Abstract
Natural killer cells play a critical role in antiviral and anti-tumor responses. While current NK cell immune therapies have focused primarily on cancer biology, many of these advances can be readily applied to target HIV/SIV-infected cells. Promising developments include recent reports that CAR NK cells are capable of targeted responses while producing less off-target and toxic side-effects than are associated with CAR T cell therapies. Further CAR NK cells derived from inducible pluripotent stem cells or cell lines may allow for more rapid ‘off-the-shelf’ access. Other work investigating the IL-15 superagonist ALT-803 (now N803) may also provide a recourse for enhancing NK cell responses in the context of the immunosuppressive and inflammatory environment of chronic HIV/SIV infections, leading to enhanced control of viremia. With a broader acceptance of research supporting adaptive functions in NK cells it is likely that novel immunotherapeutics and vaccine modalities will aim to generate virus-specific memory NK cells. In doing so, better targeted NK cell responses against virus-infected cells may usher in a new era of NK cell-tuned immune therapy.
A. Introduction:
Ever since the identification of B and T cells as crucial components of adaptive immunity [1] the research community has been trying to exploit how best to elicit targeted humoral and cell-mediated responses. While these approaches have led to the development of numerous vaccine candidates and therapeutics, most of these approaches only engage innate immune cells as a means to augment the adaptive response, rather than to generate an independent protective innate response. This is in part due to the innate immune response lacking the antigen specificity of B and T cells, and that innate immune responses appeared to lack memory-recall potential, both classical defining traits that distinguish the innate from adaptive immune systems [2, 3]. Rather, the scope of innate immune activation has been generally restricted to the development of adjuvants that engage Toll-like receptors (TLRs), or elicit a broad, non-specific inflammatory response in order to promote an enhanced adaptive cell-mediated or humoral response [4, 5].
Through a balance of inhibitory and activating receptor engagement, natural killer (NK) cells recognize and eliminate tumor and virus-infected cells as a primary effector of the innate immune system. Classically, NK cells are described as non-specific because they develop antigen receptors independently of RAG [6]. Nevertheless experimental data from mice, non-human primates and humans has recently indicated that NK cells may also possess the ability to quickly respond in an antigen specific manner – suggesting the presence of memory properties [7–11]. Through several paradigm shifting works, NK cells are now gaining acceptance to have adaptive features, especially in the context of cytomegalovirus (CMV) [12, 13]. Adaptive NK cells have now also been recently described specific to HIV and SIV/SHIV antigens [11, 14]. These particularly exciting findings suggest it may be possible to use HIV-specific NK cells as better immune therapies and perhaps even as a functional cure for HIV.
Above all other viral infections studied in the context of adaptive NK cells, CMV is probably the most well understood. In mice, Ly-49h+ NK cells expand after infection with murine CMV (mCMV) by recognizing CMV protein m157, and respond more potently after reactivation or new infection with mCMV [15]. Likewise, in humans and non-human primates CMV/rhesus cytomegalovirus (rhCMV) infections drive the expansion of NKG2C+ NK cells [16, 17]. Whether or not NKG2C is specifically recognizing CMV antigens in vivo is unclear, nevertheless it has been shown that NKG2C exhibits preferential binding preference to some CMV peptides, especially when presented on HLA-E [18]. These adaptive NK cell populations are long-lived and display more maturation markers than classical NK cells, including CD57, and proliferative and cytotoxic functions upon encountering the same antigen are enhanced [16, 19]. While NKG2C is a prototypical marker used to delineate antigen-specific NK cells in humans, other receptors may be involved. Activating KIRs may promote HCMV-induced NK cell differentiation [20] especially because an expansion of mature NK cells expressing functional activating KIR has been observed in patients with a homozygous deletion of NKG2C [21]. Another subset of adaptive NK cells are induced by cytokine milieus. They were most clearly delineated by Cooper and colleagues who showed that re-stimulation of murine NK cells induced greater IFN-γ production if they were pre-treated with IL-12 and IL-15 [22]. Cytokine-induced adaptive NK cells are being used for immunotherapies in the cancer biology field [23, 24] and their enhanced potency could also be considered for viral infections. Finally, a third subset of adaptive NK cells express elevated levels of Fc receptors such as CD16 on their surface, while also lacking expression of the associated intracellular γ signaling chain [25]. Similar to the NKG2C+ NK cell population, γ chain-deficient (ΔG) NK Cells are expanded in HCMV-seropositive individuals and also become long-lived after an initial stimulation through antibody binding. Interestingly, despite lacking the γ chain, antibody-dependent cellular cytotoxicity (ADCC) and the proliferative potential of ΔG NK cells are enhanced [26, 27]. Further, memory-like NK cells appear to have enhanced binding to antibodies against herpes simplex virus, influenza, HCV, and even HIV [13].
In HIV-1 and SIV infection of nonhuman primate models, various genetic, epidemiological, and functional studies have shown that NK cells are among the first immune populations to expand following infection, and they may be directly involved in preventing virus replication and disease [28, 29]. Our group and others have shown that the NKG2C+ NK cell population is increased following infection with SIV/HIV, though the contribution of CMV to this increase is unclear [30, 31]. Furthermore, NK cells play an important role in controlling non-pathogenic SIV infection by migrating into lymph node follicles, a likely viral reservoir [32, 33]. This phenomenon is heavily dependent on the NK cell growth cytokine IL-15. Interestingly, NK cells from HIV-positive donors can be strongly stimulated with IL-15 in order to improve their antiviral and cytotoxic potential and, more importantly, enhance their ability to clear HIV-infected cells [34]. The first demonstration that primates mobilize adaptive NK cells against HIV/SIV antigens was provided by our group [11]. In this study our group showed that purified NK cells from the spleens and livers of rhesus macaques infected with simian–human immunodeficiency virus (SHIV) demonstrated antigen-specific NK cell responses against viral proteins. An even more striking finding was that NK cells from macaques vaccinated 5 years prior with ENV- or GAG-expressing Adenovirus vectors were able to efficiently lyse matched presented antigens [11]. While these responses were NKG2-dependent, delineating the full mechanisms of primate NK cell memory will require further study.
