] [Google Scholar]
- 10. Reiber H, Peter JB. Cerebrospinal fluid analysis: disease-related data patterns and evaluation programs. J Neurol Sci 2001;184:101–22. 10.1016/S0022-510X(00)00501-3 [DOI] [PubMed] [Google Scholar]
- 11. Linde A, Klapper PE, Monteyne P, et al. Specific diagnostic methods for herpesvirus infections of the central nervous system: a consensus review by the European Union Concerted Action on Virus Meningitis and Encephalitis. Clin Diagn Virol 1997;8:83–104. 10.1016/S0928-0197(97)00015-9 [DOI] [PubMed] [Google Scholar]
- 12. Florczak-Wyspiańska J, Nawotczyńska E, Kozubski W. Yellow fever vaccine-associated neurotropic disease (YEL-AND) - A case report. Neurol Neurochir Pol 2017;51:101–5. 10.1016/j.pjnns.2016.09.002 [DOI] [PubMed] [Google Scholar]
- 13. Beck A, Tesh RB, Wood TG, et al. Comparison of the live attenuated yellow fever vaccine 17D-204 strain to its virulent parental strain Asibi by deep sequencing. J Infect Dis 2014;209:334–44. 10.1093/infdis/jit546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Khromava AY, Eidex RB, Weld LH, et al. Yellow fever vaccine: an updated assessment of advanced age as a risk factor for serious adverse events. Vaccine 2005;23:3256–63. 10.1016/j.vaccine.2005.01.089 [DOI] [PubMed] [Google Scholar]
- 15. Centres for Disease Control and Prevention. Adverse events associated with 17D-derived yellow fever vaccination-United States, 2001-2002. MMWR Morb Mortal Wkly Rep 2002;51:989–93. [PubMed] [Google Scholar]
- 16. Kitchener S. Viscerotropic and neurotropic disease following vaccination with the 17D yellow fever vaccine, ARILVAX. Vaccine 2004;22(17-18):2103–5. 10.1016/j.vaccine.2004.01.026 [DOI] [PubMed] [Google Scholar]
- 17. Biscayart C, Carrega ME, Sagradini S, et al. Yellow fever vaccine-associated adverse events following extensive immunization in Argentina. Vaccine 2014;32:1266–72. 10.1016/j.vaccine.2014.01.015 [DOI] [PubMed] [Google Scholar]
- 18. Barrett ADT. The reemergence of yellow fever. Science 2018;361:847–8. 10.1126/science.aau8225 [DOI] [PubMed] [Google Scholar]