Abstract
Granulocytes are the major type of phagocytes constituting the front line of innate immune defense against bacterial infection. In adults, granulocytes are derived from hematopoietic stem cells in the bone marrow. Alcohol is the most frequently abused substance in human society. Excessive alcohol consumption injures hematopoietic tissue, impairing bone marrow production of granulocytes through disrupting homeostasis of granulopoiesis and the granulopoietic response. Because of the compromised immune defense function, alcohol abusers are susceptible to infectious diseases, particularly septic infection. Alcoholic patients with septic infection and granulocytopenia have an exceedingly high mortality rate. Treatment of serious infection in alcoholic patients with bone marrow inhibition continues to be a major challenge. Excessive alcohol consumption also causes diseases in other organ systems, particularly severe alcoholic hepatitis which is life threatening. Corticosteroids are the only therapeutic option for improving short-term survival in patients with severe alcoholic hepatitis. The existence of advanced alcoholic liver diseases and administration of corticosteroids make it more difficult to treat serious infection in alcoholic patients with the disorder of granulopoieis. This article reviews the recent development in understanding alcohol-induced disruption of marrow granulopoiesis and the granulopoietic response with the focus on progress in delineating cell signaling mechanisms underlying the alcohol-induced injury to hematopoietic tissue. Efforts in exploring effective therapy to improve patient care in this field will also be discussed.
Keywords: Alcohol abuse, bone marrow, granulopoiesis, the granulopoietic response, immune defense, stem cells, progenitor cells, cell signaling, leukopenia, bacterial infection
1. Introduction
Granulocytes are a population of leukocytes characterized by the presence of granules in their cytoplasm. This heterogeneous cell population includes neutrophils, eosinophils, and basophils. Representing the front line of innate immune defense, neutrophils are the most abundant type of granulocytes in the body of humans. Since eosinophils and basophils comprise only a very small portion of the population, the term of “granulocytes” is also used to loosely imply neutrophilic granulocytes (or polymorphonuclear leukocytes, PMNs) (Babior & Golde, 2001). Granulocytes are derived from hematopoietic stem cells (HSCs). In adults, HSCs are rare event cells residing in the bone marrow, which possess the unique capacity of self-renewal and differentiation into all types of blood cells (Kondo, et al., 2003; Shizuru, Negrin, & Weissman, 2005). Under the homeostatic condition, the process of HSC self-renewal, differentiation, and commitment to granulopoiesis is tightly controlled in order to maintain the optimal pool of HSCs and the balanced production of granulocytes as well as other blood cell types (Akala & Clarke, 2006; Kondo, et al., 2003). In humans, HSCs display the lineage (lin)−CD34+ surface marker (Bernstein, at al., 1994; Engelhardt, et al., 2002; Hogan, et al. 2002; Murray, et al., 1994; 1995; 1996; Chang, et al., 2000; Tian & Zhang, 2016). A subpopulation of dormant human HSCs are lin−CD34− (Dao and Nolta, 2000), which appear to be long-term repopulating HSCs (LTR-HSCs) (Sonoda, 2008). These lin−CD34− HSCs can give rise to short-term repopulating HSCs (STR-HSCs) which likely carry the surface marker of lin−CD34+. In mice, the bone marrow lin−stem cell growth factor receptor (c-kit)+stem cell antigen-1 (Sca-1)+ (LKS) cell population is enriched with hematopoietic stem cells (Okada, et al., 1992; Osawa, et al., 1996b). Mouse LTR-HSCs are also CD34 low/negative (Osawa, et al., 1996a). The CD34 positive LKS cell population consists of relatively downstream STR-HSCs and multipotent progenitor cells (MPPs). More committed progenitors such as common myeloid progenitors (CMPs) derived from MPPs bear lin−c-kit+Sca-1− surface marker (Okada, et al., 1992; Osawa, et al., 1996b). CMPs sequentially differentiate into myelomonocytic progenitor cells (GMP or granulocyte/macrophage colony forming unit, CFU-GM) and granulocytic progenitor cells (CFU-G). CFU-G cells then stepwisely differentiate into myeloblasts, promyelocytes, myelocytes, metamyelocytes, and eventually granulocytes. Mitotic capability is lost after the stage of myelocytes. Therefore, cell development along the granulocytic lineage can be roughly divided into two compartments, i.e. the mitotic and post-mitotic compartments. Terminally differentiated mature granulocytes are commonly retained in the storage pool of bone marrow before being released into the systemic circulation. The marrow storage pool houses approximately 96–99% of total body neutrophilic granulocytes in humans and in mice (Furze & Rankin, 2008; Strydom & Rankin, 2013; Summers, et al., 2010). On average, the transit time for granulocytes to pass through the post-mitotic compartment/storage pool is around 5 days in humans (Summers, et al., 2010).
Upon bacterial infection or host confrontation with an inflammatory challenge, mobilization of granulocytes from the bone marrow is enhanced. The level of circulating granulocytes can increase over 10 fold in hours with the concomitant reduction of granulocyte storage in the bone marrow (Furze & Rankin, 2008; Strydom & Rankin, 2013). In the meantime, marrow quickly initiates the granulopoietic response to enhance production of granulocytes at the expense of other blood cell lineage development (Barthlen, et al., 1999; Ueda, et al., 2005). During the granulopoietic response, hematopoietic stem/progenitor cells (HSPCs) are activated for proliferation. Reprograming of these hematopoietic precursors at the transcriptional and post-transcriptional levels takes place to promote their commitment toward granulocytic lineage development (Melvan, et al., 2011; Shi, et al., 2013; 2017a; 2018; Zhang, et al., 2008). With these enhanced activities, the transit time for granulocytic lineage cells to pass through both the mitotic and post-mitotic compartments is markedly shortened (Terashima, er al., 1996). The substantial capacity of bone marrow for producing large amount of granulocytes through the granulopoietic response is critically important for reinforcing host immune defense against invading pathogens.
Alcohol is the most frequently abused substance in human society (Merikangas & McClair, 2012). Excessive alcohol consumption injures the bone marrow and impairs homeostasis of granulopoiesis as well as the granulopoietic response (Melvan, et al., 2011; 2012; Raasch, et al., 2010; Shi, et al., 2017a; Zhang, et al., 2009). Alcohol exposure also impairs functional activities of granulocytes (Szabo & Mandrekar, 2009; Zhang, et al., 2002). The resulted defects in immune defense significantly increase the host susceptibility to serious infections, particularly pneumonia and septicemia (Cook, 1998; de Wit, et al., 2010; MacGregor & Louria, 1997; Mehta, 2016; O’Brien, et al., 2007; Zhang, et al., 2002; 2008). Clinical studies on large cohorts of patients treated in the ICU and emergency medical settings have shown that 12–26% of total cases are alcohol dependent or have a high blood alcohol level at admission (O’Brien et al., 2007; Plurad, et al., 2010). Alcoholic patients have significantly higher rates of septic complications including septic shock. One third to half of hospitalized patients with pulmonary infections are alcoholics (Dorff, et al, 1973; Goss, et al., 2003; Winterbauer, et al., 1969). Lung infections in alcohol abusers are frequently complicated with septicemia (Fernández-Solá, et al., 1995; Hammond, et al., 1993; Musher, et al., 2000; Ruiz, et al., 1999; Saitz, et al., 1997). A striking feature of alcoholic patients with septic infection is that they often present with granulocytopenia (Jong, et al., 1995; MacGregor & Louria, 1997;). Mortality rates in alcoholic patients with septic infection and granulocytopenia continue to be exceedingly high at 83–100% (Jong, et al., 1995; Perlino, et al., 1985). Excessive alcohol consumption causes severe injury in the liver. Patients with alcoholic liver diseases are also frequently complicated with serious infections, which are the main cause of death in these individuals (Bruns, et al., 2014; Karakike, et al., 2017). This review will focus on discussing the recent development in studies on alcohol-induced injury to marrow granulopoiesis and the granulopoietic response. The progress in delineating the underlying cell signaling mechanisms will be highlighted. Efforts in exploring effective therapy to improve patient care in this field will also be addressed.
2. Alcohol injures hematopoietic stem/progenitor cells
2.1. Cell toxicity of alcohol
Excessive alcohol consumption causes damage to hematopoietic tissue (Ballard, 1997; Heermans, 1998; Michot & Gut, 1987). Morphological examinations have revealed that bone marrow samples from alcohol abusers exhibit a significant reduction of mature granulocytes with vacuolization in myeloid progenitor cells (Ballard, 1980; Yeung, et al., 1988). This alcohol-induced vacuolization in hematopoietic precursor cells is not restricted in the granulocytic lineage, the erythroid and megakaryocytic lineages are also involved (Latvala, et al., 2004; Roselle, et al., 1986; Yeung, et al., 1988). Ultrastructural examination discloses that the vacuoles are present in the cytoplasm and free of organized structure (Yeung, et al., 1988). In vitro culture of marrow cells from normal individuals in nutrient medium containing alcohol can induce cytoplasmic vacuolization (Yeung, et al., 1988). The critical alcohol concentration for inducing vacuolization is 62.5 mg/dl. The proportion of cells developing vacuoles appears correlating with the concentration of alcohol. In the clinic, vacuolization in peripheral blood leukocytes including granulocytes and lymphocytes has also been observed in patients with acute alcohol intoxication (Davidson & McPhie, 1980). In addition to causing vacuolization in hematopoietic precursor cells, alcohol exposure also leads to formation of vacuolar inclusions in a variety of other cell types, including neurons (Goldstein, et al., 1983), inner ear hair cells (Nordemar, 1988), ovary granulosa and theca cells (Laura, et al., 2003), myocardial cells (Rajbanshi & Pandanaboina, 2014), pancreas acinar cells (Werner, et al., 2002), as well as uterine tube epithelial cells (Martinez, et al., 1999).
Alcohol-induced formation of vacuoles in hematopoietic precursor cells is a sign of cell stress. At the present time, however, knowledge about the effects of alcohol-induced vacuolization on functional activities of hematopoietic cells remains limited. Cytoplasmic vacuolization is a morphological change frequently occurring in cells following exposure to various natural and artificial low-molecular-weight compounds as well as infection with bacterial or viral pathogens (Aki, et al., 2012; Shubin, et al., 2016). Vacuolization may primarily reflect an adaptive response for cell survival (Henics & Wheatley, 1999), which subsequently has the potential to lead to distinctive forms of cell death subsequently (Aki, et al., 2012; Henics & Wheatley, 1999; Shubin, et al., 2016). Recent studies have revealed that a variety of inducers can cause cell vacuolization leading to specific types of cell death through different pathways (Aki, et al., 2012; Shubin, et al., 2016). Exposure to weakly basic amine-containing lipophilic compounds can induce cell vacuolization (Marceau, et al., 2012; Shubin, et al., 2016). These lipophilic bases are uncharged in neutral extracellular fluid, allowing them to enter into cells via simple diffusion and/or active transportation. After entering acidic endosomal-lysosomal organelles and Golgi apparatus in the cell, they become positively charged through protonation rendering them unable to diffuse out through the organelle membrane. The trapped weak bases with positive charge increase the osmotic pressure, which drives diffusion of water into the organelles to form vacuoles. Ethanol is a slightly charged water-soluble polar molecule diffusible to cytoplasmic membrane. At the present time, nevertheless, there is no evidence to suggest if any alteration of osmotic pressure in the organelles occurs due to physicochemical interactions of ethanol during the process of cell vacuolization. Disruption of various metabolic pathways can induce formation of vacuoles in different cellular compartments irrelevant to their acidic/basic environments. Vacuolization of endoplasmic reticulum (ER) and swelling of mitochondria are associated with paraptosis-like cell death (PLCD) (Shubin, et al., 2016). Impairment of either endoplasmic reticulum-associated protein degradation (ERAD) or ER-localized big conductance calcium-activated potassium channels (BKCa) mediates PLCD. Oxidative stress, impairment of protein folding in the ER, and disruption of ubiquitin-proteasome system cause ER stress and vacuolization. Excessive production of reactive oxygen species (ROS) interrupts the function of BKCa system leading to mitochondrial swelling. Alcohol has been shown to evoke oxidative stress (Das & Vasudevan, 2007), disrupt protein folding in the ER (Ji, 2015), and inhibit ubiquitin-proteasome activity (Donohue & Thomes, 2014) in cells. These negative effects of alcohol on cell functional processes may potentially contribute to the formation of vacuoles. Methuosis is a type of cell death associated with vacuolization of macropinosomes (Maltese & Overmeyer, 2014), during which, failure of macropinosomes to fuse with other organelles of the endocytic pathway leads to macropinosome accumulation in the cytoplasm, fusing with each other to form vacuoles. Ultrastructural examinations of bone marrow samples from subjects with alcohol intoxication have shown that surface invagination of the cell membrane in erythroblasts leads to endocytosis and consequently vacuole formation (Yeung, et al., 1988). It remains to be defined if this type of alcohol-induced vacuole formation shares a similar mechanism as seen in abnormal macropinocytosis during methuosis.
In addition to inducing vacuolization-associated injury, alcohol causes cell death through the apoptosis pathway. Studies have shown that alcohol exposure promotes apoptosis in stem/progenitor cells of both the embryonic and adult tissue origins, including human and murine embryonic stem cells (Arzumnayan, et al., 2009; Nash, et al., 2012), human placental trophoblast cells (Bolnick, et al., 2014), human neural stem cells (Hao, et al., 2003), and human bone marrow-derived mesenchymal stem cells (MSCs) (Chen, et al., 2013). HSCs appear to be more resistant to alcohol than neural stem cells in the induction of apoptosis. In vitro incubation of human neural stem cells with 5 mM of ethanol causes apoptosis, whereas HSCs are unaffected by even 20 mM ethanol (Hao, et al., 2003). However, blood alcohol concentration can reach much higher levels than 20 mM in alcohol abusers with acute or binge intoxication. Further studies are warranted to define if alcohol at high levels frequently seen in the blood of heavy drinkers would induce apoptosis in HSCs. In addition, it seems also worthwhile to further verify if long-term exposure to low levels of ethanol would exert any negative effects on HSC survival as well as other functional activities.
2.2. Toxic products from alcohol metabolism
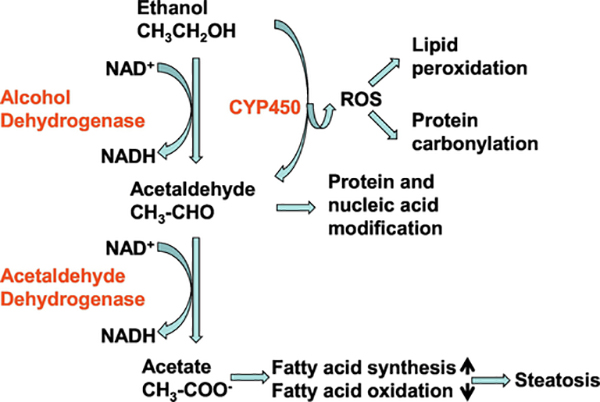
Except for the direct toxicity of alcohol to hematopoietic precursor cells (Guthrie & Beckman, 1983; Liu, 1980), cellular metabolites of ethanol can exert negative effects on HSPCs. Nucleated bone marrow cells metabolize ethanol (Bond & Wickramasinghe, 1983; Wickramasinghe, et al., 1981; Xu, et al., 1989). Acetaldehyde, the immediate metabolite from ethanol through both the alcohol dehydrogenase (ADH) and cytochrome P450 pathways (Smith, 2015; Lu & Cederbaum, 2008), is cytotoxic. It can bind and form covalent adducts with macromolecules, including proteins and nucleic acids, either on cell surface membrane or in intracellular constituents (Latvala, et al., 2001; Niemelä, 2001; Xu, et al., 1989; Yu, et al., 2010). Alcohol exposure causes oxidative stress in cells. ROS generated during metabolism of ethanol cause damage to cell membrane lipids as well as cellular proteins and nucleic acids (Koch, et al., 2004; Niemelä, 2001; Wu, et al., 2003; 2006). Molecular modifications of lipids, proteins, and nucleic acids mediated by metabolites of alcohol can subsequently lead to disruption and/or impairment of HSPC structural integrity, signaling regulation, metabolism, survival, proliferation, as well as differentiation (Garaycoechea, et al., 2018; Latvala, et al., 2001; Van Wassenhove, et al., 2016). In addition, DNA damage caused by cellular accumulation of acetaldehyde is carcinogenic, which may play a role in inducing the malignant transformation of HSPCs (Smith, et al., 2015; Yu, et al., 2010). Figure 1 illustrates generation of harmful metabolites from ethanol metabolism.
