Abstract
Objective:
Examine associations of hip abductor strength with i) cartilage damage worsening in the tibiofemoral and patellofemoral compartments 2 years later, and ii) poor function and disability outcomes 5 years later.
Methods:
Participants had knee osteoarthritis (K/L≥2) in at least one knee. Hip abductor strength was measured using Biodex Dynamometry. Participants underwent 3.0T MRI of both knees at baseline and 2 years later. Baseline-to-2-year cartilage damage progression, defined as any worsening of WORMS cartilage damage score, was assessed at each tibiofemoral and patellofemoral surface. LLFDI (Late-Life Function and Disability Instrument) and Chair-Stand-Rate were recorded at baseline and 5-year follow-up; outcomes analyzed using quintiles. Poor outcomes were defined as remaining in the same low-function quintiles or being in a worse quintile at 5-year follow-up. We analyzed associations of baseline hip abductor strength with cartilage damage worsening and function and disability outcomes using multivariable log-binomial models.
Results:
275 knees from 164 persons [age=63.7 (SD=9.8) years, 79.3% women] comprised the structural outcome sample, and 187 persons [age=64.2 (9.7), 78.6% women] the function and disability outcomes sample. Greater baseline hip abductor strength was associated with reduced risks of baseline-to-2-year medial patellofemoral and lateral tibiofemoral cartilage damage worsening [adjusted relative risks (RRs) range: 0.80–0.83) and with reduced risks of baseline-to-5-year poor outcomes for Chair-Stand-Rate and LLFDI Basic Lower-Extremity Function and Disability Limitation (adjusted RRs range: 0.91–0.94).
Conclusion:
Findings support a beneficial role of hip abductor strength for disease modification and for function and disability outcomes, and as a potential therapeutic target in managing knee osteoarthritis.
Keywords: Knee OA, Strength, MRI, Function, Disability
INTRODUCTION
Functional impairments and disability are common among persons with knee osteoarthritis (OA)1,2. In the elderly, knee OA contributes to as much chronic disability as cardiovascular disease3. There is no disease-modifying therapy for OA; practice guidelines recommend strength training as an effective intervention to reduce symptoms and preserve function4,5. The quadriceps muscle has long been a focus, although any effect of quadriceps strengthening on delaying structural progression has not been confirmed6,7. In addition, observational studies have not consistently revealed an association between quadriceps strength and risk of subsequent progression8–10. Novel insights on alternative targets for disease modification and function preservation will help refine physical and rehabilitative therapy for knee OA.
Lower extremity muscle weakness has been proposed as a contributing factor to function decline and structural progression11. In daily activities, hip abductor activation is required to maintain balance and postural stability during walking and transfers12,13. Hip abductor weakness has been linked to poor function in persons with knee OA14 and after knee arthroplasty15. Impaired hip abductor force generation may impact movement mechanics during weightbearing activities and potentially lead to altered joint loading and structural progression. Compared with age-matched healthy individuals, persons with knee OA had an approximately 20% hip abductor strength deficit16,17. Whether hip abductor weakness is a consequence of knee OA and/or a modifiable risk factor for disease progression remains debatable16. We reported that greater internal hip abduction moment during gait was associated with a reduced likelihood of subsequent medial tibiofemoral (TF) disease worsening18. Although the internal hip abduction moment reflects dynamic activity of hip abductors during walking, it is a net joint moment representing the contribution of both hip abductors and adductors, and could be influenced by other variables such as limb inertia and body mass19,20.
Hip abductor strength, in contrast, is easily interpreted, clinically translatable, and enhanced by exercise. Hip strengthening exercise lessened pain and improved function in the short-term in persons with predominantly medial TF OA21–24, but its long-term effect on disease progression and function preservation is unclear. The role of hip abductor strength in patellofemoral (PF) OA is unknown. It is established that individuals with PF pain demonstrate weak hip abductors and hip strengthening is an integral component in managing PF pain25–27. Early MRI-detected change in patellar cartilage28 and elevated PF joint stress29 found in persons with PF pain suggest a disease continuum between PF pain and PF OA30,31. In theory, greater hip abductor strength may protect against OA progression in the PF compartment.
According to Nagi’s disablement model32 and the World Health Organization’s International Classification of Functioning, Disability, and Health (ICF)33, function limitation pertains to the inability or limitation to perform discrete physical tasks, such as ambulation, climbing stairs, or reaching; disability refers to the inability or limitation in major life tasks or social roles within a typical sociocultural and physical environment, such as personal care, household management, job, or hobbies. Ideally, both function and disability should be considered.
The central role of hip abductors in activities and the potential for strong hip muscles to prevent function decline, disability progression, and structural worsening make hip abductors an attractive target in the management of knee OA. We tested the hypotheses that greater baseline hip abductor strength is associated with i) a reduced risk of cartilage damage worsening in the TF and PF compartments 2 years later, and ii) a reduced risk of poor function and disability outcomes 5 years later.
METHODS
Participants.
Study participants were from a prospective, longitudinal, observational cohort study of knee OA, the MAK-3 Study (Mechanical Factors in Arthritis of the Knee-Study 3)34. They were recruited from the community using advertising in periodicals targeting older persons, neighborhood organizations, letters to members of the registry of the Buehler Center on Aging, Health, and Society at Northwestern University, and via medical center referrals. Inclusion criteria were: definite TF osteophyte presence [Kellgren/Lawrence (K/L) radiographic grade ≥ 2] in one or both knees; and Likert category of at least “a little difficulty” for 2 or more items in the WOMAC (Western Ontario and McMaster Universities Osteoarthritis Index) physical function scale. Exclusion criteria were: corticosteroid injection within the previous 3 months; history of avascular necrosis, rheumatoid or other inflammatory arthritis, periarticular fracture, Paget’s disease, villonodular synovitis, joint infection, ochronosis, neuropathic arthropathy, acromegaly, hemochromatosis, gout, pseudogout, osteopetrosis, or meniscectomy; or exclusion criteria for MRI. Approval was obtained from the Institutional Review Boards of Northwestern University and NorthShore University HealthSystem Evanston Hospital. All participants provided written consent.
Baseline hip abductor and knee extensor strength.
We used a computer-driven Biodex System 3 PRO dynamometer (Biodex Medical, Shirley, NY, USA) to measure bilateral baseline isometric hip abductor strength, quantified as torque in the unit of Newton-meter. The Biodex System is widely used for measuring strength and has good reliablity and validity35. After calibration, participants stood in a padded height-and-weight-adjustable standing frame with forearm supports and handgrips. The thigh resistance pad was placed on the lateral thigh, proximal to the knee. The axis of rotation was aligned at the ipsilateral anterior superior iliac spine, with the hip joint in a neutral position. Participants practiced pushing against the pad at submaxial effort 2 times, then performed 3 repetitions of maximal isometric hip abduction, holding for 5 seconds with 60-second rest between repetitions. We tested right and left hip strength sequentially. To investigate the additional impact of knee extensor strength in the relationship between hip abductor strength and poor outcomes, we measured bilateral knee extensor isometric strength in a seated position, at 60-degree knee flexion. An experienced examiner conducted all testing. Test-retest reliability was excellent [intraclass correlation coefficient (ICC) = 0.91]. For analysis, we averaged peak hip abductor and knee extensor torque from 3 repetitions and normalized to body weight.
