Abstract
Purpose:
In older women, breast cancer and its treatment can have profound impact on their physical, mental, and social health, especially in frail patients. This study evaluated the association between frailty and long-term health-related quality of life (HRQOL) in older women undergoing breast cancer treatment.
Methods:
Using the Carolina Senior Registry (CSR), participants with breast cancer were contacted to complete a follow-up HRQOL questionnaire (median 4 years). Baseline Geriatric Assessment (GA) variables were used to calculate the Carolina Frailty Index (CFI) and categorize participants as robust, pre-frail, or frail. Outcomes included HRQOL domains of physical function, social roles, fatigue, depression, anxiety, pain, and sleep disturbance assessed using PROMIS® instruments. Regression modeling compared outcomes between frailty groups using adjusted mean differences (AMD).
Results:
Of 190 eligible patients, 63 completed follow-up HRQOL survey. Mean age was 70 years (range 65–86) and 91% were white. Based on the CFI, 49 (78%) patients were robust, 11 (18%) pre-frail, and 3 (5%) frail. After controlling for age and cancer stage, patients identified as pre-frail/frail reported worse physical function (AMD −9.2, p <0.001) and social roles (AMD −7.2, p= 0.002), and more fatigue (AMD 7.6, p= 0.008), depression (AMD 5.6, p= 0.004) and sleep disturbance (AMD 6.9, p=.008) compared to robust patients at follow-up.
Conclusions:
Frailty in older women with breast cancer was associated with worse long-term HRQOL outcomes. Further research is needed to develop interventions for frail patients at-risk for reduced HRQOL.
Keywords: frailty, health-related quality of life, geriatric assessment, breast cancer, geriatric oncology
Introduction
With over 255,000 new cases per year, breast cancer is the most common cancer and the second most common cause of cancer mortality among women in the US [1]. Breast cancer risk increases with age, and nearly half of new breast cancer diagnoses are in older women [2]. Although cancer survivors over the age of 65 years represent the fastest growing segment of the cancer population [3], a vast gap in knowledge exists regarding critical outcomes that are most important to them. Older adults with cancer tend to value their quality of life as more important than incremental gains in survival when making treatment decisions [4], yet few studies incorporate and assess health-related quality of life (HRQOL) as a cancer care outcome. Breast cancer and its many treatments impact the physical, mental, and social health of women [5]. Frail older adults have less reserve than healthier older adults [6] and are potentially less able to recover from treatment toxicities resulting in a persistent loss in HRQOL domains.
There is great heterogeneity in the health status of older adults of similar age [7]. Geriatric assessment (GA) goes beyond chronological age to comprehensively appraise the overall health status of an older adult and identify potential vulnerabilities such as frailty. GA is recommended for the evaluation of older adults with cancer and has been shown to be predictive of survival in a variety of oncologic settings, predictive of severe treatment-related toxicity, and able to detect impairments that might otherwise go unnoticed in a routine history and physical examination [8]. However, the ability of GA to predict patient-reported outcomes such as HRQOL has not been evaluated to date. The objective of our study was to evaluate whether a GA-derived frailty index could predict long-term HRQOL in older women undergoing breast cancer treatment.
Methods
Participants
The sample for this study was derived from the Carolina Seniors Registry (CSR) (NCT01137825), launched at the North Carolina Cancer Hospital in 2009 as a large observational cancer registry to collect GA data on older adults (≥65 years) with a cancer diagnosis. Using the CSR, we identified older women with breast cancer as those who had completed the GA either before or during treatment through August 2015. These CSR participants (N=190) were then linked to their electronic medical record to obtain their latest-available contact information and vital status. This information was then used to re-contact patients that were identified as still living in order to complete a follow-up HRQOL questionnaire. CSR participants with contact information were contacted by phone and explained the details of the follow-up study and assessment. Interested participants provided oral consent to participate in the HRQOL follow-up study, and the follow-up questionnaire was administered by phone. All contacts and interviews were conducted by trained interviewers employed by the UNC Health Registry/Cancer Survivorship Cohort. Patients were re-contacted 3 times by phone on different days prior to determining them to be a non-responder. This study was approved by the Institutional Review Board of the University of North Carolina (IRB #15–2032) and informed consent was obtained from all individual participants included in the study.
