Abstract
Our previous study has revealed 4-(4-(4-chlorophenyl)-1,4-diazepan-1-yl)-1-(4-fluorophenyl)butan-1-one-2HCI (SYA013) 1 as a sigma ligand with moderate selectivity for the sigma-2 receptor. Given the overexpression of sigma receptors in solid tumors and reports of sigma ligands with anticancer activities, we selected 1 for evaluation in several solid tumor cell lines. In addition, we have synthesized new analogs of 1 and now report that several of them bind preferentially at the sigma-2 receptor and have shown inhibition of several cancer cell lines including MDA-MB-231, MDA-MB-486, A549, PC-3, MIA PaCa-2 and Panc-1 cells. In particular, compounds 1 and 12 have demonstrated sub-micromolar activity against the Panc-1 cell line. It has also been observed that several of these compounds demonstrate selective toxicity toward cancer cells, when compared to normal cells.
Keywords: Sigma receptors, sigma-2 receptor, indanone, homopiperazine, oxime, anticancer activity
1. Introduction
Sigma (σ) receptors are distinct proteins incorporated in plasma, mitochondrial and endoplasmic reticulum membranes of tissues in the brain, kidneys, endocrine, liver, immune system, and reproductive organs [1]. Currently, there are two well-defined sigma receptor subtypes; sigma-1 (σ1) and sigma-2 (σ2) [2]. The σ1 receptor has a molecular weight of 25 kDa and the (σ2) receptor has a molecular weight of 18 – 21.5 kDa and both have been cloned [3]. There are several recent reviews that address the importance of sigma receptors as targets for drug development [4]. Until recently, interest in sigma receptor ligands was focused on their use as CNS agents however, research has shown that both σ1 and σ2 receptors are widely expressed in various cell lines [5], but non-malignant cells from the same tissue displayed less sigma receptor expression than malignant cells [6]. It has also been shown that there is an expression of σ1 and σ2 receptors in breast cancer and other cell lines, with increased levels of σ2 receptors versus the σ1 receptor subtype, [7]. In addition, several lines of evidence have suggested that the proportion of σ2 receptors in fast multiplying cells is higher than in dormant cells with fast multiplying cells expressing up to ten times more σ2 receptors than resting cells [8]. Furthermore, it has been noted that o receptors were abundant in human breast tumor biopsy tissue, but practically lacking in normal breast tissue [8],[9]. Thus, σ receptors, and in particular σ2 receptors, have a great potential to serve as targets for anticancer drug development due to their high concentration in several tumor cell types with fast proliferation [10].
Three receptors are commonly targeted in anti-breast cancer drug design; these are estrogen receptors (ER), progesterone receptors (PR) and human epidermal growth factor receptor 2 (HER2). However, it has been shown that there is a subtype of breast cancers which is estrogen receptor-negative, progesterone receptor-negative and HER2-negative, designated “triple negative breast cancer” (TNBC) [11]. Depending on the stage of its diagnosis, TNBC can be particularly aggressive, and more likely to recur than other subtypes of breast cancers. Black women have the highest risk factor for TNBC. According to the Breastcancer.org website, an analysis has found that African American women are three times as likely as white women to be diagnosed with TNBC [12] which has a poorer prognosis. Given the absence of targeted receptors in TNBC, sigma ligands may serve as a useful target for anti-TNBC drug development [7].
We have previously demonstrated that SYA013, a homopiperazine analog of haloperidol, binds with moderate selectivity to σ2 receptors (σ1 = Ki = 24 and σ2 Ki = 5.6 nM), and that its deoxygenated analog 2 (Fig. 1) resulted in increased affinity for the σ1 receptor and a loss of selectivity for the σ2 receptor [13]. Thus, we hypothesize that TNBC, which overexpresses sigma receptors, could be an interesting target for the design of new SYA013 analogs.
Fig. 1.
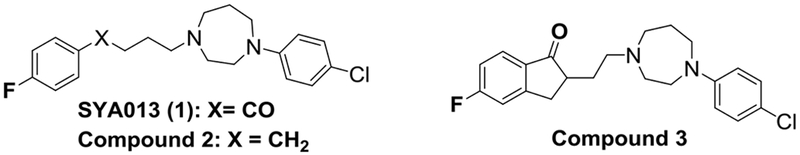
Structures of SYA013, analog 2 and 3
The aim of the current study therefore was to design and synthesize a limited number of new SYA013 analogs, investigate the structural elements that impact σ2 selectivity and evaluate a select number of analogs for activity against TNBC and subsequently other cell lines. We now report the synthesis of several such analogs of SYA013, including selected oximes, that bind with high affinity to σ2 receptors and display anticancer activity against TNBC and other cancer cell lines.
2. Results and discussion
2.1. Chemistry
Compound 3 was previously synthesized and reported by us [14]. The synthetic methods leading to alkylating agents 14, 15, 16 and 17 were also previously reported [14], [15], [16]. Compounds 4 – 8 were synthesized as described in Scheme 1a. The synthetic methods and conditions had been fairly well studied in order to identify conditions that could improve the yields of the products. The final synthesis employed a one-step coupling reaction of alkylating agents (14 – 17) with amines (18 – 20) in toluene under refluxing conditions. Compound 9 was synthesized using the same method and conditions as described in Scheme 1b by coupling of alkylating agent 17 and 1-(4-chlorophenyl)piperazine.
Scheme 1a.
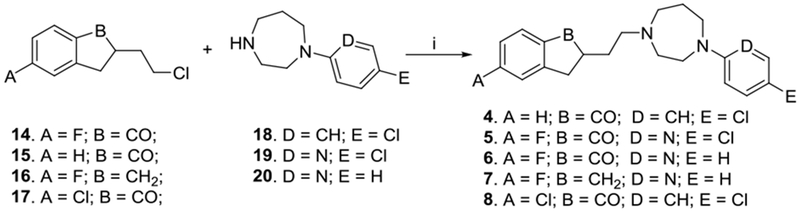
Synthesis of indanone analogs of SYA013. Reagents and conditions: i) Kl, NaHCO3, toluene, reflux.
Scheme 1b.
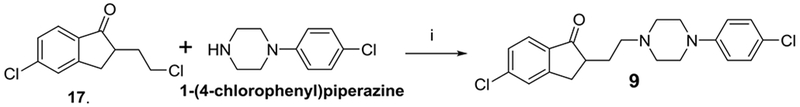
Synthesis of compound 9. Reagents and conditions: i) Kl, NaHCO3, toluene, reflux.
Alkylating agents 21 and 22 were synthesized from the corresponding 2-(2-chloroethyl)-5-fluoro-2,3-dihydro-1 H-inden-1-one 14 and 5-chloroindanone counterpart 17 in a 2-step process, as depicted in Scheme 2.
Scheme 2.
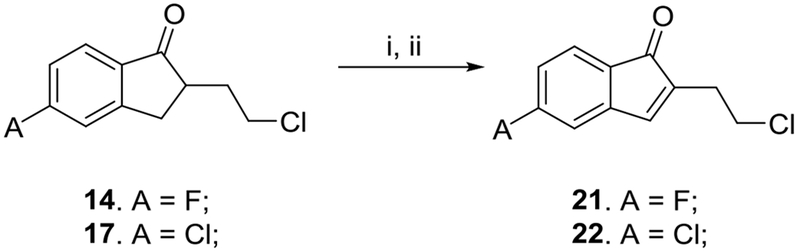
Preparation of alkylating agents 21, 22. Reagents and conditions: i) Br2, CH2Cl2, rt; ii) Li2CO3, decane, 180 °C, 12 hr.
Reaction of alkylating agents 21 and 22 with 1-(4-chlorophenyl)-1,4-diazepane 18 afforded analogs 10, 11 (Scheme 3). A crucial step is the use of toluene as the solvent for alkylation, since isopropanol and other polar solvents yielded multiple spots with low yields which may be due to the competing Michael addition to the indenone [17].
Scheme 3.
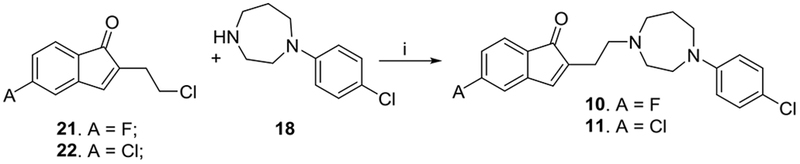
Synthesis of analogs 10, 11. Reagents and conditions: i) Kl, NaHCO3, toluene, reflux.
Oxime 12, 13 were prepared by refluxing ketone 1 and indanone 3 with hydroxylamine hydrochloride under a basic condition in ethanol (Scheme 4).
Scheme 4.