Figure 1.
Generation of harmful metabolites from ethanol metabolism. EYP450: cytochrome P450
The capacity of tolerance to alcohol-induced disruption in cell functional activities may vary among HSPCs at different stages of differentiation. HSCs and the upstream multipotent progenitors are relatively more resistant to the negative effects exerted by ethanol and acetaldehyde in comparison to committed myeloid and the downstream progenitor cells (Smith, et al., 2015). Aldehyde dehydrogenase (ALDH) catalyzes the conversion of acetaldehyde to acetate, which is a key step in alcohol metabolism. ALDH activity is higher in HSCs than in the relatively downstream progenitors and most differentiated types of hematopoietic cells (Storms, et al., 1999). This uniqueness in high ALDH activity seems beneficial for detoxification of reactive aldehydes in order to maintain the functional integrity of primitive hematopoietic precursor cells. The physiologic role of ALDH activity in modulation of HSC function has been observed across species ranging from zebrafish to humans (Chute, et al. 2006; Garaycoechea, et al., 2012; Gasparetto, et al., 2012; Ma, et al., 2010; Muramoto, et al., 2010). The ALDH family contains 19 isoenzyme members with different substrate specificities, subcellular localizations, and patterns of expression (Jackson, et al., 2011). In mice with deficiency in both ALDH1A1 and ALDH3A1, a wide range of hematopoietic abnormalities have been observed, including reduction in HSC number along with changes in cell cycling, intracellular signaling, as well as gene expression (Gasparetto, et al., 2012). In this respect, however, inconsistent observations also exist. Studies on competitive repopulation have reported that inhibition of ALDH in 34−LKS cells with diethylaminobenzaldehyde (DEAB) causes an increase in repopulating activity of these LTR-HSCs in lethally irradiated mice (Muramoto, et al., 2010). Further investigation on ALDH isoforms involved in the process will provide critical information for understanding the current controversy. Mice with combined inactivation of aldehyde catabolism (ALDH2 knockout) and the Fanconi anemia DNA-repair pathway (Fancd2 knockout) display a predisposition to leukemia, and are susceptible to the toxic effects of ethanol (Langevin, et al., 2011). In humans, deficiency in ALDH2 expression and function caused by a single nucleotide substitution (ALDH2*2) is associated with marrow failure (Van Wassenhove, et al., 2016). People with polymorphisms in genes responsible for metabolism of alcohol derived reactive aldehydes and repair of their DNA adducts in HSCs and other hematopoietic cells have been postulated to be specifically vulnerable to ethanol-induced disorders of hematopoiesis (Smith, et al., 2015).
Enzymes involved in the metabolism of alcohol have certain overlap with those catalyzing the synthesis of retinoic acid (RA), a small lipophilic molecule playing an important role in the regulation of HSPC activities. RA regulates cell function through its entering the nucleus to activate nuclear RA receptors (RAR). Binding of RAR/retinoid X receptor heterodimer to the RA response element triggers transcriptional activation of retinoid-responsive genes (Vassalli, 2019). RA is derived from retinol (vitamin A). The initial step of reversible retinol oxidation to retinaldehyde is catalyzed by either alcohol dehydrogenases (ADH1, ADH3, and ADH4) or retinol dehydrogenases (Kumar, et al., 2012). Retinaldehyde is then irreversibly metabolized to RA by cytosolic ALDH isozymes, such as ALDH1A1, ALDH1A2, and ALDH1A3, in a tightly regulated manner (Duester et al., 2003). HSCs from human and murine origins express high levels of ALDH1A1 (Smith, et al., 2015; Vassalli, 2019). Studies have shown that inhibition of ALDH1A1 delays RA-mediated differentiation of murine HSCs (Muramoto, et al., 2010). RA plays a central role in initiation of RARα-cyclin-dependent kinase-activating kinase signaling to mediate granulocytic differentiation (Luo, et al., 2007). Inhibition of ALDH activity impedes granulocytic differentiation of hematopoietic precursor cells in the in vitro culture system. In alcohol metabolism, cytosolic ADH, such as ADH1 and ADH4, catalyzes the conversion of ethanol to acetaldehyde that is subsequently converted to acetate by mitochondrial ALDH2 and cytosolic ALDH (Cheung, et al., 2003; Holmes, 1994; Sladek, et al., 1989). Under the normal condition, enzyme forms most efficient for retinol metabolism are different from those most efficient for ethanol metabolism (Duester, 1998). In the ADH family, for example, ADH1 is the most efficient one for catalyzing ethanol oxidation, while ADH4 serves as the best candidate for a retinol dehydrogenase. Among ALDH family members, similarly, oxidation of acetaldehyde is performed most efficiently by ALDH2, whereas ALDH1 functions efficiently as a retinal dehydrogenase. Nevertheless, the overlap of enzymatic activities involved in both ethanol metabolism and RA synthesis implies the possibility that the intoxicated level of ethanol may competitively inhibit the activity of RA synthetic pathway (Duester, 1998). Indeed, in vitro experiments have shown that ethanol competitively inhibits ADH-catalyzed retinol oxidation (Julià, et al., 1986; Mezey & Holt, 1971). Exposure to high level (100 mM) of ethanol leads to a significant decrease in RA detection in 7.5-day-old embryos (Deltour, et al., 1996). Therefore, further delineating the potential interplay between alcohol metabolism and RA signaling in the circumstance of cell exposure to the intoxicated level of ethanol will be helpful for improving knowledge about molecular mechanisms underlying alcohol-induced disruption of HSPC commitment to homeostatic granulopoiesis and the granulopoietic response.
Alcohol disrupts homeostasis of granulopoiesis
Leukopenia is common in heavy alcohol drinkers, which can occur along with other hematological abnormalities including lymphopenia, anemia, and thrombocytopenia (Latvala, et al., 2004; Liu, 1980; Panasiuk & Kemona, 2001). In patients referred for bone marrow examinations due to occult abnormalities in peripheral blood cells, forty percent of them excessively consume alcohol (Latvala, et al., 2004). Further observations have unveiled that the effects of alcohol consumption on leukocyte level in the peripheral circulation may vary depending on the quantity, duration, and pattern of alcohol consumption. Under certain circumstances, alcohol abusers may even exhibit a transient increase in granulocyte counts in the circulation. In a report of 45 healthy volunteers and 300 chronic drinkers with or without recent excessive drinking, alcoholics with recent excessive drinking have higher levels of circulating neutrophils as compared to healthy controls or alcoholics without recent drinking (Li, et al., 2017). In another prospective study on 88 patients with alcoholic liver cirrhosis, model for end stage liver disease (MELD) scoring is used to divide them into five different groups, in which group 1 to 5 constitute patients with MELD scores of 1–9, 10–19, 20–29, 30–39 and >40, respectively. (Jain, et al., 2016). All patients in group 1 have normal leukocyte count. Leukocytosis predominates in MELD group 2 and 3 patients. In group 4, leukopenia is more prevalent. All patients in group 5 have leukopenia. Concomitantly, a progressive fall in hemoglobin level and an increase in the instance of thrombocytopenia have been observed in patients with the increase in their MELD scores. In experimental studies, a reduction of leukocyte counts in the blood along with decreased levels of hematocrit and hemoglobin has been detected in dogs fed on diet containing ethanol (Beard, et al., 1963) In an study on rats with oral administration of alcohol at a high dose (2 mL per animal per day) for 10 to 22 weeks, it has been observed that despite overall leukopenia following alcohol ingestion, the absolute neutrophil count in the blood is increased along with a significant reduction of absolute lymphocyte count in alcohol-treated animals (Kanwar & Tikoo, 1992).
The level of granulocytes in the systemic circulation can be affected by multiple factors, including the granulopoietic activity of hematopoietic precursor cells, the storage capacity for mature granulocytes in the marrow storage pool, the mobilization of granulocytes from bone marrow into the systemic circulation, the removal of granulocytes from the circulation by macrophages in the liver, bone marrow stroma, and marginal zone of the spleen under the homeostatic circumstance, as well as the exit of granulocytes from the blood stream into tissue sites of inflammation during the inflammatory reaction. Alcohol may exert diverse effects on each of these processes.
3.1. Disturbance of homeostatic granulopoiesis
As mentioned previously, bone marrow samples from individuals with heavy alcohol consumption frequently exhibit hypocellularity with few mature granulocytes (Ballard, 1980; Liu, 1980; Nakao, et al., 1991). This morphological feature implies the existence of impaired granulopoietic activity of hematopoietic precursor cells. Alcohol and its metabolites are toxic to hematopoietic precursor cells, particularly myeloid progenitors. Bone marrow cells from alcohol abusers with osteonecrosis exhibit a significantly reduced activity of CFU-GM in comparison to those from normal volunteers and patients with bone-marrow grafting for a nonunion orthopedic problem (Hernigou & Beaujean, 1997). However, observations about the in vitro effect of alcohol on the granulocyte/macrophage colony forming activity in normal bone marrow cells appear inconsistent. In an investigation on human marrow cells, culturing cells in the soft agar medium containing alcohol has shown that ethanol at concentrations commonly seen in the blood of intoxicated patients (>200 mg/100 ml) sharply inhibits CFU-GM activity (Tisman & Herbert, 1973). While studies from other groups have reported that the CFU-GM activity in human marrow cells cultured in either the soft agar or methylcellulose medium system is resistant to the negative effect of alcohol even at the high concentration of ethanol (>600 mg/100 ml) (Imperia, et al., 1984; Meagher, et al., 1982). In animal experiments carried out by our group, we have used 5-bromo-2-deoxyuridine (BrdU) incorporation to determine the activity of proliferation in mouse bone marrow LKS cells. Neither acute alcohol intoxication (via intraperitoneal injection with 20% alcohol in saline at a dose of 5 g alcohol/kg body weight) alone nor chronic feeding on the Lieber-DeCarli low-fat liquid alcohol diet (LED supplies 36% of calories as ethanol) for 5 weeks plus binge alcohol intoxication causes significant change in marrow LKS proliferative activity under the homeostatic condition (Shi, et al., 2017a; Zhang, et al., 2009). In addition, in vitro exposure to ethanol at concentrations of 50 and 100 mM does not affect marrow c-kit+Sca-1+ proliferation in the culture system as reflected by their BrdU incorporation (Shi, et al., 2017a). LKS cells are rare premitive precursors containing enriched HSCs in mice (Okada, et al. 1992). Under the homeostatic condition, most of HSCs are in the dormant status (Kohli & Passegué, 2014). Only a small fraction of them enter into cell cycle for self-renewal and/or lineage commitment. In addition, the high cellular activity of ALDH may also help HSCs to resist the negative effect of alcohol on proliferation. Another possible reason for the inconsistency of observations from ex vivo cultures and animal experiments in relation to the morphological feature of bone marrow in alcoholic patients may be the duration of alcohol exposure. As described earlier, the typical hypocellularity of bone marrow biopsy and reduced CFU-GM activity are usually seen in individuals with chronically heavy alcohol consumption.
In adults, the hematopoietic niche environment in the bone marrow provides critical signals for regulating HSPC activities. The hematopoietic niche consists an interdependent network of osteolineage cells, endothelial cells, pericytes, CXC chemokine ligand 12 (CXCL12)-abundant reticular (CAR) cells, MSCs, sympathetic nerve fibers, nonmyelinating Schwann cells, and other hematopoietic cells (Calvi & Link, 2015; Wei & Frenette, 2018). Excessive alcohol consumption has been shown to cause structural damage and functional interruption of the marrow niche environment, which may serves as a potential mechanism underlying the disturbance of homeostatic granulopoiesis following alcohol exposure. In humans, long term heavy alcohol consumption causes decrease in bone mass and bone mineral density (Maurel, et al., 2012a). An experimental study on rats fed with a diet containing high content of alcohol (35% v/v) for 17 weeks has shown a significant decrease in bone mineral density and trabecular thickness (Maurel, et al., 2011). Human, animal, and cell culture studies have demonstrated that alcohol has a dose-dependent toxic effect on osteoblast activity. Alcohol exposure causes increase in osteocyte apoptosis and accumulation of lipid droplets within the osteocytes, leading to the development of osteopenia and decrease in bone formation (Chakkalakal, 2005; Maurel, et al., 2012b). Alcohol also enhances adipogenic activity in the bone marrow and in the cortical bone microvessels (Maurel, et al., 2012b; 2014; Wezeman & Gong, 2001). Marrow MSCs are multipotent and capable of differentiation into osteoblasts as well as adipocytes. Studies on MSCs have suggested that signaling pathways regulating adipogenic and osteogenic differentiations of MSCs may exist a potentially inverse relationship, such that cell signaling promotes adipogenesis at the expense of osteogenesis and vice versa (Yuan, et al., 2016). Peroxisome proliferator-activated receptor γ (PPARγ) is a master transcriptional regulator of adipocyte differentiation, which inhibits osteoblast differentiation. While bone morphogenetic protein (BMP) and Wnt signals promote osteogenic differentiation, which inhibit the function of PPARγ transactivation during MSC differentiation toward adipocytes. In vitro culture of human MSCs with alcohol at 50 mM promotes their differentiation toward adipocytes (Wezeman & Gong, 2004). Alcohol up-regulates PPARγ2 at the point of MSC lineage commitment and increases adipocyte P2 synthesis for cell lipid transport as well as storage. It has been reported that mice chronically fed on diet containing high dose of alcohol, bone marrow MSC osteogenic differentiation is reduced with the enhancement of adipogenic differentiation, leading to osteopenia in these animals (Liu, et al., 2016b). This biased osteo/adipogenic lineage differentiation of marrow MSCs is correlated to the activation of phosphatidylinositide 3-kinase/protein kinase B (AKT)/mammalian target of rapamycin (mTOR) signaling, resulting in downregulation of runt-related transcription factor 2 and upregulation of PPARγ via activation of p70 ribosomal protein S6 kinase. Transient transfection of human bone marrow MSCs with small interfering RNAs (siRNA) targeting PPARγ in the culture system containing 50 mM alcohol significantly represses the adipogenic effect of alcohol on MSCs as reflected by lower adipocyte number, increased matrix mineralization, reduced adipogenic gene markers, upregulated osteogenic gene marker, and enhanced bone matrix protein synthesis (Huang, et al., 2010). Simultaneous downregulation of PPARγ and upregulation of calcitonin gene-related peptide, a neuropeptide gene closely associated with bone regeneration, can also efficiently suppress adipogenic differentiation of marrow MSCs and promoting their osteogenic differentiation (Li, et al., 2014). In addition, in vivo blockage of the mTOR pathway by rapamycin treatment has been shown to ameliorate alcohol-induced osteopenia by rescuing impaired osteo/adipogenic lineage differentiation of marrow MSCs (Liu, et al., 2016b).
Alcohol exposure not only changes the cell corporation of hematopoietic niche, but alters the cue of marrow niche environment as well. Marrow niche cells express a variety of cell membrane bound molecules as well as soluble factors to regulate hematopoietic precursor cell activities by providing either stimulatory or inhibitory signals. These cell membrane bound and soluble mediators including ligands for receptors associated with Wnt and hedgehog signaling pathways, stem cell factor (SCF, c-kit ligand, or steel factor), transforming growth factor-β (TGF-β), granulocyte-colony stimulating factor (G-CSF), granulocyte-macrophage colony-stimulating factor (GM-CSF), as well as CXCL12 (stromal cell-derived factor 1 or SDF-1). The complexity of niche signaling regulation is denoted by the feature that a specific signaling molecule can be expressed by a wide range of niche cell types at different extent. Similarly, each niche cell type can express different signaling molecules either simultaneously or dynamically. Currently, knowledge about the integrated niche regulation of granulopoiesis under the homeostatic condition remains insufficient. Information regarding the effects of excessive alcohol consumption on the niche signaling regulation of granulopoiesis remains limited and superficial. It has been reported that cell-free conditioned media from the culture of normal human T cells exposed to ethanol at the concentration greater than 100 mg% exhibit a significant reduction in capacity to stimulate proliferation of CFU-GM in human or rat bone marrow cells (Imperia, et al., 1984). In a nonhuman primate model of ethanol self-administration for 12 months, it has been observed that ethanol exposure inhibits activation-induced production of G-CSF along with ethanol-dependent upregulation of distinct microRNAs (miR-181a and miR-221) in peripheral blood mononuclear cells (PBMCs) (Asquith, et al., 2014). Alcohol-induced up-regulation of these microRNAs appears being involved in mediating the reduction of G-CSF production by PBMCs through inhibiting the expression of transcription factors signal transducer and activator of transcription 3 (STAT3) as well as aryl hydrocarbon receptor nuclear translocator (ARNT). Further investigations on the effects of alcohol on the marrow niche signaling regulation of HSPC function will provide critical insight into mechanisms underlying the disruption of granulopoiesis under homeostatic conditions in hosts who excessively consume alcohol.
