Abstract
Objective:
Lateral wedge shoe insoles decrease medial knee loading, but trials have shown no effect on pain in medial knee osteoarthritis (OA). However, insoles’ loading effects are inconsistent, and they can increase patellofemoral loading. We hypothesized that insoles would reduce pain in preselected patients.
Methods:
In persons with painful medial knee OA, we excluded those with patellofemoral OA and those with pain <4/10. We further excluded participants who, in a gait laboratory using lateral wedges, did not show at least a 2% reduction in knee adduction moment (KAM) compared with their shoes and a neutral insole. We then randomized subjects to lateral wedge vs. neutral insole for 8 week periods separated by an 8 week washout. Primary outcome was knee pain over the past week (0–10) and secondary outcomes nominated activity pain and KOOS pain. We carried out mixed model analyses adjusted for baseline pain.
Results:
Of 83 participants, 21 (25%) were excluded because of insufficient reduction in KAM. Of 62 included, mean age was 64.2 years (SD 9.1); 37.1% were women. Lateral wedge insoles produced a greater reduction in knee pain than neutral insoles (difference 0.7 on 0–10 scale; 95%CI 0.1, 1.2; p = 0.02). Secondary outcomes showed mixed findings.
Conclusions:
In persons prescreened to eliminate those with patellofemoral OA and biomechanical non-responders, lateral wedge insoles reduced knee pain, but the effect of treatment was small and is likely of clinical significance in only a minority of patients. Targeting patients may identify those who respond to this treatment.
Roughly 12% of persons aged 60 and over have painful knee osteoarthritis (OA) (1). Rates of knee replacement have been rising in large part because of the failure of medical and rehabilitative treatments. New treatments for knee OA are badly needed.
Lateral wedge insoles placed inside shoes shift loading across the knee laterally during walking. They reduce load across the medial knee where most affected persons have either isolated disease or disease combined with involvement of the patellofemoral joint. Lateral wedge insoles reduce the external knee adduction moment (KAM), a measure of the load across the medial vs. lateral compartment, by 5–6% (2, 3). Unfortunately, in trials, lateral wedges have not reduced knee pain compared with neutral insoles. In a meta-analysis by Parkes et al. (4), all eight randomized controlled trials comparing lateral wedge to neutral insoles were null, and the effect size on pain reduction was 0.03 (95% confidence interval −0.18, 0.22). A subsequently published trial also was negative (5).
In 25% of patients, the wedge does not reduce medial load (6, 7). Furthermore, OA in the patellofemoral joint may get worse as load is shifted laterally. We therefore hypothesized that if we selected persons with painful medial compartment knee OA who showed a biomechanical response to wedge insoles and did not have painful patellofemoral OA, they would experience a reduction in knee pain compared with neutral insoles.
Because use of a shoe insole is a simple, low-cost intervention, its efficacy in reducing pain could translate into large public health benefit and possibly widespread use. Further, medial knee OA is highly prevalent not only in Western countries but in developing ones where knee replacements are not widely available. We conducted a crossover trial testing a 5° lateral wedge insole, the same insole we and others tested previously (8).
METHODS
This was a randomized trial testing lateral wedge insoles vs. neutral insoles in persons with painful medial knee osteoarthritis. The clinical trial registration number was ISRCTN55059760.
Recruitment and Eligibility
Recruitment
Subjects were recruited from general practices and by way of advertisements in Manchester, England from 1/2016 through 6/2017
Inclusion Criteria
We instituted the following eligibility criteria: age 40–85; severity of overall knee pain in the last week of >4 on a 0–10 numerical grading scale (overall knee pain is the primary outcome of the trial), Kellgren & Lawrence grade 2–4 in the painful knee (as scored by a musculoskeletal radiologist) on a PA or AP radiograph obtained within the last 2 years that showed definite medial but no definite lateral narrowing. Patellofemoral osteoarthritis must be less severe than medial OA and could not be Kellgren & Lawrence grade 3 or higher there.
Additional inclusion criteria included: medial joint line tenderness on examination by an experienced physical therapist (MJC) with tenderness over the patella less than medial tenderness; stable medication regimen for three months; and willingness to wear insoles in shoes at least 4 hours daily.