MRI acquisition and assessment of cartilage damage worsening.
At baseline and 2-year follow-up, MRIs of both knees were obtained using a commercial knee coil and 1 of 2 whole-body scanners, a 3T Verio (Siemens Healthcare, Erlangen, Germany) or a 1.5T Avanto (Siemens Healthcare, Erlangen, Germany); the same scanner was used at both evaluations. The protocol included coronal T1-weighted spin-echo [TR/TE/FOV/Matrix/Slice thickness = 3 s/20 ms/14 cm, 256×256, 3 mm at 3T; TR/TE/FOV/Matrix/Slice thickness = 3 s/18 ms/14 cm, 256×256, 3 mm at 1.5T], and sagittal axial, and coronal fat-suppressed proton density-weighted turbo spin echo sequences [TR/TE/Turbo Factor/FOV/Matrix/Slice thickness = 500 ms/11 ms/7/12 cm, 320×320, 3 mm at 3T; TR/TE/Turbo Factor/FOV/Matrix/Slice thickness = 600 ms/11 ms/7/12 cm, 320×320, 3 mm at 1.5T]. Each knee was scored using the Whole-Organ MRI Score (WORMS) method36, by 1 of 2 expert musculoskeletal radiologists (inter-rater ICC = 0.98 for medial cartilage morphology)36. Baseline and 2-year scans were evaluated as pairs, with known chronology as suggested for longitudinal studies in knee OA37, but blinded to all other data. Three subregions (anterior, central, and posterior) of the medial and lateral femoral condyles and tibial plateaus were each scored separately for cartilage damage (0–6 scale), where 0 = normal cartilage thickness and signal, 1 = increased intrasubstance signal intensity without morphologic defect, 2 = solitary focal partial thickness defect, 2.5 = solitary full thickness defect, 3 = multiple partial-thickness loss, 4 = diffuse partial thickness loss, 5 = multiple areas of full-thickness loss, and 6 = diffuse full-thickness loss. The medial and lateral patellar surfaces were each scored separately on the same 0–6 scale.
We assessed cartilage damage worsening as medial TF, lateral TF, and any TF and medial PF, lateral PF, and any PF. Baseline-to-2-year cartilage damage worsening in the medial TF compartment was defined as WORMS score worsening in any of the 5 medial TF subregions (central and posterior medial femoral; and anterior, central, and posterior medial tibial); lateral TF worsening as worsening in any of the 5 lateral TF subregions (central and posterior lateral femoral; and anterior, central, and posterior lateral tibial); any TF worsening as any worsening in medial or lateral TF compartments. Medial PF cartilage damage worsening was defined as WORMS score worsening in either the medial anterior (trochlear) femoral or medial patellar subregion, lateral PF worsening as worsening in either the lateral anterior (trochlear) femoral or lateral patellar, and any PF worsening as worsening in either medial or lateral PF38–40.
Baseline-to-5-year function and disability outcomes.
At baseline and 5-year follow-up, participants completed the Late-Life Function and Disability Instrument (LLFDI)41,42, a self-reported measure that assesses functional limitations and disability. The LLFDI Function component consists of 32 items that rate task difficulty. We examined 3 relevant domains: Total Function, Basic Lower Extremity Function, and Advanced Lower Extremity Function. These 3 distinct measures separately capture physical function as a whole and in basic and advanced tasks involving the lower extremity, which are often compromised in persons with chronic knee symptoms. Sample questions in the Basic Lower Extremity Function include walking around one floor of home and stepping up and down from a curb; in the Advanced Lower Extremity Function questions include taking a 1-mile brisk walk without stopping or going up and down 3 flights of stairs with handrail. The LLFDI Disability component consists of 16 items that rate both task difficulty and frequency of participation. We examined 3 distinct disability domains; Frequency of Participation (i.e., how often does one do a particular activity), Limitation (i.e., to what extent does one feel limited in doing a particular activity), and Instrumental Role Limitation (i.e., to what extent does one feel limited in the ability to move around the home and the community). Scores in each domain are scaled as 0–100; higher scores indicate better function and less disability. The LLFDI was constructed using factor analysis and Rasch analytic techniques, and its validity and test–retest reliability have been evaluated in ethnically and racially diverse older adults with a range of functional limitations and chronic health conditions41,42. We also assessed Chair-Stand-Rate, a performance-based function measure, at baseline and 5-year follow-up. Time required for 5 repetitions of rising from a chair and sitting down was converted to a rate (number of stands per minute); higher rate indicates better function. The use of rate allows the inclusion of individuals who could not complete the test (i.e., those with a rate of 0).
To determine baseline-to-5-year poor outcome in each of the 6 LLFDI domains and in Chair-Stand-Rate, participants were categorized by baseline quintile, ranging from worst to best scores. Poor outcome was defined as remaining within the same low-function group (the worst two quintiles) or moving into a worse function quintile at 5-year follow-up43–46.
Baseline assessment of covariates, radiographic disease severity and pain.
All participants underwent bilateral, antero-posterior, weightbearing knee radiographs at baseline in the semi-flexed position with fluoroscopic confirmation of superimposition of the anterior and posterior tibial plateau lines and centering of the tibial spines within the femoral notch47. TF disease severity was assessed using the K/L system, 0 = normal, 1 = possible osteophytes, 2 = definite osteophytes, possible joint space narrowing, 3 = moderate osteophytes, definite joint space narrowing, some sclerosis, possible attrition, and 4 = large osteophytes, marked joint space narrowing, severe sclerosis, definite attrition48. To visualize the PF compartment, weight-bearing, 30° flexion, axial (skyline) views were obtained according to a protocol that specified participant positioning and technical acquisition parameters47. Medial and lateral PF compartments were graded separately using the OARSI (Osteoarthritis Research Society International) atlas-based scales49, with 0 = no joint space narrowing, 1 = possible narrowing, 2 = definite narrowing, 3 = severe narrowing. Radiographs were obtained in a single unit by 2 trained technicians. The reliability of the radiographic grading for the single x-ray reader was high with a Kappa coefficient of 0.86.
Pain was assessed by self-report using the WOMAC pain subscale (Likert format; range 0–20, higher worse). The reliability, validity, and responsiveness of WOMAC scores have been well established in studies of knee OA50,51. Pain during hip strength testing was recorded as none, mild, moderate, or severe.
Statistical analyses.
