Abstract
Understanding of the comparative bleeding risks of oral anticoagulant (OAC) therapies for the primary treatment of venous thromboembolism (VTE) is limited. Therefore, among anticoagulant-naïve VTE patients, we conducted comparisons of apixaban, rivaroxaban and warfarin on the rate of hospitalised bleeding within 180 days of OAC initation. MarketScan databases for the time-period from 2011 to 2016 were used and, for each OAC comparison, new users were matched with up to 5 initiators of a different OAC. The final analysis included 83,985 VTE patients, who experienced 1,944 hospitalised bleeding events. In multivariable-adjusted Cox regression models, rate of hospitalised bleeding was lower among new users of apixaban when compared to new users of rivaroxaban [hazard ratio (95% confidence interval): 0.58 (0.41–0.80)] or warfarin [0.68 (0.50–0.92)]. Overall, the hospitalised bleeding rate was similar when comparing new users of rivaroxaban to new users of warfarin [0.98 (0.68–1.11)], though there was some suggestion that rivaroxaban was associated with lower bleeding risk among younger individuals. Findings from this large real-world population concur with results from the randomised trial which found lower bleeding risk with apixaban versus warfarin and, for the first time, reveal a lower risk of bleeding in a comparison of apixaban versus rivaroxaban.
Keywords: venous thromboembolism, comparative effectiveness, oral anticoagulant, rivaroxaban, apixaban
Introduction
Venous thromboembolism (VTE), consisting of deep vein thrombosis (DVT) and pulmonary embolism (PE), annually affects 10 million individuals globally.(Raskob et al., 2014) Direct oral anticoagulants (DOACs), specifically dabigatran, rivaroxaban, apixaban and edoxaban(Schulman et al., 2009, Einstein Investigators, 2010, Buller et al., 2013, Agnelli et al., 2013, Schulman et al., 2014, Einstein-PE Investigators, 2012) are now included – together with warfarin – as standards of care for VTE treatment in current guidelines.(Kearon et al., 2016)
The first 3–6 months of anticoagulant treatment for VTE is generally viewed as active treatment of the initial thrombosis (primary treatment). In phase III randomised controlled trials (RCTs) of VTE primary treatment(Schulman et al., 2009, Einstein Investigators, 2010, Buller et al., 2013, Agnelli et al., 2013, Schulman et al., 2014) the DOACs were each shown to be non-inferior to warfarin in the prevention of recurrent VTE and VTE-related death. In addition, in meta-analyses of the phase III RCTs(van Es et al., 2014, Gómez-Outes et al., 2014) DOACs have had a lower risk of most bleeding complications when compared to warfarin. However, no randomised study has conducted comparisons between the DOACs and bleeding risk in the context of VTE treatment. Furthermore, despite the promising results from RCTs, whether DOACs are as effective and safe when used in the general VTE patient population as in clinical trials is unknown. Although RCTs are considered the gold standard for evaluating the efficacy of new drugs, they generally do not conduct dirct comparisons of novel treatments, but instead compare novel treatements to established therapies, and have important limitations. They recruit selected (possibly nongeneralisable) patients, their size usually does not allow for the detection of infrequent adverse effects or the evaluation of patient subgroups, and results might not reflect the effectiveness of a new treatment in usual practice.(Basu, 2012, Kunz et al., 2012) For instance, concerns have been raised about use of DOACs among patients with kidney disease, because impaired renal function may lead to DOAC accumulation and increased bleeding risk.(Lega et al., 2014) Compliance to DOACs in real-world settings may also be poorer than typically exhibited among RCT participants.(Shore et al., 2015, Shore et al., 2014, Tsai et al., 2013, Garkina et al., 2016, Manzoor et al., 2017) Furthermore, no VTE RCTs have conducted comparisons between DOACs.
Using VTE patient data from a routine practice setting, we characterised the rate of major hospitalised bleeding events associated with use of apixaban, rivaroxaban and warfarin during the VTE primary treatment period. We also evaluated associations in subgroups of particular interest. Comparisons with dabigatran and edoxaban were not made, because these OACs were prescribed relatively infrequently in our study time-period, from 2011–2016.
Methods
Study population
This retrospective cohort study utilised health claims data obtained from the MarketScan data warehouse (Truven Health Analytics, Inc., Ann Arbor, MI; Hansen, 2017) for the time-period from 1 January 2011 to 31 December 2016. We used data from both the MarketScan Commercial Database, which consists of employer and health plan sourced data, and the Medicare Supplemental database, which includes retirees with Medicare supplemental insurance paid for by employers. These databases contain detailed inpatient and outpatient medical claims that are linked to outpatient prescription drug claims and person-level enrollment information, are de-identified, compliant with the Health Insurance Portability and Accountability Act, and commercially available. As such, the University of Minnesota Institutional Review Board deemed this analysis exempt from review.
