Abstract
Objective:
To determine the CT findings and pattern in interstitial pneumonia with autoimmune features (IPAF) and to assess whether imaging can predict survival in IPAF.
Materials and Methods:
This retrospective study was HIPAA compliant and approved by our institutional review board. We included 136 subjects who met criteria for IPAF and had diagnostic quality chest CT scans from 2006 to 2015; 74 of these subjects had pathology available for review within one year of chest CT. CT findings and usual interstitial pneumonitis (UIP) pattern of disease were assessed as was the pattern of UIP on pathology. Analysis of chest CT findings associated with survival was performed using standard univariate and multivariable Cox proportional hazards methodology as well as the unadjusted log rank test; survival was presented visually using the Kaplan-Meier survival curve estimator.
Results:
The majority of IPAF subjects had a high confidence UIP pattern on CT (57.4%, 78/136). A substantial minority of subjects had an inconsistent with UIP pattern on CT (28.7%, 39/136). A UIP pattern on CT was associated with smoking (<p-value 0.01), male sex (<p-value 0.01), and older age (p-value <0.001). Approximately, ¼ of subjects had a nonspecific interstitial pneumonitis pattern on CT. Interestingly, nearly a tenth of subjects had a CT pattern most consistent with hypersensitivity pneumonitis rather than customary CT patterns ascribed to lung disease from connective tissue disease. Most subjects with a possible UIP pattern on CT had UIP on pathology (83.3%). Focused multivariate analysis demonstrated that honeycombing (HR 2.17, 95% CI 1.05–4.47) and pulmonary artery (PA) enlargement (HR 2.08, 95% CI 1.02–4.20) on CT were independent predictors of survival.
Conclusions:
IPAF most often presents with a UIP pattern on CT and is associated with worse survival when there is concomitant honeycombing or PA enlargement.
Keywords: interstitial pneumonia with autoimmune features, connective tissue disease, CT, survival, usual interstitial pneumonitis
It has been recognized that a substantial proportion of patients with idiopathic interstitial pneumonia (IIP) have signs and symptoms suggestive of an underlying autoimmune process but do not meet defined criteria for a specific connective tissue disease (CTD). The differences in interstitial lung disease (ILD) between diagnosed or suspected CTD as compared to other types of ILD is an ongoing field of study. A multi-society task force recently introduced new research criteria for interstitial pneumonia with autoimmune features (IPAF) in an attempt “to derive a uniform name and set of classification criteria for patients with IIP and an autoimmune flavor” separate from those with ILD who do not have evidence for autoimmune components or a defined CTD (1). The proposed criteria include traditional clinical and serologic features of CTD, as well as morphologic features consistent with CTD from the subdomains of chest radiographic imaging and histopathology, and physiologic features from pulmonary function tests.
The imaging patterns and prognostic significance of the IPAF classification have yet to be fully defined. Previous studies in patients with disease classifications similar to IPAF (e.g. lung-dominant CTD, undifferentiated CTD-associated ILD [UCTD-ILD], and autoimmune-featured ILD) have shown differing results, likely related to differences in the criteria for diagnosis [1–4]. One of the goals of the IPAF classification is to develop a consistent platform from which to rigorously study this specific group of patients.
The 1st purpose of this study was to document in detail the CT imaging features of IPAF patients, based on a systematic review of a large number of CT scans and corresponding medical records at our medical center. Given that most patients with CTD-ILD (with the exception of rheumatoid arthritis) have non-specific interstitial pneumonia (NSIP) and/or organizing pneumonia (OP) morphology, and that many CT imaging findings inconsistent with UIP [5] support the diagnosis of IPAF, we expected that the CT appearance would be most often inconsistent with UIP. The 2nd purpose of this study was to assess which, if any, CT findings were predictive of survival in IPAF. Imaging findings have been shown to predict survival in the setting of IIP; in CTD, the significance of imaging findings is less clear and in IPAF, unknown [6]. We hypothesized that, similar to idiopathic ILD, a UIP pattern and macroscopic CT findings of pulmonary fibrosis (honeycombing, reticulation, and traction bronchiectasis) would be associated with decreased survival.
Methods
Subjects and Clinical Information
This retrospective study was HIPAA compliant and approved by our institutional review board (#14163-A). Our ILD research registry was utilized to identify adult subjects evaluated in our ILD clinic from 2006–2015. Patients with idiopathic pulmonary fibrosis (IPF), unclassifiable IIP, biopsy-proven idiopathic NSIP, and biopsy-proven OP were identified [5]. Patients with an interim diagnosis of UCTD-ILD based on previous criteria were also identified [2]. ILD diagnosis was achieved using a multi-disciplinary approach. Follow-up time was censored on 12/01/15. Subjects were excluded from the study if they had a known cause for ILD, declined to give informed consent, or did not undergo the necessary tests (serological assessment, chest CT, or surgical lung biopsy) needed to achieve a confident IPAF diagnosis. Of the 1045 patients in the ILD registry, 144 met criteria for IPAF as previously described [7]. Of these, 136 subjects had chest CT scans of diagnostic quality available for review. This prior article described the presenting features of an IPAF cohort from the clinical pulmonary perspective whereas in this manuscript we report on the specific CT imaging findings of IPAF as well as the CT findings associated with survival in IPAF. The prior article analyzed survival based mainly on pathological findings.
