Abstract
During pregnancy and postpartum, women in high HIV prevalence regions continue to be at high risk for acquiring HIV, due to both behavioral and biological mechanisms, despite declines in coital frequency as a pregnancy advances. We estimated differences in rates of partnership concurrency for men with and without pregnant or postpartum sexual partners. We used monthly retrospective panel data from Ghana from three perspectives: couple-level data, female reports of pregnancy and male partner concurrency, and male reports of concurrent partnerships and female partner pregnancy. Coital frequency increased during the first trimester and then declined with advancing pregnancy. However, in all three analyses, there was no compelling evidence that men with pregnant or postpartum partners had additional concurrent partnerships. Our findings suggest that even though women’s sexual activity likely declines during pregnancy and postpartum, they may not be at increased risk of HIV/STI due to their partners seeking additional partnerships.
Keywords: HIV/STI, multiple concurrent partnerships, Agbogbloshie, pregnant, postpartum
INTRODUCTION
During pregnancy and postpartum, women in high HIV prevalence regions continue to be at high risk for acquiring HIV(1–5), although there is no consensus as to whether this increased risk is due to behavior change alone, or also biological mechanisms. Reduced condom use before and during pregnancy has been documented, which would increase risk of HIV/STI. However, women’s sexual activity typically declines during pregnancy and immediacy postpartum before it begins to increase again (4, 6). Biological factors, such as high levels of estrogen and progesterone that accompany pregnancy, can lead to changes in the genital tract such as increased inflammation and are associated with increased HIV susceptibility (6, 7). Further research is needed to examine additional behavioral mechanisms that could put women at increased risk of HIV acquisition.
Pregnant women may be at higher risk of exposure to HIV if their male partners have additional outside sexual partnerships. Since pregnant women’s sexual activity tends to decline over the course of a pregnancy, male partners of pregnant and postpartum women may therefore seek additional concurrent partnerships if their pregnant partner is unable or unwilling to engage in sexual activity. Male partner’s concurrent partnerships could significantly increase risk of HIV acquisition for pregnant and postpartum women once they re-engage in sexual activity with their partner.
Concurrent sexual partnerships are defined as overlapping sexual partnerships where sexual intercourse with one partner occurs between two acts of intercourse with another partner (8, 9). Most evidence suggests that concurrent sexual partnerships significantly influence HIV transmission within heterosexual populations in sub-Saharan Africa (10–13). Controlling for the number of partners, having overlapping partnerships increases exposure to partners, but not the individual who is having concurrent partners. Thus, concurrency is a transmission, not an acquisition, risk factor. However, disparate findings on concurrency and HIV risk have arisen in the literature, partly due to challenges in study design and indirect measurement (14, 15). Concurrency and other sexual network factors are challenging to measure, since they often rely on self-reports of relationship timing, or perceived outside partnerships from one perspective.
In this paper, we used three different perspectives to test whether men with pregnant or postpartum partners had higher rates of concurrent partnerships compared to men with partners who are not pregnant or postpartum. We hypothesized that the probability of concurrency will be higher for men who have pregnant partners. Since coital frequency may decline with pregnancy and postpartum, men may seek additional partnerships. Second, we hypothesized that once controlling for coital frequency, pregnancy will not be associated with rates of concurrency. If our hypotheses were true, then extra prevention interventions, such as pre-exposure prophylaxis, would be needed to protect pregnant and post-partum women from HIV infection.
DATA & METHODS
This analysis used data from the Migration and HIV in Ghana (MHG) study, a cross-sectional study of sexually active adults in Agbogbloshie, Ghana in 2012 (16). Agbogbloshie is an urban resource-poor area in the capital city of Accra, selected for this study based on its hypothesized high levels of migration and HIV infection. The MHG used a 3-stage cluster randomized sampling scheme to obtain a probability sample of the population. Eligibility criteria to participate were current residence in the selected household, age 18–49 years, and lifetime history of consensual sexual intercourse. Overall, 590 subjects participated in the study, of whom 106 were referred partners in linked cohabiting partnerships (see detailed description below). Our recruitment completion rate was 70%. Key reasons for non-recruitment were lack of success locating the subject (14.1%) and ineligibility for the study due to age or lack of sexual activity (6.6%). Less than 2% of non-recruitment was due to refusal of participation. Interviews were conducted separately for each member of a linked partnership; responses were confidential and not shared with the other member. Additional details of the study protocol, questionnaire, and recruitment have been published elsewhere (16). The Institutional Review Boards of the University of Washington and University of Ghana approved all procedures.
The survey focused on demographics, migration and travel, sexual network characteristics and sexual behavior. We used an event history calendar to collect detailed partnership data for up to three partners in the past 12 months, with responses for each month during that period. For each partner, data included the duration of the partnership and the likelihood that the partner had additional partners. Month-by-month partnership data also included coital frequency and pregnancy status of female partners. For this analysis, we used three different sub-samples: couple-level data, female reports, and male reports.