The relationships between baseline hip abductor strength and baseline-to-2-year TF and PF cartilage damage worsening were examined using log-binomial models52 with generalized estimating equations to account for correlations between the 2 limbs of each individual. The relationships between baseline hip abductor strength (worse limb) and poor baseline-to-5-year function and disability outcomes were examined using person-level log-binomial models. Unadjusted and adjusted (adjusting for baseline age, sex, pain, and disease severity by K/L grade) relative risks (RRs) and associated 95% confidence intervals (CIs) were calculated; a 95% CI that excludes 1.0 was considered to be statistically significant. We chose adjustment variables (e.g., age, sex, pain, and radiographic disease severity) based on our understanding of the disease course and previous cohort studies in knee OA and guided by underlying plausible interrelationships of the predictor, outcome, and covariates for each of the strength-structure and strength-function associations. Body mass index (BMI) was not included in the models because hip abductor strength was normalized to body weight. We performed sensitivity analyses adjusting for baseline PF joint space narrowing score (instead of K/L grade) for cartilage damage worsening outcomes in the respective compartment of any PF, medial PF, and lateral PF. To investigate the influence of knee extensor strength in these relationships, we examined these associations stratified by baseline median knee extensor strength.
RESULTS
Hip abductor strength and cartilage damage worsening 2 years later.
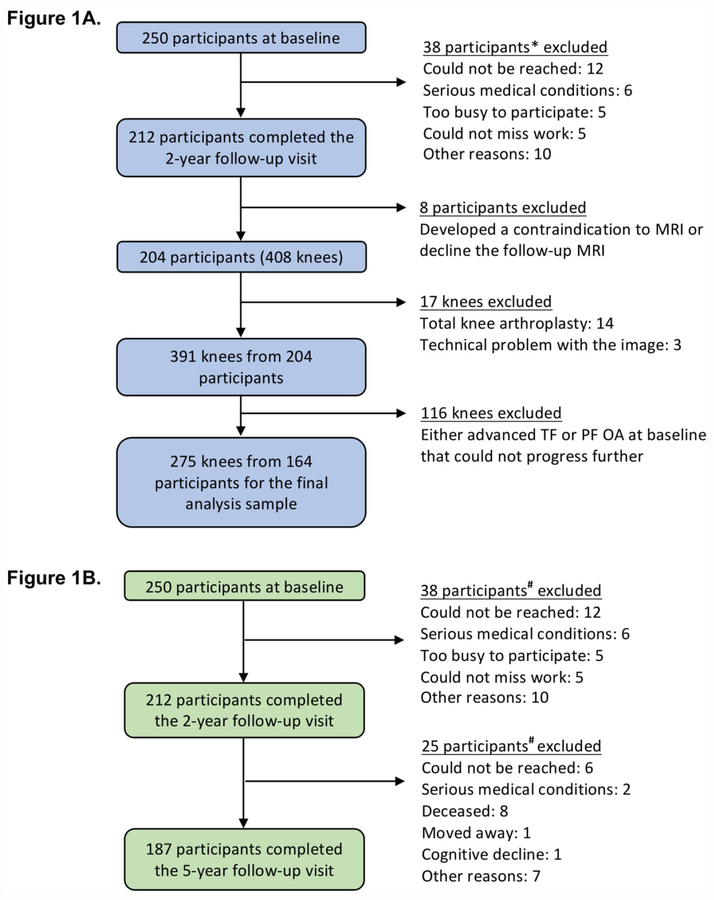
The derivation of the analysis sample (275 knees from 164 persons) for this 2-year outcome is shown in Figure 1A. Table 1 summarizes baseline characteristics of the sample. Table 2 shows the number of knees that had a poor outcome (baseline-to-2-year cartilage damage worsening) in the TF and PF compartments. As shown in Table 2, greater baseline hip abductor strength was significantly associated with reduced risks of medial PF (adjusted RR = 0.80, 95% CI: 0.67, 0.95) and lateral TF (adjusted RR = 0.83, 95% CI: 0.71, 0.98) cartilage damage worsening 2 years later. For every 0.1 Nm/kg increase in body-weight-normalized hip abductor strength, there was a 17–20% reduction in risk of medial PF and lateral TF cartilage damage worsening. Adjusted RRs for each compartment were in the protective range (i.e., each RR < 1.0), but most were not statistically significant (Table 2). Sensitivity analyses where pain and K/L grade were removed as covariates minimally altered the effect estimates. Sensitivity analyses that adjusted for baseline PF joint space narrowing score (instead of K/L grade) for cartilage damage worsening outcomes in the respective compartment of any PF, medial PF, and lateral PF yielded minimally different RRs and 95% CIs (data not shown). Similar trends were noted when only women were included in the models (Supplemental Table A). The number of men (n=34) was insufficient for a separate men-only analysis.
Figure 1. Derivation of Analysis Samples for the 2-year cartilage damage worsening (1A) and 5-year poor function and disability outcomes (1B).
* Compared to those who completed the 2-year visit (n=212), those (n=38) not completing the 2-year visit did not differ statistically with respect to sex distribution (71.7% female in non completers versus 76.5% in completers), BMI (mean 28.9 kg/m2, SD 5.1 versus 28.5, 5.7, respectively), or disease severity by K/L grade (38.1% had K/L grade 3 or 4 versus 31.4%), but were statistically slightly older (mean 68.1 years, SD 11.1 versus 64.2, 10.0).
# Compared to those who completed the 5-year visit (n=187), those (n=63) not completing the 5-year visit did not differ statistically with respect to sex distribution (66.7% female in noncompleters versus 78.6% in completers), BMI (mean 28.3 kg/m2, SD 4.9 versus 28.6, 5.8), or disease severity by K/L grade (39.7% had K/L grade 3 or 4 versus 29.9%), but were statistically slightly older (mean 67.4 years, SD 11.6 versus 64.2, 9.7).
Table 1.
Baseline characteristics of the baseline-to-2-year cartilage damage worsening analysis sample (n = 275 knees from 164 persons)
| Person-based characteristics (n = 164): | Mean (SD) or number (%) |
|---|---|
| Age (years) | 63.7 (9.8) |
| Female | 130 (79.3%) |
| BMI (kg/m2) | 28.0 (5.3) |
| WOMAC pain (0–20), higher indicating more severe pain | 4.5 (3.5) |
| WOMAC function (0–68), higher indicating worse function | 12.9 (11.1) |
| Knee-based characteristics (n = 275): | |
| K/L grade | |
| 0 | 16 (5.8%) |
| 1 | 72 (26.2%) |
| 2 | 143 (52.0%) |
| 3 | 44 (16.0%) |
| PF joint space narrowing score | |
| 0 | 101 (36.7%) |
| 1 | 103 (37.5%) |
| 2 | 71 (25.8%) |
| Hip abductor strength (Nm/kg) | 0.84 (0.24) |
| Knee extensor strength (Nm/kg) | 1.01 (0.29) |
Abbreviations: SD, Standard Deviation; BMI, Body Mass Index; WOMAC, Western Ontario and McMaster Universities Osteoarthritis Index; K/L, Kellgren/Lawrence; PF, patellofemoral
Table 2.