A diagnosis of IPAF was achieved using the current research guidelines comprised of clinical, serological, and morphological (imaging, pathology, physiology) features [8]. We extracted clinical data from the initial clinic visit, including demographics, history, medications, physical examination findings, and laboratory findings including a comprehensive serologic assessment. Anti-CADM (MDA-5) and anti PM-Scl were not included in the routine ILD evaluation at our medical center and, therefore, could not be assessed. Pulmonary function test data were also assessed, including percent predicted forced vital capacity, percent predicted total lung capacity, and percent predicted diffusion capacity of the lung for carbon monoxide.
CT Evaluation
The earliest chest CT scan of diagnostic quality for each subject was scored. Outside hospital studies were reviewed if image quality was adequate (18 of the 136 CT scans evaluated). The criteria for diagnostic quality included thin acquisition/reconstruction (<2.0mm) through the whole thorax and absence of significant motion artifact which obscured the lung parenchymal detail. HRCT scans performed at our medical center were performed on various scanners (Philips Brilliance 16–64-slice scanners or Brilliance iCT 256-slice scanner). A supine helical CT acquisition was performed during full inspiration at 120 kVp, 220 mAs; images were reconstructed using a 512 × 512-pixel image matrix. Transverse (axial) images were reconstructed contiguously at 1 mm and 3 mm slice thickness using a standard lung kernel. Coronal and sagittal images were reconstructed at 2.5 mm thickness. End-expiratory phase helical CT images were also obtained (120 kVp, 60 mAs, 3 mm contiguous reconstruction), as were transverse 1 mm prone images during full inspiration (1.2mm thick x 10mm).
Chest CT scans were evaluated by 2 dedicated thoracic radiologists (SMM and JHC with 32 and 11 years of thoracic imaging experience, respectively) by consensus, blinded to other data. CT scans were scored for reticulation, CT honeycombing, traction bronchiectasis, air-trapping or mosaic attenuation, and ground-glass opacity. The preponderant distribution of lung disease was also scored in the zonal (diffuse, upper, mid, or lower) and transverse (diffuse, bronchovascular, peripheral, or peripheral with subpleural sparing) planes. Reticulation, CT honeycombing, traction bronchiectasis, air-trapping or mosaic attenuation, and ground-glass opacity were scored as present or absent; the percentile of lung involvement in regards to these categories was scored to the nearest 5%. Multi-compartment involvement [8] was systematically evaluated on chest CT by assessing for the presence of pleural effusion or thickening, pericardial effusion or thickening, mosaic attenuation in the absence of visible emphysema, and non-traction bronchiectasis, as well as pulmonary artery (PA) diameter [9]. We corrected mosaic attenuation for emphysema (as a marker of substantial smoking injury to the lungs) given that small airways disease is common in smoking and may have confounded statistical analyses [10]. A threshold of 3.3 cm for PA enlargement was used to increase the specificity of this finding [11, 12].
Level of confidence for CT diagnosis of IPAF relative to UIP was scored as per guidelines (UIP, possible UIP, and inconsistent with UIP) (Figures 1–3) [5, 13–17]. If the pattern on CT was not definitely UIP, the readers by consensus also selected the best overall alternative imaging diagnosis including the whole spectrum of the IIPs, hypersensitivity pneumonitis (HP), sarcoidosis, obliterative bronchiolitis, asbestosis, silicosis, and cellular bronchiolitis, with level of confidence (possible, probable, or definite).
Figure 1:
75-year-old man with interstitial pneumonia with autoimmune features. Axial (A) and coronal (B) images from chest CT demonstrate peripheral and basilar predominant pulmonary fibrosis characterized by reticulation, traction bronchiectasis, traction bronchiolectasis, and subpleural honeycombing consistent with UIP.
Figure 3:
79-year-old woman with interstitial pneumonia with autoimmune features. Axial (A) and coronal (B) images from chest CT demonstrate axially diffuse and zonally upper preponderant lung disease characterized by ground-glass opacity and reticulation diagnostic of an inconsistent with UIP pattern given the degree of ground-glass opacity and upper lung preponderance.