Geriatric Assessment
The CSR utilizes a validated GA tool designed specifically for use in older adults with cancer [9,10]. The GA is comprised of both a healthcare professional portion and patient-reported measures. The healthcare provider section includes an assessment of objective physical function through the Timed Up and Go (TUG) test, cognition using the Blessed Orientation Memory and Concentration (BOMC), Karnofsky Performance Status (KPS), and weight loss. The patient-reported section includes an assessment of instrumental activities of daily living (IADL), medications, comorbidities, social support, physical health, self-reported falls, and self-rated KPS. For a more detailed description of the CSR including sampling methods, recruiting procedures, and the performance of assessments, please see Williams et al [10].
Frailty Index
Using GA data from the CSR, we recently developed a 36-item Carolina Frailty Index (CFI) based on the principles of deficit accumulation [11] that have been previously validated to predict mortality in community-dwelling elders [12,13]. The CFI includes multiple items relating to limitations in IADL, comorbidities, cognition, social activity, falls, and nutrition. Each deficit item is rated between 0 and 1, where a higher score indicates greater frailty. A list of CFI variables is provided in Supplemental Table 1. A score is calculated by dividing the total number of deficits by the total number of variables assessed. For instance, if 9 deficits are identified in a patient from a list of 36 possible deficits, then that person’s frailty index is 9/36= 0.25. The CFI categorizes older adults into three groups based on their deficit count (robust [0–0.2], pre-frail [0.2–0.35], and frail [>0.35]) [12,13]. In a multivariable model using the CFI, increased frailty in older persons with cancer was significantly associated with increasing age, African American race, lower education, increasing number of daily medications, and lower Karnofsky Performance Status [12]. The CFI has also been shown to be predictive of all-cause mortality in older adults with cancer independent of age, cancer type/stage, and comorbidity, as well as related to inflammatory markers and measures of skeletal muscle in older adults [13–15]. Using the GA data from enrollment into the CSR, we calculated a CFI for each participant.
Health-Related Quality of Life
HRQOL is defined as the impact of a medical condition or its treatment on a person’s physical, emotional, and social well-being [16]. The measures used in our study consist of multiple individual HRQOL domains. Survey instruments were selected from the National Institutes of Health (NIH) Patient-Reported Outcomes Measurement Information System® (PROMIS®) (http://www.HealthMeasures.net). All PROMIS measures are scored on a T-score metric, with a mean of 50 and standard deviation of 10 For most domains, the mean of 50 references the US general population. Higher PROMIS scores indicate higher levels of the domain the instrument is measuring. For example, higher scores on the PROMIS Fatigue measure indicate more fatigue whereas higher scores on the PROMIS Physical Functioning measure indicate better function. We chose PROMIS instruments related to Fatigue (4 items), Physical Function (10 items), Pain Interference (4 items), Social Roles (4 items), Anxiety (4 items), Depression (4 items), and Sleep Disturbance (4 items). The recommended minimally important difference for PROMIS T-scores in cancer populations varies per instrument and ranges from 2.5 to 6 points [17]. The HRQOL measures were collected only as part of the follow-up assessment and were not included in the baseline GA.
Statistical analysis
Descriptive statistics summarize the baseline characteristics of the sample. Study participants were included only if they had data on at least half of the 36 CFI variables (excluded n=8, 11%) similar to previous usage of CFI [13,15]. Given the low number of patients in the pre-frail and frail groups, these two groups were combined for all further analyses similar to prior publications [15,18]. Linear regression was used to compare HRQOL differences between pre-frail/frail patients and robust patients adjusting for age and cancer stage using adjusted mean differences (AMD). SAS statistical software version 9.4 (SAS Institute Inc., Cary, NC) was used for all analyses.