Associations of baseline BW-normalized hip abductor strength (per 0.1 Nm/kg) with baseline-to-2-year tibiofemoral and patellofemoral cartilage damage worsening outcomes [Relative Risk (95% CI)] (n = 275 knees from 164 persons)
| Baseline Hip Abductor Strength (Nm/kg) | Any TF | Medial TF | Lateral TF | Any PF | Medial PF | Lateral PF |
|---|---|---|---|---|---|---|
| Number of knees (%) with poor outcome | 65 (23.6) | 38 (13.8) | 30 (10.9) | 46 (16.7) | 27 (9.8) | 23 (8.4) |
| Unadjusted | 0.92 (0.84, 1.01) | 0.95 (0.84, 1.08) | 0.84* (0.73, 0.97) | 0.90 (0.78, 1.04) | 0.83* (0.70, 0.99) | 0.94 (0.78, 1.13) |
| Adjusteda | 0.94 (0.86, 1.04) | 0.95b (0.84, 1.08) | 0.83*b (0.71, 0.98) | 0.88 (0.75, 1.02) | 0.80* (0.67, 0.95) | 0.92 (0.75, 1.13) |
Abbreviations: BW, body weight; CI, confidence interval; TF, tibiofemoral; PF, patellofemoral; WOMAC, Western Ontario and McMaster Universities Osteoarthritis Index; K/L, Kellgren/Lawrence
Relative risk (RR) with associated 95% CI that excludes 1.0 was considered to be statistically significant
Adjusted for age, sex, WOMAC pain, and K/L grade
Adjusted for age and sex. Models became unstable when adjusting for age, sex, WOMAC pain, and K/L grade
We further examined these relationships stratified by baseline knee extensor strength. In knees with better baseline extensor strength, baseline hip abductor strength was generally associated with a reduced likelihood of TF cartilage damage worsening. Women-only analyses had similar results (Supplemental Table B).
Hip abductor strength and poor function and disability outcomes 5 years later.
The derivation of the analysis sample (187 persons) is shown in Figure 1B. Table 3 illustrates LLFDI Total Function baseline cutpoints for each quintile and quintile movement between baseline and 5-year follow-up. Poor outcome was defined as remaining within the same low-function group (the worst two quintiles) or moving into a worse function quintile at 5-year follow-up. Similar methods were used to define poor outcomes in the other 5 LLFDI domains and for Chair-Stand-Rate. Table 4 summarizes the baseline characteristics of this sample. Of the 187 persons, 12 did not complete the Chair-Stand test at 5-year follow-up. Table 5 shows the number of persons with a poor outcome for each function or disability scale. As shown in Table 5, greater baseline hip abductor strength was significantly associated with reduced risks of poor outcomes for Chair-Stand-Rate (adjusted RR = 0.91, 95% CI: 0.83, 0.99), LLFDI Basic Lower Extremity Function (adjusted RR = 0.94, 95% CI: 0.88, 0.99), and LLFDI Disability Limitation (adjusted RR = 0.92, 95% CI: 0.85, 0.99) over the baseline-to-5-year follow-up interval. Every 0.1 Nm/kg increase in baseline body-weight-normalized hip abductor strength was associated with a 6–9% reduction in risk of poor outcomes. Sensitivity analyses where pain and K/L grade were removed as covariates minimally altered the effect estimates. Similar trends were noted in women-only models (Supplemental Table C).
Table 3.
LLFDI Total Function baseline quintiles and quintile movements between baseline and 5-year follow-up
| LLFDI Total Function quintile group at 5-year follow-up (n = 187) | ||||||
|---|---|---|---|---|---|---|
| LLFDI Total Function auintile aroup at baseline (n = 187) | Q1 (worst) | Q2 | Q3 | Q4 | Q5 (best) | |
| Q1 (worst) (≤ 54.14) |
30 | 8 | 2 | 0 | 1 | 41 |
| Q2 (> 54.14, ≤ 59.92) |
15 | 17 | 3 | 1 | 0 | 36 |
| Q3 (> 59.92, ≤ 65.02) |
3 | 16 | 11 | 8 | 4 | 42 |
| Q4 (> 65.02, ≤ 69.6) |
1 | 5 | 10 | 11 | 9 | 36 |
| Q5 (best) (> 69.6) |
0 | 1 | 7 | 6 | 18 | 32 |
| 49 | 47 | 33 | 26 | 32 | 187 | |
Abbreviations: LLFDI, Late-Life Function and Disability Instrument; Q1 to Q5 define quintile ranges used for the analysis. Shaded cells are numbers of participants with poor baseline-to-5-year outcome, defined as remaining within the same low-function group (the worse two groups) or moving into a worse function group at the 5-year follow-up.
Table 4.
Baseline characteristics of the baseline-to-5-year poor function and disability outcomes analysis sample (n = 187 persons)
| Person-based (n = 187): | Mean (SD) or number (%) |
|---|---|
| Age (years) | 64.2 (9.7) |
| Female | 147 (78.6%) |
| BMI (kg/m2) | 28.6 (5.8) |
| K/L grade of the knee with worse baseline hip abductor strength | |
| 0 | 12 (6.4%) |
| 1 | 30 (16.0%) |
| 2 | 89 (47.6%) |
| 3 | 27 (14.4%) |
| 4 | 29 (15.5%) |
| PF joint space narrowing score of the knee with worse baseline hip abductor strength | |
| 0 | 52 (27.8%) |
| 1 | 61 (32.6%) |
| 2 | 41 (21.9%) |
| 3 | 33 (17.6%) |
| WOMAC pain (0–20), higher indicating more severe pain | 4.5 (3.5) |
| WOMAC function (0–68), higher indicating worse function | 13.3 (11.3) |
| Hip abductor strength (worse of the two limbs) (Nm/kg) | 0.77 (0.22) |
| Knee extensor strength (worse of the two limbs) (Nm/kg) | 0.82 (0.28) |
| Chair-Stand-Rate (#/min) | 20.40 (6.58) |
| LLFDI Total Function (0–100), higher indicating better function | 61.88 (9.35) |
| LLFDI Basic LE Function (0–100) | 71.56 (13.25) |
| LLFDI Advanced LE Function (0–100) | 54.80 (12.94) |
| LLFDI Disability Frequency of Participation (0–100), higher indicating less disability | 55.46 (6.69) |
| LLFDI Disability Limitation (0–100) | 74.03 (12.84) |
| LLFDI Disability Instrumental Role Limitation (0–100) | 73.28 (13.41) |
Abbreviations: SD, Standard Deviation; BMI, Body Mass Index; K/L, Kellgren/Lawrence; PF, patellofemoral; WOMAC, Western Ontario and McMaster Universities Osteoarthritis Index; LLFDI, Late Life Function Disability Instrument
Table 5.