B. Current NK cell therapies and HIV infection
HIV infection leads to systemic immune activation and immunosuppression, having an impact on NK cells which commonly show signs of dysregulation and exhaustion [35, 36]. NK cells normally recognize several receptors or ligands presented on the surfaces of target cells, including MHC molecules, Fc regions of antibodies or self-proteins, that engage either inhibitory or activating NK receptors. As a result, potential avenues for NK cell based therapies tend to exploit these mechanisms, both for NK cell enhancement and reversal of dysfunction. Another approach, ‘immune restoration’, involves preventing the elimination of uninfected CD4+ T cells via NK cells using blocking antibodies against gp41. This preventing the interaction between gp41/gp120 and CD4 receptor which results in the upregulation of an NK-activating ligand, NKp44L, on CD4+ T cells which are subsequently eliminated by NK cells (ClinicalTrials.gov ID: NCT01549119, NCT02041247 and [37]). Currently, there are several clinical trials that are investigating the role of NK cells in either controlling HIV through direct or indirect NK cell modulation. In order to enhance targeting of virally infected cells several groups have attempted harness NK cells in combination with broadly neutralizing antibodies (bNAbs) (ClinicalTrials.gov ID: NCT02018510), TLR agonists to elicit enhanced killing of virally infected cells (ClinicalTrials.gov ID: NCT02077868, NCT02200081and NCT02668770), or via targeting of various immune checkpoints or inhibitory molecules (ClinicalTrial.gov ID numbers: NCT02921685, NCT02643550, NCT02399917, NCT02599649, NCT02252263, NCT02481297, NCT01687387, NCT01714739, NCT01592370, NCT01750580), and discussed below.
bNAbs, NK cell activation and SIV/HIV
The majority of functions mediated by bNAbs are via cells expressing Fc receptors, which can transduce intracellular signaling in multiple effector cell types. NK cells are one of the principal mediators of antibody dependent cellular cytotoxicity (ADCC) (reviewed in [38]) due to their expression of CD16 (FcγRIII). In fact, Fc receptor expression levels in humanized mice [39] and rhesus macaques [40] seem to directly influence the ability of bNAbs to mediate the control of viral replication. This role of Fc receptors seems to be even more important than complement binding in regulating viral infection [41]. Unsurprisingly, non-neutralizing HIV-1 antibodies have been shown to lack ADCC breadth [42]. Thus, it is becoming increasingly evident that the elimination of virus-infected cells may require non-neutralizing functions mediated through interaction with Fc receptors, particularly those on NK cells [43]. Data from a clinical trial with bNAb antibody cocktails showed very modest results with only 2 out of 8 participants showing moderate delays in viral rebound [44]. Several bNAbs have since been developed against HIV (reviewed in [45]) with the goal of eliciting potent immune responses in HIV-1 infected patients. A recent Phase I clinical trial with the bNAb 3BNC117 that targets CD4 binding sites showed that it acted as a highly efficacious agent for reducing HIV-1 viremia [46]. Follow-up studies of 3BNC117 mediated clearing of virus from mice by the same group suggested the requirement of FcγR expressing NK cells to engage the antibody [47]. These observations have also been confirmed by multiple follow-up studies [39, 48–50].
Toll like receptors (TLR) agonists to activate NK cells in HIV/SIV infections
TLRs are pathogen recognition receptors (PRR) that identify molecular patterns in pathogenic microbes and are expressed in NK cells and other innate immune cells including DCs, macrophages and B cells. The role of TLR agonists in direct NK cell activation is relatively underexplored and somewhat controversial since some studies indicate only indirect activation of NK cells through DC, monocyte, macrophage, or B cell activation following TLR stimulation [51–53]. Regardless of the mode of activation, TLR-mediated stimulation using agonists that engage TLR3, 7, 8 and 9 is a major focus of anti-HIV therapy and thus provides an opportunity to examine the roles that NK cells contribute to antiviral responses.
TLR3 recognition of viral RNA leads to potent inflammatory responses mediated, in part, by interferon activation. In the context of HIV infection, TLR3 agonists have been used to enhance polyfunctional responses in NK cells from exposed seronegative (ESN) individuals. In a study using HIV serodiscordant couples, Lima et al. showed that there was an elevated expression of the activation marker CD38, along with IFN-γ, TNFα and CD107a in ESN individuals in response to TLR3 stimulation [54].
Agonists that engage both TLR7 and TLR8 lead to either direct or indirect NK activation via DC signaling [53]. Currently, two small molecule agonists of TLR7, Vesatolimod (GS-9620) and its analog, GS-986, are being used in SIV-infected/ART-treated rhesus macaques [55]. This dual therapy of ART and TLR agonist showed reduction in the viral reservoir with transient viremia and immunopotentiation as demonstrated by a rapid activation of NK and T cell responses to infection. Recently Borducchi EN et al. showed that treatment with PGT121 and GS-9620 in SHIV-infected rhesus macaques delayed viral rebound following ART discontinuation due to activation of NK cells and monocytes leading to antibody mediated clearance of virus infected cells [56]. Treatment with the TLR8 agonist, VTX2337, led to both direct and indirect activation of NK cells via DC mediated production of inflammatory cytokines and chemokines including IL-12, and TNF-α [57]. Direct NK cell activation by VTX2337 resulted in increased levels of IFN-γ secretion, enhanced NK cell cytotoxicity against K562 target cells, as well as enhanced ADCC activity against other tumor targets [57].
The TLR9 agonist Lefitolimod (MGN1703) is used in studies to reactivate the latent viral reservoir via ‘shock and kill’ therapy. TLR9 activation of NK cells is primarily due to activation of pDCs, with subsequent secretion of IFN-α, leading to the recruitment of cytotoxic NK and effector CD8+ T cells [58]. In addition to immune stimulation, MGN1703 also induces reactivation of latent HIV, allowing for targeting via NK and CD8+ T cell dependent cytotoxicity. The specific effects of MGN1703 stimulation on NK cells include NKp46 upregulation, which has been associated with NK cell lysis of HIV-infected CD4+ T cells, NKG2A upregulation on CD56dim cells, elevated expression of the activation/tissue marker CD69 and increased antiviral functions as evidenced by CD107a and IFN-γ expression when cultured with K562 and latently HIV-1 infected ACH2 cells as well as autologous HIV-1 infected CD4+T cells in vitro [59]. MGN 1703 is already in phase III testing for treatment of metastatic colorectal cancer (ClinicalTrials.gov ID: NCT02077868) and is currently also being investigated for small cell lung cancer and melanoma (ClinicalTrials.gov ID number: NCT02200081 and NCT02668770, respectively).
