Abstract
The ε4 allele of the APOE gene is associated with poorer cognition in later life. This study aimed to advance understanding of how environments potentially moderate this genetic risk by focusing on childhood socioeconomic status (SES). Previous research across diverse national contexts has found that older adults from higher-SES families in childhood demonstrate better cognitive functioning than their lower-SES counterparts. Nevertheless, few studies have examined whether higher childhood SES might also promote later life cognition by mitigating the effects of ε4 carrier status. To address this gap, we used data from 3017 participants in the Wisconsin Longitudinal Study, which has followed a random sample of people who graduated from Wisconsin high schools in 1957. Childhood SES included parents’ educational attainment, father’s occupational status, and household income in adolescence. We constructed measures of memory and of language/executive functioning using scores from neurocognitive tests administered when participants were approximately ages 65 and 72. APOE ε4 status was measured through saliva samples. Results from cross-controlled multilevel models indicated that APOE ε4 status—and not childhood SES—independently predicted memory, whereas childhood SES—and not APOE ε4 status—independently predicted language/executive functioning. Moreover, a statistical interaction between APOE ε4 status and childhood SES for memory indicated that at baseline, higher childhood SES protected against the risk of APOE ε4 status, whereas lower childhood SES exacerbated the risk of APOE ε4 status. However, by follow-up, these moderating effects dissipated, and APOE ε4 status alone was associated with memory. We interpret these results in light of theorizing on differential susceptibility for poorer cognition across the life course.
Keywords: Apolipoprotein E, Cognitive aging, Differential susceptibility, Gene-by-environment interactions, Life course perspective, Socioeconomic status, U.S., Wisconsin longitudinal study
1. Introduction
Cholesterol contributes to neurophysiological development—including myelin growth, synapse formation, and neuronal remodeling—which is instrumental to learning, memory formation, and other cognitive processes (Leduc et al., 2010). Proteins produced by the apolipoprotein E (APOE) gene transport cholesterol within and between the organs of the body, particularly the central nervous system. APOE has three isoforms, each of which metabolizes cholesterol somewhat differently due to its biochemical structure (Huang and Mahley, 2014). Accordingly, carrying one or two copies of the ε4 allele is associated with a 3- and 12-fold increase in risk, respectively, of Alzheimer’s disease (AD) in later life, as well as with developing AD younger than persons who carry only ε2 or ε3 alleles (Corder et al., 1993; Saunders et al., 1993). The ε4 allele is not only associated with AD, but also with other neuropsychiatric disorders and memory deficits from approximately age 60 onward (Forero et al., 2016).
Although this genetic risk has been well established for more than 25 years, research also has demonstrated that carrying APOE ε4 does not guarantee cognitive problems in later life. Environmental factors can potentially reduce or neutralize the effects of the gene’s protein on cognitive functioning (Reynolds et al., 2014). Most studies of gene-by-environment interactions have investigated behaviors and environments exclusively in adulthood. Yet there is growing consensus among researchers and clinicians that circumstances as early as infancy have potential implications for later life cognitive health and that neurophysiological abnormalities manifest decades before the onset of cognitive impairment (Ritchie et al., 2015). A large and growing body of empirical evidence indicates that childhood socioeconomic (SES)—as indicated by conditions such as parents’ occupation, education, and income—is associated with individuals’ cognition decades later (Anderson et al., 2017; Rogers et al., 2009). Integrating this literature with theorizing on gene-by-environment interactions and differential susceptibility (Belsky et al., 2007), we used data from the Wisconsin Longitudinal Study (WLS), which is one of the longest-running cohort studies in the U.S., to examine the extent to which childhood SES modifies associations between genetic risk from APOE ε4 and later life cognition.
1.1. Dual risk and differential susceptibility models of gene-by-environment interaction
Much of the prior research on the confluence of environmental and genetic effects on later life conditions has adopted a “diathesis-stress,” “double jeopardy,” or “dual risk” perspective (Ellis et al., 2011). This perspective focuses on mechanisms of vulnerability whereby risks are cumulative and heighten the likelihood of developmental problems. A study by Petkus et al. (2012) demonstrates this idea within the field of cognitive aging. Using data from the Rancho Bernardo Study of Healthy Aging, they found that carriers of the APOE ε4 allele who had also experienced sexual assault were at a multiplicatively higher level of risk for both earlier and faster decline in executive functioning than those with experience of sexual assault alone. The authors interpreted this finding in line with the idea that people who both carry genetic risk factors and face social adversity realize the poorest outcomes throughout compounded vulnerability.
More recent theorizing on gene-by-environment interactions, however, suggests other processes through which genetics and environments jointly contribute to developmental outcomes. One such perspective has been articulated by Belsky and colleagues as the differential susceptibility perspective (Belsky et al., 2009). This perspective suggests that individuals with particular genotypes are more susceptible to social-environmental influences—for better or for worse (Mitchell et al., 2013). Accordingly, certain alleles render people more likely to be jeopardized by disadvantageous environments—but also more likely to benefit from advantageous environments. Non-carriers, by contrast, are less responsive or entirely non-responsive to environmental conditions.
APOE may be among these “plasticity” genes. At the cellular level, ε4 carriers show impaired neural plasticity compared to non-carriers (Chen et al., 2010). That is, the ε2 and ε3 variants of APOE help the brain to develop and change in response to environmental stimuli, whereas the ε4 variant does not. This finding would suggest that carriers of ε2 and ε3 might demonstrate greater susceptibility to environments, while carriers of ε4 would demonstrate less (Reynolds et al., 2014). There is some empirical evidence to support this hypothesis. For example, studies have found that carriers of ε2 and ε3 are more susceptible to a range of environments from dietary fat intake (T. L. Huang et al., 2005) to cognitive stimulation (Runge et al., 2014). However, many gene-by-environment interaction studies find the opposite: ε4 carriers are most responsive to environments, while environmental effects are much less pronounced or not significant for non-carriers. These studies also cover a range of environments from obesity (Rajan et al., 2014) to educational attainment (Cook and Fletcher, 2015). Despite inconsistent findings, the evidence is nonetheless suggestive that environments interact with APOE.
Notably, the bulk of APOE-by-environment studies examine environments in midlife or older adulthood. Yet new evidence suggests that the neurological effects of APOE may first manifest in young adulthood (Forero et al., 2016), and that indeed, early-life environments might continue to have effects on cognitive aging (Reynolds et al., 2014). Accordingly, a new body of studies has begun to examine childhood environments as potential moderators of association between APOE ε4 and later life cognition (e.g., Petkus et al., 2012). Below, we focus our review on studies that have focused specifically on childhood SES as a moderator of associations between APOE ε4 and later life cognition.
