Abstract
Objective:
To compare the transcriptome of articular cartilage from knees with meniscus tears to knees with end-stage osteoarthritis (OA).
Design:
Articular cartilage was collected from the non-weight bearing medial intercondylar notch of knees undergoing arthroscopic partial meniscectomy (APM; N=10, 49.7±10.8 years, 70% females) for isolated medial meniscus tears and knees undergoing total knee arthroplasty (TKA; N=10, 66.0±7.0 years, 50% females) due to end-stage OA. RNA preparation was subjected to SurePrint-G3 human 8×60K RNA microarrays to probe differentially expressed (DE) transcripts followed by computational exploration of underlying biological processes. Real-time PCR amplification was performed on selected transcripts to validate microarray data.
Results:
81 transcripts were significantly (FDR≤0.05) differentially expressed (45 elevated, 36 repressed) between APM and TKA samples (≥2-fold). Among these, CFD, CSNIS1, TSPAN11, CSF1R and CD14 were elevated in the TKA group, while CHI3L2, HILPDA, COL3A1, COL27A1 and FGF2 were highly expressed in APM samples. A few long intergenic non-coding RNAs (lincRNAs), small nuclear RNAs (snoRNAs) and antisense RNAs were also differentially expressed between the two groups. Transcripts up-regulated in TKA cartilage were enriched for protein localization and activation, chemical stimulus, immune response, and toll-like receptor signaling pathway. Transcripts up-regulated in APM cartilage were enriched for mesenchymal cell apoptosis, epithelial morphogenesis, canonical glycolysis, extracellular matrix organization, cartilage development, and glucose catabolic process.
Conclusions:
This study suggests that APM and TKA cartilage express distinct sets of OA transcripts. The gene profile in cartilage from TKA knees is an end-stage OA whereas APM knees are clearly earlier in the degenerative process.
Keywords: Partial meniscectomy, Microarrays, Knee arthroplasty, lincRNAs, extracellular matrix organization, immune response
Introduction
Osteoarthritis (OA) is the most common form of arthritis and is characterized primarily by the degeneration and loss of articular cartilage. OA is considered a heterogeneous disease with a variety of pathogenic factors, all of which result in similar patterns of cartilage degeneration. Meniscal tears are likely to be an important early event in the initiation and propagation of degenerative changes in the knee[1] and are known to predispose about 50% of individuals to develop knee OA within 10 to 20 years after injury[2, 3]. However, the molecular basis for the initiation and progression of OA following meniscus injury still remains largely unknown. While the relationship between meniscus lesions and OA is complex, it is thought that meniscus tears in healthy knee may lead to OA and OA can also lead to meniscus tears that may exacerbate the OA process[3]. Yet, little is known about the biological basis of this relationship. Recently, we have reported that a number of transcripts (including CSN1S1, COL10A1, WIF1, SPARCL1, POSTN and VEGFA) with potential relevance to the pathogenesis of OA are differentially expressed in OA and non-OA meniscus providing some molecular clues to the relationship between the meniscus and OA [4].
Little data is available on cartilage from knees with meniscus tears but no OA compared to knees with end-stage OA. Gene expression profiles of human cartilage procured from patients undergoing total knee arthroplasty (TKA) and injured meniscus procured from patients during arthroscopic partial meniscectomy (APM) identified transcripts differentiating between hyaline and fibrocartilage tissues[5]. However, we are not aware of any existing study that has directly compared gene expression in the cartilage from APM knees and TKA knees.
A few studies compared degenerated cartilage with the “normal” either taken from within the same diseased (e.g. arthritis, anteromedial gonarthrosis) joints[6–9], normal joints[10, 11] or from trauma patients[12] providing important insights into the changes in the gene expression pattern. However, “normal” cartilage in most of these studies was largely excised from areas of grossly intact cartilage in an end-stage degenerated joint, which was exposed to the inflammatory and catabolic mediators found in OA synovium and synovial-fluid. In addition, normal-looking and degenerated cartilage is not always taken from the same location in the joint, a potentially important confounder of any comparison. We have previously reported that cartilage from some patients with a meniscus tear, but no OA, exhibits a pre-OA phenotype[13] compared to data coming from OA and non-OA cartilage[10] as well as from genome-wide association studies[14].
Here, we aim to investigate the molecular differences between cartilage from knees undergoing APM and TKA. We hypothesize that cartilage from knees undergoing APM has a degenerative phenotype earlier in the disease process than end-stage OA based on transcript-signatures and exhibits a repair-response while cartilage from OA patients demonstrates a distinct and more advanced degenerative phenotype. Comparing the molecular phenotypes of cartilage from knees undergoing APM and TKA is an important step to elucidate how the molecular biology of cartilage changes after meniscus injury along the pathway to OA. We believe that transcriptome differences between these types of cartilage may shed light on the very early response of the knee joint to meniscus injury.
Methods
Patients
The Institutional Review Board at the study institution approved the protocol. Eligible patients undergoing APM or TKA provided written and signed informed consent prior to surgery. The operating surgeon (RHB or RWW) collected the cartilage samples during the procedure. Male and female patients of any age and body-mass-index (BMI) were included (Table-1). All patients had preoperative X-rays which were reviewed and assessed using the Kellgren-Lawrence (K-L) scale for OA.
Table 1:
Characteristics of study patients
| Sample ID | Group | Age (years) | Sex | BMI (kg/m2) | Side | Surgery | K-L score | Onset of symptoms | Tear pattern | Smoking | Diabetes | Chondrosis |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| P4-001 | APM | 37 | Female | 21.92 | Left | PMM | 0 | Nonspecific | Complex | No | No | No |
| P4-002 | APM | 45 | Male | 28.84 | Right | PMM | 0 | Nonspecific | Complex | No | No | No |
| P4-003 | APM | 62 | Female | 27.76 | Left | PMM | 0 | Nonspecific | Complex | No | No | No |
| P4-004$ | APM | 58 | Female | 20.52 | Left | PMM/Ch | 0 | Injury | Complex | No | No | Yes |
| P4-005$ | APM | 31 | Female | 24.27 | Left | PMM | 0 | Nonspecific | Complex | No | No | No |
| P4-007 | APM | 53 | Male | 26.62 | Left | PMM/Ch | 0 | Acute | Complex | No | No | Yes |
| P4-008$ | APM | 50 | Male | 28.48 | Right | PMM/Ch | 0 | Nonspecific | Complex | No | No | No |
| P4-009$ | APM | 65 | Female | 27.07 | Left | PMM | 0 | Injury | Complex | No | No | No |
| P4-010* | APM | 53 | Male | 31.99 | Right | PMM | 0 | Injury | Oblique | No | Yes | No |
| P4-011$ | APM | 43 | Male | 34.43 | Right | PMM | 0 | Acute | Radial | No | No | No |
| P4-012* | APM | 40 | Male | 24.54 | Left | PMM | 0 | Acute | Oblique | No | No | No |
| P4-013$ | APM | 53 | Male | 26.32 | Left | PMM | 0 | Injury | Complex | No | No | No |
| P4-102$ | TKA | 57 | Male | 31.80 | Left | TKA | 3 | - | - | No | No | - |
| P4-103$ | TKA | 64 | Female | 28.72 | Right | TKA | 3 | - | - | Yes | No | - |
| P4-104* | TKA | 53 | Female | 30.04 | Left | TKA | 3 | - | - | No | No | - |
| P4-105$ | TKA | 62 | Female | 46.51 | Right | TKA | 4 | - | - | No | Yes | - |
| P4-106 | TKA | 80 | Male | 30.42 | Right | TKA | 4 | - | - | No | No | - |
| P4-107 | TKA | 67 | Female | 38.62 | Right | TKA | 4 | - | - | No | No | - |
| P4-108* | TKA | 70 | Female | 38.47 | Left | TKA | 4 | - | - | No | No | - |
| P4-109$ | TKA | 64 | Female | 44.62 | Right | TKA | 4 | - | - | No | No | - |
| P4-111 | TKA | 61 | Male | 31.46 | Left | TKA | 3 | - | - | No | No | - |
| P4-112 | TKA | 62 | Female | 46.05 | Left | TKA | 4 | - | - | No | No | - |
| P4-113$ | TKA | 79 | Female | 31.28 | Left | TKA | 4 | - | - | No | No | - |
| P4-114$ | TKA | 64 | Female | 37.29 | Left | TKA | 4 | - | - | No | No | - |
APM = arthroscopic partial meniscectomy; TKA = total knee arthroplasty; BMI = body mass index; PMM = partial medial meniscectomy; Ch = chondroplasty; K-L = Kellgren-Lawrence;
excluded from microarray analysis;
used for real-time polymerase chain reaction
Patients undergoing APM had a tear in the medial meniscus posterior horn and were clinically indicated for surgical intervention based on history and physical examination. We noted whether each patient had a specific trauma or an acute onset of symptoms without a specific injury. All APM patients had preoperative bilateral Rosenberg weight-bearing views, bilateral Merchant views and a lateral view of the involved knee as well as an MRI of the knee. The X-rays and MRIs were reviewed as part of routine clinical practice by a musculoskeletal radiologist and an orthopedic surgeon. None of the patients had any degenerative changes on X-ray or any evidence for chondral damage or other injury on MRI prior to their surgery. Patients who were considered for inclusion in the study based on pre-operative imaging were excluded if there was any chondral damage in the tibiofemoral compartments or more than focal grade 2 changes in the patellofemoral joint at the time of APM. The pattern of each meniscus tear was also recorded at the time of surgery. TKA patients met the American College of Rheumatology criterion for knee OA, had moderate to severe pain and functional limitations, and had failed non-operative measures.