Histopathological Evaluation and Radiology-pathology Correlation
In 74 of the 136 CT scans, there was pathology available for assessment within 1 year of the scored CT scan. Pathological samples from surgical lung biopsies were reviewed by a pulmonary pathologist (ANH), with expertise in ILD, as previously described [7]. The biopsies were evaluated for pathologic patterns of ILD such as UIP, NSIP, OP, LIP, and chronic HP, as well as, other evidence of underlying autoimmune diseases
Statistical Analysis
CT findings were summarized descriptively as mean (standard deviation) or median (range) as appropriate. A two-tailed Fisher exact test or chi square test was used to compare proportions. Average values for continuous variable between groups were compared using a two-tailed Student t test (parametric) or Mann-Whitney U (nonparametric) test. Analysis of chest CT findings associated with survival was performed using standard univariate and multivariable Cox proportional hazards methodology as well as the unadjusted log rank test. Survival was presented visually using the Kaplan-Meier survival curve estimator. Time from diagnosis to death or lung transplantation were used as endpoints. The multivariate analysis of survival included chest CT variables thought to affect survival in biologically plausible independent pathways; thus, only honeycombing (as the major proxy for end-stage fibrosis), mosaic attenuation (as a proxy for small airways disease), and ground-glass opacity (as a proxy for pulmonary inflammation) were included. The analysis was adjusted for GAP score (sex, age, and physiology [FVC and DLCO]) [18], and positivity of the clinical domain as defined for IPAF classification [8]. All statistical studies were performed using Stata (StataCorp. 2015. Release 14. College Station, TX).
Results
The average age was 63.5 (10.9) years. There were near-equal numbers of men and women (50.7% women). Most of the cohort was comprised of non-Hispanic whites (70.6%) with fewer African American (17.6%), Hispanic (7.4%), and Asian (4.4%) subjects.
CT Findings
An average of 22.5% (12.5%) of the lung volume was affected by ILD. The vast majority of subjects had basilar (96.0%, 121/136) and peripheral (79.5%, 100/136) predominant distribution of disease. A diffuse distribution was the next most common pattern both in the zonal (8.7%) and axial (15.1%) planes. Approximately, 60.2% of subjects had honeycombing on CT. In those with honeycombing, 3.1% (4.3%) of the lung volume was affected. Ground-glass opacity was present in 27.9% of subjects and affected an average of 15.9% (17.1%) of the lung volume. Air-trapping was present in 10.3% of subjects; 12.1% (5.6%) of the lung volume was affected on average. Almost all subjects had traction bronchiectasis (96.3%, 131/136) with an average of 11.4% (7.1%) of the lung volume affected. Multi-compartment involvement as defined by IPAF criteria was common. Pleural effusion or thickening was present in 13.2% of subjects (18/136), pericardial effusion or thickening was present in 1.5% (2/136). Mosaic attenuation without CT evidence of emphysema was present in 34.6% of subjects (47/136), and PA enlargement was present in 27.2% (37/136).
UIP Pattern and Diagnosis on CT
The majority of IPAF subjects had a UIP or possible UIP (65.4%, 89/136) pattern on CT [Table 1]. A substantial minority of subjects had an inconsistent with UIP pattern on CT (34.6%, 47/136). The most common finding in CT scans considered inconsistent with UIP was the presence of a significant degree of ground-glass opacity (53.2%, 25/47). Non-basilar and/or non-peripheral predominant distribution was present in 23.4% (11/47) of CT scans inconsistent with UIP.
Table 1:
CT UIP patterns in IPAF.
| CT UIP Pattern | N (percentage) |
|---|---|
| UIP | 70 (51.5) |
| Possible UIP | 19 (14.0) |
| Inconsistent with UIP | 47 (34.6) |
In regard to the single best diagnosis based solely on imaging pattern, UIP was the single best diagnosis in approximately half of subjects followed by NSIP in a quarter of subjects (Table 2). Nearly a tenth of subjects had an imaging pattern most consistent with HP rather than the typical CT patterns ascribed to CTD-ILD.
Table 2:
CT patterns in IPAF.
| CT Pattern | N | Percentage |
|---|---|---|
| UIP | 70 | 51.5 |
| NSIP | 37 | 27.2 |
| HP | 11 | 8.1 |
| NSIP OP | 9 | 6.6 |
| OP | 5 | 3.7 |
| Other | 4 | 2.9 |
The UIP pattern was statistically more common compared to the inconsistent with UIP pattern on CT in smokers or former smokers and in men (Table 3). Those with UIP on CT were also were older than those with an inconsistent with UIP pattern on CT. No apparent difference in ethnicity was noted though this may have been a function of limited power.
Table 3:
Demographic variables relative to high confidence CT diagnoses.
| CT inconsistent with UIP (N=47) | CT UIP (N=70) |
Percentage CT inconsistent with UIP | Percentage CT UIP | p-value | |
|---|---|---|---|---|---|
| Smoker | 19 | 42 | 40.4 | 60.0 | 0.041* |
| White | 31 | 51 | 66.0 | 72.9 | 0.537 |
| Male | 16 | 41 | 34.0 | 58.6 | 0.014* |
Statistically significant
CT and pathology correlation
The strongest agreement between CT and pathology in regard to UIP diagnosis was when CT scans were scored as UIP (93.8% UIP on pathology). There was slightly lower agreement between CT and pathology when CT scans were scored as possible for UIP on CT (83.3% UIP on pathology), though this was not statistically different when compared to UIP on CT (Table 3). There was much lower agreement between CT and pathology when CT scans were scored as inconsistent with UIP (50.0% UIP on pathology). When compared to the other CT categories, there was a significant difference in CT and pathology concordance in the inconsistent with UIP subjects (p-value < 0.001).