Results
Of 190 patients with breast cancer identified from the CSR, 71 patients completed the follow-up HRQOL questionnaire (see Figure 1 for full consort diagram). Reasons for not completing the follow-up questionnaire included 47 patients deceased, 15 declined participation, and 57 lost to follow-up (either no contact information available or unable to be contacted by phone). Of the 71 patients that completed the follow-up HRQOL questionnaire, 63 had sufficient data to calculate the CFI. There were no statistically significant differences in mean age (p=0.49), race (p=0.65), education (p=0.10), or marital status (p=0.59) between those who completed/declined the follow up questionnaire and those who were deceased or lost to follow-up; however, 28% of those who completed/declined the follow-up questionnaire were prefrail/frail compared to 42% of those who were deceased/lost to follow-up (p=0.08). The follow-up questionnaire was completed an average of 4 years (range 0.61–5.45 years) after completion of the initial GA.
Figure 1:
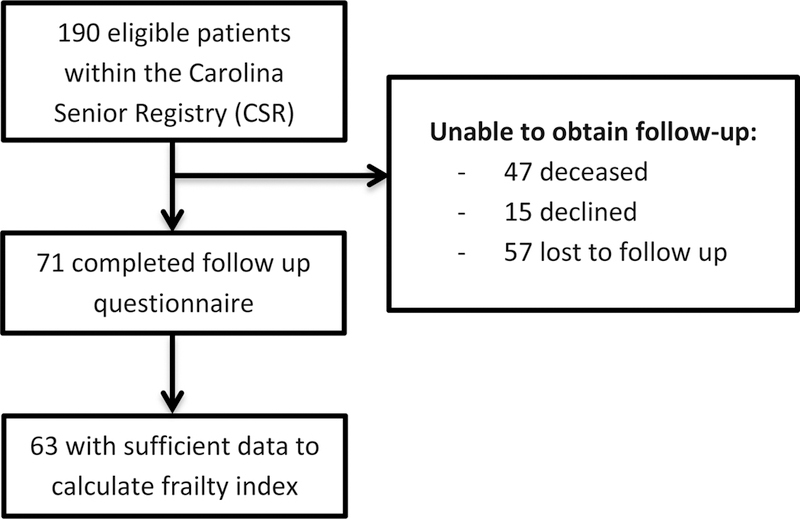
Consort Diagram
For the 63 patients that comprised the final study sample, the mean age was 70 years (range 65–86), 91% were white, 62% had at least some college education, and 56% were married (see Table 1). The majority of participants had early stage breast cancer (71%), underwent surgical resection (94%), received radiation therapy (77%), and endocrine therapy (60%). Sixteen percent of participants reported an impairment in instrumental activities of daily living (IADL) and 77% identified at least one limitation in physical function. Most patients (92%) assessed themselves as 80% or higher on the KPS scale, and 23% of patients reported a fall within the last 6 months. Based on the CFI calculation, 49 (78%) patients were robust and 14 (23%) were pre-frail/frail [11 (18%) pre-frail and 3 (5%) frail]. Mean scores for the follow-up HRQOL outcome measures were Physical Functional 46 (Standard Deviation [SD] 8), Social Roles 57 (SD 8), Fatigue 46 (SD 9), Depression 46 (SD 7), Anxiety 45 (SD 8), Pain Interference 50 (SD 8), and Sleep Disturbance 46 (SD 8).
Table 1.