Exclusion Criteria
Subjects were excluded for the following reasons: a history of high tibial osteotomy, other realignment surgery or a knee replacement in the painful knee; a knee arthroscopy within the last 6 months or an intraarticular injection of either steroid or viscosupplementation in that knee within the last 3 months. Persons with the following disorders or conditions were excluded: rheumatoid arthritis or other inflammatory arthritis, diabetic neuropathic pain or fibromyalgia; foot or ankle problems that contraindicated the use of footwear load-modifying interventions; or severe coexisting medical morbidities. Further exclusions included: inability to walk unaided without a crutch, cane or walker; BMI >= 35; and persons who currently used or required foot orthoses. We also excluded those unable to retain information regarding study procedures or who were unable to walk 100 meters without stopping. A secondary outcome of the study was change in MRI features, and those with contraindications to MRI were also excluded, as were those who planned knee surgery within the next 6 months.
Evaluation and Treatment
For eligible subjects, a gait laboratory appointment was made. There, subjects were randomized remotely to the order of testing of insoles in their shoes (no insole, neutral insole, lateral wedge insole). A 10 camera Qualisys (Gothenburg, Sweden) Proreflex motion analysis system operating at 100 Hz and two Kistler (Alton, UK) force plates operating at 1000 Hz was used to measure kinematics and kinetics. Each participant completed a minimum of five successful trials (a successful trial was when a single foot contacted the force plate). The CAST marker set technique (9) was employed (8). Retroreflective markers were mounted securely to the participants’ shoes with the foot modelled as a rigid segment. Ankle and knee joint centers were calculated as midpoints between the malleoli and femoral epicondyles respectively. The hip joint center was calculated using the regression model of Bell et al (10) based on anterior and posterior superior iliac spine markers. Participants walked at their self-selected speed and this did not differ across conditions. Using an inverse dynamic approach Visual 3D (C-Motion, Rockville, Maryland), we calculated the first peak KAM normalized to the participant’s mass (Nm/kg) averaged across the 5 trials. In addition, subjects were asked about comfort of each condition and whether they noted any immediate effect on knee pain.
We characterized a subject as a biomechanical responder if, for the study knee, there was at least a 2% reduction of their KAM in the lateral wedge insole compared to both the KAM in their own shoe and the KAM when they used the neutral insole. Biomechanical responders were eligible for the study. We chose a 2% reduction as this is above the minimal detectable difference in KAM (11) and was a reasonable approach to ensure biomechanical response
Eligible subjects were randomized by a statistician who had no contact with study staff and created sealed opaque envelopes for each study ID number opened when a subject entered the study. The randomized allocation list used to create the envelopes was a single-allocation computer-generated list of balanced permuted blocks with a block size of 6. Study staff were blinded to block size. Subjects were randomized to one of two treatment sequences 1:1 (AB or BA) in a two-period crossover trial. Each randomized participant was provided either (A) a 5° lateral wedge insole or (B) a neutral insole, for 8 weeks before an 8 week washout. Then, they switched to the other treatment for 8 weeks. The initial treatment was taken away at the last visit of the assigned treatment period, preventing treatment contamination. Both insoles had a density of 70 Shore A.
Participants attended the clinic for 5 visits: a screening visit (−2 weeks), baseline visit (week 0), post-treatment visit (8 weeks), post-washout visit/second baseline visit (16 weeks), and second post treatment visit (24 weeks).
Participants were asked to wear insoles at least 4 hours per day but could wear them for as long as they wanted.
Bone marrow lesions (BMLs) appear on knee MRI in regions where excessive loading has produced bone damage. Medial lesions predominate in those with medial OA. BMLs in the patellofemoral joint shrank over 6 weeks when focal loading there was reduced (12), suggesting BMLs may respond to unloading treatments. We evaluated subjects for change in BML volume in the medial joint. At baseline, subjects underwent MRI of their study knee and then obtained MRI’s again at 8, 16 and 24 weeks. Using a 3 T Philips Achieva scanner (Philips, Best Netherlands), we obtained sagittal images with SPAIR fat suppression, repetition time (TR) 4300ms, echo time (TE) 50ms, Field of View (FOV) 16×24cm, 212×220 acq matrix, slice thickness 3mm with 0.3mm gap BW 621–655 Hz/pixel.
Outcomes: Pain
The primary pre-specified symptom outcome was overall pain in the knee in the past week scored using a numerical rating scale (0–10) as per recommendations of the IMMPACT group (13). Also, at baseline, subjects were asked to identify the activity that provoked the most knee pain and were asked to score pain during this activity at each visit) (14). Lastly, we administered the KOOS questionnaire at each visit (15).