Survival and CT findings
Univariate statistically significant CT findings associated with worse survival included percent reticulation (HR 1.04, 95% CI 1.01–1.06), presence of honeycombing on CT (HR 2.60, 95% CI 1.33–5.07), mosaic attenuation corrected for presence of CT emphysema (HR 2.17, 95% CI 1.19–3.95), and PA enlargement (HR 2.23, 95% CI 1.22–4.05) [Table 4]. UIP pattern on CT and distribution of pulmonary fibrosis were not statistically significant predictors of survival. Corresponding KM survival curves with log rank test p-values are shown for presence of honeycombing on CT, mosaic attenuation on CT corrected for presence of CT emphysema, and PA enlargement in Figures 4–6, respectively. Focused multivariate analysis demonstrated that only honeycombing (HR 2.17, 95% CI 1.05–4.47) and PA enlargement (HR 2.08, 95% CI 1.02–4.20) were independent predictors of survival but percent reticulation and mosaic attenuation corrected for CT emphysema were not (Table 5).
Table 4:
Radiology and pathology correlation for UIP diagnosis.
| CT Diagnosis | Pathology: Not UIP | Pathology: UIP | % UIP |
|---|---|---|---|
| Inconsistent with UIP | 12 | 12 | 50% |
| Possible UIP | 3 | 15 | 83.3% |
| UIP | 2 | 30 | 93.8% |
| Total | 17 | 57 |
Figure 4:
Kaplan–Meier survival curves of CT honeycombing and no CT honeycombing shows that those with CT honeycombing had significantly worse survival than those without CT honeycombing.
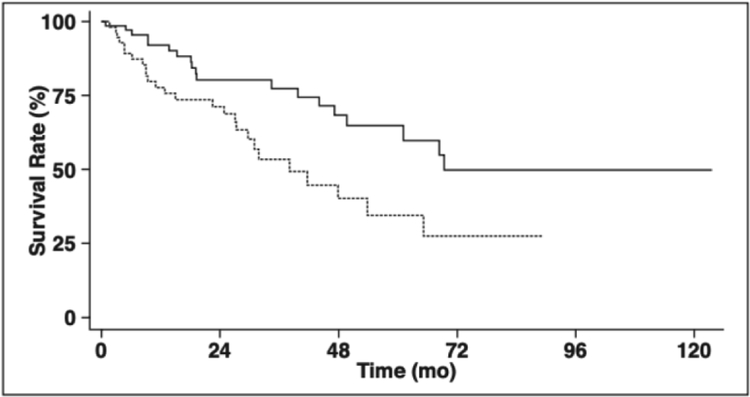
Figure 6:
Kaplan–Meier survival curves of presence and absence of pulmonary artery enlargement on CT shows that those with a PA larger than or equal to 3.3cm had significantly worse survival.
Table 5:
Cox unadjusted and adjusted HRCT prognostic features in interstitial pneumonia with autoimmune features.
| Unadjusted (n=136) | Adjusted* (n=136) | ||||||
|---|---|---|---|---|---|---|---|
| Variable | HR | p-value | 95% CI | HR | p-value | 95% CI | |
| Honeycomb pattern | 2.60 | 0.005 | 1.33 – 5.07 | 2.17 | 0.037 | 1.05 – 4.47 | |
| Reticulation (% involvement) | 1.04 | 0.001 | 1.01 – 1.06 | 1.01 | 0.386 | 0.98 – 1.05 | |
| Multicompartment features | |||||||
| Mosaic attenuation excluding emphysema^ | 2.17 | 0.011 | 1.19 – 3.95 | 1.79 | 0.117 | 0.87 – 3.70 | |
| Pulmonary artery enlargement | 2.23 | 0.009 | 1.22 – 4.05 | 2.08 | 0.043 | 1.02 – 4.20 | |
| UIP Pattern** | |||||||
| Possible UIP | 0.99 | 0.982 | 0.36 – 2.73 | ||||
| Definite UIP | 1.57 | 0.172 | 0.82 – 2.98 | ||||
| Mosaic attenuation | 1.63 | 0.102 | 0.91 – 2.94 | ||||
| Ground-glass opacities | 0.99 | 0.968 | 0.52 – 1.88 | ||||
| Axial distribution of fibrosis*** | |||||||
| Peripheral | 1.40 | 0.575 | 0.43 – 4.56 | ||||
| Peripheral with subpleural sparing | 0.32 | 0.325 | 0.03 – 3.09 | ||||
| Diffuse | 1.29 | 0.722 | 0.32 – 5.17 | ||||
| Pleural or pericardial effusion or thickening | 1.77 | 0.146 | 0.82 – 3.81 | ||||
adjusted for age, gender, fvc, dlco and presence of clinical domain
compared to ‘Inconsistent with UIP’
compared to central/bronchovascular pattern
using a pulmonary artery diameter cutoff of 33mm
multicompartment features excluding patients with smoking history: univariate analysis HR=1.83; p=0.047
Discussion
The main findings of this study were as follows: (1) contrary to our initial hypothesis, most IPAF subjects had a UIP pattern of pulmonary fibrosis on CT; (2) 1/10 of IPAF subjects had an imaging pattern most consistent with HP; (3) age, smoking, and male sex were associated with a UIP pattern; (4) possible UIP on CT is strongly associated with UIP on pathology; and (5) honeycombing and PA enlargement on CT were independent predictors of survival in IPAF.