Blocking inhibitory NK cell receptors during infection
NKG2A is an inhibitory molecule present on both NK and CD8+ T cells. It forms a heterodimer on the cell surface along with CD94 and has been shown to interact with HLA-E in the context of presented peptides, and has preferential affinity to bind HLA-E as compared to the activating NKG2C/CD94 heterodimer [60, 61]. However, CD94/NKG2A activation triggers a negative feedback loop preventing NK cell activation and thereby can promote immune escape [62]. Several studies targeting NKG2A in order to enhance NK and CD8+ T cell functions using the NKG2A blocking antibody, Monalizumab, are now underway for cancer therapy [63, 64]. Anti-NKG2A antibody treatment has also been shown to improve NK cell cytotoxicity and viral clearance in patients with chronic hepatitis B as well as in mouse models of hepatitis B [65]. Currently there are several clinical trials examining the effects of blocking NKG2A using Monalizumab in patients with squamous cell carcinoma or following allogeneic stem cell transplantation (ClinicalTrials.gov ID numbers: NCT02921685, NCT02643550).
KIR molecules play a crucial role in determining the activation status of NK cells, specifically through their interaction with MHC molecules [66]. The anti-KIR mAb Lirilumab (IPH2102) binds to inhibitory KIR2DL1,2 and 3 receptors and blocks its interaction with HLA-C. This blockade mimics a KIR mismatch and improves responses against virus-infected or tumor cells. In vitro, IPH2102 treatment was shown to augment NK cell-mediated killing of autologous HLA-C expressing leukemic cells [67]. Further, this therapy has been shown to be well tolerated in both hematological malignancies and solid tumors, and may also be used as a monotherapy or in combination with other agents [68]. IPH2102 is currently undergoing phase I/II clinical trials as monotherapy or in combination across a range of hematologic and solid tumors (ClinicalTrial.gov ID numbers: NCT02399917, NCT02599649, NCT02252263, NCT02481297, NCT01687387, NCT01714739, NCT01592370, NCT01750580). Similar blockades of inhibitory receptors in HIV infections could be an attractive approach to augment immunotherapies or curative strategies.
IL-15 and IL-15 superagonists to augment NK cell function
ALT-803 (now N803) is a fusion complex of IL-15 and IL-15Rα with IgG1 Fc that has enhanced IL-15 biological activity through a single amino acid mutation in IL-15 [69, 70]. ALT-803 has been shown to be well tolerated in patients with relapsed hematologic malignancies with augmented NK and T cell responses (ClinicalTrials.gov ID: NCT01885897 and [71]). Further, ALT-803 is being tested in concert with other checkpoint inhibitors such as anti-PD-1 therapy in patients with metastatic non-small cell lung cancer [70]. Because this agonist is capable of activating both CD8+ T and NK cells, there is substantial interest in utilizing ALT-803 in SIV/HIV infection [72]. Recent work from Garrido C. et al. show that ex vivo culture of NK cells with IL-15 and in the presence of Vorinostat, a histone deacetylase inhibitor and potent latency reversal agent, enhance NK cell mediated killing of HIV-infected target cells [34]. As IL-15 treatment enhances ADCC and can sometimes be used in concert with other cytokines, this approach may also be able to elicit adaptive ΔG NK or cytokine-induced NK cells, though this remains to be shown. This approach may prove pivotal for the development of functional cure therapies for HIV.
Modification of NK cells using CAR technology
The substantial investment in development of chimeric antigen receptor (CAR) T cells has driven advances in the cancer field over the past decade [73–75]. More recently, several groups are developing CAR NK cells originating from the NK-92 cell line [76], stem cells [77, 78] or derived from cord blood (ClinicalTrial.gov ID: NCT03056339) [79] in order to treat multiple cancers [80]. Particularly interesting is that some CAR NK cell therapeutic interventions seem to have less significant toxicity associated with treatment [81] than has been observed in CAR T cell therapies, where cytokine release syndrome can lead to potentially lethal outcomes [82]. Several studies suggest that using CAR-T cells could be an asset in HIV therapy and cure work. Much of the focus revolves around the development of CARs target gp120 and/or gp41 via expression of CD4ζ (described below), bNAbs and bi-specific antibodies [83–85]. These developments, and in particular CAR NK cells derived from induced pluripotent stem cells (iPSC) [78] have the potential to be clinically scalable in order to provide ‘off-the-shelf’ treatment for cancer patients [86]. Since the targets for CAR NK cells are customizable, it is quite probable that this technology will be highly relevant to developing targeted NK cell therapy to eliminate SIV/HIV-virally infected cells. In fact, there have been several attempts at targeting HIV-infected CD4+ T cells using CAR-NK cells. In one approach a chimeric antigen receptor comprising of CD4 fused with the CD3ζ chain, termed CD4ζ, provides NK cells with the ability to bind to gp120 from HIV and signal via the CD3ζ chain. While CD4ζ CAR-NK cells showed increased inhibition of HIV replication in CD4+ T cells in vitro, there was no significant difference in in vivo studies when comparing these cells to unmodified NK cells [87–89]. These data provide enthusiasm for the continued development of HIV-specific CAR-NK cells which will likely become a crucial component of HIV cure therapies.
C. Concluding remarks and future directions:
NK cell-based therapies are still in a relative infancy especially for treatment of viral infections. This is in contrast to the substantial advances in cancer immunotherapy, perhaps as a result of greater regulatory freedom/ willingness to try relatively risky therapeutics in otherwise terminally ill patients [90]. Nevertheless, several exciting developments in cancer biology, including the development of CAR NK cells, and activation of NK cells using IL-15 superagonists like N803 show substantial promise for being quickly implemented in HIV antiviral therapy. Further, with the identification of adaptive NK cell responses against HIV and SIV, it is possible that targeting MHC-E peptide presentation to NKG2C on NK cells could be achieved with vaccination approaches that promote enhanced presentation via MHC-E, or via specific cytokine signatures to produce cytokine-dependent memory NK responses through the development of more targeted adjuvants. Exploiting NK cells and NK cell memory responses could be the decisive turning point in augmenting prophylactic and therapeutic modalities against HIV.