Couple-level reports
Out of the 106 linked partnerships, 102 couples had complete data for, and 1,224 couple-months. Only married or cohabiting couples were eligible to be included in this sub-sample. The index respondents (n = 51) in the couple-level analysis are also included in either the male- and female-reports analysis.
The main outcome in the analyses was male sexual partnership concurrency per couple-month, measured as having more than one ongoing partnership at any point during the month. Sequence and timing of partnerships were reported directly from the male partner in the couple. Women reported the likelihood that their male partner had other partners, but this was not used for the main concurrency outcome in the couple-level analysis. For months in which concurrency could not be verified, such as months with multiple short-duration (less than one month) partnerships or transitional partnerships in which one ended and a new partnership began within the month, we conservatively assumed that an overlap in partnerships did not occur.
The main predictor variable was pregnancy status, as reported by the female partner in the couple. Pregnancy (yes/no) was reported each month for the last 12 months. Using this information, we calculated pregnancy month (1–9) and postpartum period (3 months post birth). If the pregnancy was left censored, then the last month of pregnancy during the year was marked as month 9, and each previous month was counted backwards. Postpartum period was marked for the three months after the last month pregnant. If the pregnancy was ongoing (right censored), then the first month pregnant during the year was marked as month 1, and each subsequent month was counted until censored. If a pregnancy started during the year and ended before month 9, a miscarriage was assumed and no postpartum period was assigned. We then created a trimester variable: pregnant 1–3 months = 1st trimester, pregnancy 4–6 months = 2nd trimester, pregnancy 7–9 months = 3rd trimester, and post-partum period = 4th trimester. Men also reported when their partner was pregnant. Other data from couples included monthly coital frequency, partnership duration, and age.
Male reports
Out of the 484 index respondents, 202 were men and 179 men reported any sexual activity in the past year, representing 1,902 person-months of men in active partnerships (person-months when men were not in a partnership were dropped). Male concurrency was calculated the same way as it was in the couple-level analysis. Female partner pregnancy status was measured dichotomously (yes/no) based on male reports. Men only reported partner pregnancy status during active relationships; therefore, we were unable to calculate trimester and postpartum period for the male-report analysis. For example, if a female partner was pregnant during a 3 month partnership, it is unclear which stage of pregnancy she was in.
Female reports
Out of the 484 index respondents, 282 were women and 236 women reported any sexual activity in the past year, representing 2,563 person-months in active partnerships. Male partner concurrency was calculated based on female reports of whether their partner had other partners while they were in a relationship. We assumed that concurrency occurred if the female reported “definitely” or “probably”, and that concurrency did not occur if the female reported “probably not” or “definitely not”. We dropped 493 person-months in which the female responded “don’t know” or refused to answer. Female pregnancy status and trimester were defined the same way as they were in the couple-level analysis.
Statistical Analysis
First, we described the socio-demographic and sexual behavior characteristics of the sample of sexually active men, women, and couples, accounting for the survey design. Second, we examined patterns of male concurrency by the pregnancy and trimester status of his female partner at the level of person-month, unadjusted by clustering within individual. Third, we assessed cross-sectional evidence for the association between male concurrent partnerships and female pregnancy and postpartum status (defined both as pregnant: yes/no; and by trimester and 3-month postpartum status) using pooled ordinary least squares regression models, with cluster-robust standard errors. These models were repeated using three data sub-samples: couple-level, female, and male reports. Then we tested whether the relationship between pregnancy and male concurrency was explained by coital frequency, by including it in the multivariate models. Lastly, we explored the feasibility of using fixed effects logistic regression models to examine variation between concurrency and pregnancy status within individuals and couples. In these models, individuals and couples are used as their own controls, and those without any variation in concurrency across months are dropped.
RESULTS
Overall, 33.6% of sexually active men (according to male-reports) reported any concurrent partnership in the last year (Table 1), compared to 6.6% of women. Among women, 21.2% reported that their most recent male partner definitely or probably had a concurrent partner at any point during the duration of their partnership, while 11.7% of men perceived that their most recent female partner was concurrent. Men reported more total partnerships in the last 12 months (1.9) and slightly more total coital acts per month (5.5) compared to women (1.2 sexual partners and 4.2 total coital acts per month). When restricted to sex acts within the most recent partnership, men reported only slightly more acts per month: 4.58 compared to 4.07. About 25% of the women reported being pregnant at any point in time over the prior 12 month, while 18.8% of the men reported their most recent partner was pregnant at any point during the duration of their partnership. HIV prevalence was higher among women compared to men (5.6% vs. 2.7% respectively). In our sample, 33% (3 out of 9) of the women diagnosed with HIV were pregnant at some point during the prior 12 months (data not shown). In comparison, the estimated adult national HIV prevalence in Ghana in 2017 was 1.7%, which was slightly lower than the prevalence among pregnant women at antenatal clinics throughout Ghana at 2.1% (17).
