Abstract
The underlying mechanisms that result in neurophysiological changes and cognitive sequelae in the context of repetitive mild traumatic brain injury (rmTBI) remain poorly understood. Animal models provide a unique opportunity to examine cellular and molecular responses using histological assessment, which can give important insights on the neurophysiological changes associated with the evolution of brain injury. To better understand the potential cumulative effects of multiple concussions, the focus of animal models is shifting from single to repetitive head impacts. With a growing body of literature on this subject, a review and discussion of current findings is valuable to better understand the neuropathology associated with rmTBI, to evaluate the current state of the field, and to guide future research efforts. Despite variability in experimental settings, existing animal models of rmTBI have contributed to our understanding of the underlying mechanisms following repeat concussion. However, how to reconcile the various impact methods remains one of the major challenges in the field today.
Keywords: Animal research, concussion, mild traumatic brain injury, repetitive head impacts, neuropathology
1. Introduction
Mild traumatic brain injury (mTBI) is a serious public health issue with roughly 42 million people affected worldwide—every year (Gardner & Yaffe, 2015). The prevalence of repetitive mTBI (rmTBI) is unclear, but prior mTBI may increase risk for future mTBI (Dams-O’Connor, et al., 2013). mTBI, also known as concussion, results from a blow to or jolt of the head and is associated with various clinical features, which may or may not include loss of consciousness (usually seconds to minutes), indicating great heterogeneity of its underlying pathology (Rosenbaum & Lipton, 2012). Typically, concussion results in altered brain function without gross neuropathology as can be seen with standard imaging techniques (Shenton, et al., 2012).
While most individuals make a full recovery within the first few days to weeks after injury (Karr, Areshenkoff, & Garcia-Barrera, 2014), some individuals (i.e., the “miserable minority”) develop permanent disability after concussion (Alexander, 1995), including headaches, dizziness, behavioral abnormalities, memory dysfunction, and motor control impairment (Lo, Shifteh, Gold, Bello, & Lipton, 2009). The underlying mechanisms that result in these detrimental effects remain poorly understood, which makes it difficult to treat and predict disease outcome.
The study of human mTBI is complicated by the heterogeneity of underlying pathology due to inter-individual differences and differences in the characteristics of each injury (Lipton, et al., 2012; Rosenbaum & Lipton, 2012). Animal models can address some of these limitations and provide valuable insights in the underlying pathology. One major advantage of animal research is that it enables reproducible trauma and the systematic variation of experimental parameters. Animal research also provides a unique opportunity to examine cellular and molecular responses using histological assessment, which can give important insights on the neurophysiological changes associated with the evolution of brain injury.
Since the 1940’s, various animal models have been developed to reproduce the biomechanical, neurological, and pathological aspects observed in human head injuries. The methods to produce head injury are generally based on weight-drop, fluid-percussion, or piston-controlled impact. Several excellent reviews discuss these TBI models in more detail, including their strengths and limitations (Shultz, et al., 2017; Xiong, Mahmood, & Chopp, 2013). In short, in weight-drop models, trauma is achieved by allowing an object to free fall from a specific height onto the animal’s head (with or without craniotomy) to produce focal (Feeney, Boyeson, Linn, Murray, & Dail, 1981) or more diffuse injury (Marmarou, et al., 1994). Manipulating the distance and weight of the falling object affects force to the head at the time of impact over the interacting surface area, or the load, and thus the injury severity. In fluid-percussion models, brain injury is produced by rapidly injecting fluid volumes into the cranial cavity causing a pressure pulse that produces elastic deformation of the brain (requires craniotomy), which is thought to resemble brain deformation after head impact (Dixon, et al., 1987; Sullivan, et al., 1976). In piston-controlled impact, initially developed in the ferret (Lighthall, 1988), a pneumatic or electromagnetic driven rod is fired directly onto the exposed cortex (requires single or bilateral craniotomy and is also known as controlled cortical impact or CCI) or closed-skull either with or without scalp retraction, with the head often immobilized in a stereotaxic frame.
Animal models of single mild traumatic brain injury typically show minimal and transient changes in cognition and behavior in the absence of abundant histopathological findings compared to sham animals (Hylin, Orsi, Zhao, et al., 2013; Meehan, Zhang, Mannix, & Whalen, 2012; B. Mouzon, et al., 2012; B. C. Mouzon, et al., 2014; Qin, et al., 2016; Winston, et al., 2016). In our own laboratory, a small sample of rats was examined with diffusion tensor imaging (DTI) and histology after a single rubber-tipped skull impact (Branch, et al., 2012). Acute changes (n=13) in corpus callosum white matter anisotropy (indicated by low fractional anisotropy and high radial diffusivity) followed by progressive recovery to pre-injury values after 2 weeks (n=9) was found in the absence of any abundant histological findings.
To date, most animal studies have modelled single mild TBI. However, individuals who participate in contact sports are often at risk for multiple head impacts. For example, 25% of retired National Football League players reported to have experienced more than one concussion in their career (Pellman, et al., 2004). Moreover, there is great potential for the study of subconcussive head impacts (e.g., soccer heading) due to its highly repetitive nature and association with altered brain structure, cognition, and CNS symptoms as found in human studies (Levitch, et al., 2018; Lipton, et al., 2013; Stewart, et al., 2017). To better understand the potential cumulative effects of multiple concussions, the focus of animal models is shifting from single to repetitive head impacts. With a growing body of literature on this subject, a review and discussion of current findings is valuable to better understand the neuropathology associated with repeat concussion, to evaluate the current state of research, and to guide future research efforts. The purpose of this review is to systematically summarize and detail the methods and pathological findings of the animal literature related to closed-skull, repetitive mild TBI, and to highlight its strengths and limitations, which can serve as important considerations for future research. We summarize key aspects of the cited articles, including animals used, model characteristics (i.e., model specifications, adverse effects, inter-injury interval, repeated anesthesia, and head immobilization techniques), primary outcome measures (i.e., behavior, histopathology, MRI), and overaraching challenges. While prior reviews have provided an excellent overview of single impact or more severe TBI modeling (Johnson, Meaney, Cullen, & Smith, 2015; Nizamutdinov & Shapiro, 2017; O’Connor, Smyth, & Gilchrist, 2011; Xiong, et al., 2013; Y. P. Zhang, et al., 2014), the focus of our review is exclusively on animal models of repetitive head impacts that fall within the mild end of the TBI spectrum.
2. Methods
A structured literature search was performed using PubMed to include all relevant articles through 2017. A detailed description of the selection process is shown in the PRISMA flow diagram (Figure 1).
Figure 1.
PRISMA flow diagram. aReasons for the exclusion of these studies are listed in section ‘2.2 Literature search criteria and keywords’.
2.1. Definition of mild traumatic brain injury animal models
In this work, we use the terms mild traumatic brain injury or “mTBI” and “concussion” synonymously to indicate the same condition. While various definitions for concussion exist, clinical concussion is typically characterized by direct-impact, non-penetrating head injury—that is, closed-skull or closed-head impact (Mayer, Quinn, & Master, 2017). For this reason, clinically relevant models of experimental concussion should use minimally invasive head impact methods, induce mild injury and exhibit symptoms in the absence of gross neuropathology (Bolouri & Zetterberg, 2015). Therefore, we limited our literature search to “closed-skull” models and excluded any impact techniques such as controlled cortical impact (CCI) and fluid percussion injury (FPI), which requires craniotomy—an invasive method—that has shown to generate profound inflammatory, morphological and behavioral damage independent of TBI (Adams, Schwarting, & Huston, 1994; Cole, et al., 2011). Blast studies were also excluded from this review as their biomechanics and pathological mechanism may be different from direct impact closed-head injuries (Angoa-Perez, et al., 2014; Kimpara & Iwamoto, 2012).
2.2. Literature search criteria and keywords
Title and abstract sections were searched to identify the literature related to mTBI (primary key words used: “mild traumatic brain injury,” “mild tbi,” “mTBI,” and “concussion”), which yielded 29,654 articles. Sixteen additional records were identified through other sources. Search results were then narrowed down to 685 articles to include studies on repetitive head impacts only (primary key words in combination with “repetitive,” “repeat,” and “multiple”). These search results where then filtered to exclude “blast” studies, “reviews,” “human studies,” “full text not available,” and “language other than English.” After this filter, a total of 64 articles remained that were individually assessed for eligibility. Given the heterogeneity in human mTBI as well as in the field of animal mTBI models, we utilized very strict inclusion and exclusion criteria to enable review of studies with relatively similar model characteristics. Thirty-two articles were further excluded for the following reasons: open skull models (n=7), pathology limited to retina/optic nerve (n=3), research without wildtype animals (n=5), exposure other than repetitive mTBI, e.g., steroids, high-fat diet, or alcohol (n=3), methodology-based reports (n=3), single mTBI studies and studies that are more severe than mild TBI (n=7), therapeutic intervention studies (n=3), in vitro studies (n=1). After we applied all these inclusion/exclusion criteria, filters and assessments, a total of 32 original animal research articles on closed-skull, repetitive mTBI were retained and systematically analyzed for this review. These articles are listed in Table 1.
Table 1.
Repetitive mild traumatic brain injury animal studies identified through the search process. Authors listed in alphabetical order.
Abbreviations: MRI, magnetic resonance imaging; HIST, histology; IHC, immunohistochemistry
3. Animal models of repetitive mild traumatic brain injury (rmTBI)
3.1. Animals
More than 2000 wild-type animals were used in the 32 studies reviewed in this work. Most studies used mice (24 studies, 75%; n mice, median ± IQR [range] = 118 ± 112 mice [18 – 327]) followed by rats (8 studies, 25%; n rats, median ± IQR [range] = 36 ± 16 rats [27–135]). Species subtype most often used for mice were C57BL/6 (22 studies, 92%) followed by Swiss Webster (1 study, 4%) and one unspecified strain (1 study, 4%). Species subtype most often used for rats were Sprague-Dawley (5 studies, 62.5%) followed by Wistar (3 studies, 37.5%). All studies exclusively used male animals, except one study that did not specify gender. At the time of testing and regardless of species, most animals were adult (21 studies, 65%), followed by young adult (4 studies, 13%), juvenile (3 studies, 9%), and unspecified age (4, 13%). Average weight of all mice was 26 ± 5 gram (range = 20 – 42 gram) versus 325 ± 53 gram (range = 230 – 500 gram) for all rats. Food and water were provided ad libitum (22 studies, 69%), as standard lab diet with water ad libitum (4 studies, 13%), or it was unspecified (6 studies, 19%). Animals were typically housed in standard conditions—that is, 12h light/dark cycles at 20–23C and 50% humidity.
Most animal studies included in this work used adult rodents, while only few studies focused on juvenile animals. Studying animals at developmental stages that correspond with human developmental stages is greatly needed, because brain injury is disproportionally high in children and adolescents (Thurman, 2016). Although an in-depth discussion of juvenile models of rmTBI is beyond the scope of this review, we believe that more studies should include juvenile animals to better understand the mechanisms associated with brain injury and adverse outcomes following mTBI in pediatric populations.
All studies included in this review used rodents (i.e., mice or rats). While the use of other animal species such as ferrets (Lighthall, 1988), swine (Smith, et al., 1997), mongrel dogs (Gurdjian, Lissner, Webster, Latimer, & Haddad, 1954), rhesus monkeys (Grubb, Naumann, & Ommaya, 1970; Kanda, et al., 1981; Ommaya, Grubb, & Naumann, 1971; Yarnell & Ommaya, 1969), cynomologous monkeys (Jane, Steward, & Gennarelli, 1985), and chimpanzees (Ommaya, Corrao, & Letcher, 1973; Ommaya & Hirsch, 1971) was more common in past TBI research, the use of rodents has become standard practice as it has clear economic advantages (Bryda, 2013) and it presents fewer ethical concerns (Coors, Glover, Juengst, & Sikela, 2010).
However, some limitations of rodent-only models should be noted. While rodent brains are structurally and functionally similar to humans, a number of crucial differences exist. Rodent brains are much smaller and have lower mass, which may affect the ability to simulate rotational acceleration of larger brains – a key feature of traumatic brain injury – and thus may not exactly replicate the tissue-level forces and neuropathology desired (Cullen, et al., 2016). Further, rodents not only have a lissenchephalic brain, they also have disproportionately less white matter than grey matter (K. Zhang & Sejnowski, 2000), which makes modeling diffuse axonal injury (DAI), the hallmark pathology of closed-head diffuse brain injury, more complicated. Also, the maturational sequence of large brain species is closer to humans than small brain species, which makes comparison of brain injury between rodents and human more challenging at specific developmental stages (Duhaime, 2006). Even within rodents (i.e., mice versus rats) substantial differences exist, especially in terms of social and cognitive behavior (Ellenbroek & Youn, 2016). For these reasons, in an attempt to bridge the gap between rodents and humans, larger-brain animals, such as minipigs or swine are re-introduced to study human disease and development, including Huntington’s Disease (Schubert, et al., 2016), cerebral white matter injury (McGuire, et al., 2017), and traumatic brain injury (Cullen, et al., 2016; Williams, et al., 2018). So, while research with rodents has clear economic, practical and ethical benefits, there are translational challenges that limit the applicability of rodent models.
