Abstract
Active cell proliferation and turnover in the growth plate is essential for embryonic and postnatal bone growth. We performed a lineage tracing of Wnt/β-catenin signaling responsive cells (Wnt-responsive cells) using Axin2CreERT2;Rosa26ZsGreen mice and found a novel cell population that resides in the outermost layer of the growth plate facing the Ranvier’s groove (RG, the perichondrium adjacent to growth plate). These Wnt-responsive cells rapidly expanded and contributed to formation of the outer growth plate from the neonatal to the growing stage, but stopped expanding at the young adult stage when bone longitudinal growth ceases. In addition, a second Wnt-responsive sporadic cell population was localized within the resting zone of the central part of the growth plate during the postnatal growth phase. While it induced ectopic chondrogenesis in the RG, ablation of β-catenin in the Wnt-responsive cells strongly inhibited expansion of their descendants toward the growth plate. These findings indicate that the Wnt-responsive cell population in the outermost layer of the growth plate is a unique cell source of chondroprogenitors involving lateral growth of the growth plate, and suggest that Wnt/β-catenin signaling regulates function of skeletal progenitors in a site- and stage-specific manner.
Keywords: Growth plate, genetic animal models, Chondrocyte and cartilage biology, Wnt/β-catenin/LRPs
Introduction
The growth plate is a major tissue responsible for long bone formation, morphogenesis and growth in embryonic and postnatal skeletogenesis. Genetic and acquired abnormality of the growth plate causes various types of skeletal disorders. [1, 2] A key role of the growth plate in skeletal growth is to provide the longitudinal and transverse space and environment to newly forming bone. There are several cellular events critical for this role in growth plate. Proliferation and columnar alignment of chondrocytes support continuous longitudinal growth. Matrix production and accumulation are also important for interstitial growth. Cell hypertrophy greatly increases the tissue space, which is suggested to be responsible for the longitudinal tissue volume.[3, 4] In addition, recent studies have indicated that growth plate chondrocytes serve as osteoprogenitors and directly contribute to trabecular and cortical bone formation.[5–7]
During long bone growth, chondroprogenitors for the growth plate may reside in several locations. Cell labeling studies using tritiated thymidine or bromodeoxyuridine have suggested that the long-term labeled cells (slow-cycling cells), one of the characteristics of stem cells, are present in in the perichondrium adjacent to the growth plate and the border between epiphyseal bone and the growth plate during postnatal growth in rodents and rabbits.[8, 9] The former region is called Ranvier’s groove (RG) or the groove of Ranvier and the later is the resting (reservoir) zone of growth plate. Previous histological and functional studies have addressed that these two regions likely provide new cells to the growth plate.[10, 11] These studies only demonstrated the stem cell properties of these cells but did not prove that growth plate chondrocytes are actually derived from them. Recent studies have clearly demonstrated presence of chondroprogenitors in the resting zone. Parathyroid hormone-related protein (PTHrP)-positive chondrocytes in the resting zone express a panel of markers for skeletal stem and progenitor cells, and uniquely possessed the properties of skeletal stem cells in cultured conditions.[12] Lineage tracing analysis revealed that PTHrP-positive cell lineage originating from resting zone continued to form growth plate column in the long term.[12]
Beta-catenin plays a main role in the canonical Wnt signaling pathway.[13–15] The Wnt/β-catenin signaling pathway regulates development of organs and tissues in embryos and maintains tissue renewal and homeostasis in adults.[15] Studies have shown that this signaling supports retention of stem cells and regulates proliferation and differentiation of progenitors in tissue renewal in a context-dependent manner.[15–17] Wnt proteins, likely mediated by β-catenin signaling, maintains embryonic stem cell phenotype in culture.[18] Embryonic and adult stem cells require the Wnt/β-catenin signaling in their survival, proliferation or differentiation in epithelial cells in several types of organs including hair follicles, [19, 20] intestines [21, 22] and mammary glands.[23] The importance of Wnt/β-catenin signaling has been demonstrated for neural development and regeneration.[24–26]
During the growth plate development, Wnt/β-catenin signaling is indispensable. Inactivation of β-catenin signaling strongly affects cartilage development and induces morphological and functional abnormality of the growth plate,[27–31] suggesting that Wnt/β-catenin signaling may participate in regulation of chondroprogenitors in the growth plate. We hypothesized that chondroprogenitors may be Wnt-regulated cells and can be visualized as Wnt/β-catenin signaling responsive cells (Wnt-responsive cells). Therefore, we performed the lineage tracing studies using the Wnt/β-catenin signaling reporter system, which has successfully identified stem/progenitors in various tissues.[23, 32, 33] Our results demonstrated a specific population involving the appositional transverse growth of the growth plate during postnatal skeletal growth.
Materials and Methods
Ethics statement
All animal experiment procedures were approved and conducted strictly according to the NIH guidelines, the Institutional Animal Care and Use Committee of the Children’s Hospital of Philadelphia and/or the University of Maryland, Baltimore.
Animals
We crossed Axin2CreERT2-, Col2CreERT-, AcanCreERT2 or CtskCre-mice with Rosa-ZsGreen (ZsG)- or Rosa-tdTomato (RFP)-mice to create Axin2CreERT2;ZsG, AcanCreERT2;RFP, Col2CreERT;ZsG, Col2CreERT2;RFP, and CtskCre;ZsG mice. β-cateninfl/fl;Axin2CreERT2;ZsG mice, which conditionally ablated β-catenin under the control of the Axin2 promoter/enhancer, were created by mating Axin2CreERT2;β-cateninfl/wt mice with β-cateninfl/fl;ZsG mice. Mice received a single intraperitoneal injection of tamoxifen (Tx) in corn oil/10% ethanol (40 mg/ Kg). The tissues (knee joints, ribs and vertebrae) were harvested at P9, P13, P20, P42 and P70, corresponding to 3, 7, 14, 36 and 64 days after the tamoxifen injection at P6. For the short labeling, the tissues were collected 3 or 7 days after a tamoxifen injection at P21, P42 and P70. Unless otherwise specified, female mice were used for experiments due to low productivity of male Axin2CreERT2 mice. The detail methods and materials for the animals are described in Supplemental information.
Tissue processing, imaging and histological assessments
Paraffin sections and frozen sections were used for analysis of ZsG-positive cells and RFP-positive cells, respectively. Fluorescence of the ZsG- or RFP-labeled cells was observed and captured under a confocal microscope (Leica TCS-LSI, Leica Biosystems Inc. Buffalo Grove, IL) or the BX-700 (Keyence, Itaska, IL) after DAPI (GeneCopia, Rockville MD) staining for nuclei. Unless otherwise indicated, we analyzed sections from at least three mice from independent experiments and showed representative images as results. Immunohistochemistry (IHC) was performed after antigen retrieval in Tris/EDTA (pH 9.0, 95°C 10 min or 0.2% Triton X-100 in PBS for 2 hours at R.T. Sections were stained with primary antibodies followed by incubation with fluorophore-conjugated or biotinylated secondary antibodies. The detail methods and materials for tissue processing, imaging, immunohistochemistry, and in situ hybridization are described in Supplemental information.
EdU staining for detecting slow-cycling cells.
To detect the slow-cycling cells, mice received daily intraperitoneal injections of EdU (5-ethynyl-20-deoxyuridine, 5mg/10ml/mouse; Life Technology, Grand Island, NY) four times from P4 to P7.[34] After 1, 2 and 3 weeks from the last injection, the knee joints were harvested and processed to prepare longitudinal sections. Sections were stained using Click-iT® EdU Imaging Kits (Life Technology, Grand Island, NY) according to the manufacturer’s instructions. Because the EdU staining procedure diminishes the ZsG fluorescence, we imaged the ZsG-positive cells before the EdU staining, then merged the EdU image with the ZsG image.
Histological quantification
Numbers of immunofluorescent- or marker-positive cells and DAPI-positive cells were quantified using NIS-Elements Microscope Imaging Software (Nikon Instruments, Tokyo, Japan) and the ratio of immunofluorescent- or marker-positive cells to DAPI-positive cells was calculated. We analyzed 3–4 different levels per sample and 3–4 samples per group.