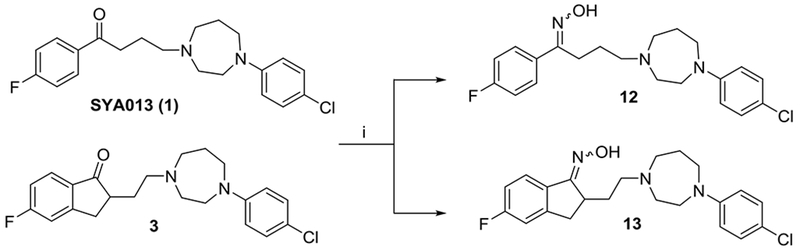
Synthesis of oximes 12 and 13. Reagents and conditions: i) hydroxylamine hydrochloride, NaOH, EtOH-H2O, reflux.
2.2. Biological evaluation
2.2.1. Radioligand binding assays
In our previous article [13], we reported that the carbonyl group in SYA013: 4-(4-(4-chlorophenyl)-1,4-diazepan-1-yl)-1-(4-fluorophenyl)butan-1-one (1) appears to play a significant role in the binding affinity of the homopiperazine analogs to sigma receptors. Thus, we wondered what effect restricting the carbonyl group into an indanone moiety as in 3, might have on binding affinity and selectivity for the σRs. It was also of interest to probe the structure-affinity relationships in the SYA013 scaffold, and so changes were made at four locations on the scaffold of the restricted analog including dehydrogenated analogs or indenones to yield compounds 3 – 11. Subsequently, the oxime analogs of 1 and 3 were synthesized for screening at the σRs and then evaluated along with several carbonyl analogs for anticancer activity against MDA-MB-231 and the compounds with activity against TNBC cells were screened against other cancer cell lines. The structures of the analogs are depicted in Fig. 2 and the binding affinity data are reported in Table 1. It is important to note that the screening activities were carried out on racemic mixtures of the compounds since the separated enantiomers were unstable under ambient conditions and reverted to the racemic mixtures thereafter.
Fig 2.
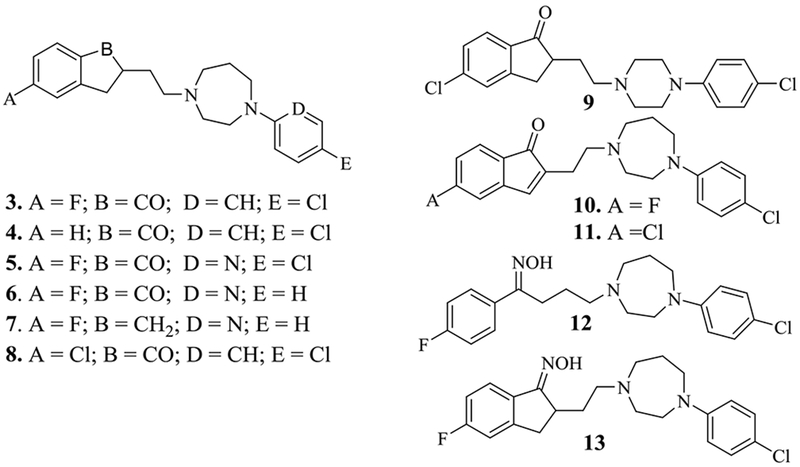
Indanone and oxime analogs of SYA013.
Table 1.
*Binding affinity constants of indanone and oxime analogs of (1).
| Compds | σ1, pKi ± SEM (Ki, nM) | σ2, pKi ± SEM (Ki, nM) | Ki(σ1)/Ki(σ2) |
|---|---|---|---|
| %1 | 7.63±0.07 (24.0) | 8.29±0.07 (5.6) | 4.3 |
| %2. | 8.44±0.05 (3.6) | 8.07±0.06 (8.5) | 0.4 |
| 3. | 7.2±0.04 (63.0) | 9.22±0.07 (0.6) | 105 |
| 4. | (21.0±3.4) | (4.4±0.8) | 4.8 |
| 5. | 7.79±0.05 (16.0) | 9.13±0.07 (0.7) | 22.9 |
| 6. | 7.36±0.06 (44) | 8.75±0.05 (1.8) | 24.4 |
| 7. | 7.65±0.08 (22.0) | 8.85±0.05 (1.4) | 15.7 |
| 8. | 7.59±0.04 (26.0) | 7.72±0.04 (19.0) | 1.4 |
| 9. | 7.68±0.04 (21.0) | 7.74±0.04 (18.0) | 1.2 |
| 10. | 7.11±0.06 (78.0) | 7.7±0.1 (20.0) | 3.9 |
| 11. | 8.30 ± 0.1 (4.9) | 7.9±0.1 (13.0) | 0.4 |
| 12. | 8.29±0.03 (5.1) | 8.46±0.04 (3.5) | 1.5 |
| 13. | 6.60 ± 0.1 (277) | 6.4±0.1 (407) | 0.7 |
| Haloperidol | 8.61±0.1 (2.46) | 8.32±0.1 (4.8) | 0.51 |
| #PB 28 | (0.38) | (0.68) | 0.56 |
Compound 3, the carbonyl restricted analog of 1, binds with more than 2-fold lower affinity for the σ1 receptor but a greater than 9-fold higher affinity for σ2 receptor compared to compound 1. Compound 3 is thus, 105-fold more selective for the σ2 receptor compared to σ1 Compound 4 is the desfluoro analog of 3 and binds with 3-fold higher affinity than compound 3 at the σ1 receptor but over 7-fold lower affinity at the σ2 receptor, suggesting the fluorinated analog is preferred at the σ2 receptor. Compound 5, the pyridine analog of 3 binds with a higher affinity at the σ1 receptor but maintains similar binding affinity at the σ2 receptor while compounds 6 and 7 display similar binding profiles as 5. It should however be noted that unlike the deoxygenated analog 2, compound 7 did not display reversal of selectivity for the σ2 receptor. Compound 8 is the dichloro analog of 3 with similar affinity at the σ1 receptor but over 31-fold reduction in affinity for the σ2 receptor. On the other hand, 4 is the deschloro analog of 8, suggesting that the para chloro has no effect on the phenone moiety in binding to σ1R but makes a significant contribution to binding to the σ2R. In compound 9, the homopiperazine ring was replaced by a piperazine ring in order to explore another isostere of the homopiperazine. Interestingly, there was no significant change in their binding affinities at both sigma receptors suggesting that the piperazine could serve as a bioisostere for the homopiperazine moiety. Compounds 10 and 11 are the indenone analogs of indanones 3 and 8 respectively. While binding affinity was similar at the σ1 receptor for 10, affinity decreased significantly at the σ2 receptor. On the other hand, affinity was enhanced at both sigma receptors when the fluorine (8) was replaced with chlorine (11). With limited data, it is unclear why the trend of binding affinities in the indanone and indenone pairs are inconsistent and we speculate that the binding modes could have changed. It should also be noted that separation of the enantiomers of the indanones was not possible because the enantiomers quickly racemize on standing producing the racemic mixture. Overall, the indanone analogs of SYA013 (compounds 3, 5, 6 and 7) display high affinity and selectivity (>10 fold) for the σ2 receptor. In fact, compound 3 has the highest binding affinity and is the most selective ligand for the σ2 receptor (σ2Ki, = 0.6 nM and σ1Ki/σ2Ki = 105) in this study.
Given the observation that oximes may enhance anticancer properties [18], [19], the oximes of compounds 1 and 3, that is 12 and 13 respectively, were synthesized and evaluated for affinity at the sigma receptors. Compound 12 binds with high affinity at both sigma receptors but compound 13 resulted in a significantly lower affinity for both receptors. This significant change in binding affinity may be associated with a change in topology of the indanone and the indenone ring systems.
2.2.2. Cell viability studies against TNBC cells
With the radioligand binding screening results of the analogs at the σRs at hand, we turned our attention to their activity against a TNBC cell line (MDA-MB-231). The original lead compound 1 and the highest affinity ligands for the σ2 receptor, i.e., compounds 3 and 5, and the oximes of compounds 1 and 3, i.e., 12 and 13, were evaluated for their anti-TNBC activity. Cisplatin, a standard anti-TNBC drug, was included in the assay as a positive control. The cell viability IC50 values are reported in Table 2. In general, the compounds were effective in suppressing the viability of the MDA-MB-231 cells, and their potencies increased with increasing treatment time as indicated by the decrease in the IC50S except for Cisplatin (Table 2).
Table 2.
