Abstract
Background:
This retrospective study compared the change in serum creatinine between African American and Caucasian Total Knee Arthroplasty (TKA) patients. The authors hypothesized that African Americans would demonstrate significantly greater change, and that a significantly greater proportion would demonstrate creatinine changes consistent with acute kidney injury (AKI).
Methods:
Primary TKAs performed at a single institution between July, 2011-June, 2016 were identified: 1035 primary TKAs met inclusion and exclusion criteria (110 African American, 925 Caucasian, excluding Hispanic and Asian patients). None were excluded based on sex, age, BMI, preoperative diagnosis or comorbidities. All patients had pre-operative and post-operative creatinine levels available in the EMR. Each patient received the same pre-op and post-op protocol for NSAID use along with other drugs administered including anesthesia. All patients received 1 gram of IV (intravenous) vancomycin with some patients additionally receiving 1 gram of vancomycin powder administered locally at the end of surgery. All patients were controlled for fluid intake and blood loss, along with no patient receiving a transfusion or IV contrast. Patient demographics and pre/postoperative serum creatinine were recorded then analyzed for presence of AKI (≥0.3mg/dL). Pre/postoperative serum creatinine concentrations were compared between African American and Caucasian patients using 2×2 repeated measures ANOVA. Prevalence of patients in each group demonstrating AKI was calculated using Fisher Exact test.
Results:
African American patients had significantly greater serum creatinine preoperatively (1.00±0.26 vs. 0.90±0.22, p<0.001) and a significantly greater increase postoperatively (0.10 vs. 0.03, p<0.001). A significantly greater number of African American patients demonstrated AKI (10.9% vs. 5.1%, p=0.03). Furthermore, a significantly greater number of African American patients stayed in the hospital an additional 2 or more days for renal issues (2.7% vs. 0.4%, p=0.03).
Conclusions:
Altered renal function was significantly more common in African American TKA patients. Future studies are necessary to determine if tailoring anti-inflammatories, perioperative medications, and preoperative comorbidities reduce the risk of renal injury and/or a longer hospital stay for this subset of patients.
Keywords: knee replacement, renal function, renal injury, race
Introduction
Osteoarthritis affects more than 4 million Americans and the current gold standard of treatment for knee osteoarthritis is total knee arthroplasty (TKA) [1]. Approximately 700,000 knee replacement procedures are performed annually in the United States and this number is projected to increase to 3.48 million procedures per year by 2030 [1]. Periprosthetic joint infections (PJI) represent what can be a devastating outcome for TKA patients and impart a significant burden of cost to the health care system [2–3]. Many methods have been investigated to decrease the incidence, and antibiotic administration within 1 hour of incision has proven to be the most effective in reducing these infections [4].
Prophylactic intravenous vancomycin therapy has been purposed as an adjunctive agent to cephalexin to prevent periprosthetic joint infection; however, there have been concerns due to its effect on kidney function [5]. Despite these concerns, periprosthetic infection can be a devastating complication following elective surgery [6]. Administration of a cephalosporin alone fails to cover patients who are carriers of MRSA. Despite decolonization strategies, 20% of patients remain MRSA carriers [7]. Thus, with administration of two antibiotics, there is the potential for renal injury. Over the past two decades, dramatic rises in the incidences of acute kidney injury (AKI) have been reported in the United states. Between 2000–2009 there were continued increases by up to 10% per year with a near tripling in absolute number of annual cases [8]. In 2011 acute and unspecified renal failure ranked #16 in the top 20 most expensive conditions billed to Medicare with an aggregate hospital cost of more than 3 billion dollars [9].