Patient characteristics at baseline
| Total Sample (n=63) |
|
|---|---|
| Age, mean (range) | 71 (65–86) |
| Time since baseline survey, mean (range) | 3.7 years (0.6–5.3) |
| Race, n (%) | |
| White | 57 (91) |
| Black/Other | 6 (9) |
| Education, n (%) | |
| High school degree or less | 24 (38) |
| Assoc./bachelor’s degree | 19 (30) |
| Advanced degree | 20 (32) |
| Marital Status, n (%) | |
| Married | 35 (56) |
| Divorced | 17 (27) |
| Widowed | 9 (14) |
| Single | 2 (3) |
| Stage, n (%) | |
| I | 28 (44) |
| II | 17 (27) |
| III | 8 (13) |
| IV | 10 (16) |
| Cancer Treatments, n (%) | |
| Surgery | 58 (94) |
| Chemotherapy | 28 (45) |
| Endocrine therapy | 37 (60) |
| Radiation therapy | 48 (77) |
| Geriatric Assessment domains*, n (%) | |
| IADLs | |
| - Independent | 52 (84) |
| - Impaired | 10 (16) |
| Physical function | |
| - Not limited | 14 (23) |
| - Limited | 47 (77) |
| KPS (patient-reported) | |
| - ≥80 | 57 (92) |
| - 60–80 | 5 (8) |
| Self-reported falls | |
| - None | 48 (77) |
| - ≥1 fall | 14 (23) |
| Timed Up and GO | |
| - < 14 seconds | 47 (75) |
| - ≥ 14 seconds | 16 (25) |
| Comorbidities | |
| - 0–4 | 54 (93) |
| - 5–8 | 4 (7) |
| Vision impairments | 9 (14) |
| Hearing impairments | 6 (10) |
| Carolina Frailty Index | |
| - Robust (0–0.2) | 49 (78) |
| - Pre-frail (0.2–0.35) | 11 (18) |
| - Frail (>0.35) | 3 (5) |
Not all cells add up to n=63 due to missing data. Abbreviations: IADLs, instrumental activities of daily living; KPS, Karnofsky Performance Status.
After controlling for age and cancer stage, patients identified as pre-frail/frail reported significantly worse Physical Function (37.6 vs. 48.4, AMD −9.2, p<.001), worse Social Roles (50.6 vs. 58.8, AMD −7.2, p=.002), more Fatigue (52.8 vs. 44.4, AMD 7.6, p=.008), more Depression (51.1 vs. 44.3, AMD 5.6, p=.004), and more Sleep Disturbance (51.8 vs. 44.8, AMD 6.9, p=.008) compared to robust patients (see figure 2). Pre-frail/frail patients also reported more Anxiety (49.24 vs. 44.31, AMD 4.1, p=0.085) and Pain Interference (53.94 vs. 49.02, AMD 4.8, p=0.062) than robust patients; however these differences were not statistically significant.
Figure 2:
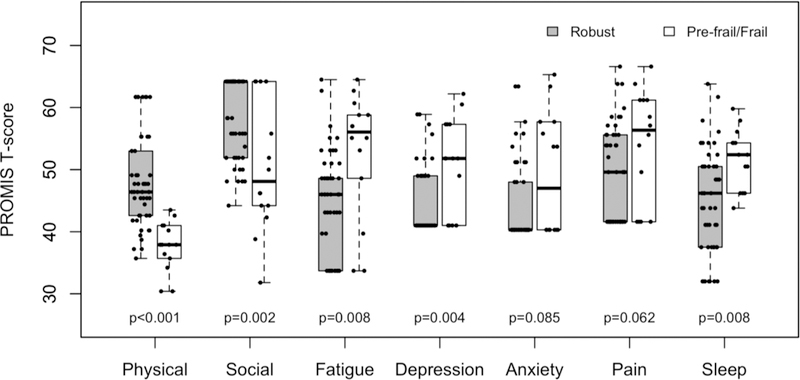
Examines the differences in Health-Related Quality of Life (HRQOL) domain scores at follow-up between participants identified as robust versus pre-frail/frail on baseline geriatric assessment. All PROMIS measures use a T-score with a mean of 50 and standard deviation of 10. Higher PROMIS Physical Function and Social Role scores indicate better HRQOL and higher PROMIS symptom scores (Fatigue, Depression, Anxiety, Pain Interference, and Sleep Disturbance) represent more severe symptom burden.