Figure 1. Therapeutic interventions and their effect on NK cell anti-HIV/SIV functions.
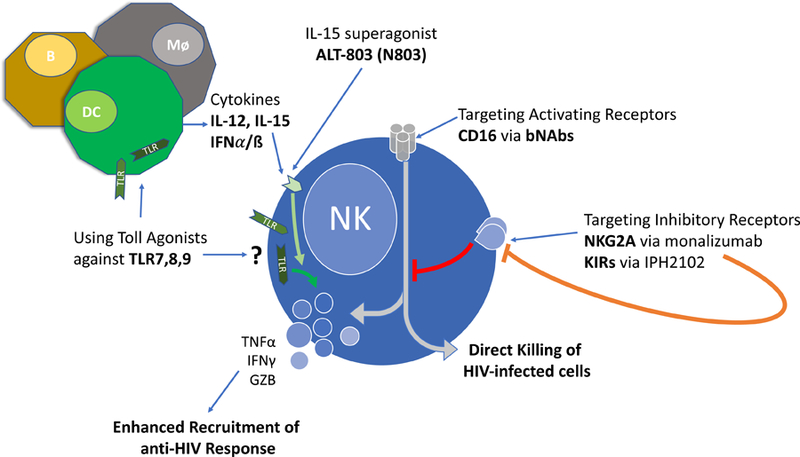
Current therapies targeting NK cells directly or indirectly serve to augment NK cell anti-HIV/SIV responses. NK cell activation through the blocking of inhibitory signaling resulting from NKG2A, or inhibitory KIRs leads to enhanced NK cell activation. TLR 7,8,9 agonists most likely lead to indirect NK cell activation via cytokine production by dendritic cells (DC), macrophages (Mø), or B cells (B). These cytokines (IL-12, IL-15, IFNα/β) in addition to the IL-15 superagonist (N803) engage the cognate receptors on NK cells (IL-12R, IL-15R, IFNAR), leading to NK cell activation and net increases in anti-HIV/SIV responses.
Acknowledgements
This work was supported by National Institutes of Health grants R01 AI120828 and R01 DE026014, both to R.K.R. D.R.R. was also supported, in part, by NIH training grant T32 AI007387–29.
Abbreviations:
- ADCC
Antibody-dependent cellular cytotoxicity
- CAR
Chimeric antigen receptor
- CMV
Cytomegalovirus
- rhCMV
Rhesus cytomegalovirus
- NK
Cell Natural killer cell
- RAG
Recombination activation gene
- iPSC
Induced pluripotent stem cell
- HIV
Human immunodeficiency virus
- SIV
Simian immunodeficiency virus
- SHIV
Simian-human immunodeficiency virus
Footnotes
Conflict of Interest Disclosure
The authors declare no conflicts of interest.
References
- 1.Berek C and Milstein C (1987) Mutation drift and repertoire shift in the maturation of the immune response. Immunol Rev 96, 23–41. [DOI] [PubMed] [Google Scholar]
- 2.Fearon DT and Locksley RM (1996) The instructive role of innate immunity in the acquired immune response. Science 272, 50–3. [DOI] [PubMed] [Google Scholar]
- 3.Janeway CA Jr. (1992) The immune system evolved to discriminate infectious nonself from noninfectious self. Immunol Today 13, 11–6. [DOI] [PubMed] [Google Scholar]
- 4.Di Pasquale A, Preiss S, Tavares Da Silva F, Garcon N (2015) Vaccine Adjuvants: from 1920 to 2015 and Beyond. Vaccines (Basel) 3, 320–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Francica JR, Zak DE, Linde C, Siena E, Johnson C, Juraska M, Yates NL, Gunn B, De Gregorio E, Flynn BJ, Valiante NM, Malyala P, Barnett SW, Sarkar P, Singh M, Jain S, Ackerman M, Alam M, Ferrari G, Tomaras GD, O’Hagan DT, Aderem A, Alter G, Seder RA (2017) Innate transcriptional effects by adjuvants on the magnitude, quality, and durability of HIV envelope responses in NHPs. Blood Adv 1, 2329–2342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Karo JM and Sun JC (2015) Novel molecular mechanism for generating NK-cell fitness and memory. Eur J Immunol 45, 1906–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sun JC and Lanier LL (2017) Is There Natural Killer Cell Memory and Can It Be Harnessed by Vaccination? NK Cell Memory and Immunization Strategies against Infectious Diseases and Cancer. Cold Spring Harb Perspect Biol [DOI] [PMC free article] [PubMed]
- 8.Holmes TD and Bryceson YT (2016) Natural killer cell memory in context. Semin Immunol 28, 368–76. [DOI] [PubMed] [Google Scholar]
- 9.Marcus A and Raulet DH (2013) Evidence for natural killer cell memory. Curr Biol 23, R817–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Paust S and von Andrian UH (2011) Natural killer cell memory. Nat Immunol 12, 500–8. [DOI] [PubMed] [Google Scholar]
- 11.Reeves RK, Li H, Jost S, Blass E, Li H, Schafer JL, Varner V, Manickam C, Eslamizar L, Altfeld M, von Andrian UH, Barouch DH (2015) Antigen-specific NK cell memory in rhesus macaques. Nat Immunol 16, 927–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Geary CD and Sun JC (2017) Memory responses of natural killer cells. Semin Immunol 31, 11–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Paust S, Blish CA, Reeves RK (2017) Redefining Memory: Building the Case for Adaptive NK Cells. J Virol 91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Peppa D, Pedroza-Pacheco I, Pellegrino P, Williams I, Maini MK, Borrow P (2018) Adaptive Reconfiguration of Natural Killer Cells in HIV-1 Infection. Frontiers in immunology 9, 474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sun JC, Beilke JN, Lanier LL (2009) Adaptive immune features of natural killer cells. Nature 457, 557–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lopez-Verges S, Milush JM, Schwartz BS, Pando MJ, Jarjoura J, York VA, Houchins JP, Miller S, Kang SM, Norris PJ, Nixon DF, Lanier LL (2011) Expansion of a unique CD57(+)NKG2Chi natural killer cell subset during acute human cytomegalovirus infection. Proc Natl Acad Sci U S A 108, 14725–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Foley B, Cooley S, Verneris MR, Curtsinger J, Luo X, Waller EK, Anasetti C, Weisdorf D, Miller JS (2012) Human cytomegalovirus (CMV)-induced memory-like NKG2C(+) NK cells are transplantable and expand in vivo in response to recipient CMV antigen. J Immunol 189, 5082–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hammer Q, Ruckert T, Borst EM, Dunst J, Haubner A, Durek P, Heinrich F, Gasparoni G, Babic M, Tomic A, Pietra G, Nienen M, Blau IW, Hofmann J, Na IK, Prinz I, Koenecke C, Hemmati P, Babel N, Arnold R, Walter J, Thurley K, Mashreghi MF, Messerle M, Romagnani C (2018) Peptide-specific recognition of human cytomegalovirus strains controls adaptive natural killer cells. Nat Immunol 19, 453–463. [DOI] [PubMed] [Google Scholar]