1.2. Childhood socioeconomic status (SES) and later life cognition
Child development research is increasingly recognizing that SES is a critical context for lifelong neurocognitive development (Richards and Wadsworth, 2004). Childhood SES is a multi-faceted construct that includes characteristics such as parental education, occupational status, and income. Scholars have theorized that higher SES affords children developmental assets, such as more cognitively complex environments, better nutrition, and higher quality schools. Lower SES, in contrast, can pose developmental liabilities, such as environmental toxins and greater chronic stress (Duncan and Magnuson, 2012). Consistent with this theorizing, studies have found that from infancy, children from higher SES families demonstrate better cognitive outcomes, on average, than children from less advantaged families (Tomalski et al., 2013).
Cognitive advantages from having high SES in childhood extend throughout childhood and into young adulthood, middle, and later life (Richards and Wadsworth, 2004). Much of this research has been based on cohort studies from diverse national contexts, including the United Kingdom, New Zealand, Sweden, China, as well as Central and Eastern Europe (Beck et al., 2018; Ericsson et al., 2017; Wang et al., 2017). Other studies have used data from large social surveys that incorporate retrospective measures of childhood SES (Moceri et al., 2000; Zhang et al., 2016). Many of these studies have found that SES in adulthood, at least in part, helps to explain why higher childhood SES is associated with better cognition: Children from wealthy, well-educated families are more likely to become wealthy and well-educated themselves, which helps to promote later life cognition. Yet most studies find that associations between childhood SES and later life cognition persist after accounting for adult SES, indicating that childhood SES might have more direct and long-lasting effects on individuals’ neurophysiological development (Rogers et al., 2009).
Studies have begun to examine how childhood SES and APOE ε4 interact in their association with later life cognition. Four studies have focused on SES in childhood, with two concluding that ε4 carriers are most disadvantaged by lower childhood SES, and two finding no differences in associations between APOE and later life cognition by childhood SES. A pair of studies examined a sample of about 500 members of a Seattle-area HMO (Moceri et al., 2001, 2000). The 2000 study, using self- or proxy-reports of childhood background (including area of residence and sibship size), found no differences in associations by APOE ε4 status. The 2001 study, which drew SES information from the U.S. Census, identified an interaction such that individuals from low SES families (i.e., father held a manual occupation; household size of seven or more persons) who carried ε4 had the highest odds of developing AD. A third study used the Aging, Demographics, and Memory Study subsample of the Health and Retirement Study (Rogers et al., 2009). Similar to the study by Moceri et al. (2001), ε4 carriers whose parents had low educational attainment had nearly four times the odds of dementia of non-carriers whose parents were highly educated. Finally, a recent study examined community-dwelling Swedes aged 75 and older, and estimated structural equation models to examine associations between cognitive reserve variables in childhood, middle adulthood, and later life within a nine-year prospective study of dementia (Wang et al., 2017). Results indicated associations between cognitive reserve in each life stage and risk of dementia, with no differences between APOE ε4 carriers and non-carriers.
1.3. Focus of the current study
Our study aimed to advance understanding of childhood SES as a potential modifier of the association between carrying the APOE ε4 allele and later life cognition by using data from the Wisconsin Longitudinal Study (WLS). The WLS affords both methodological and conceptual advantages. Most notably, the WLS has measures of household income, parental occupational status, and parental education collected when participants were in adolescence. This feature eliminates the possibility of recall bias and other sources of error in retrospective measures of childhood. Also, the WLS offers multiple measures of cognitive functioning in later life, which allow for testing whether gene-by-environment interactions are specific to particular aspects of later life cognition, as well as whether the nature of the interactions changes over ontogenetic time.
Accordingly, we examine the extent to which childhood SES moderates associations between APOE ε4 and later life cognitive function, with particular attention to the nature of potential interactions. For example, does higher childhood SES simply eliminate the genetic risk of carrying ε4, or does lower childhood SES also exacerbate genetic risk? We also explore the extent to which the interaction between childhood SES and genetic risk differs across multiple domains of cognitive functioning, and whether the nature of potential moderation changes between baseline and 7-year follow-up.
2. Methods
2.1. Data
The Wisconsin Longitudinal Study (WLS) has followed a random sample of one-third of the men and women who graduated from Wisconsin high schools in 1957 (N = 10,317). Data collection over time has included in-person, telephone, and mail surveys; genetic assays of salivary data; and collection of state and local administrative records. Survey administration occurred at ages 18 (1957), 36 (1975; 90% response rate), 54 (1993; 87% survey response rate), 65 (2004; 86% survey response rate), and 72 (2011; 74% survey response rate). (Survey response rates exclude deceased participants.) Cognitive measures were administered in 2004 - here, called baseline - and 2011 - here, called follow-up. From the original sample of 10,317, we excluded several groups of participants, including (a) the 2831 who had left the study before “baseline” because of death or other reasons; (b) the 2412 who did not have sufficient measures of cognitive function at baseline (because they were not randomly selected for neurocognitive testing as part of the study protocol or completed half or fewer of the tests); and (c) the 2057 who declined to provide saliva for genetic assay. (We extensively examined the available data for evidence of selective attrition; methods and results are in Appendix A and also discussed below.) Our final analytic sample was 3017 participants.
As the WLS data reflect, in 1960, fewer than 3% of Wisconsin residents were nonwhite (Wisconsin Legislative Reference Bureau, 2017). Only a handful of African American, Asian, Latina/o and Native American participants were among the original WLS sample and in insufficient numbers for statistical analysis. Therefore, the WLS is a white sample, and this racial/ethnic homogeneity precludes broader population inferences.
2.2. Cognitive function
Participants were asked to complete six cognitive tests at baseline and then again at follow-up. At baseline, these tests were asked over the phone, and at follow-up, they were asked in person. First, the letter fluency task asked participants to name as many words as they could, beginning with the letter “L” or “F” in 60 s, without naming any proper names, repeats, or same words with different endings (Tombaugh et al., 1999). Similarly, the category fluency task requested individuals to list as many words that fit in either the category “food” or “animals” in 60 s (Tombaugh et al., 1999). Next, participants were presented with items from a subscale of the Wechsler Adult Intelligence Scale Revised (WAIS-R) (Wechsler, 1981), which included naming how two items were similar for six sets of items (e.g., a table and a chair). Each answer was then scored ranging from 0 to 2, with 2 as the top score. Participants were then asked to complete immediate and delayed recall tasks, which asked them to list back as many of the 10 words that had just been read to them and then asked again, on average, 12 min later, to repeat as many of the 10 words as they could remember. The final cognitive task, digit ordering, was a modified protocol of the WAIS-III digit backward subtest (Wechsler, 1997). It involved reorganizing in ascending order a series of numbers read aloud. This task began with three sets of numbers and continued to increase in difficulty by adding an additional digit.