The present analysis includes individuals aged 18 to 99 years with incident VTE (DVT or PE), at least one prescription for OAC within the 31.5 days before or after their first VTE claim (note that hospital discharge dates are used) and ≥3 months of continuous enrollment prior to their first OAC prescription. VTE was defined as having at least one inpatient claim for VTE or 2 outpatient claims for VTE, 7 to 185 days apart, based on International Classification of Diseases, 9th Revision, Clinical Modification (ICD-9-CM) and 10th Revision (ICD-10-CM) codes. ICD-9-CM and ICD-10-CM codes to define VTE are provided in Table S1, and could occur in any position. The positive predictive value (PPV) was 91% in a recent validation study, which empoloyed a definition to that used herin, inclusive of both inpatient and outpatient encounters and additionally required treatment.(Sanfilippo et al., 2015)
The initial sample included 472,757 VTE patients aged 18 to 99 years. The analytic sample was 367,886 once it was restricted to individuals ever prescribed an OAC between 1 January 2011 and 31 December 2016; 231,189 after requiring the first OAC prescription by ±31 days of the VTE date; and 173,779 after requiring ≥3 months of continuous enrollment before the first OAC prescription. These 173,779 were our eligible study population.
Anticoagulant use
Prescriptions for DOACs and warfarin were identified from outpatient pharmaceutical claims data, which includes information on the National Drug Code (NDC), the prescription fill date, and the number of days supplied. Validity of warfarin claims in administrative databases is excellent (sensitivity: 94%, PPV: 99%).(Garg et al., 2011) Validation studies of DOAC claims have not yet been conducted.
The analysis focused on ‘new users’ of OACs, meaning that the VTE patients did not have any prior exposure to OACs during the time period under observation. Patients were categorised according to the first OAC prescription filled, and remained in this initial category for the entire analysis, in order to mimic the intent-to-treat approach of a RCT.(Hernán et al., 2008)
Identification of hospitalised bleeding events
Hospitalised bleeding events were identified based on hospitalisation discharge codes indicating intracranial haemorrhage, gastrointestinal bleeding and other major bleeding. Intracranial haemorrhage was defined by ICD-9-CM codes 430, 431 or 432 as the primary discharge diagnosis in an inpatient claim;(Cunningham et al., 2011) the PPV of this definition is estimated to be >90%.(Andrade et al., 2012, Fang et al., 2011) Gastrointestinal bleeding and other major bleeding events were defined using the algorithm determined by (Cunningham et al (2011), which is based on inpatient claims as primary and secondary diagnoses, and presence of transfusion codes. The PPV for gastrointestinal bleeding is estimated to be 86%,(Cunningham et al., 2011) and for other bleeding (including genitourinary bleeding, haemopericardium, haemoperitoneum, haemarthrosis, epistaxis, haemoptysis, haemorrhage from throat and unspecified haemorrhage) the PPV was similar.(Cunningham et al., 2011) Crosswalks, with review for face validity, were used to translate ICD-9-CM codes to ICD-10-CM codes.
Assessment of pre-specified covariates
Information prior to the OAC initiation date (minimum 90 days) from all data sources in MarketScan (i.e., demographic data, inpatient, outpatient, and pharmacy claims) was used to derive pre-specified covariates. We identified 16 pre-specified comorbidies using validated algorithms(Cunningham et al., 2011, Quan et al., 2005) applied to the inpatient and outpatient data, and 8 different medication categories based on pharmacy prescription fills were used to identify usage of 8 different medication categories. These pre-specified covariates are listed in Table I, and the codes are listed in Table S2. ICD-9-CM comorbidity codes were translated to ICD-10-CM codes using cross-walks, with review of face-validity.
Table I.
Characteristicsa of venous thromboembolism patients by anticoagulant use, MarketScan databases, 2011 to 2016
| New users | |||
|---|---|---|---|
| Warfarin | Apixaban | Rivaroxaban | |
| N= | 46,217 | 6,786 | 30,982 |
| Age, years | 58.4 ± 15.9 | 60.4 ± 16.2 | 56.4 ± 15.4 |
| Female, % | 50.1 | 50.3 | 49.2 |
| Comorbidities,b % | |||
| Hypertension | 59.8 | 64.2 | 55.6 |
| Diabetes | 24.4 | 24.9 | 20.7 |
| Myocardial infarction | 7.8 | 8.5 | 5.5 |
| Heart failure | 14.9 | 17.1 | 10.7 |
| Ischaemic stroke/TIA | 13.7 | 10.2 | 10.3 |
| Haemorrhagic stroke | 2.3 | 5.9 | 1.7 |
| Peripheral artery disease | 14.7 | 15.3 | 11.7 |
| Dementia | 2.0 | 3.1 | 1.4 |
| Chronic pulmonary disease | 30.1 | 26.7 | 27.1 |
| Renal Disease | 12.7 | 13.2 | 7.1 |
| Liver disease | 10.5 | 9.7 | 9.6 |
| Malignancy | 18.8 | 17.0 | 16.1 |
| Metastatic cancer | 6.5 | 5.9 | 5.8 |
| Depression | 19.3 | 19.6 | 17.8 |
| Haematological disorders | 17.8 | 13.7 | 13.9 |
| Alcohol abuse | 0.9 | 1.9 | 0.9 |
| Medications, % | |||
| Antiplatelets | 6.8 | 7.0 | 5.1 |
| ACE inhibitors | 25.5 | 24.6 | 22.2 |
| Angiotensin receptor blockers | 15.0 | 17.1 | 14.5 |
| Beta-blockers | 27.9 | 30.2 | 23.9 |
| Calcium channel blockers | 20.5 | 21.5 | 17.5 |
| Statins | 34.1 | 34.8 | 30.6 |
| Diabetes medications | 7.5 | 7.0 | 5.5 |
| SSRIs | 29.7 | 29.8 | 28.6 |
| Follow-up Information | |||
| Number of bleeding events | 1,266 | 74 | 604 |
| Cumulative incidence | 2.74% | 1.09% | 1.95% |
| Person-years | 19,910 | 2,480 | 12,984 |
| Rate per 6 monthsc | 3.2% | 1.5% | 2.3% |
Values correspond to mean ± standard deviation or percentage.
