Abstract
Rationale:
Epidemiological data suggest that menthol may increase vulnerability to cigarette/nicotine use and relapse. While menthol’s sensory properties are often attributed as the underlying cause of the enhanced vulnerability, an alternative possibility is that they are mediated via pharmacological interactions with nicotine.
Objective:
This study addressed the possibility that menthol enhances nicotine intake and relapse vulnerability via pharmacological interactions with nicotine using a concurrent intravenous menthol/nicotine self-administration procedure.
Methods:
Following acquisition, adolescent rats were given 23-hr/day access to nicotine (0.01 mg/kg/infusion), nicotine plus menthol (0.16, 0.32, or 0.64 mg/kg/infusion), or menthol alone (0.16, 0.32, 0.64 mg/kg/infusion) for a total of 10 days. Nicotine-seeking was assessed using an extinction/cue-induced reinstatement procedure following 10-days of forced abstinence. We also assessed the effect of menthol (0.32 mg/kg/infusion) on progressive-ratio responding for nicotine (0.01 mg/kg/infusion).
Results:
Menthol decreased PR responding for nicotine, but did not affect self-administration under extended access conditions. The low dose of menthol tended to decrease subsequent extinction responding, and was not different from menthol alone, whereas, the high dose decreased reinstatement responding. Although not significant, the highest levels of extinction responding were observed in a minority of rats in the moderate and high menthol-nicotine groups; rats in these groups also took longer to extinguish.
Conclusions:
Taken together, these results demonstrate that pharmacological interactions of menthol with nicotine reduce, rather than increase, nicotine’s reinforcing effects and some measures of relapse vulnerability. Importantly, however, moderate and high menthol doses may increase some aspects of relapse vulnerability in a minority of individuals.
Keywords: Extended Access, Menthol, Nicotine-Seeking, Progressive-Ratio, Reinstatement, Self-Administration
Introduction
Menthol additive is widely used in cigarettes to maximize their appeal by reducing the harshness of cigarette smoke and enhancing the flavor (WHO 2016). Although there has been a gradual decrease in the sale of regular cigarettes, use of menthol cigarettes is increasing, especially among adolescent and young adult smokers (Courtemanche et al. 2017; Villanti et al. 2016a; WHO 2016). Menthol is also commonly used as a flavorant in e-cigarettes (Yingst et al. 2017), which are now more commonly used than cigarettes with particularly high rates of use in adolescent and young adult populations (Rath et al. 2017; Villanti et al. 2016b). Adolescents appear to be particularly vulnerable to use menthol tobacco products with results showing that 80% of adolescents initiate smoking with menthol cigarettes, and 60% prefer to continue to use menthol cigarettes (Villanti et al. 2012). There is also evidence suggesting that adolescent menthol cigarette smokers are more vulnerable than adolescent non-menthol cigarette smokers to progress to regular cigarette use (Azagba et al. 2014). Adolescent menthol cigarette smokers also smoke more cigarettes per day, have a shorter time to first cigarette of the day, and report higher levels of craving as compared to adolescent non-menthol cigarette smokers (Hersey et al. 2006; Wackowski and Delnevo 2007) suggesting that menthol may increase smoking and craving/relapse vulnerability. While few studies have examined this possibility in adolescent populations, results from adult smokers consistently show an enhanced risk of relapse in menthol cigarette smokers as compared to non-menthol cigarette smokers (Ahijevych and Garrett 2010; Levy et al. 2011; Pletcher et al. 2006). These epidemiological data indicate that menthol may increase vulnerability to cigarette/nicotine use and relapse; however, it is not yet known whether such effects are caused by menthol products or inherent to the populations using them (e.g., adolescents).
Menthol in cigarettes adds the sensation of coolness and minty taste, which produce a positive sensory experience in some smokers (Anderson 2011; Klausner 2011; Lawrence et al. 2011). It has been suggested that these sensory properties serve as powerful cues that become associated with smoking and serve to influence smoking intensity as well as nicotine craving and relapse (Ahijevych and Garrett 2010; Kreslake et al. 2008b; Perkins and Karelitz 2014). There is also mounting evidence suggesting that some of these effects may be mediated independent of sensory cues effects via pharmacological interactions of menthol and nicotine (Ashoor et al. 2013a; Brody et al. 2013; Hans et al. 2012; Thompson et al. 2018). For example, as compared to non-menthol cigarette smokers, menthol cigarette smokers have a higher density of nAChRs at subtypes that are associated with enhanced smoking intensity and relapse (i.e., α4β2; Brody et al. 2013). In vitro findings further demonstrate that menthol is a negative allosteric modulator of nicotinic acetylcholine receptors (nAChRs; Ashoor et al. 2013a; Hans et al. 2012), the chief site of action for nicotine which underlies its reinforcing effects and craving/relapse (Alsharari et al. 2015; Ashoor et al. 2013a; Brody et al. 2013; Ton et al. 2015). Recent findings also show that chronic systemic menthol administration, on its own, increases the number of lower sensitivity nAChR subunits (α4 and α6), and decrease the development of nicotine-induced conditioned place preference, a measure of its rewarding effects (Henderson et al. 2016). These effects are in contrast to those observed following chronic nicotine exposure (Hilario et al. 2012), and suggest that menthol’s pharmacological effects may oppose those of nicotine. However, evidence indicates that chronic systemic menthol administration with nicotine producing the opposite effect and enhances the development of a nicotine-induced conditioned place preference (Henderson et al. 2017). While these findings demonstrate pharmacological interactions of menthol and nicotine, it’s not clear whether such effects impact nicotine use and relapse.
Animal models of nicotine addiction provide an ideal platform for determining a causal effect of menthol on nicotine use and relapse vulnerability. Several recent studies have examined the effects of menthol on nicotine self-administration, with variable results. For example, Wang et al. (2014) showed that rats that self-administered intravenous (IV) infusions of nicotine paired with oral menthol deliveries during 3-hr sessions self-administered more nicotine and responded at higher levels during extinction and cue-induced reinstatement testing as compared to rats self-administering IV nicotine or oral menthol alone (Wang et al. 2014). In contrast, Wickham et al. (2018) showed that oral menthol did not affect IV nicotine self-administration during 1-hr sessions under fixed-ratio (FR) or progressive-ratio (PR) schedules. However, they did find that oral menthol reduced the aversive effects of oral nicotine solutions (Wickham et al. 2018), indicating that its efficacy, at least for oral consumption, may be mediated via sensory cue effects (i.e., masking bitter taste). Indeed, the ability of oral menthol to augment IV nicotine intake (Wang et al. 2014) and to offset the aversive effects of oral nicotine (Fan et al. 2016) appears to be mediated, at least in part, via sensory cue effects (i.e., induction of a cooling sensation via TRPM8 channels).
The possibility that effects of menthol on nicotine self-administration are mediated through pharmacological interactions with nicotine, independent of sensory cue effects, has also been examined in recent studies through the use of systemic menthol administration (intraperitoneal, IP). These findings have also been variable, however, with one study reporting that menthol increased IV nicotine self-administration under FR and PR schedules during 1-hr sessions (Biswas et al. 2016); whereas, another study showing that menthol did not affect IV nicotine self-administration under a FR schedule during 1-hr sessions, but increased subsequent nicotine-seeking (Harrison et al. 2017). While these findings indicate that menthol may enhance vulnerability to nicotine use and seeking through pharmacological interactions with nicotine, one caveat is that menthol was administered as a single bolus injection rather than concurrently with nicotine as is the case in human smokers (i.e., with each puff on a cigarette). This is important since pharmacological effects are likely to be very different between a single systemic pretreatment and repeated, concurrent menthol/nicotine exposure (Ahmed 2015). It is also not clear whether effects observed under these short access conditions translate to human tobacco/nicotine use disorder (Cohen et al. 2015b).