Associations of baseline BW-normalized hip abductor strength (per 0.1 Nm/kg) with baseline-to-5-year poor outcomes by Chair-Stand-Rate and LLFDI function and disability scores [Relative Risk (95% CI)] (n = 187 persons)
| Baseline Hip Abductor Strength (Nm/kg) | Chair-Stand-Rate | LLFDI Total Function | LLFDI Basic Lower Extremity Function | LLFDI Advanced Lower Extremity Function | LLFDI Disability Frequency of Participation | LLFDI Disability Limitation | LLFDI Disability Instrumental Role Limitation |
|---|---|---|---|---|---|---|---|
| Number of persons (%) with poor outcome | 71 (40.6) | 111 (59.4) | 107 (57.2) | 122 (65.2) | 91 (48.7) | 85 (45.5) | 86 (46.0) |
| Unadjusted | 0.90* (0.83, 0.98) | 0.95 (0.90, 1,00) | 0.93* (0.88, 0.99) | 0.95* (0.90, 0.99) | 0.94 (0.88, 1.01) | 0.94 (0.87, 1.01) | 0.96 (0.90, 1.03) |
| Adjusteda | 0.91* (0.83, 0.99) | 0.99 (0.95, 1.02) | 0.94* (0.88, 0.99) | 0.98 (0.95, 1.02) | 0.94 (0.88, 1.00) | 0.92* (0.85, 0.999) | 0.95 (0.88, 1.02) |
Abbreviations: BW, body weight; LLFDI, Late Life Function Disability Instrument; CI, confidence interval; K/L, Kellgren/Lawrence
Relative risk (RR) with associated 95% CI that excludes 1.0 was considered to be statistically significant
Adjusted for age, sex, hip pain during strength testing, and K/L grade of the knee with worse baseline hip abductor strength
We further examined these relationships stratified by baseline knee extensor strength. In persons with better baseline knee extensor strength, hip abductor strength had additional protective effects on function decline and disability, especially in Chair-Stand-Rate and LLFDI Disability Frequency of Participation. Women-only analyses yielded similar findings (Supplemental Table D).
DISCUSSION
In persons with knee OA, these findings suggest an association of greater baseline hip abductor strength and reduced risks of both baseline-to-2-year knee MRI-detected structural worsening and poor baseline-to-5-year function and disability outcomes. For every 0.1 Nm/kg increase in baseline body-weight-normalized hip abductor strength, there was a 17–20% reduced risk of 2-year medial PF and lateral TF compartment cartilage damage worsening and a 6–9% reduced risk of poor 5-year function and disability outcomes. Prior clinical trials of hip strengthening in the setting of knee OA have shown abductor strength improvements ranging from 0.14 to 0.25 Nm/kg21,22. An increase of 0.1 Nm/kg is reasonably attainable for individuals with knee OA. These findings support a beneficial role of hip abductor strength for disease modification and function preservation and as a therapeutic target to be incorporated in conservative management of knee OA.
Individuals with knee OA have hip abductor strength deficits when compared with healthy older adults14–17. These studies were cross-sectional, and unable to determine whether hip abductor weakness is a risk factor for disease worsening. To our knowledge, the current study, a prospective cohort study, is the first to report a potential benefit of hip abductor strength on subsequent cartilage damage worsening. The mean isometric hip abductor strength in our participants was 0.84 (SD 0.24) Nm/kg, comparable to published reports of 0.86 (SD 0.29) Nm/kg among 89 persons16 and 0.83 (SD 0.16) Nm/kg among 99 persons53 with knee OA.
The mechanisms by which greater hip abductor strength protects against progression are unclear and warrant further investigation. Trials21–23,54 have consistently found short-term clinical improvements in pain and function after hip-focused strengthening programs, of 4 to 12 weeks duration. The structural effects of such interventions have not been examined. Excessive knee load during walking has been shown to accelerate disease progression55,56, but it remains unclear whether hip strengthening alters knee loading. A 4-week hip abductor strength training program23 resulted in a reduction in the external knee adduction moment (KAM), a determinant of medial knee load during gait, in persons with knee OA. Hip abductor and external rotator strengthening for 6 weeks57 reduced KAM during running in healthy women. In contrast, despite improved symptoms, function, and strength, KAM change was not observed following 8 or 12 weeks of a supervised home hip-focused exercise program21,22. KAM, although a commonly used marker for knee load, may not fully capture the overall loading condition at the knee58. A probabilistic approach in musculoskeletal modeling revealed that weakening hip abductor force-generating capacity during gait elevated first and second peak knee contact force by 0.2 and 0.5 times body weight, respectively59, supporting the idea that diminished hip abductor strength could increase knee load.
In previous studies of persons with PF pain, hip abductor strengthening has resulted in symptom relief and normalized lower limb movement patterns and PF joint mechanics60–62. In theory, greater hip abductor strength may contribute to better local mechanical environment for the PF compartment. As a stabilizing muscle in the frontal plane, greater hip abductor strength may help normalize load distribution between the medial and lateral TF compartment, preventing TF cartilage deterioration. It is also plausible that the beneficial effect of hip strength operates through a combination of load moderation and other unidentified mechanisms.
To our knowledge, our study is the first long-term study reporting a protective effect of hip abductor strength on poor lower extremity function and disability outcomes in persons with knee OA. Our definition of poor outcome did not rely on change. In OA, change in function or disability measures may require several years. A focus on change ignores those with persistently high or low function, effectively lumping these individuals together in the same group. In a chronic disease that is slow to evolve, factors related to persistent low- or high-function states are of particular importance to the development of prevention or intervention programs. While clinical trials21–23,54 of hip-focused strengthening have consistently demonstrated short-term benefits, long-term associations have not been examined. Considering the critical role of the hip musculature in maintaining balance and postural stability during walking and transfers, our findings are not surprising. A recent study also demonstrated the benefit of adding hip abductor strengthening to a standard rehabilitation program in performance-based function measures 1 year after total knee arthroplasty63.
Interestingly, in the setting of better knee extensor strength, greater hip abductor strength appeared to confer additional beneficial effects on both joint health and long-term function and disability, suggesting that stronger hip abductors could further protect against poor structural and function outcomes compared to strong knee extensors alone. Our findings align with the conclusion of a recent meta-analysis64 suggesting that combined hip and knee strengthening over an average of 6 weeks resulted in greater pain relief and self-reported activity when compared with knee strengthening alone in individuals with PF pain. The improvements of combined hip and knee strengthening were maintained beyond the intervention period, with moderate-to-large effect sizes, suggesting possible long-term effects64.
The current study has limitations. Knee structural worsening was assessed between baseline and 2-year follow-up. A longer follow-up time may further elucidate the role of hip abductor strength in protecting against cartilage deterioration. Nearly 80% of our study participants were women, limiting the ability to perform analyses in men only. It is unclear whether these results can be generalized to men. The functional threshold for knee extensor strength in persons with knee OA has not been established. Therefore, we used median values as cutpoints to dichotomize knee strength in subgroup analyses. Although clinically relevant and important, subgroup analyses by knee extensor strength and by sex are likely to have limited power for many comparisons of interest.