IC50 (μM) values of synthetic compounds against MDA-MB-231 cell line as determined by cell viability studies.
| Compound | 1 | 3 | 5 | 12 | 13 | Cisplatin |
|---|---|---|---|---|---|---|
| IC50 μM; (24 h) | 36.0±7.0 | 33.6±1.9 | ND | 17.0±0.4 | ND | ND |
| IC50 μM; (48 h) | 18.0±0.4 | 23.5±1.8 | 58.0±16.0 | 8.0±0.2 | 8.0±0.2 | 50.0±9.8 |
| IC50 μM; (72 h) | 15.0±1.1 | 20.1±0.2 | ND | 7.0±0.3 | ND | 64.2±4.3 |
ND = Not determined.
As shown in Table 2 and Fig. 3, compound 3 exhibited both concentration-dependent as well as time-dependent suppression of cell viability and inhibits MDA-MB-231 cells with an IC50 of 23.5 μM compared to cisplatin (Table 2, Fig. 4), with an IC50 of 50.0 μM at 48 hours. Thus 3 is at least two times more potent than cisplatin in this assay. Among the five compounds evaluated, compounds 12 and 13 were the most potent against the TNBC cells which is consistent with reports by others [18], [19] indicating that the oxime analog displayed a much higher anticancer activity than the carbonyl counterpart. Among the carbonyl analogs, compound 1 has the highest anti-TNBC activity with an IC50 value of 15.0 μM in 72 h compared to that of cisplatin (64.2 μM) while compound 5, a higher affinity ligand for σ2R, had the lowest anti-TNBC activity. Thus, the available data suggest that the σ2R binding affinity of the compounds in general, did not directly correlate with their anticancer activities.
Fig 3:
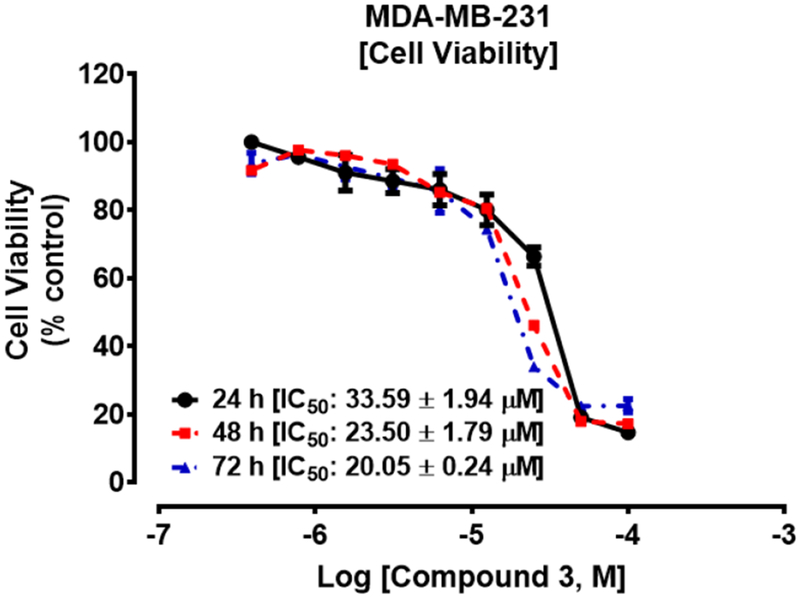
Compound 3 suppressed the viability of MDA-MB-231 cells. Cells were cultured and seeded in 96-well plates at a density of 2 × 104 per well and allowed to attach overnight at 37°C in 5% CO2/95% humidified air. Cells were then treated with compound 3 (0 – 200 μM) for 24 h, 48 h and 72 h as described in the methods. Cell viability was determined after the final treatment by fluorescence using the resazurin reduction assay.
Fig 4:
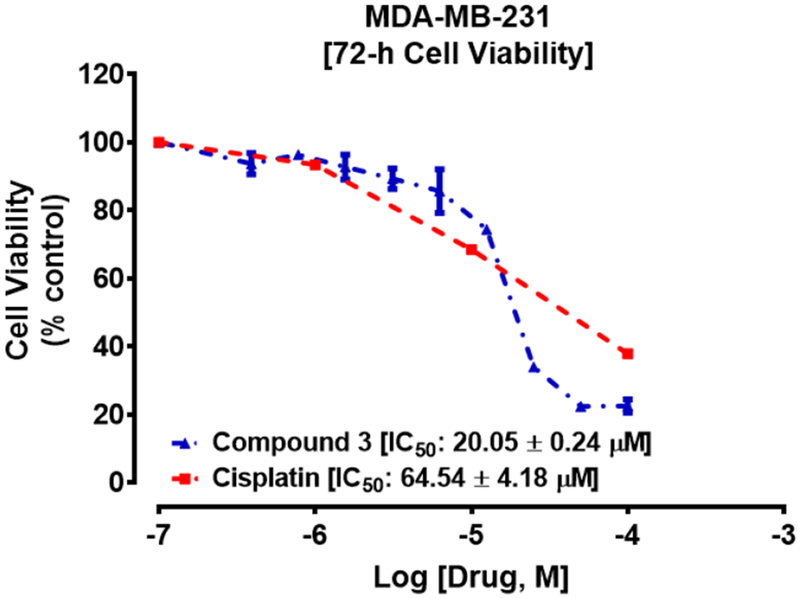
Cisplatin (IC50 64.2±4.2 μM) appears to be relatively less potent compared to compound 3 (IC50 20.1±0.2 μM) on MDA-MB-231 cells after treatment for 72 h. Each point represents the mean ± SEM relative to the control untreated cells.
2.2.3. Cell viability studies against normal cells (HEK293), comparisons
In addition, the IC50 value of 3 against MDA-MB-231 cells was compared to its effect against normal embryonic kidney cell lines, (Human Embryonic Kidney 293 cells or HEK 293 cells) in order to evaluate its selective toxicity (Fig. 5 and Table 3). As shown in Fig 3, compound 3 demonstrated a two-fold selectivity for inhibiting the cancer cells (IC50 = 20.1 μM) compared to HEK293 cells with an IC50 value of 41.3 μM (Table 3).
Fig 5.
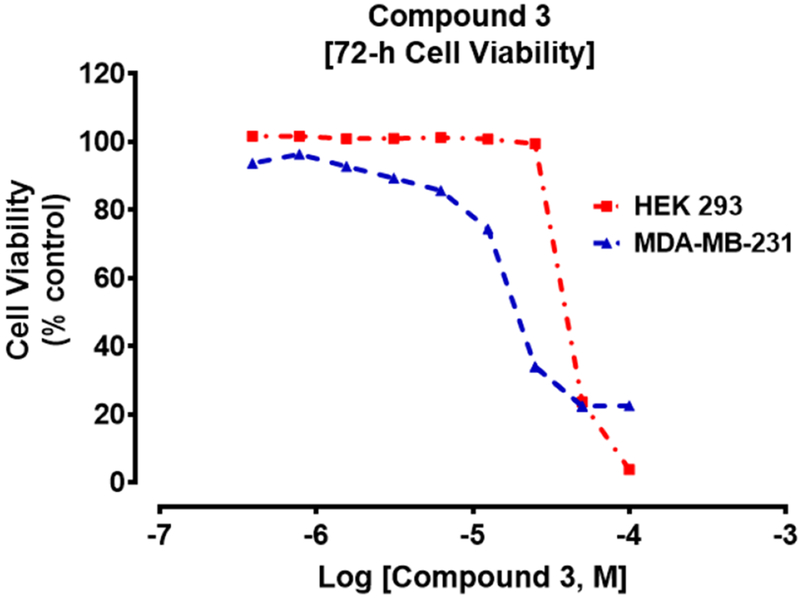
Effect of 3 on TNBC or HEK 293 cells.
Table 3.
IC50 (μM) values for TNBC and HEK293 cells
| σ2R Ligand | IC50 (μM) |
|
|---|---|---|
| MDA-MB-231 | HEK 293 | |
| 3 | 20.1±0.2 | 41,3±0.2 |
2.2.4. Cell viability studies against MDA-MB-468, A549, PC-3, MIA PaCa-2, and Panc-1 cell lines
Based on the initial results, we conducted further cell viability studies on other solid tumor cell lines known to overexpress σRs including the other TNBC cell line MDA-MB-468 [20], human alveolar basal epithelial adenocarcinoma A549 cells [21], prostate cancer PC-3 cells [22], the pancreatic cancer cell lines, MIA PaCa-2 [23], and Panc-1 [23]. The results are shown in Fig. 6 and Table 4.
Fig. 6.