- 19.O’Sullivan TE and Sun JC (2015) Generation of Natural Killer Cell Memory during Viral Infection. J Innate Immun 7, 557–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Beziat V, Liu LL, Malmberg JA, Ivarsson MA, Sohlberg E, Bjorklund AT, Retiere C, Sverremark-Ekstrom E, Traherne J, Ljungman P, Schaffer M, Price DA, Trowsdale J, Michaelsson J, Ljunggren HG, Malmberg KJ (2013) NK cell responses to cytomegalovirus infection lead to stable imprints in the human KIR repertoire and involve activating KIRs. Blood 121, 2678–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Della Chiesa M, Falco M, Bertaina A, Muccio L, Alicata C, Frassoni F, Locatelli F, Moretta L, Moretta A (2014) Human cytomegalovirus infection promotes rapid maturation of NK cells expressing activating killer Ig-like receptor in patients transplanted with NKG2C−/− umbilical cord blood. J Immunol 192, 1471–9. [DOI] [PubMed] [Google Scholar]
- 22.Cooper MA, Elliott JM, Keyel PA, Yang L, Carrero JA, Yokoyama WM (2009) Cytokine-induced memory-like natural killer cells. Proc Natl Acad Sci U S A 106, 1915–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Berrien-Elliott MM, Wagner JA, Fehniger TA (2015) Human Cytokine-Induced Memory-Like Natural Killer Cells. J Innate Immun 7, 563–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Romee R, Rosario M, Berrien-Elliott MM, Wagner JA, Jewell BA, Schappe T, Leong JW, Abdel-Latif S, Schneider SE, Willey S, Neal CC, Yu L, Oh ST, Lee YS, Mulder A, Claas F, Cooper MA, Fehniger TA (2016) Cytokine-induced memory-like natural killer cells exhibit enhanced responses against myeloid leukemia. Sci Transl Med 8, 357ra123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang T, Scott JM, Hwang I, Kim S (2013) Cutting edge: antibody-dependent memory-like NK cells distinguished by FcRgamma deficiency. J Immunol 190, 1402–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee J, Zhang T, Hwang I, Kim A, Nitschke L, Kim M, Scott JM, Kamimura Y, Lanier LL, Kim S (2015) Epigenetic modification and antibody-dependent expansion of memory-like NK cells in human cytomegalovirus-infected individuals. Immunity 42, 431–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shah SV, Manickam C, Ram DR, Kroll K, Itell H, Permar SR, Barouch DH, Klatt NR, Reeves RK (2018) CMV Primes Functional Alternative Signaling in Adaptive Deltag NK Cells but Is Subverted by Lentivirus Infection in Rhesus Macaques. Cell Rep 25, 2766–2774 e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jost S and Altfeld M (2012) Evasion from NK cell-mediated immune responses by HIV-1. Microbes Infect 14, 904–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Scully E and Alter G (2016) NK Cells in HIV Disease. Curr HIV/AIDS Rep 13, 85–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gondois-Rey F, Cheret A, Granjeaud S, Mallet F, Bidaut G, Lecuroux C, Ploquin M, Muller-Trutwin M, Rouzioux C, Avettand-Fenoel V, Moretta A, Pialoux G, Goujard C, Meyer L, Olive D (2017) NKG2C(+) memory-like NK cells contribute to the control of HIV viremia during primary infection: Optiprim-ANRS 147. Clin Transl Immunology 6, e150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ram DR, Manickam C, Hueber B, Itell HL, Permar SR, Varner V, Reeves RK (2018) Tracking KLRC2 (NKG2C)+ memory-like NK cells in SIV+ and rhCMV+ rhesus macaques. PLoS Pathog 14, e1007104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Huot N, Bosinger SE, Paiardini M, Reeves RK, Muller-Trutwin M (2018) Lymph Node Cellular and Viral Dynamics in Natural Hosts and Impact for HIV Cure Strategies. Front Immunol 9, 780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Huot N, Jacquelin B, Garcia-Tellez T, Rascle P, Ploquin MJ, Madec Y, Reeves RK, Derreudre-Bosquet N, Muller-Trutwin M (2017) Natural killer cells migrate into and control simian immunodeficiency virus replication in lymph node follicles in African green monkeys. Nat Med 23, 1277–1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Garrido C, Abad-Fernandez M, Tuyishime M, Pollara JJ, Ferrari G, Soriano-Sarabia N, Margolis DM (2018) Interleukin-15-Stimulated Natural Killer Cells Clear HIV-1-Infected Cells following Latency Reversal Ex Vivo. J Virol 92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Alter G, Teigen N, Davis BT, Addo MM, Suscovich TJ, Waring MT, Streeck H, Johnston MN, Staller KD, Zaman MT, Yu XG, Lichterfeld M, Basgoz N, Rosenberg ES, Altfeld M (2005) Sequential deregulation of NK cell subset distribution and function starting in acute HIV-1 infection. Blood 106, 3366–3369. [DOI] [PubMed] [Google Scholar]