Race has been previously identified as a risk factor related to complication and readmission rates after primary TKA [10] and African American patients have been previously reported to be at greater risk of renal disease due to higher rates of diabetes, hypertension, and cardiovascular disease while being almost four times as likely as Caucasians to develop acute renal failure [11–12]. Few studies have explored if racial differences in acute renal function exist following primary TKA. Because of the known detrimental effects of vancomycin on kidney function, our hypothesis was that African Americans would demonstrate significantly greater changes in serum creatinine, and that a significantly greater proportion of African American patients would demonstrate creatinine changes consistent with acute kidney injury (AKI) according to the Kidney Disease Improving Global Outcomes KDIGO criteria [13]. The purpose of this study was to compare the pre- to postoperative change in serum creatinine between African American and Caucasian TKA patients and secondarily to ascertain if this leads to an increased cost on the healthcare system.
Materials and Methods
As part of this IRB-approved retrospective study we identified 1071 primary TKAs performed at a single institution between July 2011 and June 2016. Twenty patients were excluded due to incomplete electronic medical records regarding demographic factors, creatinine, and/or antibiotic prophylaxis. Due to small numbers in our sample data, Hispanic (n=9), Asian (n=4), and patients with unknown or other race (n=3) were excluded from this analysis. Patients were not excluded based on sex, age, BMI, preoperative diagnosis or other medical comorbidities in order to be as otherwise wholly inclusive as possible. After application of inclusion and exclusion criteria, 1035 primary TKAs were identified, 110 were African American and 925 were Caucasian patients.
Demographic information, which included age, body mass index (BMI), sex, and race, was collected for each patient, and ASA grade was utilized as a surrogate measure for preoperative comorbidities. Per the standard of care at our institution, pre- and postoperative serum creatinine values were collected, and patient charts were individually reviewed to determine any incidences of extended hospital stay due specifically to renal issues.
In order to limit outside influence on kidney function, a number of factors were controlled for. All patients had their creatinine measured pre-operatively to establish a baseline and once again day 1 post-operatively when admitted to the hospital. If the patient stayed multiple days creatinine was checked daily, however the post-op day 1 creatinine value was used for analysis. Each patient received the same pre-op and post-op protocol for NSAID use along with other drugs administered including anesthesia. Patients were not screened for MRSA colonization given the lack of evidence demonstrating cost effectiveness [7,14,15]. As high as 20% of patients who undergo decolonization protocol remain colonized with MRSA [7]. Thus, institutional protocol was for all patients to receive 1 gram of IV (intravenous) vancomycin and weight-based dosing of cephalosporin or clindamycin with some patients additionally receiving 1 gram of vancomycin powder administered locally at the end of surgery. The intra-wound application of vancomycin was given based on recent studies demonstrating reduced infection rates with local administration [16–19]. All patients were controlled for fluid intake and blood loss, along with no patient receiving a transfusion or IV contrast.
Statistical Analyses
Pre- and post-op serum creatinine concentrations were compared between African American and Caucasian patients using a 2 × 2 repeated measures ANOVA with antibiotic prophylactic regimen included as a covariate. The prevalence of AKI was determined using the KDIGO definition for Stage I AKI: Increase in SCr ≥0.3 mg/dl within 48h or ≥50% within 7 days. (Table 1) [13]. The prevalence of patients in each group that demonstrated acute renal failure (ARF) was calculated using the Fisher Exact test. Similar methods were then used to compare the frequency of patients whose hospital stay was extended 2 or more days due to renal-related issues. Analyses were performed with SPSS Statistics 23 (IBM, Armonk, NJ) and MedCalc (Ostend, Belgium), and p ≤ 0.05 was considered statistically significant for all analyses.
Table 1.