ICD codes used to define these comorbidities are provided in Table S1.
The rate is calculated per 6 months of follow-up since the analysis was restricted to the primary treatment phase, which we defined as the first 6 months following OAC prescription. Rate per 6 months can be calculated as follows: [(N events/person-years)/2].
Abbreviations: ACE: angiotensin-converting enzyme; ICD: International Classification of Diseases; N: number; OAC: oral anticoagulant; SSRI: selective serotonin reuptake inhibitors; TIA: transient ischaemic attack
Statistical analysis
A total of 173,779 VTE patients met the inclusion criteria and were eligible for matching. Each new OAC user was matched with up to 5 initiators of the comparing OAC by age (± 3 years), sex, time since database enrollment (± 90 days) and drug initiation date (± 90 days). Matching was done separately for each OAC comparison, using an automated greedy matching algorithm.(Bergstralh, 2003) The matched dataset included 83,985 participants. The majority of unmatched participants were warfarin users (96.6%).
After matching, for each OAC comparison, a propensity score for exposure to the OAC was created via logistic regression, using comorbidities and medications determined a priori (Table I). Cox proportional hazards regression was used to estimate the association between OACs and risk of incident bleeding. Follow-up began at the date of the first OAC prescription filled. Person-time was accrued until incident hospitalised bleeding, health plan disenrollment, the end of study follow-up or 180 days, whichever came first. Cox models were adjusted for age (continuous), sex and the propensity score (continuous) for OAC exposure. Multiplicative interactions were evaluated between OAC use and sex, age (<65 vs. ≥65 years), and kidney disease status (yes vs. no).
In sensitivity analyses, we adjusted for empirically-defined high-dimensional propensity scores (HDPS)(Schneeweiss et al., 2009) instead of the propensity scores created using the pre-specfied comorbidities and medications. We have detailed our MarketScan HDPS approach previously.(Lutsey et al., 2018) Separate high-dimensional propensity scores were calculated for each of the 3 comparisons, and for the periods before and after ICD-10 adoption (1 October 2015). To estimate one effect over these two study periods, an indicator variable for ICD-9 or ICD-10 study period was included in the model. There was no evidence of multiplicative interactions in the association between OACs and bleeding risk by ICD-9 or ICD-10 study period.
Additional sensitivity analyses were also conducted a) to correspond to a 3-month primary treatment period, by ending follow-up 90 days after the OAC initiation date, b) stratifying according to whether the initial VTE event was managed on an inpatient or outpatient basis, and c) requiring ≥6 months of continuous enrollment before the first OAC prescription (≥3 months was used for the primary analysis). All statistical analyses were performed with SAS v 9.3 (SAS Institute Inc., Cary, NC).
Results
Characteristics of the 83,985 matched VTE patients, according to initial filled OAC prescription, are provided in Table I. Mean age (standard deviation) of these VTE patients was 57.9 (15.8) years and 50% were female. The final number matched in each OAC category was: warfarin 46,217 patients; apixaban 6,786 patients; rivaroxaban 30,982 patients. Overall, the distribution of covariates was relatively similar across OAC options, though rivaroxaban users tended to have fewer comorbid conditions. VTE events were initially managed in an inpatient setting for 61% of the sample.
A total of 1,944 hospitalised bleeding events occurred during the first 180 days after OAC initiation; 74 occurred among apixaban users, 604 among rivaroxaban users and 1,266 among warfarin users. The cumulative incidence of hospitalised bleeding was 1.1% among apixaban users, 2.0% among rivaroxaban users and 2.7% among warfarin users (Table I).
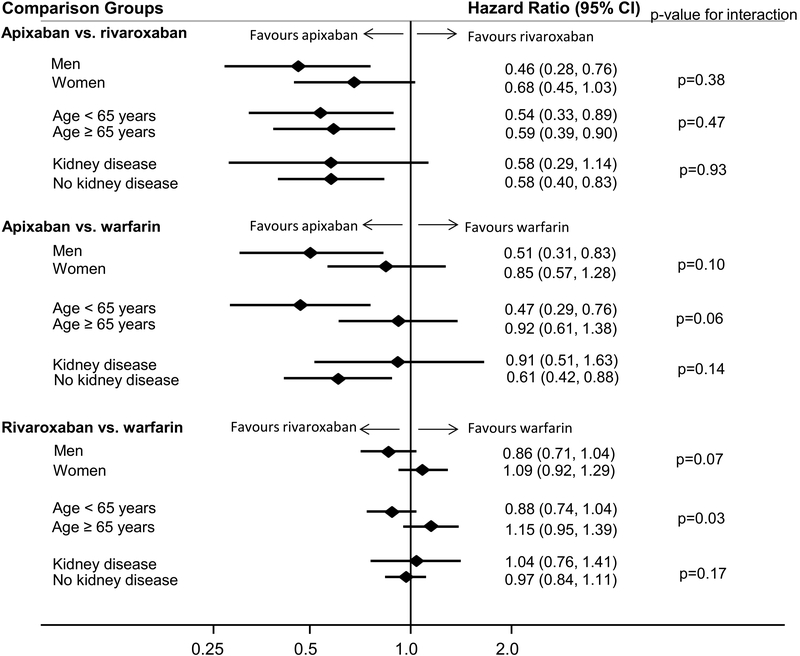
The rate of hospitalised bleeding was lower among apixaban users, as compared to rivaroxaban users [hazard ratio (HR) (95% confidence interval (CI)): 0.58 (0.41, 0.80)] (Table II). Results were similar across subgroups defined according to sex, age (< vs. ≥65 years) and prevalent kidney disease (Figure 1). Findings were also similar in analyses that limited follow-up to 90 days (Table III). Likewise, apixaban was associated with lower hospitalised bleeding risk than rivaroxaban in a number of sensitivity analyses, including adjusting for the empirically-derived HDPS, restricting to VTE cases initially managed as inpatients, and when requiring 6 months of continuous enrollment prior to anticoagulant initiation (Tables S3–S5).