3.2. Impediment of marrow capacity for storage of granulocytes
Alcohol negatively affects the marrow capacity of storing mature granulocytes, which may serve as another mechanism underlying the development of hypocellularity with the loss of mature granulocytes in the bone marrow in alcohol abusers (Ballard, 1980; Liu, 1980). In this respect, the specific pattern of chronic plus binge alcohol consumption appears to cause the most striking disturbance. Alcoholics with recent excessive drinking have significantly higher levels of circulating neutrophils compared to healthy controls or alcoholics without recent drinking (Li, et al., 2017). Experimental studies on mice with chronic plus binge alcohol administration have also shown substantial increases in the number of circulating granulocytes 9–24 h after the binge intoxication (Li, et al., 2017; Shi, et al., 2017a). This chronic plus binge alcohol administration induced elevation of circulating granulocyte levels include increases in both percentage of neutrophils in white blood cells and absolute counts of neutrophils in the systemic circulation (Shi, et al. 2017b). Nevertheless, chronic feeding with alcohol diet for 5 weeks alone does not alter the granulocyte level in the blood. Binge alcohol intoxication alone only causes a moderately increase in the granulocyte level in the circulation. Analysis of the bone marrow has revealed that chronic plus binge alcohol administration substantially reduces the marrow storage pool of granulocytes without any significant change in hematopoietic stem/progenitor cell populations. These observations indicate that excessive alcohol consumption, particularly chronic plus binge drinking, reduces the marrow storage capacity for granulocytes by promoting release of them into the systemic circulation.
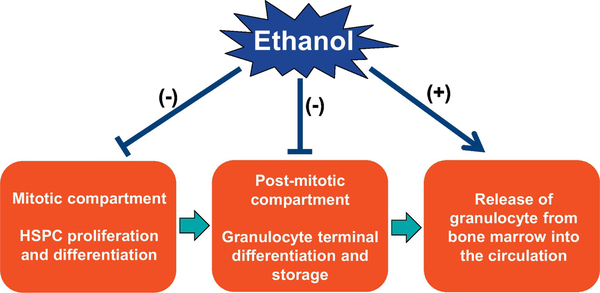
At the present time, knowledge about mechanisms by which excessive alcohol consumption reduces marrow storage pool of granulocytes and increases granulocyte release from the bone marrow into the circulation remains limited. Chronic plus binge alcohol exposure causes exacerbated tissue injury in the liver (and likely in other organ systems) with a strong inflammatory reaction (Bertola, et al., 2013; Li, et al., 2017; Maricic, et al., 2015; Xu, et al., 2015). The generated proinflammatory cytokines and chemokines apparently promote marrow release of granulocytes into the circulation as well as infiltration of granulocytes into tissue sites of inflammation. In addition to the effects on generating inflammatory mediators, alcohol exposure also exerts an impact on activities of granulocytes and other nucleated cell types either in the circulation or in the bone marrow. Studies have shown that feeding with liquid alcohol diet for 5 weeks alone is able to increase caspase-1 activation in blood neutrophils in mice (Shi, et al., 2017b). This increase in caspase-1 activation is further enhanced in mice with chronic-plus-binge alcohol administration. Both chronic alcohol diet feeding alone and chronic-plus-binge alcohol administration cause a significant increase in caspase-1 activation in neutrophils in the bone marrow. In addition, either chronic alcohol diet feeding alone or chronic-plus-binge alcohol administration causes a significant increase in caspase-1 activation in non-neutrophilic cell types in both the systemic circulation and the bone marrow. Alcohol-induced inflammatory reaction and activation of the caspase-1 inflammasome pathway in marrow cells likely participate in mediating the release of granulocytes from marrow storage pool into the circulation. In the bone marrow, immature myeloid cells bind to the stromal network through interactions between oligosaccharides and lectins as well as between adhesion molecules. During the maturation process, cell surface molecules are altered and the ability for multivalent binding to stroma components or cells reduces gradually. Mobilization of marrow cells involves the remodeling of marrow matrix and the basement membranes of the bone marrow sinuses by activities of marrow proteases, particularly matrix metalloproteinases (MMPs) (Pelus, et al., 2004; Starckx, et al., 2002; Yu & Han, 2006). Mature granulocytes can secrete considerable amounts of latent MMPs, including neutrophil procollagenase (pro-matrix metalloproteinase-8 or proMMP-8) and progelatinase B (pro-matrix metalloproteinase-9 or proMMP-9) (Opdenakker, 2001). Activation of these MMPs is achieved via proteolysis, induced by extracellular proteinases including plasmin and urokinase (Yu & Han, 2006). Activated MMPs can further activate other pro-MMPs, constituting a feedforward mechanism in the loop of activation. Proinflammatory cytokines interleukin-1β (IL-1β) and IL-18 produced through activation of the caspase-1 inflammasome pathway may mediate the local inflammatory reaction (Kopitar-Jerala, 2017; Jha, et al., 2017), which would activate related extracellular proteinases to facilitate the activation of MMPs via proteolysis in the marrow hematopoietic environment (Borkowska, et al., 2014; Del Rosso, et al., 2008; 2011; Syrovets, et al., 2012). Further investigations on alcohol-induced inflammatory reaction in relation to the functional alteration of molecules regulating retention of granulocytes in the marrow storage pool will improve understanding about mechanisms underlying marrow release of granulocytes following excessive alcohol exposure, particularly following chronic plus acute alcohol consumption. The adverse effects of alcohol on homeostasis of granulopoiesis and marrow storage of granulocytes are summarized in Figure 2.
Figure 2.
The adverse effects of alcohol on homeostasis of granulopoiesis and marrow storage of granulocytes.
4. Alcohol impairs the granulopoietic response
In response to infection, particular acute bacterial infection, the bone marrow can quickly release a large amount of granulocytes from its storage pool into the systemic circulation. In the meantime, activation of HSPCs occurs to promote granulocyte generation in order to reinforce host defense for eliminating invading pathogens. Any defects in this process will compromise host immunity. Alcohol abuse, especially acute intoxication or chronic plus binge drinking, suppresses the granulopoietic response. Clinical investigations have shown that the occurrence of serious infection in alcoholic patients is frequently preceded by an episode of very heavy drinking (Dorff, et al., 1973). Bone marrow analysis of alcoholic patients during the neutropenic stage has demonstrated that virtually none of the neutrophil precursors has matured beyond an early developmental stage (Ballard, 1997). Further, the neutrophil storage in the bone marrow is depleted more rapidly in active alcoholics than in healthy control subjects. Activation of HSPC proliferation and transcriptional reprograming of primitive hematopoietic precursor cells for enhancing their commitment toward granulocytic lineage development are critically important in the granulopoietic response (Shi, et al., 2013; 2018; Zhang, et al., 2008). Alcohol intoxication disrupts several key signaling mechanisms regulating the activation of HSPCs during the granulopoietic response.
4.1. Inhibition of HSPC proliferative activation
Because in the undifferentiated status, HSCs commonly do not share the same profile of signaling mechanisms with the downstream fully differentiated cell types for the regulation of their functional activities. However, primitive hematopoietic precursor cells express pattern recognition receptors and receptors for most proximal proinflammatory cytokines (Du, et al., 2012; McKinstry, et al., 1997; Nagai, et al., 2006; Sawamiphak, et al., 2014; Schuettpelz, et al., 2014; Takizawa, et al., 2017). Therefore, they are able to respond to ligand stimulations including those mediated by pathogen-associated molecular patterns (PAMPs), damage-associated molecular patterns (DAMPs), and certain types of cytokines. In mice, challenge with gram-negative bacteria, gram-positive bacteria, or fungal pathogens, causes activation of HSPCs through various toll-like receptor (TLR)-associated signaling cascades (Raasch, et al., 2010; Shi, et al., 2011; 2013). Lipopolysaccharide (LPS), the cell wall component of gram-negative bacteria, is a potent PAMP ligand. Binding of LPS to TLR4 activates the p44/42 mitogen-activated protein kinase (MAPK) or extracellular-signal-regulated kinase1/2 (ERK1/2) pathway through activation of the Ras-c-Raf-mitogen-activated protein kinase ERK kinase 1/2 (MEK1/2) cascades (Guha & Mackman, 2001; Guha, et al., 2001). Phosphorylation of p44/42 leads to its nuclear translocation where it activates several ERK targets (including transcription factors such as Elk-1) and induces expression of the first class of G1 cyclins, cyclin D. Upregulation of cyclin D-cyclin-dependent kinase 4/6 (CDK4/6) activity promotes S phase entry during cell cycling (Meloche & Pouysségur, 2007; Torii, et al., 2006). Mice with Escherichia coli bacteremia show a rapid expansion of marrow LKS cell population (Shi, et al., 2013; 2017a; Zhang, et al., 2008; 2009). E. coli infection induces activation of p44/42 MAPK and upregulation of cyclin D1 expression in marrow cells (Shi, et al., 2017a), which are accompanied with enhancement of LKS cell proliferation as reflected by their increase in BrdU incorporation (Shi, et al., 2017a; Zhang, et al., 2008; 2009). TLR4 gene knockout impaired the increase in BrdU incorporation into marrow LKS cells during E. coli infection (Shi, et al., 2013). In vitro stimulation of isolated HSPCs with LPS also activates their proliferation in the culture system (Shi, et al., 2013; 2017a). This TLR4-p44/42 MAPK-cyclin D1 signaling mediated activation of cell proliferation plays an important role in the expansion of marrow LKS cell pool during the granulopoietic response. Acute alcohol intoxication or chronic plus binge alcohol administration inhibits the activation of TLR4-p44/42 MAPK-cyclin D1 signaling and suppresses proliferative activation of LKS cells in the bone marrow (Shi, et al., 2017a; Zhang, et al. 2009). It is well known that activation of the TLR4-p38 MAPK-nuclear factor-κB (NF-κB) pathway mediates cell expression of proximal proinflammatory cytokine tumor necrosis factor-α (TNF-α) (Hochdörfer, et al., 2013; Togbe, et al., 2007). Mice with E. coli bacteremia show a marked early increase in TNF-α level in the systemic circulation (Zhang, et al., 2008; 2009). TNF-α also activates p44/42 MAPK signaling cascades (Sabio & Davis, 2014; Winston, et al., 1995a; 1995b). Intravenous challenge with recombinant TNF-α evokes expansion of marrow LKS cell population in mice (Zhang, et al., 2009). Alcohol intoxication suppresses both tissue production of TNF-α in response to bacterial infection (Zhang, et al., 2009 PMID: 19630706) and TNF-α-induced activation of LKS cells during the granulopoietic response (Zhang, et al., 2009). Activation of the TLR4-p44/42 MAPK-cyclin D1 pathway not only promotes proliferation of HSCs, but enhances cell cycling in myeloid progenitor and granulocytic progenitor cells as well (Melvan, et al., 2012; Shi, et al., 2017a). Both acute alcohol intoxication and chronic plus binge alcohol administration suppress this signaling regulation in enhancement of proliferation in myeloid/granulocytic lineage-committed progenitor cells during the granulopoietic response to bacterial infection.
4.2. Obstruction of HSPC phenotypic conversion and reprogramming
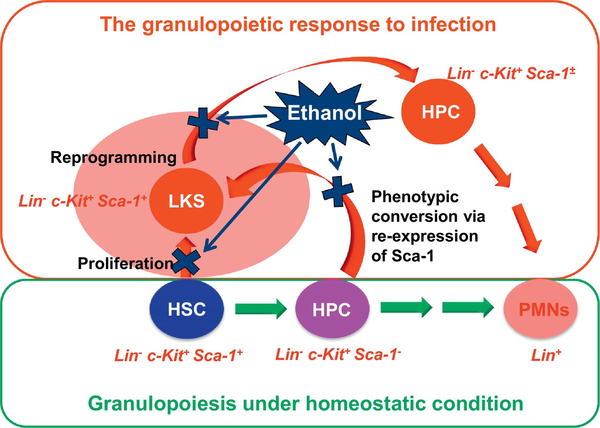
Recent studies on the HSPC response to bacterial infection in murine models have revealed another crucial process, termed “phenotypic conversion,” for the rapid expansion of marrow LKS cell pool. In this process, downstream myeloid progenitor cells baring lin−c-kit+Sca-1− phenotypic marker re-express Sca-1 to convert themselves back to the upstream LKS (lin−ckit+Sca-1+) phenotype (Raasch, et al., 2010; Shi, et al., 2013; 2017a; Zhang, et al., 2008; 2009 ). Approximately 70–85% of total LKS cells in the expanded marrow LKS cell pool are generated through this phenotypic conversion pathway during the early stage of bacterial infection. At the present time, it remains unclear if LKS cells generated from the phenotypic conversion would regain the same capacity of multipotency for development of all blood cell lineages as the true LKS cells under the homeostatic condition. Regardless, the expanded population of LKS cells evidently serves as a platform for HSPC reprograming to enhance their commitment toward granulocytic lineage development during the granulopoietic response. A unique feature in the activation of HSPCs is the marked upregulation of Sca-1 expression by these precursor cells at both the transcriptional and protein levels (Melvan, et al., 2011; Shi, et al., 2013). Sca-1 is an 18-kDa mouse glycosyl phosphatidylinositol (GPI)-anchored cell surface protein of the Ly6 gene family, which plays a pivotal role in signaling stem/progenitor cell functional activities (Bradfute, et al., 2005; Ito, et al., 2003). Since lacking either an identified ligand or directly associated intracellular signaling pathway, Sca-1 most likely acts as a coregulator of lipid raft signal to mediate changes in stem/progenitor cell functional activities. Sca-1 has been proposed to alter lipid raft composition via weak protein-protein interactions, sequestering or obstructing key signaling molecules in the vicinity of their receptors or even promoting raft clustering (Holmes & Stanford, 2007). Sca-1 may play a role in several lipid raft-mediated signaling pathways, including those involving receptor tyrosine kinases and Src family kinases (Stefanová, et al., 1991). Sca-1 and lipid rafts also have close associations with c-kit signaling in HSPCs (Bradfute, et al., 2005; Ito, et al., 2003). In mice with systemic E. coli infection, the upregulation of Sca-1 expression is in correlation with the activation of p44/42 MAPK signaling and enhancement of proliferation in HSPCs (Melvan, et al., 2011; 2012). Transcriptional activation of genes for granulocytic lineage development, such as CCAAT enhancer-binding protein-β (CEBP–β), Spi-1 proto-oncogene (Spi-1 or PU.1), and granulocyte colony-stimulating factor receptor (G-CSFR) occurs in parallel with the increase in Sca-1 expression in HSPCs during the granulopoietic response (Shi, et al., 2013; 2017a). Further, antibody cross-linking Sca-1expressed on hematopoietic precursor cells in response to LPS stimulation in the in vitro culture system not only generates a feedforward signal for enhancing Sca-1itself expression, but also upregulates PU.1 expression by these cells (Shi, et al., 2013). HSPCs from mice with Sca-1 gene knockout exhibit a decrease in granulocyte colony-forming activity (Ito, et al., 2003). Mice with Sca-1 knockout also show impairment of the granulopoietic response to serious bacterial infection (Melvan, et al., 2011; 2012; Shi, et al., 2013).