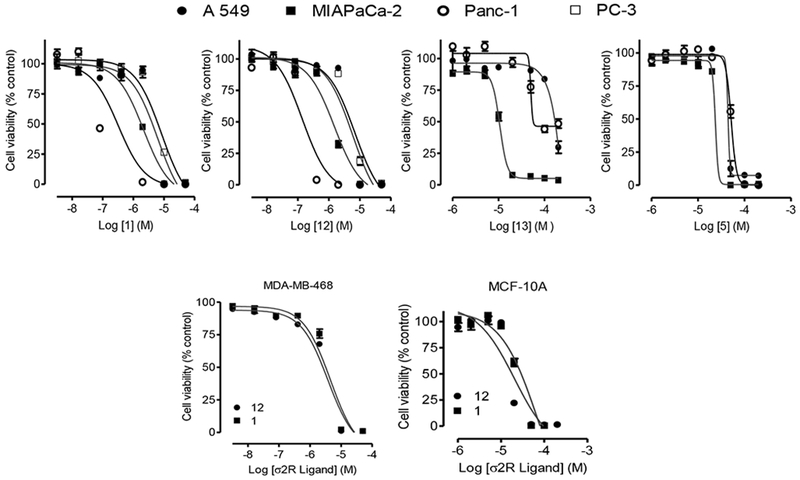
Sigma-2 ligands inhibit diverse cancer cell viability. (Top panel) Human lung cancer A549 cells (●), human prostate cancer PC-3 cells (□), human pancreatic cancer MIAPaCa-2 (■) and Panc-1 (○) cells were seeded into 96-wells tissue culture plates at 20,000 cells per well overnight in experimental media. Cells were then treated with the indicated σ2R ligands as described in the methods. (Bottom panel) Similar tests were conducted on human breast cancer, MDA-MB-468 cells and the non-tumorigenic epithelial cell line, MCF-10A using compounds 1 and 12. Cell viability was determined after the final treatments by fluorescence using the resazurin reduction assay. Each point represents the mean ± SEM relative to the control untreated cells.
Table 4.
IC50 (μM) values of synthetic compounds against various cancer cell lines and the non-tumorigenic epithelial cell line, MCF-10A
| σ2R Ligands | IC50 (μM) |
|||||
|---|---|---|---|---|---|---|
| MDA-MB-468 | MCF-10A | A549 | PC-3 | MIA PaCa-2 | Panc-1 | |
| 12 | 4.0±0.8 | 20.0 ± 7.0 | 5.8 ± 2.0 | 6.9 ± 2.0 | 1.5 ± 0.24 | 0.14 ± 0.1 |
| 1 | 5.0±1.5 | 22 ± 1.3 | 6.2 ± 2.0 | 8.2 ± 2.0 | 2.0 ± 0.3 | 0.4 ± 0.2 |
| 13 | ND | ND | >200 | ND | 10.0 ± 0.3 | 76.0 ± 0.3 |
| 5 | ND | ND | 52.0 ± 1.2 | ND | 42.0 ± 15.0 | 51.0 ± 35.0 |
ND: Not determined
Overall, the results demonstrate that 1 and 12 display marked selective toxicity against MDA-MB-468 when compared to the non-tumorigenic epithelial MCF-10A cells. In particular, while compounds 1 and 12 have activity against all the cell lines evaluated, they are especially effective against the pancreatic cancer cell lines, (MIA PaCa-2 and Panc-1 cell lines) as shown in Table 4. These two compounds exhibited sub-micromolar IC50 values against Panc-1 cells, which therefore suggest a need to take a closer look at them.
It is important to note that there are suggestions that pan-SR ligands might be more effective as anticancer agents [24]. This however, has not been the focus of this manuscript. In addition, the functional status of the current agents have not been evaluated and may very well explain why there was no correlation between the sigma-2 binding affinity of the reported compounds and inhibition of cancer cells.
3. Conclusions
In summary, we have shown in this study that several of the sigma ligands screened displayed some selectivity for the σ2 receptor verses the sigma-1 receptor. Preliminary cell viability studies have demonstrated that not only do these compounds inhibit TNBC cells but they appear to show selective toxicity toward cancer cells when compared to normal cell lines. In particular, compounds 1 and 12 have shown significant inhibition of pancreatic cancer Panc-1 cells with sub-micromolar IC50 values of 0.4 and 0.14 μM, respectively. These encouraging results will be further evaluated in future studies.
4. Experimental
4.1. Chemistry
4.1.1. General
Melting points were determined on a Gallenkamp (UK) apparatus and are uncorrected. All NMR spectra were obtained on either a Varian 300 MHz Mercury Spectrometer or Bruker 600 MHz Spectrometer. The TopSpin 3.5 software running on the Bruker NMR has a multiplet analysis tool (MANA) that identifies and defines multiplets automatically or interactively. Elemental analyses were carried out by Atlantic Microlab, Inc., Norcross, GA, and are within 0.4% of theory unless otherwise noted. Flash chromatography was performed on Isolera I (Biotage) instrument on silica gel (Davisil grade 634) with gradient elution varying from 100% Hexane to 100% EtOAc as a standard procedure except where otherwise indicated. Starting materials were obtained from Sigma–Aldrich and were used without further purification. Yields reported in the manuscript are not optimized yields.
4.1.2. Synthesis of indenones 21 and 22)
4.1.2.1. 1 2-(2-chloroethyl)-5-fluoro-1 H-inden-1-one (21)
To a solution of 2-(2-chloroethyl)-5-fluoro-2,3-dihydro-1H-inden-1-one 14 (1 g, 4.7 mmol) in CH2CI2 (15 mL) was added dropwise a solution of Br2 (800 mg, 5 mmol) in CH2CI2 (5 mL). After stirring for 3 h, the solvent was removed and 2-bromo-2-(2-chloroethyl)-5-fluoro-2,3-dihydro-1H-inden-1-one was obtained. The solid was dried under vacuum for 12 h and used as such for the next step. 2-Bromo-2-(2-chloroethyl)-5-fluoro-2,3-dihydro-1H-inden-1-one (1.2 g, 4.1 mmol), Li2CO3 (2 g, 27 mmol) in decane (30 mL) was heated at 180 ºC overnight. After cooling to room temperature, the mixture was purified by flash chromatography on silica gel using gradient elution from Hexane (100%) to EtOAc (100%) to give 2-(2-chloroethyl)-5-fluoro-1H-inden-1-one 21, 250 mg, in a yield of 25.3%.
1H NMR (300 MHz, CDCI3): 8.30 (1H, dd, J = 5.7, 8.7 Hz), 7.19 (1H, dt, J = 2.7, 8.4 Hz), 7.06 (1H, dd, J = 2.7, 8.7 Hz), 6.37 (1H, s), 3.86 (2H, t, J = 6.6 Hz), 2.98 (2H, t, J = 6.6 Hz).
4.1.2.2. 2-(2-chloroethyl)-5-chloro-1H-inden-1-one (22)
Using the above procedure, 22 was obtained in 21% yield. 1H NMR (300 MHz, CDCl3): 8.20 (1H, d, J = 8.4 Hz), 7.45 (1H, d, J = 8.4 Hz), 7.38-7.40 (1H, m), 6.32 (1H, d, J =3.2 Hz), 3.85 (2H, t, J = 6.6 Hz), 3.69 (2H, t, J = 6.6 Hz), 3.08 (1H, t, J = 6.6 Hz), 2.98 (1H, t, J = 6.6 Hz).
4.1.3. Synthesis of analogs (4 – 11)
4.1.3.1. General Procedure I
A mixture of 2-(2-chloroethyl)-5-fluoro-2,3-dihydro-1H-inden-1-one, 14 (or 15-17) (5.2 mmol), 1-(5-chloropyridin-2-yl)-1,4-diazepane, 18 (or 19-20) (5.7 mmol), Kl (150 mg), NaHCO3 (11.9 mmol) in Toluene (10 mL) was heated to reflux under N2 for 12 h. After cooling to rt, the mixture was diluted with EtOAc (500 mL) followed by washing with H2O (2x300 mL). The organic layer was pooled, dried over Na2SO4 and filtered. The filtrate was concentrated in vacuo to dryness followed by column chromatography on silica gel to afford the final product.
4.1.3.2. 2-(2-(4-(4-chlorophenyl)-1,4-diazepan-1-yl)ethyl)-2,3-dihydro-1H-inden-1-one (4)
Using 14 and 18, resulted in the free base 4, which was converted to the HCI salt, and recrystallized from MeOH-Et2O to afford the pure HCI salt, in a yield of 36%, mp 209-210 °C. 1H NMR (300 MHz, DMSO-d6): 10.96 (1H, brs), 7.69 (1H, d, J = 7.2 Hz) (indanone), 7.64 (1H, d, J = 7.2 Hz) (indanone), 7.57 (1H, d, J = 7.5 Hz) (indanone), 7.42 (1H, m) (indanone), 7.19 (2H, d, J = 9.0 Hz), 6.74 (2H, d, J = 9.0 HZ), 3.76 (2H, m), 3.49 (2H, m), 3.37 (5H, m), 3.10 (2H, m), 2.82 (1H, m), 2.74 (1H, m), 2.34 (1H, m), 2.23 (1H, m), 2.13 (1H, m), 1.86 (1H, m).