- 36.Schafer JL, Li H, Evans TI, Estes JD, Reeves RK (2015) Accumulation of Cytotoxic CD16+ NK Cells in Simian Immunodeficiency Virus-Infected Lymph Nodes Associated with In Situ Differentiation and Functional Anergy. J Virol 89, 6887–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vieillard V, Gharakhanian S, Lucar O, Katlama C, Launay O, Autran B, Ho Tsong Fang R, Crouzet J, Murphy RL, Debre P (2016) Perspectives for immunotherapy: which applications might achieve an HIV functional cure? Oncotarget 7, 38946–38958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mikulak J, Oriolo F, Zaghi E, Di Vito C, Mavilio D (2017) Natural killer cells in HIV-1 infection and therapy. AIDS 31, 2317–2330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bournazos S, Klein F, Pietzsch J, Seaman MS, Nussenzweig MC, Ravetch JV (2014) Broadly neutralizing anti-HIV-1 antibodies require Fc effector functions for in vivo activity. Cell 158, 1243–1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ko SY, Pegu A, Rudicell RS, Yang ZY, Joyce MG, Chen X, Wang K, Bao S, Kraemer TD, Rath T, Zeng M, Schmidt SD, Todd JP, Penzak SR, Saunders KO, Nason MC, Haase AT, Rao SS, Blumberg RS, Mascola JR, Nabel GJ (2014) Enhanced neonatal Fc receptor function improves protection against primate SHIV infection. Nature 514, 642–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hessell AJ, Hangartner L, Hunter M, Havenith CE, Beurskens FJ, Bakker JM, Lanigan CM, Landucci G, Forthal DN, Parren PW, Marx PA, Burton DR (2007) Fc receptor but not complement binding is important in antibody protection against HIV. Nature 449, 101–4. [DOI] [PubMed] [Google Scholar]
- 42.Bruel T, Guivel-Benhassine F, Lorin V, Lortat-Jacob H, Baleux F, Bourdic K, Noel N, Lambotte O, Mouquet H, Schwartz O (2017) Lack of ADCC Breadth of Human Nonneutralizing Anti-HIV-1 Antibodies. J Virol 91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bruel T, Guivel-Benhassine F, Amraoui S, Malbec M, Richard L, Bourdic K, Donahue DA, Lorin V, Casartelli N, Noel N, Lambotte O, Mouquet H, Schwartz O (2016) Elimination of HIV-1-infected cells by broadly neutralizing antibodies. Nat Commun 7, 10844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Trkola A, Kuster H, Rusert P, Joos B, Fischer M, Leemann C, Manrique A, Huber M, Rehr M, Oxenius A, Weber R, Stiegler G, Vcelar B, Katinger H, Aceto L, Gunthard HF (2005) Delay of HIV-1 rebound after cessation of antiretroviral therapy through passive transfer of human neutralizing antibodies. Nat Med 11, 615–22. [DOI] [PubMed] [Google Scholar]
- 45.McCoy LE and Burton DR (2017) Identification and specificity of broadly neutralizing antibodies against HIV. Immunol Rev 275, 11–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Caskey M, Klein F, Lorenzi JC, Seaman MS, West AP Jr., Buckley N, Kremer G, Nogueira L, Braunschweig M, Scheid JF, Horwitz JA, Shimeliovich I, Ben-Avraham S, Witmer-Pack M, Platten M, Lehmann C, Burke LA, Hawthorne T, Gorelick RJ, Walker BD, Keler T, Gulick RM, Fatkenheuer G, Schlesinger SJ, Nussenzweig MC (2015) Viraemia suppressed in HIV-1-infected humans by broadly neutralizing antibody 3BNC117. Nature 522, 487–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lu CL, Murakowski DK, Bournazos S, Schoofs T, Sarkar D, Halper-Stromberg A, Horwitz JA, Nogueira L, Golijanin J, Gazumyan A, Ravetch JV, Caskey M, Chakraborty AK, Nussenzweig MC (2016) Enhanced clearance of HIV-1-infected cells by broadly neutralizing antibodies against HIV-1 in vivo. Science 352, 1001–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gooneratne SL, Richard J, Lee WS, Finzi A, Kent SJ, Parsons MS (2015) Slaying the Trojan horse: natural killer cells exhibit robust anti-HIV-1 antibody-dependent activation and cytolysis against allogeneic T cells. J Virol 89, 97–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kramski M, Stratov I, Kent SJ (2015) The role of HIV-specific antibody-dependent cellular cytotoxicity in HIV prevention and the influence of the HIV-1 Vpu protein. AIDS 29, 137–44. [DOI] [PubMed] [Google Scholar]
- 50.Wren LH, Chung AW, Isitman G, Kelleher AD, Parsons MS, Amin J, Cooper DA, investigators A. s. c., Stratov I, Navis M, Kent SJ (2013) Specific antibody-dependent cellular cytotoxicity responses associated with slow progression of HIV infection. Immunology 138, 116–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Adib-Conquy M, Scott-Algara D, Cavaillon JM, Souza-Fonseca-Guimaraes F (2014) TLR-mediated activation of NK cells and their role in bacterial/viral immune responses in mammals. Immunol Cell Biol 92, 256–62. [DOI] [PubMed] [Google Scholar]
- 52.Ram DR, Kroll K, Reeves RK (2018) Indirect activation of rhesus macaque (Macaca mulatta) NK cells in oral and mucosal draining lymph nodes. J Med Primatol 47, 302–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Schlaepfer E and Speck RF (2008) Anti-HIV activity mediated by natural killer and CD8+ cells after toll-like receptor 7/8 triggering. PLoS One 3, e1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lima JF, Oliveira LMS, Pereira NZ, Duarte AJS, Sato MN (2017) Polyfunctional natural killer cells with a low activation profile in response to Toll-like receptor 3 activation in HIV-1-exposed seronegative subjects. Sci Rep 7, 524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lim SY, Osuna CE, Hraber PT, Hesselgesser J, Gerold JM, Barnes TL, Sanisetty S, Seaman MS, Lewis MG, Geleziunas R, Miller MD, Cihlar T, Lee WA, Hill AL, Whitney JB (2018) TLR7 agonists induce transient viremia and reduce the viral reservoir in SIV-infected rhesus macaques on antiretroviral therapy. Sci Transl Med 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Borducchi EN, Liu J, Nkolola JP, Cadena AM, Yu WH, Fischinger S, Broge T, Abbink P, Mercado NB, Chandrashekar A, Jetton D, Peter L, McMahan K, Moseley ET, Bekerman E, Hesselgesser J, Li W, Lewis MG, Alter G, Geleziunas R, Barouch DH (2018) Antibody and TLR7 agonist delay viral rebound in SHIV-infected monkeys. Nature [DOI] [PMC free article] [PubMed]