Alcohol intoxication suppresses the upregulation of Sca-1 expression by HSPCs during the granulopoietic response (Shi, et al., 2017a; Zhang, et al., 2009). The increase in Sca-1 expression is primarily regulated at the transcriptional level. The promoter region of Sca-1 gene contains multiple binding sites for activating protein-1 (AP-1) transcription factor (Melvan, et al., 2011). C-Jun is the most potent transcriptional activator in the AP-1 family (Shaulian & Karin, 2002). Activation of either TLR4 or TNF receptor (TNFR) following ligand (such as LPS or TNF-α) engagement actives c-Jun N-terminal kinases (JNKs) and subsequently enhances the transcriptional activity of c-Jun by phosphorylation of its N-terminal activation domain (Khan, et al., 2017; Medvedev, et al., 2007; Reinhard, et al., 1997; 2007; Song, et al., 1997). Systemic bacterial infection in murine models quickly induces JNK activation in bone marrow cells (Melvan, et al., 2011). Inhibition of JNK activation with specific JNK inhibitor suppresses up-regulation of Sca-1 expression by cultured hematopoietic precursor cells in response to LPS or TNF-α (Shi, et al., 2017a; Zhang, et al., 2009). Alcohol intoxication inhibits JNK activation in marrow cells in response to E. coli bacteremia (Melvan, et al., 2011), which may serve as the mechanism underlying alcohol-induced suppression of Sca-1 upregulation in HSPCs. Alcohol-induced inhibition of Sca-1 signaling couples with impairment of proliferative activation in HSPCs and disruption of HSPC reprograming for enhancing commitment toward granulocytic lineage development (Melvan, et al., 2011; 2012; Raasch, et al., 2010; Shi, et al., 2011; 2017a, Zhang, et al., 2009). Consequently, the granulopoietic response to bacterial infection is impaired in experimental animals with excessive alcohol exposure. In this context, the chronic plus binge alcohol consumption pattern apparently exerts the most destructive effect on granulocyte-mediated immune defense in hosts. This typical pattern of alcohol consumption exhausts the marrow reserve of mature granulocytes by inducing granulocyte release from the marrow storage pool into the circulation in the homeostatic state. When host confronts the challenge of serious infection, it suppresses the granulopoietic response rendering the host incapable of reinforcing the phagocytic defense against invading pathogens.
4.3. Disruption of major granulopoietic signals
G-CSF is a lineage-specific growth factor stimulating granulopoiesis (Dale, et al., 1995). During bacterial infection, tissue cells at sites of infection and inflammation produce G-CSF, leading to the significant increase in G-CSF level in the circulation (Kragsbjerg, et al., 1995; Pauksen, et al., 1994; Quinton, et al., 2002; Shahbazian, et al., 2004). This increase in G-CSF level promotes the granulopoietic response in the bone marrow and enhances mobilization of granulocytes into the circulation (Dale, et al., 1995; Semerad, et al., 2002; Zhang, et al., 2005). Binding of G-CSF to its receptor activates the p44/42 MAPK-cyclin D signaling pathway, which mediates activation of HSPC proliferation in both homeostatic and emergency conditions (Marino & Roguin, 2008). Engagement of G-CSF with G-CSFR also triggers activation of the STAT3 signaling cascades (Marino & Roguin, 2008; Zhang, et al., 2010). STAT3 directly controls G-CSF-dependent expression of C/EBPβ (Zhang, et al., 2010). Both STAT3 and C/EBPβ regulate c-Myc oncogene through interactions with the c-myc promoter during demand-driven granulopoiesis. C-Myc transcription has been identified as a normal event in granulopoiesis linked to proliferative activity as well as to the primitive stage in development (Gowda, et al., 1986). Activation of the STAT3 pathway also mediates expression of the CDK inhibitor p27Kip1 which causes G1 cell cycle arrest to turn into terminally granulocytic differentiation (de Koning, et al., 2000; McArthur, et al., 2002; Mangan & Reddy, 2005; Rane, et al., 2002). Alcohol intoxication disrupts G-CSFR signaling in the regulation of the granulopoietic response at different steps. Alcohol suppresses G-CSF production by tissue cells in response to infectious challenges (Bagby, et al., 1998), reducing ligand stimulation for the initial activation of G-CSFR. Further, alcohol exposure significantly increases STAT3 phosphorylation in marrow cells of experimental animals with bacterial infection and enhances G-CSF-induced activation of the STAT3-p27Kip1 pathway in cultured murine myeloid progenitor cell line 32D-G-CSFR cells (Siggins, et al., 2011). These alcohol-induced disruptions of G-CSFR signaling have been shown to accompany the inhibition of HSPC proliferative activation and marrow production of granulocytes in response to bacterial infection (Bagby, et al., 1998; Siggins, et al., 2011). Because of impairing host immune defense, alcohol administration causes an increase in bacterial loads in the systemic circulation and vital organ tissues in animal models of bacterial infection (Bagby, et al., 1998; Zhang, et al., 2009). The infection-associated mortality rates are also significantly increased in animals with alcohol intoxication. Figure 3 shows the negative effects of alcohol on activation of HSPCs during the granulopoietic response in murine models.
Figure 3.
The negative effects of alcohol on activation of HSPCs during the granulopoietic response in murine models.
Recent studies have shown that other signaling mechanisms, including the sonic hedgehog (SHH)-glioma-associated oncogene 1 (Gli1) (Shi, et al., 2018), TLR4-toll-like receptor adaptor molecule 1 (TRIF)-ROS-p38 MAPK (Takizawa, et al., 2017), and granulocyte-macrophage colony-stimulating factor receptor (GM-CSFR)-STAT5A/B (Kimura, et al., 2009) signaling cascades are involved in the regulation of granulopoiesis under homeostatic conditions and during the granulopoietic response to stressful challenges. Further investigation on the potential interplays between alcohol and these cell signaling pathways will provide deeper insight into mechanisms underlying alcohol-induced impairment of marrow granulocyte production for maintaining and/or reinforcing host phagocytic defense. It is worth noting that most of studies on alcohol-induced disruption of cell signaling regulation in the granulopoietic response are conducted on animal models thus far, particular in murine models. As described previously, human HSPCs do not share the same set of surface markers with those from the murine origin. Accordingly, signaling regulations in human HSPCs may not be identical to those observed from studies on experimental animals. Further efforts in verification of signaling mechanisms underlying the regulation of functional activities in human HSPCs will be helpful for developing targeted therapy to effectively treat impairment of the granulopoietic response in alcohol abusers.
5. Progress in therapeutic approaches
5.1. Treatment of alcohol addiction and abuse
Generally, alcohol-induced injury to marrow granulocytic lineage in adults is reversible (Michot & Gut, 1987; Nakao, et al., 1991). Both in the absence and presence of infection, the reduction of marrow cellularity and impairment of granulocytic lineage development in alcohol abusers can usually return to normal following a short period of abstinence. Granulocytopenia in alcoholic patients with bacterial infection can be transient with a possible “rebound” granulocytosis between 5 and 10 days of hospitalization, if the patient survives (Ballard, 1997; Eichner, 1973; Lindenbaum, 1987). The cytoplasm vacuoles in myeloid precursors disappear following abstinence for 6 to 10 days (Liu, 1973). The marrow granulocyte reserve is also able to return to the normal level following two weeks of abstinence. Along with the injury to granulopoiesis, however, alcohol abuse also causes abnormalities in erythropoiesis and thrombocytopoiesis, leading to pancytopenia in the peripheral circulation (Ballard, 1997; Eichner, 1973; Latvala, et al., 2004). There is a concern that long term heavy alcohol consumption may increase the risk of developing myelodysplastic syndrome (MDS), a heterogeneous group of myeloid disorders leading to dysplasia and ineffective hematopoiesis with the characterization of peripheral blood cytopenias (Montalban-Bravo & Garcia-Manero, 2018). Some patients with MDS may even have a transformation into acute myeloid leukemia (AML). Currently, clinical reports about this risk remain highly controversial (Dalamaga, et al., 2002; Du, et al., 2010; Jin, et al., 2014; Liu, et al., 2016a; Pekmezovic, et al., 2006; Ugai, et al., 2017; Strom, et al., 2005; 2008). Several sets of case-control studies have shown that alcohol consumption is a risk factor for MDS in adults (Avgerinou, et al., 2017; Pekmezovic, et al., 2006). Further, a meta analysis has suggested that alcohol intake may increase the risk of MDS in a dose dependent manner (Jin, et al., 2014). A stronger association of alcohol with MDS has been detected in individuals who consume ≥10 g/day. In contrast, other case-control studies and meta-analyses have failed to identify any role of alcohol intake alone in MDS etiology (Dalamaga, et al., 2002; Du, et al., 2010). Also, some case-control and cohort studies have reported that alcohol consumption is associated with a decrease in the risk of MDS (Liu, et al., 2016a; Strom, et al., 2005; Ugai, et al., 2017). These inconsistencies are most likely resulted from various populations of individuals with different drinking patterns and durations included in each study, which suggests the necessity for further strengthening the quality control of investigations on the long-term effect of heavy alcohol consumption on the development of myelodysplastic disorders. Acetaldehyde metabolites generated from alcohol consumption contribute to formation of various DNA adducts, some of which have been demonstrated to be carcinogenic (Yu, et al., 2010). As described previously, certain ALDH isoforms may metabolize reactive aldehydes in HSCs. Loss of these ALDHs leads to perturbation of many cell processes which may predispose HSCs to disorders in growth and leukemic transformation. It has been postulated that polymorphisms in genes responsible for metabolizing reactive aldehydes and repairing their DNA adducts in HSPCs may predispose to the development of acute leukemia in heavy alcohol users (Smith, et al., 2015). Currently, regardless, reports about the risk of excessive alcohol consumption in developing acute myeloid leukemia remain inconsistent. A recently completed systematic review with meta-analysis of 18 investigations, including 10 case-control and 8 cohort studies carried out in a total of 7142 leukemia cases from America, Europe, and Asia, respectively, fails in defining an increased risk of leukemia among alcohol drinkers (Rota, et al., 2014).
Since alcohol-induced disorders of granulopoiesis are generally reversible, application of the optimal therapy including the selected or combined pharmacological treatments with psychosocial as well as psychotherapeutic approaches to treat alcohol addiction and abuse should be the primary choice for treatment (Johnson, 2010). Successful maintenance of long-term abstinence will not only allow recovery of marrow injury caused by previous alcohol exposure, but will be helpful for limiting the potential risk for developing MDS as well as other types of myeloid malignances.
5.2. Treatment with G-CSF
Excessive alcohol consumption predisposes the host to develop serious bacterial infection, particularly septic infection. Treatment of alcoholic patients who have septic infection in the presence of granulocytopenia remains a major challenge. Standard treatment of these patients consists of antimicrobial chemotherapy, vitamin supplement, and intensive care support. Even with the standard treatment, the mortality rate remains exceedingly high. Efforts of stimulating marrow granulopoietic activity with G-CSF have been deployed in the treatment of serious infection in hosts with excessive alcohol exposure. In experimental studies, administration of G-CSF for 2 days prior to alcohol intoxication and pulmonary infection with Klebsiella pneumoniae has been shown to significantly attenuate alcohol-induced inhibition of granulocyte recruitment into the infected lung (Nelson, et al., 1991) G-CSF enhances bactericidal activity in the lung and substantially improves survival of alcohol intoxicated animals following pulmonary infection. In a rabbit model of gram-negative sepsis complicated by leukopenia, administration of G- CSF in combination with penicillin G at 24 h post the infection has been reported to significantly increase the level of circulating leukocytes and improve survival over treating with antibiotics alone (Smith, et al., 1995). The application of G-CSF for treatment of infectious diseases has been approached cautiously because G-CSF promotes both production of granulocytes and functional activities of these immune effector cells. The concern exists about if granulocytes might non-selectively amplify the body’s inflammatory response leading to inadvertent tissue injury (Morstyn, et al., 1997). In a swine model of trauma plus septic infection, intravenous G-CSF administration at the time of resuscitation has been shown to improve survival without potentiating PMN-mediated lung reperfusion injury (Patton, et al., 1998). A clinical trial of 756 patients with community-acquired pneumonia has also shown that administration of G-CSF (300 μg/day) to patients for up to 10 days causes a 3-fold increase in the number of circulating PMNs (Nelson, et al., 1998). G-CSF treatment is well-tolerated by these patients. Patients treated with G-CSF exhibit a faster resolution of X-ray abnormalities and fewer complications including the adult respiratory distress syndrome and disseminated intravascular coagulation. Another extensive analysis of six clinical studies on G-CSF as an adjunct to antibiotics in the treatment of pneumonia in adults involving a total of 2018 people has demonstrated that G-CSF use appears safe with no increase in the incidence of total serious adverse events or organ dysfunction (Cheng, et al. 2007). These observations suggest that G-CSF may be useful for treatment of serious infections in immunocompromised patients, such as individuals who abuse alcohol. Indeed, one case report has described that receiving 300 microgram G-CSF daily in adjunction to the standard therapy, a 32-year-old woman with alcoholism, leukopenia, and pneumococcal sepsis is able to recover from her leukopenia and septic infection before being discharged in good condition (Grimsley, 1995).
Excessive alcohol consumption frequently causes serious liver diseases including severe alcoholic hepatitis and alcoholic cirrhosis. Severe alcoholic hepatitis is life-threatening with the acute mortality rate around 30%. Corticosteroids are the only therapeutic option for improving short-term survival at the present time (Gustot, et al., 2017; Karakike, et al., 2017). Corticosteroids have been shown to exert various effects on marrow HSPC activities and the defense function of immune effector cells (Dror, et al., 2000; Family, et al., 2018; Hernigou & Beaujean, 1997). Advanced liver diseases are also frequently accompanied with granulocytopenia (Jain, et al., 2016; Ng, et al., 2002; Qamar, et al., 2009) and impairment of neutrophil function (Karakike, et al., 2017; Rolas, et al., 2013). Infectious complications including septic infection occur in approximately 50% of patients with alcoholic liver diseases, which are the main causes of death in these individuals (Karakike, et al., 2017; Bruns, et al., 2014). Because of the complexity in pathological changes associated with alcohol abuse, liver damage, corticosteroid therapy, granulocytopenia, and serious infection, treatment of these patients requires a specific caution. Administration of G-CSF in adjunction to the standard therapy has been shown to substantially increase the survival of patients with either severe alcoholic hepatitis or alcoholic liver failure (Chavez-Tapia, et al., 2015; Garg, et al., 2012; Moreau & Rautou, 2014; Singh, et al., 2014). G-CSF treatment increases leukocyte and neutrophil counts, improves liver function, as well as reduces the occurrence of septic infection and scores of sequential organ failure (Garg, et al., 2012; Moreau & Rautou, 2014). Mechanisms by which G-CSF therapy improves survival in this patient population remain to be clarified. Experimental and clinical studies have observed that G-CSF administration induces release of HSCs from the bone marrow (Garg, et al., 2012; Gordon, et al., 2006; Liu, et al., 2006; Singh, et al., 2014; Spahr, et al., 2008). One postulation is that the marrow-derived stem cells may promote liver regeneration (Liu, et al., 2006; Pai, et al., 2008; Spahr, et al., 2008; Yannaki, et al., 2005). Regardless, G-CSF stimulates granulopoiesis and promotes marrow production of granulocytes (Basu, et al., 2002; Bugl, et al., 2012; Christopher & Link, 2007). G-CSF also enhances functional activities of mature granulocytes (Pitrak, 1997; Rapoport, et al., 1992; Spiekermann, et al., 1997). Obviously, the beneficial effects of G-CSF on marrow granulopoiesis and neutrophil-mediated phagocytic defense against invading pathogens may play an important role in improving survival of patients with advanced alcoholic liver disease in the presence of bacterial infection (Moreau & Rautou, 2014). Further investigation on the interplays between G-CSF administration and corticosteroid treatment in relation to granulopoiesis and functional activities of granulocytes will provide critical information for developing novel therapeutic strategies to effectively treat life-threatening infection in patients with advanced alcoholic liver diseases and bone marrow injury.