The purpose of this study was to determine the effects of menthol on nicotine self-administration and relapse vulnerability under conditions simulate the concurrent menthol/nicotine exposure conditions observed in human menthol cigarette smokers. In order to do so, menthol was added directly to the IV nicotine solutions which allowed for fast and concurrent nicotine/menthol exposure with precisely controlled doses to simulate the low, moderate, and high menthol dose exposure conditions that are observed in humans (Carchman and Southwick 1990; Kreslake et al. 2008b; Stowe 1976). This procedure also allowed for an examination of pharmacological effects independent of sensory cue effects (such as taste and smell; Rose et al. 2000; Westman et al. 1996). Given the apparent vulnerability in adolescents to menthol cigarette use (Azagba et al. 2014; Villanti et al. 2012), and recent findings showing that the effects of menthol exposure differ between adolescents and adults (Fait et al. 2017; Thompson et al. 2018), we used an adolescent-onset model of nicotine addiction. We used an extended access paradigm that allows 23-hr/day access to nicotine and results in levels of nicotine intake that are compared to those observed in humans (0.18 to 1.38 mg/kg/day; Valentine et al. 1997). Such conditions also produce high levels of nicotine-seeking following abstinence (Abdolahi et al. 2010; Sanchez et al. 2014). We also examined the effect of menthol on nicotine’s reinforcing effects as assessed under a PR schedule in a separate group of animals. Based on previous findings in human adolescent menthol cigarette smokers, and previous preclinical findings for the effects of menthol in adults, we hypothesized that menthol would increase nicotine’s reinforcing effects, levels of nicotine self-administration, and subsequent nicotine-seeking/relapse vulnerability.
Materials and Methods
Subjects
Male Sprague-Dawley rats (N=95) were purchased from Charles Rivers Laboratories (Portage, ME, USA). As in our previous studies (Abdolahi et al. 2010; Sanchez et al. 2014), rats arrived at the animal facility on postnatal day (PND) 22 and were individually housed in operant conditioning chambers in a temperature and humidity controlled environment on a 12-h light/dark cycle (lights on at 7AM). Food and water were provided ad libitum. After a 2-day habituation period, rats were pre-trained to lever-press for sucrose pellets (45-mg) using a brief training protocol as previously described (FR1; 2 consecutive days ≥50 deliveries; Lynch 2008). Animal weight was monitored prior to surgery, and then every Monday, Wednesday, and Friday throughout the experiment. All procedures were conducted in accordance with the guidelines set by the University of Virginia Animal Care and Use Committee.
Drugs
Nicotine bitartrate (Sigma-Aldrich, St. Louis, MO, USA) was dissolved in sterile 0.9% saline, pH adjusted to 7.4 (± 0.5) using NaOH, and then filtered through a 0.22 mm syringe filter with dose expressed as the free base weight (Fischer Scientific, Pittsburg, PA, USA). We focused on the effects of DL-menthol (racemic (±) menthol) since the majority of menthol tobacco products contain this form of menthol (FDA 2011; Heck 2010). This form of menthol has also been used in other preclinical studies examining its pharmacological effects (Ashoor et al. 2013a; Ashoor et al. 2013b; Willis et al. 2011). Menthol was purchased from Sigma-Aldrich (St. Louis, MO, USA), dissolved in DMSO, and then diluted with sterile 0.9% saline (with or without nicotine) to a final concentration of 1.58, 3.16 and 6.32 mg/ml. The mixture was stirred at 60°C for 30 minutes, pH adjusted to 7.4 using 0.1 N NaOH, and then filtered through a 0.22 mm syringe filter. Solutions were kept at room temperature until used. Considering potential menthol and/or nicotine mass loss during the heating process, the final concentration of each was determined using a precipitation method of gravimetric analysis for mass loss. Findings from this analysis showed that there was a 6% mass loss of menthol, but no loss of nicotine, following the heating process. As such, the final dose for nicotine was 0.01 mg/kg/infusion and 0.16, 0.32, and 0.64 mg/kg/infusion for the low, moderate, and high concentration of menthol, respectively. These doses also approximate levels of menthol exposure in humans. Specifically, using dose conversion guidelines from (Nair and Jacob 2016; Reagan-Shaw et al. 2008) to determine equivalent dosing between human and rat (i.e., a conversion factor of 6.2), we selected the low dose to approximate the level of menthol exposure observed in humans following a single puff of a mentholated cigarette (~10 mg/cigarette; Ai et al. 2018). This dose range also included a moderate and high dose to approximate levels of exposure observed among moderate and heavy smokers (Appleton 1986). These doses have been also shown to be safe for intravenous infusion (Appleton 1986; Rodger and Lewis 1979). The same concentration of nicotine, menthol, and their combinations were maintained throughout the study with the infusion duration adjusted three times/week (based on changes in body weight; 1.3 s/200 g) to maintain a constant mg/kg dose within each group/dose condition.
Apparatus
Self-administration sessions were carried out in operant conditioning chambers (31 cm × 24 cm × 21 cm) equipped with two levers (an active drug-associated lever and an inactive lever) each with a stimulus light above it, a water bottle, ventilation fan, feeder that dispensed sucrose pellets, and a house light that was illuminated from 7AM to 7PM (Med Associates, St. Albans, VT, USA). Each operant conditioning chamber was placed in a sound-attenuating ventilated box (ENV-018 M; Med Associates) that held an infusion pump (PHM-100, Med Associates) containing a drug syringe. The drug syringe was connected to Tygon tubing that attached to a swivel (Instech Laboratories Inc., Plymouth Meeting, PA, USA) embedded in a counterbalanced metal arm above the operant conditioning chamber. A polyethylene tube encased in a metal spring (C313CS; PlasticsOne, Roanoke, VA, USA) was attached to the swivel and to a 22-gauge guide (C313G; PlasticsOne) embedded within an infusion harness (CIH95AB; Instech Laboratories Inc.) the animal wore following surgery and thereafter until study completion. Experimental events and data collection were managed by Schedule Manager Software version 3.13 (Med Associates).
Surgery
Rats underwent surgery on PND 28 and were anesthetized using a mixture of ketamine (40 mg/kg, IP) and dexmedetomidine (0.2 mg/kg, IP). A chronic indwelling jugular catheter (OD = 0.94 mm, ID = 0.51 mm; Dow Corning Corporation, Midland, MI, USA) was implanted into the right jugular vein with the tip terminating at the opening of the right atrium, and was secured to surrounding tissue with sutures. The catheter exited from the back of the animal and connected to a metal cannula embedded in the center of an external silicone harness. Analgesic (ketoprofen, 2 mg/kg, subcutaneous) and antibiotic (cefazolin, 12 mg/kg, IV) was provided immediately after the surgery and for the following two days. Catheters were flushed every Monday, Wednesday, and Friday with heparinized saline prior to the self-administration session to verify and help maintain patency. If a catheter was leaking, no blood appeared in the line, or pressure prevented flushing, the catheter was considered no longer patent. Rats that lost catheter patency prior to completing extended access self-administration or that did not acquire nicotine self-administration within 6 days (as detailed below) were not included in the study (10 of 95).
Nicotine self-administration training
All rats were trained to self-administer nicotine (0.01 mg/kg/infusion) under a FR1 schedule beginning on PND 30. This dose of nicotine was selected based on our previous results showing that adolescent rats rapidly acquire nicotine self-administration (within 5–6 days) at this dose (Sanchez et al. 2013; 2014). This was important because it allowed us to examine the effects of menthol on levels of intake beginning at the same time-point during adolescence (PND 35–36) in rats that already acquired self-administration and had a similar level of exposure to nicotine (5–6 days, up to 20 infusions/day). Daily self-administration sessions were conducted using methods previously described (Sanchez et al. 2013; 2014). Briefly, daily sessions began at 12PM by the extension of the active lever into the operant conditioning chamber and the delivery of one non-contingent infusion of nicotine. Each response on the active lever during the session was reinforced with a nicotine infusion which was paired with the illumination of a light above the active lever and the sound of the pump (between 0.8 and 1.3 sec depending on body weight). Sessions ended after all 20 infusions that were available were obtained, or until 11:50AM. At this time, the active lever retracted from the operant conditioning chamber and remained retracted until the next daily session. The inactive lever remained extended throughout the study. These training sessions continued for a total of 5–6 days.