Table II:
Adjusted hazard ratios (95% confidence intervals)* for hospitalised bleeding within 180 days comparing new users of specific oral anticoagulants among patients with venous thromboembolism, MarketScan databases, 2011 to 2016
| Apixaban vs. Rivaroxaban | Rivaroxaban | Apixaban |
| Number matched | 14,751 | 6,638 |
| Number of bleeding events | 170 | 49 |
| Person-years | 5,824 | 2,432 |
| HR (95% CI) | 1 (reference) | 0.58 (0.41, 0.80) |
| Apixaban vs. Warfarin | Warfarin | Apixaban |
| Number matched | 13,595 | 6,441 |
| Number of bleeding events | 191 | 52 |
| Person-years | 5,548 | 3,055 |
| HR (95% CI) | 1 (reference) | 0.68 (0.50, 0.92) |
| Rivaroxaban vs. Warfarin | Warfarin | Rivaroxaban |
| Number matched | 46,154 | 28,010 |
| Number of bleeding events | 719 | 367 |
| Person-years | 19,886 | 11,962 |
| HR (95% CI) | 1 (reference) | 0.98 (0.86, 1.11) |
Analyses matched on age, sex and time since database enrollment, and adjusted for age, sex, and a comorbidity score (created using prevalence of 16 common diagnoses and 8 medications).
Abbreviations: CI: confidence interval; HR: hazard ratio.
Figure 1.
Adjusted hazard ratios (95% confidence intervals)a of incident hospitalised bleeding within 180 days, according to initial oral anticoagulant therapy for the treatment of patients with venous thromboembolism, stratified by subgroups of interest
aAnalyses matched on age, sex and time since database enrollment, and adjusted for age, sex, and a comorbidity score (created using prevalence of 16 common diagnoses and 8 medications). The diamonds represent hazard ratios and the horizontal lines represent confidence intervals.
Abbreviations: CI, confidence interval.
Table III:
Adjusted hazard ratios (95% confidence intervals) for hospitalised bleeding within 90 days comparing new users of specific oral anticoagulants among patients with venous thromboembolism, MarketScan databases, 2011 to 2016
| Apixaban vs. Rivaroxaban | Rivaroxaban | Apixaban |
| Number matched | 14,751 | 6,638 |
| Number of bleeding events | 132 | 38 |
| Person-years | 3,280 | 1,421 |
| HR (95% CI) | 1 (reference) | 0.59 (0.41, 0.85) |
| Apixaban vs. Warfarin | Warfarin | Apixaban |
| Number matched | 13,595 | 6,441 |
| Number of bleeding events | 131 | 39 |
| Person-years | 2,405 | 1,394 |
| HR (95% CI) | 1 (reference) | 0.69 (0.48, 0.99) |
| Rivaroxaban vs. Warfarin | Warfarin | Rivaroxaban |
| N matched | 46,154 | 28,010 |
| Number matched | 490 | 283 |
| Number of bleeding events | 10,650 | 6,447 |
| HR (95% CI) | 1 (reference) | 1.09 (0.94, 1.26) |
Analyses matched on age, sex and time since database enrollment, and adjusted for age, sex, and a comorbidity score (created using prevalence of 16 common diagnoses and 8 medications).
Abbreviations: CI, confidence interval; HR, hazard ratio.
In comparisons of apixaban versus warfarin, apixaban was associated with lower risk of hospitalised bleeding after both 180 days of follow-up [HR (95% CI): 0.68 (0.50, 0.92); Table II] and 90 days of follow-up (Table III). Although not statistically significant on the multiplicative scale, there was some evidence that this association was stronger among men than women (pinteraction = 0.10), and among VTE patients aged <65 years than ≥65 years (pinteraction = 0.06; Figure 1). When adjusting for HDPS instead of the a priori propensity score, the association was attenuated [HR 95% CI: 0.84 (0.60, 1.18); Table S3]. Results were similar to the main findings in additional sensitivity analyses restricting to VTE patients managed in-hospital and requiring 6 months of continuous enrollment (Tables S4, S5).