Statistics
Statistical analysis was performed using Excel 2007 (Microsoft, Redmond, WA) and GraphPad Prism 7 (GraphPad Software, San Diego, CA). The values are average ± 1 standard deviation (SD). Kruskal-Wallis one-way analysis of variance was used to detect any significant differences among the groups, and the Dunn’s method (post hoc test) was used to analyze the differences among each experiment of the groups. The threshold of significance is defined as follows for all analyses performed: *P < 0.05.
Results
The Wnt-responsive cells in epiphyseal cartilage of the early postnatal mice
The Axin2CreERT2 mice were bred with the Cre-responsive ZsGreen (ZsG) reporter mouse strain. The resulting Axin2CreERT2;ZsG mice received a single tamoxifen injection at postnatal 6 days of age (P6) and subjected to histological inspection for ZsG-labeled cells at P9, corresponding to 3 days after tamoxifen injection (Fig. 1A). Three days following tamoxifen injection, ZsG-labeled cells were limitedly localized in and near Ranvier’s groove (RG), the perichondrium adjacent to the growth plate (Fig. 1A, arrows) and in the periphery of the articular cartilage in the proximal tibia (Fig. 1A, arrowheads). Distribution of the ZsG-positive (ZsG+) cells was similar to that of the reporter-positive cells in the Axin2LacZ/LacZ mouse as previously reported [35]. Because the RG is known to contains several different types of cell populations including osteo- and chondro-progenitor cells [10], we decided to focus on this area in detail (Fig. 1D and Supplemental Fig. S1A).
Fig. 1. The Wnt-responsive cells in epiphyseal cartilage of the early postnatal Axin2CreERT2;ZsGreen mice.
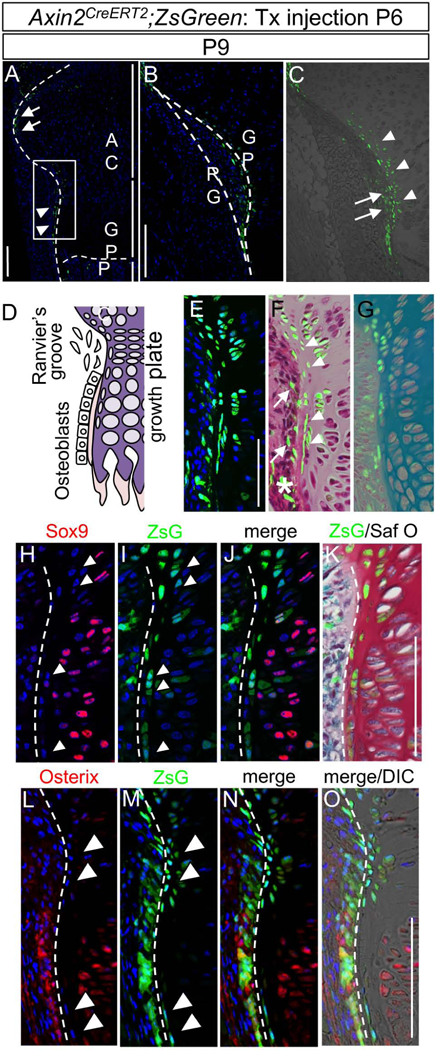
Female mice carrying Axin2CreERT2;RosaZsGreen (Axin2CreERT2;ZsG) received a tamoxifen injection at postnatal day 6 (P6). The Ranvier’s groove (RG) and growth plate (GP) of the tibial proximal epiphysis were histologically examined 3 days post injection (P9). The images represent the posterior side of the proximal tibia. (A) A small aggregation of ZsGreen-positive (ZsG+, green) cells were detected in the periphery of articular cartilage (arrows) and Ranvier’s groove (RG, arrowheads). AC, articular cartilage; GP, growth plate; POC, primary ossification center. (B and C) Magnified image of square white box in A. B, ZsG (green) and DAPI (blue) images in the posterior region of the tibial growth plate. GP, growth plate; RG, Ranvier’s groove. C, ZsG (green) and bright field. ZsG+ cells were found in both perichondrium (RG, Ranvier’s groove, arrows) and periphery of the growth plate (arrowheads). (D) Schematic representation of the RG and neighboring GP chondrocytes. (E-G) Histological analysis. E, ZsG and DAPI. F, ZsG and HE. G, ZsG and Toluidine blue staining. Note that ZsG+ cells are found in both the RG (arrows) and outermost layer of GP (arrowheads). Some of osteoblasts contain ZsG+ cells (asterisk). Representative images of at least five animals per time points are shown. (H-K) Immunohistological analyses for Sox9 expression. H, Sox9 immunostaining (red) and DAPI (blue). I, ZsG (green) and DAPI (blue). J, Sox9 immunostaining (red), ZsG (green) and DAPI (blue). K, ZsG (green) and safranin O staining (Saf O, cartilage is stained in red)). (L-O) Immunohistological analysis for Osterix expression. L, Osterix immunostaining (red) and DAPI (blue). M, ZsG (green) and DAPI (blue). N, Osterix immunostaining (red), ZsG (green), and DAPI (blue). O, Osterix immunostaining (red), ZsG (green), DAPI (blue) and DIC. The sections were stained with Saf O after the fluorescence image was captured, and the fluorescent image and Saf O image were superimposed. Note the ZsG+ cells in outermost layer of the growth plate (arrowhead) were embedded in cartilage matrix, but little or no staining for Sox 9 and Osterix. Bars = 100 μm.
Magnified images showed that ZsG+ cells were not only in the fibrous perichondrium (Figs. 1B and 1C, arrows) but also in the outermost layer of the growth plate facing the perichondrium (Fig. 1C, arrowheads). Merged images with ZsG and Hematoxylin-Eosin staining (HE) or Toluidine Blue staining (TB) confirmed that the ZsG+ cells are distributed in both periphery of the growth plate (Fig. 1F, arrows) and inside the growth plate near the RG surrounded by cartilage matrix (Fig. 1F, arrowheads), 3 days after tamoxifen induction at P6. In situ hybridization revealed that Axin2, Wnt4 and Wnt10b transcripts were mainly expressed in both RG and outermost layer of the growth plate of P9 C57BL/6 mice (Supplemental Fig. S1B, arrowheads), which is consistent with the previous reports.[32] IHC using anti-β-catenin antibody shows nuclear β-catenin expression in chondrocyte exist in outermost layer of the growth plate, and the co-localization of nuclear β-catenin and ZsG was confirmed in this specific area of P9 Axin2CreERT2;ZsG mice, 3 days after tamoxifen induction at P6 (Supplemental Fig. S1C, arrowheads). These results indicate that ZsG cells are responding to Wnts produced in this region and active status in Wnt/β-catenin signaling pathway.
To characterize the ZsG+ cells in the outermost growth plate, we conducted histological and immunohistological analysis on tibia of P9 Axin2CreERT2;ZsG mice, which received tamoxifen induction at P6. The ZsG+ cells in the peri-growth plate were small in size and hardly expressed detectable levels of Sox9 and Osterix proteins (Figs. 1H-K and Figs. 1L-O, arrowheads), indicating their undifferentiated nature. [36–38]
Lineage tracing of Wnt-responsive cells in epiphyseal cartilage from the early postnatal to the young adult stage
Next, to reveal the possible role of the ZsG+ Wnt-responsive cells in cartilage growth, we conducted the lineage tracing experiment using Axin2CreERT2;ZsG mice. The Axin2CreERT2;ZsG mice received a single tamoxifen injection at postnatal 6 days of age (P6) and were subjected to histological inspection for ZsG-labeled cells at P13, P20, P42 and P70, corresponding to 7 days to 64 days after tamoxifen injection, in addition of inspection at P9 (Supplemental Fig. S2A).