13C NMR (150 MHz, DMSO-d6): 207.2, 154.0, 147.7, 136.3, 135.6, 129.2 (2), 128.3, 127.3, 120.5, 113.9 (2), 54.7, 54.3, 53.5, 47.2, 44.7, 43.9, 32.5, 25.6, 23.7.
Calcd for C22H25CIN2O•HCl: C 65.19, H 6.46, N 6.91; Found: C 65.18, H 6.55, N 6.90.
4.1.3.3. 2-(2-(4-(5-chloropyridin-2-yl)-1,4-diazepan-1-yl)ethyl)-5-fluoro-2,3-dihydro-1H-inden-1-one (5)
Using 14 and 19, resulted in the free base 5, which was converted into HCI salt, followed by crystallization from MeOH-Et2O to afford pure HCI salt in a yield of 28%, mp 195-197 °C.
1H NMR (300 MHz, DMSO-d6): 11.04 (1H, s), 8.28 (1H, brs), 8.08 (1H, d, J = 2.4 Hz), 7.70 (1H, dd, J = 5.4 (H-F coupling), 8.4 Hz) (indanone), 7.64 (1H, dd, J = 2.7 (H-F coupling), 9.0 Hz) (indanone), 7.43 (1H, dd, J = 1.8, 9.0 Hz), 7.26 (1H, dt, J = 2.4, 9.0 Hz), 6.78 (1H, d, J = 9.0 Hz) (indanone), 4.21-4.28 (1H, m), 3.68-3.78 (1H, m), 3.45-3.58 (4H, m), 3.24-3.37 (2H, m), 3.04-3.19 (3H, m), 2.76-2.86 (2H, m), 2.36-2.41 (1H, m), 2.10-2.26 (2H, m), 1.82-1.92 (1H, m).
13C NMR (75 MHz DMSO-d6): 205.5, 167.1 (d, J = 255 Hz), 157.2 (d, J = 10.5 Hz), 155.0, 142.4,139.6, 132.9 (d, J = 1.6 Hz), 126.3 (d, J = 11.2 Hz), 118.7, 116.2 (d, J = 23.2 Hz), 113.9 (d, J = 22 Hz), 110.0, 54.6, 53.9, 53.3, 46.7, 45.0, 42.1, 32.5, 25.4, 23.4.
Calcd for C21H23ClFN3O•2HCI: C 54.74, H 5.47, N 9.12; Found: C 54.96, H 5.51, N 9.14.
4.1.3.4. 5-fluoro-2-(2-(4-(pyridin-2-yl)-1,4-diazepan-1-yl)ethyl)-2,3-dihydro-1H-inden-1-one (6)
Using 14 and 20, resulted in the free base 6, which was converted into the HCI salt, followed by crystallization from MeOH-Et2O to afford the pure salt in a yield of 18.5 %, mp 242-243 °C.
1H NMR (300 MHz, DMSO-d6): 14.36 (1H, brs), 11.45 (1H, s), 8.04 (1H, d, J = 6.3 Hz), 7.99 (1H, t, J = 8.1 Hz), 7.75 (1H, dd, J = 5.4 (H-F coupling), 8.7 Hz) (indanone), 7.47 (1H, d, J = 8.7 Hz) (indanone), 7.27 (2H, m), 6.96 (1H, t, J = 6.6 Hz) (indnaone), 4.23 (1H, m), 4.06 (1H, m), 3.64 (5H, m), 3.30 (4H, m), 2.86 (2H, m), 2.53 (1H, m), 2.22 (2H, m), 1.88 (1H, m).
13C NMR (150 MHz, DMSO-d6): 205.4, 167.0 (d, J = 251.9 Hz), 157.2 (d, J = 10.1 Hz), 152.8, 143.4, 138.3, 132.9, 126.3 (d, J = 10.8 Hz), 116.1 (d, J = 23.9 Hz), 113.8 (d, J = 22.1 Hz), 112.9, 112.1, 54.6, 53.3, 52.7, 47.7, 44.9, 43.1, 32.4, 25.2, 23.0.
Calcd for C21H24FN3O•2H2)O:C 58.66, H 6.19, N 9.77; Found: C 58.69, H 6.23, N 9.67.
4.1.3.5. 1-(2-(5-fluoro-2,3-dihydro-1H-inden-2-yl)ethyl)-4-(pyridin-2-yl)-1,4-diazepane (7)
Using 16 and 20 resulted in the free base 7, which was converted into the HCI salt followed by crystallization from MeOH-Et2O to afford the pure salt, in a yield of 27.5%, mp 172-173 °C.
1H NMR (300 MHz, DMSO-d6): 11.39 (1H, brs), 8.03 (1H, m), 7.99 (1H, m), 7.26 (1H, m), 7.17 (1H, dd, J = 5.8 (H-F coupling), 8.4 Hz) (indanone), 6.91 (3H, m) (overlap, 2 of 3 indanone), 4.21 (1H, m), 4.06 (1H, m), 3.70 (3H, m), 3.57 (2H, m), 3,21 (2H, m), 3.14 (2H, m), 2.99 (2H, m), 2.56 (2H, m), 2.41 (1H, m), 2.18 (1H, m), 1.90 (2H, m).
13C NMR (75 MHz, CDCI3) for free base 7: 161.9 (d, J = 241.6 Hz), 158.2, 147.9, 145.5 (d, J = 8.0 Hz), 138.6, 137.2, 125.0 (d, J = 8.0 Hz), 112.7 (d, J = 23.0 Hz), 111.4 (d, J = 20.5 Hz), 111.3, 105.4, 56.7, 55.2, 55.0, 46.6, 46.5, 39.4, 39.0, 38.4, 33.3, 27.7.
Calcd for C21H26FN3•2HCl•0.7 H2O: C 59.35, H 6.64, N 9.89; Found: C 59.35, H 6.96, N 9.72.
4.1.3.6. 5-chloro-2-(2-(4-(4-chlorophenyl)-1,4-diazepan-1-yl)ethyl)-2,3-dihydro-1H-inden-1-one (8)
Using 17 and 18 resulted in the free base 8, which was converted into the HCI salt followed by crystallization from MeOH-Et2O to afford the pure salt in a yield of 27%, mp 189-191 °C.
1H NMR (600 MHz, DMSO-d6): 10.78 (1H, brs), 7.73 (1H, s) (indanone), 7.67 (1H, d, J = 8.1 Hz) (indanone), 7.51 (1H, dd, J = 1.7, 8.1 Hz) (indanone), 7.21 (2H, d, J = 9.0 Hz), 6.77 (2H, d, J = 9.0 Hz), 3.72-3.82 (2H, m), 3.41-3.53 (3H, m), 3.31-3.39 (3H, m), 3.17-3.29 (2H, m), 3.07-3.11 (1H, m), 2.80-2.88 (2H, m), 2.32-2.35 (1H, m), 2.23-2.27 (1H, m), 22.14-2.17 (1H, m), 1.84-1.93 (1H, m).
13C NMR (75, MHz DMSO-d6): 205.9, 155.9, 147.7, 140.4, 135.0, 129.2 (2), 128.5, 127.3, 125.3, 120.4, 113.9 (2), 54.6, 54.5, 47.1, 44.8, 44.0, 32.3, 32.2, 25.4, 23.8.
Calcd for C22H24Cl2N2O•HCl•0.2 H2O C 59.59, H 5.77, N 6.32; Found: C 59.41, H 5.69, N 6.24.
4.1.3.7. 5-chloro-2-(2-(4-(4-chlorophenyl)piperazin-1-yl)ethyl)-2,3-dihydro-1H-inden-1-one (9)
Using 17 and 1-(4-chlorophenyl)piperazine resulted in the free base 9, which was converted into the HCI salt followed by crystallization from MeOH-Et2O to afford the pure salt in a yield of 34%, mp 211-213 °C.
1H NMR (600 MHz, DMSO-d6): 11.00 (1H, brs), 7.75 (1H, s) (indanone), 7.68 (1H, d, J = 8.0 Hz) (indanone), 7.51 (1H, d, J = 8.0 Hz) (indanone), 7.29 (2H, d, J = 8.9 Hz), 7.02 (2H, d, J = 8.9 Hz), 3.81 (2H, brs), 3.57 (2H, brs), 3.31-3.41 (2H, m), 3.21-3.24 (1H, m), 3.12-3.16 (4H, m), 2.84-2.89 (2H, m), 2.26-2.29 (1H, m), 1.91-1.94 (1H, m).