Comparison of patient demographics
| African American | Caucasian | p value | |
|---|---|---|---|
| Patients | 110 | 927 | |
| Mean Age | 59.8 | 61.8 | 0.03 |
| Mean BMI | 35.3 | 34.0 | 0.06 |
| Sex | 0.64 | ||
| Female | 69 | 560 | |
| Male | 41 | 367 | |
| ASA Grade | 0.46 | ||
| 1 | 1 | 18 | |
| 2 | 29 | 269 | |
| 3 | 78 | 603 | |
| 4 | 2 | 37 |
Results
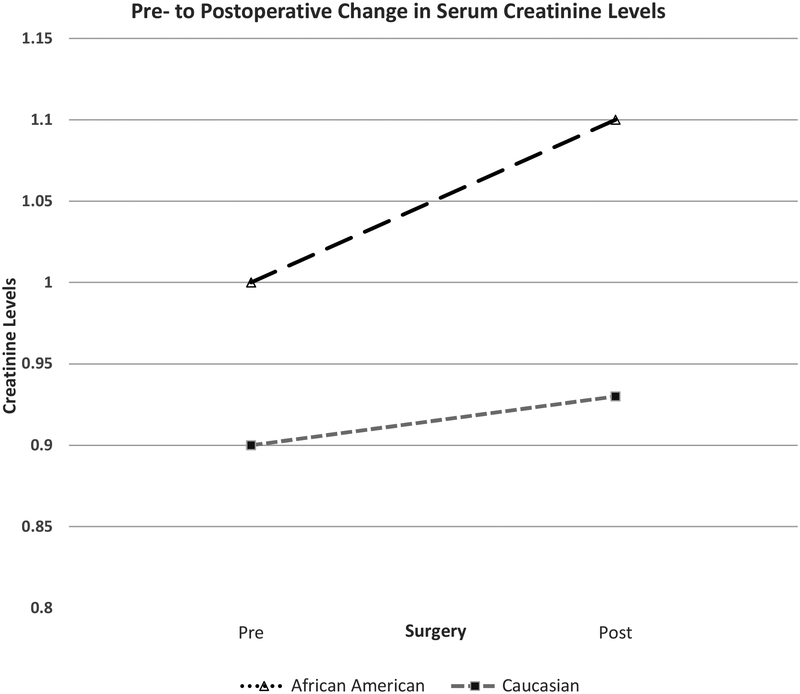
African American patients were found on average to have significantly greater serum creatinine pre-operatively 1.00 ± 0.26 vs. 0.90 ± 0.22, p<0.001 which was to be expected given the known association between African American’s and higher serum creatinine levels at baseline [20]. However, a significantly greater increase in serum creatinine postoperatively was observed in African American patients versus Caucasian patients (pre- to postoperative change = 0.10 vs. 0.03, p < 0.001) (Figure 1). African Americans demonstrated a significantly greater rate of AKI according to the aforementioned KDIGO criteria showing a 10.9% incidence (12/110) compared to a 5.1% incidence (47/925) in the Caucasian population (p =0.03) (Table 3). A significantly greater number of African American patients had hospital stays extended by an extra 2 days or more specifically for renal issues when compared to Caucasian patients (3/110, 2.7% vs. 4/925, 0.4%, p = 0.03) (Table 4). African American patients were significantly more likely to have an extended stay of 2 or more days in the hospital attributed to renal issues than Caucasians (Odds Ratio = 6.5, p < 0.02, [95%CI 1.4–29.2])
Figure 1.
The pre- to postoperative change in serum creatinine levels was significantly greater for African American than Caucasian subjects (p < 0.001)
Table 3. Comparison of acute kidney injury between African American and Caucasian subjects.
African Americans demonstrated a significantly greater rate of AKI according to the aforementioned KDIGO criteria showing an incidence of 10.9% (12/110) compared to a 5.1% incidence (47/925) in the Caucasian population (p =0.03).
| Stage 1 AKI | No AKI | AKI Incidence | |
|---|---|---|---|
| African American | 12 | 98 | 10.9% |
| Caucasian | 47 | 878 | 5.1% |
Table 4. Comparison of hospital stay between African American and Caucasian subjects.