Cartilage sampling
The techniques for collecting articular cartilage were the same as described in a previous study [13]. During APM, a ring curette was used to collect a small fragment of cartilage from a non-weight bearing portion of the medial intercondylar-notch. During TKA, a size-matched fragment of cartilage was collected from a similar area of the medial intercondylar-notch. The samples were limited to articular cartilage without collecting any subchondral bone. Immediately after harvest, the cartilage was placed in a tube of RNAlater solution (Thermo-Fisher-Scientific) for quick transport to the laboratory.
RNA preparation
The RNA was prepared using a previously described technique [4]. Briefly, isolation was performed using both TRIzol:Chloroform method and Minispin columns (Qiagen). The cartilage samples were immersed in liquid nitrogen and homogenized using Mikro-dismembrator (B. Braun, Biotech International). One milliliter of TRIzol-Reagent (Invitrogen) was added before transferring the solution to a microfuge tube for incubation at room-temperature for 5 minutes. After the addition of 200-μL chloroform, the solution was mixed vigorously and incubated at room-temperature for 5 additional minutes before being transferred to a phase-lock gel tube. Once the gel collected at the bottom, the tube was centrifuged for 15 minutes at 12000 rpm and 4°C. The upper aqueous-phase containing RNA was decanted into a clean microfuge tube. Same volume of 70% RNase-free ethyl-alcohol was mixed before applying to RNeasy spin-columns (Qiagen) and centrifuged for 15 seconds at 8000 rpm at room-temperature. The flow-through was decanted and prior to washing the column four-times with the supplied wash buffers (each buffer twice). The resulting RNA was eluted in 30-μL of RNase-free water. Nanodrop ND-1000 (Thermo-Fisher-Scientific) was used to determining the RNA concentrations and the quality of the total RNA samples was assessed by Agilent 2100 Bioanalyzer (Agilent-Technologies).with the using the RNA integrity numbering system.
Microarray hybridization
Microarray hybridization procedure was essentially the same as we reported previously[4]. Briefly, A Sigma WTA2 kit (Sigma-Aldrich) was used to amplify 20-ng of RNA and the resulting cDNA was labelled with the Kreatech-ULS RNA-labeling kit (Kreatech-Diagnostics). Three milligrams of cDNA was mixed with Kreatech-labeling buffer and Kreatech cyanine-5 (Cy5)/DY-ULS, incubated for 15 minutes in the dark at 85°C, and then quenched on ice for 3 minutes. Columns (Qiagen) were used to purify labelled cDNA. The amplified RNA was suspended in Agilent Gene-Expression buffer, blocking-agent, and Kreablock (Agilent) for hybridization. The hybridization solution was applied to SurePrint-G3 Human 8×60K microarrays (Agilent) and incubated with streptavidin Cy5 for 20 hours at 65°C. Washing procedures were performed according to the Agilent Gene-Expression protocol. An Agilent C-class Microarray scanner was used to laser-scan the hybridized arrays to detect Cy5 fluorescence. Fluorescent Cy5 signals were recorded in focal units for each spotted probe bound to the array surface. Each spot on the array consists of oligos made up of a known 60 base-pair sequence for a gene of interest and the intensity in focal units corresponded to the number of Cy5-labeled cDNA fragments hybridized to the spot of oligos bound to the surface of the array. The focal units were interpreted as relative measures of expression as the intensity represents the relative-abundance of a given gene in the sample. Feature-Extraction software (Agilent) was used to perform gridding and image analysis. RNA samples were randomized on the microarrays to avoid expression/detection bias.
Data mining and statistical analysis
The function read.maimages was used to import the Agilent Feature-Extraction data into the R/Bioconductor package Limma. The intensity measures were transformed to the log2 scale, background-subtracted, filtered for reliable signals above background (10% greater than the 95th percentile of negative control probes), and then quantile-normalized to account for array-to-array variance. The probes were then filtered again to only include probe ID’s that matched known and current Ensembl gene ID’s, limiting analysis to probes. An additive generalized-linear-model including coefficients accounting for patient status, sex, BMI, age, and two latent unknown effects as derived by the R-package SVA was then created.
A Spearman correlation-matrix and multidimensional-scaling plots were used to assess the sample performance. Gene/transcript performance was assessed with plots of residual standard-deviation of every gene to their average log-count with a robustly fitted trend-line of the residuals. Gene-level analysis was performed assuming that log2 focal unit intensity was an independent observation for that gene and that the data was nearly Gaussian in distribution. Therefore, a generalized-linear-model (Limma GLM) fitted to the two conditions of interest along with blocking factors for other known or confounding sources of variation yielded genes whose low P-values correspond to the difference in variance within versus across conditions and low uncertainty under the null-hypothesis that there was no difference in relative-expression between the two groups due to TKA or APM alone. We calculated post-hoc statistical power on specimens using the R/Bioconductor package sizepower and found that we had a final statistical power of 94%. In the case of whole transcriptome analysis, statistical power of a study by itself does not serve as an accurate means of determining the uncertainty of the results. Instead, we controlled for false-positives by limiting our focus to genes whose Benjamini-Hochberg multiple testing correction false-discovery-rate (FDR) adjusted P≤0.05 and whose reported fold-changes were in excess of an absolute value of 1.5. Data were shown in fold-change for each comparison with 95% confidence-interval (CI, lower limit to upper limit).
All microarrays were performed by the Genome Technology Access Center at the study institution. The raw array data are accessible through the accession number GSE117999 at http://www.ncbi.nlm.nih.gov/proiects/geo). Based on our prior experience with Agilent microarray studies where RNA-mass and quality are not limited, the correlation of technical replicates for high quality samples was typically >95% and mirrors the reported technical reproducibility described in Agilent’s technical literature and of that reported by MAQC consortium[15].
Gene-ontology (GO) analysis
The R/Bioconductor packages GAGE and Pathview were used to elucidate global transcriptomic changes in known GO from the biological interpretation of the large-set of features found in the Limma results. GAGE measures perturbations in GO terms based on changes in observed log2 fold-changes for the genes within that term versus the background log2 fold-changes observed across features not contained in the respective term as reported by Limma. For GO terms with a statistical significance of P<0.05, heatmaps were automatically generated for each respective term to show how genes co-vary/co-express across the term in relation to a given biological-process or molecular-function.
Real-time PCR
Real time PCR was used to validate the expression pattern of 12 transcripts (Table-2), including 6 randomly selected patients in the APM group and 6 randomly selected patients in the TKA group. Briefly, 160-ng of RNA was subjected to DNase-I (Life-Technologies) to remove genomic DNA contamination. Random-hexamers and the SuperScript-II First-Strand Synthesis System (Invitrogen) were used to synthesize first-strand cDNA. PCR was performed on a 7500 Fast Real-Time PCR-System (Applied-Biosystems). PPIA acted as the housekeeping gene for normalization of fluorescence threshold (Ct) values of target genes using SYBR Green PCR Master-Mix (Applied-Biosystems). This housekeeping gene demonstrated stable expression with negligible variation across samples and has been used as a housekeeping gene in cartilage for both OA-related and non-OA related studies and exhibited no differential-expression in impacted and control cartilage specimens[16]. Amplification steps were essentially the same as reported previously[4]. Ct values were normalized to PPIA for each sample (ΔCt) and then log2 transformed. GraphPad-Prism (GraphPad Software) was used to detect the significant deference via the Mann-Whitney U test.