In summary, greater baseline hip abductor strength was associated with a reduced risk of knee cartilage damage worsening, particularly in the medial PF and lateral TF compartments, and poor function and disability outcomes. Stronger hip abductors may confer additional structural and functional benefits in the presence of strong knee extensors. Incorporating hip abductor strengthening into nonpharmacological management of knee OA may help to optimize patient outcomes and slow structural progression. Our findings highlight the need for future clinical trials to assess the effects of hip-focused strengthening on long-term pain, function and disability outcomes as well as on structural preservation, and to determine the optimal exercise prescription of strengthening frequency, intensity, and duration.
Supplementary Material
ACKNOWLEDGEMENTS
The authors would like to thank Ms. Laura Belisle, Ms. September Cahue, and Mr. Clifton Saurel for assistance in data collection and all study participants for their contribution to the study.
FUNDING SOURCE:
NIH/NIAMS P30 AR072579 and P60 AR064464
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
CONFLICT OF INTEREST:
Ali Guermazi is President of Boston Imaging Core Lab (BICL), LLC and Consultant to Pfizer, GE, AstraZeneca, TissueGene, Roche, Galapagos, MerckSerono. None of the other authors have declared any conflict of interest.
REFERENCES
- 1.Cross M, Smith E, Hoy D, Nolte S, Ackerman I, Fransen M, et al. The global burden of hip and knee osteoarthritis: estimates from the Global Burden of Disease 2010 study. Ann Rheum Dis. 2014;73(7):1323–1330. doi: 10.1136/annrheumdis-2013-204763 [DOI] [PubMed] [Google Scholar]
- 2.Barbour KE, Helmick CG, Boring M, Brady TJ. Prevalence of doctor-diagnosed arthritis and arthritis-attributable activity limitation — United States, 2013–2015. MMWR Morb Mortal Wkly Rep. 2017;66(9):246–253. doi: 10.15585/mmwr.mm6609e1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Litwic A, Edwards M, Dennison E, Cooper C. Epidemiology and Burden of Osteoarthritis. Br Med Bull. 2013;105:185–199. doi: 10.1093/bmb/lds038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hochberg MC, Altman RD, April KT, Benkhalti M, Guyatt G, McGowan J, et al. American College of Rheumatology 2012 recommendations for the use of nonpharmacologic and pharmacologic therapies in osteoarthritis of the hand, hip, and knee. Arthritis Care Res. 2012;64(4):455–474. [DOI] [PubMed] [Google Scholar]
- 5.McAlindon TE, Bannuru RR, Sullivan MC, Arden NK, Berenbaum F, Bierma-Zeinstra SM, et al. OARSI guidelines for the non-surgical management of knee osteoarthritis. Osteoarthritis Cartilage. 2014;22(3):363–388. doi: 10.1016/j.joca.2014.01.003 [DOI] [PubMed] [Google Scholar]
- 6.Mikesky AE, Mazzuca SA, Brandt KD, Perkins SM, Damush T, Lane KA. Effects of strength training on the incidence and progression of knee osteoarthritis. Arthritis Rheum. 2006;55(5):690–699. doi: 10.1002/art.22245 [DOI] [PubMed] [Google Scholar]
- 7.Bennell KL, Hinman RS. A review of the clinical evidence for exercise in osteoarthritis of the hip and knee. J Sci Med Sport. 2011;14(1):4–9. doi: 10.1016/j.jsams.2010.08.002 [DOI] [PubMed] [Google Scholar]
- 8.Sharma L, Dunlop DD, Cahue S, Song J, Hayes KW. Quadriceps strength and osteoarthritis progression in malaligned and lax knees. Ann Intern Med. 2003;138(8):613–619. [DOI] [PubMed] [Google Scholar]
- 9.Amin S, Baker K, Niu J, Clancy M, Goggins J, Guermazi A, et al. Quadriceps strength and the risk of cartilage loss and symptom progression in knee osteoarthritis. Arthritis Rheum. 2009;60(1):189–198. doi: 10.1002/art.24182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Segal NA, Glass NA, Torner J, Yang M, Felson DT, Sharma L, et al. Quadriceps weakness predicts risk for knee joint space narrowing in women in the MOST cohort. Osteoarthritis Cartilage. 2010;18(6):769–775. doi: 10.1016/j.joca.2010.02.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Roos EM, Herzog W, Block JA, Bennell KL. Muscle weakness, afferent sensory dysfunction and exercise in knee osteoarthritis. Nat Rev Rheumatol. 2011;7(1):57–63. doi: 10.1038/nrrheum.2010.195 [DOI] [PubMed] [Google Scholar]
- 12.MacKinnon CD, Winter DA. Control of whole body balance in the frontal plane during human walking. J Biomech. 1993;26(6):633–644. [DOI] [PubMed] [Google Scholar]
- 13.Pandy MG, Lin Y-C, Kim HJ. Muscle coordination of mediolateral balance in normal walking. J Biomech. 2010;43(11):2055–2064. doi: 10.1016/j.jbiomech.2010.04.010 [DOI] [PubMed] [Google Scholar]
- 14.Costa RA, Oliveira LM de, Watanabe SH, Jones A, Natour J. Isokinetic assessment of the hip muscles in patients with osteoarthritis of the knee. Clin Sao Paulo Braz. 2010;65(12):1253–1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Piva SR, Teixeira PEP, Almeida GJM, Gil AB, DiGioia AM, Levison TJ, et al. Contribution of hip abductor strength to physical function in patients with total knee arthroplasty. Phys Ther. 2011;91(2):225–233. doi: 10.2522/ptj.20100122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hinman RS, Hunt MA, Creaby MW, Wrigley TV, McManus FJ, Bennell KL. Hip muscle weakness in individuals with medial knee osteoarthritis. Arthritis Care Res. 2010;62(8):1190–1193. doi: 10.1002/acr.20199 [DOI] [PubMed] [Google Scholar]
- 17.Deasy M, Leahy E, Semciw AI. Hip Strength Deficits in People With Symptomatic Knee Osteoarthritis: A Systematic Review With Meta-analysis. J Orthop Sports Phys Ther. 2016;46(8):629–639. doi: 10.2519/jospt.2016.6618 [DOI] [PubMed] [Google Scholar]
- 18.Chang A, Hayes K, Dunlop D, Song J, Hurwitz D, Cahue S, et al. Hip abduction moment and protection against medial tibiofemoral osteoarthritis progression. Arthritis Rheum. 2005;52(11):3515–3519. doi: 10.1002/art.21406 [DOI] [PubMed] [Google Scholar]
- 19.Henriksen M, Aaboe J, Simonsen EB, Alkjaer T, Bliddal H. Experimentally reduced hip abductor function during walking: Implications for knee joint loads. J Biomech. 2009;42(9):1236–1240. doi: 10.1016/j.jbiomech.2009.03.021 [DOI] [PubMed] [Google Scholar]