Because the six tests were scored on different metrics, we calculated the percent of maximum possible scores for each test (Cohen et al., 1999). We conducted a factor analysis using these six cognitive scores, which resulted in a two-factor solution: memory and language/execu- tive function (results presented in Appendix B). We averaged test scores within each domain. The domain of memory included the scores from the immediate recall, delayed recall, and digit ordering tasks. The domain of language/executive function included the scores from the WAIS-R similarities, letter fluency, and category fluency tasks.
2.3. APOE status
Following data collection at baseline, participants were asked to provide saliva for DNA assay. The WLS assayed for two single nucleotide polymorphisms (SNPs) of APOE, rs429358 and rs7412. We then used these two assayed SNPs to determine each individual’s APOE genotype. We initially grouped participants into three categories: having no ε4 alleles (i.e., ε2/ε2, ε2/ε3, or ε3/ε3), having one ε4 allele (i.e., ε2/ε4 or ε3/ε4), or having two ε4 alleles. The group of individuals who had two ε4 alleles was small (n = 88) and thus underpowered to detect significant statistical differences. Therefore, we created a dichotomous indicator of any copies of ε4. APOE was in Hardy-Weinberg equilibrium in this sample.
2.4. Childhood SES
In 1957, participants reported both mother’s and father’s educational attainment on a scale ranging from 7 (no high school) to 18 (has graduate degree) years. Father’s occupation and household income were drawn from annual state tax filings from the years 1954 through 1957. Father’s occupational status was scored using the 1950 Duncan Socioeconomic Index (SEI), which is a composite score of levels of occupational education and income as reported in the 1950 Census (Hauser and Warren, 1997). The scores range from 1 to 100, with higher scores representing greater occupational prestige. These scores were then averaged over a four-year period. Household income was also averaged over the four-year period, and then was recoded into quartiles to correct for skewness. Global childhood SES was then created by standardizing each of the four measures and averaging them. To correct for skew in this composite score, we took the natural log.
2.5. Covariates
Scholars have identified family structure (Yi et al., 2007), family size (Moceri et al., 2000), intelligence (Glymour et al., 2008), and geographic setting (Borenstein et al., 2006) as early life conditions associated with both childhood SES and later life cognition. We included measures of these factors to account for potential confounding in associations among childhood SES, APOE ε4, and later life cognition. Moreover, emerging evidence suggests that children of lower SES families demonstrate different auditory processing patterns than children of higher SES families (Skoe et al., 2013). Accordingly, we also included a measure of self-reported hearing problems, especially because the neurocognitive tests relied upon auditory stimuli.
Family structure was a binary measure indicating whether the participant lived with both parents most of the time until 1957 (0 = no). Family size was a measure of the participant’s total number of siblings. IQ was a raw score from the Henmon-Nelson Test of Mental Ability, a test all high school students in Wisconsin were required to take in their third year of high school (Henmon and Nelson, 1954). The WLS obtained scores from schools’ administrative records. The original scores ranged from 61 to 145, and we standardized the scores for ease of interpretation. An additional covariate was a categorical measure of adolescent geographic setting that assessed the population of the town in which the participant attended high school: rural (reference category; 9999 residents or fewer); suburban (10,000–49,999 residents); and urban (50,000 residents or greater). We also included a dichotomous variable for gender (0 = male) and self-reported hearing problems (0 = none) at baseline and/or follow-up (assessed using the Health Utilities Index Mark 3; Horsman et al., 2003).
2.6. Analytic strategy
Statistical approach
We used multilevel regression models to examine the 5717 observations from 3017 participants that comprised our two waves of data. Multilevel models allowed us to estimate level and change simultaneously, and are equivalent to latent change score models (Rabe-Hesketh and Skrondal, 2012). The approach allowed us to retain both the 2700 participants who were active throughout the study as well as the 317 persons who completed surveys at baseline and contributed genetic data, but who dropped out of the study before follow-up. We modeled time as a linear function and allowed for random intercepts (i.e., between-person variation in baseline cognitive scores).
We modeled memory and language/executive function at baseline and follow-up as a function of APOE ε4 carrier status and childhood SES. We estimated both a main effects model and an interaction model for each outcome. All models adjusted for the full set of covariates, as well as for the other domain of cognition. Controlling for the other domain of cognition accounts for the non-independence of the outcomes; that is, memory and language/executive functioning are elements of overall cognitive function and are correlated. We followed the steps presented by Belsky et al. (2007) to probe the nature of models with statistically significant interaction terms, described in further detail below.
Missing data
Within the analytic sample of 3017 participants, there were missing data only on the measures of family structure, number of siblings, and hearing problems. Hearing problems was the measure with the most missing data (5% of observations). There were no patterns evident in the missing data. We conducted multiple imputation by chained equations, estimated our models, and combined the estimates across the five datasets using Rubin’s (1987) rules. Results estimated using listwise deletion were substantively similar and are not shown here.
3. Results
3.1. Descriptive statistics
As Table 1 displays, approximately one quarter (27%) of participants in the analytic sample were carriers of the APOE ε4 allele. Carriers had significantly lower memory scores at follow-up than participants who did not have any ε4 alleles. Those with and without an ε4 allele did not differ significantly on any other measure in the study, including memory scores at baseline.
Table 1.