Bleeding risk was similar in analyses comparing rivaroxaban to warfarin. In the primary analysis, with 180 days of follow-up, the HR (95% CI) was 0.98 (0.86, 1.11). Results were similar when follow-up was restricted to 90 days (Table III), and in all sensitivity analyses (Tables S3–S5). However, there was a significant multiplicative interaction (p=0.03), whereby rivaroxaban was associated with lower bleeding risk among VTE patients <65 years [HR 95% CI: 0.88 (0.74, 1.04)] but not among those older than ≥65 years [HR 95% CI: 1.15 (0.95, 1.39)] (Figure 1). Though not significant, there was also some suggestion that rivaroxaban may be more protective than warfarin in men versus women (pinteraction = 0.07).
Discussion
In this real-world population of 83,985 patients prescribed OACs for the primary treatment of VTE, subsequent risk of hospitalised bleeding was lower among apixaban users than among rivaroxaban or warfarin users. Overall, bleeding risk was similar when comparing rivaroxaban versus warfarin. The present findings provide clinically relevant information about OACs and hospitalised bleeding risk in VTE patients by i) providing a comparison of apixaban versus rivaroxaban, ii) reporting bleeding risk for apixaban versus warfarin in a usual practice patient sample and iii) demonstrating that, among those with known kidney disease, bleeding risk was generally similar among DOAC and warfarin users.
Comparison of apixaban vs. rivaroxaban
Our observation that apixaban was associated with the lowest risk of hospitalised bleeding is consistent with the known pharmokinetics of the anticoagulants as well as observations in other populations. Apixaban is dosed twice daily throughout treatment, versus once daily for rivaroxaban starting on day 22 of treatment. This is despite rivaroxaban having a shorter half-life than apixaban.(Mueck et al., 2014) To achieve equivalence to warfarin(Einstein Investigators, 2010, Agnelli et al., 2013) the peak anticoagulant effect of rivaroxaban could be higher than apixaban to accommodate the once daily dosing(Salem et al., 2015). Other evidence that bleeding risk may be greater with rivaroxaban than apixaban comes from two network meta-analysis of phase III RCTs, which provided indirect evidence of higher major bleeding risk for rivaroxaban versus apixaban, though precision was poor (Sterne et al., 2017, Mantha and Ansell, 2015), an observational study that did not restrict to the 6-month primary treatment period (Dawwas et al., 2019), comparisons conducted in the context of atrial fibrillation,(Norby and Alonso, 2017) and the observation of increased menstrual bleeding with rivaroxaban versus apixaban.(Myers and Webster, 2016)
Apixaban and rivaroxaban versus warfarin
Hospitalised bleeding risk was lower among VTE patients using apixaban than among warfarin users. This is consistent with results from the Apixaban for the Initial Management of Pulmonary Embolism and Deep-Vein Thrombosis as First-Line Therapy (AMPLIFY) Phase III randomised controlled trial,(Agnelli et al., 2013) which reported 6-month major bleeding in 0.6% of patients in the apixaban group and 1.8% of patients in the warfarin group, and a relative risk of 0.31 (95% CI: 0.17–0.55) when comparing apixaban to warfarin. Our results from a real-world population of VTE patients complement those from AMPLIFY. In the present analysis of MarketScan data, 1.1% of participants prescribed apixaban and 2.7% of participants prescribed warfarin had hospitalised bleeding in the primary treatment period, resulting in a HR (95% CI) of 0.68 (0.50–0.92). VTE patients included in the present analysis may be more representative of all VTE patients than those included in AMPLIFY, given that AMPLIFY excluded individuals with low haemoglobin concentrations, platelet counts or serum creatinine, whereas the MarketScan data included all enrollees in health plans reporting to MarketScan. The MarketScan findings also have greater precision than those of AMPLIFY because only 15 major bleeding events were reported in the apixaban group in AMPLIFY, whereas the MarketScan data identified 74 events among VTE patients prescribed apixaban. Together, these findings strongly suggest that apixaban is preferential to warfarin in terms of bleeding risk.
In contrast, overall there was no difference between rivaroxaban and warfarin in relation to risk of hospitalised bleeding in the present analysis [HR (95% CI): 0.98 (0.86–0.92)]. There was, however, suggestive evidence that rivaroxaban may be advantageous among VTE patients <65 years. The efficacy of rivaroxaban versus warfarin was evaluated in non-inferiority trials of DVT (EINSTEIN-DVT)(Einstein Investigators, 2010) and PE (EINSTEIN-PE)(Einstein-PE Investigators, 2012) patients, respectively. Among DVT patients, risk of first major or clinically relevant nonmajor bleeding was similar [HR (95% CI): 0.97 (0.76, 1.22)] though there was some suggestion that rivaroxaban may be beneficial when looking only at major bleeding events [HR (95% CI): 0.65 (0.33, 1.30)]. Similarly, in EINSTEIN PE the HR (95% CI) for major or clinically relevant nonmajor bleeding was 0.90 (0.76, 1.07), while for major bleeding it was 0.49 (0.31, 0.79) (Einstein-PE Investigators, 2012). Cumulative incidences for major bleeding in the rivaroxaban group in EINSTEIN-DVT (Einstein Investigators, 2010), EINSTEIN-PE (Einstein-PE Investigators, 2012) and MarketScan were 0.8%, 1.1% and 2.0%, respectively while in the warfarin group they were 1.2%, 2.2% and 2.7%, respectively. The EINSTEIN trials both had numerous exclusion criteria, yielding patient populations that were probably healthier than individuals included in the present MarketScan analysis.