Effect of menthol on nicotine self-administration under extended access conditions
Following nicotine self-administration training, 67 rats were randomly assigned to one of five groups for extended access self-administration: nicotine alone (Nic; n=11), menthol alone (Ment; n=11), nicotine plus the low dose of menthol (Nic+Mentlow; n=13), nicotine plus the moderate dose of menthol (Nic+Mentmod; n=16), or nicotine plus the high dose of menthol (Nic+Menthigh; n=16). The Ment group was comprised of rats tested at the low (n=4), moderate (n=3), and high (n=4) dose of menthol. Given that no differences were observed between the different Ment groups with regard to the number of infusions obtained during the extended access self-administration period (average infusions: Mentlow, 21.9 ± 0.04; Mentmod, 20.9 ± 10.9; Menthigh, 18.2 ± 1.8), or for responding during extinction (total responses: Mentlow, 43.5 ± 9.8; Mentmod, 47 ± 13.6; Menthigh, 46.6 ± 20.0) and reinstatement testing (total responses: Mentlow, 12 ± 6.3; Mentmod, 16 ± 6.1; Menthigh, 19.6 ± 6.2 ), data were pooled to make a single Ment control group. The 23-hr/day extended access sessions began at 12PM on PND 35 or 36. Sessions were conducted using the same conditions as described for nicotine self-administration training, except that now there was no limit to the number of infusions that could be obtained. Sessions were run for a total of 10 days.
A 10-day forced abstinence period began after the last extended access self-administration session on PND 45 or 46, wherein animals remained in their operant conditioning chambers without access to the active lever or drug. The duration of the abstinence period was based on our previous results showing that rats had moderate to high levels of extinction and cue-induced reinstatement responding following 10 days of extended access nicotine self-administration and 10 days of abstinence (Sanchez et al. 2013; 2014).
Effect of menthol on subsequent extinction and cue-induced reinstatement of nicotine-seeking
Rats underwent extinction and reinstatement testing after the 10th day of abstinence on PND 55 or 56 using a within-session procedure and methods previously described (Sanchez et al. 2013; 2014). This procedure allowed us to examine both extinction and reinstatement responding in one session at an abstinence time-point associated with high levels of drug seeking (Sanchez et al. 2013; 2014). Extinction was examined in a minimum of five, 1-hr sessions separated by a 5-min timeout period. Each session began with the extension of the formerly active lever into the chamber; responses were recorded but had no programmed consequence. These sessions continued until responding extinguished (defined as fewer than 15 responses in the last session; 5–8 sessions).
The 1-hr cue-induced reinstatement session began 5-min after the last extinction session with the extension of the formerly active lever into the chamber and a presentation of the cues formerly associated with the drug (light above the formerly active lever and the sound of the pump; between 1.3 and 1.8 sec, depending on body weight). Each response on the formerly active lever led to presentation of these same cues but did not produce an infusion. Data from one of the rats in the Nic group were not available for the extinction or the reinstatement session (n=10) and data from one of the rats in the Nic+Mentmod group (n=15) was not available for the reinstatement session due to a technical issue. A summary of the experimental events is depicted in Figure 1a.
Figure 1:

Summary of experimental events as a function of postnatal day (PND). (a) Effect of menthol on extended access nicotine self-administration (SA) and subsequent relapse vulnerability: Rats arrived at the laboratory on PND 22, underwent catheter implantation surgery on PND 28, and began self-administering nicotine on PND 30 (20 infusions/day). Following the 5–6 day training period, rats were given extended 23-hr/day access to infusions of nicotine (Nic; n=11), menthol (Ment; n=11), or nicotine plus a low (Nic+Mentlow; n=13), moderate (Nic+Mentmod; n=16), or high dose of menthol (Nic+Menthigh; n=16) for a total of 10 days. Nicotine-seeking was assessed following a 10-day abstinence period on PND 55 or 56. (b) Effect of menthol on nicotine’s reinforcing effects: Following surgery (PND 28), and nicotine self-administration training (20 infusions/day; PND 30–36), rats were tested under a progressive-ratio (PR) schedule beginning on PND 35 or 36. Once responding stabilized (3–7 sessions), rats in the Nic group (n=9) continued to have access to nicotine infusions for an additional 3 sessions under the PR schedule, whereas, rats assigned to the Nic+Mentmod group (n=9) were given access to nicotine plus menthol infusions for 3 PR test sessions.
Effect of menthol on nicotine self-administration under a PR schedule
In order to determine how menthol impacts nicotine’s reinforcing effects, 18 rats were randomly assigned to be tested on responding for nicotine alone (n=9) versus nicotine plus a moderate dose of menthol (0.32 mg/kg/infusion; n=9) under a PR schedule. This dose of menthol was selected based on pilot results indicating that it reduced nicotine intake under both extended access and PR testing conditions. Although the final data did not reveal a significant effect of this dose on intake under extended access conditions or on subsequent extinction or reinstatement responding (as detailed in the results), it did produce the most variability in responding during extinction and reinstatement testing. These rats underwent lever pre-training, surgery, and nicotine self-administration training using the same conditions as those described above.
Following nicotine self-administration training, rats were tested under a PR schedule wherein the response requirement to obtain an infusion of nicotine increased progressively each session in the following steps: 1, 2, 4, 6, 9, 12, 15, 20, 25, 32, 40, 50, 62, 77, 95, etc (Sanchez et al. 2015). The final ratio reached, which is believed to be a sensitive measure of both reinforcing efficacy and motivation to obtain the drug (Arnold and Roberts 1997), was measured daily. PR sessions began at 12PM on PND 35 with the extension of the active lever into the operant conditioning chamber and one non-contingent infusion of nicotine. Sessions ended at 11:50AM the next day with the retraction of the active lever. All rats were tested on responding for nicotine infusions for a minimum of 3 stable sessions under the PR schedule. Following stability (defined as no progressive increase or decrease in the number of infusions obtained over 3 consecutive sessions), rats randomly assigned to the Nic group were tested on responding for nicotine infusions for an additional 3 sessions under the PR schedule, whereas, rats assigned to the Nic+Mentmod group were tested on responding for nicotine plus menthol for an additional 3 sessions. A summary of the experimental events is depicted in Figure 1b.
Data Analysis
The effect of menthol on patterns and levels of extended access nicotine self-administration was examined by comparing the number of infusions obtained during each of the 10 extended access sessions, and during the first versus the last three (average) extended access sessions using two-way mixed models ANOVA with self-administration group as the between-subjects factor and session as the within-subjects factor. Percent change in the number of infusions obtained from the first to the last three (average) extended access sessions was also compared between groups using a univariate ANOVA. The mean number of responses on the inactive levers were averaged over the extended access period and compared self-administration groups using a univariate ANOVA. The effect of menthol on the extinction and reinstatement of nicotine-seeking was examined by comparing the number of responses made on formerly active lever during the first five extinction sessions, during the first versus the fifth extinction session, and during the last extinction session versus the reinstatement session using separate two-way mixed models ANOVA with self-administration group as the between-subjects factor and session as the within-subjects factor. The total number of responses on the inactive lever during extinction and reinstatement were compared between self-administration groups using separate univariate ANOVAs. The total number of extinction sessions run were compared between groups using the non-parametric Kruskal-Wallis test since these are ordinal data. Because the number of extinction sessions run differed between subjects and groups (as detailed in the results section), we also compared total extinction responding for all 5–8 sessions completed using univariate ANOVA. A Spearman correlation coefficient was computed to determine the association between the number of extinction sessions run and reinstatement responding. The effect of menthol on body weight was examined by comparing each of the group’s weights (as measured three times/week) beginning prior to the first extended access session (session 1), and then during extended access self-administration (sessions 3, 5, 8, and 10) and abstinence (day 2, 5, 7, and 9) using two-way mixed models ANOVA with self-administration group as the between-subjects factor and session as the within-subjects factor. Following a significant overall or interactive effect of group, post-hoc comparison to controls were first made to the Ment group as negative control (versus each of the groups that self-administered nicotine alone or with menthol), and then to the Nic group as a positive control (versus each of the groups that self-administered nicotine plus menthol) using separate Dunnett’s t-tests (or Dunn’s test following the Kruskal-Wallis). This multiple comparison procedure has been used in previous studies for the comparison of positive versus negative controls since they address conceptually different questions (Li et al. 2005; Ohlsson et al. 2014). Comparisons of effects over time/sessions were made using Bonferroni-adjusted paired t-tests.