Descriptive statistics for all variables in analyses, by APOE ε 4 carrier status.
| Total Sample N = 3017 | APOE ε4 Carrier N = 815 | APOE ε4 Non-carrier N = 2202 | Test Statistic For Group Comparison | |
|---|---|---|---|---|
| Mean (SD) or % | Mean (SD) or % | Mean (SD) or % | ||
| Cognitive Function | ||||
| Memory (baseline) | 54.42 (16.41) | 53.91 (16.84) | 54.61 (16.25) | 1.03 |
| Memory (7-year follow-up) | 49.29 (13.58) | 47.57 (14.05) | 49.92 (13.35) | 4.03*** |
| Language/executive function (baseline) | 47.14 (12.10) | 47.21 (12.50) | 47.11 (11.96) | −0.19 |
| Language/executive function (7-year follow-up) | 44.95 (12.20) | 44.86 (12.78) | 44.98 (11.97) | 0.22 |
| Components of Gene-by-Environment Interaction | ||||
| Has APOE ε4 | 27.01 | - | - | - |
| Childhood SES (SD) | 0.05 (0.99) | 0.07 (0.98) | 0.03 (1.00) | −1.36 |
| Covariates | ||||
| IQ | 102.89 (14.59) | 102.85 (14.71) | 102.91 (14.55) | 0.10 |
| Female | 51.61 | 52.27 | 51.36 | 0.20 |
| Urban adolescence (50,000 or more residents) | 23.30 | 22.70 | 23.52 | 0.23 |
| Suburban adolescence (10,000–49,999 residents) | 24.66 | 23.56 | 25.07 | 0.73 |
| Rural adolescence (9999 or fewer residents) | 52.04 | 53.74 | 51.41 | 1.30 |
| Did not live with both parents until high school graduation | 9.71 | 11.06 | 9.22 | 2.29 |
| Number of siblings | 3.00 (2.23) | 2.95 (2.26) | 3.02 (2.22) | 0.77 |
| Hearing problems baseline | 3.67 | 3.84 | 3.61 | 0.09 |
| Hearing problems follow-up | 7.50 | 6.79 | 7.77 | 0.41 |
Notes: For continuous measures: means, standard deviations, and t-tests are shown. For categorical measures: proportions and chi-square tests are shown.
p < .05
p < .01
p < .001.
On average, participants came from households in which childhood SES was relatively low. An examination of the four individual elements of childhood SES (not shown) revealed that the average mother and father had not finished high school, that the average father had an occupation in the lowest third of prestige, and that the median household income-$5500 per year-was lower than the U.S. median of $5620 in 1957 (Bureau of Labor Statistics, 2006).
3.2. Multilevel model findings
Overall, the results of the multilevel models differed for memory versus language/executive function, as presented in Table 2. Average decline between baseline and follow-up was 9.50 percentage points (p < .001) for memory and 6.51 percentage points (p < .001) for language/executive function. Model 1 demonstrates that being a carrier of the APOE ε4 allele was associated with a significant (p < .01) additional decline in memory (1.76 percentage points) over the seven-year observation period. Based on the average rate of decline, this effect renders ε4 carriers approximately a year and four months “older,” or poorer-performing, than non-carrier counterparts by follow-up (i.e., 9.50/7 years *1.3 years = 1.76). APOE ε4 had no associations with language/executive function at baseline nor on change by follow-up. Childhood SES had no associations with memory, but was associated with poorer language/executive function performance at baseline, such that a standard deviation increase in childhood SES improved language/executive function by 0.76 percentage points (p < .001). This effect is equivalent to approximately 10 months of average change (i.e., 6.51/7*0.83 = 0.77). Childhood SES was not associated with change in language/executive function.
Table 2.
Multilevel models indicating associations between APOE ε4 carrier status, childhood socioeconomic status, and cognition (in percent of maximum possible) at age 65 baseline and change across a seven-year period (N = 3017).
| Memory | Language/Executive Function | |||
|---|---|---|---|---|
| Model 1 | Model 2 | Model 1 | Model 2 | |
| B (SE) | B (SE) | B (SE) | B (SE) | |
| Fixed Components | ||||
| Change over 7 years | − 9.50*** (1.54) | − 9.56*** (1.54) | −6.51*** (0.93) | −6.47*** (0.93) |
| APOE ε4 carrier (baseline) | − 0.73 (0.57) | − 0.85 (0.57) | 0.11 (0.42) | 0.12 (0.42) |
| APOE ε4 carrier (change) | − 1.76** (0.69) | − 1.63* (0.69) | 0.04 (0.45) | −0.02 (0.45) |
| Childhood SES (baseline) | 0.46 (0.28) | − 0.03 (0.32) | 0.76*** (0.21) | 0.79** (0.24) |
| Childhood SES (change) | − 0.37 (0.34) | 0.12 (0.39) | 0.16 (0.22) | −0.05 (0.25) |
| Carrier* childhood SES (baseline) | – | 1.80** (0.57) | - | −0.13 (0.43) |
| Carrier* childhood SES (change) | – | − 1.91** (0.70) | - | 0.81 (0.45) |
| Random Components | ||||
| Person-level intercept | 7.67 (0.25) | 7.68 (0.25) | 7.17 (0.16) | 7.17 (0.16) |
| Observation-level intercept | 11.46 (0.16) | 11.44 (0.16) | 7.40 (0.10) | 7.40 (0.10) |
| Deviance; df | 45,953; 21 | 45,942; 23 | 42,172; 21 | 42,169; 23 |
Note. Childhood SES is standardized. All models include the effects on baseline and change of: the other domain of cognitive function; IQ; gender; geographic setting in adolescence; family structure; number of siblings; and hearing problems.
p < .001
p < .01
p < .05.
Model 2 includes the statistical interaction between childhood SES and APOE ε4. Results indicated a statistically significant interaction of 1.80 percentage points (p < .01) per standard deviation of childhood SES for memory at baseline, as well as a 1.91 percentage point decline (p < .01) per standard deviation of childhood SES between baseline and follow-up. Based on the average rate of decline, these values make an ε4 carrier whose childhood SES was one standard deviation above average approximately a year and four months “younger,” or higher-performing, than a carrier of average SES at baseline (9.56/7*1.3 = 1.78), but this advantage is entirely lost by follow-up. Interactions did not achieve statistical significance (p < .05) in models for language/executive functioning.