Table 2:
Primers used for quantitative PCR validation
| Gene symbol | Accession # | Gene name | Forward primer (5’ - 3’) | Location | Reverse primer (5’ - 3’) | Location | Amplicon size (base-pair) | ||
|---|---|---|---|---|---|---|---|---|---|
| from | to | from | to | ||||||
| CFD | NM_001928 | Complement factor D | CTCCAAGCGCCTGTACGAC | 301 | 319 | CAGTGTGGCCTTCTCCGAC | 409 | 391 | 109 |
| CSN1S1 | NM_001025104.1 | Casein alpha S1 | CTCACCTGTCTTGTGGCTGT | 65 | 84 | GGCTCACTGCTCTCTGATGG | 156 | 137 | 92 |
| TSPAN11 | NM_001080509.2 | Tetraspanin 11 | TACTTGTCATGGTGACCGGC | 319 | 338 | ATGACGAGCAACAGGCAGAA | 412 | 393 | 94 |
| CD14 | NM_000591.3 | CD14 molecule | AGAACCTTGTGAGCTGGACG | 445 | 464 | TGCAGACACACACTGGAAGG | 541 | 522 | 97 |
| HOXC8 | NM_022658.3 | Homeobox C8 | CCTCCGCCAACACTAACAGT | 526 | 545 | GCTGTAAGTTTGCCGTCCAC | 650 | 631 | 125 |
| TMEM176A | NM_018487.2 | Transmembrane protein 176A | GCTCGAGTGACTGGAACACT | 542 | 561 | CATGGCCTGAAGGGTTCTGA | 660 | 641 | 119 |
| CHI3L2 | NM_004000.2 | Chitinase 3 like 2 | GCTGGACCCATCACAGAGTC | 945 | 964 | GGAACCTGCTGATCCTGGAG | 1045 | 1026 | 101 |
| HILPDA | NM_013332.3 | Hypoxia inducible lipid droplet associated | TGCAGAGGAGTAGGGTCCTT | 237 | 256 | AGGCGATGGGCTCTCTAGTA | 370 | 351 | 134 |
| COL3A1 | NM_000090.3 | Collagen type III alpha 1 chain | TCGAGGCAGTGATGGTCAAC | 1092 | 1111 | TTTGAACCAGGAGACCCTGC | 1202 | 1183 | 111 |
| COL27A1 | NM_032888.3 | Collagen type XXVII alpha 1 chain | TGGACAGACGTGTCTCAAGC | 5587 | 5606 | AGTGGATGGTGATGTGCTGG | 5705 | 5686 | 119 |
| FGF2 | NM_002006.5 | Fibroblast growth factor 2 | GTGCTAACCGTTACCTGGCT | 700 | 719 | TCAGTGCCACATACCAACTG | 849 | 830 | 150 |
| COL5A1 | NM_000093.4 | Collagen type V alpha 1 chain | GACACCGCAGTACCTGACAC | 1116 | 1135 | GGGCTCCTTCCCTAGGTCTT | 1253 | 1234 | 138 |
| PPIA | NM_021130.4 | Peptidylprolyl isomerase A | TCTGCACTGCCAAGACTGAG | 430 | 449 | TGGTCTTGCCATTCCTGGAC | 546 | 527 | 117 |
Results
Study patients
Our initial query with principal-component-analysis (PCA) indicated that two patients from APM group (P4-010, P4-012) and two patients from TKA group (P4-104, P4-108) were extreme outliers as they were far from the cluster of majority and were therefore excluded (Table-1). These samples also had low Spearman-correlations or confounding deviations in hierarchical-clustering analysis. The final study cohort included 10 patients without radiographic OA (K-L Score=0) undergoing APM and 10 patients with radiographic OA (K-L score=3-4) undergoing TKA. None of the APM patients had any chondrosis in the medial- or lateral-compartment at the time of surgery. Only 3/10 APM patients had any chondrosis in the knee at the time of surgery, always limited to Grade-2 changes involving the patellofemoral-compartment. Age [mean±standard-deviation years] was significantly (P=0.001) different between APM (49.7±10.8) and TKA (66.0±7.6) groups. BMI [mean (kg/m2) ± standard-deviation] was significantly (P=0.001) higher in TKA (36.4±7.0) than APM (26.6±3.9) group. Similarly, K-L score (mean±standard-deviation) was significantly (P<0.0001) higher in TKA (3.7±0.5) than APM (0.00±0.00) group. The distribution by sex (70% female TKA cohort, 50% female APM cohort) was not (P=0.650). The clinical history and tear pattern varied for the APM cohort, with the majority of patients lacking a specific injury or acute onset of symptoms and complex tears were most common.
Quantitative transcriptomic differences
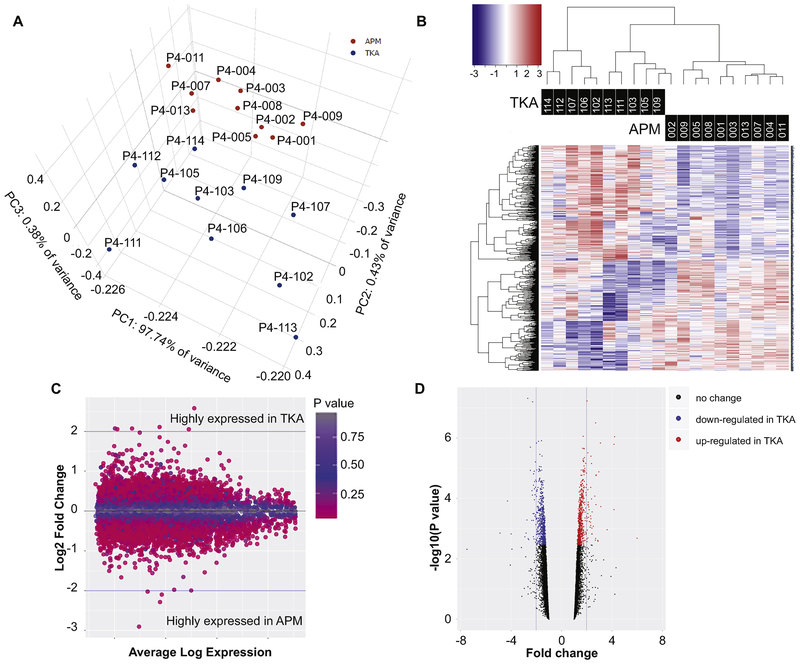
PCA revealed that patients were clustered into two distinct clusters: one cluster comprised of samples from APM patients and the other cluster had samples from TKA patients based on PC1 (Fig-1A). Patients were clustered by condition based on gene expression signatures on hierarchical-clustering heatmaps (Fig-1B). Expression fold-change and averaged-expression level of differentially-expressed transcripts are shown as MA-plot (Fig-1C). Furthermore, we displayed the differentially-expressed transcripts concurrently through volcano-plots (Fig-1C) to indicate the trend of transcription expression in magnitude (fold-change) and significance (P-value). The transcripts shown in the upper right (up-regulated) and left (down-regulated) corners are statistically most significant (lowest P-value) and are greatest in magnitude (fold-change). Both the MA- and Volcano-plots serve to illustrate the relationship of the statistical uncertainty and the observed changes in expression where data points of individual genes on the extremes (y-axis for the MA-plot and x-axis for the Volcano-plot) represent genes with the greatest statistical certainty and highest practical/biological-relevance for gene expression changes between the two cohorts. The GAGE GO analysis where we measured the changes in log2 fold-change expression within well characterized gene sets versus the background of all other genes tested serves as our interpretation of biological relevance.
Figure 1.
A). Principal components analysis of 10 APM and 10 TKA samples showed clear distinction between two groups. Each dot represents one patient. B). Normalized gene expression level (z-score) of DE transcripts between APM and TKA patients were used to generate heatmaps. Color bar above heatmaps indicates patients’ metadata in which patients were mainly clustered. Based on DE transcripts, TKA and APM samples were distinctly separated. C). Expression fold-change (FC) and averaged expression level of DE transcripts between APM and TKA patients are shown in the form of an MA plot. D). The differentially expressed transcripts at ≥ 2 fold-change are depicted in volcano plots to pictorially represent trend of expression by P-value (y-axes) and fold-change (x-axes).