The effect of menthol on PR responding for nicotine was examined by comparing the average number of infusions obtained at baseline (averaged across the three stable PR sessions) versus those obtained during each of the three PR test sessions between the two self-administration groups (Nic versus Nic+Mentmod) using two-way mixed models ANOVA. Percent change from the average number of infusions obtained at baseline as compared to during each of the test sessions was also compared between the two groups using two-way mixed models ANOVA. The effect of menthol on body weight was examined by comparing the weight of each rat prior to the first test session (i.e., during baseline) versus at the end of the testing phase using two-way mixed models ANOVA. Unpaired t-tests were used for comparisons between the two groups, and Bonferroni-adjusted paired t-tests were used to examine effects over time/sessions within each group. Differences were considered statistically significant at P < 0.05. All data were analyzed using SPSS-24.
Results
Effect of menthol on nicotine self-administration under extended access conditions
Levels of self-administration were highest during the first day of extended access self-administration, but decreased to lower levels over the 10-day period (Figure 2a). Levels of self-administration were also higher in the groups self-administering nicotine (alone or with menthol) as compared to the Ment group (Figure 2b). A two-way mixed models ANOVA comparing the number of infusions obtained during the extended access self-administration period between self-administration groups revealed a significant overall effect of day (F (9, 558) = 9.43, P < 0.001) and group (F (4, 62) = 10.65, P < 0.001). Post-hoc comparison of the average number of infusions obtained over the 10-day extended access period revealed significant differences between the Ment group and all other groups (P < 0.05). No significant differences, however, were observed in comparison to the Nic group (P’s > 0.05). We also observed a significant interaction of day by group (F (36, 558) = 1.52, P < 0.05); however, this effect also appears to be attributable to the Ment group as all post-hoc comparisons within the first day and the last three days were significantly different from the Ment group (P’s < 0.05), whereas, no differences were observed as compared to the Nic group (P’s > 0.05). Levels of inactive lever responding over the 10-day extended access period were relatively low (average responses: 21.0 ± 9.9, 21.8 ± 8.0, 20.9 ± 5.7, 17.5 ± 6.5, and 14 ± 6.1 for the Nic, Nic+Mentlow, Nic+Mentmod, Nic+Menthigh, and Ment groups), and did not differ significantly between groups (F (4, 62) = 0.166, P > 0.05). An analysis of percent change in the number of infusions from the first day to the average of the last three days also revealed a non-significant effect of group (P > 0.05) indicating that menthol did not impact the pattern of nicotine self-administration. Thus, menthol did not affect patterns or levels of nicotine self-administration under extended access conditions.
Figure 2:
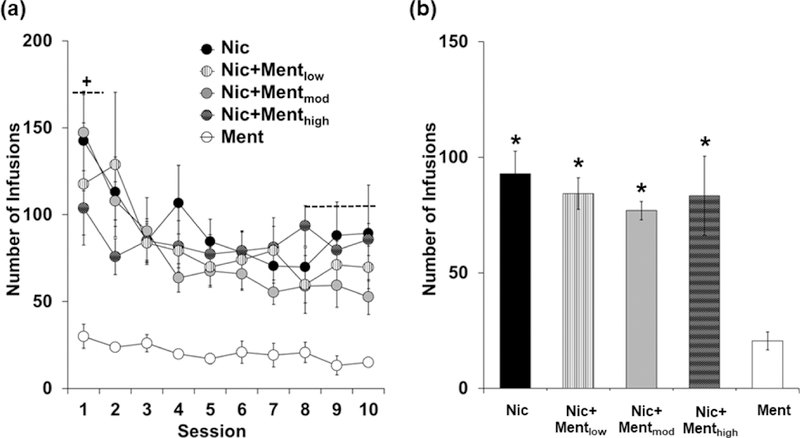
Menthol does not impact patterns or levels of nicotine self-administration under extended access conditions. (a) Mean (± SEM) number of infusions self-administered for the Nic (n=11; black circles), Ment (n=11; white circles), Nic+Mentlow (n=13; white circles with black stripes), Nic+Mentmod (n=16; light gray circles) and Nic+Menthigh (n=16; dark gray circles with grid pattern) groups during each of the 10 extended access sessions. A plus (+) sign with dashed lines indicates a significant difference in the number of infusions obtained in the first extended access session as compared to the last three (average) sessions. (b) Mean (± SEM) number of infusions obtained averaged over the 10 extended access sessions for the Nic (black bar), Ment (white bar), Nic+Mentlow (white bar with black stripes), Nic+Mentmod (light gray bar) and Nic+Menthigh (dark gray bar with grid pattern) groups. An asterisk (*) indicates a significant difference from the Ment group.
Effect of menthol on subsequent extinction and cue-induced reinstatement of nicotine-seeking
Although menthol did not affect subsequent patterns of extinction responding, there was a tendency for it to decrease levels of extinction responding, particularly at the low dose (Figure 3a, b). Results from the repeated measures ANOVA revealed a significant overall effect of extinction hour (F (4, 244) = 17.88, P < 0.001) and group (F (4, 61) = 3.82, P < 0.01), but a non-significant interaction of extinction hour by group (P > 0.05). In all groups, levels of extinction responding were highest during the first hour of extinction, but decreased progressively over time with post-hoc comparison of responding during the first hour versus the fifth hour of extinction revealing a significant difference (P < 0.001). Post-hoc comparison to the Ment group revealed that levels of responding across the five extinction sessions were significantly higher in the Nic (P < 0.01), the Nic+Mentmod (P < 0.05), and the Nic+Menthigh groups (P < 0.05), but not in the Nic+Mentlow group (P > 0.05). Subsequent comparison to the Nic group also revealed a trend for lower levels of extinction responding in the Nic+Mentlow group (P = 0.09). No differences were observed between the Nic group and the Nic+Mentmod or the Nic+Menthigh groups (P’s > 0.05). Similar findings were observed for total levels of extinction responding during each of the sessions completed (5 to 8 sessions) with results from the univariate ANOVA revealing a significant effect of group (F (4, 61) = 3.10, P < 0.05), and post-hoc comparisons to Ment revealing significantly higher levels of responding in each of the groups (P < 0.05) except the Nic+Mentlow group (P = 0.58). However, no significant differences were observed for total levels of extinction responding in comparison to the Nic group (P’s > 0.05). Levels of inactive lever responding during each of the extinction sessions were relatively low (total responses: 7.4 3.1, 5.2 ± 1.3, 7.4 ± 2.4, 6.4 ± 2.6, and 5.8 ± 1.7, for Nic, Nic + Mentlow, Nic + Mentmod, Nic + Menthigh, and Ment, respectively) and did not differ significantly between groups (F (4, 66) = 1.46, P > 0.05). Thus, while a history of menthol-nicotine self-administration did not affect subsequent extinction responding, self-administration of the low menthol dose in combination with nicotine tended to decrease subsequent extinction responding to a level comparable to that observed in the Ment group.