- 20.Rutherford DJ, Hubley-Kozey C. Explaining the hip adduction moment variability during gait: Implications for hip abductor strengthening. Clin Biomech. 2009;24(3):267–273. doi: 10.1016/j.clinbiomech.2008.12.006 [DOI] [PubMed] [Google Scholar]
- 21.Bennell KL, Hunt MA, Wrigley TV, Hunter DJ, McManus FJ, Hodges PW, et al. Hip strengthening reduces symptoms but not knee load in people with medial knee osteoarthritis and varus malalignment: a randomised controlled trial. Osteoarthritis Cartilage. 2010;18(5):621–628. doi: 10.1016/j.joca.2010.01.010 [DOI] [PubMed] [Google Scholar]
- 22.Sled EA, Khoja L, Deluzio KJ, Olney SJ, Culham EG. Effect of a home program of hip abductor exercises on knee joint loading, strength, function, and pain in people with knee osteoarthritis: a clinical trial. Phys Ther. 2010;90(6):895–904. doi: 10.2522/ptj.20090294 [DOI] [PubMed] [Google Scholar]
- 23.Thorp LE, Wimmer MA, Foucher KC, Sumner DR, Shakoor N, Block JA. The biomechanical effects of focused muscle training on medial knee loads in OA of the knee: a pilot, proof of concept study. J Musculoskelet Neuronal Interact. 2010;10(2):166–173. [PubMed] [Google Scholar]
- 24.Foroughi N, Smith RM, Lange AK, Baker MK, Fiatarone Singh MA, Vanwanseele B Lower limb muscle strengthening does not change frontal plane moments in women with knee osteoarthritis: A randomized controlled trial. Clin Biomech. 2011;26(2):167–174. doi: 10.1016/j.clinbiomech.2010.08.011 [DOI] [PubMed] [Google Scholar]
- 25.Barton CJ, Lack S, Malliaras P, Morrissey D. Gluteal muscle activity and patellofemoral pain syndrome: a systematic review. Br J Sports Med. 2013;47(4):207–214. doi: 10.1136/bjsports-2012-090953 [DOI] [PubMed] [Google Scholar]
- 26.Davis IS, Powers CM. Patellofemoral pain syndrome: proximal, distal, and local factors, an international retreat, April 30-May 2, 2009, Fells Point, Baltimore, MD. J Orthop Sports Phys Ther. 2010;40(3):A1–A48. doi: 10.2519/jospt.2010.0302 [DOI] [PubMed] [Google Scholar]
- 27.Witvrouw E, Callaghan MJ, Stefanik JJ, Noehren B, Bazett-Jones DM, Willson JD, et al. Patellofemoral pain: consensus statement from the 3rd International Patellofemoral Pain Research Retreat held in Vancouver, September 2013. Br J Sports Med. 2014;48(6):411–414. doi: 10.1136/bjsports-2014-093450 [DOI] [PubMed] [Google Scholar]
- 28.Thuillier DU, Souza RB, Wu S, Luke A, Li X, Feeley BT. T1ρ imaging demonstrates early changes in the lateral patella in patients with patellofemoral pain and maltracking. Am J Sports Med. 2013;41(8):1813–1818. doi: 10.1177/0363546513495167 [DOI] [PubMed] [Google Scholar]
- 29.Farrokhi S, Keyak JH, Powers CM. Individuals with patellofemoral pain exhibit greater patellofemoral joint stress: a finite element analysis study. Osteoarthritis Cartilage. 2011;19(3):287–294. doi: 10.1016/j.joca.2010.12.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hinman RS, Lentzos J, Vicenzino B, Crossley KM. Is patellofemoral osteoarthritis common in middle-aged people with chronic patellofemoral pain?: Patellofemoral OA in chronic patellofemoral knee pain. Arthritis Care Res. 2014;66(8):1252–1257. doi: 10.1002/acr.22274 [DOI] [PubMed] [Google Scholar]
- 31.Crossley KM. Is patellofemoral osteoarthritis a common sequela of patellofemoral pain? Br J Sports Med. 2014;48(6):409–410. doi: 10.1136/bjsports-2014-093445 [DOI] [PubMed] [Google Scholar]
- 32.Nagi S Disability concepts revisited: implication for prevention In: Pope AM, Tarlov AR, eds. Disability in America: Toward a National Agenda for Prevention. Washington, D.C: National Academies Press; 1991:309–327. [Google Scholar]
- 33.International Classification of Functioning, Disability, and Health: ICF. Geneva: World Health Organization; 2001. [Google Scholar]
- 34.Chang AH, Chmiel JS, Moisio KC, Almagor O, Zhang Y, Cahue S, et al. Varus thrust and knee frontal plane dynamic motion in persons with knee osteoarthritis. Osteoarthritis Cartilage. 2013;21(11):1668–1673. doi: 10.1016/j.joca.2013.08.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Drouin JM, Valovich-mcLeod TC, Shultz SJ, Gansneder BM, Perrin DH. Reliability and validity of the Biodex system 3 pro isokinetic dynamometer velocity, torque and position measurements. Eur J Appl Physiol. 2004;91(1):22–29. doi: 10.1007/s00421-003-0933-0 [DOI] [PubMed] [Google Scholar]
- 36.Peterfy CG, Guermazi A, Zaim S, Tirman PFJ, Miaux Y, White D, et al. Whole-Organ Magnetic Resonance Imaging Score (WORMS) of the knee in osteoarthritis. Osteoarthritis Cartilage. 2004;12(3):177–190. doi:14972335 [DOI] [PubMed] [Google Scholar]
- 37.Felson DT, Nevitt MC. Blinding images to sequence in osteoarthritis: evidence from other diseases. Osteoarthritis Cartilage. 2009;17(3):281–283. doi: 10.1016/j.joca.2008.09.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Felson DT, Lynch J, Guermazi A, Roemer FW, Niu J, McAlindon T, et al. Comparison of BLOKS and WORMS Scoring Systems Part II. Longitudinal Assessment of Knee MRIs for Osteoarthritis and Suggested Approach Based on their Performance: Data from the Osteoarthritis Initiative. Osteoarthritis Cartilage. 2010;18(11):1402–1407. doi: 10.1016/j.joca.2010.06.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hunter DJ, Zhang W, Conaghan PG, Hirko K, Menashe L, Reichmann WM, et al. Responsiveness and reliability of MRI in knee osteoarthritis: a meta-analysis of published evidence. Osteoarthritis Cartilage. 2011;19(5):589–605. doi: 10.1016/j.joca.2010.10.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Roemer FW, Hunter DJ, Crema MD, Kwoh CK, Ochoa-Albiztegui E, Guermazi A. An illustrative overview of semi-quantitative MRI scoring of knee osteoarthritis: lessons learned from longitudinal observational studies. Osteoarthritis Cartilage. 2016;24(2):274–289. doi: 10.1016/j.joca.2015.08.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Haley SM, Jette AM, Coster WJ, Kooyoomjian JT, Levenson S, Heeren T, et al. Late Life Function and Disability Instrument: II. Development and evaluation of the function component. J Gerontol A Biol Sci Med Sci. 2002;57(4):M217–222. [DOI] [PubMed] [Google Scholar]