Outcomes : Structure
Technicians at iMorphics, blinded to treatment assignment, manually segmented BML volumes in paired images from each subject’s knee. BMLs were outlined on each MRI slice and the volume integrated over all slices. For sagittal images, on which we based results, they segmented BMLs in patella, femur and tibia. Interobserver reliability for BML volume was ICC = 0.91 (p <0.001) (12).
The primary structural outcome was change in medial BMLs. We defined these as BMLs involving either the tibia medial to the cruciate ligaments or femur medial to the notch using regions derived from the WORMS scale (16).
Analysis:
The analysis followed an intention-to-treat approach. Multiple imputation by chained equations (MICE) was used to correct estimates for bias due to missing data, assuming data was missing at random.
To assess the difference in treatment effects between the two insoles, we used maximum likelihood mixed-effects multiple linear regression models. The primary analysis model used overall pain in the last week, measured at the end of each treatment visit as the outcome and adjusted for baseline (pre-treatment visit) pain scores. Using the baseline value as a covariate (the ANCOVA approach) is a recommended methodology with less bias and greater power to detect differences (17). Participant ID was included as a random effect. To test for carry-over effects, we included three additional covariates: a treatment term, a period term, and, the term testing for carryover, a treatment-by-period interaction term. We ran a second model identical to the first that removed the period, and treatment-by-period interaction terms.
The same regression models were used to analyze the secondary outcomes, using the same terms, but replacing overall knee pain in the last week with the secondary outcome of interest.
In addition to evaluating the response to insoles on a continuous scale, we also defined responders as those who achieved an accepted minimally important difference in overall knee pain of 1 on the 0–10 point scale (18). We examined what the percentage of trial participants achieved responder status as the improvement in pain from baseline pain score in that treatment period to the pain score at the end of the period.
Sample Size
We aimed to detect a treatment effect size (delta) of 0.4 SDs with 80% power (two sided alpha = 0.05). Based on a within-person SD of 2.4 for overall pain in the last week (19) testing a lateral wedge insole, this effect size represents a difference of 1.0 point on this knee pain rating scale and translates to a sample size of 52 subjects completing the trial. We assumed 10% loss to follow-up and aimed to randomize 58 subjects.
The protocol was approved by National Research Ethics Service Committee North West (Preston).
RESULTS
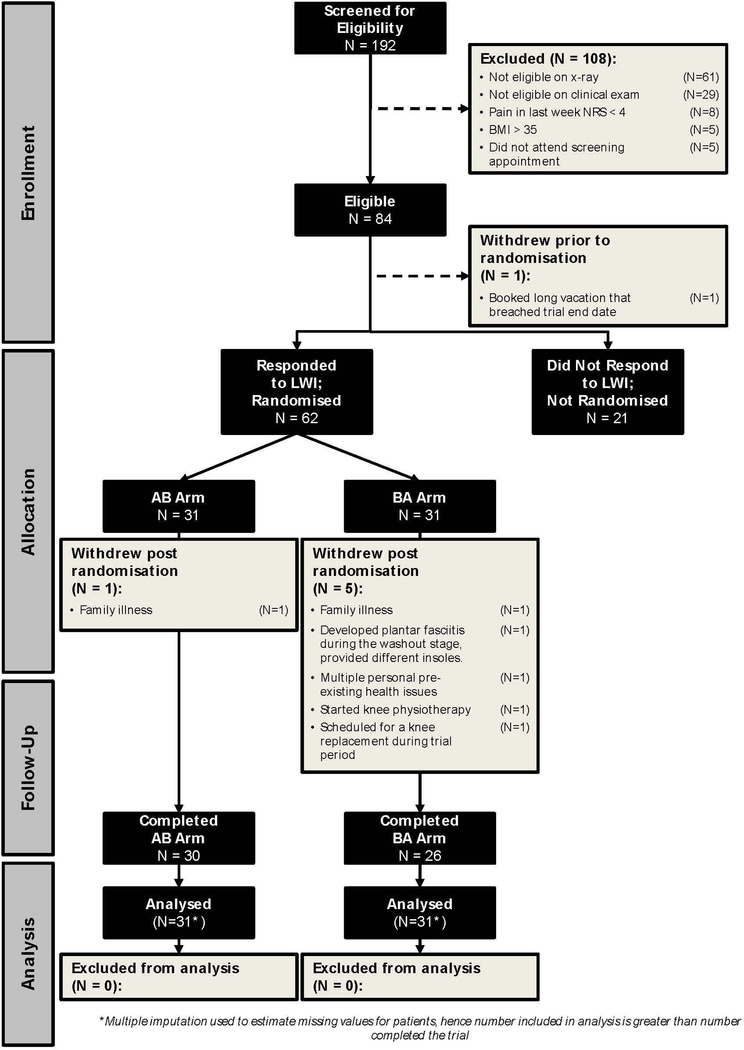
Of 83 participants who satisfied inclusion criteria and were evaluated in the gait laboratory (Figure 1), 62 were biomechanical responders. In this group, the mean reduction in KAM compared with the neutral insole was 6.6% and compared with their own shoe was 7.5% (Table 1). In contrast, 21 participants (25.3%) were biomechanical non-responders and, on average, their KAM was 2% higher with the lateral wedge insoles than with their own shoes (Table 1). There were no differences between responders and non-responders in immediate knee pain on walking in the lab or in the comfort of their own shoes vs. inserts. Randomized participants were on average 64 years old, had an average BMI of 28, and the majority were men (Table 2). Of the 62 randomized, 59 completed the first and 56 the second treatment period.