13C NMR (150 MHz, DMSO-d6): 206.0, 156.0, 148.9, 140.5, 135.1, 129.3 (2), 128.6, 127.4, 124.0, 117.9 (2), 54.0, 51.1, 45.7 (2), 44.9 (2), 32.4, 25.1.
Calcd for C21H22Cl2N2O•HCl: C 59.24, H 5.45, N 6.58; Found: C 59.24, H 5.26, N 6.65.
4.1.3.8. 2-(2-(4-(4-chlorophenyl)-1,4-diazepan-1-yl)ethyl)-5-fluoro-1 H-inden-1-one (10)
Using 21 and 18 resulted in the free base 10, which was converted into the HCI salt followed by crystallization from MeOH-Et2O to afford the pure salt in a yield of 32%, mp 234-235 •C.
1H NMR (300 MHz, DMSO-d6): 11.11 (1H, s), 8.17 (1H, dd, J = 5.7 (H-F coupling), 8.7 Hz) (indanone), 7.36-7.48 (2H, m) (indanone), 7.19 (2H, d, J = 9.0 Hz), 6.75 (2H, d, J = 9.0 Hz), 6.69 (1H, s), 3,68-3.84 (2H, m), 3.54-3.59 (2H, m), 3.33-3.47 (3H, m), 3.19-3.25 (1H, m), 3.01-3.14 (4H, m), 2.31-2.41 (1H, m), 2.11-2.20 (1H, m).
13C NMR (150 MHz, DMSO-d6): 166.5 (d, J = 252.2 Hz), 161.1, 154.8, 147.7, 140.2 (d, J = 11.4 Hz), 132.9 (d, J = 10.4 Hz), 129.2 (2), 120.6, 117.1, 116.9, 113.9 (2), 111.9 (d, J = 22.8 Hz), 104.6, 54.6, 53.7, 53.3, 47.2, 44.0, 28.4, 23.8.
Calcd for C22H22ClFN2O•2HCl: C 57.72, H 5.28, N 6.12; Found: C 57.68, H 5.21, N 6.20.
4.1.3.9. 5-chloro-2-(2-(4-(4-chlorophenyl)-1,4-diazepan-1-yl)ethyl)-1H-inden-1-one (11)
Using 22 and 18 resulted in the free base 11, which was converted into the HCI salt followed by crystallization from MeOH-Et2O to afford the pure salt in a yield of 28%, mp 242-243 °C.
1H NMR (300 MHz, DMSO-d6): 10.93 (1H, s), 8.10 (1H, d, J = 8.7 Hz) (indanone), 7.72 (1H, s) (indanone), 7.61(1H, dd, J = 2.7, 8.7 Hz) (indanone), 7.21 (2H, d, J = 9.0 Hz), 6.77 (2H, d, J = 9.0 Hz), 6.69 (1H, s), 3.74-3.80 (2H, m), 3.54-3.60 (2H, m), 3.38-3.48 (4H, m), 3.21-3.27 (1H, m), 3.07-3.14 (3H, m), 2.28-2.35 (1H, m), 2.15-2.21 (1H, m).
13C NMR (150 MHz, DMSO-d6): 161.3, 154.9, 147.7, 140.7, 139.0, 131.4, 129.2 (2), 129.0, 125.6, 120.5, 118.9, 113.9 (2), 104.2, 54.6, 53.7, 53.2, 47.2, 44.0, 28.4, 23.7.
Calcd for C22H22CI2N2O’HCl •1.0H2O C 57.97, H 5.53, N 6.15; Found: C 57.86, H 5.18, N 6.16.
4.1.4. Synthesis of oximes (12 and 13)
4.1.4.1. General procedure II
To a solution of 4-(4-(4-chlorophenyl)-1,4-diazepan-1-yl)-1-(4-fluorophenyl)butan-1-one (1.33 mmol), hydroxylamine hydrochloride (2.0 mmol) in EtOH (10 ml) was added a solution of NaOH (5 mL, 1M). The resulting mixture was refluxed overnight. Water (100 mL) was added which resulted in the formation of a solid. The solid was dried under vacuum overnight and subsequently recrystallized from MeOH to give, 4-(4-(4-chlorophenyl)-1,4-diazepan-1-yl)-1-(4-fluorophenyl)butan-1-one oxime.
4.1.4.2. 4-(4-(4-chlorophenyl)-1,4-diazepan-1-yl)-1-(4-fluorophenyl)butan-1-one oxime (12)
Using procedure II, compound 1 was converted to the oxime, 12 in a yield of 42%, mp 203-204 °C.
1H NMR (300 MHz, DMSO-d6): 11.42 (1H, s), 7.68-7.73 (2H, m) (indanone), 7.16-7.25 (4H, m) (overlap, 2 of 4 indanone), 6.73 (2H, d, J = 9.0 Hz), 3.68-3.74 (2H, m), 3.36- 3.48 (4H, m), 3.04-3.18 (4H, m), 2.69-2.74 (2H, m), 2.26-2.32 (1H, m), 2.05-2.13 (1H, m), 1.82-1.92 (2H, m).
13C NMR (75 MHz, DMSO-d6): 162.9 (d, J = 244.6 Hz), 154.9, 147.7, 132.4 (d, J = 3.3 Hz), 129.2 (2), 128.3 (2, d, J = 8.2 Hz), 120.4, 115.8 (2, d, J = 21.5 Hz), 113.8 (2), 56.2, 54.4, 53.6, 47.1, 43.8, 23.7, 22.6, 21.1.
Calcd for C21H25ClFN3O: C 64.69, H 6.46, N 10.78. Found: C 64.70, H 6.38, N 10.66.
4.1.4.3. 2-(2-(4-(4-chlorophenyl)-1,4-diazepan-1-yl)ethyl)-5-fluoro-2,3-dihydro-1H-inden-1-one oxime (13)
Using procedure II, compound 3 was converted to the oxime 13, in a 34% yield, mp 185-186 °C.
1H NMR (300 MHz, DMSO-d6): 10.97 (1H, s), 8.28 (1H, dd, J = 5.4 (H-F coupling), 8.4 Hz) (indanone), 7.02-7.16 (4H, m) (overlap, 2 of 4 indanone), 6.64 (2H, d, J = 8.4 Hz), 3.37-3.44 (4H, m), 3.10 (1H, dd, J = 8.4, 16.8 Hz), 2.93-3.00 (1H, m), 2.63-2.70 (3H, m), 2.49-2.53 (4H, m), 1.76-1.89 (3H, m), 1.46-1.54 (1H, m).
13C NMR (150 MHz, DMSO-d6): 163.6 (d, J = 246 Hz), 159.2, 150.6 (d, J = 8.8 Hz), 147.9, 130.8 (d, J = 9.4 Hz), 130.6 (d, J = 2.2 Hz), 129.1 (2), 118.9, 114.2 (d, J = 22 Hz), 113.2 (2), 112.7 (d, J = 21.5 Hz), 54.8, 54.6, 54.1, 49.2, 48.1, 39.6, 35.7, 32.4, 27.2.
Calcd for C22H25ClFN3O 0.4H2O: C 64.59, H 6.36, N 10.27; Found: C 64.79, H 6.09, N 10.24.
4.2. Biological testing
4.2.1. Receptor binding studies
Binding affinities (Ki, nM) reported in Tables 1 were conducted by the National Institute of Mental Health Psychoactive Drug Screening Program (NIMH-PDSP). Details of the methods and the radioligands used for the binding assays at each receptor were previously reported [25].
4.2.2. Material and methods for cell culture and cell viability studies
Human adenocarcinoma MDA-MB-231, MIAPaCa-2, Panc-1, A549, MDA-MB-468 and HEK293, MCF-10A cell lines were purchased from American Type Culture Collection (Manassas,VA. USA). Dulbecco’s modified eagle medium (DMEM), high glucose, GlutaMax, Ham’s F-12K, fetal bovine serum (FBS), penicillin-streptomycin-neomycin antibiotic mixture (PSN) were obtained from Life Technologies; Thermo Fisher (Grand Island, NY. USA). DMEM/F-12 was from Gibco. Hank’s balanced salt solution was from (Sigma Aldrich, St. Louis, MO. USA); and PBS was purchased from Genesee Scientific (San Diego, CA. USA).