Figure 3:
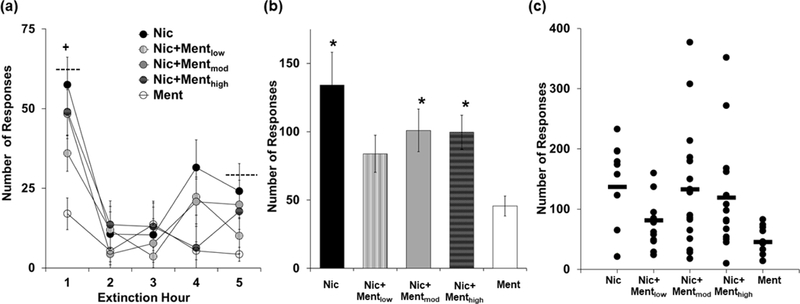
Menthol modestly impacts extinction responding. (a) Mean (± SEM) number of responses during each of the 5 extinction sessions for the Nic (n=10; black circles), Ment (n=11; white circles), Nic+Mentlow (n=13; white circles with black stripes), Nic+Mentmod (n=16; light gray circles) and Nic+Menthigh (n=16; dark gray circles with grid pattern) groups. A plus (+) sign with dashed lines indicates a significant difference in the number of responses in the first versus the fifth extinction session. (b) Mean (± SEM) total number of responses during each of the 5–8 completed extinction sessions for the Nic (black bar), Ment (white bar), Nic+Mentlow (white bar with black stripes), Nic+Mentmod (light gray bar) and Nic+Menthigh (dark gray bar with grid pattern) groups. An asterisk (*) indicates a significant difference from the Ment group. (c) Scatter plot of the total number of extinction responses for each animal within each of the groups.
It’s important to note that although the moderate and high doses of menthol did not significantly impact subsequent levels or patterns of extinction responding, we did observe the highest levels of responding in these groups (Figure 3c). Specifically, 2 animals in the Nic+Mentmod responded 308 and 377 times during extinction, which is approximately 125 and 175% higher than the average observed in the Nic group, and 2 of the animals in the Nic+Menthigh group responded 272 and 352 times, which is approximately 100 and 157% higher than the average observed in the Nic group. The average number of extinction sessions run was also highest in the Nic+Mentmod and Nic+Menthigh groups (6.2 ± 0.3 and 5.7 ± 0.3, respectively) as compared to the Nic (5.4 ± 0.2), Nic+Mentlow (5.3 ± 0.1), and Ment groups (5.1 ± 0.1), and although these differences were not significant in comparison to the Nic group (P > 0.05), the overall effect of group from the Kruskal–Wallis analysis was significant (H(4) = 11.33, P < 0.05). Post-hoc comparison to the Ment group was also significant for the Nic+Mentmod (P < 0.05), but not for the Nic+Menthigh group (P > 0.05). It is important to note, however, that all animals were at the same level of non-responsiveness (less than 15 responses/session) prior to reinstatement testing (as described below). There was also no significant association between number of extinction sessions run and reinstatement responding (P > 0.05). Thus, while the effects of the moderate and high menthol doses were not different overall from those observed following nicotine self-administration alone, these doses may increase subsequent nicotine-seeking in a minority of individuals.
Each of the groups showed similar low levels of responding during the last extinction session, and as expected, showed an increase in responding during the cue-induced reinstatement session (Figure 4a). Levels of reinstatement responding were particularly high for the Nic group, and were dose-dependently decreased in rats that formerly self-administered menthol in combinationwith nicotine (Figure 4b). Results from the repeated measures ANOVA showed a significant main effects of session (F (1, 60) = 65.96, P < 0.001), and group (F (4,60) = 2.80, P < 0.05). We also observed a non-significant trend for an interaction of session and group (F (4,60) = 2.21, P = 0.079) indicating that the increase from extinction to reinstatement was similar between the groups. Indeed, comparison of responding during the last extinction session versus the reinstatement session within each group revealed a significant increase for each of the groups (Nic: t (9) = 4.45, P < 0.001; Nic+Mentlow: t (12) = 4.00, P < 0.01; Nic+Mentmod: t (14) = 2.92, P < 0.05; Nic+Menthigh: t (15) = 3.59, P < 0.001; Ment: t (10) = 3.65, P < 0.01). Although no significant group difference was observed during the last extinction session (P > 0.05), a significant effect was observed within the reinstatement session (F (4,60) = 2.54, P < 0.05) with post-hoc comparison to Ment revealing significantly higher levels of responding in the Nic group (P < 0.05), but no other significant differences (P > 0.05). Comparison to the Nic group revealed that reinstatement responding was significantly decreased in the Nic+Menthigh group (P < 0.05). Levels of inactive lever responding during the reinstatement session were relatively low (total responses: 2.0 ± 0.9, 2.8 ± 1.2, 2.2 ± 0.8, 2.0 ± 0.8, and 2.4 ± 0.9, for Nic, Nic + Mentlow, Nic + Mentmod, and Nic + Menthigh, and Ment, respectively) and did not differ significantly between groups (F = 0.14, P > 0.05). Thus, a history of menthol-nicotine self-administration decreased subsequent cue-induced reinstatement of nicotine-seeking, with the high menthol dose producing a significant decrease.
Figure 4:

Menthol dose-dependently decreases cue-induced reinstatement. (a) Mean (± SEM) number of responses during the last extinction session and the reinstatement session for the Nic (n=10; black bars), Ment (n=11; white bars), Nic+Mentlow (n=13; white bars with black stripes), Nic+Mentmod (n=15; light gray bars) and Nic+Menthigh (n=16; dark gray bars with grid pattern) groups. A plus (+) sign with dashed lines indicates a significant difference in the number of responses during the last extinction session versus the reinstatement session. An asterisk (*) indicates a significant difference from the Ment group. A number sign (#) indicates a significant difference from the Nic group. (b) Scatter plot of the number of reinstatement responses for each animal within each of the groups.
Menthol did not systemically affect body weight during extended access self-administration or abstinence (Figure 5a). While significant effects of day (F (8,496) = 992.3, P < 0.05), and day by group (F (32,496) = 209.7, P < 0.05) were observed from the overall analysis of body weights, there was no significant effect of group (P > 0.05). Subsequent comparison to menthol and nicotine within each of the days also revealed non-significant effects (P’s > 0.05). Menthol also did not systemically affect weight gain over time, and each of the groups gained a similar amount of weight as compared to the Ment and Nic groups (Figure 5b). The two-way mixed models ANOVA comparing percent change in body weight at the start of the extended access self-administration period to the end of abstinence revealed a significant effect of day (F (1,62) = 1431.1, P < 0.05), but non-significant effects of group (F (4,62) = 0.371, P > 0.05) and day and group (F (4,62) = 0.276, P > 0.05). Thus, menthol-nicotine self-administration did not differentially impact weight gain over time as compared to nicotine or menthol self-administration alone.
Figure 5:
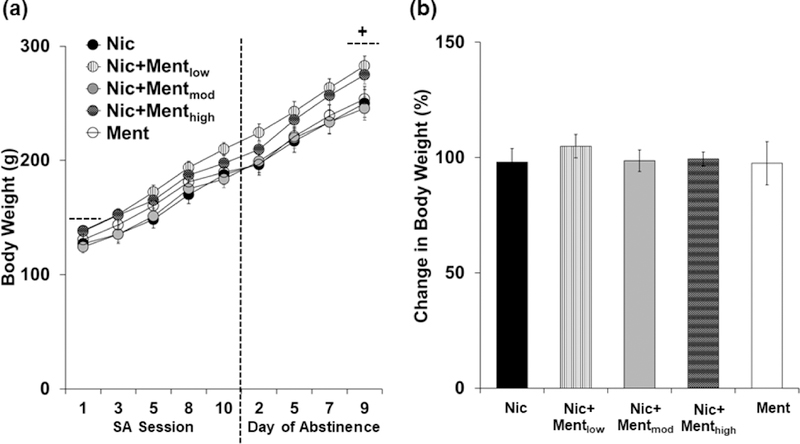
Menthol does not impact body weight during extended access self-administration or abstinence. (a) Mean (± SEM) body weights prior to the first extended access session (session 1), and then throughout extended access self-administration (sessions 3,5,8,10) and abstinence (days 2,5,7,9) for the Nic (n=11; black circles), Ment (n=11; white circles), Nic+Mentlow (n=13; white circles with black stripes), Nic+Mentmod (n=16; light gray circles) and Nic+Menthigh (n=16; dark gray circles with grid pattern) groups. A plus (+) sign with dashed lines indicates a significant difference in the body weights prior to the first extended access session versus the last day of abstinence. (b) Mean (± SEM) percent change in body weight from the first extended access session to the last day of abstinence for the Nic (black bar), Ment (white bar), Nic+Mentlow (white bar with black stripes), Nic+Mentmod (light gray bar) and Nic+Menthigh (dark gray bar with grid pattern) groups.