Among all human RNAs spotted on the microarray chip (40,146), Limma analysis generated a list of 1301 (3.24%) transcripts differentially-expressed between APM and TKA at FDR≤0.05 regardless of fold-change. While these differentially-expressed genes were largely protein-coding, eight lincRNAs (long-intergenic non-coding RNAs, a class of long non-coding RNAs that do not overlap with the bodies of known protein-coding genes), 10 snoRNAs (small-nuclear RNAs, a class of small non-protein coding RNA molecules that primarily guide site-specific chemical modifications of other RNAs) and three antisense RNAs (is a single-stranded RNA that is complementary to a protein coding mRNA with which it hybridizes, and thereby blocks its translation into protein) were also detected as differentially-expressed.
Differentially-expressed transcripts (mRNAs)
582 protein-coding transcripts showed at least ≥1.5-fold magnitude of difference between APM and TKA cartilage at FDR≤0.05 (Supplemental-Table-1). At 2-fold, 81 transcripts (45 elevated in TKA, 36 elevated in APM) were differentially-expressed (Table-3). Notably, CFD (5.99-fold [95% CI, 2.15— 16.67], FDR=0.040), CSN1S1 (4.21-fold [95% CI, 2.81—6.33], FDR=0.003), TSPAN11 (4.16-fold [95% CI, 2.74—6.34], FDR=0.003), CSF1R (3.62-fold [95% CI, 1.83—7.16], FDR=0.029) and CD14 (2.96-fold [95% CI, 2.02—4.34], FDR=0.005) were highly elevated in TKA group while CHI3L2 (−4.87-fold [95% CI, −3.53 — −1.04], FDR=0.033), HILPDA (−3.99-fold [95% CI, −3.10 — −0.89], FDR=0.034), COL3A1 (−2.91-fold [95% CI, −2.42 — −0.66], FDR=0.038), COL27A1 (−2.81-fold [95% CI, −2.31 — −0.67], FDR=0.033) and FGF2 (−2.60-fold [95% CI, −2.19 — −0.57], FDR=0.042) were repressed in TKA.
Table 3:
Gene transcripts differentially expressed between AMP and TKA cartilage*
| Gene symbol | Gene name | P value | FDR | Fold change | 95% CI | |
|---|---|---|---|---|---|---|
| Lower limit | Upper limit | |||||
| Gene transcripts elevated in TKA cartilage | ||||||
| CFD | complement factor D | 0.002 | 0.040 | 5.99 | 2.15 | 16.67 |
| CSN1S1 | casein alpha s1 | <0.001 | 0.003 | 4.21 | 2.81 | 6.33 |
| TSPAN11 | tetraspanin 11 | <0.001 | 0.003 | 4.16 | 2.74 | 6.34 |
| CSF1R | colony stimulating factor 1 receptor | 0.001 | 0.029 | 3.62 | 1.83 | 7.16 |
| C1QB | complement C1q B chain | <0.001 | 0.014 | 3.11 | 1.88 | 5.15 |
| INCENP | inner centromere protein | <0.001 | 0.003 | 3.09 | 2.21 | 4.32 |
| CD14 | CD14 molecule | <0.001 | 0.005 | 2.96 | 2.02 | 4.34 |
| GFPT2 | glutamine-fructose-6-phosphate transaminase 2 | 0.003 | 0.044 | 2.88 | 1.54 | 5.40 |
| TPPP | tubulin polymerization promoting protein | 0.002 | 0.043 | 2.87 | 1.55 | 5.35 |
| PPP6R1 | protein phosphatase 6 regulatory subunit 1 | <0.001 | 0.006 | 2.74 | 1.90 | 3.95 |
| HOXC8 | homeobox C8 | <0.001 | 0.013 | 2.74 | 1.76 | 4.25 |
| C1orf61 | chromosome 1 open reading frame 61 | <0.001 | 0.006 | 2.74 | 1.90 | 3.95 |
| LEO1 | LEO1 homolog, Paf1/RNA polymerase II complex component | <0.001 | 0.001 | 2.70 | 2.06 | 3.52 |
| NCF1 | neutrophil cytosolic factor 1 | 0.002 | 0.040 | 2.69 | 1.52 | 4.76 |
| CYP1B1 | cytochrome P450 family 1 subfamily B member 1 | 0.002 | 0.043 | 2.68 | 1.50 | 4.79 |
| C11orf96 | chromosome 11 open reading frame 96 | 0.001 | 0.025 | 2.52 | 1.56 | 4.08 |
| TMEM176A | transmembrane protein 176A | <0.001 | 0.016 | 2.43 | 1.61 | 3.67 |
| UHRF2 | ubiquitin like with PHD and ring finger domains 2 | 0.001 | 0.034 | 2.43 | 1.48 | 3.97 |
| DEFB1 | defensin beta 1 | 0.001 | 0.027 | 2.40 | 1.51 | 3.83 |
| CRIP1 | cysteine rich protein 1 | 0.001 | 0.034 | 2.39 | 1.47 | 3.90 |
| Gene transcripts elevated in APM cartilage | ||||||
| CHI3L2 | chitinase 3 like 2 | 0.001 | 0.033 | −4.87 | −3.53 | −1.04 |
| HILPDA | hypoxia inducible lipid droplet associated | 0.001 | 0.034 | −3.99 | −3.10 | −0.89 |
| COL3A1 | collagen type III alpha 1 chain | 0.002 | 0.038 | −2.91 | −2.42 | −0.66 |
| COL27A1 | collagen type XXVII alpha 1 chain | 0.001 | 0.033 | −2.81 | −2.31 | −0.67 |
| CCDC80 | coiled-coil domain containing 80 | 0.001 | 0.020 | −2.74 | −2.17 | −0.74 |
| CLEC18B | C-type lectin domain family 18 member B | <0.001 | 0.000 | −2.68 | −1.76 | −1.08 |
| C10orf10 | chromosome 10 open reading frame 10 | 0.002 | 0.041 | −2.65 | −2.22 | −0.59 |
| FGF2 | fibroblast growth factor 2 | 0.002 | 0.042 | −2.60 | −2.19 | −0.57 |
| RHOBTB3 | Rho related BTB domain containing 3 | <0.001 | 0.005 | −2.47 | −1.77 | −0.85 |
| ZRANB2 | zinc finger RANBP2-type containing 2 | <0.001 | 0.003 | −2.46 | −1.70 | −0.90 |
| INSIG1 | insulin induced gene 1 | 0.001 | 0.021 | −2.36 | −1.85 | −0.62 |
| PFKFB3 | 6-phosphofructo-2-kinase/fructose-2,6-biphosphatase 3 | <0.001 | 0.009 | −2.34 | −1.72 | −0.73 |
| UCKL1 | uridine-cytidine kinase 1 like 1 | <0.001 | 0.010 | −2.31 | −1.70 | −0.71 |
| DUT | deoxyuridine triphosphatase | <0.001 | <0.001 | −2.28 | −1.48 | −0.90 |
| ITM2C | integral membrane protein 2C | <0.001 | 0.017 | −2.25 | −1.72 | −0.62 |
| LYNX1 | Ly6/neurotoxin 1 | 0.001 | 0.021 | −2.23 | −1.74 | −0.58 |
| COL5A1 | collagen type V alpha 1 chain | 0.002 | 0.038 | −2.23 | −1.82 | −0.49 |
| CD72 | CD72 molecule | <0.001 | 0.008 | −2.23 | −1.60 | −0.71 |
| SLC25A37 | solute carrier family 25 member 37 | 0.002 | 0.041 | −2.18 | −1.78 | −0.47 |
| VSIG10 | V-set and immunoglobulin domain containing 10 | <0.001 | 0.017 | −2.18 | −1.65 | −0.59 |
only top 20 elevated/repressed gene transcripts are shown;
FDR = false discovery rate
Biological interpretation
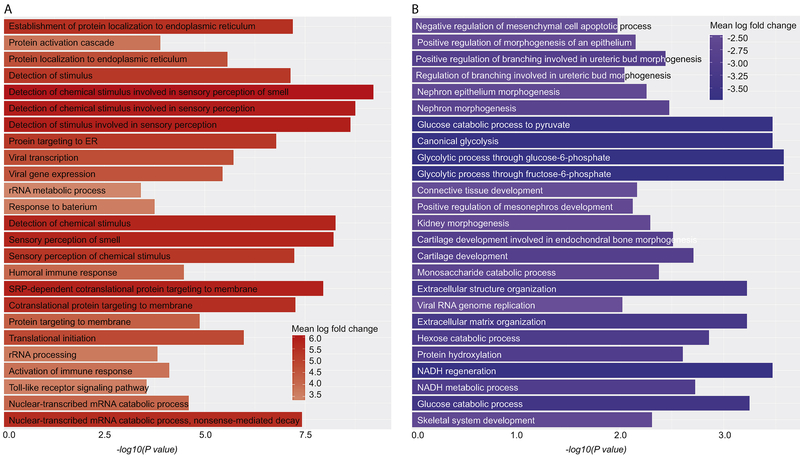
GO analysis revealed that a large number of differentially-expressed transcripts belong to numerous enriched biological processes. Transcripts up-regulated in TKA cartilage were enriched for protein-localization to endoplasmic-reticulum, protein-activation cascade, chemical-stimulus, immune-response, toll-like receptor signaling pathway and nuclear-transcribed mRNA catabolic process (Fig-2A). Transcripts up-regulated in APM cartilage were enriched for mesenchymal-cell apoptosis, epithelial-morphogenesis, canonical-glycolysis, connective-tissue development, cartilage-development, extracellular-structure organization and glucose-catabolic process (Fig-2B).