6. Conclusive remarks
Excessive alcohol consumption impairs granulocytic lineage development and marrow production of granulocytes. Ethanol and its metabolites induce disorder of granulopoiesis through their toxicity to HSPCs and disruption of hematopoietic niche environment as well as cell signaling regulations. Alcohol-induced defects of granulocytic defense predispose the host to developing infectious diseases, particularly septic infection. Patients with alcoholism, septic infection, and granulocytopenia have an exceedingly high mortality rate. Alcohol abuse injures the liver, which may lead to developing advanced liver diseases. Treatment of alcoholic liver diseases with corticosteroids may further compromise host immune defense. G-CSF stimulates granulopoiesis and enhances functional activities of granulocytes. G-CSF administration in adjunction to standard therapy has been shown to be beneficial in the treatment of alcoholic patient with septic infection and granulocytopenia. G-CSF treatment can also improve the survival rates of patients with advanced alcoholic liver disease in the absence and presence of infectious complications. Further efforts in exploring protection of granulopoietic activity and the granulopoietic response will promote developing novel approaches for effective prevention and treatment of serious infections in alcohol abusers.
Acknowledgments
Role of the Funding Source
This work is supported by NIH grant R01AA022816 and Watanakunakorn Chair Endowment Fund. The funding sources has no involvement in study design, the collection, analysis and interpretation of data, in the writing the report, and in the decision to submit the paper for publication.
Abbreviations
- ADH
alcohol dehydrogenases
- AKT
protein kinase B
- ALDH
Aldehyde dehydrogenase
- AML
acute myeloid leukemia
- AP-1
activating protein-1
- ARNT
aryl hydrocarbon receptor nuclear translocator
- BKCa
big conductance calcium-activated potassium channels
- BMP
bone morphogenetic protein
- BrdU
5-bromo-2-deoxyuridine
- CAR CXC
chemokine ligand 12-abundant reticular
- CDK
cyclin-dependent kinase
- CEBP–β
CCAAT enhancer-binding protein-β
- CFU-G
granulocytic progenitor cells
- CFU-GM
granulocyte/macrophage colony forming unit
- c-kit
stem cell growth factor receptor
- CMPs
common myeloid progenitors
- cMyc
c-Myc oncogene
- CXCL12
CXC chemokine ligand 12
- DAMPs
damage-associated molecular patterns
- DEAB
diethylaminobenzaldehyde
- ER
endoplasmic reticulum
- ERAD
endoplasmic reticulum-associated protein degradation
- ERK
extracellular-signal-regulated kinase
- G-CSF
granulocyte-colony stimulating factor
- G-CSFR
granulocyte colony-stimulating factor receptor
- Gli1
g lioma-associated oncogene 1
- GM-CSF
granulocyte-macrophage colony-stimulating factor
- GM-CSFR
granulocyte-macrophage colony-stimulating factor receptor
- GMPs
myelomonocytic progenitor cells
- GPI
glycosyl phosphatidylinositol
- HSCs
hematopoietic stem cells
- HSPCs
hematopoietic stem/progenitor cells
- IL-1β
interleukin-1β
- IL-18
interleukin-18
- JNKs
c-Jun N-terminal kinases
- Lin
lineage
- LED
Lieber-DeCarli low-fat liquid alcohol diet
- LKS
lin-c-kit+Sca-1+
- LPS
lipopolysaccharide
- LTR-HSCs
long-term repopulating HSCs
- MAPK
mitogen-activated protein kinase
- MDS
myelodysplastic syndrome
- MEK
mitogen-activated protein kinase ERK kinase
- MELD
model for end stage liver disease
- MMP-8
matrix metalloproteinase-8
- MMP-9
matrix metalloproteinase-9
- MMPs
matrix metalloproteinases
- MPPs
multipotent progenitor cells
- MSCs
mesenchymal stem cells
- mTOR
mammalian target of rapamycin
- NF-κB
nuclear factor-κB
- PAMPs
pathogen-associated molecular patterns
- PBMCs
peripheral blood mononuclear cells
- PLCD
paraptosis-like cell death
- PMNs
polymorphonuclear leukocytes
- PPARγ
Peroxisome proliferator-activated receptor γ
- PU.1
Spi-1 proto-oncogene
- RA
retinoic acid
- RAR
retinoic acid receptor
- ROS
reactive oxygen species
- Sca-1
stem cell antigen-1
- SCF
stem cell factor
- SDF-1
stromal cell-derived factor 1
- SHH
sonic hedgehog siRNA small interfering RNAs
- Spi-1
Spi-1 proto-oncogene
- STAT3
transcription factors signal transducer and activator of transcription 3
- STR-HSCs
short-term repopulating HSCs
- TGF-β
transforming growth factor-β
- TLR
toll-like receptor
- TNF-α
tumor necrosis factor-α
- TNFR
TNF receptor
- TRIF
TIR-domain-containing adapter-inducing interferon-β (TLR4-toll-like receptor adaptor molecule 1)
Footnotes
Conflict of Interest Statement
The authors declare that there are no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Akala OO & Clarke MF (2006). Hematopoietic stem cell self-renewal. Current Opinion in Genetics and Development 16, 496–501. [DOI] [PubMed] [Google Scholar]
- Aki T, Nara A, & Uemura K (2012). Cytoplasmic vacuolization during exposure to drugs and other substances. Cell Biology and Toxicology 28, 125–131. [DOI] [PubMed] [Google Scholar]
- Arzumnayan A, Anni H, Rubin R, & Rubin E (2009). Effects of ethanol on mouse embryonic stem cells. Alcoholism Clinical & Experimental Research 33, 2172–2179. [DOI] [PubMed] [Google Scholar]
- Asquith M, Pasala S, Engelmann F, Haberthur K, Meyer C, Park B, Messaoudi I (2014). Chronic ethanol consumption modulates growth factor release, mucosal cytokine production, and microRNA expression in nonhuman primates. Alcoholism Clinical & Experimental Research 38, 980–993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avgerinou C, Giannezi I, Theodoropoulou S, Lazaris V, Kolliopouloum G, Zikos P, Symeonidis A. (2017). Occupational, dietary, and other risk factors for myelodysplastic syndromes in Western Greece. Hematology 22, 419–429. [DOI] [PubMed] [Google Scholar]
- Babior BM & Golde DW (2001). Production, distribution, and fate of neutrophils In Beutler E, Lichtman MA, Coller BS, Kipps TJ, & Seligsohn U (eds), Williams Hematology Sixth Edition (pp753–759). New York: McGraw-Hill Companies, Inc. [Google Scholar]
- Bagby GJ, Zhang P, Stoltz DA, & Nelson S (1998). Suppression of the granulocyte colony-stimulating factor response to Escherichia coli challenge by alcohol intoxication. Alcoholism Clinical & Experimental Research 22, 1740–1745. [PubMed] [Google Scholar]
- Ballard HS (1980) Alcohol-associated pancytopenia with hypocellular bone marrow. American Journal of Clinical Pathology 73, 830–834. [DOI] [PubMed] [Google Scholar]
- Ballard HS (1997). The hematological complications of alcoholism. Alcohol Health and Research World 21, 42–52. [PMC free article] [PubMed] [Google Scholar]
- Barthlen W, Zantl N, Pfeffer K, Heidecke CD, Holzmann B, & Stadler J (1999). Impact of experimental peritonitis on bone marrow cell function. Surgery 126, 41–47. [DOI] [PubMed] [Google Scholar]
- Basu S, Dunn A, & Ward A (2002). G-CSF: function and modes of action (Review). International Journal of Molecular Medicine 10, 3–10. [PubMed] [Google Scholar]
- Beard JD, Barlow G, & Tuttle A (1963). Observations of peripheral blood elements during chronic ethanol administration in dogs. Physiologist 6, 163. [Google Scholar]
- Bernstein ID, Andrews RG, & Rowley S (1994). Isolation of human hematopoietic stem cells. Blood Cells 20, 15–23. [PubMed] [Google Scholar]
- Bertola A, Park O, & Gao B (2013). Chronic plus binge ethanol feeding synergistically induces neutrophil infiltration and liver injury in mice: a critical role for E-selectin. Hepatology 58, 1814–1823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolnick JM, Karana R, Chiang PJ, Kilburn BA, Romero R, Diamond MP, Armant DR (2014). Apoptosis of alcohol-exposed human placental cytotrophoblast cells is downstream of intracellular calcium signaling. Alcoholism Clinical & Experimental Research 38, 1646–1653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bond AN & Wickramasinghe SN (1983). Investigations into the production of acetate from ethanol by human blood and bone marrow cells in vitro. Acta Haematologica 69, 303–313. [DOI] [PubMed] [Google Scholar]
- Borkowska S, Suszynska M, Mierzejewska K, Ismail A, Budkowska M, Salata D, Ratajczak MZ (2014). Novel evidence that crosstalk between the complement, coagulation and fibrinolysis proteolytic cascades is involved in mobilization of hematopoietic stem/progenitor cells (HSPCs). Leukemia 28, 2148–2154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradfute SB, Graubert TA, & Goodell MA (2005). Roles of Sca-1 in hematopoietic stem/progenitor cell function. Experimental Hematology 33, 836–843. [DOI] [PubMed] [Google Scholar]
- Bruns T, Zimmermann HW, & Stallmach A (2014). Risk factors and outcome of bacterial infections in cirrhosis. World Journal of Gastroenterology 20, 2542–2554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bugl S, Wirths S, Müller MR, Radsak MP, & Kopp HG (2012). Current insights into neutrophil homeostasis. Annals of the New York Academy of Sciences 1266:171–178. [DOI] [PubMed] [Google Scholar]
- Calvi LM & Link DC (2015). The hematopoietic stem cell niche in homeostasis and disease. Blood 126, 2443–2451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakkalakal DA (2005). Alcohol-induced bone loss and deficient bone repair. Alcoholism Clinical & Experimental Research 29, 2077–2090. [DOI] [PubMed] [Google Scholar]
- Chang H, Jensen LA, Quesenberry P, & Bertoncello I (2000). Standardization of hematopoietic stem cell assays: a summary of a workshop and working group meeting sponsored by the National Heart, Lung, and Blood Institute held at the National Institutes of Health, Bethesda, MD on September 8–9, 1998 and July 30, 1999. Experimental Hematol 28, 743–752. [DOI] [PubMed] [Google Scholar]
- Chavez-Tapia NC, Mendiola-Pastrana I, Ornelas-Arroyo VJ, Noreña-Herrera C, Vidaña-Perez D, Delgado-Sanchez G, Barrientos-Gutierrez T (2015). Granulocyte-colony stimulating factor for acute-on-chronic liver failure: systematic review and meta-analysis. Annals of Hepatology 14, 631–641. [PubMed] [Google Scholar]
- Chen Y, Gao H, Yin Q, Chen L, Dong P, Zhang X, Kang J (2013). ER stress activating ATF4/CHOP-TNF-α signaling pathway contributes to alcohol-induced disruption of osteogenic lineage of multipotential mesenchymal stem cell. Cellular Physiology and Biochemistry 32, 743–754. [DOI] [PubMed] [Google Scholar]
- Cheng AC, Stephens DP, & Currie BJ (2007). Granulocyte-colony stimulating factor (G-CSF) as an adjunct to antibiotics in the treatment of pneumonia in adults. Cochrane Database of Systematic Reviews 2:CD004400, 1–21. [DOI] [PubMed] [Google Scholar]
- Cheung C, Davies NG, Hoog JO, Hotchkiss SA, & Smith Pease CK (2003). Species variations in cutaneous alcohol dehydrogenases and aldehyde dehydrogenases may impact on toxicological assessments of alcohols and aldehydes. Toxicology 184, 97–112. [DOI] [PubMed] [Google Scholar]
- Christopher MJ & Link DC (2007). Regulation of neutrophil homeostasis. Current Opinion in Hematology 14, 3–8. [DOI] [PubMed] [Google Scholar]
- Chute JP, Muramoto GG, Whitesides J, Colvin M, Safi R, Chao NJ, & McDonnell DP (2006). Inhibition of aldehyde dehydrogenase and retinoid signaling induces the expansion of human hematopoietic stem cells. Proceedings of the National Academy of Sciences of the United State of America 103, 11707–11712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook RT (1998). Alcohol abuse, alcoholism, and damage to the immune system--a review. Alcoholism Clinical & Experimental Research 22, 1927–1942. [PubMed] [Google Scholar]
- Dalamaga M, Petridou E, Cook FE, & Trichopoulos D (2002). Risk factors for myelodysplastic syndromes: a case-control study in Greece. Cancer Causes & Control 13, 603–608. [DOI] [PubMed] [Google Scholar]
- Dale DC, Liles WC, Summer WR, & Nelson S (1995). Review: granulocyte colony-stimulating factor: role and relationships in infectious diseases. The Journal of Infectious Disease 172, 1061–1075. [DOI] [PubMed] [Google Scholar]
- Dao MA & Nolta JA (2000). CD34: to select or not to select? That is the question. Leukemia 14, 773–776. [DOI] [PubMed] [Google Scholar]
- Das SK & Vasudevan DM (2007). Alcohol-induced oxidative stress. Life Sciences 81, 177–187. [DOI] [PubMed] [Google Scholar]
- Davidson RJ & McPhie JL (1980). Cytoplasmic vacuolation of peripheral blood cells in acute alcoholism. Journal of Clinical Pathology 33, 1193–1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Koning JP, Soede-Bobok AA, Ward AC, Schelen AM, Antonissen C, van Leeuwen D, Touw IP (2000). STAT3-mediated differentiation and survival and of myeloid cells in response to granulocyte colonystimulating factor: role for the cyclin-dependent kinase inhibitor p27(Kip1). Oncogene 19, 3290–3298. [DOI] [PubMed] [Google Scholar]
- de Wit M, Jones DG, Sessler CN, Zilberberg MD, & Weaver MF (2010) Alcohol-use disorders in the critically ill patient. Chest 38, 994–1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Rosso M, Fibbi G, Pucci M, Margheri F, & Serrati S (2008). The plasminogen activation system in inflammation. Frontiers in Bioscience 13, 4667–4686. [DOI] [PubMed] [Google Scholar]
- Del Rosso M, Margheri F, Serratì S, Chillà A, Laurenzana A, & Fibbi G (2011). The urokinase receptor system, a key regulator at the intersection between inflammation, immunity, and coagulation. Current Pharmaceutical Design 17, 1924–1943. [DOI] [PubMed] [Google Scholar]
- Deltour L, Ang HL, & Duester G (1996). Ethanol inhibition of retinoic acid synthesis as a potential mechanism for fetal alcohol syndrome. The FASEB Journal 10, 1050–1057. [PubMed] [Google Scholar]
- Donohue TM Jr. & Thomes PG (2014). Ethanol-induced oxidant stress modulates hepatic autophagy and proteasome activity. Redox Biology 3:29–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorff GJ, Rytel MW, Farmer SG, & Scanlon G (1973). Etiologies and characteristic features of pneumonias in a municipal hospital. The American Journal of the Medical Sciences 266, 349–358. [DOI] [PubMed] [Google Scholar]
- Dror Y, Ward AC, Touw IP, & Freedman MH (2000). Combined corticosteroid/granulocyte colony-stimulating factor (G-CSF) therapy in the treatment of severe congenital neutropenia unresponsive to G-CSF: Activated glucocorticoid receptors synergize with G-CSF signals. Experimental Hematology 28, 1381–1389. [DOI] [PubMed] [Google Scholar]
- Du J, Wang J, Kong G, Jiang J, Zhang J, Liu Y, Zhang J (2012). Signaling profiling at the single-cell level identifies a distinct signaling signature in murine hematopoietic stem cells. Stem Cells 30, 1447–1454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du Y, Fryzek J, Sekeres MA, & Taioli E (2010). Smoking and alcohol intake as risk factors for myelodysplastic syndromes (MDS). Leukemia Research 34, 1–5. [DOI] [PubMed] [Google Scholar]
- Duester G (1998). Alcohol dehydrogenase as a critical mediator of retinoic acid synthesis from vitamin A in the mouse embryo. The Journal of Nutrition 128, 459S–462S. [DOI] [PubMed] [Google Scholar]