A significantly greater number of African American patients had hospital stays extended by an extra 2 days or more specifically for renal issues when compared to Caucasian patients (3/110, 2.7% vs. 4/925, 0.4%, p = 0.03) (Table 4).
| Extended stay | No Extended Stay | |
|---|---|---|
| African American | 3 | 110 |
| Caucasian | 4 | 925 |
Discussion
Recent medical advances in anesthetic and surgical techniques have led to decreased morbidity and mortality, allowing patients with more significant comorbidities to undergo elective TKA procedures [21–23]. Despite these advances, AKI persists as a perioperative problem evidenced by epidemiological studies citing renal injury occurences with a 0.3–0.4 mg/dL rise in serum creatinine increased hospital-based mortality by 70% [21,24–25]. Due to limited treatment options, it is essential to identify at risk populations with the goal of preventing the occurrence of perioperative AKI.
Our study demonstrated African American TKA patients exhibited greater increases in serum creatinine, increased prevalence of AKI, and more frequent occurrence of prolonged hospital stays attributed to renal issues. Treating orthopaedists may need to consider modifying standard TKA protocols to include strategies to prevent insults to the kidneys including a risk stratification system to better balance the risk of kidney insult with the need for antibiotic prophylaxis and prevention of PJI. Similar to national trends aimed at preoperatively optimizing patients by reducing BMI, A1C, blood pressure, and tobacco use, surgery could be delayed in favor of medically optimizing patients known risk factors for AKI in order to facilitate better outcomes while still allowing for routine antibiotic administration [26]. Improved perioperative pain management, renally dosed anti-inflammatories, and other pre- or peri-operative treatments such as early aggressive volume status optimization may assist in reducing the risk of renal injury and increased hospital stay in this subset of patients [27].
Future studies are necessary in order to determine if either medically optimizing patients or modifying pre- and perioperative clinical care pathways can reduce the risk of AKI in African American TKA patients. Not only would successful intervention lessen the disparity in TKA outcomes for African American patients, but with changing healthcare reimbursement, there are financial ramifications for failing to reduce preventable postoperative complications. In addition, identifying and implementing interventions for other patient groups at increased risk of AKI are needed. Perioperative dehydration, history of diabetes mellitus, perioperative shock, administration of non-steroid anti-inflammatory drugs, and nephrotoxic antibiotics have been shown to be statistically significantly correlated with development of postoperative kidney disease and failure to regain preoperative renal function [28]. It has also been theorized that diminished intravascular volume, secondary to an inability of certain patients to manage fluid shifts that occur during TKA, may be a potential cause of hemoconcentration and subsequent AKI [21,29–30]. Furthermore, multiple studies have observed a connection between angiotensin-converting enzyme inhibitor (ACE-I) medications and AKI in both cardiac and gastric bypass patients [31–35]. While a possible connection between these needs to be further investigated in arthroplasty patients, ACE-I have been purported to have contributed to a case of AKI in a TKA patient [21].
This study was not without limitations. It was a single center study and future studies replicating the results are needed. Due to the retrospective nature of the study, it was subject to transcription errors, omissions of reporting, and other known limitations of this study design. An a priori power analysis was not run prior to the analysis; however, the study was adequately powered to detect group differences, and the racial makeup of the sample was consistent with local demographics. The 2016 US census data reported African Americans represent 14.1% [36] of our local population compared with the 10.7% representation by African Americans in the dataset. The rates of comorbidities such as hypertension, diabetes, and cardiovascular disease were not collected during our data collection in favor of ASA. The decision to utilize ASA was based off the finding by Courney et al [5] that ASA was an independent predictor of AKI postoperatively which was consistent with previous literature. However we acknowledge this is a limitation of the study as it does not provide assurance that differences in these other comorbidities could be affecting the relative rates of AKI in both groups rather than race itself. Finally, potential treatments that patients were receiving for preoperative renal disease were not taken into account, which could have potentially influenced the current results.