Most studies in this review used mice (75%). The use of mouse models has several advantages, including the availability of techniques for genetic manipulation (Battey, Jordan, Cox, & Dove, 1999; Y. P. Zhang, et al., 2014), even though the availability of genetic tools for the rat has increased in recent years (Ellenbroek & Youn, 2016; Hamilton, et al., 2014). Not surprisingly, the Sprague Dawley rat and the C57BL/6 mouse were the most used animal strains. The use of these strains in the research community is widespread, possibly due to easier handling and more favorable breeding characteristics compared to other strains.
Of important note, none of the reviewed studies indicated the use of female animals in their study of rmTBI. Relying on male only animals improves reproducibility due to limited hormonal changes, but ignores the clinically relevant diversity in response to brain injury and outcome in men and women (Bazarian, Blyth, Mookerjee, He, & McDermott, 2010; Gallagher, et al., 2018). In accordance with newly developed NIH policies (Clayton & Collins, 2014), future research should balance male and female animals in their study design as much as possible.
3.2. Model characteristics to generate multiple mTBIs
See Table 2 for a detailed summary of model specifications, and Supplemental Table 1 for a more detailed summary of model specifications for each study included in this review. In short, all studies included in this review used a closed-skull impact approach. In other words, craniotomy was not performed for the impact procedure, although 13 studies (41%) used scalp incision and retraction to expose the skull for direct impact or disc placement. Piston-controlled impact (18 studies, 56%) and weight-drop (13 studies, 41%) were the methods most often used to generate rmTBI. Both methods are popular for good reasons: In piston-controlled impact, the impact parameters can be precisely set and adjusted through the electromagnetic or pneumatic driven impactor device, which enables high impact reproducibility. On the other hand, weight-drop methods require simple and inexpensive materials that are easy to setup. One study (Viano, Hamberger, Bolouri, & Saljo, 2009) used projectile or ballistic impact (1 study, 3%).
Table 2.
Model characteristics of the 32 studies included in this review.
| Median ± IQR [min – max] | |
|---|---|
| Number of wildtype animals per study | 80 ± 112 [18 – 327] |
| Number of repetitive impacts | 4 ± 3 [2–42] |
| Inter-injury interval (days) | 1 ± 2 [3 min – 30 days] |
| Episodes of anesthesia | 4 ± 3 [0–30] |
| N studies (%) | |
| Species: mice / rats (% mice) | 24 / 8 (75%) |
| Impact methods | |
| Piston-controlled (% impact methods) | 18 (56%) |
| Weight drop (% impact methods) | 13 (41%) |
| Projectile impact (% impact methods) | 1 (3%) |
| Closed-skull impact | 32 (100%) |
| Intact scalp (% shaved) | 14 (36%) |
| Skull bone (scalp retraction) (% closed-skull impact) | 8 (25%) |
| Helmet (% disc placed on intact scalp)a | 10 (50%) |
| General anesthetics for the impact procedureb | 30 (94%) |
| Isoflurane / otherc (% isoflurane) | 25 / 5 (83%) |
| Head not rigidly fixed (% studies) | 22 (72%) |
Two studies (Petraglia, Plog, Dayawansa, Chen, et al., 2014; Petraglia, Plog, Dayawansa, Dashnaw, et al., 2014) specified a disc-to-head attachment method (elastic band and tape), whereas 3 studies (Bolouri, et al., 2012; Creeley, et al., 2004; Qin, et al., 2016) did not. All studies specified the use of a special adhesive to secure the disc to the skull bone, except one (Weil, et al., 2014).
Two studies (Petraglia, Plog, Dayawansa, Chen, et al., 2014; Petraglia, Plog, Dayawansa, Dashnaw, et al., 2014) used no anesthesia for the impact procedure. One study (Hylin, Orsi, Rozas, et al., 2013) reported the use of a partially awake animal, as assessed by the recovery of the tail-pinch response, i.e., 40 seconds after discontinuation of anesthesia, an impact was applied to the skull.
Other anesthetics used include halothane/propofol, pentobarbital, and diazepam.
Among all studies, the number of repetitive impacts was 4 ± 3 hits (median ± IQR) and ranged from 2 to 42 impacts. The inter-injury interval was 1 ± 2 days (median ± IQR) and ranged from a minimum of 3 minutes to a maximum of 30 days between impacts. All studies used general anesthesia for the impact session in the form of isoflurane (n=25, 83%) or other anesthetics (i.e., halothane/propofol, n=2, 7%; pentobarbital, n=2, 7%; or diazepam, n=1, 3%), except for two studies that reported the use of awake animals (Petraglia, Plog, Dayawansa, Chen, et al., 2014; Petraglia, Plog, Dayawansa, Dashnaw, et al., 2014). Animals underwent 4 ± 3 episodes of anesthesia (median ± IQR) with a minimum of 0 and a maximum of 30 episodes. The median impact velocity for piston-controlled, weight-drop, and projectile impact was 5 m/s, 4.35 m/s, and 10.25 m/s, respectively.
The majority of studies included in this review reported the impact location to be centrally on the head (n=22 studies), often defined as “on the midline between bregma and lambda” or similar. All other studies (n=10 studies) reported left-sided impact, often defined as “left hemisphere between lambda and bregma up to but not crossing the midline” or similar. While a central location may be the most intuitive choice for impact, a good reason to opt for lateral impact is that, in addition to linear acceleration, it can also induce rotational acceleration of the brain, which is a key feature of concussion. Of further note, most animal models use the same location for multiple impacts (whether central or lateral), which improves the reproducibility of results, but may limit the translational potential of the model given that repeated head impacts in settings such as sports typically vary in impact location on the head. For this reason, future studies should consider varying impact location.
It should be noted that the level of experimental variability among these studies is substantial, which introduces a number of issues in respect to reproducibility of results among laboratories and translational potential of models – an issue especially valid for closed-head injury models due to the many factors that affect the mechanical response of the head (e.g., impact method and type of head support) (Namjoshi, et al., 2013). The experimental variability among studies and potential solutions are discussed in more detail later in this review (see section “5. Overarching challenges of rmTBI animal models”).
3.3. Adverse effects
A number of studies reported increased mortality (reported by 2 studies, and up to 10% of impacted animals (Kane, et al., 2012)), skull fractures (reported by 7 studies, and up to 25% of impacted animals (Hylin, Orsi, Rozas, et al., 2013)), and intracranial bleeding (reported by 5 studies, and up to 10% of impacted animals (Kane, et al., 2012)). One study reported the euthanization of 12 out of 50 animals before the end of the study due to significant motor deficits (Buckley, et al., 2015). Animals that displayed any of these adverse effects were reportedly removed from the respective study. While the incidence of adverse effects following head impacts was low, all of these findings are extremely rare in human studies of concussion, which suggests a potential mismatch between injury severity used in the animal models versus the typical injury experienced in the clinic. These findings represent a limitation of the animal studies in terms of clinical significance and also highlight the variability between animal models utilized to date.
3.4. Inter-injury interval
Time between impacts, also known as inter-injury interval, is an important factor in the development of brain injury and cognitive outcome after repeated head impacts (Meehan, et al., 2012; Vagnozzi, et al., 2007). Of all studies included in this review, the typical inter-injury interval was 1 day (median), but it ranged from 3 minutes to 30 days between impacts. One study reported that a shorter inter-injury interval resulted in more evident axonal injury and vascular dysfunction, although this was also dependent on impact intensity (Fujita, Wei, & Povlishock, 2012). Bolton and colleagues (Bolton & Saatman, 2014) studied the effects of inter-injury interval and reported increased acute pathology (neuron death and glial reactivity) following 5 head impacts at 24 hours interval compared to a single head impact, but when the inter-injury interval was increased from 24 to 48 hours, the resulting pathology was comparable to that of a single mTBI. However, in their follow up study to determine long-term outcomes (Bolton Hall, Joseph, Brelsfoard, & Saatman, 2016), persistent behavioral dysfunction and chronic pathological changes were observed independent of inter-injury interval.
It has been suggested that the inter-injury interval in animal models of repetitive mTBI should be scaled to clinically relevant inter-injury intervals (Shultz, et al., 2017). For example, contact sport athletes are often exposed to multiple head impacts per training or competitive event. Therefore, animal models with short inter-injury intervals and multiple head impacts per day or session may be relevant to athletes with repetitive sports-related concussion or subconcussion. Alternatively, long inter-injury intervals of weeks or more could be considered to study the effects of a repeat concussion after a period of rest. Further research efforts are needed to clarify the role of inter-injury interval after repetitive concusion.
3.5. Repeated episodes of general anesthesia
It is known that general anesthesia has both neuro-protective (Deng, et al., 2014; Karmarkar, Bottum, & Tischkau, 2010; Tawfeeq, Halawani, Al-Faridi, Aal-Shaya, & Taha, 2009) and neuro-destructive properties (Dong, Wu, Xu, Zhang, & Xie, 2012; Le Freche, et al., 2012; B. Mouzon, et al., 2012). All studies of repetitive mTBI included in this review, except for 2 reports (Petraglia, Plog, Dayawansa, Chen, et al., 2014; Petraglia, Plog, Dayawansa, Dashnaw, et al., 2014), used between 2 and 30 episodes of general anesthesia. Most studies used sham injured animals or “anesthesia controls” that underwent the same procedures and anesthesia duration—but did not receive the head impact—to control for the effects of repeated anesthesia. While the use of general anesthesia may seem necessary for trauma induction, the use of un-anesthetized animals has been described in this work (Petraglia, Plog, Dayawansa, Chen, et al., 2014; Petraglia, Plog, Dayawansa, Dashnaw, et al., 2014) and should be considered in future TBI research to avoid the potential confounding effects of repeated anesthesia on the evolution of brain injury pathology and outcome measures. A recent review (Wojnarowicz, Fisher, Minaeva, & Goldstein, 2017) also underscores this point by listing various anesthesia-related artifacts that complicate animal modeling and provide justification for anesthesia-free experiments where ethically possible. However, it should be noted that the elimination of anesthesia introduces a new potential confounder: stress due to repeated restraint and injury. Repeated stress followed by mTBI has been associated with altered brain metabolism and more severe behavioral deficits in rats compared to those with mTBI alone (Xing, et al., 2013). Nonetheless, it can be argued, at least from a translational perspective, that stress is a lesser confound than anesthesia given that exposure to stress is a plausible cofactor to real world concussion, certainly more so than repeated episodes of anesthesia.
3.6. Head immobilization during impact
Another important point of consideration in the design of animal TBI models is the choice of head immobilization technique. In 22 of 32 studies in this review, the animal’s head was allowed to move after impact—that is, the head was supported by a foam pad or similar material, but not completely immobilized in a stereotaxic frame with ear bars as was the case in the other studies (these were all piston-controlled models, but not all piston-controlled models used full head immobilization techniques). While full head immobilization enables precise impact localization and reproducibility, it does not account for the important mechanical forces that act on the head and brain after impact—including linear and rotational acceleration—that contribute to diffuse injury associated with mild TBI (McKee & Daneshvar, 2015). The unrestricted movement of the head is a key characteristic of head impact, and therefore, animal models of rmTBI should involve rapid head rotation and acceleration (opposed to the head fixed in place), especially when serving as models of sports-related head injury (Angoa-Perez, et al., 2014). Furthermore, some minor variability in impact location, as a result of the non-immobilized head technique, may actually be relevant to the real world of multiple head impacts. For these reasons, future studies of repetitive mild TBI should consider the use of a compliant head support rather than full head immobilization.
4. Assessment of pathology
To get an idea of the evolution of pathology associated with repeated head impacts, findings in this review are grouped by injury phase: “acute” refers to assessment 1–2 days after final injury, “subacute” refers to assessment 3–7 days after final injury, “short-term” refers to assessment 1–4 weeks after final injury, and “long-term” refers to assessment more than one month after final injury.
4.1. Histological assessment and immunohistochemistry
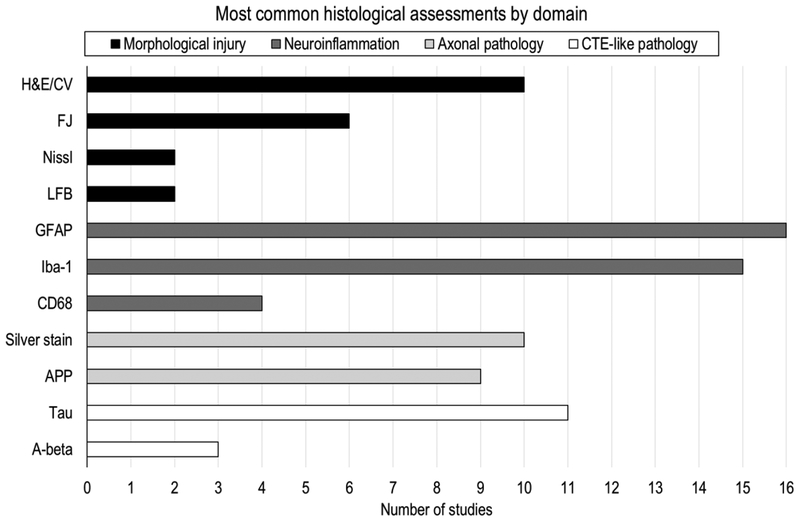
Animal research provides a unique opportunity, not possible in human subjects, to examine cellular and molecular responses using histological assessment and immunohistochemistry, which can give important insights on the neurophysiological changes associated with (repetitive) concussion. Nearly all studies included in this review (29/32, 91%) used histological assessment or immunohistochemistry to examine cellular and molecular pathology. The most common assays used include glial fibrillary acidic protein (GFAP) to assess reactive astrocytosis (16 studies), ionized calcium-binding adapter molecule 1 (iba-1) to assess microgliosis (15 studies), tau to assess CTE-like pathology (11 studies), hematoxylin and eosin staining (H&E) to assess gross morphological pathology (10 studies), and amyloid precursor protein (APP) to assess axonal pathology (9 studies) (see Figure 2 and Table 3).