Figure 2: Barplots showing different biological processes.
Gene ontology biological process are shown for transcripts elevated in TKA group (A) and for transcripts elevated in APM group (B). Top 25 biological processes are shown in each category. ER = endoplasmic reticulum, rRNA = ribosomal ribonucleic acid, SRP = signal recognition particle, mRNA = messenger ribonucleic acid, NADH = nicotinamide adenine dinucleotide
Differentially-expressed lincRNAs, snoRNAs and antisense RNAs
Eight lincRNAs were differentially-expressed between APM and TKA (two elevated, six repressed in TKA). There were 10 snoRNAs that showed a differential-expression between APM and TKA (all elevated in TKA). Finally, three antisense RNAs (all elevated in TKA) were also detected as differentially-expressed between the two groups (Table-4).
Table 4:
Antisense, lincRNAs and snoRNAs differentially expressed between APM and TKA cartilage
| Gene symbol | Gene biotype | Gene name | P value | FDR | Fold change | 95% CI | Description | |
|---|---|---|---|---|---|---|---|---|
| Lower limit | Upper limit | |||||||
| RAD21-AS1 | antisense | RAD21 antisense RNA 1 | 0.002 | 0.035 | 1.66 | 1.24 | 2.21 | Up in TKA |
| C1QTNF9B-AS1 | antisense | C1QTNF9B antisense RNA 1 | 0.003 | 0.044 | 1.51 | 1.18 | 1.94 | Up in TKA |
| WT1-AS | antisense | WT1 antisense RNA | <0.001 | 0.019 | 1.51 | 1.24 | 1.84 | Up in TKA |
| PWRN1 | lincRNA | Prader-Willi region non-protein coding RNA 1 | 0.001 | 0.023 | 1.69 | 1.29 | 2.21 | Up in TKA |
| FAM157A | lincRNA | Family With Sequence Similarity 157 Member A | <0.001 | 0.019 | 1.52 | 1.24 | 1.87 | Up in TKA |
| TTTY12 | lincRNA | testis-specific transcript, Y-linked 12 (non-protein coding) | <0.001 | 0.016 | −1.50 | −1.80 | −1.24 | Up in APM |
| C9orf163 | lincRNA | chromosome 9 open reading frame 163 | 0.001 | 0.031 | −1.53 | −1.93 | −1.21 | Up in APM |
| C22orf34 | lincRNA | chromosome 22 open reading frame 34 | <0.001 | 0.004 | −1.54 | −1.77 | −1.34 | Up in APM |
| MGC45922 | lincRNA | uncharacterized LOC28 | 0.001 | 0.021 | −1.88 | −2.58 | −1.37 | Up in APM |
| C10orf91 | lincRNA | chromosome 10 open reading frame 91 | <0.001 | 0.016 | −1.98 | −2.72 | −1.44 | Up in APM |
| MEG3 | lincRNA | maternally expressed 3 (non-protein coding) | <0.001 | 0.012 | −4.28 | −7.87 | −2.32 | Up in APM |
| SNORA23 | snoRNA | small nucleolar RNA, H/ACA box 23 | <0.001 | 0.015 | 4.16 | 2.19 | 7.90 | Up in TKA |
| SNORD114-14 | snoRNA | small nucleolar RNA, C/D box 114-14 | 0.001 | 0.030 | 1.73 | 1.28 | 2.34 | Up in TKA |
| SNORD116-28 | snoRNA | small nucleolar RNA, C/D box 116-28 | <0.001 | 0.013 | 1.60 | 1.30 | 1.98 | Up in TKA |
| SNORD3B-1 | snoRNA | small nucleolar RNA, C/D box 3B-1 | 0.001 | 0.023 | 1.58 | 1.25 | 2.00 | Up in TKA |
| SNORA54 | snoRNA | small nucleolar RNA, H/ACA box 54 | 0.002 | 0.034 | 1.57 | 1.22 | 2.02 | Up in TKA |
| SNORD116-29 | snoRNA | small nucleolar RNA, C/D box 116-29 | <0.001 | 0.008 | 1.57 | 1.32 | 1.86 | Up in TKA |
| SNORA36A | snoRNA | small nucleolar RNA, H/ACA box 36A | <0.001 | 0.018 | 1.55 | 1.25 | 1.91 | Up in TKA |
| SNORD116-10 | snoRNA | small nucleolar RNA, C/D box 116-10 | <0.001 | 0.018 | 1.52 | 1.24 | 1.86 | Up in TKA |
| SNORD116-19 | snoRNA | small nucleolar RNA, C/D box 116-19 | 0.001 | 0.025 | 1.52 | 1.22 | 1.89 | Up in TKA |
| SNORD116-26 | snoRNA | small nucleolar RNA, C/D box 116-26 | <0.001 | 0.013 | 1.51 | 1.26 | 1.81 | Up in TKA |
FDR = false discovery rate; CI = confidence interval; APM = arthroscopic partial meniscectomy; TKA = total knee arthroplasty
Real-time PCR
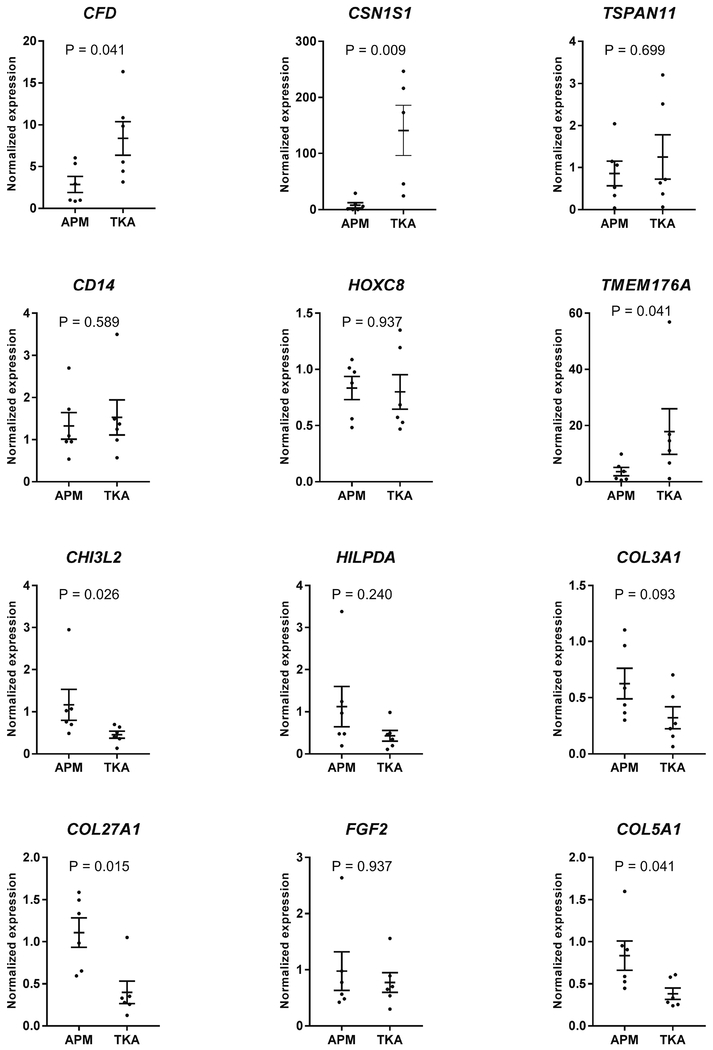
Real-time PCR confirmed that all the transcripts tested (total 12, 6 elevated and 6 repressed in TKA) showed a concordance between microarrays and real-time PCR. Notably, the expression-pattern was same between the two assays with many genes showing statistical-significance between APM and TKA even in a subset (N=6 each group) of samples (Fig-3).
Figure 3: Validation of transcripts by real-time PCR.