Figure 1.
Trial patient Flow Diagram
Table 1.
Percent difference in responders vs. non-responders in external Knee Adduction Moment (KAM) using lateral wedge insoles vs. neutral insoles and vs. participant’s own shoes
| Own Shoe | Neutral Insole |
Lateral Wedge Insole | Difference Between Own Shoe and LWI | Difference Between Neutral Insole and LWI | ||
|---|---|---|---|---|---|---|
| mean (SD) | mean (SD) | mean (SD) |
Adjusted mean (95% CI) |
adjusted mean (95% CI) |
||
| Non-Responders (N=21) | Absolute Values | 0.54 (0.17) | 0.54 (0.16) | 0.55 (0.17) | 0.01 (0.00 to 0.02) | 0.00 (−0.01 to 0.02) |
| % Change | - | - | - | 1.98 (−0.07 to 4.03) | 0.64 (−2.12 to 3.39) | |
| Immediate Pain | 2.86 (2.46) | 2.33 (2.35) | 2.05 (2.09) | −0.78 (−1.28 to −0.28) | −0.27 (−0.77 to 0.23) | |
| Immediate Comfort* | 7.29 (1.74) | 7.10 (2.21) | 7.62 (1.77) | 0.35 (−0.18 to 0.88) | 0.22 (−0.32 to 0.75) | |
| Responders (N=62) | Absolute Values | 0.50 (0.15) | 0.49 (0.15) | 0.46 (0.14) | -0.04 (−0.04 to −0.03) | -0.03 (−0.04 to −0.03) |
| % Change | - | - | - | −7.54 (−8.53 to −6.55) | −6.56 (−7.69 to −5.42) | |
| Immediate Pain | 3.03 (2.21) | 2.84 (2.06) | 2.48 (1.89) | −0.28 (−0.57 to 0.02) | −0.18 (−0.48 to 0.11) | |
| Immediate Comfort* | 7.00 (1.87) | 7.32 (1.94) | 7.44 (1.77) | 0.14 (−0.21 to 0.49) | 0.12 (−0.22 to 0.47) |
Immediate pain and comfort graded on a 0–10 scale but for pain, lower numbers represent less pain and for comfort, higher scores represent more comfort.
Table 2.
Characteristics of responders and non-responders
| Variable | Responders (N=62) |
Non-Responders (N=21) | |
|---|---|---|---|
| Age in years | mean (SD) | 64.18 (9.10) | 65.86 (10.03) |
| BMI | mean (SD) | 28.21 (3.44) | 28.56 (3.99) |
| No. females (%) | 23 (37.10) | 9 (42.86) | |
| HAD* Anxiety Score | mean (SD) | 12.17 (2.24) | 12.48 (1.57) |
| HAD* Depression Score | mean (SD) | 9.10 (1.24) | 8.33 (0.97) |
| Overall Knee Pain in last week NRS (0–10) | mean (SD) | 5.26 (1.63) | 5.24 (1.87) |
| Pain on nominated activity NRS (0–10) | mean (SD) | 6.18 (1.54) | 6.05 (1.66) |
| KOOS Pain Subscale Score (0–100)ɸ | mean (SD) | 55.20 (13.45) | 58.07 (12.07) |
| Kellgren and Lawrence Grade of Studied Knee (%) |
|||
| -Grade 2 | 17 (27.4%) | ||
| -Grade 3 | 37 (59.7%) | ||
| -Grade 4 | 8 (12.9%) |
Hospital Anxiety and Depression Scale. Scores range from 0–21 with higher scores (above 11) indicating either depression or anxiety
KOOS scores range from 100 to 0 where 100 represents no pain/difficulty.