This report also provides novel information about the comparative effectiveness of individual OACs in key VTE patient subgroups, namely according to sex, age category and known kidney disease. Overall, findings from the primary analyses were robust across these subgroups. However, both rivaroxaban and apixaban appeared to perform better among younger than older VTE patients, relative to warfarin. This observation is not entirely surprising; bleeding profile is known to vary by age, with risk and antithrombotic drug-related bleeding increasing with advancing age.(Patti et al., 2017)
Strengths & limitations
The present analysis has numerous strengths. First, the sample size of OAC users was large, resulting in a relatively large number of hospitalised bleeding events, and the ability to conduct analyses was restricted to key patient subgroups (e.g., those with known kidney disease). Second, we conducted comparisons between DOACs, thus providing information useful for clinical decision-making. Third, we evaluated these associations the context of routine clinical practice. As such, our findings may be less prone to problems of external validity than RCTs, which may not be generalisable across all patient and provider profiles, nor representative of routine clinical practice.
This analysis also has important limitations. Foremost is the possibility of uncontrolled confounding. Of particular concern is the possibility that prescription patterns differed by VTE patient health, for instance, with more ill patients having a greater likelihood of being prescribed a certain OAC (e.g., warfarin), and also having greater bleeding risk. While risk factors for bleeding might impact choice of warfarin versus the DOACs, the choice between DOACs, which are all newer therapies, should be less likely to be based on patient conditions – there is no theoretical reason why providers would select apixaban versus rivaroxaban based on patient charcteristics. Nonetheless, we attempted to control for confounding through matching on initial patient characteristics, and adjusting (separately) for an a priori-defined propensity score, and an empirically derived HDPS that utilised the wealth of information available in the MarketScan databases. The HDPS approach has been shown to be effective at controlling for confounding.(Schneeweiss et al., 2009) Misclassification is another concern, as with all analyses of administrative data. However, the algorithms for VTE, hospitalised bleeding and warfarin prescription fills all have high PPVs. The PPV for DOAC prescription fills has not yet been quantified, but may be similar to warfarin. As a final limitation, edoxaban and dabigatran were not included in the present analysis, as there were too few users of these OACs to make meaningful comparisons. Despite the noteworthy limitations of administrative data, when used with rigorous analytic methods, these data can provide timely answers about the comparative effectiveness of different treatment strategies.(Parks and Redberg, 2017, Corrigan-Curay et al., 2018)
Conclusions
In the present analysis of a real-world insured patient population in the United States, risk of hospitalised bleeding in the VTE primary treatment phase was lower among users of apixaban versus users of rivaroxaban or warfarin. These findings were generally consistent across patient subgroups of sex, age and known kidney disease. Given that none of the phase III RCTs showed benefit for DOACs over warfarin for the outcomes of VTE recurrence and mortality, safety concerns, such as bleeding risk, and convenience, such as once or twice daily dosing, will probably drive anticoagulant choice. Of course, patients themselves may have different preferences for anticoagulant choice, such as easy reversibility and the ability to measure levels(Kearon et al., 2016) which should be addressed when providers treat VTE.
Supplementary Material
Acknowledgements
This work was supported by NIH National Heart Lung and Blood Institute grants R01-HL131579 and R01-HL122200.
Footnotes
Conflicts of Interest
No conflicts of interest exist.
References
- AGNELLI G, BULLER HR, COHEN A, CURTO M, GALLUS AS, JOHNSON M, MASIUKIEWICZ U, PAK R, THOMPSON J, RASKOB GE, WEITZ JI. 2013. Oral apixaban for the treatment of acute venous thromboembolism. N Engl J Med, 369, 799–808. [DOI] [PubMed] [Google Scholar]
- ANDRADE SE, HARROLD LR, TJIA J, CUTRONA SL, SACZYNSKI JS, DODD KS, GOLDBERG RJ & GURWITZ JH 2012. A systematic review of validated methods for identifying cerebrovascular accident or transient ischemic attack using administrative data. Pharmacoepidemiology and Drug Safety, 21 Supplement 1, 100–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BASU A 2012. Patient-centered or ‘central’ patient: Raising the veil of ignorance over randomization. Stat Med, 31, 3057–9; discussion 3066–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BERGSTRALH EKJ 2003. GMATCH macro [Online]. Available: http://www.mayo.edu/research/departments-divisions/department-health-sciences-research/division-biomedical-statistics-informatics/software/locally-written-sas-macros [Accessed].