Conclusions
In a sample of older women with predominantly early stage breast cancer, we found that baseline frailty was associated with worse HRQOL at follow-up across the majority of HRQOL measures. Older women that are frail or pre-frail at or near the time of their breast cancer diagnosis are at-risk for long-term worse physical function and reduced social roles, as well as more fatigue, depression, and sleep disturbances. As HRQOL is often prioritized over survival by older patients with cancer [4], our results suggest that frail and pre-frail patients are at increased risk for poor long-term HRQOL and should be targeted for interventions such as enhanced management of GA deficits to address their medical and supportive care needs [19].
Frailty and its impact on outcomes in older adults with cancer has become an area of increasing interest. Recent evidence from the Cancer and Aging Research Group has shown that prefrail/frail older adults are more likely to have grade 3/4 chemotherapy toxicity and are at increased risk for hospitalization and drug discontinuation [18]. In addition, our research team has shown that frailty is associated with overall mortality independent of age, sex, cancer type and stage, and comorbidities frail patients had a more than 2-fold increased risk of all-cause mortality compared to robust patients (adjusted Hazards Ratio 2.36) [13]. In a recent secondary analyses of CALGB 369901 of older adults with breast cancer, pre-frail and frail patients also had an elevated long-term all-cause and breast cancer specific mortality [20]. However, the relationship of frailty with HRQOL in older adults undergoing cancer therapy remains largely unexplored. The results of our study are consistent with frailty in the larger geriatric non-cancer populations, where frailty phenotypes have been associated with lower scores on both physical and mental HRQOL after adjusting for sociodemographic and health-related covariates [21,22].
Our study should be considered in the context of its limitations. Our sample was limited to breast cancer participants within the CSR and our results may not be applicable to other cancer types nor representative of the overall breast cancer population. A majority of our sample (91%) were non-Hispanic white, and further work is needed to address this question in minority populations. In addition, we were only able to contact and perform the HRQOL follow-up survey in a third of the patients identified in the CSR. Our comparison of those who completed/declined the follow up HRQOL survey and those who were either deceased or lost to follow-up were not significantly different in mean age, race, education, or marital status; however, a higher proportion were prefrail/frail among those either lost to follow up/deceased compared to those that completed the follow-up survey (28% vs 42%). This is consistent with the concept that frailty is associated with increased mortality [13]. Furthermore, we had to exclude an additional 8 patients that did not sufficiently complete the GA to calculate a frailty index. Given the limited sample size, we were unable to examine the impact of specific treatments on long-term HRQOL, and we propose this as an important area requiring further examination in older adult cancer survivors. Lastly, there are some notable areas of overlap between our GA-based frailty index and the domains of HRQOL. The GA utilized in our registry consists of several questions regarding IADL (7 questions) and physical functioning (5 questions) that are among the domains included in our frailty-index; therefore, associations with worse long-term physical functioning are not surprising.
Older adults place a high priority on HRQOL in making treatment decisions; therefore, including HRQOL assessments in treatment trials and the overall care of older adults with cancer is critical [23,24]. Our results demonstrate that frail patients are at increased risk for poor long-term HRQOL across multiple domains, and highlight the importance of incorporating GA into the management and evaluation of older adults with cancer. Further validation of our results in a larger and more diverse sample of older persons with cancer and developing/piloting interventions focused on long-term HRQOL are necessary.
Supplementary Material
Acknowledgments
Funding: Supported in part by the UNC Oncology Clinical Translational Research Training Program (NCI 5K12CA120780-07), the Breast Cancer Research Foundation (New York, NY), the University Cancer Research Fund at UNC, and the Clinical and Translational Science Award program of the National Center for Advancing Translational Sciences, National Institutes of Health (1UL1TR001111).
Footnotes
Compliance with Ethical Standards: This study complied with the laws and ethical standards of the United States and was approved by the Institutional Review Board of the University of North Carolina (IRB #15-2032).
Conflicts of Interest: Author Sanoff has received research funding from Merck and Bayer, and no other relevant conflicts of interest to declare.