4.2.2.1. Cell culture
MDA-MB-231, MIAPaCa-2, Panc-1, MDA-MB-468 and HEK 293 were cultured in DMEM, high glucose, GlutaMax; MCF-10A cells were maintained in DMEM/F-12; and A549 cells were cultured in Ham’s F-12K medium. The DMEM/F-12 was supplemented with insulin (10 μg/mL), epidermal growth factor (EGF, 20ng/ml), cholera toxin (100 ng/ml), hydrocortisone (0.5 μg/mL), horse serum (5%), and penicillin/streptomycin (100 U/ml/100 μg/mL); while the other media were supplemented with 100 U/ml penicillin, 100 U/ml streptomycin, and 10% heat inactivated FBS. The cells were incubated in a humidified incubator with atmosphere of 95% CO2 set at 37 °C and sub-cultured when approximately 80-90% confluent. Unless stated otherwise, assays were performed with experimental media containing 5% FBS.
4.2.2.2. Cell viability studies
Cells were seeded into 96-well plates at 20,000 cells per well and incubated overnight in experimental media. Upon confirmation of cells being attached, they were treated for 24 h, 48 h, and 72 h with varying concentrations of the σ2R ligands (σ2RL) in a 1:9 mixture of DMSO/acetone or Cisplatin dissolved in 0.9% sodium chloride (normal saline). Control cells for σ2RL were treated with equivalent volumes of the 1:9 mixture of DMSO/acetone, while the controls of Cisplatin were treated with normal saline. The treatments were repeated at the 24th h for the 48-h assay and at the 24th and 48th h for the 72-h assay.
To determine and quantify the effect of the compounds on cell viability, resazurin reagent that is metabolized by live cells to the fluorescent resorufin product was used. Briefly, 20 μL of 0.02% resazurin reagent prepared in PBS was added to each well and incubated for 2-3 h. The fluorescence was measured at 560 nm excitation wavelength and a detection wavelength of 590 nm using the FLx 800 Microplate Fluorescence Reader from BioTek (Winooski, VT. USA). Using GraphPad Prism 5 software (San Diego, CA. USA), cell viability was expressed as the percentage of the fluorescence in the treated cells relative to that of the controls and the IC50 values were determined from the plots of the non-linear regression of the logs of σ2RL or Cisplatin concentrations.
Acknowledgements
We acknowledge the continuing financial support of the NIH/NIGMS SCORE grant number 2SC1GM116724 and a Title III Grant to Florida A&M University. The work was also supported in part by the Pharmaceutical Research Center NIH/ NCRR 1C06-RR12512-01 Grant. Ki determinations and receptor binding profiles were generously provided by the National Institute of Mental Health’s Psychoactive Drug Screening Program, Contract # HHSN-271-2013-00017-C (NIMH PDSP). The NIMH PDSP is directed by Bryan L. Roth, MD, PhD, at the University of North Carolina at Chapel Hill and Project Officer Jamie Driscoll at NIMH, Bethesda MD, USA. Funding sources acknowledged had no involvement in the study design, data collection and interpretation, or article preparation and submission of this manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].(a) Quirion R, Bowen WD, Itzhak Y, Junien JL, Musacchio JM, Rothman RB, Su TP, Tam SW, Taylor DP, A proposal for the classification of sigma binding sites, Trends Pharmacol. Sci., 13 (1992) 85–86; [DOI] [PubMed] [Google Scholar]; (b) Rousseaux CG, Greene SF, Sigma receptors [sigmaRs]: biology in normal and diseased states, J. Recept. Signal Transduct. Res., (2015) 1–62; [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Su TP, Sigma receptors. Putative links between nervous, endocrine and immune systems, Eur. J. Biochem, 200 (1991) 633–642. [DOI] [PubMed] [Google Scholar]
- [2].Hellewell SB, Bruce A, Feinstein G, Orringer J, Williams W, Bowen WD, Rat liver and kidney contain high densities of sigma 1 and sigma 2 receptors: characterization by ligand binding and photoaffinity labeling, Eur. J. Pharmacol, 268 (1994) 9–18. [DOI] [PubMed] [Google Scholar]
- [3].(a) Hanner M, Moebius FF, Flandorfer A, Knaus HG, Striessnig J, Kempner E, Glossmann H, Purification, molecular cloning, and expression of the mammalian sigma-binding site, Proc. Natl. Acad. Sci. U. S. A, 93 (1996) 8072–8077; [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Seth P, Leibach FH, Ganapathy V, Cloning and structural analysis of the cDNA and the gene encoding the murine type 1 sigma receptor, Biochem. Biophys. Res. Commun, 241 (1997) 535–540. [DOI] [PubMed] [Google Scholar]; c) Alon A, Schmidt, Wood MD, Sahn JJ, Martin SF, Kruse AC, Identification of the gene that codes for the σ2 receptor. Proc Natl Acad Sci USA 114 (2017) 7160–7165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].(a) Jia H, Zhang Y, Fluang Y, Imaging sigma receptors in the brain: New opportunities for diagnosis of Alzheimer’s disease and therapeutic development, Neurosci Lett., 691 (2019) 6913–6910. [DOI] [PubMed] [Google Scholar]; b) Grundman M, Morgan R, Lickliter JD, Schneider LS, DeKosky S, Izzo NJ, Guttendorf R, Higgin M, Pribyl J, Mozzoni K, Safferstein H, Catalano SM A phase 1 clinical trial of the sigma-2 receptor complex allosteric antagonist CT1812, a novel therapeutic candidate for Alzheimer’s disease. Alzheimers Dement (N Y). 5 (2019) 20–26. [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Georgiadis MO, Karoutzou O, Foscolos AS, Papanastasiou I, Sigma Receptor (σR) Ligands with Antiproliferative and Anticancer Activity. Molecules. 22 (2017) pii: E1408. [DOI] [PMC free article] [PubMed] [Google Scholar]; d) Abate C, Niso M, Berardi F, Sigma-2 receptor: past, present and perspectives on multiple therapeutic exploitations, Future Med Chem. 10 (2018) 1997–2018. [DOI] [PubMed] [Google Scholar]; e) Blass BE, Rogers JP, The sigma-2 (σ-2) receptor: a review of recent patent applications: 2013–2018 Expert Opin Ther Pat. 28 (2018) 655–663. [DOI] [PubMed] [Google Scholar]; f) Zeng C, Mach RH The Evolution of the Sigma-2 (σ2) Receptor from Obscure Binding Site to Bona Fide Therapeutic Target. Adv Exp Med Biol. 964 (2017) 49–61. [DOI] [PubMed] [Google Scholar]; g) Smith SB, Introduction to Sigma Receptors: Their Role in Disease and as Therapeutic Targets. Adv Exp Med Biol. 964 (2017) 1–4. [DOI] [PubMed] [Google Scholar]; h) Izzo NJ, Xu J, Zeng C, et al. , Alzheimer’s therapeutics targeting amyloid beta 1-42 oligomers II: Sigma-2/PGRMC1 receptors mediate Abeta 42 oligomer binding and synaptotoxicity. PLoS One. 9(2014), e111899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Vilner BJ, John CS, Bowen WD, Sigma-1 and sigma-2 receptors are expressed in a wide variety of human and rodent tumor cell lines, Cancer Res., 55 (1995) 408–413. [PubMed] [Google Scholar]