- 57.Lu H, Dietsch GN, Matthews MA, Yang Y, Ghanekar S, Inokuma M, Suni M, Maino VC, Henderson KE, Howbert JJ, Disis ML, Hershberg RM (2012) VTX-2337 is a novel TLR8 agonist that activates NK cells and augments ADCC. Clin Cancer Res 18, 499–509. [DOI] [PubMed] [Google Scholar]
- 58.Vibholm L, Schleimann MH, Hojen JF, Benfield T, Offersen R, Rasmussen K, Olesen R, Dige A, Agnholt J, Grau J, Buzon M, Wittig B, Lichterfeld M, Petersen AM, Deng X, Abdel-Mohsen M, Pillai SK, Rutsaert S, Trypsteen W, De Spiegelaere W, Vandekerchove L, Ostergaard L, Rasmussen TA, Denton PW, Tolstrup M, Sogaard OS (2017) Short-Course Toll-Like Receptor 9 Agonist Treatment Impacts Innate Immunity and Plasma Viremia in Individuals With Human Immunodeficiency Virus Infection. Clin Infect Dis 64, 1686–1695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Offersen R, Nissen SK, Rasmussen TA, Ostergaard L, Denton PW, Sogaard OS, Tolstrup M (2016) A Novel Toll-Like Receptor 9 Agonist, MGN1703, Enhances HIV-1 Transcription and NK Cell-Mediated Inhibition of HIV-1-Infected Autologous CD4+ T Cells. J Virol 90, 4441–4453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Braud VM, Allan DS, O’Callaghan CA, Soderstrom K, D’Andrea A, Ogg GS, Lazetic S, Young NT, Bell JI, Phillips JH, Lanier LL, McMichael AJ (1998) HLA-E binds to natural killer cell receptors CD94/NKG2A, B and C. Nature 391, 795–9. [DOI] [PubMed] [Google Scholar]
- 61.Lee N, Llano M, Carretero M, Ishitani A, Navarro F, Lopez-Botet M, Geraghty DE (1998) HLA-E is a major ligand for the natural killer inhibitory receptor CD94/NKG2A. Proc Natl Acad Sci U S A 95, 5199–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Borrego F, Ulbrecht M, Weiss EH, Coligan JE, Brooks AG (1998) Recognition of human histocompatibility leukocyte antigen (HLA)-E complexed with HLA class I signal sequence-derived peptides by CD94/NKG2 confers protection from natural killer cell-mediated lysis. J Exp Med 187, 813–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.McWilliams EM, Mele JM, Cheney C, Timmerman EA, Fiazuddin F, Strattan EJ, Mo X, Byrd JC, Muthusamy N, Awan FT (2016) Therapeutic CD94/NKG2A blockade improves natural killer cell dysfunction in chronic lymphocytic leukemia. Oncoimmunology 5, e1226720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ruggeri L, Urbani E, Andre P, Mancusi A, Tosti A, Topini F, Blery M, Animobono L, Romagne F, Wagtmann N, Velardi A (2016) Effects of anti-NKG2A antibody administration on leukemia and normal hematopoietic cells. Haematologica 101, 626–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Li F, Wei H, Wei H, Gao Y, Xu L, Yin W, Sun R, Tian Z (2013) Blocking the natural killer cell inhibitory receptor NKG2A increases activity of human natural killer cells and clears hepatitis B virus infection in mice. Gastroenterology 144, 392–401. [DOI] [PubMed] [Google Scholar]
- 66.Beziat V, Hilton HG, Norman PJ, Traherne JA (2017) Deciphering the killer-cell immunoglobulin-like receptor system at super-resolution for natural killer and T-cell biology. Immunology 150, 248–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Romagne F, Andre P, Spee P, Zahn S, Anfossi N, Gauthier L, Capanni M, Ruggeri L, Benson DM Jr., Blaser BW, Della Chiesa M, Moretta A, Vivier E, Caligiuri MA, Velardi A, Wagtmann N (2009) Preclinical characterization of 1–7F9, a novel human anti-KIR receptor therapeutic antibody that augments natural killer-mediated killing of tumor cells. Blood 114, 2667–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Vey N, Karlin L, Sadot-Lebouvier S, Broussais F, Berton-Rigaud D, Rey J, Charbonnier A, Marie D, Andre P, Paturel C, Zerbib R, Bennouna J, Salles G, Goncalves A (2018) A phase 1 study of lirilumab (antibody against killer immunoglobulin-like receptor antibody KIR2D; IPH2102) in patients with solid tumors and hematologic malignancies. Oncotarget 9, 17675–17688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Rhode PR, Egan JO, Xu W, Hong H, Webb GM, Chen X, Liu B, Zhu X, Wen J, You L, Kong L, Edwards AC, Han K, Shi S, Alter S, Sacha JB, Jeng EK, Cai W, Wong HC (2016) Comparison of the Superagonist Complex, ALT-803, to IL15 as Cancer Immunotherapeutics in Animal Models. Cancer immunology research 4, 49–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wrangle JM, Velcheti V, Patel MR, Garrett-Mayer E, Hill EG, Ravenel JG, Miller JS, Farhad M, Anderton K, Lindsey K, Taffaro-Neskey M, Sherman C, Suriano S, Swiderska-Syn M, Sion A, Harris J, Edwards AR, Rytlewski JA, Sanders CM, Yusko EC, Robinson MD, Krieg C, Redmond WL, Egan JO, Rhode PR, Jeng EK, Rock AD, Wong HC, Rubinstein MP (2018) ALT-803, an IL-15 superagonist, in combination with nivolumab in patients with metastatic non-small cell lung cancer: a non-randomised, open-label, phase 1b trial. Lancet Oncol 19, 694–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Romee R, Cooley S, Berrien-Elliott MM, Westervelt P, Verneris MR, Wagner JE, Weisdorf