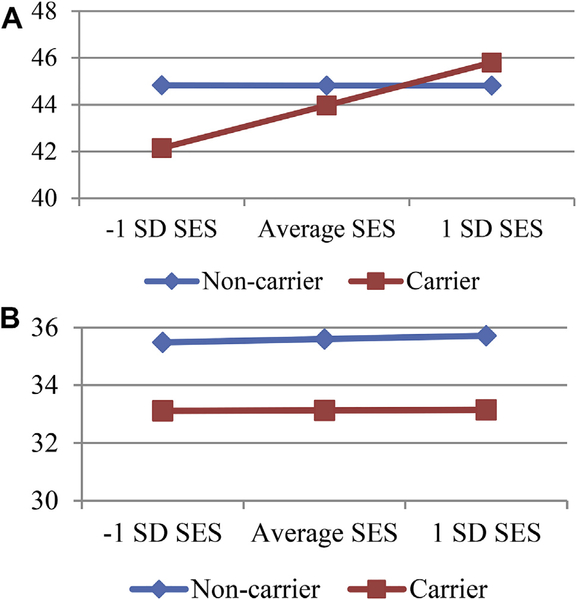
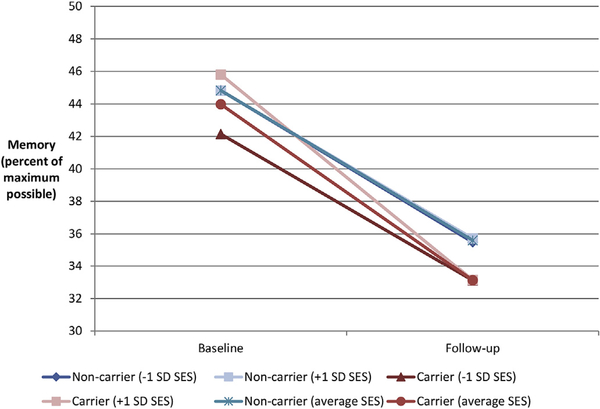
The interactions for memory are illustrated for each time point in Fig. 1. At baseline (Panel A), childhood SES was associated with memory scores for APOE ε4 carriers only. Carriers experienced better memory when they had lived in advantaged households in childhood and poorer memory when they had lived in disadvantaged households in childhood. The memory scores of non-carriers were unrelated to childhood SES. However, by follow-up, memory scores were unrelated to childhood SES for both groups. APOE ε4 carriers had uniformly poorer memory scores than non-carriers, regardless of their childhood SES. The time points are graphed together in Fig. 2, which demonstrates that all participants experienced some memory decline over the 7-year period, but that carriers’ scores on memory were differentiated by childhood SES at baseline.
Fig. 1.
Gene-by-environment interaction of APOE ε4 carrier status and childhood socioeconomic status for memory, by wave.
Note. Panel A shows the interaction at baseline. Panel B shows the interaction at 7-year follow-up. Results are from Table 2, Model 2.
Fig. 2.
Gene-by-environment interaction of APOE ε4 carrier status and childhood socioeconomic status over a seven-year period.
Note. Results from Table 2, model 2.
Belsky et al. (2007) specified three statistical conditions to conclude that differential susceptibility is present in a sample, and our results meet all conditions at baseline. First, Model 2 demonstrates a statistically significant interaction. Second, the two interacting factors, APOE ε4 status and childhood SES, are independent. The correlation between the two is 0.01 (p = .17), or alternatively, APOE ε4 status is not significantly related to childhood SES in a regression model. Third, APOE ε4 status is independent of the outcome, as seen in the non-significant main effect of APOE ε4 on memory in Models 1 and 2. However, there is no longer evidence for differential susceptibility by the time the participants are 72: There is no significant statistical interaction between APOE ε4 and childhood SES. We conclude that differential susceptibility is present at baseline, but not at follow-up.
3.3. Sensitivity analyses
We conducted a series of tests to ensure that our results were robust to alternative analytic choices, sample characteristics, measurement choices, and model assumptions. First, analyses for selective attrition and item non-response are found in Appendix A. The key result-the interaction between childhood SES and APOE ε4 for memory at baseline but not at follow-up-was robust to multiple alternative assumptions about the nature of the missing data. This suggests that results are not driven by the characteristics of participants who died or otherwise left the study. Second, we used the results of our best-fitting factor model (see Appendix B) to calculate factor scores as outcomes. Results from these analyses were consistent with the results of our analysis of mean scores, except that APOE ε4 was not significantly related to change in memory in either Model 1 or Model 2. Third, given the recent availability of polygenetic measures in the WLS (Okbay et al., 2018), we conducted an exploratory analysis that estimated the models using a polygenic score for cognitive performance in place of APOE status. The results for the polygenic score did not yield the same statistically significant results as those for APOE ε4 specifically. In Appendix C, we present these analyses and offer both methodological and conceptual interpretations of why results for APOE ε4 and a polygenic measure differ. Finally, we re-estimated our models excluding people with the ε2/ε4 profile from the sample, because some literature suggests that ε2 counteracts the effects of ε4. The results were consistent with the findings presented here.
4. Discussion
This study examined cognition (i.e., memory and language/executive function) among over 3000 men and women who graduated from Wisconsin high schools in 1957. Approximately a quarter of the participants carried one or more ε4 alleles of the gene APOE, a risk factor for cognitive impairment in later life (Corder et al., 1993; Saunders et al., 1993). Our primary aim was to examine the extent to which childhood SES moderates associations between being an ε4 carrier and cognition in later life—both in terms of baseline levels of cognition and change in cognition over 7 years. We found that APOE ε4 was not associated with baseline levels or change in language/executive function, but it was associated with declines in memory. Findings further indicated that being an APOE ε4 carrier was associated with a differential susceptibility to childhood SES: ε4 carriers from disadvantaged families had the poorest memory at baseline, whereas ε4 carriers from advantaged families had the best memory at baseline. Nevertheless, this moderating effect of childhood SES was not present at follow-up, with ε4 carriers demonstrating poorer memory, on average, than non-carriers, regardless of childhood SES. We further interpret these results below.
4.1. Specificity of cognitive domains
First, it is noteworthy that we found a different pattern of results with respect to memory in comparison to language/executive functioning. Specifically, we found evidence of associations between APOE ε4 and memory, but not language/executive functioning.
This finding is consistent with prior research that similarly has found associations between ε4 status and memory among non-demented older adults, but not language (Forero et al., 2016). These studies also have found associations between ε4 and executive functioning that did not manifest in the current study, possibly because our measure was a hybrid of two cognitive domains, executive functioning and language. Overall, these findings suggest that neurological substrates for memory are perhaps more sensitive to APOE ε4 than those underlying language/executive functioning. This finding also corroborates reports of memory decline as a common symptom that predates diagnosis of AD (Bature et al., 2017).
On the flipside, we found main associations between childhood SES and baseline levels of language/executive functioning, but not memory. However, our missing data analyses (see Appendix A) indicate that childhood SES would have been associated with both baseline and change in memory in a larger sample. Finding a more powerful effect in regards language/executive functioning compared to memory is consistent with studies of children’s neurological development, which has found that childhood SES is a more powerful predictor of language and executive functioning than other domains (Noble et al., 2007). Taken as a whole, these results indicate the importance of continuing to conceptualize cognition as a multidimensional construct, with some components likely more sensitive to particular genetic and social conditions than others.