Table 1:
Socio-demographic and behavioral characteristics of sexually active adults in Agbogbloshie, Accra, Ghana, 2012 (n = 415 individuals)
| Total | Men | Women | ||||
|---|---|---|---|---|---|---|
| %/ mean | 95% CI | %/ mean | 95% CI | %/ mean | 95% CI | |
| Sex | ||||||
| Male | 42.6 | 36.9–48.5 | ||||
| Female | 57.3 | 51.6–63.1 | ||||
| Age | ||||||
| 18 – 29 | 55.8 | 49.9–61.6 | 50.5 | 41.8–59.2 | 59.8 | 52.0–67.2 |
| 30 – 39 | 29.1 | 24.2–34.6 | 33 | 25.2–41.8 | 26.3 | 20.3–33.3 |
| 40 – 49 | 15.0 | 11.3–19.6 | 16.5 | 10.9–24.2 | 13.9 | 9.5–20.1 |
| Marital status | ||||||
| Never married | 40.6 | 34.9–46.6 | 39.4 | 31.4–48.0 | 41.6 | 33.8–49.8 |
| Cohabiting | 21.5 | 16.7–27.2 | 19.9 | 13.5–28.4 | 22.6 | 16.2–30.7 |
| Married | 31.4 | 26.2–37.0 | 34.1 | 26.1–43.0 | 29.4 | 23.0–36.7 |
| Widowed/separated/divorced | 6.5 | 4.4–9.5 | 6.6 | 3.7–11.4 | 6.4 | 3.8–10.8 |
| Income | ||||||
| Mean (in Ghana Cedi) | 2039 | 1812–2265 | 2655 | 2246–3063 | 1572 | 1349–1795 |
| Median (Ghana Cedi) | 1580 | 1920 | 1200 | |||
| Pregnancy status | ||||||
| Ever pregnant (self* or most recent partner) | 18.8 | 12.6–27.1 | 24.9 | 18.9–31.9 | ||
| Sexual history | ||||||
| Total partners* | 1.48 | 1.33–1.63 | 1.9 | 1.57–2.22 | 1.17 | 1.09–1.25 |
| Concurrency | ||||||
| Any concurrent partnership* | 18.0 | 14.0–22.9 | 33.6 | 25.8–42.4 | 6.6 | 3.7–11.1 |
| Most recent partner ever concurrent | 17.2 | 12.8–22.8 | 11.7 | 6.3–20.8 | 21.2 | 15.1128.9 |
| Coital frequency | ||||||
| Acts per month, total | 4.77 | 4.27–5.28 | 5.52 | 4.59–6.45 | 4.22 | 3.69–4.75 |
| Acts per month with most recent partner | 4.28 | 4.14–4.43 | 4.58 | 4.33–4.83 | 4.07 | 3.90–4.26 |
| HIV-1 Infection | ||||||
| Infected | 4.4 | 1.8–7.0 | 2.7 | 0.0–5.4 | 5.6 | 1.6–9.7 |
Reported in in last 12 months
How many sexual partners, including spouse, in last 12 months
Among the married and cohabiting partnerships, 30% of men and 4% of women reported having a concurrent partnership at any point in the last 12 months (Table 2). For 70% of the couples, the female report of her male partner’s concurrency matched the male self-report. For ~22% of the couples, the male partner reported concurrency but the female did not report that he had one, while the rest (~7%) were couples in which the female said the male partner was concurrent but the male reported that he was not. Mean coital frequency within the partnership was around 4.5 acts per month; male and female reports were similar. Men reported slightly more total coital acts due to a distribution with a much longer right tail; these included acts outside of the main partnership. Median total coital acts were the same (data not shown). More than a third of women in the couple sub-sample were pregnant at any point in the last year, and men slightly under-reported their female partner’s pregnancy. Average partnership duration was around 9 years, with men reporting slightly longer durations. Lastly, men were on average 5 years older than their female partner.