- BULLER HR, DECOUSUS H, GROSSO MA, MERCURI M, MIDDELDORP S, PRINS MH, RASKOB GE, SCHELLONG SM, SCHWOCHO L, SEGERS A, SHI MG, VERHAMME P, WELLS P & HOKUSAI VTEI 2013. Edoxaban versus Warfarin for the Treatment of Symptomatic Venous Thromboembolism. New England Journal of Medicine, 369, 1406–1415. [DOI] [PubMed] [Google Scholar]
- CORRIGAN-CURAY J, SACKS L & WOODCOCK J 2018. Real-world evidence and real-world data for evaluating drug safety and effectiveness. JAMA, 320, 867–868. [DOI] [PubMed] [Google Scholar]
- CUNNINGHAM A, STEIN CM, CHUNG CP, DAUGHERTY JR, SMALLEY WE & RAY WA 2011. An automated database case definition for serious bleeding related to oral anticoagulant use. Pharmacoepidemiology and Drug Safety, 20, 560–566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAWWAS GK, BROWN J, DIETRICH E & PARK H (2019). Effectiveness and safety of apixaban versus rivaroxaban for prevention of recurrent venous thromboembolism and adverse bleeding events in patients with venous thromboembolism: a retrospective population-based cohort analysis. Lancet Hematology. 6, e20–e28. [DOI] [PubMed] [Google Scholar]
- EINSTEIN INVESTIGATORS. 2010. Oral rivaroxaban for symptomatic venous thromboembolism. N Engl J Med, 363, 2499–510. [DOI] [PubMed] [Google Scholar]
- EINSTEIN-PE INVESTIGATORS. 2012. Oral rivaroxaban for the treatment of symptomatic pulmonary embolism. N Engl J Med, 366, 1287–97. [DOI] [PubMed] [Google Scholar]
- FANG MC, GO AS, CHANG Y, BOROWSKY LH, POMERNACKI NK, UDALTSOVA N & SINGER DE 2011. A new risk scheme to predict warfarin-associated hemorrhage: The ATRIA (Anticoagulation and Risk Factors in Atrial Fibrillation) Study. J Am Coll Cardiol, 58, 395–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GARG RK, GLAZER NL, WIGGINS KL, NEWTON KM, THACKER EL, SMITH NL, SISCOVICK DS, PSATY BM & HECKBERT SR 2011. Ascertainment of warfarin and aspirin use by medical record review compared with automated pharmacy data. Pharmacoepidemiology and Drug Safety, 20, 313–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GARKINA SV, VAVILOVA TV, LEBEDEV DS & MIKHAYLOV EN 2016. Compliance and adherence to oral anticoagulation therapy in elderly patients with atrial fibrillation in the era of direct oral anticoagulants. Journal of Geriatric Cardiology : JGC, 13, 807–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GÓMEZ-OUTES A, TERLEIRA-FERNÁNDEZ AI, LECUMBERRI R, SUÁREZ-GEA ML & VARGAS-CASTRILLÓN E 2014. Direct oral anticoagulants in the treatment of acute venous thromboembolism: A systematic review and meta-analysis. Thrombosis Research, 134, 774–782. [DOI] [PubMed] [Google Scholar]
- HANSEN L 2017. The Truven Health MarketScan Databases for life sciences researchers [Online]. Truven Health Analytics, IBM Watson Health. Available: https://truvenhealth.com/Portals/0/Assets/2017-MarketScan-Databases-Life-Sciences-Researchers-WP.pdf [Accessed 24 August 2017].
- HERNÁN MA, ALONSO A, LOGAN R, GRODSTEIN F, MICHELS KB, WILLETT WC, MANSON JE & ROBINS JM 2008. Observational studies analyzed like randomized experiments. An application to postmenopausal hormone therapy and coronary heart disease. Epidemiology, 19, 766–779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KEARON C, AKL EA, ORNELAS J, BLAIVAS A, JIMENEZ D, BOUNAMEAUX H, HUISMAN M, KING CS, MORRIS TA, SOOD N, STEVENS SM, VINTCH JR, WELLS P, WOLLER SC & MOORES L 2016. Antithrombotic Therapy for VTE Disease: CHEST Guideline and Expert Panel Report. Chest, 149, 315–52. [DOI] [PubMed] [Google Scholar]
- KUNZ LM, YEH RW & NORMAND SL 2012. Comparative effectiveness research: does one size fit all? Stat Med, 31, 3062–5; discussion 3066–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEGA JC, BERTOLETTI L, GREMILLET C, BOISSIER C, MISMETTI P & LAPORTE S 2014. Consistency of safety profile of new oral anticoagulants in patients with renal failure. Journal of Thrombosis and Haemostasis, 12, 337–343. [DOI] [PubMed] [Google Scholar]
- LUTSEY PL, NORBY FL, ZAKAI NA, MACLEHOSE RF, CHEN LY, SHAH S, DATTA YH & ALONSO A 2018 Oral anticoagulation therapy and subsequent risk of venous thromboembolism in atrial fibrillation patients. Current Medical Research and Opinion, 2018. October 26:1–20. doi: 10.1080/03007995.2018.1541445. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- MANTHA S & ANSELL J 2015. Indirect comparison of dabigatran, rivaroxaban, apixaban and edoxaban for the treatment of acute venous thromboembolism. Journal of Thrombosis and Thrombolysis, 39, 155–165. [DOI] [PubMed] [Google Scholar]
- MANZOOR BS, LEE TA, SHARP LK, WALTON SM, GALANTER WL & NUTESCU EA 2017. Real-World Adherence and Persistence with Direct Oral Anticoagulants in Adults with Atrial Fibrillation. Pharmacotherapy: The Journal of Human Pharmacology and Drug Therapy, 37, 1221–1230. [DOI] [PubMed] [Google Scholar]