4.2. Differential susceptibility
The results of this study corroborate prior research in the “dual risk” tradition that indicates that people who both carry genetic and face social risk factors realize the poorest outcomes throughout compounded vulnerability. For example, the poorest memory outcomes at baseline were for participants who both carried APOE ε4 and also experienced low SES in childhood. However, participants who carried APOE ε4 and experienced high SES in childhood had the best memory at baseline, on average. These results suggest a flip-side to dual risk, which theorists have called “differential susceptibility” (Ellis et al., 2011). This concept emphasizes the possibility that “plasticity genes” do not confer risk only, but rather a more general sensitivity to environmental conditions (Belsky et al., 2009). Scholars have theorized on plasticity genes from an evolutionary perspective, positing that genetic factors that pose the possibility of a beneficial or detrimental outcome would not only remain in the gene pool, but also could be a significant advantage in times of rapid social or ecological change.
APOE is a candidate plasticity gene because one of its cellular-level actions is to modulate synaptic plasticity (Reynolds et al., 2014). The alleles ε2 and ε3 make the brain more efficient and effective at adapting to environmental change, whereas the allele ε4 impairs change (Chen et al., 2010). However, our present results are consistent with the body of literature that finds, counterintuitively, that it is ε4 carriers who respond most to different environments. Further research will need to establish why some studies, including this one, have found ε4 carriers to be most susceptible to environmental influences, when the allele’s biological effects suggest that ε4 carriers should be less environmentally-influenced. It is theoretically plausible, for example, that the extent to which ε4 carriers are more or less susceptible to environmental factors depends on the nature of the environmental condition, as well as the broader genetic context. Advancing understanding of this complexity (i.e., investigating beyond single-gene-and-single- environment interactions) is essential for advancing social and health behavior interventions that are especially likely to optimize cognitive outcomes among people facing the highest levels of environmental and genetic risk.
4.3. Change over time
Findings from this study indicated that the moderating effect of childhood SES on genetic risk for poorer memory was no longer present by follow-up. In other words, memory performance at follow-up among ε4 carriers was uniformly poorer than that of non-carriers across childhood SES groups. This finding is consistent with twin and sibling research indicating that genetics, moreso than environments, increasingly explain differences in cognitive function as the adult life course progresses (Reynolds et al., 2014).
This finding suggests the importance of attending to age, or ontogenetic time, in gene-by-environment research, which is congruent with prior evolutionary theorizing on the developmental outcomes of carrying specific genes. Antagonistic pleiotropy describes cases in which a particular allele of a gene functions in ways that enhance reproductive fitness among adults of child-bearing age, but then in later life, functions in ways associated with negative outcomes (Williams, 1957). It is theorized that natural selection eliminates from the gene pool alleles that produce outcomes that hinder survival to childbearing ages. But there is little evolutionary pressure on survival after childbearing: The allele may produce undesirable outcomes, but by the time they appear, the parent has already passed the allele on to offspring. There is some evidence that antagonistic pleiotropy may be characteristic of APOE ε4 (Forero et al., 2016). For example, in one study, ε4 carriers under the age of 35 demonstrated superior attentional skills both behaviorally and on MRI (Rusted et al., 2013). However, the meaning of such results is in dispute, since studies have not consistently yielded evidence for benefits of carrying ε4 among younger adults (Ihle et al., 2012).
4.4. Limitations and conclusion
This study is limited in several ways. First, the analyses drew upon only two waves of cognitive data. Additional waves of data are necessary for further examining the ages over which differential susceptibility dissipates, the variation in rates of change in later life cognition between individuals, and whether rates of change on particular cognitive outcomes are non-linear. Second, there were not enough participants who carried two copies of ε4 for there to be sufficient statistical power to distinguish between the effects of one versus two copies, making our study unable to examine childhood SES as a moderator at different levels of genetic risk. Third, the cognitive tests given in the WLS did not allow us to separate the domains of language and executive function. These domains are conceptually distinct, and more sensitive measures may have revealed different relationships with APOE and childhood SES. Fourth, it is possible, although we believe unlikely, that missing data on APOE status might have biased results (See Appendix A for further discussion.). Finally, our sample does not allow for addressing knowledge gaps on the function of APOE ε4 among people of nonEuropean ancestry. This is an especially important gap to address given that AD and other dementias are more common among African American and Latina/o adults than among whites (Mayeda et al., 2016), and that persons of color in the U.S. face disadvantages, including discrimination (Zhang et al., 2016).
Additionally, the statistical estimates reported in this study indicate small effect sizes, although these effects are consistent with other research that spans multiple decades of the life course (Gow et al., 2008). These findings might be limited in their immediate utility for meaningful clinical directions, although they could be useful for the development of broader population health and prevention purposes (Canevelli et al., 2016 for a thoughtful discussion of converting research results into practice approaches).
Despite these limitations, this study provides further evidence that later life memory is especially sensitive to the genetic risk of carrying APOE ε4. Higher childhood SES served as a buffer against this genetic risk, while lower childhood SES exacerbated the risk, however, only at baseline. By follow-up, APOE ε4 status was associated with poorer memory, regardless of childhood SES. Findings suggest the continued importance of studies on gene-by-environment interactions and cognitive aging to consider the dynamics of timing of risk and protective factors across the life course—both by life stage (i.e., childhood versus adulthood) and within life stages (i.e., earlier versus later periods of later life).
Supplementary Material
Acknowledgements
This research was funded by NIA R01 AG 057491.We also received invaluable support from the Genomics for Social Scientists workshop at the University of Michigan, funded by NIA R25 AG 053227. Wisconsin Longitudinal Study data are available to researchers at https://www.ssc.wisc.edu/wlsresearch/data/
Footnotes
Appendix A. Supplementary data
Supplementary data related to this article can be found at https://doi.org/10.1016/j.socscimed.2018.07.025.