Figure 2.
An overview, of the most common histological assessments performed by the rmTBI animal studies in this review, stratified by domain. Of note, not listed are assessments reported by only a single study (i.e., ILB-4, iba-1/iNOS, iba-1/arginase-1, SMI31, MAP-2, Caspase-3, NeuN, Hoechst, MHC-II, dapi, NF200). Abbreviations: H&E, Hematoxylin and Eosin; CV, Cresyl Violet; FJ, Fluoro-Jade; LFB, Luxol Fast Blue; GFAP, glial fibrillary acidic protein; iba-1, ionized calcium-binding adapter molecule 1; CD68, Cluster of Differentiation 68; APP, Amyloid Precursor Protein; A-beta, Amyloid beta.
Table 3.
Summary of histological findings by domain and injury phase.
| Injury phase | ||||
|---|---|---|---|---|
| Acute (1–2 days) |
Sub-acute (3–7 days) |
Short-term (1–4 weeks) |
Long-term (>1 month) |
|
| Domain | Findingsa | |||
| Morphological injuryb | 5/8 | 0/5 | - | 2/8 |
| Neuroinflammationc | 13/14 | 10/11 | 11/14 | 16/20 |
| Axonal damaged | 7/13 | 6/8 | 5/6 | 3/5 |
| CTE-like pathologye | 2/3 | 2/2 | 2/2 | 3/11 |
Numerators indicate the number of studies reporting evident histological findings. Denominators represent the total number of studies that performed histological analysis in these domains, including those that did not find evident changes.
Morphological injury assessed by H&E, FJ, NISSL, or LFB staining.
Neuroinflammation assessed by GFAP, iba-1 or CD68 staining.
Axonal damage assessed by APP or silver staining.
CTE-like pathology assessed by tau or amyloid-beta staining.
4.1.1. Examination of gross morphological pathology
About one third of studies reviewed in this work (11 out of 32) reported incidents of truly gross pathology, such as skull fracture or hemorrhage, which disqualified the animal from further study as described earlier in section “Adverse effects.” However, more subtle morphological injury was assessed using standard staining methods and included the use of Hematoxylin and Eosin (H&E), Cresyl Violet (CV), Luxol Fast Blue (LFB), or Fluoro-Jade (FJ). These dyes typically visualize morphological features of the neuron such as the cell body, dendrites, axons, and axon terminals, as well as finer morphological features such as large proteins, nuclei, Nissl substance, or myelin.
In the acute to short-term injury phase after final impact, studies typically show no overt structural abnormalities or neuronal loss (Fidan, et al., 2016; Laurer, et al., 2001; Meehan, et al., 2012; B. Mouzon, et al., 2012; Prins, Hales, Reger, Giza, & Hovda, 2010; Shitaka, et al., 2011; Xu, et al., 2016), although one study reported hemorrhagic lesions in the entorhinal cortex at 24 hours after the fifth concussion (Bolton & Saatman, 2014), and another study (Winston, et al., 2016) reported increased FJ-B staining, suggestive of axonal abnormalities and degenerating cells, 24 hours after the final of 30 impacts (over 6 weeks). These findings confirm the idea that acute concussive injury is not associated with gross morphological injury, but rather with microstructural damage.
In the long-term, studies confirm the absence of overt neuropathology (Bolton Hall, et al., 2016; Laurer, et al., 2001; Luo, et al., 2014; Meehan, et al., 2012; Weil, Gaier, & Karelina, 2014), but some have observed significantly reduced neuron count in the cortex and dentate gyrus (Qin, et al., 2016), and gradual loss of the body of the corpus callosum at 6 and 12 months (B. C. Mouzon, et al., 2014). Neurodegeneration that arises after an extended period may be the result of secondary injury mechanisms, such as excitotoxicity, oxidative stress, mitochondrial dysfunction, chronic neuroinflammation and apoptosis (Gupta & Sen, 2016).
4.1.2. Examination of neuroinflammation
Immunolabeling with GFAP and iba-1 are the most common methods used to examine neuroinflammation after rmTBI. Increased level of GFAP—the primary intermediate filament protein found in astrocytes and believed to play a role in the mechanical strength and shape of the cell (Liedtke, et al., 1996)—is an indication of reactive astrogliosis. The upregulation of iba-1—a protein believed to play a role in activated microglia after injury (Ito, et al., 1998)—is an indication of microgliosis.
Strong evidence of widespread astrogliosis through increased GFAP staining has been reported by multiple studies at all injury phases. As early as 1 to 2 days after injury, reactive astrogliosis has been observed in the optic tract (Winston, et al., 2016), cortex, corpus callosum, hippocampus (Chen, Desai, & Kim, 2017; B. Mouzon, et al., 2012; Yang, et al., 2015), entorhinal cortex, cerebellum, and brain stem (Bolton & Saatman, 2014). Using qualitive assessment, Prins and colleagues (Prins, et al., 2010) also found clear morphological changes in the astrocytes around the site of impact one day after rmTBI. Furthermore, increased and widespread GFAP-positive staining has been reported in the sub-acute stage (Chen, et al., 2017; Kane, et al., 2012; Petraglia, Plog, Dayawansa, Dashnaw, et al., 2014; Xu, et al., 2016; Yang, et al., 2015), short-term stage (Chen, et al., 2017; Hylin, Orsi, Rozas, et al., 2013; Weil, et al., 2014; Yang, et al., 2015), and long term (from months up to 1 year after final injury) (Chen, et al., 2017; Luo, et al., 2014; Mannix, et al., 2014; Mannix, et al., 2013; B. C. Mouzon, et al., 2014; Petraglia, Plog, Dayawansa, Dashnaw, et al., 2014; Weil, et al., 2014).
Similarly, widespread and persistent iba-1 positive cells have been observed for all injury phases (Bennett, Mac Donald, & Brody, 2012; Chen, et al., 2017; Fidan, et al., 2016; Mannix, et al., 2014; B. Mouzon, et al., 2012; B. C. Mouzon, et al., 2014; Namjoshi, et al., 2014; Ojo, et al., 2015; Shitaka, et al., 2011; Winston, et al., 2016; Xu, et al., 2016). For instance, quantification of iba-1 positive cells in the optic tract by Winston and colleagues (Winston, et al., 2016) revealed a 206% increase in microglia 1 day after 30 repetitive hits (over 6 weeks), which continued to increase to 249% above sham levels at 60 days, and was still significantly increased at 365 days—albeit at lower magnitude and with few bushy microglia remaining. Further, Fidan and colleagues (Fidan, et al., 2016) assessed microglial subtypes by performing double immunostaining and found pro-inflammatory M1-phenotype microglia in the external capsule underlying the impact and immunosuppressive M2-phenotype microglia in bilateral amygdala 7 days after rmTBI in the developing rat brain (PND 18), which disappeared when assessed at 21 and 92 days after repetitive injury. In addition to GFAP and iba-1, studies that immunostained brain sections for CD68 to test for activated microglia reported persistent increases in activated microglia (Winston, et al., 2016; Xu, et al., 2016) detectable at 10 weeks (in visual pathway independent of inter-injury interval) (Bolton Hall, et al., 2016) and up to 6 months (Petraglia, Plog, Dayawansa, Dashnaw, et al., 2014) after rmTBI.
Furthermore, from the studies included in this review, there is strong evidence that the degree of neuroinflammation is correlated with injury severity and number of head impacts (Bolton & Saatman, 2014; Hylin, Orsi, Rozas, et al., 2013; Luo, et al., 2014; B. Mouzon, et al., 2012; B. C. Mouzon, et al., 2014; Petraglia, Plog, Dayawansa, Dashnaw, et al., 2014; Prins, et al., 2010; Shitaka, et al., 2011; Weil, et al., 2014; Winston, et al., 2016). For example, one study (Bolton & Saatman, 2014) reported significantly more iba-1 staining after 5 impacts compared with a single impact, particularly with shorter inter-injury intervals. In another study, a dose-dependent increase in astrogliosis was found with increasing impact velocities (3, 4, and 5 m/s) as well as with increasing number of head impacts, although mice receiving five impacts did not show further increase in astrogliosis compared with those receiving three impacts (Luo, et al., 2014).
Increased GFAP and iba-1 staining has been reported in all injury phases, but positive staining of neuroinflammatory markers may have different interpretations. Acute post-TBI neuroinflammation has been associated with several neurodegenerative processes, including blood-brain barrier damage, brain edema, chemotaxis, phagocytosis, neutrophil respiratory burst, B-cell activation, necrosis and apoptosis (Schmidt, Heyde, Ertel, & Stahel, 2005). In line with this idea, suppression of proinflammatory cytokines and chemokines following TBI may decrease neuronal dysfunction, as has been demonstrated in a mouse model of closed head injury (Lloyd, Somera-Molina, Van Eldik, Watterson, & Wainwright, 2008). However, the role of neuroinflammation after TBI may be two-sided (Morganti-Kossmann, Rancan, Stahel, & Kossmann, 2002). Acute microglial activation has also been found to play a neuroprotective and regenerative role through the secretion of brain-derived neurotropic factor (BDNF) and insulin-like growth factor (IGF-1) (J. Neumann, et al., 2006), debris clearance, facilitating the reorganization of neuronal circuits, and triggering repair (Lalancette-Hebert, Gowing, Simard, Weng, & Kriz, 2007; H. Neumann, Kotter, & Franklin, 2009). The pathophysiology and neuroinflammatory cascade after TBI is complex and remains not fully understood. To aid in the development of efficacious therapeutics, more research is needed to better understand how neuroinflammation plays a deleterious or beneficial role after repetitive mild TBI.
4.1.3. Examination of axonal pathology
Labeling of amyloid precursor protein (APP) and silver staining are the most common methods to assess for the presence of axonal damage. APP is a trans-membrane protein with truly multifunctional properties that is highly expressed within the central nervous system (Muller, Deller, & Korte, 2017). APP is conveyed by fast axonal transport and dramatically upregulated after disruption of the cytoskeleton, as shown in the brains of experimental rats (Bramlett, Kraydieh, Green, & Dietrich, 1997; Otsuka, Tomonaga, & Ikeda, 1991) and humans (McKenzie, et al., 1996). Earlier work has shown that multifocal intra-axonal accumulation of APP has been detected as early as 1 hour after (single) traumatic brain injury in the rat (Pierce, Trojanowski, Graham, Smith, & McIntosh, 1996) and typically peaks within 2 weeks after which it diminishes (Gao, et al., 2017; Huh, Widing, & Raghupathi, 2008).
Qualitative and quantitative analysis of APP deposition has provided evidence for axonal injury in various animal models of rmTBI (Chen, et al., 2017; B. Mouzon, et al., 2012; Prins, et al., 2010; Shitaka, et al., 2011), which have found to be more prominent with shorter inter-injury interval (Bolton & Saatman, 2014; Fujita, et al., 2012), but, by contrast, a number of studies have not been able to replicate these findings (Bennett, et al., 2012; Meehan, et al., 2012; Winston, et al., 2016; Xu, et al., 2016). Two rmTBI studies confirmed the presence of APP after an extended period—that is, in ipsilateral thalamus at 56 days (Laurer, et al., 2001) and in the corpus callosum at 6 and 12 months (B. C. Mouzon, et al., 2014), which suggests continued progression of neuropathology long after the initial injury in these models. There are several reasons that could explain the variability in APP findings among rmTBI animal models: 1) variability in APP staining protocols among laboratories could result in different findings; 2) despite axonal pathology, APP is not accumulating; 3) the microtubule network is disrupted in some models, but not in others; 4) even though the level of injury severity of animal models included in this review is similar, it is not identical, which results in different levels of axonal pathology and APP staining; and 5) axonal assessment may have taken place outside the “window of opportunity” to detect such changes. The upregulation of APP may occur as early as 30 minutes after head impact (Van den Heuvel, et al., 1999) and may persist for at least 2 weeks for select brain regions, such as the thalamus, but not the corpus callosum (Pierce, et al., 1996). Since assessments often take place after the final impact—which could be weeks or months after the initial injury—this could result in insensitivity to the early pathological changes. Interestingly, APP may have a neuroprotective, rather than a detrimental role in TBI through the APP-derivate sAPPα, which has neuroprotective and neurotropic functions following trauma (Plummer, Van den Heuvel, Thornton, Corrigan, & Cappai, 2016). Thus, while the presence of APP typically indicates axonal injury, it’s function may be beneficial following TBI and further research is needed to examine APP as a potential therapeutic agent.