- Duester G, Mic FA, & Molotkov A (2003). Cytosolic retinoid dehydrogenases govern ubiquitous metabolism of retinol to retinaldehyde followed by tissue-specific metabolism to retinoic acid. Chemico-Biological Interactions 143–144, 201–210. [DOI] [PubMed] [Google Scholar]
- Eichner ER (1973). The hematologic disorders of alcoholism. The American Journal of Medicine 54, 621–630. [DOI] [PubMed] [Google Scholar]
- Engelhardt M, Lübbert M, & Guo Y (2002). CD34(+) or CD34(−): which is the more primitive? Leukemia 16, 1603–1608. [DOI] [PubMed] [Google Scholar]
- Family L, Li Y, Chen LH, Page JH, Klippel ZK, & Chao C (2018). A Study of Novel Febrile Neutropenia Risk Factors Related to Bone Marrow or Immune Suppression, Barrier Function, and Bacterial Flora. Journal of the National Comprehensive Cancer Network 16, 1201–1208. [DOI] [PubMed] [Google Scholar]
- Fernández-Solá J, Junqué A, Estruch R, Monforte R, Torres A, & Urbano-Márquez A (1995). High alcohol intake as a risk and prognostic factor for community-acquired pneumonia. Archives of Internal Medicine 155, 1649–1654. [DOI] [PubMed] [Google Scholar]
- Furze RC & Rankin SM (2008). Neutrophil mobilization and clearance in the bone marrow. Immunology 125, 281–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garaycoechea JI, Crossan GP, Langevin F, Daly M, Arends MJ, & Patel KJ (2012). Genotoxic consequences of endogenous aldehydes on mouse haematopoietic stem cell function. Nature 489, 571–575. [DOI] [PubMed] [Google Scholar]
- Garaycoechea JI, Crossan GP, Langevin F, Mulderrig L, Louzada S, Yang F, Patel KJ (2018). Alcohol and endogenous aldehydes damage chromosomes and mutate stem cells. Nature 553, 171–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garg V, Garg H, Khan A, Trehanpati N, Kumar A, Sharma BC, Sarin SK (2012). Granulocyte colony-stimulating factor mobilizes CD34(+) cells and improves survival of patients with acute-on-chronic liver failure. Gastroenterology 142, 505–512. [DOI] [PubMed] [Google Scholar]
- Gasparetto M, Sekulovic S, Brocker C, Tang P, Zakaryan A, Xiang P, … Smith C (2012). Aldehyde dehydrogenases are regulators of hematopoietic stem cell numbers and B-cell development. Experimental Hematology 40, 318–329. [DOI] [PubMed] [Google Scholar]
- Goldstein B, Maxwell DS, Ellison G, & Hammer RP (1983). Dendritic vacuolization in the central nervous system of rats after long-term voluntary consumption of ethanol. Journal of Neuropathology & Experimental Neurology 42, 579–589. [DOI] [PubMed] [Google Scholar]
- Gordon MY, Levicar N, Pai M, Bachellier P, Dimarakis I, Al-Allaf F, Habib NA (2006). Characterization and clinical application of human CD34+ stem/progenitor cell populations mobilized into the blood by granulocyte colony-stimulating factor. Stem Cells 24, 1822–1830. [DOI] [PubMed] [Google Scholar]
- Goss CH, Rubenfeld GD, Park DR, Sherbin VL, Goodman MS, & Root RK (2003). Cost and incidence of social comorbidities in low-risk patients with community-acquired pneumonia admitted to a public hospital. Chest 124, 2148–2155. [DOI] [PubMed] [Google Scholar]
- Gowda SD, Koler RD, & Bagby GC Jr. (1986). Regulation of C-myc expression during growth and differentiation of normal and leukemic human myeloid progenitor cells. The Journal of Clinical Investigation 77, 271–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimsley EW (1995). Granulocyte colony stimulating factor in the treatment of alcohol abuse, leukopenia, and pneumococcal sepsis. The Southern Medical Journal 88, 220–221. [DOI] [PubMed] [Google Scholar]
- Guha M & Mackman N (2001). LPS induction of gene expression in human monocytes. Cellular Signalling 13, 85–94. [DOI] [PubMed] [Google Scholar]
- Guha M, O’Connell MA, Pawlinski R, Hollis A, McGovern P, Yan SF, Mackman N (2001). Lipopolysaccharide activation of the MEK-ERK1/2 pathway in human monocytic cells mediates tissue factor and tumor necrosis factor alpha expression by inducing Elk-1 phosphorylation and Egr-1 expression. Blood 98, 1429–1439. [DOI] [PubMed] [Google Scholar]
- Gustot T, Fernandez J, Szabo G, Albillos A, Louvet A, Jalan R, Moreno C (2017). Sepsis in alcohol-related liver disease. Journal of Hepatology 67, 1031–1050. [DOI] [PubMed] [Google Scholar]
- Guthrie TH Jr. & Beckman JB (1983). The direct hematopoietic toxicity of ethyl alcohol. Journal of the Medical Association of Georgia 72, 323–326, 328. [PubMed] [Google Scholar]
- Hammond JM, Lyddell C, Potgieter PD, & Odell J (1993). Severe pneumococcal pneumonia complicated by massive pulmonary gangrene. Chest 104, 1610–1612. [DOI] [PubMed] [Google Scholar]
- Hao HN, Parker GC, Zhao J, Barami K, & Lyman WD (2003). Differential responses of human neural and hematopoietic stem cells to ethanol exposure. Journal of Hematotherapy and Stem Cell Research 12, 389–399. [DOI] [PubMed] [Google Scholar]
- Heermans EH (1998). Booze and blood: the effects of acute and chronic alcohol abuse on the hematopoietic system. Clinical laboratory science 11, 229–232. [PubMed] [Google Scholar]
- Henics T & Wheatley DN (1999). Cytoplasmic vacuolation, adaptation and cell death: a view on new perspectives and features. Biology of the Cell 91, 485–498. [DOI] [PubMed] [Google Scholar]
- Hernigou P & Beaujean F (1997). Abnormalities in the bone marrow of the iliac crest in patients who have osteonecrosis secondary to corticosteroid therapy or alcohol abuse. The Journal of Bone and Joint Surgery (American Volume) 79, 1047–1053. [DOI] [PubMed] [Google Scholar]
- Hochdörfer T, Tiedje C, Stumpo DJ, Blackshear PJ, Gaestel M, & Huber M (2013). LPS-induced production of TNF-α and IL-6 in mast cells is dependent on p38 but independent of TTP. Cellular Signalling 25, 1339–1347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogan CJ, Shpall EJ, & Keller G (2002). Differential long-term and multilineage engraftment potential from subfractions of human CD34+ cord blood cells transplanted into NOD/SCID mice. Proceedings of the National Academy of Sciences of the United States of America 99, 413–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes C & Stanford WL (2007). Concise review: stem cell antigen-1: expression, function, and enigma. Stem Cells 25, 1339–1347. [DOI] [PubMed] [Google Scholar]
- Holmes RS (1994). Alcohol dehydrogenases: a family of isozymes with differential functions. Alcohol and Alcoholism Supplement 2, 127–30. [PubMed] [Google Scholar]
- Huang Q, Zhang H, Pei FX, Chen ZY, Wang GL, Shen B, Kong QQ (2010). Use of small interfering ribonucleic acids to inhibit the adipogenic effect of alcohol on human bone marrow-derived mesenchymal cells. International Orthopaedics 34, 1059–1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imperia PS, Chikkappa G, & Phillips PG (1984). Mechanism of inhibition of granulopoiesis by ethanol. Proceedings of the Society for Experimental Biology and Medicine 175, 219–225. [DOI] [PubMed] [Google Scholar]
- Ito CY, Li CY, Bernstein A, Dick JE, & Stanford WL (2003). Hematopoietic stem cell and progenitor defects in Sca-1/Ly-6A-null mice. Blood 101, 517–523. [DOI] [PubMed] [Google Scholar]
- Jackson B, Brocker C, Thompson DC, Black W, Vasiliou K, Nebert DW, & Vasiliou V (2011). Update on the aldehyde dehydrogenase gene (ALDH) superfamily. Humman Genomics 5, 283–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain D, Aggarwal HK, Rao A, Dahiya S, & Singla S (2016). Hematological spectrum in patients with alcoholic liver cirrhosis: a model of end-stage liver disease score based approach. International Journal of Advances in Medicine 3, 234–240 [Google Scholar]
- Jha S, Brickey WJ, & Ting JP (2017). Inflammasomes in Myeloid Cells: Warriors Within. Microbiology Spectrum 5, doi: 10.1128/microbiolspec.MCHD-0049-2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji C (2015). Advances and New Concepts in Alcohol-Induced Organelle Stress, Unfolded Protein Responses and Organ Damage. Biomolecules 5, 1099–1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin J, Yu M, Hu C, Ye L, Xie L, Chen F, Tong H (2014). Alcohol consumption and risk of myelodysplastic syndromes: A meta-analysis of epidemiological studies. Molecular and Clinical Oncology 2, 1115–1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson BA (2010). Medication treatment of different types of alcoholism. The American Journal of Psychiatry 167, 630–639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jong GM, Hsiue TR, Chen CR, Chang HY, & Chen CW (1995). Rapidly fatal outcome of bacteremic Klebsiella pneumoniae pneumonia in alcoholics. Chest 107, 214–217. [DOI] [PubMed] [Google Scholar]
- Julià P, Farrés J, & Parés X (1986). Ocular alcohol dehydrogenase in the rat: regional distribution and kinetics of the ADH-1 isoenzyme with retinol and retinal. Experimental Eye Research 42, 305–314. [DOI] [PubMed] [Google Scholar]
- Kanwar KC & Tikoo A (1992). Hematological lesions in rat following heavy alcohol ingestion. Journal of Environmental Pathology, Toxicology and Oncology 11, 241–245. [PubMed] [Google Scholar]
- Karakike E, Moreno C, & Gustot T (2017). Infections in severe alcoholic hepatitis. Annals of Gastroenterology 30, 152–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan MA, Farahvash A, Douda DN, Licht JC, Grasemann H, Sweezey N, & Palaniyar N (2017). JNK Activation Turns on LPS- and Gram-Negative Bacteria-Induced NADPH Oxidase-Dependent Suicidal NETosis. Scientific Reports 7:3409, doi: 10.1038/s41598-017-03257-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura A, Rieger MA, Simone JM, Chen W, Wickre MC, Zhu BM, Hennighausen L (2009). The transcription factors STAT5A/B regulate GM-CSF-mediated granulopoiesis. Blood 114, 4721–4728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch OR, Pani G, Borrello S, Colavitti R, Cravero A, Farrè S, & Galeotti T (2004). Oxidative stress and antioxidant defenses in ethanol-induced cell injury. Molecular Aspects of Medicine 25, 191–198. [DOI] [PubMed] [Google Scholar]
- Kohli L & Passegué E (2014). Surviving change: the metabolic journey of hematopoietic stem cells. Trends in Cell Biology 24, 479–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondo M, Wagers AJ, Manz MG, Prohaska SS, Scherer DC, Beilhack GF, … Weissman IL (2003). Biology of hematopoietic stem cells and progenitors: implications for clinical application. Annual Review of Immunology 21, 759–806. [DOI] [PubMed] [Google Scholar]
- Kopitar-Jerala N (2017). The Role of Interferons in Inflammation and Inflammasome Activation. Frontiers in Immunology 8:873, doi: 10.3389/fimmu.2017.00873. eCollection 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kragsbjerg P, Jones I, Vikerfors T, & Holmberg H (1995). Diagnostic value of blood cytokine concentrations in acute pneumonia. Thorax 50, 1253–1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar S, Sandell LL, Trainor PA, Koentgen F, & Duester G (2012). Alcohol and aldehyde dehydrogenases: retinoid metabolic effects in mouse knockout models. Biochimica et Biophysica Acta 1821, 198–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langevin F, Crossan GP, Rosado IV, Arends MJ, & Patel KJ (2011). Fancd2 counteracts the toxic effects of naturally produced aldehydes in mice. Nature 475, 53–58. [DOI] [PubMed] [Google Scholar]
- Latvala J, Parkkila S, Melkko J, & Niemelä O (2001). Acetaldehyde adducts in blood and bone marrow of patients with ethanol-induced erythrocyte abnormalities. Molecular Medicine 7, 401–405. [PMC free article] [PubMed] [Google Scholar]
- Latvala J, Parkkila S, & Niemelä O (2004). Excess alcohol consumption is common in patients with cytopenia: studies in blood and bone marrow cells. Alcoholism Clinical & Experimental Research 28, 619–624. [DOI] [PubMed] [Google Scholar]
- Laura IA, Martinez M, Padovani CR, & Martinez FE (2003). Ultrastructural and morphometric analysis on the ovary of Wistar rats after chronic ethanol ingestion. Journal of submicroscopic cytology and pathology 35, 167–176. [PubMed] [Google Scholar]
- Li M, He Y, Zhou Z, Ramirez T, Gao Y, Gao Y, Gao B (2017). MicroRNA-223 ameliorates alcoholic liver injury by inhibiting the IL-6-p47phox-oxidative stress pathway in neutrophils. Gut 66, 705–715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Wang Y, Li Y, Sun J, & Zhao G (2014). The effect of combined regulation of the expression of peroxisome proliferator-activated receptor-γ and calcitonin gene-related peptide on alcohol-induced adipogenic differentiation of bone marrow mesenchymal stem cells. Molecular and Cellular Biochemistry 392, 39–48. [DOI] [PubMed] [Google Scholar]
- Lindenbaum J (1987). Hematologic complications of alcohol abuse. Seminars in Liver Disease 7, 169–181. [DOI] [PubMed] [Google Scholar]
- Liu F, Pan X, Chen G, Jiang D, Cong X, Fei R, & Wei L (2006). Hematopoietic stem cells mobilized by granulocyte colony-stimulating factor partly contribute to liver graft regeneration after partial orthotopic liver transplantation. Liver Transplantation 12, 1129–1137. [DOI] [PubMed] [Google Scholar]
- Liu P, Holman CD, Jin J, & Zhang M (2016a). Alcohol consumption and risk of myelodysplastic syndromes: a case-control study. Cancer Causes & Control 27, 209–216. [DOI] [PubMed] [Google Scholar]
- Liu YK (1973). Leukopenia in alcoholics. The American Journal of Medicine 54, 605–610. [DOI] [PubMed] [Google Scholar]
- Liu YK (1980). Effects of alcohol on granulocytes and lymphocytes. Seminars in Hematology 17, 130–136. [PubMed] [Google Scholar]
- Liu Y, Kou X, Chen C, Yu W, Su Y, Kim Y, Liu Y (2016b). Chronic High Dose Alcohol Induces Osteopenia via Activation of mTOR Signaling in Bone Marrow Mesenchymal Stem Cells. Stem Cells 34, 2157–2168. [DOI] [PubMed] [Google Scholar]
- Lu Y, & Cederbaum AI (2008). CYP2E1 and oxidative liver injury by alcohol. Free Radical Biology & Medicine 44, 723–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo P, Wang A, Payne KJ, Peng H, Wang JG, Parrish YK,Wu L (2007). Intrinsic retinoic acid receptor alpha-cyclin-dependent kinase-activating kinase signaling involves coordination of the restricted proliferation and granulocytic differentiation of human hematopoietic stem cells. Stem Cells 25, 2628–2637. [DOI] [PubMed] [Google Scholar]
- Ma AC, Chung MI, Liang R, Leung, & A.Y. (2010). A DEAB-sensitive aldehyde dehydrogenase regulates hematopoietic stem and progenitor cells development during primitive hematopoiesis in zebrafish embryos. Leukemia 24, 2090–2099. [DOI] [PubMed] [Google Scholar]
- MacGregor RR & Louria DB (1997). Alcohol and infection. Current clinical topics in infectious diseases 17, 291–315. [PubMed] [Google Scholar]
- Maltese WA & Overmeyer JH (2014). Methuosis: nonapoptotic cell death associated with vacuolization of macropinosome and endosome compartments. The American Journal of Pathology 184, 1630–1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangan JK & Reddy EP (2005). Activation of the Jak3 pathway and myeloid differentiation. Leukemia & Lymphoma 46, 21–27. [DOI] [PubMed] [Google Scholar]