In the current study, a UIP or possible UIP pattern on CT was present in the majority of IPAF subjects, found in 65.4% of this IPAF cohort. Recent evidence suggests that the vast majority of patients with a possible UIP pattern on CT have UIP on pathology as in those with a UIP pattern on CT [13, 19–21]. Previous studies in UCTD have shown that UIP is the most common pattern though NSIP is also quite common, depending on diagnostic criteria used [2, 4, 22, 23]. In the setting of CTD, NSIP is the most common type of lung disease. An exception to this rule is rheumatoid arthritis, where UIP pattern is most common [24, 25]. The high prevalence of UIP in IPAF suggests that these patients may mimic rheumatoid arthritis patients in regard to their underlying pathophysiology and prognosis—a subject worthy of future investigation. In our cohort, those with a UIP pattern of pulmonary fibrosis more often had a history of smoking, were older, and were more often men as compared to those with an inconsistent with UIP pattern. In the setting of IPF; smoking, age, and male sex are major risk factors for the development of UIP [5]. Similar phenomena maybe present in patients with IPAF and should be explored further.
The presence of autoimmune features in the setting of HP has only recently been formally recognized; up to 15% of patients with HP may have associated autoimmune features [26]. A similar proportion of subjects in our study with IPAF demonstrated a CT pattern consistent with HP. Though not diagnostic of HP (which requires multidisciplinary discussion), our findings suggest that there is relationship between autoimmunity and HP whether pathophysiologic or phenotypic. Only a small minority of those exposed to antigens known to cause HP develop significant ILD, implying presence of an underlying genetic susceptibility. Inhaled substances may trigger autoimmune disease in susceptible individuals as development of CTD have been described in individuals exposed to cigarette smoke, silica and coal dust, as well as dust and chemicals from the World Trade Center [26–30]. Alternatively, while the CT appearance of IPAF has yet to be formally defined, HP morphology may be one of the phenotypes of IPAF. In addition, there may be yet undefined IPAF imaging patterns, as suggested by the substantial minority of subjects who could not be classified in traditional UIP categories on CT.
Multiple previous studies in different clinical settings have demonstrated that a CT pattern suggestive of UIP--but not meeting strict criteria for UIP--is almost always associated with a UIP pattern on pathology [14, 19, 20, 31]. Flaherty, et al. showed that all 11 patients with “probable UIP” on HRCT (defined as CT findings consistent with UIP except for absence of basilar distribution) had UIP on pathology in a cohort of 96 patients selected for histological UIP and NSIP [14]. Another study, as part of a larger genome wide association study, showed that “probable UIP” (defined as CT findings suggestive of UIP but absence of honeycombing) was associated with a UIP diagnosis on pathology in 82.4% of cases [13]. In 2 separate IPF studies, Raghu, et al. and Yagihashi, et al. found that 94.0% of 84 subjects and 93.8% of 64 subjects who met CT criteria for possible UIP had UIP confirmed on histology, respectively [19, 20]. This suggests that these cases may not require biopsy for accurate diagnosis. Current guidelines direct patients with a possible UIP pattern on CT to surgical lung biopsy for more definitive diagnosis. Given the morbidity and mortality risk of surgical lung biopsy in patients with ILD, an alternative pathway that minimizes the number of patients sent for surgical lung biopsy is attractive. Our data and previous studies suggest that patients with possible UIP on CT could be managed like those with a high confidence UIP pattern on CT rather than pursuing biopsy.
Survival ramifications of the IPAF classification have yet to be fully delineated. Studies evaluating prognostic factors in UCTD (or similar designations) have suggested that those with autoimmune interstitial pneumonias who do not meet strict criteria for a defined CTD likely have similar survival to IPF and may have worse survival compared to those with defined CTD [2–4]. Honeycombing on CT was independently associated with worse survival in the current study, which supports the well-accepted relationship between disease severity and poor survival in pulmonary fibrosis [6, 32]. On the other hand, the absence of a survival difference relative to UIP CT classification was somewhat unexpected. NSIP is associated with superior survival as compared to UIP in the idiopathic setting [33, 34]. Therefore, one would expect those with a high confidence UIP pattern on CT to have worse survival compared to those with an inconsistent with UIP pattern.