Conclusion:
African American TKA patients demonstrated greater increases in serum creatinine, an increased prevalence of AKI, and more frequent increased hospital stays attributed to renal issues. Future studies are necessary to determine if the instance is preventable or can be reduced in order to better outcomes and reduce cost.
Supplementary Material
Table 2:
Kidney Disease Improving Global Outcomes (KDIGO)
| KDIGO Criteria |
|---|
| Stage 1: Increased of ≥0.3 mg/dl or to 1.5–1.9 times baseline |
| Stage 2: To 2–2.9 times baseline |
| Stage 3: To 3.0 times baseline or at least 4.0 mg/dl or initiation of RRT |
Conflicts of Interest and Source of Funding:
PSMRF: The project is supported by the National Center for Advancing Translational Sciences: UL1TR000117/UL1TR001998 and UKHealthCare.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Dillon CF, Rasch EK, Gu Q, Hirsch R. Prevalence of knee osteoarthritis in the United States: arthritis data from the Third National Health and Nutritional Examination Survey 1991–1994. J Rheumatol 2006;33:2271–9. [PubMed] [Google Scholar]
- [2].Kurtz SM, Lau E, Schmier JK, Ong KL, Zhao K, Parvizi J. Infection burden for hip and knee arthroplasy in the United States. J Arthroplasty 2008;23:984–91. doi: 10.1016/j.arth.2007.10.017. [DOI] [PubMed] [Google Scholar]
- [3].Kurtz SM, Lau E, Watson H, Schmier JK, Parvizi J. Economic burden of periprosthetic joint infection in the United States. J Arthroplasty 2012;27:(8 suppl):61–5. doi: 10.1016/j.arth.2007.10.017. [DOI] [PubMed] [Google Scholar]
- [4].Marculescu CE, Osmon DR, Antibiotic prophylaxis in orthopaedic prosthetic surgery. Infect Dis Clinic N Am 2005;19:931–46. [DOI] [PubMed] [Google Scholar]
- [5].Courney PM, Melnic CM, Zimmer Z, Anari J, Lee GC. Addition of Vancomycin to Cefazolin prophylaxis is associated with acute kidney injury after primary joint arthroplasty. Clin Orthop Relat Res 2015;473:2197–2203. doi: 10.1007/s11999-014-4062-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Lum ZC, Natsuhara KM, Shelton TJ, Giordani M, Pereira GC, Meehan JP. Mortality During Total Knee Periprosthetic Joint Infection. J Arthroplasty 2018;33:3783–8. doi: 10.1016/j.arth.2018.08.021. [DOI] [PubMed] [Google Scholar]
- [7].Baratz MD, Hallmark R, Odum SM, Springer BD. Twenty Percent of Patients May Remain Colonized With Methicillin-resistant Staphylococcus aureus Despite a Decolonization Protocol in Patients Undergoing Elective Total Joint Arthroplasty. Clin Orthop Relat Res 2015;473:2283–90. doi: 10.1007/s11999-015-4191-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Siew ED, Davenport A. The growth of acute kidney injury: a rising tide or just closer attention to detail? Kidney Int 2015; 87:46–61. 10.1038/ki.2014.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Torio CM, Moore BJ. National Inpatient Hospital Costs: The Most Expensive Conditions by Payer, 2013: Statistical Brief #204. 2016; Healthcare Cost and Utilization Project (HCUP) Statistical Briefs [Internet]. Rockville (MD): Agency for Healthcare Research and Quality (US); 2006. February-. https://www.ncbi.nlm.nih.gov/books/NBK368492/ [PubMed] [Google Scholar]
- [10].Roche M, Law TY, Sultan AA, Umpierrez E, Khlopas A, Rosas S, Kurowicki J, Wang K, Mont MA. Racial Disparities in Revision Total Knee Arthroplasty: Analysis of 125,901 Patients in National US Private Payer Database. J Racial Ethn Health Disparities. 2018. June 18. doi: 10.1007/s40615-018-0504-z. [DOI] [PubMed] [Google Scholar]