Effect of menthol on nicotine self-administration under a PR schedule
When menthol was added to the nicotine solutions for the Nic+Mentmod group, the number of infusions decreased from approximately 8 to 6 infusions (~30% decrease); whereas, no difference was observed for the group that continued to self-administer nicotine alone (Nic; Figure 6a). A two-way mixed models ANOVA comparing the average number of infusions obtained at baseline versus the average obtained during the testing phase revealed a significant effect of session (F (3,48) = 7.24, P < 0.001), interaction of session and group (F (3,48) = 3.87, P < 0.05), but a non-significant effect of group (P > 0.05). Although no significant differences were observed between the two groups for the number of infusions obtained at baseline or during testing (P’s > 0.05), subsequent analysis within each group revealed a significant effect of session for the Nic+Mentmod group (F (3,48) = 14.8, P < 0.001), but not for the Nic group (P > 0.05). Further analysis within the Nic+Mentmod group revealed significant decreases from baseline for each of the test sessions (session 1: t (8) = 4.83, P < 0.01; session 2: t (8) = 5.45, P < 0.01; session 3: t (8) = 6.03, P < 0.001). Similarly, an analysis of percent change from baseline revealed a significant effect of group (F (1,16) = 14.4, P < 0.01), but non-significant effects of session and session x group (P’s > 0.05) indicating that the addition of menthol induced a similar decrease in nicotine self-administration across each of the test sessions (Figure 6b). Thus, menthol decreased nicotine’s reinforcing effects.
Figure 6:
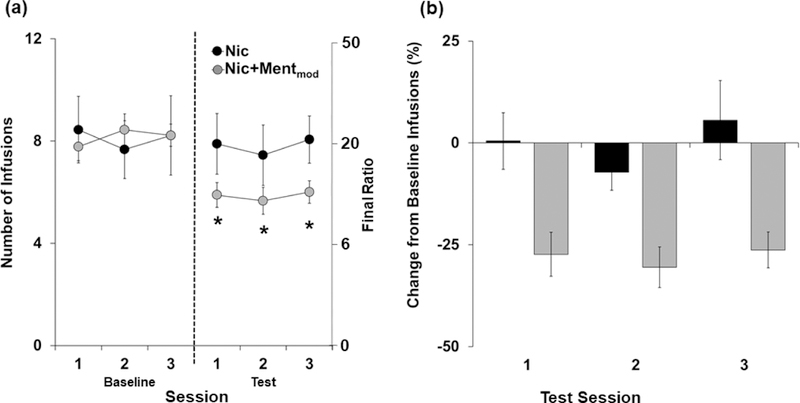
Menthol decreases nicotine’s reinforcing effects as assessed under a progressive ratio (PR) schedule. (a) Mean (± SEM) number of infusions obtained during the three baseline sessions versus the three test sessions for the Nic (n=9; black circles) and Nic+Mentmod (n=9; light gray circles) groups. An asterisk (*) indicates a significant difference from baseline for the Nic+Mentmod group. (b) Mean (± SEM) percent change from the baseline number of infusions for each of the test sessions and groups (nicotine = black bar; Nic+Mentmod = gray bar).
The Nic and Nic+Mentmod groups also gained a similar amount of weight from the start to the end of the test period (27 ± 5 g versus 30 ± 5 g, respectively), and no significant overall or interactive effects of group were observed for body weights at baseline versus test (P’s > 0.05).
Discussion
Here we assessed whether menthol, at one of three doses added into IV nicotine solutions, would enhance nicotine’s reinforcing effects, levels of intake, and subsequent relapse vulnerability. In contrast to our hypothesis, concurrent delivery of menthol and nicotine did not cause an increase in nicotine self-administration under extended access conditions; intake was similar between each of the groups that self-administered nicotine (alone or in combination with menthol), and each of these groups obtained more infusions as compared to the menthol alone group. Menthol at the moderate dose also decreased nicotine self-administration under the PR schedule indicating that it decreases nicotine reinforcing effects. Also in contrast to our hypothesis, menthol dose-dependently decreased subsequent reinstatement responding, with the high dose inducing a significant decrease. Effects on extinction responding were more variable, and although not significant, the low dose tended to decrease extinction responding and was not significantly different from menthol alone. However, we did observe the highest levels of extinction responding in rats that formerly self-administered nicotine in combination with the moderate and high doses of nicotine, and although not significant, rats in these groups also took longer to extinguish responding. These effects of menthol do not appear to be due to anorectic or other general health effects as menthol-nicotine self-administration did not significantly impact body weights as compared to nicotine or menthol self-administration alone. Taken together, these results demonstrate that pharmacological interactions of menthol with nicotine reduce, rather than increase, nicotine’s reinforcing effects and some measure of nicotine-seeking/relapse vulnerability. Importantly, however, moderate and high menthol doses may increase measures of relapse vulnerability in a minority of individuals.
Our findings show that under conditions that mimic the concurrent menthol/nicotine exposure conditions observed in human, menthol reduces nicotine’s reinforcing effects and some measures of relapse vulnerability. These findings are in contrast to recent studies using IP administration to examine pharmacological effects of menthol on IV nicotine self-administration where menthol increased nicotine’s reinforcing effects and/or nicotine-seeking (Biswas et al. 2016; Harrison et al. 2017). While there are numerous procedural differences between these previous studies and the current study, one major difference is the use of short versus extended access nicotine self-administration. Short versus extended access nicotine self-administration is known to induce different behavioral and neurobiological effects. For example, rats given extended versus short access to nicotine show greater intake and motivation for nicotine following protracted abstinence (Cohen et al. 2015a;b), and a prolonged time-course for withdrawal signs (O’Dell et al. 2007), suggesting that extended, but not short access nicotine self-administration, induces an addicted phenotype (Paterson and Markou 2004). However, since we also observed decreases in nicotine’s reinforcing effects under the PR schedule following short access self-administration, it seems unlikely that stage of addiction accounts for the differential behavioral effects observed in the previous studies versus the current study.
A more likely explanation for the discrepancies between our findings with menthol and previous findings is the menthol dosing regimen. Specifically, the concurrent IV delivery of menthol and nicotine used here is likely to induce different pharmacological effects as compared to those induced following the single bolus injection of menthol prior to the nicotine self-administration session used in the previous studies (e.g. Ahmed 2015, Curry 1990). The IV route, like the smoked route, results in rapid central delivery not subject to the first-pass metabolism that systemic administration of drugs undergoes (Curry 1980). Indeed, recent evidence indicates that the menthol delivery method is critical for determining its pharmacological effects and its ability to enhance or reduce nicotine’s rewarding effects as assessed under the conditioned place preference test (Henderson et al. 2017; Henderson et al. 2016). For example, when menthol was administered via an osmotic minipump, which would induce sustained levels of menthol throughout the session, it resulted in the stabilization of lower-sensitivity nAChRs and reduced dopamine neuron excitability; under these conditions, menthol decreased nicotine’s rewarding effects. In contrast, when menthol was administered IP, which would result in moderate initial levels that progressively decrease throughout the session, it upregulated nAChRs in the midbrain dopamine neurons and enhanced neuronal excitability (Henderson et al. 2017); under these conditions, menthol enhanced nicotine’s rewarding effects (Henderson et al. 2016). Our results suggest that when menthol is concurrently delivered with nicotine, it antagonizes nicotine’s reinforcing effects. This interpretation is also consistent with previous in vitro work showing that menthol is a negative allosteric modulator of nAChRs (Ashoor et al. 2013a; Hans et al. 2012), that can inhibit the function of low affinity nAChR subtypes (e.g. α7 nAChR; Ashoor et al.2013) that that are involved in cue-induced reinstatement of nicotine-seeking (Liu 2014). It is also consistent with findings showing that it reduces nicotine’s rewarding effects in the CPP model (Henderson et al. 2016). While these findings suggest that interactions between menthol and nicotine at nAChRs may underlie the attenuating effects of menthol on nicotine use and some measures of relapse vulnerability, future studies are needed further examine this possibility and address the receptor subtypes involved.