Silver impregnation techniques are used for the detailed visualization of the neuron, including the cell body, most of the dendritic arbor, the dendritic spines, the axon, the axon collaterals, and the synapses (Baloyannis, 2015). Even though APP immunostaining is considered to be a more sensitive technique to detect axonal damage than silver staining (Gentleman, et al., 1995), silver staining has yielded more consistent findings following experimental rmTBI. Acute axonal injury in various white matter regions indicated by argyrophilic structures and significant silver uptake compared to sham has been observed after as many as 30 mTBIs over 6 weeks (Winston, et al., 2016), as well as after only 2–3 mTBIs spaced 1 day apart (Creeley, Wozniak, Bayly, Olney, & Lewis, 2004; Namjoshi, et al., 2014). Prominent changes are also observed after 7 days in various regions, including the corpus callosum, optic tract, cerebellar lobule 9, external capsule, cortex underlying the impact site, and thalamus (Fidan, et al., 2016; Namjoshi, et al., 2014; Shitaka, et al., 2011; Xu, et al., 2016). Of all regions, the corpus callosum may have the most prominent and persistent course of silver staining abnormalities with evident staining at 7 days, as reported earlier, at 14 days (Hylin, Orsi, Rozas, et al., 2013) and up to 49 days after injury (Shitaka, et al., 2011). By contrast, other brain regions have shown more transient effects—that is, a lower magnitude of silver staining at 21 days (Fidan, et al., 2016) and near complete resolution by 28 to 49 days (Shitaka, et al., 2011). Ten weeks post-injury, one study (Bolton Hall, et al., 2016) reported positive silver staining in the visual pathway and pyramidal tracts of some animals (5 rmTBIs spaced at 24 or 48 hours), however, the density of pyramidal tract silver staining in their injured animals did not significantly correlate with worsened motor coordination as assessed by the beam walking test. Using transmission electron microscopy, one study (Ojo, et al., 2015) also reported notable populations of axons undergoing neurodegeneration in the corpus callosum three months after the final of 5 impacts (48 hours apart), illustrated by balloon-shaped axoplasm, accumulation of neurofilaments, lysosome accumulation, electron-dense structures, demyelination, empty translucent axoplasm, and disrupted or redundant myelin sheaths—findings also confirmed in quantitative analyses in the same study. Of final note, a tract that becomes permeable to silver does not necesarrily mean that the axon itself has had severe enough injury to rupture the microtuble network and induce APP accumulation. In short, successful assessment of axonal injury following repeat concussion depends on both qualitative and quantitative tissue examination using multiple markers (e.g., APP, silver staining, NF200 labeling), early follow up assessment, and correlation with behavioral or neuroimaging measurements, if available.
4.1.4. Examination of tau pathology
Tau is an intra-cellular, microtubule-stabilizing protein primarily located in axons. Hyper-phosphorylation of the protein can result in insoluble aggregates and axonal degeneration, which are involved in the pathogenesis of Alzheimer’s Disease and other tauopathies (Hampel, et al., 2010). Repetitive TBI has been linked to the development of chronic traumatic encephalopathy (CTE) (Asken, Sullan, DeKosky, Jaffee, & Bauer, 2017; Kiernan, Montenigro, Solomon, & McKee, 2015; Solomon & Zuckerman, 2015) as levels of hyper-phosphorylated tau (p-tau) have been associated with neurofibrillary tangles observed in post-mortem brains from athletes exposed to repetitive concussive injury (McKee, et al., 2009). Therefore, a growing number of animal models of repeated concussion examine the presence of tau aggregates.
Namjoshi and colleagues (Namjoshi, et al., 2014) reported increased endogenous tau phosporylation in half-brain homogenates at 6 hours, 12 hours and 2 days post-injury (2 impacts spaced 1 day apart) followed by a return to sham levels after 1 week. In line with these results, Yang and colleagues (Yang, et al., 2015) followed the temporal progression of tau in serum and in the brain after 4 consecutive closed-head impacts, 72 hours apart. They found that serum p-tau levels gradually increased from day 1 to 7 and maintained high levels up to 14 days followed by a strong decrease. In contrast to p-tau, t-tau (total tau—both modified and unmodified proteins) levels increased to 30 days. In the cortex of mice who sustained rmTBI, p-tau immune-positive cells were more pronounced than sham at 24 hours, as well as 7 and 30 days after injury. P-tau in hippocampal CA3 pyramidal neurons was also evident at 1 and 7 days, but less at 30 days. Furthermore, Petraglia and colleagues (Petraglia, Plog, Dayawansa, Dashnaw, et al., 2014) found significantly increased p-tau at 7 days, 1 month and 6 months in the cortex, amygdala, and hippocampus following highly repetitive impacts (42 impacts in 7 days). Some studies that assessed tau at one instance in time found increased p-tau in the cortex of mice exposed to 5 head impacts 30 days after injury (Kane, et al., 2012), and in the corpus callosum, amygdala, hippocampus, and septal nuclei of mice exposed to 3 head impacts 6 months after the last injury (Luo, et al., 2014). What makes these animal models unique that could explain these tau positive findings? First, there may be an association between the number of impacts (i.e., generally speaking injury severity) and the level of tau over time—that is, 2 mTBIs (Namjoshi, et al., 2014), 4 mTBIs (Yang, et al., 2015) and 42 mTBIs (Petraglia, Plog, Dayawansa, Dashnaw, et al., 2014) are reportedly associated with increased tau phosphorylation detectable up to 2 days, 30 days and 6 months post-injury, respectively. Second, tau pathology and neuroinflammation may go hand-in-hand, as tau pathology is prone to induce microglial/astrocytic activation, which in turn favors the development of pathological tau (Laurent, Buee, & Blum, 2018). Therefore, it is not surprising that most animal models of rmTBI with tau positive findings also report extensive neuroinflammation.
However, not all studies in this review observed increased tau levels after rmTBI. In fact, several studies found no evidence for increased levels of hyper-phosphorylated tau either after acute (Bolton & Saatman, 2014) or long-term assessment (Bolton Hall, et al., 2016; Chen, et al., 2017; Laurer, et al., 2001; Mannix, et al., 2013; B. C. Mouzon, et al., 2014; Xu, et al., 2016). While CTE-linked tauopathy has been demonstrated in a mouse model of blast-related neurotrauma (Goldstein, et al., 2012), the relationship between repetitive concussive head impacts and the development of tauopathy remains complex as the animal literature has yielded contrasting findings. This conclusion is consistent with findings from a recent review on CTE-modeling in animals (Ojo, Mouzon, & Crawford, 2016), which notes that despite the presence of t-tau or p-tau in some studies, there is a lack of convincing evidence for the presence of other essential components of CTE-like pathology (e.g., neurofibrillary tangles, astroglial tangles and the presence of tau in the depths of the sulci that define CTE in humans) among animal studies that assess tau after TBI. Furthermore, some of the challenges of tau identification may be related to the characteristics of tau deposition. For example, in addition to phosphorylation, tau aggregates are subject to other post-translational modifications, including acetylation, glycosylation, nitration, glycation, ubiquitination, and truncation, which affects the ultrastructural conformation of the aggregates and thus the ability of specific tracers to bind to tau (Villemagne, Fodero-Tavoletti, Masters, & Rowe, 2015). Finally, despite similarties in brain features, the human brain may respond differently to TBI than the rat brain due to differences in brain size, tissue architecture and other anatomical features, and therefore modeling CTE in rodents may be more challenging.
4.2. Behavioral assessment
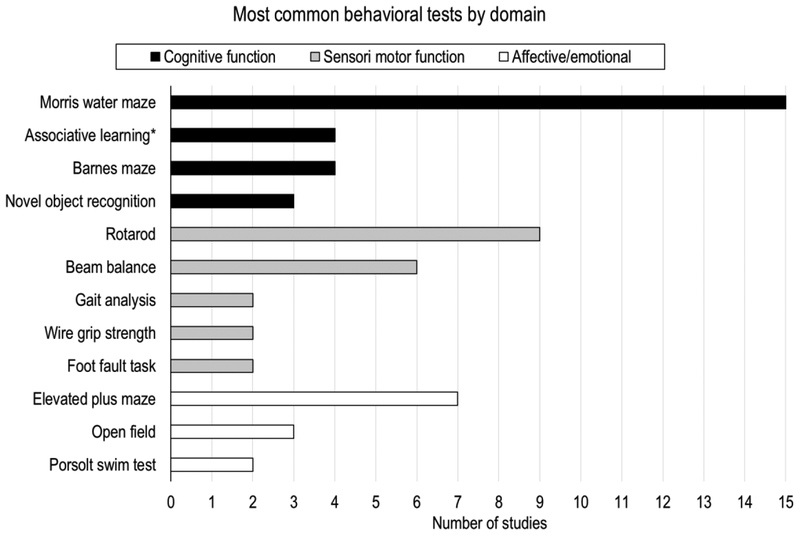
Most studies included in this review (24/32, 75%) performed behavioral assessment after repetitive mild TBI. The Morris water maze, rotarod, and elevated plus maze were most often used (see Figure 3). Behavioral assessment after experimental TBI is essential to evaluate injury severity and to mimic human cognitive, sensorimotor and emotional function.
Figure 3.
An overview of the most common behavioral assessments performed by the rmTBI animal studies reviewed in this work, stratified by domain. Of note, not listed are assessments reported by only a single study (i.e., Y-maze, radial arm maze, platform test, walking initiation, ledge test, inclined plane test, tail suspension test, elevated zero test). *Associative learning through cued and contextual fear conditioning.
4.2.1. Morris water maze (MWM)
The Morris Water Maze is one of the most widely used behavioral tests to assess learning and memory in rodents after experimental traumatic brain injury (Tucker, Velosky, & McCabe, 2018). In the typical paradigm, the rodent is placed into a pool of water, which contains an escape platform hidden a few millimeters below the water surface. Visual cues, such as colored shapes, are placed around the pool in plain sight of the animal. The rodent uses these distal cues to locate the submerged escape platform. Spatial learning is assessed across repeated trials and reference memory is determined by preference for the platform area when the platform is absent (Vorhees & Williams, 2006).
Most studies report impaired MWM performance after rmTBI. In the short-term, some studies report impaired MWM performance on both the learning and memory parts of the test (Bolouri, Saljo, Viano, & Hamberger, 2012; Hylin, Orsi, Rozas, et al., 2013; Mannix, et al., 2014; Petraglia, Plog, Dayawansa, Chen, et al., 2014; Shitaka, et al., 2011) while others report impaired spatial learning only (i.e., non-impaired memory performance) (Buckley, et al., 2015; Creeley, et al., 2004; Mannix, et al., 2013) or simply no significant changes at all (Fidan, et al., 2016; Laurer, et al., 2001; Meehan, et al., 2012; Winston, et al., 2016; Yang, et al., 2015). Meehan and colleagues (Meehan, et al., 2012) also reported that impaired MWM performance was apparent only after daily or weekly, but not monthly head impacts, which suggests that inter-injury interval—the timeframe between consecutive head impacts—may play a role in functional outcome (see earlier section on “inter-injury interval” for a more detailed discussion). Further, long-term impaired MWM performance has been reported at 1 month (Chen, et al., 2017), 3 months (Mannix, et al., 2014), 6 months (Chen, et al., 2017; Petraglia, Plog, Dayawansa, Chen, et al., 2014), and up to 1 year (Mannix, et al., 2013) following repeat concussion.
4.2.2. Rotarod
The rotarod is a sensitive and efficient test for assessing motor impairment produced by brain injury. The latency to fall from a rotating rod is dependent on factors such as rod rotating speed and brain injury severity (Hamm, Pike, O’Dell, Lyeth, & Jenkins, 1994). Most animal models of rmTBI observe acute and subacute deficits in rotarod performance (Bolton Hall, et al., 2016; Kane, et al., 2012; Laurer, et al., 2001; Mannix, et al., 2014; B. Mouzon, et al., 2012; Namjoshi, et al., 2014; Yang, et al., 2015). However, normal rotarod performance in the acute phase (Winston, et al., 2016) and impaired rotarod performance in the long term (10 weeks up to 1 year post-injury) are also reported (Mannix, et al., 2014; Namjoshi, et al., 2014; Winston, et al., 2016). These findings are mostly consistent with data from athletes who have experienced multiple concussions and who show acute postural equilibrium impairment after injury (Guskiewicz, 2011). The ability of experimental animal models to replicate functional aspects of human TBI is crucial for its ability to investigate the underlying pathophysiology associated with repeat concussion.
4.2.3. Elevated Plus Maze (EPM)
The elevated plus maze is the most frequently used anxiety test in animal research (Haller, Aliczki, & Gyimesine Pelczer, 2013). It is a test of anxiety in which the animal is placed in the center of an elevated, 4-arm maze where only two of the arms are enclosed. Anxiety-related behavior can be measured as preference for the closed versus open arms, displacement activity, freezing, immobility, and defecation (Pellow, Chopin, File, & Briley, 1985).