Table 2:
Socio-demographic and behavioral characteristics of sexually active cohabiting or married couples in Agbogbloshie, Accra, Ghana, 2012 (n = 102 couples)
| Couple | He Said | She Said | ||||
|---|---|---|---|---|---|---|
| n / mean | % / s.d. | n / mean | % / s.d. | n / mean | % / s.d. | |
| Partnership characteristics | ||||||
| Duration of partnership (years) | 9.05 | 6.41 | 8.52 | 6.41 | ||
| Difference& (years) | 0.722 | 4.573 | ||||
| Age | ||||||
| Individually reported (years) | 35.54 | 7.26 | 30.04 | 7.17 | ||
| Age difference (male - female age) (years) | 5.51 | 5.15 | ||||
| Pregnancy status | ||||||
| Female ever pregnant* | 30 | 29.41 | 37 | 36.27 | ||
| Agreement | 85 | 83.33 | ||||
| Different: male says pregnant | 5 | 4.9 | ||||
| Different: female says pregnant | 12 | 11.76 | ||||
| Concurrency | ||||||
| Any concurrent partnership*, individually reported on self | 31 | 30.39 | 4 | 3.92 | ||
| Agreement: both say male concurrent | 72 | 70.59 | ||||
| Different: female says male concurrent | 7 | 6.86 | ||||
| Different: female says male not concurrent | 23 | 22.55 | ||||
| Coital frequency | ||||||
| Total acts per month | 5.22 | 5.70 | 4.71 | 3.64 | ||
| Difference in acts per month& | 0.50 | 4.67 | ||||
| Acts per month within partnership | 4.51 | 4.41 | 4.70 | 3.56 | ||
| Difference in acts per month within partnership& | −0.26 | 3.94 | ||||
Reported in in last 12 months
Male minus female reports
All couples consist of one woman and one man
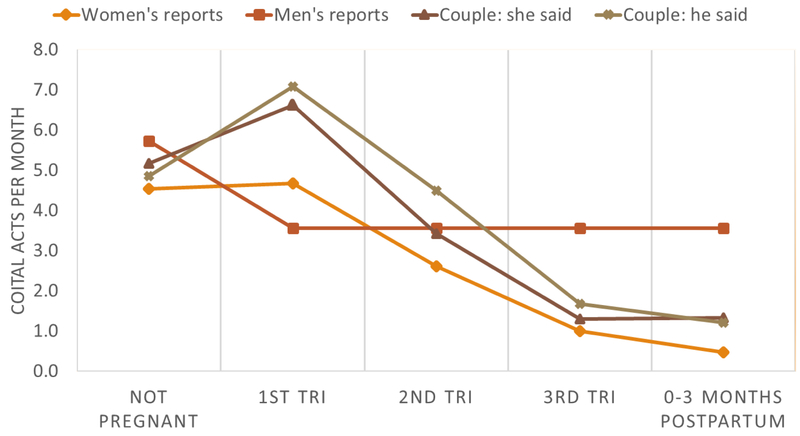
Coital frequency varied significantly by pregnancy status, and the shape of the distribution was similar across report-type. Average coital frequency varied from 4.5 to 5.7 acts per month in months in which the female partner was not reported to be pregnant (Figure 1). Coital frequency was reported to be higher in the first trimester of pregnancy (ranging from 4.7 – 7.1), and then declined in the second and third trimester. Reports of coital frequency were the lowest during the post-partum period (0.5 – 1.3). Since female partner’s trimester could not be accurately determined from the data, male reports of coital frequency were stratified by whether the partner was pregnant or not pregnant. On average, men’s reports of coital frequency were lower during times that his partner was pregnant (5.7 vs. 3.6 acts/month).
Figure 1:
Coital frequency by pregnancy and post-partum status, reported by men, women, and men and women in cohabiting or married partnerships.
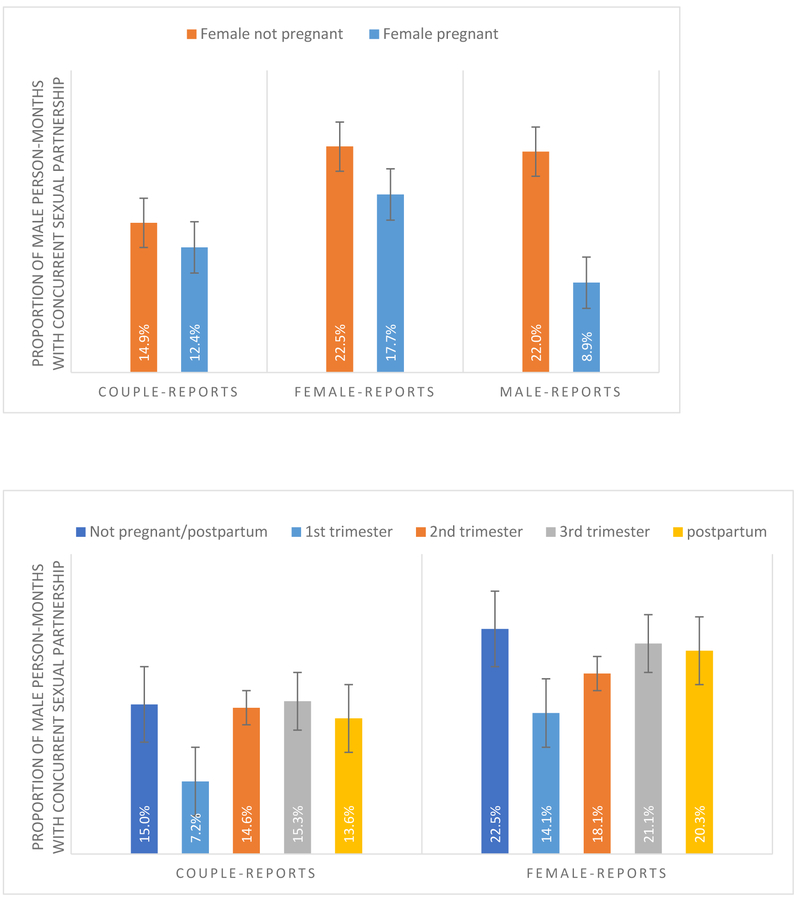
The proportion of person-months in which men in an active relationship engaged in a concurrent partnership (i.e. degree>1) ranged from 14.5% (reported by men in a married or cohabiting couple), to 20.7% (reported by men) and 21.9% (reported by female married or cohabiting partners). When stratified by the pregnancy status of female partnerships, concurrency rates were always lower for men with pregnant partners compared to men with non-pregnant partners (Figure 2a). This difference was largest and statistically significant according to the male-reports: 8.9% vs. 22.0% (Chi-square test p = 0.001). Rates of male partner’s concurrency varied by trimester of pregnancy (Figure 2b), but were not significantly different. Men’s rates of concurrency appeared to be the lowest when their female partner was in the 1st trimester of pregnancy. Rates increased in the 2nd and 3rd trimester and during the postpartum period, but were either slightly lower or the same as rates when women were not pregnant or postpartum.