During the postnatal growth, distribution of the ZsG+ cells rapidly expanded in the epiphyseal cartilage from the periphery of the growth plate after tamoxifen injection and constituted the outer portion of the growth plate over time (Figs. 2A–L). The labeled cells, previously characterized as osteoprogenitors,[32] were also sparsely found in the primary ossification center (Figs. 2A-H, asterisk). When we injected tamoxifen in Col2CreERT;RFP and AcanCreERT2;RFP mouse lines under the same experimental time course and condition, the epiphyseal cartilage was broadly labeled (Supplemental Fig. S2). Considering previous findings with the same mouse lines,[7, 39] we conclude that sufficient amount of tamoxifen has been reached to the entire growth plate. Tamoxifen-independent Cre-recombination was found sporadically in a few cells in the tibiae of P42 female Axin2CreERT2;ZsG mice (Supplemental Fig. S2C).
Fig. 2. Fate mapping of Wnt-responsive cells in Ranvier’s groove and growth plate of the tibia during postnatal growth.
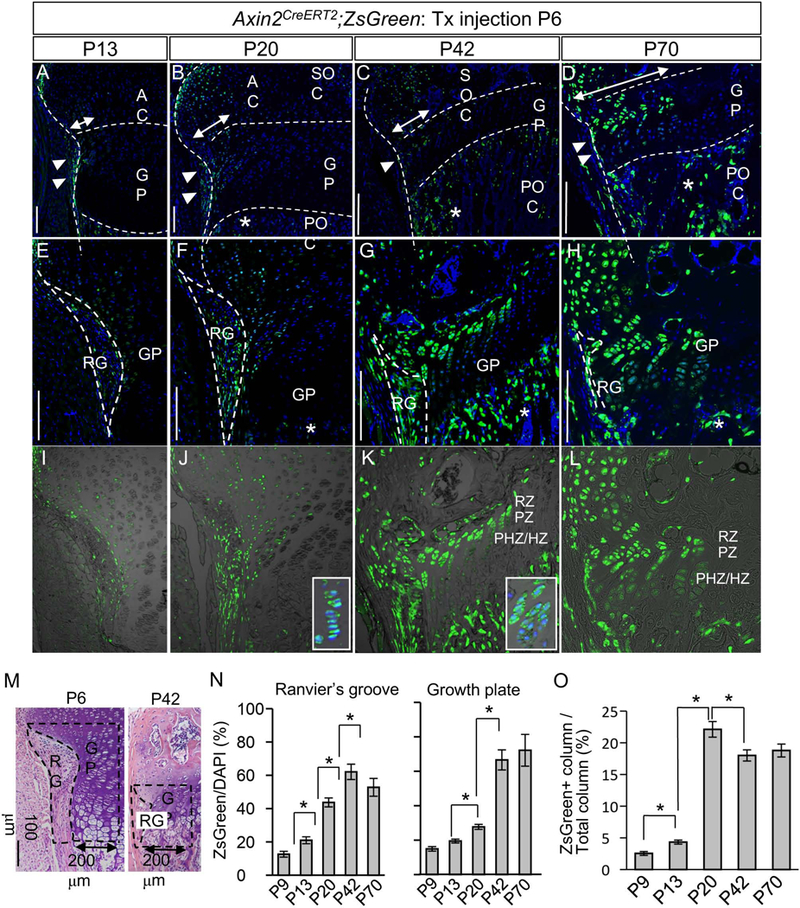
Female mice carrying Axin2CreERT2;RosaZsGreen (Axin2CreERT2;ZsG) received a tamoxifen injection at postnatal day 6 (P6). The Ranvier’s groove (RG) and growth plate (GP) of the tibial proximal epiphysis were histologically examined 7 to 64 days post injection (P13 to P70). The images represent the RG and GP of the posterior side of the proximal tibia. (A, E and I) At P13, the number of ZsG+ cells were increased in the epiphyseal cartilage, compare to P9 (Fig. 1A–C). (B, F and J) At P20, the ZsG+ cells were further expanded toward the epiphysis and also evident in GP columns (inset in J). (C, G and K) At P42, the ZsG+ cells constituted the outer columns in the GP (inset in K). (D, H and L) At P70, the ZsG+ cells occupied the epiphysis and outer GP. (A-H) ZsG (green) and DAPI (blue). (I-L) ZsG (green) and DIC. RG, Ranvier’s groove; GP, growth plate; AC, articular cartilage; POC, primary ossification center; Asterisk, ZsG+ cell in POC; RZ, resting zone; PZ, proliferative zone; PHZ/HZ, prehypertrophic and hypertrophic zone. Bar = 100 μm. Representative images of at least five animals per time points are shown. (M) ZsG+ cells were counted in RG (perichondrium adjacent to the growth plate) and GP within 200 μm range adjacent to the RG. Broken lines illustrate the RG and GP examined. (N) The percentage of number of ZsG+ cells to number of DAPI-positive cells in the RG and GP. The values and error bars represent average and standard deviations. *, p < 0.05. (O) The percentage of number of ZsG+ GP columns to number of DAPI-positive GP columns. ZsG+ GP column occupied about 20% outer portion of the GP columns at P20. The values and error bars represent average and standard deviations. *, p < 0.05.
Quantitative analysis of the labeled cells revealed that about 70% cells were ZsG-positive in both RG and the growth plate within 200 μm range adjacent to the RG by P42 (Figs. 2M and 2N) and that the labeled cells occupied about 20% portion of the growth plate column (Fig. 2O). At 10 weeks of age (P70), ZsG+ cells in the growth plate were also observed at a similar extent to that at 6 weeks of age (P42) (Fig2. 2M and 2O).
A similar distribution of ZsG-labeled cells was found in other long bone elements such as distal femur, distal ulna and radius, the growth plate of vertebrae (Supplemental Figs. S3A-F) and ribs (Supplemental Figs. S3G-L). Scanning of the clearing distal ulna by two-photon excitation microscopy followed by 3D reconstruction demonstrated that ZsG-labeled Wnt-responsive cells entirely surround the ulna (Supplemental Fig. S3M) although their distribution was variable.
Characterization of the Wnt-responsive cells in the outermost layer of the growth plate
Because the lineage tracing experiments suggested the contribution of the Wnt-responsive cells to the growth plate growth, we next evaluated the proliferation ability of these cells around the RG. During postnatal development, the overall size and morphology of the RG changes, and became smaller in size by P42 and much less evident at P70 compared to that at P6-P21 (Supplemental Fig. S4A, upper panels). IHC against Ki67, cell proliferation marker, revealed the Ki67-positive cells in the RG (Fig. 3A-C arrowheads). A short-term EdU injection revealed the cell proliferation in P9-P20, but decreased in P70 (Supplemental Fig. S4A. lower panels). The reduced size and activity of the RG correlated with decreased long bone growth over time (Supplemental Fig. S4B and C). Interestingly, there were less Ki67-positive cells in the periphery of the growth plate compare to that in center part of the growth plate (Fig. 3B arrows), and did not co-localize with ZsG+ cell in the periphery of the growth plate (Fig. 3C). A short-term EdU injection confirmed this result (Supplemental Fig. S4A. arrowheads in lower panel). The lack of Ki67 staining (Figs. 3A-C arrow) and EdU incorporation (Supplemental Fig. S4A, arrowheads) indicated the low proliferative activity of these cells. We also performed long-term cell labeling by multiple injection of EdU starting at P4 (Fig. 3D) to evaluate the slow-cycling cells, which are a phenotypic trait of stem/ progenitor cells.[33] Slow-cycling cells were found in the RG and the outermost growth plate chondrocytes (Fig. 3E-G). Some of the ZsG+ cells in the outermost layer of the growth plate of P28 tibiae were labeled by EdU (Fig. 3E, arrowheads), suggesting that ZsG+ cell includes slow-cycling cell population. These findings can be envisioned as a circumstantial evidence for the contribution of ZsG+ Wnt-responsive cells in the outermost layer of the growth plate to its appositional growth of the growth plate, which is one of the distinct modes of growth at a surface or interface.[9]
Fig. 3. Cell proliferation and slow-cycling cells of Wnt-responsive cells around the Ranvier’s groove.