References
- Anderson EL, Heron J, Ben-Shlomo Y, Kuh D, Cooper R, Lawlor DA, Howe LD, 2017. Adversity in childhood and measures of aging in midlife: findings from a cohort of British women. Psychol. Aging 32 (6), 521–530. 10.1037/pag0000182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bature F, Guinn B, Pang D, Pappas Y, 2017. Signs and symptoms preceding the diagnosis of Alzheimer’s disease: a systematic scoping review of literature from 1937 to 2016. BMJ Open 7 (8) e015746 10.1136/bmjopen-2016-015746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck A, Franz CE, Xian H, Vuoksimaa E,Tu X, Reynolds CA, Kremen WS, 2018. Mediators of the effect of childhood socioeconomic status on late midlife cognitive abilities: a four decade longitudinal study. Innovation in Aging 2 (1). 10.1093/geroni/igy003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belsky J, Bakermans-Kranenburg MJ, Ijzendoorn M. H. van, 2007. For better and for worse: differential susceptibility to environmental influences. Curr. Dir. Psychol. Sci 16 (6), 300–304. 10.1111/j.1467-8721.2007.00525.x. [DOI] [Google Scholar]
- Belsky J, Jonassaint C, Pluess M, Stanton M, Brummett B, Williams R, 2009. Vulnerability genes or plasticity genes? Mol. Psychiatr. 14 (8), 746–754. 10.1038/mp.2009.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borenstein AR, Copenhaver CI, Mortimer JA, 2006. Early-life risk factors for alzheimer disease. Alzheimer Dis. Assoc. Disord. 20 (1), 63–72. 10.1097/01.wad.0000201854.62116.d7. [DOI] [PubMed] [Google Scholar]
- Bureau of Labor Statistics, 2006. 100 years of U.S. consumer spending: data for the nation, New York City, and Boston: Retrieved January 9, 2018, from https://www.bls.gov/opub/uscs/. [Google Scholar]
- Canevelli M, Lucchini F, Quarata F, Bruno G, Cesari M, 2016. Nutrition and dementia: evidence for preventive approaches? Nutrients 8 (3), 144 10.3390/nu8030144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Durakoglugil MS, Xian X, Herz J, 2010. ApoΕ4 reduces glutamate receptor function and synaptic plasticity by selectively impairing ApoE receptor recycling. Proc. Natl. Acad. Sci. U.S.A 107 (26), 12011–12016. 10.1073/pnas.0914984107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen P, Cohen J, Aiken LS, West SG, 1999. The problem of units and the circumstance for POMP. Multivariate Behav. Res. 34 (3), 315–346. 10.1207/S15327906MBR3403_2. [DOI] [Google Scholar]
- Cook CJ, Fletcher JM, 2015. Can education rescue genetic liability for cognitive decline? Soc. Sci. Med 127, 159–170. 10.1016/j.socscimed.2014.06.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corder EH, Saunders AM, Strittmatter WJ, Gaskell PC, Roses AD, Petricak- Vance MA, Haines JL, 1993. Gene dose of apolipoprotein E type 4 allele and the risk of Alzheimer’s disease in late onset families. Science 261 (5123), 921–923. 10.1126/science.8346443. [DOI] [PubMed] [Google Scholar]
- Duncan GJ, Magnuson K, 2012. Socioeconomic status and cognitive functioning: moving from correlation to causation. Wiley Interdisciplinary Reviews: Cognitive Science 3 (3), 377–386. 10.1002/wcs.1176. [DOI] [PubMed] [Google Scholar]
- Ellis BJ, Boyce WT, Belsky J, Bakermans-Kranenburg MJ, Ijzendoorn M. H. van, 2011. Differential susceptibility to the environment: an evolutionary-neurodevelopmental theory. Dev. Psychopathol 23 (1), 7–28. 10.1017/S0954579410000611. [DOI] [PubMed] [Google Scholar]
- Ericsson M, Lundholm C, Fors S, Aslan AKD, Zavala C, Reynolds CA, Pedersen NL, 2017. Childhood social class and cognitive aging in the Swedish Adoption/Twin Study of Aging. Proc. Natl. Acad. Sci. Unit. States Am 201620603. 10.1073/pnas.1620603114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forero DA, López-León S, González-Giraldo Y, Dries DR, Pereira-Morales AJ, Jiménez KM, Franco-Restrepo JE, 2016. APOE gene and neuropsychiatric disorders and endophenotypes: a comprehensive review. Am. J. Med. Genet. Part B: Neuropsychiatric Genetics 10.1002/ajmg.b.32516. [DOI] [PubMed] [Google Scholar]
- Glymour MM, Kawachi I, Jencks CS, Berkman LF, 2008. Does childhood schooling affect old age memory or mental status? Using state schooling laws as natural experiments. J. Epidemiol. Community 62 (6), 532–537. 10.1136/jech.2006.059469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gow AJ, Johnson W, Pattie A, Whiteman MC, Starr J, Deary IJ, 2008. Mental ability in childhood and cognitive aging. Gerontology 54 (3), 177–186. 10.1159/000118098. [DOI] [PubMed] [Google Scholar]
- Hauser RM, Warren JR, 1997. Socioeconomic indexes for occupations: a review, update, and critique. Socio. Meth 27 177–298. [Google Scholar]
- Henmon VAC, Nelson MJ, 1954. The Henmon-Nelson Tests ofMental Ability. Manual for Administration. Houghton-Mifflin Company, Boston. [Google Scholar]
- Horsman J, Furlong W, Feeny D, Torrance G, 2003. The Health Utilities Index (HUI®): Concepts, measurement properties and applications. Health Qual. Life Outcome 1 (54). 10.1186/1477-7525-1-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang TL, Zandi PP, Tucker KL, Fitzpatrick AL, Kuller LH, Fried LP, Carlson MC, 2005. Benefits of fatty fish on dementia risk are stronger for those without APOE £4. Neurology 65 (9), 1409–1414. 10.1212/01.wnl.0000183148.34197.2e. [DOI] [PubMed] [Google Scholar]
- Huang Y, Mahley RW, 2014. Apolipoprotein E: structure and function in lipid metabolism, neurobiology, and Alzheimer’s diseases. Neurobiol. Dis 72, 3–12. 10.1016/j.nbd.2014.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ihle A, Bunce D, Kliegel M, 2012. APOE ε4 and cognitive function in early life: a metaanalysis. Neuropsychology 26 (3), 267–277. 10.1037/a0026769. [DOI] [PubMed] [Google Scholar]
- Leduc V, Jasmin-Bélanger S, Poirier J, 2010. APOE and cholesterol homeostasis in Alzheimer’s disease. Trends Mol. Med 16 (10), 469–477. 10.1016/j.molmed.2010.07.008. [DOI] [PubMed] [Google Scholar]
- Mayeda ER, Glymour MM, Quesenberry CP, Whitmer RA, 2016. Inequalities in dementia incidence between six racial and ethnic groups over 14 years. Alzheimer’s Dementia: The Journal of the Alzheimer’s Association 12 (3), 216–224. 10.1016/j.jalz.2015.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell C, McLanahan S, Brooks-Gunn J, Garfinkel I, Hobcraft J, Notterman D, 2013. Genetic differential sensitivity to social environments: implications for research. Am. J. Publ. Health 103 (S1), S102–S110. 10.2105/AJPH.2013.301382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moceri VM, Kukull WA, Emanual I, van Belle G, Starr JR, Schellenberg GD, Larson EB, 2001. Using census data and birth certificates to reconstruct the early- life socioeconomic environment and the relation to the development of Alzheimer’s disease. Epidemiology 12 (4), 383–389. [DOI] [PubMed] [Google Scholar]
- Moceri VM, Kukull WA, Emanuel I, Belle G. van, Larson EB, 2000. Early-life risk factors and the development of Alzheimer’s disease. Neurology 54 (2) 415–415. 10.1212/WNL.54.2.415. [DOI] [PubMed] [Google Scholar]
- Noble KG, McCandliss BD, Farah MJ, 2007. Socioeconomic gradients predict individual differences in neurocognitive abilities. Dev. Sci 10 (4), 464–480. 10.1111/j.1467-7687.2007.00600.x. [DOI] [PubMed] [Google Scholar]
- Okbay A, Benjamin DJ, Visscher PM, 2018. Documentation. Retrieved July3, 2018, from. https://www.ssc.wisc.edu/wlsresearch/documentation/GWAS/Lee_et_al_(2018)_PGS_WLS.pdf.