Figures 2a & 2b:
Proportion of person-months in which the male partner has concurrent partnerships, by female-partner pregnancy (yes/no, Figure 2a) and by female trimester and post-partum status (Figure 2b) using couple-data, female-, and male-reports.
Results from the cross-sectional regression analysis confirmed the trends described earlier. Regardless of the sub-sample examined, no statistical evidence of an association between male concurrency and female pregnancy or trimester emerged in the pooled logistic models after accounting for dependency in the observations (Table 3). Additionally, coital frequency was not independently associated with male concurrency. For the female reports, partner type was associated with concurrency. The odds of the male partner having concurrent partners was nearly five times higher if he was a boyfriend, casual, or one-time partner compared to if he was a cohabiting partner, fiancé or spouse (OR = 4.62; 95% CI: 1.93–11.1). The older the woman, the more likely the partner was reported to be concurrent as well. For the male reports, having a pregnant partner was associated with lower odds of concurrency, but this finding was not statistically significant. In the couple sub-sample, similar to the others, pregnancy was not associated with an increase in concurrency. However, the odds of a concurrency partnership were 13% higher for every additional year difference in age between the male and female (OR = 1.03, 95%CI: 1.02–1.24].
Table 3:
Cross-sectional multivariate logistic regression analysis, with robust standard errors adjusted for clustering, to examine the association between pregnancy and male sexual partnership concurrency in a given month. (OR = odds ratio, CI = confidence interval) Unit of analysis is person-months.
| Model 1 | Model 2 | Model 3 | Model 4 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| OR | 95% Cl | P value | OR | 95% Cl | P value | OR | 95% Cl | P value | OR | 95% Cl | P value | |
| Female reports | ||||||||||||
| Partner pregnant (Reference: not pregnant) | 0.74 | 0.29–1.90 | 0.534 | 1.00 | 0.39–2.54 | 0.999 | ||||||
| 1st trimester | 0.56 | 0.20–1.59 | 0.28 | 0.7 3 | 0.27–2.03 | 0.552 | ||||||
| 2nd trimester | 0.76 | 0.24–2.43 | 0.64 | 1.0 5 | 0.32–3.50 | 0.931 | ||||||
| 3rd trimester | 0.92 | 0.33–2.53 | 0.87 | 1.3 2 | 0.45–3.86 | 0.616 | ||||||
| 4th trimester (postpartum) (Reference: not pregnant or postpartum) | 0.88 | 0.29–2.68 | 0.82 | 1.29 | 0.39–4.27 | 0.682 | ||||||
| Coital frequency | 0.99 | 0.92–1.06 | 0.713 | 0.9 9 | 0.92–1.06 | 0.786 | ||||||
| Partner is boyfriend/casual/one-time (not cohabiting) (Reference: fiance/cohabiting partner/spouse) | 4.62 | 1.93–11.1 | 0.001 | 4.7 3 | 1.95–11.5 | 0.001 | ||||||
| Age | 1.0 7 | 1.02–1.13 | 0.008 | 1.0 7 | 1.02–1.13 | 0.008 | ||||||
| Male reports | ||||||||||||
| Partner pregnant (Reference: not pregnant) | 0.84 | 0.60–1.17 | 0.304 | 0.70 | 0.34–1.33 | 0.254 | ||||||
| Coital frequency | 1.06 | 0.91–1.24 | 0.442 | |||||||||
| Partner is boyfriend/casual/one-time (not cohabiting) (Reference: fiance/cohabiting partner/spouse) | 1.16 | 0.53–2.53 | 0.718 | |||||||||
| Age | 1.02 | 0.98–1.06 | 0.392 | |||||||||
| Couple reports | ||||||||||||
| Couple Pregnant (Reference: not pregnant) | 0.81 | 0.28–2.37 | 0.71 | 0.94 | 0.33–2.65 | 0.911 | ||||||
| 1st trimester | 0.44 | 0.10–2.05 | 0.30 | 0.43 | 0.09–2.13 | 0.303 | ||||||
| 2nd trimester | 0.97 | 0.32–2.95 | 0.96 | 1.12 | 0.34–3.74 | 0.844 | ||||||
| 3rd trimester | 1.02 | 0.31–3.36 | 0.97 | 1.40 | 0.40–4.90 | 0.600 | ||||||
| 4th trimester (postpartum) (Reference: not pregnant or postpartum) | 0.89 | 0.26–3.01 | 0.85 | 1.20 | 0.30–4.75 | 0.795 | ||||||
| Coital frequency -- female report | 1.05 | 0.96–1.16 | 0.293 | 1.06 | 0.96–1.17 | 0.224 | ||||||
| Age -- female | 0.99 | 0.92–1.07 | 0.874 | 0.99 | 0.92–1.07 | 0.865 | ||||||
| Age difference (male-female) | 1.13 | 1.02–1.24 | 0.017 | 1.13 | 1.02–1.24 | 0.016 | ||||||
| Duration of partnership (years) | 1.01 | 0.92–1.11 | 0.870 | 1.01 | 0.92–1.11 | 0.864 | ||||||
Slightly different patterns emerged when we examined the association between male concurrency and female partner pregnancy status within individuals and couples using fixed effects logistic regression models (Table 4). Since these models use the individual or couple as its own control, time-invariant controls were omitted as all heterogeneity was already accounted for. However, this also meant that confidence intervals were large when individuals (or couples) who did not have variability across months were dropped. Therefore, these results should be interpreted with caution due to small bin sizes in the model. We first ran the models with pregnancy or trimester as the main predictor, then re-ran the models including coital frequency to see whether the association between pregnancy remained the same or was mediated.