Most studies that used the EPM have reported short and long-term behavioral disturbances. Interestingly, these changes are characterized by both increased (Mannix, et al., 2014; Petraglia, Plog, Dayawansa, Chen, et al., 2014) and decreased activity in the open arm of the test (Petraglia, Plog, Dayawansa, Chen, et al., 2014; Winston, et al., 2016; Yang, et al., 2015). Increased activity in the open arm may be interpreted as increased risk-taking, while decreased activity has been linked to anxiety and depressive-like behavior. Some studies, however, find no changes in behavior on this test (Fidan, et al., 2016; B. C. Mouzon, et al., 2014; Winston, et al., 2016).
While the EPM is one of the most widely used tests, some have argued that it does not provide unequivocal measures of anxiety, which undermines the validity of the test (Ennaceur & Chazot, 2016). However, rmTBI animals have also demonstrated deficits on other tests of anxiety and affective behavior, such as in the Open Field, Porsolt forced swim, and tail suspension tests, consistent with anxiety and depression-like behavior at 1 to 3 months (Mannix, et al., 2014; Petraglia, Plog, Dayawansa, Chen, et al., 2014).
4.3. Magnetic Resonance Imaging (MRI)
Although most animal studies of repetitive concussion perform histological and behavioral assessment after injury, only a few studies have taken advantage of MRI. A total of five studies included in this review used MRI (4 in-vivo and 1 ex-vivo MRI). Due to the low number of MRI studies and various MRI outcomes reported, MRI findings are summarized by study, rather than by group (see Table 4).
Table 4.
MRI findings after repetitive mild TBI.
| Authors | MRI protocol | MRI timing | Summary of findings | Positive findings | Negative findings |
|---|---|---|---|---|---|
| (Bennett, et al., 2012) |
|
24h and 7 days after first injury | No acute, but delayed DTI changes in wm. In contrast, acute DTI changes in cortex that resolved after 1 week. |
|
|
| (Goddeyne, et al., 2015) |
|
2 weeks after final impact | Cortical thinning and ventriculomegaly 2 weeks after final injury compared to sham. |
|
|
| (Mannix, et al., 2013) |
|
6 months after final injury | No evidence of white matter injury or structural correlates on MRI 6 months after injury. | None |
|
| (Qin, et al., 2016) |
|
1, 3, 5, 7, 10, 14, 18, 24, and 38 days after first impact* | Cortical and subcortical atrophy without reversal. Subtle changes in DTI occurring rapidly after repeated impact followed by recovery soon after cessation of injury. |
|
|
| (Yang, et al., 2015) |
|
1, 7 and 30 days after final impact | Acute reduction in ADC, which improved after 7 days and disappeared after long-term recovery (30 days). |
|
|
daily impacts up to day 10
Abbreviations: wm, white matter; gm, gray matter; LV, lateral ventricle; FA, fractional anisotropy; RA, relative anisotropy; AD, axial diffusivity; RD, radial diffusivity; MD, mean diffusivity; CC, corpus callosum; EC, external capsule; MTR, magnetization transfer ratio; ADC, apparent diffusion coefficient; ROI, region of interest; CP, cerebral peduncle; HC, hippocampus; CT, cortex.
In the absence of truly gross pathology, such as skull fractures or hemorrhaging, two studies reported obvious morphological changes—that is, one study found cortical thinning and ventriculomegaly 2 weeks after the final of 5 impacts (24 hours apart) (Goddeyne, Nichols, Wu, & Anderson, 2015), and another study confirmed these findings through the observation of gradual cortical and subcortical atrophy plus lateral ventricle enlargement over a period of 30 days after the final of 10 impacts (24 hours apart) (Qin, et al., 2016). In human studies, computed tomography (CT) and conventional MRI typically fail to detect any evidence of macrostructural brain injury after concussion (Shenton, et al., 2012). Therefore, animal models with findings of gross morphological changes may indicate a more severe injury model than mild TBI. On the other hand, the cumulative effects of multiple concussions may cause more pronounced injury over time.
Three studies used diffusion tensor imaging (DTI), which is an advanced MRI technique to quantify microstructural alterations in white matter (Basser, Mattiello, & LeBihan, 1994). Diffusion-weighted imaging and DTI have the potential to identify grades of tissue damage, to track the evolution of changes, and to provide prognostic markers for clinical outcome (Hulkower, Poliak, Rosenbaum, Zimmerman, & Lipton, 2013). Fractional anisotropy (FA) and mean diffusivity (MD) are the most commonly used DTI-derived metrics believed to reflect overall white matter health, maturation, and organization (Basser, et al., 1994; Basser & Pierpaoli, 1996), while axial diffusivity (AD), which may reflect axon integrity, and radial diffusivity (RD), which reflects axolemma and myelin sheath integrity, can be useful in understanding the underlying physiology (Budde, et al., 2007).
One study reported subtle changes in DTI after 10 repeated impacts (24h apart), including decreased corpus callosum FA 7 to 18 days after the first impact, followed by recovery to normal levels by approximately one month after cessation of injury (Qin, et al., 2016). Following 2 impacts (24h apart), another study (Bennett, et al., 2012) reported acutely (24h after first impact) increased MD, AD, and RD in the cortex, and more delayed (7 days after final impact) decreased white matter MD and AD. Finally, one study (7 impacts in 9 days) used DTI 6 months after final impact and found no evidence of white matter injury (Mannix, et al., 2013).
These findings of microstructural white matter injuries are in line with the idea that more advanced neuroimaging techniques are needed to detect subtle neuropathological changes after concussive injury (Bigler, 2017; Shin, Bales, Edward Dixon, & Hwang, 2017; Strauss, et al., 2015). The acute but transient DTI findings are in parallel with the selective axonal injury observed by silver staining, which is evident in the acute stage and peaks around 1 week, particularly in the corpus callosum, as reported earlier in this review. This association was also observed by Bennet and colleagues (Bennett, et al., 2012) who reported a significant correlation between the density of silver staining in the corpus callosum and external capsule with relative anisotropy (RA)—a DTI measure of white matter integrity. Although more MRI studies are needed to confirm these initial findings, neuroimaging techniques, such as functional MRI and diffusion tensor imaging, have great potential to complement the existing methods to study the neuropathological changes associated with concussive and subconcussive head impacts, also discussed in another review (Dashnaw, Petraglia, & Bailes, 2012). Finally, MRI has the potential as a translational tool to help in the design of clinically relevant animal models. In this approach, animal models which yield MRI findings that parallel findings from human MRI studies, the animal model is used to characterize neuropathology relative to human exposure and dysfunction.
4.4. Other assessments
Vagnozzi (Vagnozzi, et al., 2007) and Tavazzi (Tavazzi, et al., 2007) used high-performance liquid chromatography to measure metabolic changes in the brain after repeat concussion (2 impacts at various inter-injury intervals). They reported peak changes in mitochondrial-related metabolic patterns, and in oxidative and nitrosative stresses when mTBIs were spaced 3 days apart.
Buckley and colleagues (Buckley, et al., 2015) used diffusive correlation spectroscopy to measure cerebral blood flow (CBF) after five concussions (spaced one day apart). Compared to pre-injury levels, they found reduced CBF at 4 hours after each concussion, which returned to pre-injury levels by 72 hours after final concussion. CBF values were also associated with Morris water maze performance, which suggest that CBF measurements early in the evolution of repetitive injury may be useful as a predictor of cognitive outcome.
Bolouri and colleagues (Bolouri, et al., 2012) measured intra-cranial pressure (ICP) using an optic fiber probe that was inserted into the brain parenchyma through a 1.3mm drilled hole in the skull bone after animals received 3 impacts (at 3 minutes interval) from a pneumatically fired projectile. ICP was measured at 6 and 10 hours, and 1, 2, 3, 5, and 7 days. It was found to be maximally elevated 10 hours after impact and returned to control levels after 7 days.
5. Overarching challenges of rmTBI animal models
One of the major challenges in the field of rodent TBI models is the variability in impact methods (e.g., weight-drop vs piston controlled impact) and study parameters (e.g., inter-injury interval, number of impacts, and head immmobilization), which is an obstacle in the summary of data and reproducibility of neurobehavioral and neuropathological findings among laboratories (Namjoshi, et al., 2013). How do we reconcile all these methods? While more consensus on the design parameters of repetitive animal models will reduce some of the variability between studies, as suggested in a prior review (Weber, 2007), it is unlikely that a single model can capture it all given the heterogeneity of underlying pathology associated with concussion. A more viable solution is to use a metric that can be scaled across studies. An option that offers great potential to unify impact methods is to quantify tissue strain and strain rate. Neuropathology following concussion is believed to be the result of rapid deformation of brain tissue (Cullen, et al., 2016), and tissue strain is a measure of deformation that is dimensionless and therefore scalable across species and brain size (Bayly, Black, Pedersen, Leister, & Genin, 2006; Margulies, Thibault, & Gennarelli, 1990). Brain deformation has been previously quantified in both animal and human samples, which has provided valuable information on the deformation of the brain that occurs due to linear and angular acceleration of the skull (Bayly, et al., 2006; Bayly, et al., 2005; Sabet, Christoforou, Zatlin, Genin, & Bayly, 2008). Measurements of brain deformation will also help to advance and validate computer simulations of brain injury mechanics (Lamy, Baumgartner, Yoganandan, Stemper, & Willinger, 2013). Computer simulations have the potential to replace human and animal experiments that are prohibitive or unethical to perform (e.g., pathology inducing head impacts in human subjects). For these reasons, we believe that future TBI studies should consider measuring tissue strain to improve study comparability. However, due to the complexity and costs associated with advanced imaging techniques required to measure tissue strain, we understand that, currently, most labs cannot achieve this on a routine basis (none of the animal studies included in this review measured strain). Nonetheless, recent improvements in the measurement of brain deformation (Alshareef, Giudice, Forman, Salzar, & Panzer, 2018; Knutsen, et al., 2014) may enable future comprehensive animal studies of whole brain deformation following head impact.
Alternatively, advanced study comparability may be achieved by estimating the load associated with the impact (i.e., the impact force per unit surface area), since increased load may result in greater injury severity (Fujita, et al., 2012). However, load is affected by many variables that are often unknown or unreported, such as the impact force, the effect of head movement, and the surface materials that support the animal—which makes load estimations unreliable. Moreover, since the mouse or rat skull is much more pliable than the human skull, force per unit surface area may not scale perfectly from small to larger brains and therefore would only compare reasonably within species.
Finally, the lack of a universal definition of clinical concussion (see review in (Rosenbaum & Lipton, 2012)) creates challenges for animal modeling of mTBI. Without a clear clinical definition, what is the animal model modeling and to what extend do experimental results apply to the human condition? In a prior review on the epidemiology of mTBI and neurodegenerative disease (Gardner & Yaffe, 2015), it was found that in the more than 100 studies reviewed, there were more than 50 different mTBI definitions. More consensus on the definition of human concussion will improve study validity, injury classification, and study design of animal mTBI models.
6. Summary and conclusions
The high prevalence of concussions worldwide in combination with the lack of current understanding of the underlying disease mechanisms and available clinical therapeutics, demands for further research efforts. The heterogeneity in the human condition and animal models point to an unavoidable truth that concussion is not a single disease. For this reason, various animal models may be needed to study the concussion spectrum. Since neuropathology and behavioral outcomes may be dependent on the characteristics of each model, it is crucial that researchers document their injury model as completely as possible. While no single animal model can capture the full pathology associated with brain injury, animal models are necessary in translational research as they contribute valuable preclinical information and improve our understanding of the underlying mechanisms following head impacts. A major challenge in the field of animal TBI research is how to reconcile the various impact methods. Load and strain measurements, computer simulation, and translational imaging offer great potential for studying brain biomechanics and means to unify impact methods so that mechanisms of pathology observed in animal models can be more easily interpreted and linked to clinical outcomes in patients following episodes of concussion or subconcussion. These approaches will have the potential to improve our ability to predict the evolution of disease and to develop more effective therapeutic strategies.
Supplementary Material
Acknowledgements
The authors are thankful to Dr. Mark Wagshul of Albert Einstein College of Medicine and Dr. Philip V. Bayly of Washington University for their contribution in the discussion of the manuscript. WSH wrote the main manuscript text and prepared all tables and figures. MLL and CAB contributed to discussion, reviewed and edited the manuscript. The authors report no potential conflicts of interest. This research was supported by NIH/National Center for Advancing Translational Science (NCATS) Einstein-Montefiore CTSA Grant Number TL1TR001072; Burroughs Wellcome Foundation Grant (PUP program); NIH/National Institute of Neurological Disorders and Stroke R01 NS082432; and a grant from the Dana Foundation.
Role of the Funding Source
The funding sources had no involvement in study design, data collection, analysis and interpretation of data, in writing of the report, and in the decision to submit the paper.
Abbreviations
- APP
amyloid precursor protein
- CBF
cerebral blood flow
- CTE
chronic traumatic encephalopathy
- DTI
diffusion tensor imaging
- GFAP
glial fibrillary acidic protein
- H&E
hematoxylin and eosin
- Iba-1
ionized calcium-binding adapter molecule 1
- IQR
inter-quartile range
- MRI
magnetic resonance imaging
- mTBI
mild traumatic brain injury
- MWM
Morris water maze
- p-tau
hyperphosphorylated-tau
- rmTBI
repetitive mild traumatic brain injury
- TBI
traumatic brain injury
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Submission declaration
The work described has not been published previously and is not under consideration for publication elsewhere. This work is approved by all authors and tacitly or explicitly by the responsible authorities where the work was carried out. If accepted, it will not be published elsewhere in the same form, in English or in any other language, without the written consent of the copyright-holder.