The expression of 12 transcripts differentially expressed between APM and TKA was validated by real-time PCR. The expression profile of the entire set of transcripts obtained from real-time PCR amplification was concordant with the microarray data. The data is presented as mean with standard error of the mean. (top two rows = genes elevated in TKA cartilage, bottom two rows = genes elevated in APM cartilage). Significance was determined by Mann-Whitney U test. APM = arthroscopic partial meniscectomy, TKA = total knee arthroplasty
Discussion
This investigation into the gene expression of cartilage from knees undergoing APM without radiographic OA demonstrates a distinct expression profile compared to cartilage from knees undergoing TKA with end-stage radiographic OA. Several transcripts were differentially-expressed between the two groups and GO analyses further revealed their significance in a number of biological processes and pathways. These differentially-expressed genes and pathways are promising targets for further investigation into their mechanistic role in OA disease process as they reflect differences in gene expression at distinct time-points during the disease process.
There are a number of interesting findings in terms of the individual transcripts differentially expressed between the two groups. While we know that cartilage from APM patients looks grossly normal and the knees have no radiographic features of OA, our recent analysis of this normal-appearing cartilage suggests that it exhibits molecular signatures reflective of OA[13]. Thus it appears that both APM and TKA cartilage, despite having a distinct gene expression profile, are both related to OA phenotype, albeit at different time points in disease progression. The expression pattern of these transcripts were confirmed by real-time PCR to be concordant with the microarray data.
Transcripts that are highly expressed in TKA knees are clearly associated with OA. CFD (adipsin) is responsible for activating the alternative pathway of the complement system. Little work has examined the role of adipsin in OA. Adipsin was higher in the synovial-fluid and serum among individuals with OA undergoing TKA compared to those without[17]. CFD was one of the top differentially-expressed gene in meniscus from TKA patients compared to APM patients[4]. Serum levels of CFD in OA patients were correlated with increased cartilage volume in the global knee and medial femur at baseline and two years[18]. CSN1S1 is known to mediate pro-inflammatory properties through the activation of GM-CSF via p38 MAPK pathway[19]. It shows higher expression in capsule from OA joints[20], OA cartilage[10] and OA synovium[21]. Moreover, another study confirmed that CSN1S1 exhibited significantly higher expression in OA cartilage and synovium than in normal tissues[22]. TSPAN11 (CD 151-like) is a member of the tetraspanins family, also called transmembrane-4 superfamily but nothing is known about its role in OA.
CSF1R blockage reduces inflammation in human and rodent-models of RA[23]. CSF through binding to the tyrosine-kinase receptor CSF1R promotes cell-survival and proliferation[24]. In RA, CSF is produced by synovial endothelial cell[25] and in-vitro by IL-1β and TNFα[26]. In mice, blockage of CSF1R stopped cartilage damage, systemic bone loss and bone erosion[27]. Inhibition of CSF1 and CSF1R is a promising target and therapeutic alternative for arthritis and related conditions[28]. CD14 is considered a reflection of inflammatory activation status of macrophages as it is abundant on monocyte and macrophages[29] and serves as a receptor for the bacterial lipopolysaccharide (LPS) and LPS-binding protein-complex[30]. Available literature suggests that pain in OA may be, due in part, to the release of CD14 from activated macrophages in inflamed joint tissues and from infiltrated macrophages in deep root ganglia. Synovial-fluid CD14 has been shown to be significantly associated with osteophyte severity and joint space narrowing[31]. Our findings appear to be in-line with the aforementioned studies.
Transcripts that were highly expressed in the APM group may arise from three plausible scenarios: (1) some genes are clearly associated with cartilage degenerations, (2) other genes have been implicated in mechanotransduction or response to injury, and (3) some have a role in joint homeostasis. CHI3L2 (YKL-39) is a novel growth and differentiation factor involved in cartilage homeostasis. It has been reported that CHI3L2 enhances colony forming activity, cell proliferation, and type II collagen expression in ATDC5 cells[32]. CHI3L2 activates phosphorylation of extracellular signal-regulated kinases ERK1/ERK2 in HEK293 and U87 MG cell lines[33]. YKL-39 mRNA has been shown to be significantly up-regulated in the cartilage of patients with OA[34]. Moreover, the level of YKL-39 mRNA expression was positively correlated with collagen type II up-regulation in both early and late stages of the disease[35]. It appears that YKL-39 is involved in a variety of physiological processes (e.g., tissue remodeling, chondrocyte repair, inflammation) and serves as specific biomarkers for OA progression[36]. Higher expression of CHI3L2 in APM cartilage likely represents a repair response and a surrogate for disease progression at an early stage e.g., after a meniscus tear. Gene expression of HILPDA decreases with age as well in cartilage from OA patients and gets stimulated in synovial fibroblasts by IL-17[37], an inflammatory cytokine synovial levels of which are negatively-correlated with OA severity[38]. This may, in part, explain why HILPDA is highly expressed in APM cartilage.
A number of transcriptome studies have identified COL3A1 as a differentially expressed gene between normal and arthritic cartilage. Consistent with our study, lower expression of COL3A1 has also been shown in degenerated cartilage compared to macroscopically intact cartilage from the same knee[39]. One study analyzing microarray data from GEO did find highly expressed COL3A1[40], although it was under-powered and under-analyzed. COL27A1 is a fibrillary collagen gene which is highly expressed in developing skeletal cartilage[41] and is known to be regulated by SOX9, as are COL2A1, COL11A2 and COL9A1[42]. Literature on the role and expression pattern of COL27A1 in OA is limited, but our findings suggest it responds to injury, as its expression was higher in APM cartilage compared to TKA cartilage, and may represent a marker for early OA. A genome-wide association study has found that a single-nucleotide-polymorphism in COL27A1 shows association (at nominal significance) with radiographic OA[43].
FGF2 was also significantly up-regulated in APM cartilage compared to TKA. It has been implicated in OA[44] and impeded anabolism and promotes catabolism potentially via up-regulation of MMP13[45]. The role of FGF2 in OA and cartilage-homeostasis, however, remains controversial as it is a novel endogenous chondroprotective agent in cartilage that suppresses ADAMTS5 and delays cartilage degradation in murine OA[46]. It also plays a functional role in chondrocyte mechanotransduction[47].
There were several transcripts found to be up-regulated in TKA cartilage compared to APM which were associated with immune response. These findings were interesting because involvement of immune-response systems has also been implicated in OA. An integrative meta-analysis of differentially-expressed genes in OA cartilage has demonstrated that immune-response was a highly-enriched GO-term[48]. Transcriptome analysis of equine cartilage[49] and human meniscus[50] has shown that biological processes related to immune-response were elevated with age, which is consistent with our current findings and previous findings from APM cartilage[13] and TKA meniscus[4]. These findings suggest that TKA cartilage has a higher tendency to express genes related to immune-response than APM cartilage.
GO-terms referring to extracellular-matrix organization, including several collagens (e.g. COL1A2, COL2A1, COL3A1), was enriched for highly-changed genes in preserved (non-OA) cartilage[8]. The repression of processes related to extracellular-matrix organization in the TKA cartilage relative to APM is a significant finding with a number of biological and clinical implications. Several studies have shown that loss of extracellular-matrix occurs in the degenerative phenotype of cartilage as a cause and consequence of OA[51]. As we did not compare cartilage between healthy knees and knees with OA, we cannot conclude that this identifies a degenerative-phenotype in the cartilage. It does, however, show that the cartilage from TKA patients had higher degeneration than cartilage from APM patients. The functional processes related to connective-tissue development were also repressed in TKA cartilage. Suppression of connective-tissue development, epithelial-morphogenesis and cartilage-development in TKA cartilage would minimize any potential for regeneration in this group.
While these results show statistically significant differences in gene expression, clinical-relevance of these findings is not immediately apparent since the downstream effects of these differences in gene expression are not known. Nevertheless, this study is an initial-step towards advancing our understanding of biological events after meniscal injury. In the light of above discussion, several transcripts and biological processes differentially represented in APM and TKA are characteristic of the OA disease-process[8, 10, 13]. These findings stress the importance of studying the knee joint as an organ, with inclusion of other tissues (bone, synovium, meniscus, ligaments) and healthy controls. Since the gene expression in the cartilage was compared between injured and diseased (OA) joints, further research is needed to compare these findings to normal cartilage in intact knees. In this study, the prevalence of females was 20% higher in TKA patients than APM patients. Considering that females have a higher risk for OA than males[52], we added patient sex in our model to account for this discrepancy in the percentage of female patients. While some studies[53] have reported sex-related differences in cartilage, we did not have a large enough sample-size to determine the sex-related differences in transcript-expression. Another limitation was the inclusion of three patients with focal grade 2 chondrosis in the patellofemoral compartment, reflecting the fact that it is extremely rare to find knees completely free of any chondral wear or damage at the time of APM. On the other hand, it is impossible to determine exactly why these patients do not have cartilage damage at the time of APM. This selected subsample of subjects may be highly resilient to cartilage wear and potentially less susceptible to the development of knee OA compared to more commonly encountered patients with concomitant meniscus tears and chondrosis.