- 42.Jette AM, Haley SM, Coster WJ, Kooyoomjian JT, Levenson S, Heeren T, et al. Late Life Function and Disability Instrument: I. Development and evaluation of the disability component. J Gerontol A Biol Sci Med Sci. 2002;57(4):M209–M216. doi: 10.1093/gerona/57.4.M209 [DOI] [PubMed] [Google Scholar]
- 43.Sharma L, Cahue S, Song J, Hayes K, Pai Y-C, Dunlop D. Physical functioning over three years in knee osteoarthritis: role of psychosocial, local mechanical, and neuromuscular factors. Arthritis Rheum. 2003;48(12):3359–3370. doi: 10.1002/art.11420 [DOI] [PubMed] [Google Scholar]
- 44.Dunlop DD, Semanik P, Song J, Sharma L, Nevitt M, Mysiw J, et al. Moving to Maintain Function in Knee Osteoarthritis: Evidence from the Osteoarthritis Initiative. Arch Phys Med Rehabil. 2010;91(5):714–721. doi: 10.1016/j.apmr.2010.01.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Holla JFM, Steultjens MPM, Roorda LD, Heymans MW, Ten Wolde S, Dekker J. Prognostic factors for the two-year course of activity limitations in early osteoarthritis of the hip and/or knee. Arthritis Care Res. 2010;62(10):1415–1425. doi: 10.1002/acr.20263 [DOI] [PubMed] [Google Scholar]
- 46.Colbert CJ, Song J, Dunlop D, Chmiel JS, Hayes KW, Cahue S, et al. Knee confidence as it relates to physical function outcome in persons with or at higher risk for knee osteoarthritis in the osteoarthritis initiative. Arthritis Rheum. 2012;64(5):1437–1446. doi: 10.1002/art.33505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Buckland-Wright C Protocols for precise radio-anatomical positioning of the tibiofemoral and patellofemoral compartments of the knee. Osteoarthritis Cartilage. 1995;3 Suppl A:71–80. [PubMed] [Google Scholar]
- 48.Kellgren JH, Lawrence JS. Radiological assessment of osteo-arthrosis. Ann Rheum Dis. 1957;16(4):494–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Altman RD, Hochberg M, Murphy WA, Wolfe F, Lequesne M. Atlas of individual radiographic features in osteoarthritis. Osteoarthritis Cartilage. 1995;3 Suppl A:3–70. [PubMed] [Google Scholar]
- 50.Bellamy N, Buchanan WW, Goldsmith CH, Campbell J, Stitt LW. Validation study of WOMAC: a health status instrument for measuring clinically important patient relevant outcomes to antirheumatic drug therapy in patients with osteoarthritis of the hip or knee. J Rheumatol. 1988;15(12):1833–1840. [PubMed] [Google Scholar]
- 51.McConnell S, Kolopack P, Davis AM. The Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC): a review of its utility and measurement properties. Arthritis Rheum. 2001;45(5):453–461. [DOI] [PubMed] [Google Scholar]
- 52.McNutt L-A, Wu C, Xue X, Hafner JP. Estimating the relative risk in cohort studies and clinical trials of common outcomes. Am J Epidemiol. 2003;157(10):940–943. [DOI] [PubMed] [Google Scholar]
- 53.Kean CO, Bennell KL, Wrigley TV, Hinman RS. Relationship between hip abductor strength and external hip and knee adduction moments in medial knee osteoarthritis. Clin Biomech. 2015;30(3):226–230. doi: 10.1016/j.clinbiomech.2015.01.008 [DOI] [PubMed] [Google Scholar]
- 54.Lun V, Marsh A, Bray R, Lindsay D, Wiley P. Efficacy of hip strengthening exercises compared with leg strengthening exercises on knee pain, function, and quality of life in patients with knee osteoarthritis. Clin J Sport Med. 2015;25(6):509–517. doi: 10.1097/JSM.0000000000000170 [DOI] [PubMed] [Google Scholar]
- 55.Bennell KL, Bowles K-A, Wang Y, Cicuttini F, Davies-Tuck M, Hinman RS. Higher dynamic medial knee load predicts greater cartilage loss over 12 months in medial knee osteoarthritis. Ann Rheum Dis. 2011;70(10):1770–1774. doi: 10.1136/ard.2010.147082 [DOI] [PubMed] [Google Scholar]
- 56.Chang AH, Moisio KC, Chmiel JS, Eckstein F, Guermazi A, Prasad PV, et al. External knee adduction and flexion moments during gait and medial tibiofemoral disease progression in knee osteoarthritis. Osteoarthritis Cartilage. 2015;23(7):1099–1106. doi: 10.1016/j.joca.2015.02.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Snyder KR, Earl JE, O’Connor KM, Ebersole KT. Resistance training is accompanied by increases in hip strength and changes in lower extremity biomechanics during running. Clin Biomech. 2009;24(1):26–34. doi: 10.1016/j.clinbiomech.2008.09.009 [DOI] [PubMed] [Google Scholar]
- 58.Walter JP, D’Lima DD, Colwell CW, Fregly BJ. Decreased knee adduction moment does not guarantee decreased medial contact force during gait. J Orthop Res. 2010;28(10):1348–1354. doi: 10.1002/jor.21142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Valente G, Taddei F, Jonkers I. Influence of weak hip abductor muscles on joint contact forces during normal walking: probabilistic modeling analysis. J Biomech. 2013;46(13):2186–2193. doi: 10.1016/j.jbiomech.2013.06.030 [DOI] [PubMed] [Google Scholar]
- 60.Lack S, Barton C, Sohan O, Crossley K, Morrissey D. Proximal muscle rehabilitation is effective for patellofemoral pain: a systematic review with meta-analysis. Br J Sports Med. 2015;49(21):1365–1376. doi: 10.1136/bjsports-2015-094723 [DOI] [PubMed] [Google Scholar]
- 61.Khayambashi K, Fallah A, Movahedi A, Bagwell J, Powers C. Posterolateral hip muscle strengthening versus quadriceps strengthening for patellofemoral pain: a comparative control trial. Arch Phys Med Rehabil. 2014;95(5):900–907. doi: 10.1016/j.apmr.2013.12.022 [DOI] [PubMed] [Google Scholar]
- 62.Earl JE, Hoch AZ. A proximal strengthening program improves pain, function, and biomechanics in women with patellofemoral pain syndrome. Am J Sports Med. 2011;39(1):154–163. doi: 10.1177/0363546510379967 [DOI] [PubMed] [Google Scholar]
- 63.Harikesavan K, Chakravarty RD, Maiya AG, Hegde SP, Shivanna YS. Hip abductor strengthening improves physical function following total knee replacement: One-year follow-up of a randomized pilot study. Open Rheumatol J. 2017;11(1):30–42. doi: 10.2174/1874312901711010030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Nascimento LR, Teixeira-Salmela LF, Souza RB, Resende RA. Hip and knee strengthening is more effective than knee strengthening alone for reducing pain and improving activity in individuals with patellofemoral pain: A systematic review with meta-analysis. J Orthop Sports Phys Ther. 2018;48(1):19–31. doi: 10.2519/jospt.2018.7365 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.