Table 4:
Fixed effects logistic regression analysis to examine the association between pregnancy and male sexual partnership concurrency in a given month, accounting for individual heterogeneity. (OR = odds ratio, CI = confidence interval) Unit of analysis is person-months.
| Model 1 | Model 2 | Model 3 | Model 4 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| OR | 95% Cl | P value | OR | 95% Cl | P value | OR | 95% Cl | P value | OR | 95% Cl | P value | |
| Male reports | ||||||||||||
| Partner pregnant (Reference: not pregnant) | 0.19 | 0.05–0.68 | 0.011 | 0.16 | 0.04–0.60 | 0.006 | ||||||
| Coital frequency | 1.06 | 0.91–1.24 | 0.442 | |||||||||
| Couple reports | ||||||||||||
| Couple Pregnant (Reference: not pregnant) | 1.34 | 0.55–3.31 | 0.521 | 2.35 | 0.85–6.49 | 0.099 | ||||||
| 1st trimester | 0.55 | 0.09–3.42 | 0.521 | 5.03 | 0.62–41.1 | 0.132 | ||||||
| 2nd trimester | 3.12 | 0.76–12.8 | 0.115 | 12.98 | 2.17–77.5 | 0.005 | ||||||
| 3rd trimester | 2.79 | 0.67–11.5 | 0.156 | 4.91 | 0.84–28.8 | 0.077 | ||||||
| 4th trimester (postpartum) (Reference: not pregnant or postpartum) | 3.33 | 0.77–14.5 | 0.108 | 5.64 | 0.93–34.3 | 0.060 | ||||||
| Coital frequency -- female report | 0.78 | 0.66–0.92 | 0.003 | 0.79 | 0.66–0.94 | 0.007 | ||||||
Lack of variation within women’s reports of their male partner’s likelihood of concurrent partnerships rendered the fixed effects models futile. In other words, although reports of male partner’s concurrency varied across women and their pregnancy status, individual women’s reports of whether her partner had other partners did not vary month-by-month when they were pregnant or not. This suggests that perceptions of male partner concurrency do not vary by pregnancy or trimester of pregnancy, according to women’s reports.
For men, the odds of reporting a concurrent partnership when his female partner was pregnant were 81% lower than when his partner was not pregnant (OR = 0.19, 95% CI: 0.05–0.68). Men’s reports of monthly coital frequency with his partner were not significantly associated with the odds of a concurrent partnership, and did not significantly moderate the effect of pregnancy.
The largest difference between the pooled sample and the fixed effects models were for the couples, but again, these rely on small sample sizes. Only 22 out of 102 couples had variation in male concurrency during the past year. Out of the 80 couples who were dropped in the fixed effects model, 71 never had concurrency and nine always had concurrency during the past 12 months. Among couples who reported variation in concurrent partnerships, minimal evidence of a positive association between pregnancy and concurrency emerged. Accounting for heterogeneity within couples resulted in a positive association between female pregnancy and male concurrency, although not statistically significant (OR = 1.34, 95% CI 0.55–3.31). Female reports of monthly coital frequency within the partnership were highly associated with male reports of concurrency. Each additional sex act was associated with 22% lower odds of male concurrency (OR = 0.78, 95% CI: 0.66–0.92). Coital frequency did not mediate the association between pregnancy and concurrency in the hypothesized way. Indeed, independent of the frequency of coital acts, the odds of concurrency while the female partner was pregnant was slightly higher but still not significant. Similar results are seen in the association between trimester of pregnancy and concurrency. Coital frequency was highly associated with concurrency, and positive but marginally significant associations between trimester and pregnancy emerged independent of coital frequency.
DISCUSSION
These findings suggest that even though women’s sexual activity declines during pregnancy and postpartum, they are not at increased risk of HIV/STI due to their partners seeking additional partnerships. We found no compelling evidence to support our hypothesis that reduced coital frequency during pregnancy could lead to increased concurrency. Concurrency was not significantly higher during pregnancy, and even though coital frequency declined after the first trimester during pregnancy, coital frequency was not independently associated with concurrency except for the limited couple-fixed effects model.