- Petkus AJ, Wetherell JL, Stein MB, Liu L, Barrett-Connor E, 2012. History of sexual assault is associated with greater declines in executive functioning in older adults with APOE ε4. J. Gerontol.: Ser. Bibliogr 67 (6), 653–659. 10.1093/geronb/gbr163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabe-Hesketh S, Skrondal A, 2012. Multilevel and Longitudinal Modeling Using Stata, Third Edition. Stata Press, College; Station, TX. [Google Scholar]
- Rajan KB, Skarupski KA, Rasmussen HE, Evans DA, 2014. Gene-environment interaction of body mass index and apolipoprotein E ε4 allele on cognitive decline. Alzheimer Dis. Assoc. Disord 28 (2), 134–140. 10.1097/WAD.0000000000000013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds CA, Finkel D, Zavala C, 2014. Gene by environment interplay in cognitive aging In: Behavior Genetics of Cognition across the Lifespan. Springer, New York, NY, pp. 169–199. 10.1007/978-1-4614-7447-0_6. [DOI] [Google Scholar]
- Richards M, Wadsworth M, 2004. Long term effects of early adversity on cognitive function. Arch. Dis. Child 89 (10), 922–927. 10.1136/adc.2003.032490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritchie K, Ritchie CW, Yaffe K, Skoog I, Scarmeas N, 2015. Is late-onset Alzheimer’s disease really a disease of midlife? Alzheimer’s Dementia: Translational Research & Clinical Interventions 1 (2), 122–130. 10.1016/j.trci.2015.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers MAM, Plassman BL, Kabeto M, Fisher GG, McArdle JJ, Llewellyn DJ, Langa KM, 2009. Parental education and late-life dementia in the United States. J. Geriatr. Psychiatr. Neurol 22 (1), 71–80. 10.1177/0891988708328220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin DR, 1987. Multiple Imputation for Nonresponse in Surveys. John Wiley & Sons, New York. [Google Scholar]
- Runge SK, Small BJ, McFall GP, Dixon RA, 2014. APOE moderates the association between lifestyle activities and cognitive performance: evidence of genetic plasticity in aging. J. Int. Neuropsychol. Soc.: JINS 20 (5), 478–486. 10.1017/S1355617714000356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rusted JM, Evans SL, King SL, Dowell N, Tabet N, Tofts PS, 2013. APOE ε4 polymorphism in young adults is associated with improved attention and indexed by distinct neural signatures. Neuroimage 65, 364–373. 10.1016/jLneuroimage.2012.10.010. [DOI] [PubMed] [Google Scholar]
- Saunders AM, Strittmatter WJ, Schmechel D, George-Hyslop PHS, Pericak-Vance MA, Joo SH, Roses AD, 1993. Association of apolipoprotein E allele ε4 with late- onset familial and sporadic Alzheimer’s disease. Neurology 43 (8) 1467–1467. 10.1212/WNL.43.8.1467. [DOI] [PubMed] [Google Scholar]
- Skoe E, Krizman J, Kraus N, 2013. The impoverished brain: disparities in maternal education affect the neural response to sound. J. Neurosci 33 (44), 17221–17231. 10.1523/JNEUROSCI.2102-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomalski P, Moore DG, Ribeiro H, Axelsson EL, Murphy E, Karmiloff-Smith A, Kushnerenko E, 2013. Socioeconomic status and functional brain development - associations in early infancy. Dev. Sci 16 (5), 676–687. 10.1111/desc.12079. [DOI] [PubMed] [Google Scholar]
- Tombaugh TN, Kozak J, Rees L, 1999. Normative data stratified by age and education for two measures of verbal fluency: FAS and animal naming. Arch. Clin. Neuropsychol. 14 (2), 167–177. 10.1016/S0887-6177(97)00095-4. [DOI] [PubMed] [Google Scholar]
- Wang H-X, MacDonald SWS, Dekhtyar S, Fratiglioni L, 2017. Association of lifelong exposure to cognitive reserve-enhancing factors with dementia risk: a community-based cohort study. PLoS Med. 14 (3) e1002251 10.1371/journal.pmed.1002251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D, 1981. Wechsler Adult Intelligence Scale-Revised (WAIS-R). Psychological Corporation. [Google Scholar]
- Wechsler D, 1997. WAIS-III: Administration and Scoring Manual: Wechsler Adult Intelligence Scale. Psychological Corporation. [Google Scholar]
- Williams GC, 1957. Pleiotropy, natural selection, and the evolution of senescence. Evolution 11 (4), 398–411. 10.2307/2406060. [DOI] [Google Scholar]
- Wisconsin Legislative Reference Bureau, 2017. 2015–2016 Wisconsin blue book. Retrieved January 11, 2018, from. http://legis.wisconsin.gov/LRB/publications/wisconsin-blue-book-2015/.
- Yi Z, Gu D, Land KC, 2007. The association of childhood socioeconomic conditions with healthy longevity at the oldest-old ages in China. Demography 44 (3), 497–518. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Hayward MD, Yu Y-L, 2016. Life course pathways to racial disparities in cognitive impairment among older Americans. J. Health Soc. Behav 57 (2), 184–199. 10.1177/0022146516645925. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.