Conflict of interest statement
The authors declare that there are no conflicts of interest.
References
- Adams FS, Schwarting RK, & Huston JP (1994). Behavioral and neurochemical asymmetries following unilateral trephination of the rat skull: is this control operation always appropriate? Physiol Behav, 55, 947–952. [DOI] [PubMed] [Google Scholar]
- Alexander MP (1995). Mild traumatic brain injury: pathophysiology, natural history, and clinical management. Neurology, 45, 1253–1260. [DOI] [PubMed] [Google Scholar]
- Alshareef A, Giudice JS, Forman J, Salzar RS, & Panzer MB (2018). A Novel Method for Quantifying Human In Situ Whole Brain Deformation under Rotational Loading Using Sonomicrometry. J Neurotrauma, 35, 780–789. [DOI] [PubMed] [Google Scholar]
- Angoa-Perez M, Kane MJ, Briggs DI, Herrera-Mundo N, Viano DC, & Kuhn DM (2014). Animal models of sports-related head injury: bridging the gap between pre-clinical research and clinical reality. Journal of Neurochemistry, 129, 916–931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asken BM, Sullan MJ, DeKosky ST, Jaffee MS, & Bauer RM (2017). Research Gaps and Controversies in Chronic Traumatic Encephalopathy: A Review. JAMA Neurol, 74, 1255–1262. [DOI] [PubMed] [Google Scholar]
- Baloyannis SJ (2015). Staining neurons with Golgi techniques in degenerative diseases of the brain. Neural Regen Res, 10, 693–695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basser PJ, Mattiello J, & LeBihan D (1994). MR diffusion tensor spectroscopy and imaging. Biophys J, 66, 259–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basser PJ, & Pierpaoli C (1996). Microstructural and physiological features of tissues elucidated by quantitative-diffusion-tensor MRI. J Magn Reson B, 111, 209–219. [DOI] [PubMed] [Google Scholar]
- Battey J, Jordan E, Cox D, & Dove W (1999). An action plan for mouse genomics. Nat Genet, 21, 73–75. [DOI] [PubMed] [Google Scholar]
- Bayly PV, Black EE, Pedersen RC, Leister EP, & Genin GM (2006). In vivo imaging of rapid deformation and strain in an animal model of traumatic brain injury. J Biomech, 39, 1086–1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayly PV, Cohen TS, Leister EP, Ajo D, Leuthardt EC, & Genin GM (2005). Deformation of the human brain induced by mild acceleration. J Neurotrauma, 22, 845–856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bazarian JJ, Blyth B, Mookerjee S, He H, & McDermott MP (2010). Sex differences in outcome after mild traumatic brain injury. J Neurotrauma, 27, 527–539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett RE, Mac Donald CL, & Brody DL (2012). Diffusion tensor imaging detects axonal injury in a mouse model of repetitive closed-skull traumatic brain injury. Neurosci Lett, 513, 160–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bigler ED (2017). Structural neuroimaging in sport-related concussion. Int J Psychophysiol. [DOI] [PubMed] [Google Scholar]
- Bolouri H, Saljo A, Viano DC, & Hamberger A (2012). Animal model for sport-related concussion; ICP and cognitive function. Acta Neurol Scand, 125, 241–247. [DOI] [PubMed] [Google Scholar]
- Bolouri H, & Zetterberg H (2015). Animal Models for Concussion: Molecular and Cognitive Assessments-Relevance to Sport and Military Concussions In Kobeissy FH (Ed.), Brain Neurotrauma: Molecular, Neuropsychological, and Rehabilitation Aspects. Boca Raton (FL). [Google Scholar]
- Bolton AN, & Saatman KE (2014). Regional Neurodegeneration and Gliosis Are Amplified by Mild Traumatic Brain Injury Repeated at 24-Hour Intervals. Journal of Neuropathology and Experimental Neurology, 73, 933–947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolton Hall AN, Joseph B, Brelsfoard JM, & Saatman KE (2016). Repeated Closed Head Injury in Mice Results in Sustained Motor and Memory Deficits and Chronic Cellular Changes. PLoS One, 11, e0159442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bramlett HM, Kraydieh S, Green EJ, & Dietrich WD (1997). Temporal and regional patterns of axonal damage following traumatic brain injury: a beta-amyloid precursor protein immunocytochemical study in rats. J Neuropathol Exp Neurol, 56, 1132–1141. [DOI] [PubMed] [Google Scholar]
- Branch CA, Ghanim H, Ooi F, Kyei K, Branch K, & Lipton ML (2012). DTI of the evolving response to very mild closed head trauma in rodents In Society for Neuroscience (Vol. Program No. 551.10/K12). New Orleans, LA; Program No. 551.10/K12. [Google Scholar]
- Bryda EC (2013). The Mighty Mouse: the impact of rodents on advances in biomedical research. Mo Med, 110, 207–211. [PMC free article] [PubMed] [Google Scholar]
- Buckley EM, Miller BF, Golinski JM, Sadeghian H, McAllister LM, Vangel M, Ayata C, Meehan WP 3rd, Franceschini MA, & Whalen MJ (2015). Decreased microvascular cerebral blood flow assessed by diffuse correlation spectroscopy after repetitive concussions in mice. J Cereb Blood Flow Metab, 35, 1995–2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budde MD, Kim JH, Liang HF, Schmidt RE, Russell JH, Cross AH, & Song SK (2007). Toward accurate diagnosis of white matter pathology using diffusion tensor imaging. Magn Reson Med, 57, 688–695. [DOI] [PubMed] [Google Scholar]
- Chen H, Desai A, & Kim HY (2017). Repetitive Closed-Head Impact Model of Engineered Rotational Acceleration Induces Long-Term Cognitive Impairments with Persistent Astrogliosis and Microgliosis in Mice. J Neurotrauma, 34, 2291–2302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clayton JA, & Collins FS (2014). Policy: NIH to balance sex in cell and animal studies. Nature, 509, 282–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole JT, Yarnell A, Kean WS, Gold E, Lewis B, Ren M, McMullen DC, Jacobowitz DM, Pollard HB, O’Neill JT, Grunberg NE, Dalgard CL, Frank JA, & Watson WD (2011). Craniotomy: true sham for traumatic brain injury, or a sham of a sham? J Neurotrauma, 28, 359–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coors ME, Glover JJ, Juengst ET, & Sikela JM (2010). SCIENCE AND SOCIETY The ethics of using transgenic non-human primates to study what makes us human. Nature Reviews Genetics, 11, 658–662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creeley CE, Wozniak DF, Bayly PV, Olney JW, & Lewis LM (2004). Multiple episodes of mild traumatic brain injury result in impaired cognitive performance in mice. Acad Emerg Med, 11, 809–819. [DOI] [PubMed] [Google Scholar]
- Cullen DK, Harris JP, Browne KD, Wolf JA, Duda JE, Meaney DF, Margulies SS, & Smith DH (2016). A Porcine Model of Traumatic Brain Injury via Head Rotational Acceleration. Methods Mol Biol, 1462, 289–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dams-O’Connor K, Gibbons LE, Bowen JD, McCurry SM, Larson EB, & Crane PK (2013). Risk for late-life re-injury, dementia and death among individuals with traumatic brain injury: a population-based study. J Neurol Neurosurg Psychiatry, 84, 177–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dashnaw ML, Petraglia AL, & Bailes JE (2012). An overview of the basic science of concussion and subconcussion: where we are and where we are going. Neurosurg Focus, 33, E5: 1–9. [DOI] [PubMed] [Google Scholar]
- Deng J, Lei C, Chen Y, Fang Z, Yang Q, Zhang H, Cai M, Shi L, Dong H, & Xiong L (2014). Neuroprotective gases--fantasy or reality for clinical use? Prog Neurobiol, 115, 210–245. [DOI] [PubMed] [Google Scholar]
- Dixon CE, Lyeth BG, Povlishock JT, Findling RL, Hamm RJ, Marmarou A, Young HF, & Hayes RL (1987). A fluid percussion model of experimental brain injury in the rat. J Neurosurg, 67, 110–119. [DOI] [PubMed] [Google Scholar]
- Dong Y, Wu X, Xu Z, Zhang Y, & Xie Z (2012). Anesthetic isoflurane increases phosphorylated tau levels mediated by caspase activation and Abeta generation. PLoS One, 7, e39386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duhaime AC (2006). Large animal models of traumatic injury to the immature brain. Dev Neurosci, 28, 380–387. [DOI] [PubMed] [Google Scholar]
- Ellenbroek B, & Youn J (2016). Rodent models in neuroscience research: is it a rat race? Dis Model Mech, 9, 1079–1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ennaceur A, & Chazot PL (2016). Preclinical animal anxiety research - flaws and prejudices. Pharmacol Res Perspect, 4, e00223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feeney DM, Boyeson MG, Linn RT, Murray HM, & Dail WG (1981). Responses to cortical injury: I. Methodology and local effects of contusions in the rat. Brain Res, 211, 67–77. [DOI] [PubMed] [Google Scholar]
- Fidan E, Lewis J, Kline AE, Garman RH, Alexander H, Cheng JP, Bondi CO, Clark RS, Dezfulian C, Kochanek PM, Kagan VE, & Bayir H (2016). Repetitive Mild Traumatic Brain Injury in the Developing Brain: Effects on Long-Term Functional Outcome and Neuropathology. J Neurotrauma, 33, 641–651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujita M, Wei EP, & Povlishock JT (2012). Intensity- and Interval-Specific Repetitive Traumatic Brain Injury Can Evoke Both Axonal and Microvascular Damage. J Neurotrauma, 29, 2172–2180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallagher V, Kramer N, Abbott K, Alexander J, Breiter H, Herrold A, Lindley T, Mjaanes J, & Reilly J (2018). The Effects of Sex Differences and Hormonal Contraception on Outcomes after Collegiate Sports-Related Concussion. J Neurotrauma. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao H, Han Z, Bai R, Huang S, Ge X, Chen F, & Lei P (2017). The accumulation of brain injury leads to severe neuropathological and neurobehavioral changes after repetitive mild traumatic brain injury. Brain Res, 1657, 1–8. [DOI] [PubMed] [Google Scholar]
- Gardner RC, & Yaffe K (2015). Epidemiology of mild traumatic brain injury and neurodegenerative disease. Mol Cell Neurosci, 66, 75–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gentleman SM, Roberts GW, Gennarelli TA, Maxwell WL, Adams JH, Kerr S, & Graham DI (1995). Axonal injury: a universal consequence of fatal closed head injury? Acta Neuropathol, 89, 537–543. [DOI] [PubMed] [Google Scholar]
- Goddeyne C, Nichols J, Wu C, & Anderson T (2015). Repetitive mild traumatic brain injury induces ventriculomegaly and cortical thinning in juvenile rats. J Neurophysiol, 113, 3268–3280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein LE, Fisher AM, Tagge CA, Zhang XL, Velisek L, Sullivan JA, Upreti C, Kracht JM, Ericsson M, Wojnarowicz MW, Goletiani CJ, Maglakelidze GM, Casey N, Moncaster JA, Minaeva O, Moir RD, Nowinski CJ, Stern RA, Cantu RC, Geiling J, Blusztajn JK, Wolozin BL, Ikezu T, Stein TD, Budson AE, Kowall NW, Chargin D, Sharon A, Saman S, Hall GF, Moss WC, Cleveland RO, Tanzi RE, Stanton PK, & McKee AC (2012). Chronic traumatic encephalopathy in blast-exposed military veterans and a blast neurotrauma mouse model. Sci Transl Med, 4, 134ra160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grubb RL Jr., Naumann RA, & Ommaya AK (1970). Respiration and the cerebrospinal fluid in experimental cerebral concussion. J Neurosurg, 32, 320–329. [DOI] [PubMed] [Google Scholar]
- Gupta R, & Sen N (2016). Traumatic brain injury: a risk factor for neurodegenerative diseases. Rev Neurosci, 27, 93–100. [DOI] [PubMed] [Google Scholar]
- Gurdjian ES, Lissner HR, Webster JE, Latimer FR, & Haddad BF (1954). Studies on experimental concussion: relation of physiologic effect to time duration of intracranial pressure increase at impact. Neurology, 4, 674–681. [DOI] [PubMed] [Google Scholar]
- Guskiewicz KM (2011). Balance assessment in the management of sport-related concussion. Clin Sports Med, 30, 89–102, ix. [DOI] [PubMed] [Google Scholar]
- Haller J, Aliczki M, & Gyimesine Pelczer K (2013). Classical and novel approaches to the preclinical testing of anxiolytics: A critical evaluation. Neurosci Biobehav Rev, 37, 2318–2330. [DOI] [PubMed] [Google Scholar]
- Hamilton SM, Green JR, Veeraragavan S, Yuva L, McCoy A, Wu Y, Warren J, Little L, Ji D, Cui X, Weinstein E, & Paylor R (2014). Fmr1 and Nlgn3 knockout rats: novel tools for investigating autism spectrum disorders. Behav Neurosci, 128, 103–109. [DOI] [PubMed] [Google Scholar]