However, previous studies suggest that the clinical significance of the UIP pattern in the setting of CTD is less certain. In a heterogeneous group of subjects with CTD, no significant difference in survival was present between those with CTD related NSIP and UIP [35]. Bouros, et al. showed little difference in survival in patients with systemic sclerosis related interstitial lung disease with NSIP compared to UIP (91% and 82%, respectively)[36].
Conversely, a small study in rheumatoid arthritis patients showed worse survival in those with UIP as compared to NSIP on pathology, though a much higher prevalence of smoking in UIP as compared to the NSIP may have been a confounding factor [37]. An imaging-based rheumatoid arthritis study showed that a definite UIP CT pattern was associated with worse survival as compared to those without this CT pattern (median survival of 3.2 years compared to 6.6 years; respectively) [25]. Similarly, Solomon, et al. showed that the CT UIP pattern was associated with worse survival as compared to the CT NSIP pattern in the setting of rheumatoid arthritis though this did not persist after multivariate analysis [24].
Our results also suggest that PA size on CT is independently associated with worse survival in IPAF. Pulmonary hypertension is a known manifestation of CTDs and has poor prognostic ramifications [38]. There is extensive literature documenting the strong correlation between PA size on CT and pulmonary hypertension [12, 39]. The most likely theory for the current result is that PA size is associated with pulmonary hypertension, which is in turn associated with poor prognosis. This theory could not be formally tested due to the observational nature of our study. However, there is little if any correlation between CT PA measurements and pulmonary hypertension in the setting of pulmonary fibrosis, particularly if more severe, possibly due to alterations of mediastinal structures secondary to underlying chronic pulmonary pathology [11, 40, 41]. Therefore, considering alternative pathophysiological mechanisms would be prudent. PA/aorta ratio on CT has been strongly associated with acute exacerbations in a large COPD cohort with external validation [42]. Perhaps, in the setting of chronic lung disease, enlargement of the PA on CT may signal an increased likelihood for pulmonary exacerbations, and eventually, pulmonary-related demise, though this is speculative.
Limitations of our study include its retrospective design as well as the relatively small number of subjects, which may limit the power of the study. However, the total number of subjects is on par with other single center studies in the setting of CTD related ILD. Also, there was some heterogeneity in CT acquisition parameters given that some of the CT scans were not performed at our medical center. We addressed this issue by only scoring outside hospital CT scans which could be accurately analyzed for findings of pulmonary fibrosis. Lastly, this study was performed at a tertiary medical center specializing in ILD diagnosis and treatment; therefore, our results may not be generalizable to the community setting.
This study demonstrates that a UIP pattern on CT was present in the majority of IPAF subjects, and was more common in those with known risk factors for idiopathic pulmonary fibrosis (older age, smoking history, and male sex). A possible UIP pattern on CT was almost always associated with UIP on pathology. Also, a HP pattern on CT was not uncommon in our IPAF cohort, which implies possible presence of a relationship between autoimmunity and HP. Further research regarding CT phenotypes in the setting of IPAF will be necessary to determine the significance of this finding. Finally, honeycombing and PA enlargement on CT are independent predictors of poor survival in IPAF.
Figure 2:
48-year-old woman with interstitial pneumonia with autoimmune features. Axial (A) and coronal (B) images from chest CT demonstrate peripheral and basilar predominant pulmonary fibrosis characterized by reticulation, traction bronchiectasis, and traction bronchiolectasis without subpleural honeycombing consistent with a possible UIP pattern.
Figure 5:
Kaplan–Meier survival curves of presence and absence of mosaic attenuation on CT shows that those with mosaic attenuation had significantly worse survival.