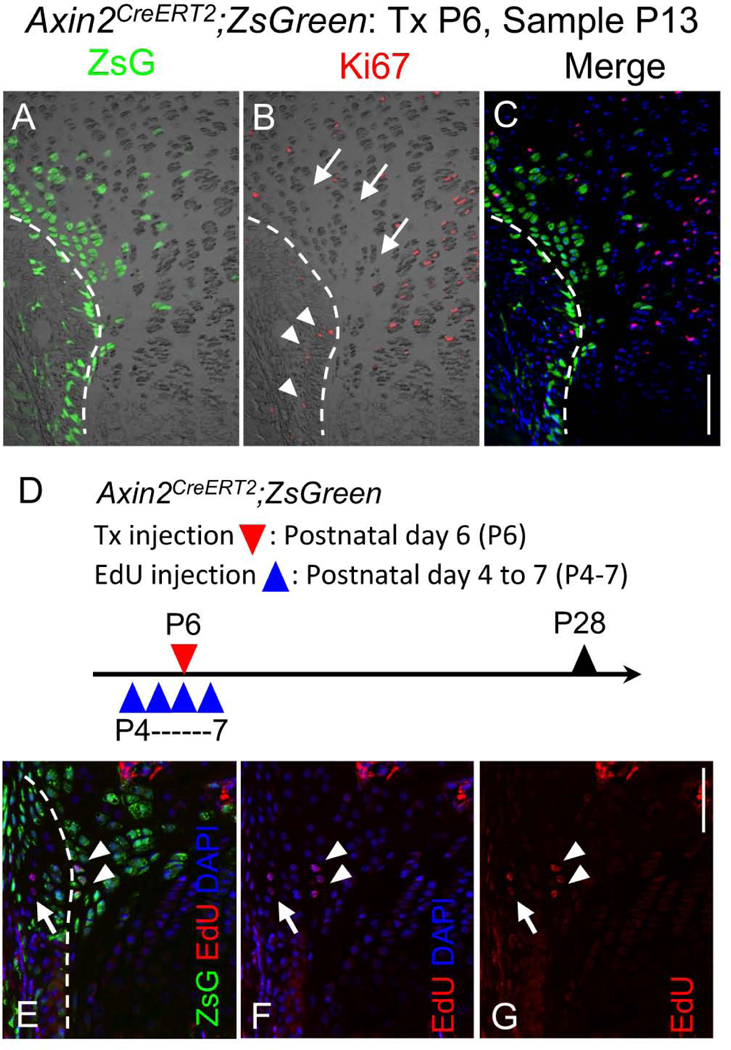
(A-C) Immunohistochemical analysis for cell proliferation marker Ki67 protein on the proximal tibia GP sections of P13 female Axin2CreERT2;ZsG mouse that had received Tx injection at P6. A, ZsG (green) and DIC, B, Ki67 immunostaining (red) and DIC, C, Ki67 immunostaining (red), ZsG (green) and DAPI (blue). A small number of Ki67 positive cells were found in the Ranvier’s groove (arrow heads). The ZsG+ cells facing to the perichondrium showed no immunoreactivity for Ki67 antibody (arrows). Bar = 100 μm. (D-G) Long-term labeled cells with EdU in ZsG+ cells. D, Female Axin2CreERT2;ZsG mice received 4 daily intraperitoneal injections of EdU (5 μg/10 μl/mouse) from P4. Tx was injected at P6. Tibia were harvested at P28 and sections were subjected to EdU staining. E, ZsG (green), EdU (red) and DAPI (blue). F, EdU (red) and DAPI (blue). G, EdU (red). EdU-labeled cells were detected in Ranvier’s groove (arrows) and in the ZsG+ cells in the outermost layer of the GP (arrowheads). Bar = 100 μm.
To evaluate the character of the ZsG+ cells around the RG as chondro- and osteo-progenitor, we used the ribs because of its simple shape. The costochondral junctions of the ribs including the RG were micro-dissected, and the perichondrium and the peri-growth plate were removed through a combination of collagenase digestion and microdissection (Supplemental Figs. S5A-C). Cells were dissociated by additional collagenase digestion and subjected to the flow cytometry analysis. We used skeletal progenitor markers to fraction the cells into subpopulations: multipotent progenitor cells, osteo- and chondro-progenitor cells and stromal progenitor cells according to previous reports (Supplemental Fig. S5D).[40, 41] ZsG-positive cells were sorted into all three populations (Supplemental Figs. S5D and E).
These data suggest that the ZsG+ cell population in the RG and periphery of the growth plate of long bone contains osteo- and chondro-progenitor cells.
Contribution of cells in the outermost layer of the growth plate to transverse growth of the growth plate.
Although the stem cell characteristic in ZsG+ Wnt-responsive cells were suggested, it is still unclear which cell population contributes to growth of the growth plate, the ZsG+ cells in the RG or the ZsG+ cells in outermost layer of the growth plate. To reveal this we compare the CtskCre;ZsG mouse system and Col2CreERT;RFP mouse system. The CtskCre;ZsG reporter mouse system enabled to label most cells in the RG at P7 (Fig. 4A) except the endothelial cells of the micro vessels (inset in Fig. 4A).[42] In this system, ZsG+ cells were also detected in the epiphyseal cartilage around P14.[42] When we chased the fate of labeled cells for longer time periods, they did not expand toward the growth plate at P28 or even at P70 (Figs. 4B and 4C) while most cells in the RG remained positive for ZsG (Fig. 4B and 4C). This is highly contrast to the results from the AxinCreERT2;ZsG mice (Figs. 2G and H). These results indicate that the cells within the RG do not supply new cells to the growth plate, while the Wnt-responsive cells in outermost layer of the growth plate labeled in the Axin2CreERT2 system are largely responsible for this role. Data obtained from Col2CreERT;RFP mice provided additional support to this conclusion. When Col2CreERT;RFP mice received a tamoxifen injection at P6 (Fig. 4D), the resultant RFP+ cells were detected in the entire epiphyseal cartilage including the growth plate, but very few were found in the RG 3 or 7 days after the injection (Figs. 4E and 4F). The entire growth plate was largely occupied with labeled cells even 6 weeks after tamoxifen injection (P42) (Fig. 4G). If non-labeled cells in the RG at P9 were a significant source of cells to the growth plate and contributed to the formation of the outer portion of the growth plate, the RFP- cells should be more evident there.
Fig. 4. Fate mapping of Cathepsin KCre and Col2a1CreERT2 lineage cells in the Ranvier’s groove and growth plate.