- Hamm RJ, Pike BR, O’Dell DM, Lyeth BG, & Jenkins LW (1994). The rotarod test: an evaluation of its effectiveness in assessing motor deficits following traumatic brain injury. J Neurotrauma, 11, 187–196. [DOI] [PubMed] [Google Scholar]
- Hampel H, Blennow K, Shaw LM, Hoessler YC, Zetterberg H, & Trojanowski JQ (2010). Total and phosphorylated tau protein as biological markers of Alzheimer’s disease. Exp Gerontol, 45, 30–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huh JW, Widing AG, & Raghupathi R (2008). Midline brain injury in the immature rat induces sustained cognitive deficits, bihemispheric axonal injury and neurodegeneration. Exp Neurol, 213, 84–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hulkower MB, Poliak DB, Rosenbaum SB, Zimmerman ME, & Lipton ML (2013). A decade of DTI in traumatic brain injury: 10 years and 100 articles later. AJNR Am J Neuroradiol, 34, 2064–2074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hylin MJ, Orsi SA, Rozas NS, Hill JL, Zhao J, Redell JB, Moore AN, & Dash PK (2013). Repeated mild closed head injury impairs short-term visuospatial memory and complex learning. J Neurotrauma, 30, 716–726. [DOI] [PubMed] [Google Scholar]
- Hylin MJ, Orsi SA, Zhao J, Bockhorst K, Perez A, Moore AN, & Dash PK (2013). Behavioral and Histopathological Alterations Resulting from Mild Fluid Percussion Injury. J Neurotrauma, 30, 702–715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito D, Imai Y, Ohsawa K, Nakajima K, Fukuuchi Y, & Kohsaka S (1998). Microglia-specific localisation of a novel calcium binding protein, Iba1. Brain Res Mol Brain Res, 57, 1–9. [DOI] [PubMed] [Google Scholar]
- Jane JA, Steward O, & Gennarelli T (1985). Axonal degeneration induced by experimental noninvasive minor head injury. J Neurosurg, 62, 96–100. [DOI] [PubMed] [Google Scholar]
- Johnson VE, Meaney DF, Cullen DK, & Smith DH (2015). Animal models of traumatic brain injury. Handb Clin Neurol, 127, 115–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanda R, Nakamura N, Sekino H, Sakai H, Masuzawa H, Mii K, Aoyagi N, Aruga T, Kono H, Sugimori T, Sugiura M, Mori N, Kikuchi A, Ono K, & Kobayashi H (1981). Experimental head injury in monkeys --concussion and its tolerance level. Neurol Med Chir (Tokyo), 21, 645–656. [DOI] [PubMed] [Google Scholar]
- Kane MJ, Angoa-Perez M, Briggs DI, Viano DC, Kreipke CW, & Kuhn DM (2012). A mouse model of human repetitive mild traumatic brain injury. J Neurosci Methods, 203, 41–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karmarkar SW, Bottum KM, & Tischkau SA (2010). Considerations for the use of anesthetics in neurotoxicity studies. Comp Med, 60, 256–262. [PMC free article] [PubMed] [Google Scholar]
- Karr JE, Areshenkoff CN, & Garcia-Barrera MA (2014). The neuropsychological outcomes of concussion: a systematic review of meta-analyses on the cognitive sequelae of mild traumatic brain injury. Neuropsychology, 28, 321–336. [DOI] [PubMed] [Google Scholar]
- Kiernan PT, Montenigro PH, Solomon TM, & McKee AC (2015). Chronic traumatic encephalopathy: a neurodegenerative consequence of repetitive traumatic brain injury. Semin Neurol, 35, 20–28. [DOI] [PubMed] [Google Scholar]
- Kimpara H, & Iwamoto M (2012). Mild traumatic brain injury predictors based on angular accelerations during impacts. Ann Biomed Eng, 40, 114–126. [DOI] [PubMed] [Google Scholar]
- Knutsen AK, Magrath E, McEntee JE, Xing F, Prince JL, Bayly PV, Butman JA, & Pham DL (2014). Improved measurement of brain deformation during mild head acceleration using a novel tagged MRI sequence. J Biomech, 47, 3475–3481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lalancette-Hebert M, Gowing G, Simard A, Weng YC, & Kriz J (2007). Selective ablation of proliferating microglial cells exacerbates ischemic injury in the brain. J Neurosci, 27, 2596–2605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamy M, Baumgartner D, Yoganandan N, Stemper BD, & Willinger R (2013). Experimentally validated three-dimensional finite element model of the rat for mild traumatic brain injury. Med Biol Eng Comput, 51, 353–365. [DOI] [PubMed] [Google Scholar]
- Laurent C, Buee L, & Blum D (2018). Tau and neuroinflammation: What impact for Alzheimer’s Disease and Tauopathies? Biomed J, 41, 21–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurer HL, Bareyre FM, Lee VM, Trojanowski JQ, Longhi L, Hoover R, Saatman KE, Raghupathi R, Hoshino S, Grady MS, & McIntosh TK (2001). Mild head injury increasing the brain’s vulnerability to a second concussive impact. J Neurosurg, 95, 859–870. [DOI] [PubMed] [Google Scholar]
- Le Freche H, Brouillette J, Fernandez-Gomez FJ, Patin P, Caillierez R, Zommer N, Sergeant N, Buee-Scherrer V, Lebuffe G, Blum D, & Buee L (2012). Tau phosphorylation and sevoflurane anesthesia: an association to postoperative cognitive impairment. Anesthesiology, 116, 779–787. [DOI] [PubMed] [Google Scholar]
- Levitch CF, Zimmerman ME, Lubin N, Kim N, Lipton RB, Stewart WF, Kim M, & Lipton ML (2018). Recent and Long-Term Soccer Heading Exposure Is Differentially Associated With Neuropsychological Function in Amateur Players. J Int Neuropsychol Soc, 24, 147–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liedtke W, Edelmann W, Bieri PL, Chiu FC, Cowan NJ, Kucherlapati R, & Raine CS (1996). GFAP is necessary for the integrity of CNS white matter architecture and long-term maintenance of myelination. Neuron, 17, 607–615. [DOI] [PubMed] [Google Scholar]
- Lighthall JW (1988). Controlled cortical impact: a new experimental brain injury model. J Neurotrauma, 5, 1–15. [DOI] [PubMed] [Google Scholar]
- Lipton ML, Kim N, Park YK, Hulkower MB, Gardin TM, Shifteh K, Kim M, Zimmerman ME, Lipton RB, & Branch CA (2012). Robust detection of traumatic axonal injury in individual mild traumatic brain injury patients: intersubject variation, change over time and bidirectional changes in anisotropy. Brain Imaging Behav, 6, 329–342. [DOI] [PubMed] [Google Scholar]
- Lipton ML, Kim N, Zimmerman ME, Kim M, Stewart WF, Branch CA, & Lipton RB (2013). Soccer heading is associated with white matter microstructural and cognitive abnormalities. Radiology, 268, 850–857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lloyd E, Somera-Molina K, Van Eldik LJ, Watterson DM, & Wainwright MS (2008). Suppression of acute proinflammatory cytokine and chemokine upregulation by post-injury administration of a novel small molecule improves long-term neurologic outcome in a mouse model of traumatic brain injury. J Neuroinflammation, 5, 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo C, Shifteh K, Gold T, Bello JA, & Lipton ML (2009). Diffusion tensor imaging abnormalities in patients with mild traumatic brain injury and neurocognitive impairment. J Comput Assist Tomogr, 33, 293–297. [DOI] [PubMed] [Google Scholar]
- Luo J, Nguyen A, Villeda S, Zhang H, Ding Z, Lindsey D, Bieri G, Castellano JM, Beaupre GS, & Wyss-Coray T (2014). Long-term cognitive impairments and pathological alterations in a mouse model of repetitive mild traumatic brain injury. Front Neurol, 5, 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mannix R, Berglass J, Berkner J, Moleus P, Qiu J, Andrews N, Gunner G, Berglass L, Jantzie LL, Robinson S, & Meehan WP 3rd. (2014). Chronic gliosis and behavioral deficits in mice following repetitive mild traumatic brain injury. J Neurosurg, 121, 1342–1350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mannix R, Meehan WP, Mandeville J, Grant PE, Gray T, Berglass J, Zhang J, Bryant J, Rezaie S, Chung JY, Peters NV, Lee C, Tien LW, Kaplan DL, Feany M, & Whalen M (2013). Clinical correlates in an experimental model of repetitive mild brain injury. Ann Neurol, 74, 65–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margulies SS, Thibault LE, & Gennarelli TA (1990). Physical model simulations of brain injury in the primate. J Biomech, 23, 823–836. [DOI] [PubMed] [Google Scholar]
- Marmarou A, Foda MA, van den Brink W, Campbell J, Kita H, & Demetriadou K (1994). A new model of diffuse brain injury in rats. Part I: Pathophysiology and biomechanics. J Neurosurg, 80, 291–300. [DOI] [PubMed] [Google Scholar]
- Mayer AR, Quinn DK, & Master CL (2017). The spectrum of mild traumatic brain injury: A review. Neurology, 89, 623–632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGuire JA, Sherman PM, Dean E, Bernot JM, Rowland LM, McGuire SA, & Kochunov PV (2017). Utilization of MRI for Cerebral White Matter Injury in a Hypobaric Swine Model-Validation of Technique. Mil Med, 182, e1757–e1764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKee AC, Cantu RC, Nowinski CJ, Hedley-Whyte ET, Gavett BE, Budson AE, Santini VE, Lee HS, Kubilus CA, & Stern RA (2009). Chronic traumatic encephalopathy in athletes: progressive tauopathy after repetitive head injury. J Neuropathol Exp Neurol, 68, 709–735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKee AC, & Daneshvar DH (2015). The neuropathology of traumatic brain injury. Handb Clin Neurol, 127, 45–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKenzie KJ, McLellan DR, Gentleman SM, Maxwell WL, Gennarelli TA, & Graham DI (1996). Is beta-APP a marker of axonal damage in short-surviving head injury? Acta Neuropathol, 92, 608–613. [DOI] [PubMed] [Google Scholar]
- Meehan WP 3rd, Zhang J, Mannix R, & Whalen MJ (2012). Increasing recovery time between injuries improves cognitive outcome after repetitive mild concussive brain injuries in mice. Neurosurgery, 71, 885–891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morganti-Kossmann MC, Rancan M, Stahel PF, & Kossmann T (2002). Inflammatory response in acute traumatic brain injury: a double-edged sword. Curr Opin Crit Care, 8, 101–105. [DOI] [PubMed] [Google Scholar]
- Mouzon B, Chaytow H, Crynen G, Bachmeier C, Stewart J, Mullan M, Stewart W, & Crawford F (2012). Repetitive mild traumatic brain injury in a mouse model produces learning and memory deficits accompanied by histological changes. J Neurotrauma, 29, 2761–2773. [DOI] [PubMed] [Google Scholar]
- Mouzon BC, Bachmeier C, Ferro A, Ojo JO, Crynen G, Acker CM, Davies P, Mullan M, Stewart W, & Crawford F (2014). Chronic neuropathological and neurobehavioral changes in a repetitive mild traumatic brain injury model. Ann Neurol, 75, 241–254. [DOI] [PubMed] [Google Scholar]
- Muller UC, Deller T, & Korte M (2017). Not just amyloid: physiological functions of the amyloid precursor protein family. Nat Rev Neurosci, 18, 281–298. [DOI] [PubMed] [Google Scholar]
- Namjoshi DR, Cheng WH, McInnes KA, Martens KM, Carr M, Wilkinson A, Fan J, Robert J, Hayat A, Cripton PA, & Wellington CL (2014). Merging pathology with biomechanics using CHIMERA (Closed-Head Impact Model of Engineered Rotational Acceleration): a novel, surgery-free model of traumatic brain injury. Mol Neurodegener, 9, 55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Namjoshi DR, Good C, Cheng WH, Panenka W, Richards D, Cripton PA, & Wellington CL (2013). Towards clinical management of traumatic brain injury: a review of models and mechanisms from a biomechanical perspective. Dis Model Mech, 6, 1325–1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumann H, Kotter MR, & Franklin RJ (2009). Debris clearance by microglia: an essential link between degeneration and regeneration. Brain, 132, 288–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumann J, Gunzer M, Gutzeit HO, Ullrich O, Reymann KG, & Dinkel K (2006). Microglia provide neuroprotection after ischemia. FASEB J, 20, 714–716. [DOI] [PubMed] [Google Scholar]
- Nizamutdinov D, & Shapiro LA (2017). Overview of Traumatic Brain Injury: An Immunological Context. Brain Sci, 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Connor WT, Smyth A, & Gilchrist MD (2011). Animal models of traumatic brain injury: a critical evaluation. Pharmacol Ther, 130, 106–113. [DOI] [PubMed] [Google Scholar]