It is also possible that the attenuating effects of menthol observed here are mediated independent of nAChRs. For example, results from both clinical and preclinical studies show that menthol inhibits the metabolism of nicotine resulting in enhanced nicotine exposure in the brain (Ahijevych and Garrett 2010; Benowitz et al. 2004; Fagan et al. 2016; Valentine et al. 2018), and this effect may contribute to findings showing that menthol cigarette smokers smoke fewer cigarettes per day, have greater nicotine dependence, and poorer smoking cessation outcomes as compared to regular cigarette smokers (Benowitz et al. 2004; Foulds et al. 2010). Such an effect is unlikely to underlie our current findings, however, since enhanced nicotine expose as the result of reduced nicotine metabolism would be expected to increase rather than decrease nicotine’s reinforcing effects and subsequent nicotine-seeking. However, future studies examining blood levels of menthol and nicotine are necessary to determine the contribution of pharmacokinetic effects of menthol on nicotine use and relapse vulnerability. This is also important since it is difficult to determine the human equivalent dose of menthol in a rat given the absence of a thorough and cross-species pharmacokinetic analysis of menthol and its metabolites.
Although the moderate and high doses of menthol in combination with nicotine did not significantly affect levels of extinction responding, it is noteworthy that the highest levels of extinction responding were observed in animals from these groups (i.e., two animals in each of these groups). Rats in these groups also took longer to extinguish responding, although these differences were also not significant. Taken together, these findings suggest that menthol may prolong extinction and enhance vulnerability through pharmacological interactions with nicotine in a minority of individuals. Individual differences in sensitivity and preference for menthol have been reported in many studies in humans, and vulnerability in humans is believed to be mediated via menthol’s peripheral sensory cue effects (DeVito et al. 2016; Kreslake et al. 2008a; Mustonen et al. 2005; Uhl et al. 2011). Our findings raise the possibility that pharmacological mechanisms, possibly through nAChRs, contribute to menthol’s ability to enhance vulnerability in certain individuals.
Our results should be interpreted with the following limitations in mind. Specifically, since the primary goal of this study was to determine whether menthol enhances vulnerability to nicotine use and relapse through pharmacological interactions, independent of sensory cues effects, both menthol and nicotine were self-administered IV. While this route of administration also mimics the concurrent rapid delivery of menthol and nicotine that is observed in humans following inhalation, effects observed in our study are unlikely to fully generalize to humans where nicotine, menthol, and other tobacco sensory cues contribute to behavior. Another limitation is that since we were primarily interested in the effects of menthol on subsequent relapse vulnerability following extended access self-administration, menthol was introduced after the acquisition of self-administration. This is in contrast to the human situation where menthol cigarette smokers generally initiate with mentholated cigarettes (Villanti et al. 2012). Future studies are thus necessary to explore the effect of menthol on the acquisition of nicotine self-administration. An additional limitation is that our study focused on just one form of menthol, DL-menthol, whereas, tobacco products contain this form as well as the L and D isomers. Recent work indicates that the pharmacological effects differ between the different isomers (Ashoor et al. 2013a), and these differences may contribute to divergent results observed with menthol in tobacco/nicotine research. Future research is thus necessary to investigate the effects of different menthol isomers on nicotine use and relapse vulnerability.
In conclusion, these findings demonstrate that pharmacologically-mediated effects of menthol reduce nicotine’s reinforcing effects as well as some measures of relapse vulnerability, and suggest that the enhanced vulnerability observed in human menthol cigarette smokers is either inherent to the populations using mentholated cigarettes or is mediated via sensory cues independent of pharmacological interactions of menthol and nicotine. Future research is needed to determine whether these pharmacological effects can be negated, or reversed, via sensory cue effects as would be expected based on the epidemiological findings. Future research is also needed to follow up on our findings indicating that pharmacologically-mediated effects of menthol may enhance some aspects of relapse vulnerability in a minority of individuals.
Acknowledgements:
We would like to acknowledge Rebecca Beiter and Elizabeth Gasteiger for technical assistance. This study was supported by the Virginia Commonwealth University’s Center for the Study of Tobacco Products Pilot Research Program, the University of Virginia’s 4-VA Innovation Grant Project (WJL), and the National Institute on Drug Abuse (R01DA024716; WJL).
Footnotes
Conflict of interest:
The authors declare that they have no conflict of interest.
References
- Abdolahi A, Acosta G, Breslin FJ, Hemby SE, Lynch WJ (2010) Incubation of nicotine seeking is associated with enhanced protein kinase A-regulated signaling of dopamine- and cAMP-regulated phosphoprotein of 32 kDa in the insular cortex. Eur J Neurosci 31: 733–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahijevych K, Garrett BE (2010) The role of menthol in cigarettes as a reinforcer of smoking behavior. Nicotine Tob Res 12 Suppl 2: S110–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed TA (2015) Pharmacokinetics of drugs following IV bolus, IV infusion, and oral administration. INTECH Chapter 3, Basic Pharmacokinetic Concepts and Some Clinical Applications
- Ai J, Taylor KM, Lisko JG, Tran H, Watson CH, Holman MR (2018) Menthol levels in cigarettes from eight manufacturers. Tob Control 27: 335–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alsharari SD, King JR, Nordman JC, Muldoon PP, Jackson A, Zhu AZX, Tyndale RF, Kabbani N, Damaj MI (2015) Effects of menthol on nicotine pharmacokinetic, pharmacology and dependence in mice. Plos One 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson SJ (2011) Menthol cigarettes and smoking cessation behaviour: a review of tobacco industry documents. Tob Control 20 Suppl 2: ii49–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Appleton S (1986) Proposal for the investigation of menthol pharmacokinetics by the intravenous, oral, and inhalation routes. RJR interoffice Memorandum Accessed on April 2014.