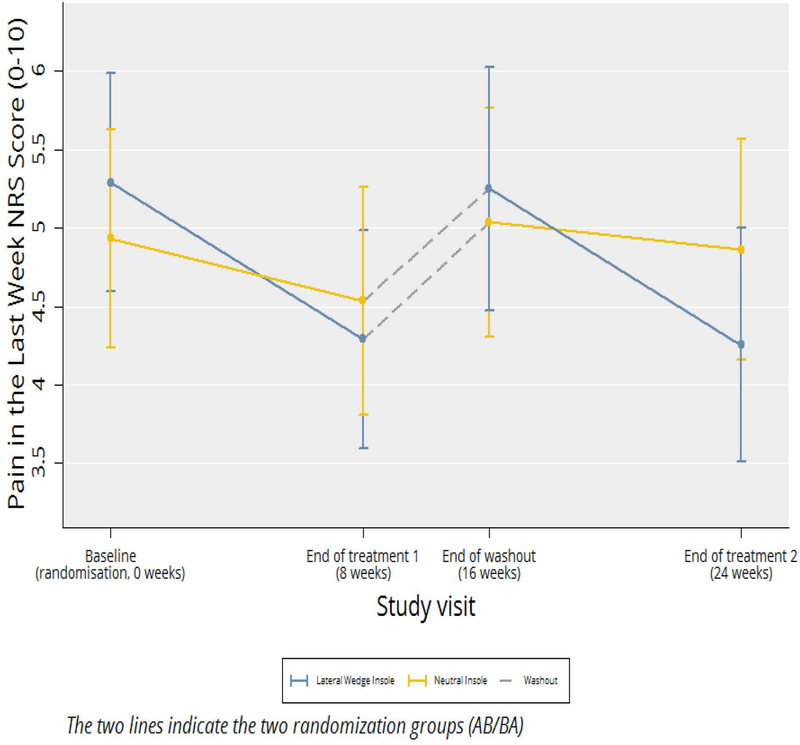
We found no significant evidence of carry-over effects for any of the outcomes of interest. When we examined our primary outcome (Figure 2 and Table 3), we found that subjects reported less knee pain when randomized to lateral wedge insoles than when on neutral insoles (difference in pain score 0.7 on a 0–10 scale (95%CI 0.1, 1.2; p = 0.02). The pain during their most painful nominated activity was also less during the period on lateral wedge insoles (Table 3). However, we did not find significant differences for the KOOS pain score or the other KOOS subscales.
Figure 2.
Cross-Over Change in Overall Knee Pain in Last Week (NRS)
Table 3.
Comparison between lateral wedge and neutral insoles after 8 weeks’ treatment**
| Post-Treatment Adjusted Mean (95% CI) |
Difference between treatments (mean, 95% CI, p) |
||
|---|---|---|---|
| Outcome | Lateral Wedge Insole | Neutral Insole | |
| Pain in last week* | 4.16 (3.69 to 4.62) | 4.85 (4.42 to 5.28) | 0.70 (0.12 to 1.27), 0.02 |
| Pain in nominated activity* | 4.80 (4.30 to 5.31) | 5.77 (5.28 to 6.26) | 0.97 (0.32 to 1.61), 0.003 |
| KOOS Pain subscale score* | 60.66 (57.21 to 64.11) | 58.82 (55.67 to 61.96) | −1.84 (−6.31 to 2.62), 0.42 |
| KOOS Symptom subscale score | 60.64 (57.59 to 63.70) | 59.41 (56.40 to 62.42) | −1.23 (−5.11 to 2.65), 0.53 |
| KOOS Activities of Daily Living subscale score | 66.29 (63.15 to 69.44) | 65.01 (61.88 to 68.14) | −1.28 (−5.19 to 2.62), 0.52 |
| KOOS Sports & Recreation subscale score | 43.57 (39.46 to 47.67) | 42.21 (37.56 to 46.86) | −1.36 (−6.97 to 4.26), 0.63 |
| KOOS Quality of Life subscale score |
44.18 (40.62 to 47.73) |
44.09 (40.80 to 47.38) |
−0.09 (−4.64 to 4.47), 0.97 |
| Total BML Volume | 12959.66 (10991.04 to 14928.29) | 11047.29 (8833.52 to 13261.05) | −1912.38 (−4602.61 to 777.86), 0.16 |
| Medial TF BML Volume | 8331.48 (6903.42 to 9759.55) | 7051.42 (5398.37 to 8704.47) | −1280.07 (−3210.91 to 650.78), 0.19 |
| Lateral TF BML Volume | 1340.93 (801.87 to 1879.99) | 1219.47 (648.63 to 1790.32) | −121.45 (−630.27 to 387.36), 0.64 |
For pain in last week and pain in nominated activity, lower scores represent pain reduction. For KOOS higher scores represent pain reduction.