- Ojo JO, Bachmeier C, Mouzon BC, Tzekov R, Mullan M, Davies H, Stewart MG, & Crawford F (2015). Ultrastructural Changes in the White and Gray Matter of Mice at Chronic Time Points After Repeated Concussive Head Injury. J Neuropathol Exp Neurol, 74, 1012–1035. [DOI] [PubMed] [Google Scholar]
- Ojo JO, Mouzon BC, & Crawford F (2016). Repetitive head trauma, chronic traumatic encephalopathy and tau: Challenges in translating from mice to men. Exp Neurol, 275 Pt 3, 389–404. [DOI] [PubMed] [Google Scholar]
- Ommaya AK, Corrao P, & Letcher FS (1973). Head injury in the chimpanzee. 1. Biodynamics of traumatic unconsciousness. J Neurosurg, 39, 152–166. [DOI] [PubMed] [Google Scholar]
- Ommaya AK, Grubb RL Jr., & Naumann RA (1971). Coup and contre-coup injury: observations on the mechanics of visible brain injuries in the rhesus monkey. J Neurosurg, 35, 503–516. [DOI] [PubMed] [Google Scholar]
- Ommaya AK, & Hirsch AE (1971). Tolerances for cerebral concussion from head impact and whiplash in primates. J Biomech, 4, 13–21. [DOI] [PubMed] [Google Scholar]
- Otsuka N, Tomonaga M, & Ikeda K (1991). Rapid appearance of beta-amyloid precursor protein immunoreactivity in damaged axons and reactive glial cells in rat brain following needle stab injury. Brain Res, 568, 335–338. [DOI] [PubMed] [Google Scholar]
- Pellman EJ, Viano DC, Casson IR, Tucker AM, Waeckerle JF, Powell JW, & Feuer H (2004). Concussion in professional football: repeat injuries--part 4. Neurosurgery, 55, 860–873; discussion 873–866. [DOI] [PubMed] [Google Scholar]
- Pellow S, Chopin P, File SE, & Briley M (1985). Validation of open:closed arm entries in an elevated plus-maze as a measure of anxiety in the rat. J Neurosci Methods, 14, 149–167. [DOI] [PubMed] [Google Scholar]
- Petraglia AL, Plog BA, Dayawansa S, Chen M, Dashnaw ML, Czerniecka K, Walker CT, Viterise T, Hyrien O, Iliff JJ, Deane R, Nedergaard M, & Huang JH (2014). The spectrum of neurobehavioral sequelae after repetitive mild traumatic brain injury: a novel mouse model of chronic traumatic encephalopathy. J Neurotrauma, 31, 1211–1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petraglia AL, Plog BA, Dayawansa S, Dashnaw ML, Czerniecka K, Walker CT, Chen M, Hyrien O, Iliff JJ, Deane R, Huang JH, & Nedergaard M (2014). The pathophysiology underlying repetitive mild traumatic brain injury in a novel mouse model of chronic traumatic encephalopathy. Surg Neurol Int, 5, 184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce JE, Trojanowski JQ, Graham DI, Smith DH, & McIntosh TK (1996). Immunohistochemical characterization of alterations in the distribution of amyloid precursor proteins and beta-amyloid peptide after experimental brain injury in the rat. J Neurosci, 16, 1083–1090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plummer S, Van den Heuvel C, Thornton E, Corrigan F, & Cappai R (2016). The Neuroprotective Properties of the Amyloid Precursor Protein Following Traumatic Brain Injury. Aging Dis, 7, 163–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prins ML, Hales A, Reger M, Giza CC, & Hovda DA (2010). Repeat traumatic brain injury in the juvenile rat is associated with increased axonal injury and cognitive impairments. Dev Neurosci, 32, 510–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin Y, Li GL, Xu XH, Sun ZY, Gu JW, & Gao FB (2016). Brain structure alterations and cognitive impairment following repetitive mild head impact: An in vivo MRI and behavioral study in rat. Behav Brain Res. [DOI] [PubMed] [Google Scholar]
- Rosenbaum SB, & Lipton ML (2012). Embracing chaos: the scope and importance of clinical and pathological heterogeneity in mTBI. Brain Imaging Behav, 6, 255–282. [DOI] [PubMed] [Google Scholar]
- Sabet AA, Christoforou E, Zatlin B, Genin GM, & Bayly PV (2008). Deformation of the human brain induced by mild angular head acceleration. J Biomech, 41, 307–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt OI, Heyde CE, Ertel W, & Stahel PF (2005). Closed head injury--an inflammatory disease? Brain Res Brain Res Rev, 48, 388–399. [DOI] [PubMed] [Google Scholar]
- Schubert R, Frank F, Nagelmann N, Liebsch L, Schuldenzucker V, Schramke S, Wirsig M, Johnson H, Kim EY, Ott S, Holzner E, Demokritov SO, Motlik J, Faber C, & Reilmann R (2016). Neuroimaging of a minipig model of Huntington’s disease: Feasibility of volumetric, diffusion-weighted and spectroscopic assessments. J Neurosci Methods, 265, 46–55. [DOI] [PubMed] [Google Scholar]
- Shenton ME, Hamoda HM, Schneiderman JS, Bouix S, Pasternak O, Rathi Y, Vu MA, Purohit MP, Helmer K, Koerte I, Lin AP, Westin CF, Kikinis R, Kubicki M, Stern RA, & Zafonte R (2012). A review of magnetic resonance imaging and diffusion tensor imaging findings in mild traumatic brain injury. Brain Imaging Behav, 6, 137–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin SS, Bales JW, Edward Dixon C, & Hwang M (2017). Structural imaging of mild traumatic brain injury may not be enough: overview of functional and metabolic imaging of mild traumatic brain injury. Brain Imaging Behav, 11, 591–610. [DOI] [PubMed] [Google Scholar]
- Shitaka Y, Tran HT, Bennett RE, Sanchez L, Levy MA, Dikranian K, & Brody DL (2011). Repetitive closed-skull traumatic brain injury in mice causes persistent multifocal axonal injury and microglial reactivity. J Neuropathol Exp Neurol, 70, 551–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shultz SR, McDonald SJ, Vonder Haar C, Meconi A, Vink R, van Donkelaar P, Taneja C, Iverson GL, & Christie BR (2017). The potential for animal models to provide insight into mild traumatic brain injury: Translational challenges and strategies. Neurosci Biobehav Rev, 76, 396–414. [DOI] [PubMed] [Google Scholar]
- Smith DH, Chen XH, Xu BN, McIntosh TK, Gennarelli TA, & Meaney DF (1997). Characterization of diffuse axonal pathology and selective hippocampal damage following inertial brain trauma in the pig. J Neuropathol Exp Neurol, 56, 822–834. [PubMed] [Google Scholar]
- Solomon GS, & Zuckerman SL (2015). Chronic traumatic encephalopathy in professional sports: retrospective and prospective views. Brain Inj, 29, 164–170. [DOI] [PubMed] [Google Scholar]
- Stewart WF, Kim N, Ifrah CS, Lipton RB, Bachrach TA, Zimmerman ME, Kim M, & Lipton ML (2017). Symptoms from repeated intentional and unintentional head impact in soccer players. Neurology, 88, 901–908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strauss S, Hulkower M, Gulko E, Zampolin RL, Gutman D, Chitkara M, Zughaft M, & Lipton ML (2015). Current Clinical Applications and Future Potential of Diffusion Tensor Imaging in Traumatic Brain Injury. Top Magn Reson Imaging, 24, 353–362. [DOI] [PubMed] [Google Scholar]
- Sullivan HG, Martinez J, Becker DP, Miller JD, Griffith R, & Wist AO (1976). Fluid-percussion model of mechanical brain injury in the cat. J Neurosurg, 45, 521–534. [PubMed] [Google Scholar]
- Tavazzi B, Vagnozzi R, Signoretti S, Amorini AM, Belli A, Cimatti M, Delfini R, Di Pietro V, Finocchiaro A, & Lazzarino G (2007). Temporal window of metabolic brain vulnerability to concussions: oxidative and nitrosative stresses--part II. Neurosurgery, 61, 390–395; discussion 395–396. [DOI] [PubMed] [Google Scholar]
- Tawfeeq NA, Halawani MM, Al-Faridi K, Aal-Shaya WA, & Taha WS (2009). Traumatic brain injury: neuroprotective anaesthetic techniques, an update. Injury, 40 Suppl 4, S75–81. [DOI] [PubMed] [Google Scholar]
- Thurman DJ (2016). The Epidemiology of Traumatic Brain Injury in Children and Youths: A Review of Research Since 1990. J Child Neurol, 31, 20–27. [DOI] [PubMed] [Google Scholar]
- Tucker LB, Velosky AG, & McCabe JT (2018). Applications of the Morris water maze in translational traumatic brain injury research. Neurosci Biobehav Rev, 88, 187–200. [DOI] [PubMed] [Google Scholar]
- Vagnozzi R, Tavazzi B, Signoretti S, Amorini AM, Belli A, Cimatti M, Delfini R, Di Pietro V, Finocchiaro A, & Lazzarino G (2007). Temporal window of metabolic brain vulnerability to concussions: mitochondrial-related impairment--part I. Neurosurgery, 61, 379–388; discussion 388–379. [DOI] [PubMed] [Google Scholar]
- Van den Heuvel C, Blumbergs PC, Finnie JW, Manavis J, Jones NR, Reilly PL, & Pereira RA (1999). Upregulation of amyloid precursor protein messenger RNA in response to traumatic brain injury: an ovine head impact model. Exp Neurol, 159, 441–450. [DOI] [PubMed] [Google Scholar]
- Viano DC, Hamberger A, Bolouri H, & Saljo A (2009). Concussion in professional football: animal model of brain injury--part 15. Neurosurgery, 64, 1162–1173; discussion 1173. [DOI] [PubMed] [Google Scholar]
- Villemagne VL, Fodero-Tavoletti MT, Masters CL, & Rowe CC (2015). Tau imaging: early progress and future directions. Lancet Neurol, 14, 114–124. [DOI] [PubMed] [Google Scholar]
- Vorhees CV, & Williams MT (2006). Morris water maze: procedures for assessing spatial and related forms of learning and memory. Nat Protoc, 1, 848–858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber JT (2007). Experimental models of repetitive brain injuries. Prog Brain Res, 161, 253–261. [DOI] [PubMed] [Google Scholar]
- Weil ZM, Gaier KR, & Karelina K (2014). Injury timing alters metabolic, inflammatory and functional outcomes following repeated mild traumatic brain injury. Neurobiol Dis, 70, 108–116. [DOI] [PubMed] [Google Scholar]
- Williams AM, Dennahy IS, Bhatti UF, Halaweish I, Xiong Y, Chang P, Nikolian VC, Chtraklin K, Brown J, Zhang Y, Zhang ZG, Chopp M, Buller B, & Alam HB (2018). Mesenchymal Stem Cell-Derived Exosomes Provide Neuroprotection and Improve Long-Term Neurologic Outcomes in a Swine Model of Traumatic Brain Injury and Hemorrhagic Shock. J Neurotrauma. [DOI] [PubMed] [Google Scholar]
- Winston CN, Noel A, Neustadtl A, Parsadanian M, Barton DJ, Chellappa D, Wilkins TE, Alikhani AD, Zapple DN, Villapol S, Planel E, & Burns MP (2016). Dendritic Spine Loss and Chronic White Matter Inflammation in a Mouse Model of Highly Repetitive Head Trauma. Am J Pathol, 186, 552–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wojnarowicz MW, Fisher AM, Minaeva O, & Goldstein LE (2017). Considerations for Experimental Animal Models of Concussion, Traumatic Brain Injury, and Chronic Traumatic Encephalopathy-These Matters Matter. Front Neurol, 8, 240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xing G, Barry ES, Benford B, Grunberg NE, Li H, Watson WD, & Sharma P (2013). Impact of repeated stress on traumatic brain injury-induced mitochondrial electron transport chain expression and behavioral responses in rats. Front Neurol, 4, 196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong Y, Mahmood A, & Chopp M (2013). Animal models of traumatic brain injury. Nat Rev Neurosci, 14, 128–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu L, Nguyen JV, Lehar M, Menon A, Rha E, Arena J, Ryu J, Marsh-Armstrong N, Marmarou CR, & Koliatsos VE (2016). Repetitive mild traumatic brain injury with impact acceleration in the mouse: Multifocal axonopathy, neuroinflammation, and neurodegeneration in the visual system. Exp Neurol, 275 Pt 3, 436–449. [DOI] [PubMed] [Google Scholar]
- Yang Z, Wang P, Morgan D, Lin D, Pan J, Lin F, Strang KH, Selig TM, Perez PD, Febo M, Chang B, Rubenstein R, & Wang KK (2015). Temporal MRI characterization, neurobiochemical and neurobehavioral changes in a mouse repetitive concussive head injury model. Sci Rep, 5, 11178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yarnell P, & Ommaya AK (1969). Experimental cerebral concussion in the rhesus monkey. Bull N Y Acad Med, 45, 39–45. [PMC free article] [PubMed] [Google Scholar]
- Zhang K, & Sejnowski TJ (2000). A universal scaling law between gray matter and white matter of cerebral cortex. Proc Natl Acad Sci U S A, 97, 5621–5626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang YP, Cai J, Shields LB, Liu N, Xu XM, & Shields CB (2014). Traumatic brain injury using mouse models. Transl Stroke Res, 5, 454–471. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.