References
- 1.Cancer Facts and Figures 2017. American Cancer Society, Atlanta, GA [Google Scholar]
- 2.Surveillance Epidemiology and End Results (SEER) Cancer Statistics Review (1975–2015) National Cancer Institute http://seer.cancer.gov/faststats/index.php.
- 3.Bluethmann SM, Mariotto AB, Rowland JH (2016) Anticipating the “Silver Tsunami”: Prevalence Trajectories and Comorbidity Burden among Older Cancer Survivors in the United States. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology 25 (7):1029–1036. 10.1158/1055-9965.EPI-16-0133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wedding U, Pientka L, Hoffken K (2007) Quality-of-life in elderly patients with cancer: a short review. Eur J Cancer 43 (15):2203–2210. 10.1016/j.ejca.2007.06.001 [DOI] [PubMed] [Google Scholar]
- 5.Stover AM, Mayer DK, Muss H, Wheeler SB, Lyons JC, Reeve BB (2014) Quality of life changes during the pre- to postdiagnosis period and treatment-related recovery time in older women with breast cancer. Cancer 120 (12):1881–1889. 10.1002/cncr.28649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fried LP, Tangen CM, Walston J, Newman AB, Hirsch C, Gottdiener J, Seeman T, Tracy R, Kop WJ, Burke G, McBurnie MA (2001) Frailty in older adults: evidence for a phenotype. The journals of gerontology Series A, Biological sciences and medical sciences 56 (3):M146–156 [DOI] [PubMed] [Google Scholar]
- 7.Balducci L, Extermann M (2000) Management of cancer in the older person: a practical approach. The oncologist 5 (3):224–237 [DOI] [PubMed] [Google Scholar]
- 8.Wildiers H, Heeren P, Puts M, Topinkova E, Janssen-Heijnen ML, Extermann M, Falandry C, Artz A, Brain E, Colloca G, Flamaing J, Karnakis T, Kenis C, Audisio RA, Mohile S, Repetto L, Van Leeuwen B, Milisen K, Hurria A (2014) International Society of Geriatric Oncology Consensus on Geriatric Assessment in Older Patients With Cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology 10.1200/JCO.2013.54.8347 [DOI] [PMC free article] [PubMed]
- 9.Hurria A, Gupta S, Zauderer M, Zuckerman EL, Cohen HJ, Muss H, Rodin M, Panageas KS, Holland JC, Saltz L, Kris MG, Noy A, Gomez J, Jakubowski A, Hudis C, Kornblith AB (2005) Developing a cancer-specific geriatric assessment: a feasibility study. Cancer 104 (9):1998–2005. 10.1002/cncr.21422 [DOI] [PubMed] [Google Scholar]
- 10.Williams GR, Deal AM, Jolly TA, Alston SM, Gordon BB, Dixon SA, Olajide OA, Chris Taylor W, Messino MJ, Muss HB (2014) Feasibility of geriatric assessment in community oncology clinics. Journal of geriatric oncology 5 (3):245–251. 10.1016/j.jgo.2014.03.001 [DOI] [PubMed] [Google Scholar]
- 11.Searle SD, Mitnitski A, Gahbauer EA, Gill TM, Rockwood K (2008) A standard procedure for creating a frailty index. BMC geriatrics 8:24 10.1186/1471-2318-8-24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guerard E, Deal A, Williams G, Jolly T, Wood W, Muss H (2015) Construction of a frailty index for older adults with cancer using a geriatric assessment. J Clin Oncol 33, 2015 (suppl; abstr 9535) 33 (supplement):abstr 9535 [Google Scholar]
- 13.Guerard EJ, Deal AM, Chang Y, Williams GR, Nyrop KA, Pergolotti M, Muss HB, Sanoff HK, Lund JL (2017) Frailty Index Developed From a Cancer-Specific Geriatric Assessment and the Association With Mortality Among Older Adults With Cancer. Journal of the National Comprehensive Cancer Network : JNCCN 15 (7):894–902. 10.6004/jnccn.2017.0122 [DOI] [PubMed] [Google Scholar]