Pain and other scores in the table are adjusted for the baseline value of that outcome which is the same for both treatment groups.
We found no effect of treatment on medial bone marrow lesion volume or on total BML volume in the knee (Table 3).
Results from complete case analyses were similar.
During their period on active treatment, 28% of participants (16/57) achieved a minimally important improvement, and on neutral insoles, 22% (13/58) experienced this level of improvement. The odds ratio for achieving important improvement on lateral wedges vs. neutral inserts was 1.35 (95% 0.58, 3.13, p = 0.49).
At the end of the first treatment period, participants reported mean insole use of 7.10 hrs/day (SD 2.72), and at the end of the second, participants reported use of 7.80 hrs/day (SD 3.17).
During the trial, 7 participants experienced side effects leading to temporary (3) or permanent (4) treatment discontinuation. Of these, 4 occurred during lateral wedge and 3 during neutral insole treatment. Of the 2 who discontinued treatment on lateral wedge insoles, one had calf pain at night and the other experienced worse knee pain. Of the 2 stopping neutral insoles, one developed a toe blister and the other had worsening knee pain.
DISCUSSION
In this trial, we prescreened subjects to select biomechanical responses to lateral wedge insoles. When we excluded persons who had either patellofemoral involvement or failed to show biomechanical responses to insoles, we detected a small effect of insoles on pain reduction that was missed in previous trials in which there was no prescreening. However, most participants in the trial did not experience a level of improvement that would qualify as minimally important based on accepted thresholds. While this trial suggests an approach to detect efficacy of this treatment, the efficacy was not sufficient to recommend this treatment and the screening approach we used. In this case, we may have found a treatment that shows statistical without clinical significance.
The small treatment effect we found may account for the lack of consistency across outcomes. While the lateral wedge insole reduced knee pain based on our primary outcome, knee pain over the past week and also led to a reduction in the patient’s nominated painful activity, it did not significantly reduce pain or self-reported function assessed using the KOOS survey, an expanded version of the Western Ontario McMaster Universities Osteoarthritis Index (WOMAC). We have reported that WOMAC is not as sensitive to change as global questions about knee pain (14) and that may partly explain the lack of effect. However, this lack of effect also suggests that the treatment effect was modest. For the primary outcome, the effect size calculated as a standardized response mean was 0.30. This effect is comparable to that found for nonsteroidal drugs vs. acetaminophen in OA (20). While a validated estimate of minimally important improvement (18) for our primary outcome is 1.0, the mean difference in pain reduction between the lateral wedge and neutral insole periods of treatment was only 0.7.
Our screening process utilized a gait laboratory to identify participants with a biomechanical response to wedges. Such laboratory evaluations are expensive and may not be widely available. Clinical screening protocols could be developed that might identify with high probability those likely to respond. We have tried to develop such a protocol without success (21), but other efforts are needed. While the KAM measures the medial vs. lateral load, another factor affecting medial joint loading is the knee flexion moment. We have previously reported that the lateral wedge used in this study does not affect this moment (22).
Once patellofemoral OA is ruled out by a simple physical examination, given the low cost and benign safety profile of these wedges, it might be argued that treating patients with these insoles is a reasonable clinical strategy rather than seeking a gait laboratory evaluation. Even so, the treatment effect is likely to be small and only a few patients will note substantial benefit.
Could the effect of lateral wedge insoles have been similar to that seen in previous trials that used WOMAC or KOOS as outcomes? In two large studies using variable stiffness shoes or lateral wedge insoles, authors (5, 19) used the same global knee pain measure and found no effect of treatment vs. control on pain.
Osteoarthritis has been challenging to treat because it combines mechanopathology with an inflammatory response to joint injury, both of which contribute to pain and disease progression. It has been unclear whether nonsurgical treatments targeting pathomechanics were likely to be major elements of the treatment armamentarium. Knee brace adherence is poor, for example. Our study offers modest promise for a simple, inexpensive treatment. Further refinement of the treatment with use of specific shoes or increases in the degree of wedging may increase efficacy.