- [11].Martins D, Agodoa L, Norris KC. Hypertensive chronic kidney disease in African Americans: strategies for improving care. Cleve Clin J Med 2012;79:726–34. doi: 10.3949/ccjm.79a.11109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].U.S. Renal Data System, USRDS 2016 Annual Data Report: Atlas of Chronic Kidney Disease and End-Stage Renal Disease in the United States, National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, Bethesda, MD, 2016. [Google Scholar]
- [13].Khwaja A KDIGO Clinical Practice Guidelines for Acute Kidney Injury. Nephron Clin Pract 2012;120:c179–84. doi: 10.1159/000339789. [DOI] [PubMed] [Google Scholar]
- [14].Williams DM, Miller AO, Henry MW, Westrich GH, Ghomrawi HMK. Cost-Effectiveness of Staphylococcus aureus Decolonization Strategies in High-Risk Total Joint Arthroplasty Patients. J Arthroplasty 2017;32:S91–S96. doi: 10.1016/j.arth.2017.01.050. [DOI] [PubMed] [Google Scholar]
- [15].Stambough JB, Nam D, Warren DK, Keeney JA, Clohisy JC, Barrack RL, Nunley RM. Decreased Hospital Costs and Surgical Site Infection Incidence With a Universal Decolonization Protocol in Primary Total Joint Arthroplasty. J Arthroplasty 2017;32:728–34. doi: 10.1016/j.arth.2016.09.041. [DOI] [PubMed] [Google Scholar]
- [16].Pérez-Prieto D, Torres-Claramunt R, Gelber PE, Shehata TM, Pelfort X, Monllau JC. Autograft soaking in vancomycin reduces the risk of infection after anterior cruciate ligament reconstruction. Knee Surg Sports Traumatol Arthrosc 2016;24:2724–8. doi: 10.1007/s00167-0143438-y. [DOI] [PubMed] [Google Scholar]
- [17].Young SW, Zhang M, Freeman JT, Mutu-Grigg J, Pavlou P, Moore GA. The Mark Coventry Award: Higher tissue concentrations of vancomycin with low-dose intraosseous regional versus systemic prophylaxis in TKA: a randomized trial. Clin Orthop Relat Res 2014;472:57–65. doi: 10.1007/s11999-013-3038-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Patel NN, Guild GN 3rd, Kumar AR. Intrawound vancomycin in primary hip and knee arthroplasty: a safe and cost-effective means to decrease early periprosthetic joint infection. Arthroplast Today 2018;4:479–83. doi: 10.1016/j.artd.2018.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Tailaiti A, Shang J, Shan S, Muheremu A. Effect of intrawound vancomycin application in spinal surgery on the incidence of surgical site infection: a meta-analysis. Ther Clin Risk Manag. 2018;14:2149–59. doi: 10.2147/TCRM.S185296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Udler MS, Nadkarni GN, Belbin G, Lotay V, Wyatt C, Gottesman O, Bottinger EP, Kenny EE, Peter I. Effect of Genetic African Ancestry on eGFR and Kidney Disease. J Am Soc Nephrol 2015. July;26(7):1682–92. doi: 10.1681/ASN.2014050474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Rosenbaum AJ, Luciano JA, Marburger R, Hume E. Acute kidney injury in the setting of knee arthroplasty: a case report and discussion investigating Angiotensin-converting enzyme inhibitors as the culprit. HSS J 2011;7:183–6. doi: 10.1007/s11420-010-9189-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Parvizi J, Holiday AD, Ereth MH, Lewallen DG. The Frank Stinchfield Award. Sudden death during primary hip arthroplasty. Clin Orthop Relat Res 1999;369:39–48. [DOI] [PubMed] [Google Scholar]