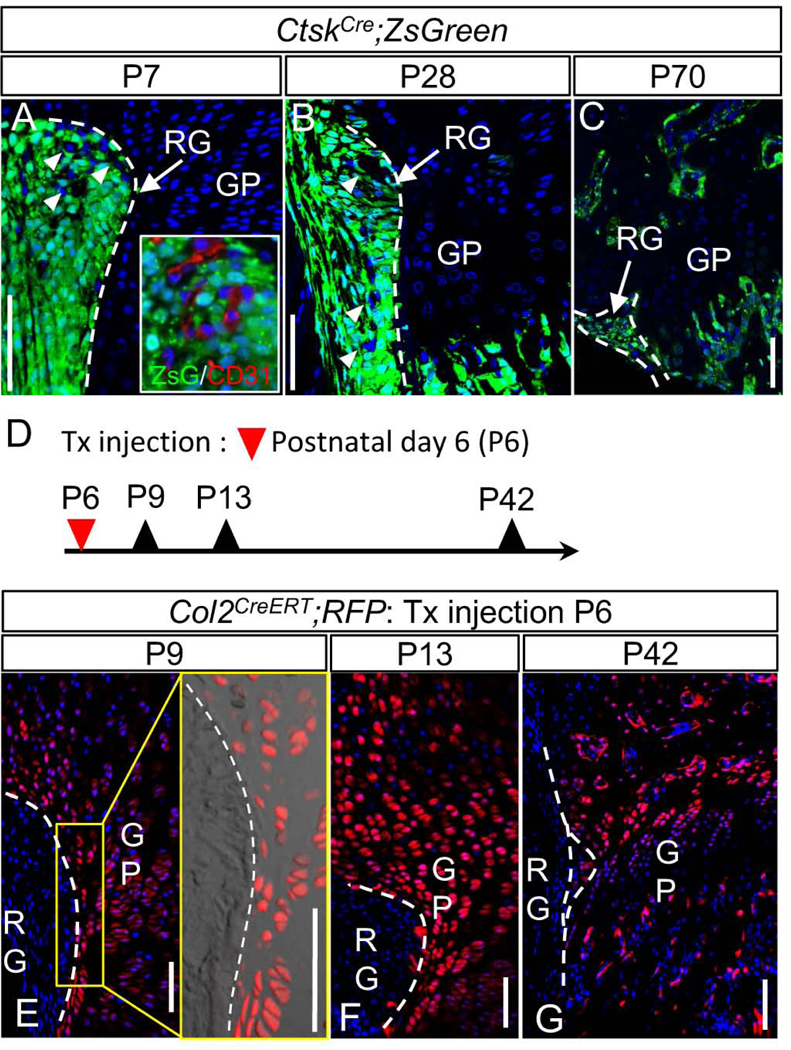
(A, B and C) Cathepsin KCre (CtskCre) mice were crossed with ZsGreen mice. Tibiae were harvested from the resulting CtskCre;ZsGreen (CtskCre;ZsG) mice at P7, P28 and P70. Representative images at P7 (A), P28 (B) and P70 (C). A majority of ZsG+ cells remain in the RG at P7, P28 and P70 and did not expand to the growth plate. A, All cells in the RG of P7 mice were ZsG+ except the blood vessels (arrowheads and inset of A). B, Yellow box is magnified. Arrowheads indicates the ZsG-negative vessels. (D-G) Col2a1CreERT (Col2CreERT) mice were crossed with Rosa-tdTomato (RFP) mice. The resulting Col2CreERT;RFP received Tx injection at P6. Tibiae were harvested at P9, P13 and P42. Representative images at P9 (E), P13 (F) and P42 (G). E, Yellow box is magnified. Note that few RFP-negative cells were found in the growth plate cartilage. F and G, a few cells were found in the RG while most of GP cells remained labeled. RG: Ranvier’s groove, GP: growth plate, Broken line: border between RG and GP. Bar = 100 μm.
Deceleration of Wnt-responsive cells toward young adult stages
To investigate changes in the number, distribution, and/or expansion of Wnt-responsive cells with age, we performed short-term labeling by injecting tamoxifen at P21 (growing stage), P42 (adolescence stage) or P70 (young adult stage) [43] and examined the distribution of ZsG+ cells 3, 7 and 14 days post-injection. Three days after the tamoxifen injection, ZsG+ cells were found in the RG, the perichondrium and growth plate near the RG in all stages. However, the area containing the ZsG+ cells became smaller with age (Figs. 5A-C, arrows). One and two week after the tamoxifen injection at P21, the number of labeled cells increased and expanded toward the articular cartilage and the growth plate (Fig. 5A, P28). This was not the case following tamoxifen injection at P42 and P70 (Fig. 5B, P49 and Fig. 5C, P77, respectively). Quantitative analysis of the labeled cells confirmed these observations (Figs. 5D-F). The findings suggest that Wnt-responsive cells reduce the contribution to growth plate formation by 6 weeks of age (P42). This timing matched the deceleration of transverse and longitudinal growth of the tibial growth plate (Supplemental Figs. S4B and C).
Fig. 5. Fate of Wnt-responsive cells around the Ranvier’s groove at different growth stages.
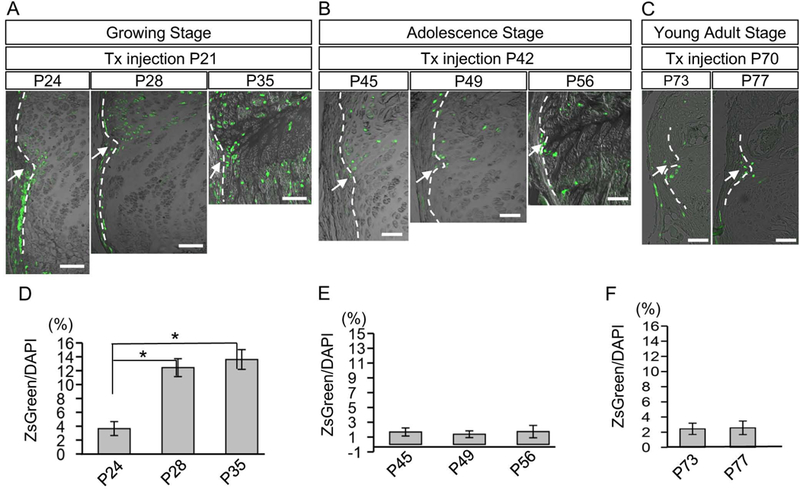
(A-C) Female mice carrying Axin2CreERT2;ZsG received Tx injection at P21 (growing stage, A), P42 (adolescence stage, B) or at P70 (young adult stage, C). The distribution of ZsG+ cells were histologically examined in the vicinity of RG after 3 days (P24, P45 or P73), 7 days (P28, P49 or P77), and 14 days (P35 or P56) post injection. Arrows represent the RG, and dotted lines indicate the border between perichondrium and growth plate. (D-F) Changes in the percentage of number of ZsG+ cells to number of DAPI positive cells in the growth plate within 200 μm range adjacent to the RG of each stage were examined. The values and error bars represent average and standard deviations. Note the significant increase of ZsG+ cells in the RG was only seen in growing stage (D). *, p < 0.05. Bar = 100 μm.
Wnt-responsive cells in the center of the growth plate
In contrast to the localization of Wnt-responsive cells in the outer part of the growth plate, we did not detect labeled cells in the center of the growth plate 3 and 7 days after Cre-induction at P6 (Supplemental Fig. S6A). Only a small number of ZsG+ cells became evident in the resting zone 2 weeks following tamoxifen injection and remained for 3 weeks (P42) (Supplemental Fig. S6A, arrowheads). A small cluster of the labeled cells was also found in a column (Supplemental Fig. S6A, arrows). However, when we injected the tamoxifen at 3 weeks of age (P21), the growth plate structure matures and the resting zone is distinguished, a significant number of Wnt-responsive cells were immediately found in the resting zone and in the proliferating zone 3 days post tamoxifen injection (Supplemental Fig. S6B, P24). These cells were increased forming small clusters in columns 7 days post injection (Supplemental Fig. S6B, P28). Moreover, the ZsG+ cells constitute an entire column between unlabeled columns 14 days post injection of tamoxifen (Supplemental Fig. S6B, P35). Although a similar distribution was also observed at 6 weeks of age (P45), 3 days post injection of tamoxifen (Supplemental Fig. S6C), expansion of Wnt-responsive cells was not detectable from 6 weeks of age (Supplemental Fig. S6D and H). These data suggest that the Wnt-responsive cells in the resting and proliferating zones may contribute to new column formation. We could trace these labeled cells to study the dynamics of the growth plate column formation in the center of growth plate at the growing stage.