- Marceau F, Bawolak MT, Lodge R, Bouthillier J, Gagné-Henley A, Gaudreault RC, & Morissette G (2012). Cation trapping by cellular acidic compartments: beyond the concept of lysosomotropic drugs. Toxicology and Applied Pharmacology 259, 1–12. [DOI] [PubMed] [Google Scholar]
- Maricic I, Sheng H, Marrero I, Seki E, Kisseleva T, Chaturvedi S, Kumar V (2015). Inhibition of type I natural killer T cells by retinoids or following sulfatide-mediated activation of type II natural killer T cells attenuates alcoholic liver disease in mice. Hepatology 61, 1357–1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marino VJ & Roguin LP (2008). The granulocyte colony stimulating factor (G-CSF) activates Jak/STAT and MAPK pathways in a trophoblastic cell line. Journal of Cellular Biochemistry 103, 1512–1523. [DOI] [PubMed] [Google Scholar]
- Martinez M, Branco Júnior AC, Cagnon VH, Mello Júnior W, Garcia PJ, & Martinez FE (1999). Ultrastructural changes on the epithelial cells of uterine tubes of Wistar rats after chronic ethanol ingestion. Journal of Submicroscopic Cytology and Pathology 31, 273–278. [PubMed] [Google Scholar]
- Maurel DB, Boisseau N, Benhamou CL, & Jaffre C (2012a). Alcohol and bone: review of dose effects and mechanisms. Osteoporosis International 23, 1–16. [DOI] [PubMed] [Google Scholar]
- Maurel DB, Benaitreau D, Jaffré C, Toumi H, Portier H, Uzbekov R, Pallu S (2014). Effect of the alcohol consumption on osteocyte cell processes: a molecular imaging study. Journal of Cellular and Molecular Medicine 18, 1680–1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurel DB, Jaffre C, Rochefort GY, Aveline PC, Boisseau N, Uzbekov R, Benhamou CL (2011). Low bone accrual is associated with osteocyte apoptosis in alcohol-induced osteopenia. Bone 49, 543–552. [DOI] [PubMed] [Google Scholar]
- Maurel DB, Pallu S, Jaffré C, Fazzalari NL, Boisseau N, Uzbekov R, Rochefort GY (2012b). Osteocyte apoptosis and lipid infiltration as mechanisms of alcohol-induced bone loss. Alcohol and Alcoholism 47, 413–422. [DOI] [PubMed] [Google Scholar]
- McArthur GA, Foley KP, Fero ML, Walkley CR, Deans AJ, Roberts JM, & Eisenman RN (2002). MAD1 and p27(KIP1) cooperate to promote terminal differentiation of granulocytes and to inhibit Myc expression and cyclin E-CDK2 activity. Molecular and Cellular Biology 22, 3014–3023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKinstry WJ, Li CL, Rasko JE, Nicola NA, Johnson GR, & Metcalf D (1997). Cytokine receptor expression on hematopoietic stem and progenitor cells. Blood. 89, 65–71. [PubMed] [Google Scholar]
- Meagher RC, Sieber F, & Spivak JL (1982). Suppression of hematopoietic-progenitor-cell proliferation by ethanol and acetaldehyde. The New England Journal of Medicine 307, 845–849. [DOI] [PubMed] [Google Scholar]
- Medvedev AE, Piao W, Shoenfelt J, Rhee SH, Chen H, Basu S, Vogel SN (2007). Role of TLR4 tyrosine phosphorylation in signal transduction and endotoxin tolerance. Journal of Biological Chemistry 282, 16042–16053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta AJ (2016). Alcoholism and critical illness: A review. World Journal of Critical Care Medicine 5, 27–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meloche S & Pouysségur J (2007). The ERK1/2 mitogen-activated protein kinase pathway as a master regulator of the G1- to S-phase transition. Oncogene 26, 3227–3239. [DOI] [PubMed] [Google Scholar]
- Melvan JN, Siggins RW, Bagby GJ, Stanford WL, Welsh DA, Nelson S, & Zhang P (2011). Suppression of the stem cell antigen-1 response and granulocyte lineage expansion by alcohol during septicemia. Critical Care Medicine 39, 2121–2130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melvan JN, Siggins RW, Stanford WL, Porretta C, Nelson S, Bagby GJ, & Zhang P (2012). Alcohol impairs the myeloid proliferative response to bacteremia in mice by inhibiting the stem cell antigen-1/ERK pathway. The Journal of Immunology 188, 1961–1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merikangas KR & McClair VL (2012). Epidemiology of substance use disorders. Human Genetics 131, 779–789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mezey E & Holt PR (1971). The inhibitory effect of ethanol on retinol oxidation by human liver and cattle retina. Experimental and Molecular Pathology 15, 148–156 [DOI] [PubMed] [Google Scholar]
- Michot F & Gut J (1987). Alcohol-induced bone marrow damage. A bone marrow study in alcohol-dependent individuals. Acta Haematologica 78, 252–257. [DOI] [PubMed] [Google Scholar]
- Montalban-Bravo G & Garcia-Manero G (2018). Myelodysplastic syndromes: 2018 update on diagnosis, risk-stratification and management. American Journal of Hematology. 93, 129–147. [DOI] [PubMed] [Google Scholar]
- Moreau R & Rautou PE (2014). G-CSF therapy for severe alcoholic hepatitis: targeting liver regeneration or neutrophil function? The Am Journal of Gastroenterology 109, 1424–1426. [DOI] [PubMed] [Google Scholar]
- Morstyn G, Foote M, & Nelson S (1997). Clinical benefits of improving host defences with rHuG-CSF. Ciba Foundation symposium 204, 78–85. [DOI] [PubMed] [Google Scholar]
- Muramoto GG, Russell JL, Safi R, Salter AB, Himburg HA, Daher P, Chute JP (2010). Inhibition of aldehyde dehydrogenase expands hematopoietic stem cells with radioprotective capacity. Stem Cells 28, 523–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray L, Chen B, Galy A, Chen S, Tushinski R, Uchida N, Vesole D (1995). Enrichment of human hematopoietic stem cell activity in the CD34+Thy-1+Lin-subpopulation from mobilized peripheral blood. Blood 85, 368–378. [PubMed] [Google Scholar]
- Murray L, DiGiusto D, Chen B, Chen S, Combs J, Conti A, Tsukamoto A (1994). Analysis of human hematopoietic stem cell populations. Blood Cells 20, 364–369. [PubMed] [Google Scholar]
- Murray LJ, Tsukamoto A, & Hoffman R (1996). CD34+Thy-1+Lin- stem cells from mobilized peripheral blood. Leukemia & Lymphoma 22, 37–42. [DOI] [PubMed] [Google Scholar]
- Musher DM, Alexandraki I, Graviss EA, Yanbeiy N, Eid A, Inderias LA, Solomon E (2000). Bacteremic and nonbacteremic pneumococcal pneumonia. A prospective study. Medicine (Baltimore) 79, 210–221. [DOI] [PubMed] [Google Scholar]
- Nagai Y, Garrett KP, Ohta S, Bahrun U, Kouro T, Akira S, Kincade PW (2006). Toll-like receptors on hematopoietic progenitor cells stimulate innate immune system replenishment. Immunity 24, 801–812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakao S, Harada M, Kondo K, Mizushima N, & Matsuda T (1991). Reversible bone marrow hypoplasia induced by alcohol. American Journal of Hematology 37, 120–123. [DOI] [PubMed] [Google Scholar]
- Nash R, Krishnamoorthy M, Jenkins A, & Csete M (2012). Human embryonic stem cell model of ethanol-mediated early developmental toxicity. Experimental Neurology 234, 127–135. [DOI] [PubMed] [Google Scholar]
- Nelson S, Belknap SM, Carlson RW, Dale D, DeBoisblanc B, Farkas S, Wilson J (1998). A randomized controlled trial of filgrastim as an adjunct to antibiotics for treatment of hospitalized patients with community-acquired pneumonia. CAP Study Group. The Journal of Infectious Diseases 178, 1075–1080. [DOI] [PubMed] [Google Scholar]
- Nelson S, Summer W, Bagby G, Nakamura C, Stewart L, Lipscomb G, & Andresen J (1991). Granulocyte colony-stimulating factor enhances pulmonary host defenses in normal and ethanol-treated rats. The Journal of Infectious Diseases 164, 901–906. [DOI] [PubMed] [Google Scholar]
- Ng YY, Lin CC, Wu SC, Hwang SJ, Ho CH, Yang WC, & Lee S,D (2002). Leukopenia and thrombocytopenia in hemodialysis patients with hepatitis B or C virus infection and non-hemodialysis patients with hepatitis cirrhosis. Clinical Nephrology, 57, 289–295. [DOI] [PubMed] [Google Scholar]
- Niemelä O (2001). Distribution of ethanol-induced protein adducts in vivo: relationship to tissue injury. Free Radical Biology and Medicine 31, 1533–1538. [DOI] [PubMed] [Google Scholar]
- Nordemar H (1988). Alcohol and ultrastructural changes in the developing inner ear. An in vitro study. Acta Oto-Laryngologica Acta Otolaryngol 105, 75–81. [DOI] [PubMed] [Google Scholar]
- O’Brien JM Jr., Lu B, Ali NA, Martin GS, Aberegg SK, Douglas IS (2007). Alcohol dependence is independently associated with sepsis, septic shock, and hospital mortality among adult intensive care unit patients. Critical Care Medicine 35, 345–350. [DOI] [PubMed] [Google Scholar]
- Okada S, Nakauchi H, Nagayoshi K, Nishikawa S, Miura Y, & Suda T (1992). In vivo and in vitro stem cell function of c-kit- and Sca-1-positive murine hematopoietic cells. Blood 80, 3044–3050. [PubMed] [Google Scholar]
- Opdenakker G (2001). New insights in the regulation of leukocytosis and the role played by leukocytes in septic shock. Verhandelingen - Koninklijke Academie voor Geneeskunde van Belgie 63, 531–538. [PubMed] [Google Scholar]
- Osawa M, Hanada K, Hamada H, & Nakauchi H (1996a). Long-term lymphohematopoietic reconstitution by a single CD34-low/negative hematopoietic stem cell. Science 273, 242–245. [DOI] [PubMed] [Google Scholar]
- Osawa M, Nakamura K, Nishi N, Takahasi N, Tokuomoto Y, Nakauchi H (1996b) In vivo self-renewal of c-Kit+ Sca-1+ Lin(low/−) hemopoietic stem cells. The Journal of Immunology 156, 3207–3214. [PubMed] [Google Scholar]
- Pai M, Zacharoulis D, Milicevic MN, Helmy S, Jiao LR, Levicar N, … Habib NA (2008). Autologous infusion of expanded mobilized adult bone marrow-derived CD34+ cells into patients with alcoholic liver cirrhosis. The Americal Journal of Gastroenterology 103, 1952–1958 [DOI] [PubMed] [Google Scholar]
- Panasiuk A & Kemona A (2001). Bone marrow failure and hematological abnormalities in alcoholic liver cirrhosis. Roczniki Akademii Medycznej w Białymstoku 46:100–105. [PubMed] [Google Scholar]
- Patton JH Jr., Lyden SP, Ragsdale DN, Croce MA, Fabian TC, & Proctor KG (1998). Granulocyte colony-stimulating factor improves host defense to resuscitated shock and polymicrobial sepsis without provoking generalized neutrophil-mediated damage. J Trauma 44, 750–758. [DOI] [PubMed] [Google Scholar]
- Pauksen K, Elfman L, Ulfgren AK, & Venge P (1994). Serum levels of granulocyte-colony stimulating factor (G-CSF) in bacterial and viral infections, and in atypical pneumonia. British Journal of Haematology 88, 256–260. [DOI] [PubMed] [Google Scholar]
- Pekmezovic T, Suvajdzic Vukovic N, Kisic D, Grgurevic A, Bogdanovic A, Gotic M, Brkic N (2006). A case-control study of myelodysplastic syndromes in Belgrade (Serbia Montenegro). Annals of Hematology 85, 514–519. [DOI] [PubMed] [Google Scholar]
- Pelus LM, Bian H, King AG & Fukuda S (2004). Neutrophil-derived MMP-9 mediates synergistic mobilization of hematopoietic stem and progenitor cells by the combination of G-CSF and the chemokines GRObeta/CXCL2 and GRObetaT/CXCL2delta4. Blood 103, 110–119. [DOI] [PubMed] [Google Scholar]
- Perlino CA & Rimland D (1985). Alcoholism, leukopenia, and pneumococcal sepsis. American Review of Respiratory Disease 132, 757–760. [DOI] [PubMed] [Google Scholar]
- Pitrak DL (1997). Effects of granulocyte colony-stimulating factor and granulocyte-macrophage colony-stimulating factor on the bactericidal functions of neutrophils. Current Opinion in Hematology 4, 183–190. [DOI] [PubMed] [Google Scholar]
- Plurad D, Demetriades D, Gruzinski G, Preston C, Chan L, Gaspard D, Cryer G (2010). Motor vehicle crashes: the association of alcohol consumption with the type and severity of injuries and outcomes. The Journal of Emergency Medicine 38, 12–17. [DOI] [PubMed] [Google Scholar]
- Qamar AA, Grace ND, Groszmann RJ, Garcia-Tsao G, Bosch J, Burroughs AK, ... Rendon G (2009). Incidence, prevalence, and clinical significance of abnormal hematologic indices in compensated cirrhosis. Clinical Gastroenterology and Hepatology 7, 689–695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinton LJ, Nelson S, Boé DM, Zhang P, Zhong Q, Kolls JK, & Bagby GJ (2002). The granulocyte colony-stimulating factor response after intrapulmonary and systemic bacterial challenges. The Journal of Infectious Diseases 185, 1476–1482. [DOI] [PubMed] [Google Scholar]
- Raasch CE, Zhang P, Siggins RW 2nd., LaMotte LR, Nelson S, & Bagby GJ (2010). Acute alcohol intoxication impairs the hematopoietic precursor cell response to pneumococcal pneumonia. Alcoholism Clinical & Experimental Research 34, 2035–2043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajbanshi SL & Pandanaboina CS (2014). Alcohol stress on cardiac tissue - ameliorative effects of Thespesia populnea leaf extract. Journal of Cardiology 63, 449–459. [DOI] [PubMed] [Google Scholar]
- Rane SG, Mangan JK, Amanullah A, Wong BC, Vora RK, Liebermann DA, Reddy EP (2002). Activation of the Jak3 pathway is associated with granulocytic differentiation of myeloid precursor cells. Blood 100, 2753–2762. [DOI] [PubMed] [Google Scholar]
- Rapoport AP, Abboud CN, & DiPersio JF (1992). Granulocyte-macrophage colony-stimulating factor (GM-CSF) and granulocyte colony-stimulating factor (G-CSF): receptor biology, signal transduction, and neutrophil activation. Blood Reviews 6, 43–57. [DOI] [PubMed] [Google Scholar]
- Reinhard C, Shamoon B, Shyamala V, & Williams LT (1997). Tumor necrosis factor alpha-induced activation of c-jun N-terminal kinase is mediated by TRAF2. The EMBO Journal 16, 1080–1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolas L, Makhezer N, Hadjoudj S, El-Benna J, Djerdjouri B, Elkrief L, Périanin A (2013). Inhibition of mammalian target of rapamycin aggravates the respiratory burst defect of neutrophils from decompensated patients with cirrhosis. Hepatology 57, 1163–1171. [DOI] [PubMed] [Google Scholar]
- Roselle GA, Mendenhall CL, Muhleman AF, & Chedid A (1986). The ferret: a new model of oral ethanol injury involving the liver, bone marrow, and peripheral blood lymphocytes. Alcoholism Clinical & Experimental Research 10, 279–284. [DOI] [PubMed] [Google Scholar]
- Rota M, Porta L, Pelucchi C, Negri E, Bagnardi V, Bellocco R, La Vecchia C (2014). Alcohol drinking and risk of leukemia-a systematic review and meta-analysis of the dose-risk relation. Cancer Epidemiology 38, 339–345. [DOI] [PubMed] [Google Scholar]
- Ruiz M, Ewig S, Torres A, Arancibia F, Marco F, Mensa J, Martinez JA (1999). Severe community-acquired pneumonia. Risk factors and follow-up epidemiology. American Journal of Respiratory and Critical Care Medicine 160, 923–929. [DOI] [PubMed] [Google Scholar]
- Sabio G & Davis RJ (2014). TNF and MAP kinase signalling pathways. Seminars in Immunology 26, 237–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saitz R, Ghali WA, & Moskowitz MA (1997). The impact of alcohol-related diagnoses on pneumonia outcomes. Archives of Internal Medicine 157, 1446–1452. [PubMed] [Google Scholar]
