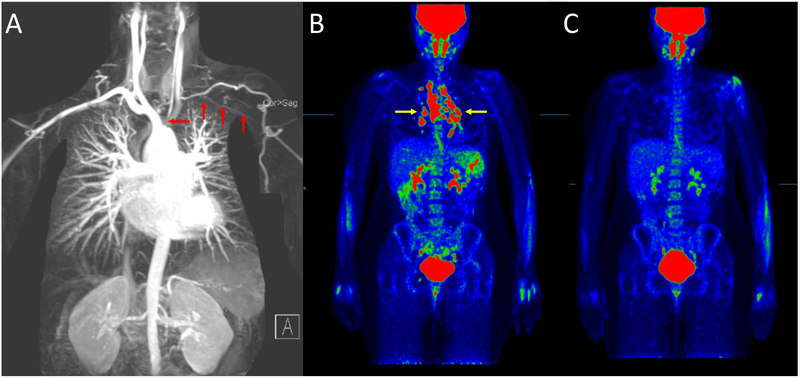
A 32-year-old woman with a history of Takayasu’s arteritis (TAK) was enrolled into an ongoing vascular imaging research study. She was diagnosed with TAK four years earlier based on vascular headache, arm claudication, and severe aortic arch disease. She had no complaints at the time of enrollment. Magnetic resonance angiography demonstrated stenosis of the left common carotid and left subclavian arteries (red arrows) that was unchanged since diagnosis (A). She was not taking medications but prior therapies included glucocorticoids and methotrexate. Physical examination revealed a decreased left carotid pulse with an associated bruit, reduced blood pressure in the left arm, and a barely palpable left radial pulse. 18Fluorodeoxyglucose (FDG)–positron emission tomography/computed tomography (PET/CT) was performed per research protocol and incidentally revealed intense FDG uptake throughout several mildly enlarged mediastinal and bilateral hilar lymph nodes (yellow arrows pointing to red nodular lesions) in a pattern strongly suggestive of sarcoidosis (B). There was no evidence of FDG activity along the walls of the large vessels to suggest active vasculitis. Transbronchial biopsy of mediastinal lymph nodes confirmed the presence of noncaseating granuloma consistent with stage 1 pulmonary sarcoidosis. The patient, who felt well, received no treatment for sarcoidosis, and a repeat FDG-PET/CT scan a year later showed complete spontaneous resolution of the intense metabolically active lymphadenopathy (C). TAK and sarcoidosis are granulomatosis diseases of unknown etiology. FDG-PET has been used for diagnostic purposes in both diseases. Patients with sarcoidosis can have concurrent large-vessel vasculitis (1). Genetic association studies have implicated IL12B in both TAK and sarcoidosis (2,3), and similar therapies can be efficacious for either condition. Without advanced molecular imaging, sarcoidosis may not have been identified in this case given the indolent course and spontaneous regression sometimes observed in stage 1 pulmonary sarcoidosis. This report raises the possibility of an under-recognized association between two diseases with overlapping pathogenic mechanisms, warranting further systematic review.
Figures: 1.
Clinical Images: Sarcoidosis concomitant with Takayasu arteritis, identified by advanced molecular imaging
Acknowledgments
Financial supports of conflicts disclosure:
This research was supported through the Intramural Research Program at the National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS). The authors declare no conflicts of interest relevant to this work.
References
- (1).Chapelon-Abric C, Saadoun D, Marie I, Comarmond C, Desbois AC, Domont F, et al. Sarcoidosis with Takayasu arteritis: a model of overlapping granulomatosis. A report of seven cases and literature review. Int J Rheum Dis 2018;21(3):740–745. [DOI] [PubMed] [Google Scholar]
- (2).Saruhan-Direskeneli G, Hughes T, Aksu K, Keser G, Coit P, Aydin SZ, et al. Identification of multiple genetic susceptibility loci in Takayasu arteritis. Am J Hum Genet 2013;93(2):298–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (3).Fischer A, Ellinghaus D, Nutsua M, Hofmann S, Montgomery CG, Iannuzzi MC, et al. Identification of Immune-Relevant Factors Conferring Sarcoidosis Genetic Risk. Am J Respir Crit Care Med 2015;192(6):727–36. [DOI] [PMC free article] [PubMed] [Google Scholar]