References
- 1.Kinder BW, Collard HR, Koth L, et al. Idiopathic nonspecific interstitial pneumonia: lung manifestation of undifferentiated connective tissue disease? Am J Respir Crit Care Med 2007; 176:691–697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Corte TJ, Copley SJ, Desai SR, et al. Significance of connective tissue disease features in idiopathic interstitial pneumonia. Eur Respir J 2012; 39:661–668 [DOI] [PubMed] [Google Scholar]
- 3.Fischer A, Pfalzgraf FJ, Feghali-Bostwick CA, et al. Anti-th/to-positivity in a cohort of patients with idiopathic pulmonary fibrosis. J Rheumatol 2006; 33:1600–1605 [PubMed] [Google Scholar]
- 4.Vij R, Noth I, Strek ME. Autoimmune-featured interstitial lung disease: a distinct entity. Chest 2011; 140:1292–1299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Raghu G, Collard HR, Egan JJ, et al. An official ATS/ERS/JRS/ALAT statement: idiopathic pulmonary fibrosis: evidence-based guidelines for diagnosis and management. Am J Respir Crit Care Med 2011; 183:788–824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lynch DA, Godwin JD, Safrin S, et al. High-resolution computed tomography in idiopathic pulmonary fibrosis: diagnosis and prognosis. Am J Respir Crit Care Med 2005; 172:488–493 [DOI] [PubMed] [Google Scholar]
- 7.Oldham JM, Adegunsoye A, Valenzi E, et al. Characterisation of patients with interstitial pneumonia with autoimmune features. Eur Respir J 2016; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fischer A, Antoniou KM, Brown KK, et al. An official European Respiratory Society/American Thoracic Society research statement: interstitial pneumonia with autoimmune features. Eur Respir J 2015; [DOI] [PubMed] [Google Scholar]
- 9.Kuriyama K, Gamsu G, Stern RG, Cann CE, Herfkens RJ, Brundage BH. CT-determined pulmonary artery diameters in predicting pulmonary hypertension. Invest Radiol 1984; 19:16–22 [DOI] [PubMed] [Google Scholar]
- 10.Lynch DA, Austin JH, Hogg JC, et al. CT-Definable Subtypes of Chronic Obstructive Pulmonary Disease: A Statement of the Fleischner Society. Radiology 2015; 277:192–205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tan RT, Kuzo R, Goodman LR, Siegel R, Haasler GB, Presberg KW. Utility of CT scan evaluation for predicting pulmonary hypertension in patients with parenchymal lung disease. Medical College of Wisconsin Lung Transplant Group. Chest 1998; 113:1250–1256 [DOI] [PubMed] [Google Scholar]
- 12.Edwards PD, Bull RK, Coulden R. CT measurement of main pulmonary artery diameter. Br J Radiol 1998; 71:1018–1020 [DOI] [PubMed] [Google Scholar]
- 13.Chung JH, Chawla A, Peljto AL, et al. CT scan findings of probable usual interstitial pneumonitis have a high predictive value for histologic usual interstitial pneumonitis. Chest 2015; 147:450–459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Flaherty KR, Thwaite EL, Kazerooni EA, et al. Radiological versus histological diagnosis in UIP and NSIP: survival implications. Thorax 2003; 58:143–148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sumikawa H, Johkoh T, Fujimoto K, et al. Pathologically proved nonspecific interstitial pneumonia: CT pattern analysis as compared with usual interstitial pneumonia CT pattern. Radiology 2014; 272:549–556 [DOI] [PubMed] [Google Scholar]
- 16.Silva CI, Muller NL, Lynch DA, et al. Chronic hypersensitivity pneumonitis: differentiation from idiopathic pulmonary fibrosis and nonspecific interstitial pneumonia by using thin-section CT. Radiology 2008; 246:288–297 [DOI] [PubMed] [Google Scholar]
- 17.Chung JH, Peljto AL, Chawla A, et al. CT Phenotypes of Pulmonary Fibrosis in the MUC5B Promoter Site Polymorphism. Chest 2016; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ley B, Ryerson CJ, Vittinghoff E, et al. A multidimensional index and staging system for idiopathic pulmonary fibrosis. Ann Intern Med 2012; 156:684–691 [DOI] [PubMed] [Google Scholar]
- 19.Yagihashi K, Huckleberry J, Colby TV, et al. Radiologic-pathologic discordance in biopsy-proven usual interstitial pneumonia. Eur Respir J 2016; 47:1189–1197 [DOI] [PubMed] [Google Scholar]
- 20.Raghu G, Lynch D, Godwin JD, et al. Diagnosis of idiopathic pulmonary fibrosis with high-resolution CT in patients with little or no radiological evidence of honeycombing: secondary analysis of a randomised, controlled trial. Lancet Respir Med 2014; 2:277–284 [DOI] [PubMed] [Google Scholar]
- 21.Gruden JF, Panse PM, Gotway MB, Jensen EA, Wellnitz CV, Wesselius L. Diagnosis of Usual Interstitial Pneumonitis in the Absence of Honeycombing: Evaluation of Specific CT Criteria With Clinical Follow-Up in 38 Patients. AJR Am J Roentgenol 2016; 206:472–480 [DOI] [PubMed] [Google Scholar]