- Sawamiphak S, Kontarakis Z, & Stainier DY (2014). Interferon gamma signaling positively regulates hematopoietic stem cell emergence. Developmental Cell 31, 640–653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuettpelz LG, Borgerding JN, Christopher MJ, Gopalan PK, Romine MP, Herman AC, Link DC (2014). G-CSF regulates hematopoietic stem cell activity, in part, through activation of Toll-like receptor signaling. Leukemia 28, 1851–1860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semerad CL, Liu F, Gregory AD, Stumpf K, & Link DC (2002). G-CSF is an essential regulator of neutrophil trafficking from the bone marrow to the blood. Immunity 17, 413–423. [DOI] [PubMed] [Google Scholar]
- Shahbazian LM, Quinton LJ, Bagby GJ, Nelson S, Wang G, & Zhang P (2004). Escherichia coli pneumonia enhances granulopoiesis and the mobilization of myeloid progenitor cells into the systemic circulation. Critical Care Medicine 32, 1740–1746. [DOI] [PubMed] [Google Scholar]
- Shaulian E & Karin M (2002) AP-1 as a regulator of cell life and death. Nature Cell Biology 4, E131–E136. [DOI] [PubMed] [Google Scholar]
- Shi X, Lin YP, Gao B, & Zhang P (2017a). Impairment of hematopoietic precursor cell activation during the granulopoietic response to bacteremia in mice with chronic-plus-binge alcohol administration. Infection and Immunity. 85, pii: e00369–17. doi: 10.1128/IAI.00369-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi X, Siggins RW, Stanford WL, Melvan JN, Basson MD, & Zhang P (2013). Toll-like receptor 4/stem cell antigen 1 signaling promotes hematopoietic precursor cell commitment to granulocyte development during the granulopoietic response to Escherichia coli bacteremia. Infection and Immunity 81, 2197–2205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi X, Wei S, Gao B, & Zhang P (2017b). Chornic-plus-binge alcohol administration activates caspase-1 activity and promotes bone marrow release of neutrophils into the systemic circulation. Alcoholism Clinical & Experimental Research 41, 208A. [Google Scholar]
- Shi X, Wei S, Simms KJ, Cumpston DN, Ewing TJ, & Zhang P (2018). Sonic hedgehog signaling regulates hematopoietic stem/progenitor cell activation during the granulopoietic response to systemic bacterial infection. Frontiers in Immunology 9:349, doi: 10.3389/fimmu.2018.00349. eCollection 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi X, Zhang P, Sempowski GD, & Shellito JE (2011), Thymopoietic and bone marrow response to murine Pneumocystis pneumonia. Infection and Immunity 79, 2031–2042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shizuru JA, Negrin RS, & Weissman IL (2005) Hematopoietic stem and progenitor cells: clinical and preclinical regeneration of the hematolymphoid system. Annual Review of Medicine 56, 509–38. [DOI] [PubMed] [Google Scholar]
- Shubin AV, Demidyuk IV, Komissarov AA, Rafieva LM, & Kostrov SV (2016). Cytoplasmic vacuolization in cell death and survival. Oncotarget 7, 55863–55889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siggins RW, Melvan JN, Welsh DA, Bagby GJ, Nelson S, & Zhang P (2011). Alcohol suppresses the granulopoietic response to pulmonary Streptococcus pneumoniae infection with enhancement of STAT3 signaling. The Journal of Immunology 186, 4306–4313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh V, Sharma AK, Narasimhan RL, Bhalla A, Sharma N, & Sharma R (2014). Granulocyte colony-stimulating factor in severe alcoholic hepatitis: a randomized pilot study. The American Journal of Gastroenterology 109, 1417–1123. [DOI] [PubMed] [Google Scholar]
- Sladek NE, Manthey CL, Maki PA, Zhang Z, & Landkamer GJ (1989). Xenobiotic oxidation catalyzed by aldehyde dehydrogenases. Drug Metabolism Reviews 20, 697–720. [DOI] [PubMed] [Google Scholar]
- Smith C, Gasparetto M, Jordan C, Pollyea DA, & Vasiliou V (2015). The effects of alcohol and aldehyde dehydrogenases on disorders of hematopoiesis. Advances in Experimental Medicine and Biology 815, 349–359. [DOI] [PubMed] [Google Scholar]
- Smith WS, Sumnicht GE, Sharpe RW, Samuelson D, & Millard FE (1995). Granulocyte colony-stimulating factor versus placebo in addition to penicillin G in a randomized blinded study of gram-negative pneumonia sepsis: analysis of survival and multisystem organ failure. Blood 86, 1301–1309. [PubMed] [Google Scholar]
- Song HY, Régnier CH, Kirschning CJ, Goeddel DV, & Rothe M (1997). Tumor necrosis factor (TNF)-mediated kinase cascades: bifurcation of nuclear factor-kappaB and c-jun N-terminal kinase (JNK/SAPK) pathways at TNF receptor-associated factor 2. Proceedings of the National Academy of Sciences of the United States of America 94, 9792–9796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonoda Y (2008). Immunophenotype and functional characteristics of human primitive CD34-negative hematopoietic stem cells: the significance of the intra-bone marrow injection. Journal of Autoimmunity 30, 136–44. [DOI] [PubMed] [Google Scholar]
- Spahr L, Lambert JF, Rubbia-Brandt L, Chalandon Y, Frossard JL, Giostra E, & Hadengue A (2008). Granulocyte-colony stimulating factor induces proliferation of hepatic progenitors in alcoholic steatohepatitis: a randomized trial. Hepatology 48, 221–229. [DOI] [PubMed] [Google Scholar]
- Spiekermann K, Roesler J, Emmendoerffer A, Elsner J, & Welte K (1997). Functional features of neutrophils induced by G-CSF and GM-CSF treatment: differential effects and clinical implications. Leukemia 11, 466–478. [DOI] [PubMed] [Google Scholar]
- Starckx S, Van den Steen PE, Wuyts A, Van Damme J, & Opdenakker G (2002). Neutrophil gelatinase B and chemokines in leukocytosis and stem cell mobilization. Leukemia & Lymphoma 43, 233–241. [DOI] [PubMed] [Google Scholar]
- Stefanová I, Horejsí V, Ansotegui IJ, Knapp W, & Stockinger H (1991). GPI-anchored cell-surface molecules complexed to protein tyrosine kinases. Science 254, 1016–1019. [DOI] [PubMed] [Google Scholar]
- Strom SS, Gu Y, Gruschkus SK, Pierce SA, & Estey EH (2005). Risk factors of myelodysplastic syndromes: a case-control study. Leukemia 19, 1912191–8. [DOI] [PubMed] [Google Scholar]
- Strom SS, Vélez-Bravo V, & Estey EH (2008). Epidemiology of myelodysplastic syndromes. Seminars in Hematology 45, 8–13. [DOI] [PubMed] [Google Scholar]
- Storms RW, Trujillo AP, Springer JB, Shah L, Colvin OM, Ludeman SM, & Smith C (1999). Isolation of primitive human hematopoietic progenitors on the basis of aldehyde dehydrogenase activity. Proceedings of the National Academy of Sciences of the United States of America 96, 9118–9123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strydom N & Rankin SM (2013). Regulation of circulating neutrophil numbers under homeostasis and in disease. Journal of Innate Immunity 5, 304–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Summers C, Rankin SM, Condliffe AM, Singh N, Peters AM, & Chilvers ER (2010). Neutrophil kinetics in health and disease. Trends in Immunology. 31, 318–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Syrovets T, Lunov O, & Simmet T (2012). Plasmin as a proinflammatory cell activator. Journal of Leukocyte Biology 92, 509–519. [DOI] [PubMed] [Google Scholar]
- Szabo G & Mandrekar P (2009). A recent perspective on alcohol, immunity, and host defense. Alcoholism Clinical & Experimental Research 33, 220–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takizawa H, Fritsch K, Kovtonyuk LV, Saito Y, Yakkala C, Jacobs K, Manz M,G (2017). Pathogen-Induced TLR4-TRIF Innate Immune Signaling in Hematopoietic Stem Cells Promotes Proliferation but Reduces Competitive Fitness. Cell Stem Cell 21, 225–240. [DOI] [PubMed] [Google Scholar]
- Terashima T, Wiggs B, English D, Hogg JC, & van Eeden SF (1996). Polymorphonuclear leukocyte transit times in bone marrow during streptococcal pneumonia. American Journal of Physiology 271, L587–L592. [DOI] [PubMed] [Google Scholar]
- Tian C & Zhang Y (2016). Purification of hematopoietic stem cells from bone marrow. Annals of Hematology 95, 543–547. [DOI] [PubMed] [Google Scholar]
- Tisman G & Herbert V (1973). In vitro myelosuppression and immunosuppression by ethanol. Journal of Clinical Investigation 52:1410–1414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Togbe D, Schnyder-Candrian S, Schnyder B, Doz E, Noulin N, Janot L, Moser R (2007). Toll-like receptor and tumour necrosis factor dependent endotoxin-induced acute lung injury. Internal Journal of Experimental Pathology 88, 387–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torii S, Yamamoto T, Tsuchiya Y, & Nishida E (2006). ERK MAP kinase in G cell cycle progression and cancer. Cancer Science 97, 697–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueda Y, Kondo M, & Kelsoe G (2005). Inflammation and the reciprocal production of granulocytes and lymphocytes in bone marrow. Journal of Experimental Medicine 201, 1771–1780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ugai T, Matsuo K, Sawada N, Iwasaki M, Yamaji T, Shimazu T, Tsugane S (2017). Smoking and alcohol and subsequent risk of myelodysplastic syndromes in Japan: the Japan Public Health Centre-based Prospective Study. British Journal of Haematology 178, 747–755. [DOI] [PubMed] [Google Scholar]
- Van Wassenhove LD, Mochly-Rosen D, & Weinberg KI (2016). Aldehyde dehydrogenase 2 in aplastic anemia, Fanconi anemia and hematopoietic stem cells. Molecular Genetics and Metabolism 119, 28–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vassalli G (2019) Aldehyde Dehydrogenases: Not Just Markers, but Functional Regulators of Stem Cells. Stem Cells International 2019:3904645, doi: 10.1155/2019/3904645. eCollection 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei Q & Frenette PS (2018). Niches for Hematopoietic Stem Cells and Their Progeny. Immunity 48, 632–648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werner J, Saghir M, Warshaw AL, Lewandrowski KB, Laposata M, Iozzo RV, Fernández-Del Castillo C (2002). Alcoholic pancreatitis in rats: injury from nonoxidative metabolites of ethanol. American Journal of Physiology Gastrointestinal and Liver Physiology 283, G65–G73. [DOI] [PubMed] [Google Scholar]
- Wezeman FH & Gong Z (2001). Bone marrow triglyceride accumulation and hormonal changes during long-term alcohol intake in male and female rats. Alcoholism Clinical & Experimental Research 25, 1515–1522. [DOI] [PubMed] [Google Scholar]
- Wezeman FH & Gong Z (2004). Adipogenic effect of alcohol on human bone marrow-derived mesenchymal stem cells. Alcoholism Clinical & Experimental Research 28, 1091–1101. [DOI] [PubMed] [Google Scholar]
- Wickramasinghe SN, Bond AN, Sloviter HA, & Saunders JE (1981). Metabolism of ethanol by human bone marrow cells. Acta Haematologica 66, 238–243. [DOI] [PubMed] [Google Scholar]
- Winston BW, Lange-Carter CA, Gardner AM, Johnson GL, & Riches DW (1995a). Tumor necrosis factor alpha rapidly activates the mitogen-activated protein kinase (MAPK) cascade in a MAPK kinase kinase-dependent, c-Raf-1-independent fashion in mouse macrophages. Proceedings of the National Academy of Sciences of the United States of America 92, 1614–1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winston BW, Remigio LK, & Riches DW (1995b). Preferential involvement of MEK1 in the tumor necrosis factor-alpha-induced activation of p42mapk/erk2 in mouse macrophages. Journal of Biological Chemistry 270, 27391–27394. [DOI] [PubMed] [Google Scholar]
- Winterbauer RH, Bedon GA, & Ball WC Jr. (1969). Recurrent pneumonia. Predisposing illness and clinical patterns in 158 patients. Annals of Internal Medicine 70, 689–700. [DOI] [PubMed] [Google Scholar]
- Wu D & Cederbaum AI (2003). Alcohol, oxidative stress, and free radical damage. Alcohol Research & Health 27, 277–284. [PMC free article] [PubMed] [Google Scholar]
- Wu D, Zhai Q, & Shi X (2006). Alcohol-induced oxidative stress and cell responses. Journal of Gastroenterology and Hepatology 21, S26–S29. [DOI] [PubMed] [Google Scholar]
- Xu MJ, Cai Y, Wang H, Altamirano J, Chang B, Bertola A Gao B (2015). Fat-Specific Protein 27/CIDEC Promotes Development of Alcoholic Steatohepatitis in Mice and Humans. Gastroenterology 149, 1030–1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu DS, Jennett RB, Smith SL, Sorrell MF, & Tuma DJ (1989). Covalent interactions of acetaldehyde with the actin/microfilament system. Alcohol and Alcoholism 24, 281–289. [DOI] [PubMed] [Google Scholar]
- Yannaki E, Athanasiou E, Xagorari A, Constantinou V, Batsis I, Kaloyannidis P, … Fassas A (2005). G-CSF-primed hematopoietic stem cells or G-CSF per se accelerate recovery and improve survival after liver injury, predominantly by promoting endogenous repair programs. Experimental Hematology 33, 108–119. [DOI] [PubMed] [Google Scholar]
- Yeung KY, Klug PP, & Lessin LS (1988). Alcohol-induced vacuolization in bone marrow cells: ultrastructure and mechanism of formation. Blood Cells 13, 487–502. [PubMed] [Google Scholar]
- Yu XF & Han ZC (2006). Matrix metalloproteinases in bone marrow: roles of gelatinases in physiological hematopoiesis and hematopoietic malignancies. Histology and Histopathology 21, 519–531. [DOI] [PubMed] [Google Scholar]
- Yu HS, Oyama T, Isse T, Kitagawa K, Pham TT, Tanaka M, & Kawamoto T (2010). Formation of acetaldehyde-derived DNA adducts due to alcohol exposure. Chemico-Biological Interactions 188, 367–375. [DOI] [PubMed] [Google Scholar]
- Yuan Z, Li Q, Luo S, Liu Z, Luo D, Zhang B, … Xiao, J. (2016). PPARγ and Wnt Signaling in Adipogenic and Osteogenic Differentiation of Mesenchymal Stem Cells. Current Stem Cell Research & Therapy 11, 216–225. [DOI] [PubMed] [Google Scholar]
- Zhang H, Nguyen-Jackson H, Panopoulos AD, Li HS, Murray PJ, & Watowich SS (2010). STAT3 controls myeloid progenitor growth during emergency granulopoiesis. Blood 116, 2462–2471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang P, Bagby GJ, Happel KI, Raasch CE, & Nelson S (2008). Alcohol abuse, immunosuppression, and pulmonary infection. Current Drug Abuse Reviews 1, 56–67. [DOI] [PubMed] [Google Scholar]
- Zhang P, Bagby GJ, Happel KI, Summer WR, & Nelson S (2002). Pulmonary host defenses and alcohol. Frontiers in Bioscience 7, d1314–d1330. [DOI] [PubMed] [Google Scholar]
- Zhang P, Nelson S, Bagby GJ, Siggins R 2nd., Shellito JE, & Welsh DA (2008). The lineage-c-Kit+Sca-1+ cell response to Escherichia coli bacteremia in Balb/c mice. Stem Cells 26, 1778–1786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang P, Quinton LJ, Gamble L, Bagby GJ, Summer WR, & Nelson S (2005). The granulopoietic cytokine response and enhancement of granulopoiesis in mice during endotoxemia. Shock 23, 344–352. [DOI] [PubMed] [Google Scholar]
- Zhang P, Welsh DA, Siggins RW 2nd., Bagby GJ, Raasch CE, Happel KI, & Nelson S (2009) Acute alcohol intoxication inhibits the lineage- c-kit+ Sca-1+ cell response to Escherichia coli bacteremia. The Journal of Immunology 182, 1568–1576. [DOI] [PMC free article] [PubMed] [Google Scholar]