Requirement of β-catenin in Wnt-responsive cells
To examine the role of Wnt/β-catenin signaling in the Wnt-responsive cell population, we generated a triple-transgenic mouse strain, β-cateninfl/fl;Axin2CreERT2;ZsG and injected tamoxifen at P13 (Fig. 6A). Efficiency of the β-catenin (ctnnb1) ablation was confirmed with quantitative real-time PCR using RNA extracted from ZsG+ cells in the RG of the transgenic and control mouse (Supplemental Fig. S7A). Although the alteration of macroscopic phenotype including length of tibia was not obvious (Supplemental Fig. S7B-D), the proximal tibiae harvested 2 weeks after tamoxifen injection showed histological abnormalities. In the β-cateninfl/fl;Axin2CreERT2;ZsG mice, ectopic cartilaginous masses appeared in the RG (Figs. 6E-G, arrowheads) and labeled with ZsG (Fig. 6H, arrowhead). The expansion of ZsG+ cells toward the growth plate column was reduced in β-catenin-deficient mutants compared to control mice (Figs. 6I and J vs Figs. 6K and L, asterisks). The ectopic cartilaginous mass in the RG showed metachromasia with Toluidine blue (TB) staining and co-stained with Sox9 (Figs. 6H) while the Sox9 positive cells were not detected in the perichondrium in AxinCreERT2; ZsG mice (Fig. 1H-J). The immunostaining results using anti-β-catenin showed reduced expression of β-catenin protein in ectopic cartilage mass (Figs. 6P-R, circle). Moreover, nuclear β-catenin expression was not detectable in ZsG+ cells in the growth plate of the target mice (Figs. 6P-R), but detectable in that of control mice (Figs. 6M-O, arrows). These findings indicate that β-catenin (ctnnb1) ablation in the Wnt-responsive cells in the growth plate inhibited their expansion toward the column formation and ectopically induced a chondrogenic phenotype in the RG.
Fig. 6. Inactivation of β-catenin in Wnt-responsive cells leads to ectopic cartilage formation.
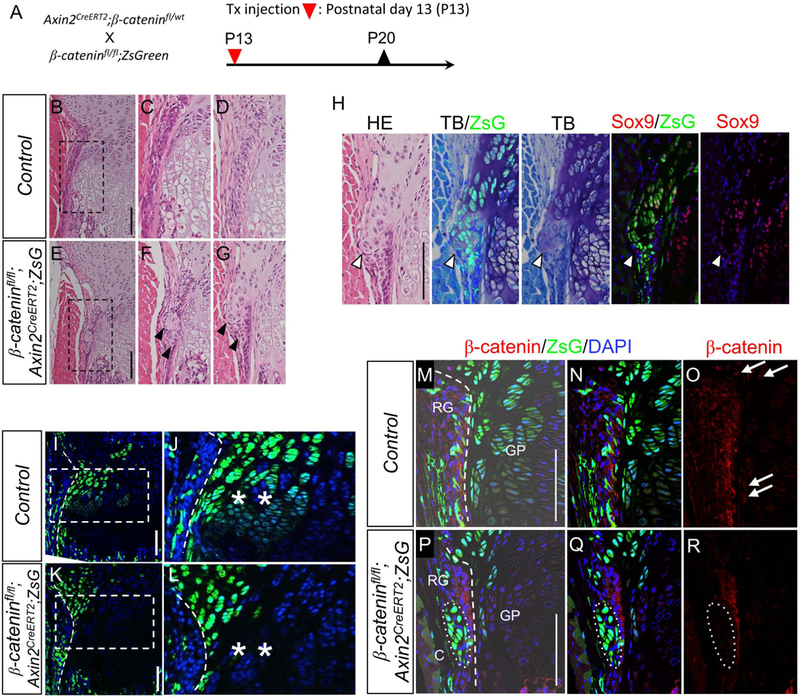
(A) β-cateninfl/fl mice possessing loxP sites in introns 1 and 6 in the β-catenin gene. Axin2CreERT2;β-cateninfl/wt were crossed with β-cateninfl/fl;ZsGreen. The resulting triple compound β-cateninfl/fl;Axin2CreERT2;ZsG mice received Tx injection at P13. Tibiae were harvested at P20. (B-G) Representative HE images of proximal tibias harvested from female mice. B-D, β-cateninfl/wt;Axin2CreERT2;ZsG (Control). E-G, β-cateninfl/fl;Axin2CreERT; ZsG. The images from two independent control (C and D) and target (F and G) mice were shown. In the β-cateninfl/fl;Axin2CreERT2;ZsG mice, the ectopic cartilaginous masses were found (arrowheads) in the groove of Ranvier (C and D). C and F are magnified images shown in squares of B and E, respectively. (H) Immunohistochemical and histochemical analysis of ectopic cartilaginous mass. HE, HE staining. TB/ZsG, Toluidin blue staining and ZsG (green). TB, Toluidin blue staining. Sox9/ZsG, Sox9 immunostaining (red), ZsG (green) abd DAPI (blue). Sox9, Sox9 immunostaining (red) and DAPI (blue). TB and TB/ZsG images were captured from the same section, and Sox9 and Sox9/ZsG images were captured from the serial section of the TB and TB/ZsG section. Note the ectopic cartilage shows cartilage matrix (stained purple) with TB and nuclear Sox9 protein expression. (I-L) Representative fluorescent images of the growth plate of proximal tibiae. I and J, β-cateninfl/wt;Axin2CreERT2;ZsG (Control). K and L, β-cateninfl/fl;Axin2CreERT2;ZsG. Note that the β-catenin-deficient mutants showed less expansion of ZsG+ cells toward the growth plate compared to the control mice (asterisks). Periphery of the growth plate (broken line) is shown in higher magnification in the right panels. Dotted line: the margin between RG and growth plate. (M-R) Efficiency of the β-catenin ablation was confirmed with immunohistochemistry using anti-β-catenin antibody. M-O, β-cateninfl/wt;Axin2CreERT2;ZsG (Control). P-R, β-cateninfl/fl;Axin2CreERT;ZsG. Note that the β-catenin-deficient mutants showed lower β-catenin protein expressions (arrowheads) compare to control mice. In the control mice, nuclear β-catenin expression was observed in the outermost cell layer of the growth plate (arrows). Bar = 100 μm.
Discussion
This study demonstrates that a small number of specific cells in the outermost layer of the growth plate near the Ranvier’s groove (RG) contribute to the appositional growth of the growth plate from the early postnatal to the growing stage in mice. These specific cells are labeled in the Axin2CreERT2;Rosa-ZsGreen mouse system after tamoxifen induction. Expression of Wnts and β-catenin nuclear localization in the RG and outermost layer of growth plate support the idea that the ZsG+ cells are in active status for Wnt/β-catenin signaling although we should recognize the limitation that the Axin2 reporter system is not an absolute tool that mirrors Wnt/β-catenin signaling activity. Furthermore, we found the Wnt-responsive cells in the resting zone and the proliferating zone constituted a new column in the center of growth plate at the growing stage. Our findings implicate that Wnt-responsive cells inside the growth plate facing the groove of Ranvier contribute to the transverse growth of growth plate in mice (Fig. 7).
Fig. 7. Schematic diagram of the proposed Wnt-responsive cell contribution to postnatal growth plate formation.
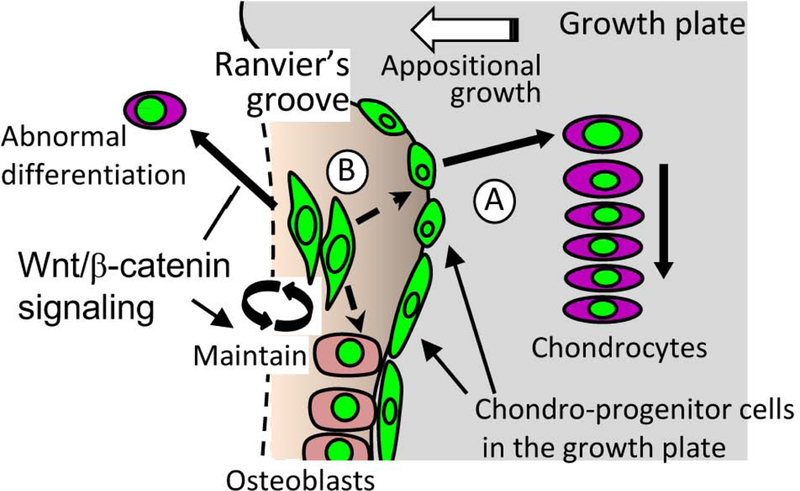
(A) Chondroprogenitors in the outermost layer of the growth plate contribute to growth plate appositional growth. (B) Wnt-responsive cell in Ranvier’s groove. These cells in the RG do not significantly supply new cells to the growth plate in postnatal growth. β-catenin may inhibit the chondrogenic differentiation in Wnt-responsive cells in the RG while it supports expansion of Wnt-responsive chondroprogenitor cells toward the growth plate.