We and others have reported that biomechanical response to lateral wedge insoles is variable (22, 24, 25). Although the reasons are unclear, one study (25) suggests that stiffness in feet and ankles in some persons may prevent the lateral ankle eversion that is necessary for knee loading to change with this treatment.
While lateral wedge insoles are thought to be safe treatments, their use occasionally (21) generates complaints of discomfort in the shoe and may cause back pain (19). We did not find major safety concerns, and no one discontinued treatment due to back pain.
We carried out a crossover trial to evaluate the effect of lateral wedge insoles. Crossover trials permit the testing of treatments more efficiently than parallel design trials and make it easier to detect modest effects of treatments.
In summary, we found, for the first time, that lateral wedge insoles may be modestly effective in reducing pain in persons with medial knee OA. However, the treatment effect was small and most treated patients did not achieve conventional levels of minimal important response. Future modifications of the treatment or of the screening strategy might offer greater levels of efficacy.
Key Messages:
Trials testing lateral wedge vs. neutral insoles in persons with painful medial knee osteoarthritis have shown no reduction in knee pain despite a proven reduction in medial loading.
The biomechanical response to lateral wedges is inconsistent with roughly 25% of persons showing no reduction in medial loading, and shifting load laterally may worsen patellofemoral loading.
This trial prescreened subjects with painful medial OA to remove those with patellofemoral disease and those who did not show a reduction in medial knee loading with lateral insoles.
While the trial showed a reduction in the primary pain outcome, this effect was small and other measures of pain showed no effect, nor was there a reduction in local bone marrow lesions on MRI, a measure of medial load.
Even after prescreening, lateral wedge insoles, at least as conventionally used, do not produce important improvement in pain in most persons.
Acknowledgements:
We appreciate the participation of all subjects and also are indebted to Helen Williams and Michelle Reading for their help with the study. We also thank Michael LaValley, Tuhina Neogi and Catherine Hill for their valuable suggestions.
Supported by the NIHR Manchester Biomedical Research Centre. Dr. Felson was supported by NIH AR47785
References
- 1.Lawrence RC, Felson DT, Helmick CG, Arnold LM, Choi H, Deyo RA, et al. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States. Part II. Arthritis Rheum 2008;58(1):26–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kerrigan DC, Lelas JL, Goggins J, Merriman GJ, Kaplan RJ, Felson DT. Effectiveness of a lateral-wedge insole on knee varus torque in patients with knee osteoarthritis. ArchPhysMedRehabil 2002;83(7):889–93. [DOI] [PubMed] [Google Scholar]
- 3.Arnold JB, Wong DX, Jones RK, Hill CL, Thewlis D. Lateral Wedge Insoles for Reducing Biomechanical Risk Factors for Medial Knee Osteoarthritis Progression: A Systematic Review and Meta-Analysis. Arthritis Care Res (Hoboken) 2016;68(7):936–51. [DOI] [PubMed] [Google Scholar]
- 4.Parkes MJ, Maricar N, Lunt M, LaValley MP, Jones RK, Segal NA, et al. Lateral wedge insoles as a conservative treatment for pain in patients with medial knee osteoarthritis: a meta-analysis. JAMA 2013;310(7):722–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hinman RS, Wrigley TV, Metcalf BR, Campbell PK, Paterson KL, Hunter DJ, et al. Unloading Shoes for Self-management of Knee Osteoarthritis: A Randomized Trial. Ann Intern Med 2016;165(6):381–9. [DOI] [PubMed] [Google Scholar]
- 6.Butler RJ, Marchesi S, Royer T, Davis IS. The effect of a subject-specific amount of lateral wedge on knee mechanics in patients with medial knee osteoarthritis. J Orthop Res 2007;25(9):1121–7. [DOI] [PubMed] [Google Scholar]