Despite these limitations, our study has several strengths. First, the comparison between injured and diseased cartilage provides a unique assessment of the condition of each tissue after knee injury versus OA. As discussed, changes consistent with the disease phenotype are seen in both groups. Second, based on initial analysis of these data, and considering published work[13], all the confounders were included in the model. Finally, our validation assay substantiated our findings on the differences seen by microarray as we observed high-concordance of expression pattern between the two assays.
In conclusion, our study clearly demonstrated that numerous transcripts were differentially expressed between cartilage from knees undergoing APM and TKA. Despite significant differences, both express genes and pathways related to OA. Therefore, the gene profile in APM cartilage likely represents an earlier stage in degeneration while TKA cartilage is end-stage. Future mechanistic studies, as well as comparison with normal (uninjured) cartilage, could shed light on how injury alters the joint-homeostasis, ultimately leading to OA and irreversible joint-destruction. Improving our knowledge of early events after meniscal injury and surgery may advance our understanding of how and why this injury impacts the knee joint as-a-whole, and what could be done to delay or prevent subsequent joint-damage.
Supplementary Material
Acknowledgements
We thank the Washington University Genome Technology Access Center for help with microarrays. We also acknowledge with thanks technical assistance by Nobuaki Chinzei.
Role of funding source
This study was supported by AOSSM/Sanofi Osteoarthritis Research Grant (PI: R. H. Brophy). Dr. Rai is supported through Pathway to Independence Award (R00-AR064837) from the National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS), National Institutes of Health (NIH). The content of this publication is solely the responsibility of the authors and does not necessarily represent the official views of the NIH, NIAMS or AOSSM.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Lewandrowski KU, Muller J, Schollmeier G. Concomitant meniscal and articular cartilage lesions in the femorotibial joint. Am J Sports Med 1997; 25: 486–494. [DOI] [PubMed] [Google Scholar]
- 2.Lohmander LS, Englund PM, Dahl LL, Roos EM. The long-term consequence of anterior cruciate ligament and meniscus injuries: osteoarthritis. Am J Sports Med 2007; 35: 1756–1769. [DOI] [PubMed] [Google Scholar]
- 3.Roos H, Adalberth T, Dahlberg L, Lohmander LS. Osteoarthritis of the knee after injury to the anterior cruciate ligament or meniscus: the influence of time and age. Osteoarthritis Cartilage 1995; 3: 261–267. [DOI] [PubMed] [Google Scholar]
- 4.Brophy RH, Zhang B, Cai L, Wright RW, Sandell LJ, Rai MF. Transcriptome comparison of meniscus from patients with and without osteoarthritis. Osteoarthritis Cartilage 2018; 26: 422–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ochi K, Daigo Y, Katagiri T, Saito-Hisaminato A, Tsunoda T, Toyama Y, et al. Expression profiles of two types of human knee-joint cartilage. J Hum Genet 2003; 48: 177–182. [DOI] [PubMed] [Google Scholar]
- 6.Dunn SL, Soul J, Anand S, Schwartz JM, Boot-Handford RP, Hardingham TE. Gene expression changes in damaged osteoarthritic cartilage identify a signature of non-chondrogenic and mechanical responses. Osteoarthritis Cartilage 2016; 24: 1431–1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Snelling S, Rout R, Davidson R, Clark I, Carr A, Hulley PA, et al. A gene expression study of normal and damaged cartilage in anteromedial gonarthrosis, a phenotype of osteoarthritis. Osteoarthritis Cartilage 2014; 22: 334–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ramos YF, den Hollander W, Bovee JV, Bomer N, van der Breggen R, Lakenberg N, et al. Genes involved in the osteoarthritis process identified through genome wide expression analysis in articular cartilage; the RAAK study. PLoS One 2014; 9: e103056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sato T, Konomi K, Yamasaki S, Aratani S, Tsuchimochi K, Yokouchi M, et al. Comparative analysis of gene expression profiles in intact and damaged regions of human osteoarthritic cartilage. Arthritis Rheum 2006; 54: 808–817. [DOI] [PubMed] [Google Scholar]
- 10.Karlsson C, Dehne T, Lindahl A, Brittberg M, Pruss A, Sittinger M, et al. Genome-wide expression profiling reveals new candidate genes associated with osteoarthritis. Osteoarthritis Cartilage 2010; 18: 581–592. [DOI] [PubMed] [Google Scholar]
- 11.Fu M, Huang G, Zhang Z, Liu J, Zhang Z, Huang Z, et al. Expression profile of long noncoding RNAs in cartilage from knee osteoarthritis patients. Osteoarthritis Cartilage 2015; 23: 423–432. [DOI] [PubMed] [Google Scholar]
- 12.Liu Q, Zhang X, Dai L, Hu X, Zhu J, Li L, et al. Long noncoding RNA related to cartilage injury promotes chondrocyte extracellular matrix degradation in osteoarthritis. Arthritis Rheumatol 2014; 66: 969–978. [DOI] [PubMed] [Google Scholar]
- 13.Rai MF, Sandell LJ, Zhang B, Wright RW, Brophy RH. RNA Microarray Analysis of Macroscopically Normal Articular Cartilage from Knees Undergoing Partial Medial Meniscectomy: Potential Prediction of the Risk for Developing Osteoarthritis. PLoS One 2016; 11:e0155373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Reynard LN, Loughlin J. Insights from human genetic studies into the pathways involved in osteoarthritis. Nat Rev Rheumatol 2013; 9: 573–583. [DOI] [PubMed] [Google Scholar]
- 15.Consortium M, Shi L, Reid LH, Jones WD, Shippy R, Warrington JA, et al. The MicroArray Quality Control (MAQC) project shows inter- and intraplatform reproducibility of gene expression measurements. Nat Biotechnol 2006; 24: 1151–1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ashwell MS, O’Nan AT, Gonda MG, Mente PL. Gene expression profiling of chondrocytes from a porcine impact injury model. Osteoarthritis Cartilage 2008; 16: 936–946. [DOI] [PubMed] [Google Scholar]
- 17.Fernandez-Puente P, Mateos J, Fernandez-Costa C, Oreiro N, Fernandez-Lopez C, Ruiz-Romero C, et al. Identification of a panel of novel serum osteoarthritis biomarkers. J Proteome Res 2011; 10: 5095–5101. [DOI] [PubMed] [Google Scholar]
- 18.Martel-Pelletier J, Raynauld JP, Dorais M, Abram F, Pelletier JP. The levels of the adipokines adipsin and leptin are associated with knee osteoarthritis progression as assessed by MRI and incidence of total knee replacement in symptomatic osteoarthritis patients: a post hoc analysis. Rheumatology (Oxford) 2016; 55: 680–688. [DOI] [PubMed] [Google Scholar]
- 19.Vordenbaumen S, Braukmann A, Petermann K, Scharf A, Bleck E, von Mikecz A, et al. Casein alpha s1 is expressed by human monocytes and upregulates the production of GM-CSF via p38 MAPK. J Immunol 2011; 186: 592–601. [DOI] [PubMed] [Google Scholar]
- 20.Campbell TM, Trudel G, Wong KK, Laneuville O. Genome wide gene expression analysis of the posterior capsule in patients with osteoarthritis and knee flexion contracture. J Rheumatol 2014; 41: 2232–2239. [DOI] [PubMed] [Google Scholar]
- 21.Ungethuem U, Haeupl T, Witt H, Koczan D, Krenn V, Huber H, et al. Molecular signatures and new candidates to target the pathogenesis of rheumatoid arthritis. Physiol Genomics 2010; 42A: 267–282. [DOI] [PubMed] [Google Scholar]
- 22.Park R, Ji JD. Unique gene expression profile in osteoarthritis synovium compared with cartilage: analysis of publicly accessible microarray datasets. Rheumatol Int 2016; 36: 819–827. [DOI] [PubMed] [Google Scholar]