- 14.Williams GR, Deal AM, Muss HB, Weinberg MS, Sanoff HK, Guerard EJ, Nyrop KA, Pergolotti M, Shachar SS (2017) Frailty and skeletal muscle in older adults with cancer. Journal of geriatric oncology 10.1016/j.jgo.2017.08.002 [DOI] [PMC free article] [PubMed]
- 15.Nishijima TF, Deal AM, Williams GR, Guerard EJ, Nyrop KA, Muss HB (2017) Frailty and inflammatory markers in older adults with cancer. Aging (Albany NY) 9 (3):650–664. 10.18632/aging.101162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cella DF (1995) Measuring quality of life in palliative care. Seminars in oncology 22 (2 Suppl 3):73–81 [PubMed] [Google Scholar]
- 17.Yost KJ, Eton DT, Garcia SF, Cella D (2011) Minimally important differences were estimated for six Patient-Reported Outcomes Measurement Information System-Cancer scales in advanced-stage cancer patients. Journal of clinical epidemiology 64 (5):507–516. 10.1016/j.jclinepi.2010.11.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cohen HJ, Smith D, Sun CL, Tew W, Mohile SG, Owusu C, Klepin HD, Gross CP, Lichtman SM, Gajra A, Filo J, Katheria V, Hurria A, Cancer, Aging Research G (2016) Frailty as determined by a comprehensive geriatric assessment-derived deficit-accumulation index in older patients with cancer who receive chemotherapy. Cancer 122 (24):3865–3872. 10.1002/cncr.30269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Magnuson A, Allore H, Cohen HJ, Mohile SG, Williams GR, Chapman A, Extermann M, Olin RL, Targia V, Mackenzie A, Holmes HM, Hurria A (2016) Geriatric assessment with management in cancer care: Current evidence and potential mechanisms for future research. Journal of geriatric oncology 7 (4):242– 248. 10.1016/j.jgo.2016.02.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mandelblatt JS, Cai L, Luta G, Kimmick G, Clapp J, Isaacs C, Pitcher B, Barry W, Winer E, Sugarman S, Hudis C, Muss H, Cohen HJ, Hurria A (2017) Frailty and long-term mortality of older breast cancer patients: CALGB 369901 (Alliance). Breast cancer research and treatment 164 (1):107–117. 10.1007/s10549-017-4222-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chang YW, Chen WL, Lin FG, Fang WH, Yen MY, Hsieh CC, Kao TW (2012) Frailty and its impact on health-related quality of life: a cross-sectional study on elder community-dwelling preventive health service users. Plos One 7 (5):e38079 10.1371/journal.pone.0038079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Moreno-Aguilar M, Garcia-Lara JM, Aguilar-Navarro S, Navarrete-Reyes AP, Amieva H, Avila-Funes JA (2013) The Phenotype of Frailty and Health-Related Quality of Life. J Frailty Aging 2 (1):2–7. 10.14283/jfa.2013.1 [DOI] [PubMed] [Google Scholar]
- 23.Fried TR, Bradley EH, Towle VR, Allore H (2002) Understanding the treatment preferences of seriously ill patients. The New England journal of medicine 346 (14):1061–1066. 10.1056/NEJMsa012528 [DOI] [PubMed] [Google Scholar]
- 24.Wildiers H, Mauer M, Pallis A, Hurria A, Mohile SG, Luciani A, Curigliano G, Extermann M, Lichtman SM, Ballman K, Cohen HJ, Muss H, Wedding U (2013) End points and trial design in geriatric oncology research: a joint European organisation for research and treatment of cancer--Alliance for Clinical Trials in Oncology--International Society Of Geriatric Oncology position article. Journal of clinical oncology : official journal of the American Society of Clinical Oncology 31 (29):3711–3718. 10.1200/JCO.2013.49.6125 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