- [6].(a) Bern WT, Thomas GE, Mamone JY, Homan SM, Levy BK, Johnson FE, Coscia CJ, Overexpression of sigma receptors in nonneural human tumors, Cancer Res., 51 (1991) 6558–6562; [PubMed] [Google Scholar]; (b) van Waarde A, Rybczynska AA, Ramakrishnan N, Ishiwata K, Elsinga PH, Dierckx RA, Sigma receptors in oncology: therapeutic and diagnostic applications of sigma ligands, Curr. Pharm. Des, 16 (2010) 3519–3537; [DOI] [PubMed] [Google Scholar]; (c) Mach RH, Zeng C, Hawkins WG, The sigma2 receptor: a novel protein for the imaging and treatment of cancer, J. Med. Chem, 56 (2013) 7137–7160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].(a) Aydar E, Onganer P, Perrett R, Djamgoz MB, Palmer CP, The expression and functional characterization of sigma (sigma) 1 receptors in breast cancer cell lines, Cancer Lett., 242 (2006)245–257; [DOI] [PubMed] [Google Scholar]; (b) Huang YS, Lu HL, Zhang LJ, Wu Z, Sigma-2 receptor ligands and their perspectives in cancer diagnosis and therapy, Med. Res. Rev, 34 (2014) 532–566. [DOI] [PubMed] [Google Scholar]; (c) Wheeler KT, Wang LM, Wallen CA, Childers SR, Cline JM, Keng PC, Mach RH, Sigma-2 receptors as a biomarker of proliferation in solid tumours, Br. J. Cancer, 82 (2000) 1223–1232; [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Pati ML, Hornick JR, Niso M, Berardi F, Spitzer D, Abate C, Hawkins W, Sigma-2 receptor agonist derivatives of 1-Cyclohexyl-4-[3-(5-methoxy-1,2,3,4-tetrahydronaphthalen-1-yl)propyl]piperazine (PB28) induce cell death via mitochondrial superoxide production and caspase activation in pancreatic cancer, BMC Cancer, 17 (2017) 51; [DOI] [PMC free article] [PubMed] [Google Scholar]; (e) Aydar E, Palmer CP, Djamgoz MB, Sigma receptors and cancer: possible involvement of ion channels, Cancer Res., 64 (2004) 5029–5035. [DOI] [PubMed] [Google Scholar]; (f) Makvandi M, Tilahun ED, Lieberman BP, Anderson RC, Zeng C, Xu K, Hou C, McDonald ES, Pryma DA, et al. The sigma-2 receptor as a therapeutic target for drug delivery in triple negative breast cancer. Biochem Biophys Res Commun, 467, (2015) 1070–1075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Mach RH, Smith CR, al-Nabulsi I, Whirrett BR, Childers SR, Wheeler KT, Sigma 2 receptors as potential biomarkers of proliferation in breast cancer, Cancer Res., 57 (1997) 156–161. [PubMed] [Google Scholar]
- [9].Zeng C, Vangveravong S, Jones LA, Hyrc K, Chang KC, Xu J, Rothfuss JM, Goldberg MP, Hotchkiss RS, Mach RH, Characterization and evaluation of two novel fluorescent sigma-2 receptor ligands as proliferation probes, Mol. Imaging, 10 (2011) 420–433. [PMC free article] [PubMed] [Google Scholar]
- [10].Perou CM, Sorlie T, Eisen MB, van de Rijn M, Jeffrey SS, Rees CA, Pollack JR, Ross DT, Johnsen H, Akslen LA, Fluge O, Pergamenschikov A, Williams C, Zhu SX, Lonning PE, Borresen-Dale AL, Brown PO, Botstein D, Molecular portraits of human breast tumours, Nature, 406 (2000) 747–752. [DOI] [PubMed] [Google Scholar]
- [11].Bauer KR, Brown M, Cress RD, Parise CA, Caggiano V, Descriptive analysis of estrogen receptor (ER)-negative, progesterone receptor (PR)-negative, and HER2-negative invasive breast cancer, the so-called triple-negative phenotype: a population-based study from the California cancer Registry, Cancer, 109 (2007) 1721–1728. [DOI] [PubMed] [Google Scholar]
- [12].http://breastcancer.about.com/od/types/tp/tnbc_risk_factors.htm, Accessed December 17, 2018
- [13].Al-Ghanim L, SYA 013 analogs as moderately selective sigma-2 (σ2) ligands: structure-affinity relationship studies, Bioorg. Med. Chem., Bioorg Med Chem. 2019. January 31 pii: S0968-0896(18)32033-9. doi: 10.1016/j.bmc.2019.01.035. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Peprah K, Zhu XY, Eyunni SV, Etukala JR, Setola V, Roth BL, Ablordeppey SY, Structure-activity relationship studies of SYA 013, a homopiperazine analog of haloperidol, Bioorg. Med. Chem, 20 (2012) 1671–1678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Ofori E, Zhu XY, Etukala JR, Bricker BA, Ablordeppey SY, Synthesis and evaluation of the structural elements in alkylated tetrahydroisoquinolines for binding to CNS receptors, Bioorg. Med. Chem, 24 (2016) 5730–5740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].(a) Ofori E, Zhu XY, Etukala JR, Peprah K Jordan KR, Adkins AA, Bricker BA, Kang HJ, Huang XP, Roth BL, Ablordeppey SY, Design and synthesis of dual 5-HT1A and 5-HT7 receptor ligands, Bioorg. Med. Chem, 24 (2016) 3464–3471. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Sampson D, Zhu XY, Eyunni SV, Etukala JR, Ofori E, Bricker B, Lamango NS, Setola V, Roth BL, Ablordeppey SY, Identification of a new selective dopamine D4 receptor ligand, Bioorg. Med. Chem, 22 (2014) 3105–3114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].(a) Fedotova A, Kondrashov E, Legros J, Maddaluno J, Yu. Rulev A, Solvent effects in the aza-Michael addition of anilines. C. R. Chimie 21 (2018) 639e643. [Google Scholar]; Wu Y-J, Michael addition of 3-bromoinden-1-one: an expedient synthesis of 5-bromo-3-trifluoroacetamido-indan-1-one Tetrahedron Letters, 47(48) (2006) 8459–8461 [Google Scholar]
- [18].Soga S, Neckers LM, Schulte TW, Shiotsu Y, Akasaka K, Narumi H, Agatsuma T, Ikuina Y, Murakata C, Tamaoki T, Akinaga S, KF25706, a novel oxime derivative of radicicol, exhibits in vivo antitumor activity via selective depletion of Hsp90 binding signaling molecules, Cancer Res., 59 (1999) 2931–2938. [PubMed] [Google Scholar]
- [19].Choi SJ, Lee JE, Jeong SY, Im I, Lee SD, Lee EJ, Lee SK, Kwon SM, Ahn SG, Yoon JH, Han SY, Kim J.l., Kim YC, 5,5’-substituted indirubin-3’-oxime derivatives as potent cyclin-dependent kinase inhibitors with anticancer activity, J. Med. Chem, 53 (2010) 3696–3706. [DOI] [PubMed] [Google Scholar]
- [20].(a) Hurvitz S, Mead M, Triple-negative breast cancer: advancements in characterization and treatment approach, Curr. Opin. Obstet. Gynecol, 28 (2016) 59–69; [DOI] [PubMed] [Google Scholar]; (b) de Ruijter TC, Veeck J, de Hoon JP, van Engeland M, Tjan-Heijnen VC, Characteristics of triplenegative breast cancer, J. Cancer Res. Clin. Oncol., 137 (2011) 183–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].(a) Giard DJ, Aaronson SA, Todaro GJ, Arnstein P, Kersey JH, Dosik H, Parks WP, In vitro cultivation of human tumors: establishment of cell lines derived from a series of solid tumors, J. Natl. Cancer Inst., 51 (1973) 1417–1423; [DOI] [PubMed] [Google Scholar]; (b) John CS, Bowen WD, Varma VM, McAfee JG, Moody TW, Sigma receptors are expressed in human non-small cell lung carcinoma, Life Sci., 56 (1995) 2385–2392; [DOI] [PubMed] [Google Scholar]; (c) Moody TW, Leyton J, John C, Sigma ligands inhibit the growth of small cell lung cancer cells, Life Sci., 66 (2000) 1979–1986. [DOI] [PubMed] [Google Scholar]
- [22].Tai S, Sun Y, Squires JM, Zhang H, Oh WK, Liang CZ, Huang J, PC3 is a cell line characteristic of prostatic small cell carcinoma, Prostate, 71 (2011) 1668–1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].(a) Gradiz R, Silva HC, Carvalho L, Botelho MF, Mota-Pinto A, MIA PaCa-2 and PANC-1 - pancreas ductal adenocarcinoma cell lines with neuroendocrine differentiation and somatostatin receptors, Sci. Rep., 6 (2016) 21648; [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Kashiwagi H, McDunn JE, Simon PO Jr., Goedegebuure PS, Xu J, Jones L, Chang K, Johnston F, Trinkaus K, Hotchkiss RS, Mach RH, Hawkins WG, Selective sigma-2 ligands preferentially bind to pancreatic adenocarcinomas: applications in diagnostic imaging and therapy, Mol. Cancer, 6 (2007) 48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Tesei A, Cortesi M, Zamagni A, Arienti C, Pignatta S, Zanoni M, Paolillo M, Curti D, Rui M, Rossi D, Collina S, Sigma Receptors as Endoplasmic Reticulum Stress “Gatekeepers” and their Modulators as Emerging New Weapons in the Fight Against Cancer Frontiers in Pharmacology 9:711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Frankowski KJ, Setola V, Evans JM, Neuenswander B, Roth BL, Aube J, Synthesis and receptor profiling of Stemona alkaloid analogues reveal a potent class of sigma ligands, Proc. Natl. Acad. Sci. U. S. A, 108 (2011)6727–6732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Berardi F, Abate C, Ferorelli S, et al. , Exploring the Importance of Piperazine N-Atoms for σ2 Receptor Affinity and Activity in a Series of Analogs of 1-Cyclohexyl-4-[3-(5-methoxy-1,2,3,4-tetrahydro-naphthalen-1-yl)propyl]piperazine(PB28), J Med Chem, 52 (2009) 7817–7828. [DOI] [PubMed] [Google Scholar]