Our results are similar to previous research. A study of Nigerian male sexual activity during pregnancy found that 28% of men engaged in extra-marital concurrent partnerships (18, 19), compared to our finding of 32%. Additionally, a study in Uganda found that pregnant women and their male partners reported significantly fewer external sexual partners than did the other groups (2). We did not find evidence of a significant reduction in partnerships; rather, we simply found no evidence to suggest that concurrency changes over periods of pregnancy. Therefore, male partners of pregnant women are not having more concurrent partnerships compared to partners of non-pregnant women, but concurrency rates are still quite high, which poses a significant risk of HIV/STI exposure for all women.
In our sample, as with many others, coital frequency varied significantly by trimester of pregnancy. However, coital frequency was not associated with concurrency in our cross-sectional models either. Only within couples with variation in concurrency was coital frequency highly predictive of concurrency. The higher the number of sex acts within the month, as reported by the female, the less likely the male reported a concurrency. Our past work has also suggested that acquiring additional partnerships does not “dilute”, or reduce the number of sex acts from the original partner (20) . Coital dilution is the reduction in the number of sex acts per partner when an additional, concurrent partner is added. Since there is no evidence of coital dilution, then we can also reject the possibility of reverse causation – that male partners of pregnant women acquire additional partners, which leads to reduced coital frequency.
One potential reason for no general increase in concurrency is pair bonding. A sexual pair bond is a behavioral and physiological bond between two individuals. This bond might be stronger when a couple is planning to have a child, and this bond may dissuade a partner from seeking additional sexual partners. Additionally, since Agbogbloshie is an urban, resource-poor setting, men might fear getting someone else pregnant concurrently since the financial costs could be high. This fear may lead to fewer additional partnerships.
Concurrency associated with pregnancy within married and cohabiting couples requires further examination. Although based on small sample sizes, our analyses suggest that among couples who ever experience concurrency, concurrency was positively associated with pregnancy even holding coital frequency constant. A possible interpretation of this finding is that it is a threshold effect. If having a concurrent sexual partnership is a possibility, or the barriers for having concurrency are low for a particular couple, then having a pregnant partner could be enough of an incentive to initiate an extramarital partnership. Nonetheless, this hypothesis would not hold true for the male fixed effects model, since among men who had variation in concurrency status, pregnancy was associated with less concurrency. Another reason why the couples fixed-effects model suggested a positive relationship between pregnancy and concurrency while the male-reports did not could be because the couples had already established a pair bond in the past. By definition, 100% of the couples were cohabiting and married while about 50% of the male-sample were married or cohabiting. Additionally, we did not have information on parity, or the number of times the women had been pregnant before. The male sample could be biased toward first-time pregnancies compared to higher-order pregnancies in the couple sample, and thus pair bonding could be stronger.
Limitations of our paper include the potential for bias in self-reports, including social desirability bias and recall bias. However, we attempted to address some of these limitations by triangulating three separate perspectives: female reports, male reports, and couple reports. Additionally, similarities in responses by the male and female within the couple sub-sample are reassuring. Recall and desirability of coital frequency may be minimal in our data, since male and female reports of coital frequency were in fact quite similar. In addition, reports of female pregnancy status by men were concordant 85% of the time. The largest potential for bias was likely female reports of male-partner concurrency, as females tended to under-report whether the male partner was concurrent in our couples sample. Another limitation is that we did not differentiate between a new concurrency and an ongoing concurrency. Further work should address the ordering of events. After a female partner gets pregnant, does coital frequency decline and consequently does a male partner acquire a new external partner?
Concurrent partnership formation is complex, and not simply a function of coital frequency. Why individuals acquire concurrent partnerships, and for how long they sustain them, is likely due to unique individual traits, dyadic characteristics, gender norms, and the social and economic setting. Nonetheless, having a sexual partner who engages in an additional concurrent partnership is a significant risk factor for HIV acquisition for women. Even though partners of pregnant and postpartum women did not have significantly more concurrent partnerships compared to patterns of non-pregnant women, overall high rates of concurrency place women at risk. Our results in context of other research suggest that heightened risk of HIV acquisition for pregnant and postpartum women compared to non-pregnant women may be due to biological rather than behavioral mechanisms. A number of public health implications thus follow. Oral pre-exposure prophylaxis (PrEP) for pregnant and postpartum women can reduce risk of HIV acquisition, and is now part of the WHO guidelines (21). Nevertheless, biological prevention messages must be accompanied by messages that promote sexual health. Community-oriented messaging on the risks of concurrent sexual partnerships should continue as well.
Acknowledgements:
This material is supported by funding from the NICHD K99/R00 HD057533. We acknowledge Dr. Francis Dodoo from The Pennsylvania State University and the University of Ghana, along with the Migration and HIV in Ghana study staff and others at the Regional Institute for Population Studies for their support. In addition, we would like to thank Ayme Tomson and Rafael Melendez-Rios for their work on this project through the NSF Network Science IGERT at the University of California Santa Barbara.