DJ, Blazar BR, Ustun C, DeFor TE, Vivek S, Peck L, DiPersio JF, Cashen AF, Kyllo R, Musiek A, Schaffer A, Anadkat MJ, Rosman I, Miller D, Egan JO, Jeng EK, Rock A, Wong HC, Fehniger TA, Miller JS (2018) First-in-human phase 1 clinical study of the IL-15 superagonist complex ALT-803 to treat relapse after transplantation. Blood 131, 2515–2527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Webb GM, Li S, Mwakalundwa G, Folkvord JM, Greene JM, Reed JS, Stanton JJ, Legasse AW, Hobbs T, Martin LD, Park BS, Whitney JB, Jeng EK, Wong HC, Nixon DF, Jones RB, Connick E, Skinner PJ, Sacha JB (2018) The human IL-15 superagonist ALT-803 directs SIV-specific CD8(+) T cells into B-cell follicles. Blood Adv 2, 76–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Miliotou AN and Papadopoulou LC (2018) CAR T-cell Therapy: A New Era in Cancer Immunotherapy. Curr Pharm Biotechnol 19, 5–18. [DOI] [PubMed] [Google Scholar]
- 74.Newick K, O’Brien S, Moon E, Albelda SM (2017) CAR T Cell Therapy for Solid Tumors. Annual review of medicine 68, 139–152. [DOI] [PubMed] [Google Scholar]
- 75.June CH, O’Connor RS, Kawalekar OU, Ghassemi S, Milone MC (2018) CAR T cell immunotherapy for human cancer. Science 359, 1361–1365. [DOI] [PubMed] [Google Scholar]
- 76.Suck G, Odendahl M, Nowakowska P, Seidl C, Wels WS, Klingemann HG, Tonn T (2016) NK-92: an ‘off-the-shelf therapeutic’ for adoptive natural killer cell-based cancer immunotherapy. Cancer Immunol Immunother 65, 485–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lowe E, Truscott LC, De Oliveira SN (2016) In Vitro Generation of Human NK Cells Expressing Chimeric Antigen Receptor Through Differentiation of Gene-Modified Hematopoietic Stem Cells. Methods Mol Biol 1441, 241–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Li Y, Hermanson DL, Moriarity BS, Kaufman DS (2018) Human iPSC-Derived Natural Killer Cells Engineered with Chimeric Antigen Receptors Enhance Anti-tumor Activity. Cell stem cell 23, 181–192 e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Mehta RS and Rezvani K (2018) Chimeric Antigen Receptor Expressing Natural Killer Cells for the Immunotherapy of Cancer. Frontiers in immunology 9, 283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Guillerey C, Huntington ND, Smyth MJ (2016) Targeting natural killer cells in cancer immunotherapy. Nat Immunol 17, 1025–36. [DOI] [PubMed] [Google Scholar]
- 81.Liu E, Tong Y, Dotti G, Shaim H, Savoldo B, Mukherjee M, Orange J, Wan X, Lu X, Reynolds A, Gagea M, Banerjee P, Cai R, Bdaiwi MH, Basar R, Muftuoglu M, Li L, Marin D, Wierda W, Keating M, Champlin R, Shpall E, Rezvani K (2018) Cord blood NK cells engineered to express IL-15 and a CD19-targeted CAR show long-term persistence and potent antitumor activity. Leukemia 32, 520–531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Neelapu SS, Tummala S, Kebriaei P, Wierda W, Gutierrez C, Locke FL, Komanduri KV, Lin Y, Jain N, Daver N, Westin J, Gulbis AM, Loghin ME, de Groot JF, Adkins S, Davis SE, Rezvani K, Hwu P, Shpall EJ (2018) Chimeric antigen receptor T-cell therapy - assessment and management of toxicities. Nat Rev Clin Oncol 15, 47–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Maldini CR, Ellis GI, Riley JL (2018) CAR T cells for infection, autoimmunity and allotransplantation. Nat Rev Immunol 18, 605–616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Haran KP, Hajduczki A, Pampusch MS, Mwakalundwa G, Vargas-Inchaustegui DA, Rakasz EG, Connick E, Berger EA, Skinner PJ (2018) Simian Immunodeficiency Virus (SIV)-Specific Chimeric Antigen Receptor-T Cells Engineered to Target B Cell Follicles and Suppress SIV Replication. Frontiers in immunology 9, 492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Sahu GK, Sango K, Selliah N, Ma Q, Skowron G, Junghans RP (2013) Anti-HIV designer T cells progressively eradicate a latently infected cell line by sequentially inducing HIV reactivation then killing the newly gp120-positive cells. Virology 446, 268–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Siegler EL, Zhu Y, Wang P, Yang L (2018) Off-the-Shelf CAR-NK Cells for Cancer Immunotherapy. Cell stem cell 23, 160–161. [DOI] [PubMed] [Google Scholar]
- 87.Ni Z, Knorr DA, Bendzick L, Allred J, Kaufman DS (2014) Expression of chimeric receptor CD4zeta by natural killer cells derived from human pluripotent stem cells improves in vitro activity but does not enhance suppression of HIV infection in vivo. Stem Cells 32, 1021–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Tran AC, Zhang D, Byrn R, Roberts MR (1995) Chimeric zeta-receptors direct human natural killer (NK) effector function to permit killing of NK-resistant tumor cells and HIV-infected T lymphocytes. J Immunol 155, 1000–9. [PubMed] [Google Scholar]
- 89.Zhen A, Kamata M, Rezek V, Rick J, Levin B, Kasparian S, Chen IS, Yang OO, Zack JA, Kitchen SG (2015) HIV-specific Immunity Derived From Chimeric Antigen Receptor-engineered Stem Cells. Mol Ther 23, 1358–1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Emens LA, Butterfield LH, Hodi FS Jr., Marincola FM, Kaufman HL (2016) Cancer immunotherapy trials: leading a paradigm shift in drug development. J Immunother Cancer 4, 42. [DOI] [PMC free article] [PubMed] [Google Scholar]