- MUECK W, STAMPFUSS J, KUBITZA D & BECKA M 2014. Clinical Pharmacokinetic and Pharmacodynamic Profile of Rivaroxaban. Clinical Pharmacokinetics, 53, 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MYERS B & WEBSTER A 2016. Heavy menstrual bleeding on Rivaroxaban ‐ Comparison with Apixaban. British Journal of Haematology, 176, 833–835. [DOI] [PubMed] [Google Scholar]
- NORBY FL & ALONSO A 2017. Comparative effectiveness of rivaroxaban in the treatment of nonvalvular atrial fibrillation. Journal of Comparative Effectiveness Research, 6, 549–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PARKS AL & REDBERG RF 2017. Dabigatran compared with rivaroxaban vs warfarin—reply. JAMA Internal Medicine, 177, 744–744. [DOI] [PubMed] [Google Scholar]
- PATTI G, LUCERNA M, PECEN L, SILLER‐MATULA JM, CAVALLARI I, KIRCHHOF P & DE CATERINA R 2017. Thromboembolic Risk, Bleeding Outcomes and Effect of Different Antithrombotic Strategies in Very Elderly Patients With Atrial Fibrillation: A Sub‐Analysis From the PREFER in AF Thromboembolic Events–E<uropean Registry in Atrial Femibrillation). Journal of the American Heart Association, 6, e005657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- QUAN H, SUNDARARAJAN V, HALFON P, FONG A, BURNAND B, LUTHI JC, SAUNDERS LD, BECK CA, FEASBY TE & GHALI WA 2005. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care, 43, 1130–9. [DOI] [PubMed] [Google Scholar]
- RASKOB GE, ANGCHAISUKSIRI P, BLANCO AN, BULLER H, GALLUS A, HUNT BJ, HYLEK EM, KAKKAR A, KONSTANTINIDES SV, MCCUMBER M, OZAKI Y, WENDELBOE A & WEITZ JI 2014. Thrombosis: A Major Contributor to Global Disease Burden. Journal of Thrombosis and Haemostasis, 34, 2363–2371. [DOI] [PubMed] [Google Scholar]
- SALEM JE, SABOURET P, FUNCK-BRENTANO C & HULOT JS 2015. Pharmacology and mechanisms of action of new oral anticoagulants. Fundam Clin Pharmacol, 29, 10–20. [DOI] [PubMed] [Google Scholar]
- SANFILIPPO KM, WANG T-F, GAGE BF, LIU W & CARSON KR 2015. Improving Accuracy of International Classification of Diseases Codes for Venous Thromboembolism in Administrative Data. Thrombosis research, 135, 616–620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHNEEWEISS S, RASSEN JA, GLYNN RJ, AVORN J, MOGUN H & BROOKHART MA 2009. High-dimensional propensity score adjustment in studies of treatment effects using health care claims data. Epidemiology, 20, 512–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHULMAN S, KEARON C, KAKKAR AK, MISMETTI P, SCHELLONG S, ERIKSSON H, BAANSTRA D, SCHNEE J, GOLDHABER SZ 2009. Dabigatran versus warfarin in the treatment of acute venous thromboembolism. N Engl J Med, 361, 2342–52. [DOI] [PubMed] [Google Scholar]
- SCHULMAN S, KAKKAR AK, GOLDHABER SZ, SCHELLONG S, ERIKSSON H, MISMETTI P, CHRISTIANSEN AV, FRIEDMAN J, LE MAULF F, PETER N, KEARON C 2014. Treatment of acute venous thromboembolism with dabigatran or warfarin and pooled analysis. Circulation, 129, 764–72. [DOI] [PubMed] [Google Scholar]
- SHORE S, CAREY EP, TURAKHIA MP, JACKEVICIUS CA, CUNNINGHAM F, PILOTE L, BRADLEY SM, MADDOX TM, GRUNWALD GK, BARON AE, RUMSFELD JS, VAROSY PD, SCHNEIDER PM, MARZEC LN & HO PM 2014. Adherence to dabigatran therapy and longitudinal patient outcomes: insights from the veterans health administration. Am Heart J, 167, 810–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SHORE S, HO PM, LAMBERT-KERZNER A, GLORIOSO TJ, CAREY EP, CUNNINGHAM F, LONGO L, JACKEVICIUS C, ROSE A & TURAKHIA MP 2015. Site-level variation in and practices associated with dabigatran adherence. JAMA, 313, 1443–50. [DOI] [PubMed] [Google Scholar]
- STERNE JA, BODALIA PN, BRYDEN PA, DAVIES PA, LOPEZ-LOPEZ JA, OKOLI GN, THOM HH, CALDWELL DM, DIAS S, EATON D, HIGGINS JP, HOLLINGWORTH W, SALISBURY C, SAVOVIC J, SOFAT R, STEPHENS-BOAL A, WELTON NJ & HINGORANI AD 2017. Oral anticoagulants for primary prevention, treatment and secondary prevention of venous thromboembolic disease, and for prevention of stroke in atrial fibrillation: systematic review, network meta-analysis and cost-effectiveness analysis. Health Technol Assess, 21, 1–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TSAI K, ERICKSON SC, YANG J, HARADA AS, SOLOW BK & LEW HC 2013. Adherence, persistence, and switching patterns of dabigatran etexilate. Am J Manag Care, 19, e325–32. [PubMed] [Google Scholar]
- van ES N, COPPENS M, SCHULMAN S, MIDDELDORP S & BÜLLER HR 2014. Direct oral anticoagulants compared with vitamin K antagonists for acute venous thromboembolism: evidence from phase 3 trials. Blood, 124, 1968–1975. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.