Footnotes
Drs. Cassels, Jenness, and Biney declare that we have no conflict of interest. The Institutional Review Boards of the University of Washington and the Noguchi Memorial Institute for Medical Research, University of Ghana, approved the study protocols. Informed consent was obtained from all individual participants included in the study.
Bibliography
- 1.Drake AL, Wagner A, Richardson B, John-Stewart G. Incident HIV during pregnancy and postpartum and risk of mother-to-child HIV transmission: a systematic review and meta-analysis. PLoS Med. 2014;11(2):e1001608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gray RH, Li X, Kigozi G, Serwadda D, Brahmbhatt H, Wabwire-Mangen F, et al. Increased risk of incident HIV during pregnancy in Rakai, Uganda: a prospective study. Lancet. 2005;366(9492):1182–8. [DOI] [PubMed] [Google Scholar]
- 3.Kinuthia J, Drake AL, Matemo D, Richardson BA, Zeh C, Osborn L, et al. HIV acquisition during pregnancy and postpartum is associated with genital infections and partnership characteristics. AIDS. 2015;29(15):2025–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kinuthia J, Kiarie JN, Farquhar C, Richardson B, Nduati R, Mbori-Ngacha D, et al. Cofactors for HIV-1 incidence during pregnancy and postpartum period. Curr HIV Res. 2010;8(7):510–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Moodley D, Esterhuizen TM, Pather T, Chetty V, Ngaleka L. High HIV incidence during pregnancy: compelling reason for repeat HIV testing. AIDS. 2009;23(10):1255–9. [DOI] [PubMed] [Google Scholar]
- 6.Thomson KA, Hughes J, Baeten JM, John-Stewart G, Celum C, Cohen CR, et al. Increased Risk of HIV Acquisition Among Women Throughout Pregnancy and During the Postpartum Period: A Prospective Per-Coital-Act Analysis Among Women With HIV-Infected Partners. J Infect Dis. 2018;218(1):16–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hapgood JP, Kaushic C, Hel Z. Hormonal Contraception and HIV-1 Acquisition: Biological Mechanisms. Endocr Rev. 2018;39(1):36–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Goodreau S, Morris M. Concurrency Tutorials 2012. [Available from: http://www.statnet.org/concurrency. [Google Scholar]
- 9.UNAIDS Reference Group. HIV: consensus indicators are needed for concurrency. Lancet. 2010;375(9715):621–2. [DOI] [PubMed] [Google Scholar]
- 10.Eaton JW, Hallett TB, Garnett GP. Concurrent sexual partnerships and primary HIV infection: a critical interaction. AIDS Behav. 2011;15(4):687–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Goodreau SM, Cassels S, Kasprzyk D, Montano DE, Greek A, Morris M. Concurrent partnerships, acute infection and HIV epidemic dynamics among young adults in Zimbabwe. AIDS Behav. 2012;16(2):312–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Leung KY, Kretzschmar M. Concurrency can drive an HIV epidemic by moving R0 across the epidemic threshold. AIDS. 2015;29(9):1097–103. [DOI] [PubMed] [Google Scholar]
- 13.Morris M, Kurth AE, Hamilton DT, Moody J, Wakefield S. Concurrent partnerships and HIV prevalence disparities by race: linking science and public health practice. Am J Public Health. 2009;99(6):1023–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lurie MN, Rosenthal S. Concurrent partnerships as a driver of the HIV Epidemic in sub-Saharan Africa? The evidence is limited. AIDS Behav. 2010;14(1):17–24; discussion 5–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tanser F, Barnighausen T, Hund L, Garnett GP, McGrath N, Newell ML. Effect of concurrent sexual partnerships on rate of new HIV infections in a high-prevalence, rural South African population: a cohort study. Lancet. 2011;378(9787):247–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cassels S, Jenness SM, Biney AA, Ampofo WK, Dodoo FN. Migration, sexual networks, and HIV in Agbogbloshie, Ghana. Demogr Res. 2014;31:861–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ghana AIDS Commission. National and Sub-National HIV and AIDS Estimates and Projections. 2017.
- 18.Lawoyin TO, Larsen U. Male sexual behaviour during wife’s pregnancy and postpartum abstinence period in Oyo State, Nigeria. J Biosoc Sci. 2002;34(1):51–63. [PubMed] [Google Scholar]
- 19.Onah HE, Iloabachie GC, Obi SN, Ezugwu FO, Eze JN. Nigerian male sexual activity during pregnancy. Int J Gynaecol Obstet. 2002;76(2):219–23. [DOI] [PubMed] [Google Scholar]
- 20.Jenness SM, Biney AA, Ampofo WK, Nii-Amoo Dodoo F, Cassels S. Minimal coital dilution in Accra, Ghana. J Acquir Immune Defic Syndr. 2015;69(1):85–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.The World Health Organization (WHO). Preventing HIV during pregnancy and breastfeeding in the context of PrEP. Geneva, Switzerland: 201710. [Google Scholar]