The RG has obtained attention as a chondro- and osteoprogenitor niche in 1900s. Shapiro et al [10] have performed careful morphological studies and radiolabel-based functional studies and advocated that the RG contains two types of skeletal progenitors. The first are osteoprogenitors, densely packed in the deepest part of the groove that differentiate into osteoblasts surrounding the epiphyseal growth-plate and the adjacent metaphysis. This population has also been demonstrated as Wnt-responsive in this AxinCreERT2 system in other studies.[32] The second type is relatively undifferentiated mesenchymal cells, presumably chondroprogenitors and fibroblasts. More recent studies have reported that the RG contains the slow-cycling cells that could be labeled with tritium thymidine or bromodeoxy uridine for long term and that some cells in this region are positive to mesenchymal stem cell markers. [8, 9] Further, lineage tracing studies have indicated that the cells in the RG expand to articular cartilage, periosteum and bone. [32, 42]
Here, we reported a novel population of Wnt-responsive cells in outermost layer of the growth plate facing the RG. Interestingly, a majority of these ZsG-labeled cells showed a low proliferation activity and no or undetectable Sox9 and Osterix expression compared to other non-labeled growth plate chondrocytes and perichondrium cells.[36–38] Moreover, this population contains slow-cycling cells. These observations indicate that the ZsG+ cells in the outermost layer of the growth plate are chondroprogenitors contributing to formation of the growth plate. Because Sox9 is an essential transcription factor in cartilage, and required to secure chondrocyte lineage commitment, promotes chondrocytes survival, and transcriptionally activates the genes for many cartilage-specific structural components including Col2A1. [36–38] Thus, it is unclear, why and how the cells in outermost layer of the growth plate can exist within cartilage matrix, and how the cells can induce Col2A1 promoter activity in Col2CreERT;ZsG, Col2CreERT2;RFP mice and AcanCreERT2;RFP. One possibility is that a low and undetectable level of Sox9 proteins are expressed in outermost layer of the growth plate chondrocytes to support a low level of type II collagen matrix production.
Expansion of the ZsG+ cells in the outermost layer of the growth plate was rapid at the neonatal stage. This capacity was gradually depleted, accompanying decreases in widening of growth plate and longitudinal bone growth. Our results indicate that supply of new cells to the growth plate from this ZsG+ population is an important mechanism regulating skeletal growth. One may argue that these cells might be descendants of the undifferentiated mesenchymal cells recruiting from the RG as previously suggested.[9, 10] However, the results from the lineage tracing with the CtskCre system and the Col2CreERT system do not support this argument. The CtskCre system enables most cells to be labeled in the RG at P10, but failed to label growth plate chondrocytes at 4 weeks of age, suggesting that cells in the RG do not significantly supply cells to the growth plate in postnatal growth. The Col2CreERT system did not label the RG after tamoxifen injection at P6, but labeled the entire epiphyseal and growth plate cartilage for over 5 weeks. The findings suggest that majority of the chondrocytes in the growth plate unlikely originate from the RG.
Previous studies have demonstrated that decreases in number and proliferation activity of the resting chondrocytes are closely linked to reduction in bone growth with age and pathological growth arrest, indicating that the resting zone contain chondroprogenitors.[11, 44] More recently lineage tracing analysis directly demonstrated a subset of cells labeled with the PTHrP reporter system form growth plate.[12] The ZsG+ Wnt-responsive cells were also found in resting zone and proliferating zone of P20 mice. It is interesting to study relation of these ZsG+ population in the center part of the growth plate with the PTHrP-lineage cells. Curiously, these ZsG+ cells were not observed in early stage (P9, P13). Although the process and origin of the ZsG+ cell in the resting zone and proliferating zone of P20 growth plate need to be further studied, we could raise two possibilities, migration of a small number of cells from the RG or outer growth plate although they are hardly detected at an early stage and migration of mesenchymal stem/progenitor cells from the secondary ossification center.
Growth plate injuries may cause closure or growth plate arrest and lead to cessation of the longitudinal elongation of the bone.[45, 46] This condition would result in imbalance in bone growth, progressive skeletal deformity and physical problems. However, the growth plate does not always induce arrest after injuries, and typically shows good healing ability, especially when the injuries are not associated with dislocation of epiphysis, articular cartilage injury, or growth plate compression. Healing is better at younger ages. The high healing capacity of the young growth plate is likely due to high proliferative capacity and potential to supply new cells.[47] The Wnt-responsive cell population that we identified in this study could be responsible for growth plate repair.
Ablation of β-catenin in Wnt-responsive cells induced ectopic chondrogenic changes in the cells in the RG. Our previous study showed that β-catenin deletion under the control of Col2CreER system showed growth plate deformity (outgrowth of the growth plate), but did not show obvious changes in the RG. [30] Taken together with our previous study, the ectopic cartilage mass or ectopic chondrocytes in the RG may be originated from undifferentiated mesenchymal cells in the RG. The findings indicate that Wnt/β-catenin signaling is an essential regulator of cell lineage of mesenchymal progenitors in the RG, a role which has been previously demonstrated in the developing limb cartilage at embryos.[28, 30] Interestingly, we found that β-catenin deletion in Wnt-responsive cells strongly inhibits expansion of the marked cells toward the epiphysis and growth plate although the current model was not able to determine the impact of inhibition of the cell expansion on the skeleton phenotype due to the additional defects in other organs. A strong up-regulation of Sox-9 staining in targeted cells after removal of β-catenin revealed that they quickly expressed a chondrogenic phenotype. Τhe data collected indicate that the Wnt-responsive cells in the growth plate likely behave as chondroprogenitors and supply new cells to the growth plate, which would be tightly controlled by Wnt/β-catenin signaling.
In conclusion, we demonstrated that Wnt-responsive cells contribute to postnatal formation of the growth plate in site and age specific manners. There are at least two types of Wnt-responsive cells in peripheral region of the growth plate (Fig. 7): A, the cells in outermost layer of the growth plate near the RG contribute to transverse appositional growth of growth plate; B, the cells in the RG do not significantly supply new cells to the growth plate in postnatal growth, but may provide new osteoblasts, and may give rise to chondrogenic cells under the pathological condition. In addition, the cells in the resting and proliferating zones can be used to trace the growth plate column formation.