- 23.Garcia S, Hartkamp LM, Malvar-Fernandez B, van Es IE, Lin H, Wong J, et al. Colony-stimulating factor (CSF) 1 receptor blockade reduces inflammation in human and murine models of rheumatoid arthritis. Arthritis Res Ther 2016; 18: 75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hume DA, MacDonald KP. Therapeutic applications of macrophage colony-stimulating factor-1 (CSF-1) and antagonists of CSF-1 receptor (CSF-1R) signaling. Blood 2012; 119: 1810–1820. [DOI] [PubMed] [Google Scholar]
- 25.Nakano K, Okada Y, Saito K, Tanikawa R, Sawamukai N, Sasaguri Y, et al. Rheumatoid synovial endothelial cells produce macrophage colony-stimulating factor leading to osteoclastogenesis in rheumatoid arthritis. Rheumatology (Oxford) 2007; 46: 597–603. [DOI] [PubMed] [Google Scholar]
- 26.Campbell IK, Ianches G, Hamilton JA. Production of macrophage colony-stimulating factor (M-CSF) by human articular cartilage and chondrocytes. Modulation by interleukin-1 and tumor necrosis factor alpha. Biochim Biophys Acta 1993; 1182: 57–63. [DOI] [PubMed] [Google Scholar]
- 27.Toh ML, Bonnefoy JY, Accart N, Cochin S, Pohle S, Haegel H, et al. Bone- and cartilage-protective effects of a monoclonal antibody against colony-stimulating factor 1 receptor in experimental arthritis. Arthritis Rheumatol 2014; 66: 2989–3000. [DOI] [PubMed] [Google Scholar]
- 28.El-Gamal MI, Al-Ameen SK, Al-Koumi DM, Hamad MG, Jalal NA, Oh CH. Recent Advances of Colony-Stimulating Factor-1 Receptor (CSF-1R) Kinase and Its Inhibitors. J Med Chem 2018; 61: 5450–5466. [DOI] [PubMed] [Google Scholar]
- 29.Landmann R, Muller B, Zimmerli W. CD14, new aspects of ligand and signal diversity. Microbes Infect 2000; 2: 295–304. [DOI] [PubMed] [Google Scholar]
- 30.Wright SD, Ramos RA, Tobias PS, Ulevitch RJ, Mathison JC. CD14, a receptor for complexes of lipopolysaccharide (LPS) and LPS binding protein. Science 1990; 249: 1431–1433. [DOI] [PubMed] [Google Scholar]
- 31.Daghestani HN, Pieper CF, Kraus VB. Soluble macrophage biomarkers indicate inflammatory phenotypes in patients with knee osteoarthritis. Arthritis Rheumatol 2015; 67: 956–965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Miyatake K, Tsuji K, Yamaga M, Yamada J, Matsukura Y, Abula K, et al. Human YKL39 (chitinase 3-like protein 2), an osteoarthritis-associated gene, enhances proliferation and type II collagen expression in ATDC5 cells. Biochem Biophys Res Commun 2013; 431: 52–57. [DOI] [PubMed] [Google Scholar]
- 33.Areshkov PA, Kavsan VM. Chitinase 3-like protein 2 (CHI3L2, YKL-39) activates phosphorylation of extracellular signal-regulated kinases ERK1/ERK2 in human embryonic kidney (HEK293) and human glioblastoma (U87 MG) cells. Tsitol Genet 2010; 44: 3–9. [PubMed] [Google Scholar]
- 34.Steck E, Breit S, Breusch SJ, Axt M, Richter W. Enhanced expression of the human chitinase 3-like 2 gene (YKL-39) but not chitinase 3-like 1 gene (YKL-40) in osteoarthritic cartilage. Biochem Biophys Res Commun 2002; 299: 109–115. [DOI] [PubMed] [Google Scholar]
- 35.Knorr T, Obermayr F, Bartnik E, Zien A, Aigner T. YKL-39 (chitinase 3-like protein 2), but not YKL-40 (chitinase 3-like protein 1), is up regulated in osteoarthritic chondrocytes. Ann Rheum Dis 2003; 62: 995–998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ranok A, Wongsantichon J, Robinson RC, Suginta W. Structural and thermodynamic insights into chitooligosaccharide binding to human cartilage chitinase 3-like protein 2 (CHI3L2 or YKL-39). J Biol Chem 2015; 290: 2617–2629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hattori T, Ogura N, Akutsu M, Kawashima M, Watanabe S, Ito K, et al. Gene Expression Profiling of IL-17A-Treated Synovial Fibroblasts from the Human Temporomandibular Joint. Mediators Inflamm 2015; 2015: 436067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liu Y, Peng H, Meng Z, Wei M. Correlation of IL-17 Level in Synovia and Severity of Knee Osteoarthritis. Med Sci Monit 2015; 21: 1732–1736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fukui N, Ikeda Y, Ohnuki T, Tanaka N, Hikita A, Mitomi H, et al. Regional differences in chondrocyte metabolism in osteoarthritis: a detailed analysis by laser capture microdissection. Arthritis Rheum 2008; 58: 154–163. [DOI] [PubMed] [Google Scholar]
- 40.Sun J, Yan B, Yin W, Zhang X. Identification of genes associated with osteoarthritis by microarray analysis. Mol Med Rep 2015; 12: 5211–5216. [DOI] [PubMed] [Google Scholar]
- 41.Pace JM, Corrado M, Missero C, Byers PH. Identification, characterization and expression analysis of a new fibrillar collagen gene, COL27A1. Matrix Biol 2003; 22: 3–14. [DOI] [PubMed] [Google Scholar]
- 42.Jenkins E, Moss JB, Pace JM, Bridgewater LC. The new collagen gene COL27A1 contains SOX9-responsive enhancer elements. Matrix Biol 2005; 24: 177–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yau MS, Yerges-Armstrong LM, Liu Y, Lewis CE, Duggan DJ, Renner JB, et al. Genome-Wide Association Study of Radiographic Knee Osteoarthritis in North American Caucasians. Arthritis Rheumatol 2017; 69: 343–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Meo Burt P, Xiao L, Dealy C, Fisher MC, Hurley MM. FGF2 High Molecular Weight Isoforms Contribute to Osteoarthropathy in Male Mice. Endocrinology 2016; 157: 4602–4614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yan D, Chen D, Cool SM, van Wijnen AJ, Mikecz K, Murphy G, et al. Fibroblast growth factor receptor 1 is principally responsible for fibroblast growth factor 2-induced catabolic activities in human articular chondrocytes. Arthritis Res Ther 2011; 13: R130. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 46.Chia SL, Sawaji Y, Burleigh A, McLean C, Inglis J, Saklatvala J, et al. Fibroblast growth factor 2 is an intrinsic chondroprotective agent that suppresses ADAMTS-5 and delays cartilage degradation in murine osteoarthritis. Arthritis Rheum 2009; 60: 2019–2027. [DOI] [PubMed] [Google Scholar]
- 47.Vincent TL, McLean CJ, Full LE, Peston D, Saklatvala J. FGF-2 is bound to perlecan in the pericellular matrix of articular cartilage, where it acts as a chondrocyte mechanotransducer. Osteoarthritis Cartilage 2007; 15: 752–763. [DOI] [PubMed] [Google Scholar]
- 48.Wang X, Ning Y, Guo X. Integrative meta-analysis of differentially expressed genes in osteoarthritis using microarray technology. Mol Med Rep 2015; 12: 3439–3445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Peffers M, Liu X, Clegg P. Transcriptomic signatures in cartilage ageing. Arthritis Res Ther 2013; 15: R98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rai MF, Patra D, Sandell LJ, Brophy RH. Transcriptome analysis of injured human meniscus reveals a distinct phenotype of meniscus degeneration with aging. Arthritis Rheum 2013; 65: 2090–2101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Squires GR, Okouneff S, Ionescu M, Poole AR. The pathobiology of focal lesion development in aging human articular cartilage and molecular matrix changes characteristic of osteoarthritis. Arthritis Rheum 2003; 48: 1261–1270. [DOI] [PubMed] [Google Scholar]
- 52.Srikanth VK, Fryer JL, Zhai G, Winzenberg TM, Hosmer D, Jones G. A meta-analysis of sex differences prevalence, incidence and severity of osteoarthritis. Osteoarthritis Cartilage 2005; 13: 769–781. [DOI] [PubMed] [Google Scholar]
- 53.Pan Q, O’Connor MI, Coutts RD, Hyzy SL, Olivares-Navarrete R, Schwartz Z, et al. Characterization of osteoarthritic human knees indicates potential sex differences. Biol Sex Differ 2016; 7: 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.