- [23].Parvizi J, Sullivan TA, Trousdale RT, Lewallen DG. Thirty-day mortality after total knee arthroplasty. J Bone Joint Surg Am 2001;83:1157–61. [DOI] [PubMed] [Google Scholar]
- [24].Chertow GM, Burdick E, Honour M, Bonventre JV, Bates DW. Acute kidney injury, mortality, length of stay, and costs in hospitalized patients. J Am Soc Nephrol 2005;16:3365–70. [DOI] [PubMed] [Google Scholar]
- [25].Thakar CV, Kharat V, Blanck S, Leonard AC. Acute kidney injury after gastric bypass surgery. Clin J Am Soc Nephrol 2007;2:426–30 [DOI] [PubMed] [Google Scholar]
- [26].Leeds IL, Canner JK, Gani F, Meyers PM, Haut ER, Efron JE, Johnston FM. Increased Healthcare Utilization for Medical Comorbidities Prior to Surgery Improves Postoperative Outcomes. Ann Surg 2018; June 1 doi: 10.1097/SLA.0000000000002851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Feng JE, Novikov D, Anoushiravani AA, Schwarzkopf R. Total Knee Arthroplasty: Improving Outcomes with a Multidisciplinary Approach. J Multidiscip Healthc 2018;11:63–73. doi: 10.2147/JMDH.S140550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Kateros K, Doulgerakis C, Galanakos SP, Sakellariou VI, Papadakis SA, Macheras GA. Analysis of kidney dysfunction in orthopaedic patients. BMC Nephrol 2012;3:101. doi: 10.1186/1471-2369-13-101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Lynch NM, Trousdale RT, Ilstrup DM. Complications after concomitant bilateral total knee arthroplasty in elderly patients. Mayo Clin Proc 1997;72:799–805. [DOI] [PubMed] [Google Scholar]
- [30].Ritter M, Mamlin LA, Melfi CA, Katz BP, Freund DA, Arthur DS. Outcome implications for the timing of bilateral total knee arthroplasties. Clin Orthop Relat Res 1997;345:99–105. [PubMed] [Google Scholar]
- [31].Benedetto U, Sciarretta S, Roscitano A, Fiorani B, Refice S, Angeloni E, Sinatra R. Preoperative Angiotensin-converting enzyme inhibitors and acute kidney injury after coronary artery bypass grafting. Ann Thorac Surg 2008;86:1160–5. doi: 10.1016/j.athoracsur.2008.06.018. [DOI] [PubMed] [Google Scholar]
- [32].Boldt J, Schindler E, Härter K, Görlach G, Hempelmann G. Influence of intravenous administration of angiotensin-converting enzyme inhibitor enalaprilat on cardiovascular mediators in cardiac surgery patients. Anesth Analg 1995;80:480–5. [DOI] [PubMed] [Google Scholar]
- [33].Cittanova ML, Zubicki A, Savu C, Montalvan C, Nefaa N, Zaier K, Riou B, Coriat P. The chronic inhibition of angiotensin-converting enzyme impairs postoperative renal function. Anesth Analg 2001;93:1111–5. [DOI] [PubMed] [Google Scholar]
- [34].Comfere T, Sprung J, Kumar MM, Draper M, Wilson DP, Williams BA, Danielson DR, Liedl L, Warner DO. Angiotensin system inhibitors in a general surgical population. Anesth Analg 2005;100:636–44. [DOI] [PubMed] [Google Scholar]
- [35].Kincaid EH, Ashburn DA, Hoyle JR, Reichert MG, Hammon JW, Kon ND. Does the combination of aprotinin and angiotensin-converting enzyme inhibitor cause renal failure after cardiac surgery? Ann Thorac Surg 2005;80:1388–93.. [DOI] [PubMed] [Google Scholar]
- [36].U.S. Census Bureau QuickFacts: Lexington-Fayette, Kentucky. https://www.census.gov/quickfacts/fact/table/lexingtonfayettekentucky/PST045217 [accessed January 05, 2018]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.