Supplementary Material
Acknowledgement
We thank Ms. Kimberly Wilson and Hongying Tang for their technical assistance, Mr. Adam Guess and Ms. Amanda Scheiber for professional editing. Research reported in this article was partially supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases of the National Institutes of Health under Award Number 9R01AR062908 and 2R01AR056837. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
References
- [1].Ballock RT, O’Keefe RJ: Physiology and pathophysiology of the growth plate. Birth Defects Res C Embryo Today 2003, 69:123–43. [DOI] [PubMed] [Google Scholar]
- [2].Ornitz DM, Legeai-Mallet L: Achondroplasia: Development, pathogenesis, and therapy. Dev Dyn 2017, 246:291–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Cooper KL, Oh S, Sung Y, et al. : Multiple phases of chondrocyte enlargement underlie differences in skeletal proportions. Nature 2013, 495:375–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Li Y, Trivedi V, Truong TV, et al. : Dynamic imaging of the growth plate cartilage reveals multiple contributors to skeletal morphogenesis. Nat Commun 2015, 6:6798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Yang L, Tsang KY, Tang HC, et al. : Hypertrophic chondrocytes can become osteoblasts and osteocytes in endochondral bone formation. Proc Natl Acad Sci U S A 2014, 111:12097–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Zhou X, von der Mark K, Henry S, et al. : Chondrocytes transdifferentiate into osteoblasts in endochondral bone during development, postnatal growth and fracture healing in mice. PLoS Genet 2014, 10:e1004820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Park J, Gebhardt M, Golovchenko S, et al. : Dual pathways to endochondral osteoblasts: a novel chondrocyte-derived osteoprogenitor cell identified in hypertrophic cartilage. Biol Open 2015, 4:608–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Ohlsson C, Nilsson A, Isaksson O, et al. : Growth hormone induces multiplication of the slowly cycling germinal cells of the rat tibial growth plate. Proc Natl Acad Sci U S A 1992, 89:9826–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Karlsson C, Thornemo M, Henriksson HB, et al. : Identification of a stem cell niche in the zone of Ranvier within the knee joint. J Anat 2009, 215:355–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Shapiro F, Holtrop ME, Glimcher MJ: Organization and cellular biology of the perichondrial ossification groove of ranvier: a morphological study in rabbits. J Bone Joint Surg Am 1977, 59:703–23. [PubMed] [Google Scholar]
- [11].Abad V, Meyers JL, Weise M, et al. : The role of the resting zone in growth plate chondrogenesis. Endocrinology 2002, 143:1851–7. [DOI] [PubMed] [Google Scholar]
- [12].Mizuhashi K, Ono W, Matsushita Y, et al. : Resting zone of the growth plate houses a unique class of skeletal stem cells. Nature 2018, 563:254–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Cadigan KM, Waterman ML: TCF/LEFs and Wnt signaling in the nucleus. Cold Spring Harb Perspect Biol 2012, 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Yang Y: Wnt signaling in development and disease. Cell Biosci 2012, 2:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Nusse R, Clevers H: Wnt/beta-Catenin Signaling, Disease, and Emerging Therapeutic Modalities. Cell 2017, 169:985–99. [DOI] [PubMed] [Google Scholar]
- [16].Bhavanasi D, Klein PS: Wnt Signaling in Normal and Malignant Stem Cells. Curr Stem Cell Rep 2016, 2:379–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Holland JD, Klaus A, Garratt AN, et al. : Wnt signaling in stem and cancer stem cells. Curr Opin Cell Biol 2013, 25:254–64. [DOI] [PubMed] [Google Scholar]
- [18].ten Berge D, Kurek D, Blauwkamp T, et al. : Embryonic stem cells require Wnt proteins to prevent differentiation to epiblast stem cells. Nat Cell Biol 2011, 13:1070–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].DasGupta R, Fuchs E: Multiple roles for activated LEF/TCF transcription complexes during hair follicle development and differentiation. Development 1999, 126:4557–68. [DOI] [PubMed] [Google Scholar]
- [20].Andl T, Reddy ST, Gaddapara T, et al. : WNT signals are required for the initiation of hair follicle development. Dev Cell 2002, 2:643–53. [DOI] [PubMed] [Google Scholar]
- [21].Fevr T, Robine S, Louvard D, et al. : Wnt/beta-catenin is essential for intestinal homeostasis and maintenance of intestinal stem cells. Mol Cell Biol 2007, 27:7551–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Kuhnert F, Davis CR, Wang HT, et al. : Essential requirement for Wnt signaling in proliferation of adult small intestine and colon revealed by adenoviral expression of Dickkopf-1. Proc Natl Acad Sci U S A 2004, 101:266–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].van Amerongen R, Bowman AN, Nusse R: Developmental stage and time dictate the fate of Wnt/beta-catenin-responsive stem cells in the mammary gland. Cell Stem Cell 2012, 11:387–400. [DOI] [PubMed] [Google Scholar]
- [24].Hari L, Brault V, Kleber M, et al. : Lineage-specific requirements of beta-catenin in neural crest development. J Cell Biol 2002, 159:867–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Chai R, Kuo B, Wang T, et al. : Wnt signaling induces proliferation of sensory precursors in the postnatal mouse cochlea. Proc Natl Acad Sci U S A 2012, 109:8167–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Wang YZ, Yamagami T, Gan Q, et al. : Canonical Wnt signaling promotes the proliferation and neurogenesis of peripheral olfactory stem cells during postnatal development and adult regeneration. J Cell Sci 2011, 124:1553–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Hartmann C, Tabin CJ: Dual roles of Wnt signaling during chondrogenesis in the chicken limb. Development 2000, 127:3141–59. [DOI] [PubMed] [Google Scholar]
- [28].Hill TP, Spater D, Taketo MM, et al. : Canonical Wnt/beta-catenin signaling prevents osteoblasts from differentiating into chondrocytes. Dev Cell 2005, 8:727–38. [DOI] [PubMed] [Google Scholar]
- [29].Guo X, Day TF, Jiang X, et al. : Wnt/beta-catenin signaling is sufficient and necessary for synovial joint formation. Genes Dev 2004, 18:2404–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Day TF, Guo X, Garrett-Beal L, et al. : Wnt/beta-catenin signaling in mesenchymal progenitors controls osteoblast and chondrocyte differentiation during vertebrate skeletogenesis. Dev Cell 2005, 8:739–50. [DOI] [PubMed] [Google Scholar]
- [31].Cantley L, Saunders C, Guttenberg M, et al. : Loss of beta-catenin induces multifocal periosteal chondroma-like masses in mice. Am J Pathol 2013, 182:917–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Tan SH, Senarath-Yapa K, Chung MT, et al. : Wnts produced by Osterix-expressing osteolineage cells regulate their proliferation and differentiation. Proc Natl Acad Sci U S A 2014, 111:E5262–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Lim X, Tan SH, Koh WL, et al. : Interfollicular epidermal stem cells self-renew via autocrine Wnt signaling. Science 2013, 342:1226–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Candela ME, Cantley L, Yasuaha R, et al. : Distribution of slow-cycling cells in epiphyseal cartilage and requirement of beta-catenin signaling for their maintenance in growth plate. J Orthop Res 2014, 32:661–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Dao DY, Yang X, Flick LM, et al. : Axin2 regulates chondrocyte maturation and axial skeletal development. J Orthop Res 2010, 28:89–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Bi W, Deng JM, Zhang Z, et al. : Sox9 is required for cartilage formation. Nat Genet 1999, 22:85–9. [DOI] [PubMed] [Google Scholar]
- [37].Henry SP, Liang S, Akdemir KC, et al. : The postnatal role of Sox9 in cartilage. J Bone Miner Res 2012, 27:2511–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Rashid H, Ma C, Chen H, et al. : Sp7 and Runx2 molecular complex synergistically regulate expression of target genes. Connect Tissue Res 2014, 55 Suppl 1:83–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Ono N, Ono W, Nagasawa T, et al. : A subset of chondrogenic cells provides early mesenchymal progenitors in growing bones. Nat Cell Biol 2014, 16:1157–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Ransom RC, Carter AC, Salhotra A, et al. : Mechanoresponsive stem cells acquire neural crest fate in jaw regeneration. Nature 2018, 563:514–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Chan CKF, Gulati GS, Sinha R, et al. : Identification of the Human Skeletal Stem Cell. Cell 2018, 175:43–56 e21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Yang W, Wang J, Moore DC, et al. : Ptpn11 deletion in a novel progenitor causes metachondromatosis by inducing hedgehog signalling. Nature 2013, 499:491–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Brust V, Schindler PM, Lewejohann L: Lifetime development of behavioural phenotype in the house mouse (Mus musculus). Front Zool 2015, 12 Suppl 1:S17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Schrier L, Ferns SP, Barnes KM, et al. : Depletion of resting zone chondrocytes during growth plate senescence. J Endocrinol 2006, 189:27–36. [DOI] [PubMed] [Google Scholar]
- [45].Peterson CA, Peterson HA: Analysis of the incidence of injuries to the epiphyseal growth plate. J Trauma 1972, 12:275–81. [DOI] [PubMed] [Google Scholar]
- [46].Schurz M, Binder H, Platzer P, et al. : Physeal injuries of the distal tibia: long-term results in 376 patients. Int Orthop 2010, 34:547–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Lui JC, Nilsson O, Baron J: Recent research on the growth plate: Recent insights into the regulation of the growth plate. J Mol Endocrinol 2014, 53:T1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