- 22.Omote N, Taniguchi H, Kondoh Y, et al. Lung-Dominant Connective Tissue Disease: Clinical, Radiologic, and Histologic Features. Chest 2015; 148:1438–1446 [DOI] [PubMed] [Google Scholar]
- 23.Kim HC, Ji W, Kim MY, et al. Interstitial pneumonia related to undifferentiated connective tissue disease: pathologic pattern and prognosis. Chest 2015; 147:165–172 [DOI] [PubMed] [Google Scholar]
- 24.Solomon JJ, Chung JH, Cosgrove GP, et al. Predictors of mortality in rheumatoid arthritis-associated interstitial lung disease. Eur Respir J 2016; 47:588–596 [DOI] [PubMed] [Google Scholar]
- 25.Kim EJ, Elicker BM, Maldonado F, et al. Usual interstitial pneumonia in rheumatoid arthritis-associated interstitial lung disease. Eur Respir J 2010; 35:1322–1328 [DOI] [PubMed] [Google Scholar]
- 26.Adegunsoye A, Oldham JM, Demchuk C, Montner S, Vij R, Strek ME. Predictors of survival in coexistent hypersensitivity pneumonitis with autoimmune features. Respir Med 2016; 114:53–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Doyle TJ, Dellaripa PF, Batra K, et al. Functional impact of a spectrum of interstitial lung abnormalities in rheumatoid arthritis. Chest 2014; 146:41–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kart L, Sarikaya S, Gurel A, et al. Rheumatoid factor seropositivity and rheumatoid symptoms in coal worker’s pneumoconiosis. Clin Rheumatol 2003; 22:365–366 [DOI] [PubMed] [Google Scholar]
- 29.Rocha MC, Santos LM, Bagatin E, et al. Genetic polymorphisms and surface expression of CTLA-4 and PD-1 on T cells of silica-exposed workers. Int J Hyg Environ Health 2012; 215:562–569 [DOI] [PubMed] [Google Scholar]
- 30.Webber MP, Moir W, Zeig-Owens R, et al. Nested case-control study of selected systemic autoimmune diseases in World Trade Center rescue/recovery workers. Arthritis Rheumatol 2015; 67:1369–1376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hunninghake GW, Lynch DA, Galvin JR, et al. Radiologic findings are strongly associated with a pathologic diagnosis of usual interstitial pneumonia. Chest 2003; 124:1215–1223 [DOI] [PubMed] [Google Scholar]
- 32.Sumikawa H, Johkoh T, Colby TV, et al. Computed tomography findings in pathological usual interstitial pneumonia: relationship to survival. Am J Respir Crit Care Med 2008; 177:433–439 [DOI] [PubMed] [Google Scholar]
- 33.Nicholson AG, Colby TV, du Bois RM, Hansell DM, Wells AU. The prognostic significance of the histologic pattern of interstitial pneumonia in patients presenting with the clinical entity of cryptogenic fibrosing alveolitis. Am J Respir Crit Care Med 2000; 162:2213–2217 [DOI] [PubMed] [Google Scholar]
- 34.Daniil ZD, Gilchrist FC, Nicholson AG, et al. A histologic pattern of nonspecific interstitial pneumonia is associated with a better prognosis than usual interstitial pneumonia in patients with cryptogenic fibrosing alveolitis. Am J Respir Crit Care Med 1999; 160:899–905 [DOI] [PubMed] [Google Scholar]
- 35.Park JH, Kim DS, Park IN, et al. Prognosis of fibrotic interstitial pneumonia: idiopathic versus collagen vascular disease-related subtypes. Am J Respir Crit Care Med 2007; 175:705–711 [DOI] [PubMed] [Google Scholar]
- 36.Bouros D, Wells AU, Nicholson AG, et al. Histopathologic subsets of fibrosing alveolitis in patients with systemic sclerosis and their relationship to outcome. Am J Respir Crit Care Med 2002; 165:1581–1586 [DOI] [PubMed] [Google Scholar]
- 37.Lee HK, Kim DS, Yoo B, et al. Histopathologic pattern and clinical features of rheumatoid arthritis-associated interstitial lung disease. Chest 2005; 127:2019–2027 [DOI] [PubMed] [Google Scholar]
- 38.Condliffe R, Kiely DG, Peacock AJ, et al. Connective tissue disease-associated pulmonary arterial hypertension in the modern treatment era. Am J Respir Crit Care Med 2009; 179:151–157 [DOI] [PubMed] [Google Scholar]
- 39.Alhamad EH, Al-Boukai AA, Al-Kassimi FA, et al. Prediction of pulmonary hypertension in patients with or without interstitial lung disease: reliability of CT findings. Radiology 2011; 260:875–883 [DOI] [PubMed] [Google Scholar]
- 40.Devaraj A, Wells AU, Meister MG, Corte TJ, Wort SJ, Hansell DM. Detection of pulmonary hypertension with multidetector CT and echocardiography alone and in combination. Radiology 2010; 254:609–616 [DOI] [PubMed] [Google Scholar]
- 41.Zisman DA, Karlamangla AS, Ross DJ, et al. High-resolution chest CT findings do not predict the presence of pulmonary hypertension in advanced idiopathic pulmonary fibrosis. Chest 2007; 132:773–779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wells JM, Washko GR, Han MK, et al. Pulmonary arterial enlargement and acute exacerbations of COPD. N Engl J Med 2012; 367:913–921 [DOI] [PMC free article] [PubMed] [Google Scholar]