- 7.Hinman RS, Bowles KA, Bennell KL. Laterally wedged insoles in knee osteoarthritis: do biomechanical effects decline after one month of wear? BMCMusculoskeletDisord 2009;10:146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jones RK, Chapman GJ, Parkes MJ, Forsythe L, Felson DT. The effect of different types of insoles or shoe modifications on medial loading of the knee in persons with medial knee osteoarthritis: a randomized trial. J Orthop Res 2015;33(11):1646–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cappozzo A, Catani F, Croce UD, Leardini A. Position and orientation in space of bones during movement: anatomical frame definition and determination. Clin Biomech (Bristol, Avon) 1995;10(4):171–8. [DOI] [PubMed] [Google Scholar]
- 10.Bell AL, Brand RA, Pedersen DR Prediction of hip joint centre location from external landmarks. Hum Mov Sci 1989;8(1):3–16. [Google Scholar]
- 11.Jones RK, Chapman GJ, Findlow AH, Forsythe L, Parkes MJ, Sultan J, et al. A new approach to prevention of knee osteoarthritis: reducing medial load in the contralateral knee. J Rheumatol 2013;40(3):309–15. [DOI] [PubMed] [Google Scholar]
- 12.Callaghan MJ, Parkes MJ, Hutchinson CE, Gait AD, Forsythe LM, Marjanovic EJ, et al. A randomized trial of a brace for patellofemoral osteoarthritis targeting knee pain and bone marrow lesions. Ann Rheum Dis 2015;74(6):1164–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dworkin RH, Turk DC, Farrar JT, Haythornthwaite JA, Jensen MP, Katz NP, et al. Core outcome measures for chronic pain clinical trials: IMMPACT recommendations. Pain 2005;113(1–2):9–19. [DOI] [PubMed] [Google Scholar]
- 14.Parkes MJ, Callaghan MJ, O’Neill TW, Forsythe LM, Lunt M, Felson DT. Sensitivity to Change of Patient-Preference Measures for Pain in Patients With Knee Osteoarthritis: Data From Two Trials. Arthritis Care Res (Hoboken) 2016;68(9):1224–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Roos EM, Roos HP, Lohmander LS, Ekdahl C, Beynnon BD. Knee Injury and Osteoarthritis Outcome Score (KOOS)--development of a self-administered outcome measure. JOrthopSports PhysTher 1998;28(2):88–96. [DOI] [PubMed] [Google Scholar]
- 16.Peterfy CG, Guermazi A, Zaim S, Tirman PF, Miaux Y, White D, et al. Whole-Organ Magnetic Resonance Imaging Score (WORMS) of the knee in osteoarthritis. OsteoarthritisCartilage 2004;12(3):177–90. [DOI] [PubMed] [Google Scholar]
- 17.Vickers AJ, Altman DG. Statistics notes: Analysing controlled trials with baseline and follow up measurements. BMJ 2001;323(7321):1123–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Salaffi F, Stancati A, Silvestri CA, Ciapetti A, Grassi W. Minimal clinically important changes in chronic musculoskeletal pain intensity measured on a numerical rating scale. Eur J Pain 2004;8(4):283–91. [DOI] [PubMed] [Google Scholar]
- 19.Bennell KL, Bowles KA, Payne C, Cicuttini F, Williamson E, Forbes A, et al. Lateral wedge insoles for medial knee osteoarthritis: 12 month randomized controlled trial. BMJ 2011;342:d2912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Verkleij SP, Luijsterburg PA, Bohnen AM, Koes BW, Bierma-Zeinstra SM. NSAIDs vs acetaminophen in knee and hip osteoarthritis: a systematic review regarding heterogeneity influencing the outcomes. Osteoarthritis Cartilage 2011;19(8):921–9. [DOI] [PubMed] [Google Scholar]
- 21.Chapman GJ, Parkes MJ, Forsythe L, Felson DT, Jones RK. Ankle motion influences the external knee adduction moment and may predict who will respond to lateral wedge insoles?: an ancillary analysis from the SILK trial. Osteoarthritis Cartilage 2015;23(8):1316–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jones RK, Chapman GJ, Forsythe L, Parkes MJ, Felson DT. The relationship between reductions in knee loading and immediate pain response whilst wearing lateral wedged insoles in knee osteoarthritis. J Orthop Res 2014;32(9):1147–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kris MG, Johnson BE, Berry LD, Kwiatkowski DJ, Iafrate AJ, Wistuba, II, et al. Using multiplexed assays of oncogenic drivers in lung cancers to select targeted drugs. JAMA 2014;311(19):1998–2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hinman RS, Bowles KA, Payne C, Bennell KL. Effect of length on laterally-wedged insoles in knee osteoarthritis. Arthritis Rheum 2008;59(1):144–7. [DOI] [PubMed] [Google Scholar]
- 25.Lewinson RT, Vallerand IA, Collins KH, Wiley JP, Lun VMY, Patel C, et al. Reduced knee adduction moments for management of knee osteoarthritis:: A three month phase I/II randomized controlled trial. Gait Posture 2016;50:60–8. [DOI] [PubMed] [Google Scholar]