- Arnold JM, Roberts DCS (1997) A critique of fixed and progressive ratio schedules used to examine the neural substrates of drug reinforcement. Pharmacol Biochem Be 57: 441–447. [DOI] [PubMed] [Google Scholar]
- Ashoor A, Nordman JC, Veltri D, Yang KH, Al Kury L, Shuba Y, Mahgoub M, Howarth FC, Sadek B, Shehu A, Kabbani N, Oz M (2013a) Menthol binding and inhibition of alpha7-nicotinic acetylcholine receptors. PLoS One 8: e67674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashoor A, Nordman JC, Veltri D, Yang KH, Shuba Y, Al Kury L, Sadek B, Howarth FC, Shehu A, Kabbani N, Oz M (2013b) Menthol inhibits 5-HT3 receptor-mediated currents. J Pharmacol Exp Ther 347: 398–409. [DOI] [PubMed] [Google Scholar]
- Azagba S, Minaker LM, Sharaf MF, Hammond D, Manske S (2014) Smoking intensity and intent to continue smoking among menthol and non-menthol adolescent smokers in Canada. Cancer Cause Control 25: 1093–1099. [DOI] [PubMed] [Google Scholar]
- Biswas L, Harrison E, Gong Y, Avusula R, Lee J, Zhang M, Rousselle T, Lage J, Liu X (2016) Enhancing effect of menthol on nicotine self-administration in rats. Psychopharmacology (Berl) 233: 3417–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brody AL, Mukhin AG, La Charite J, Ta K, Farahi J, Sugar CA, Mamoun MS, Vellios E, Archie M, Kozman M, Phuong J, Arlorio F, Mandelkern MA (2013) Up-regulation of nicotinic acetylcholine receptors in menthol cigarette smokers. Int J Neuropsychopharmacol 16: 957–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carchman RA, Southwick MA (1990) Chemical senses research a research and development perspective. Phillip Morris
- Cohen A, Soleiman MT, Talia R, Koob GF, George O, Mandyam CD (2015a) Extended access nicotine self-administration with periodic deprivation increases immature neurons in the hippocampus. Psychopharmacology (Berl) 232: 453–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen A, Treweek J, Edwards S, Leão RM, Schulteis G, Koob GF, George O (2015b) Extended access to nicotine leads to a CRF1 receptor dependent increase in anxiety-like behavior and hyperalgesia in rats. Addict Biol 20(1):56–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courtemanche CJ, Palmer MK, Pesko MF (2017) Influence of the flavored cigarette ban on adolescent tobacco use. Am J Prev Med 52: e139–e146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curry SH (1980) Drug Disposition and Pharmacokinetics Oxford: Blackwell Scientific. [Google Scholar]
- DeVito EE, Valentine GW, Herman AI, Jensen KP, Sofuoglu M (2016) Effect of menthol-preferring status on response to intravenous nicotine. Tob Regul Sci 2: 317–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fait BW, Thompson DC, Mose TN, Jatlow P, Jordt SE, Picciotto MR, Mineur YS (2017) Menthol disrupts nicotine’s psychostimulant properties in an age and sex-dependent manner in C57BL/6J mice. Behav Brain Res 334: 72–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan L, Balakrishna S, Jabba SV, Bonner PE, Taylor SR, Picciotto MR, Jordt SE (2016) Menthol decreases oral nicotine aversion in C57BL/6 mice through a TRPM8-dependent mechanism. Tob Control 25: ii50–ii54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FDA (2011) The physiological effects of menthol cigarettes, Food and Drug Administration Chapter 3 1–12.
- Hans M, Wilhelm M, Swandulla D (2012) Menthol suppresses nicotinic acetylcholine receptor functioning in sensory neurons via allosteric modulation. Chem Senses 37: 463–469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison E, Biswas L, Avusula R, Zhang M, Gong Y, Liu X (2017) Effects of menthol and its interaction with nicotine-conditioned cue on nicotine-seeking behavior in rats. Psychopharmacology (Berl) [DOI] [PMC free article] [PubMed]
- Heck JD (2010) A review and assessment of menthol employed as a cigarette flavoring ingredient. Food and Chemical Toxicology 48: S1–S38. [DOI] [PubMed] [Google Scholar]
- Henderson BJ, Wall TR, Henley BM, Kim CH, McKinney S, Lester HA (2017) Menthol enhances nicotine reward-related behavior by potentiating nicotine-induced changes in nAChR function, nAChR upregulation, and DA neuron excitability. Neuropsychopharmacology 42: 2285–2291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson BJ, Wall TR, Henley BM, Kim CH, Nichols WA, Moaddel R, Xiao C, Lester HA (2016) Menthol alone upregulates midbrain nAChRs, alters nAChR subtype stoichiometry, alters dopamine neuron firing frequency, and prevents nicotine reward. J Neurosci 36: 2957–2974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hersey JC, Ng SW, Nonnemaker JM, Mowery P, Thomas KY, Vilsaint MC, Allen JA, Haviland ML (2006) Are menthol cigarettes a starter product for youth? Nicotine Tob Res 8: 403–413. [DOI] [PubMed] [Google Scholar]
- Hilario MR, Turner JR, Blendy JA (2012) Reward sensitization: effects of repeated nicotine exposure and withdrawal in mice. Neuropsychopharmacology 37: 2661–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klausner K (2011) Menthol cigarettes and smoking initiation: a tobacco industry perspective. Tob Control 20 Suppl 2: ii12–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreslake JM, Wayne GF, Alpert HR, Koh HK, Connolly GN (2008a) Tobacco industry control of menthol in cigarettes and targeting of adolescents and young adults. Am J Public Health 98: 1685–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreslake JM, Wayne GF, Connolly GN (2008b) The menthol smoker: tobacco industry research on consumer sensory perception of menthol cigarettes and its role in smoking behavior. Nicotine Tob Res 10: 705–15. [DOI] [PubMed] [Google Scholar]
- Lawrence D, Cadman B, Hoffman AC (2011) Sensory properties of menthol and smoking topography. Tob Induc Dis 9 Suppl 1: S3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy DT, Blackman K, Tauras J, Chaloupka FJ, Villanti AC, Niaura RS, Vallone DM, Abrams DB (2011) Quit attempts and quit rates among menthol and nonmenthol smokers in the United States. Am J Public Health 101: 1241–1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li SM, Newman AH, Katz JL (2005) Place conditioning and locomotor effects of N-substituted, 4’,4’’-difluorobenztropine analogs in rats. J Pharmacol Exp Ther 313: 1223–30. [DOI] [PubMed] [Google Scholar]
- Lynch WJ (2008) Acquisition and maintenance of cocaine self-administration in adolescent rats: effects of sex and gonadal hormones. Psychopharmacology (Berl) 197: 237–46. [DOI] [PubMed] [Google Scholar]
- Mustonen TK, Spencer SM, Hoskinson RA, Sachs DPL, Garvey AJ (2005) The influence of gender, race, and menthol content on tobacco exposure measures. Nicotine & Tobacco Research 7: 581–590. [DOI] [PubMed] [Google Scholar]
- Nair AB, Jacob S (2016) A simple practice guide for dose conversion between animals and human. J Basic Clin Pharm 2: 27–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Dell LE, Chen SA, Smith RT, Specio SE, Balster RL, Paterson NE, Markou A, Zorrilla EP, Koob GF (2007) Extended access to nicotine self-administration leads to dependence: Circadian measures, withdrawal measures, and extinction behavior in rats. J Pharmacol Exp Ther 320: 180–93. [DOI] [PubMed] [Google Scholar]
- Ohlsson C, Engdahl C, Fak F, Andersson A, Windahl SH, Farman HH, Moverare-Skrtic S, Islander U, Sjogren K (2014) Probiotics protect mice from ovariectomy-induced cortical bone loss. PLoS One 9: e92368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paterson NE, Markou A (2004) Prolonged nicotine dependence associated with extended access to nicotine self-administration in rats. Psychopharmacology (Berl) 173: 64–72. [DOI] [PubMed] [Google Scholar]
- Perkins KA, Karelitz JL (2014) Sensory Reinforcement-Enhancing Effects of Nicotine Via Smoking. Exp Clin Psychopharm 22: 511–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pletcher MJ, Hulley BJ, Houston T, Kiefe CI, Benowitz N, Sidney S (2006) Menthol cigarettes, smoking cessation, atherosclerosis, and pulmonary function - The Coronary Artery Risk Development in Young Adults (CARDIA) Study. Arch Intern Med 166: 1915–1922. [DOI] [PubMed] [Google Scholar]
- Rath JM, Teplitskaya L, Williams VF, Pearson JL, Vallone DM, Villanti AC (2017) Correlates of e-cigarette ad awareness and likeability in U.S. young adults. Tob Induc Dis 15: 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reagan-Shaw S, Nihal M, Ahmad N (2008) Dose translation from animal to human studies revisited. Faseb J 22: 659–661. [DOI] [PubMed] [Google Scholar]
- Rodger A, Lewis TL (1979) The registry of toxic effects of chemical substances. NIOSH v2: 34. [Google Scholar]
- Rose JE, Behm FM, Westman EC, Johnson M (2000) Dissociating nicotine and nonnicotine components of cigarette smoking. Pharmacol Biochem Be 67: 71–81. [DOI] [PubMed] [Google Scholar]
- Sanchez V, Lycas MD, Lynch WJ, Brunzell DH (2015) Wheel running exercise attenuates vulnerability to self-administer nicotine in rats. Drug Alcohol Depend 156: 193–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez V, Moore CF, Brunzell DH, Lynch WJ (2013) Effect of wheel-running during abstinence on subsequent nicotine-seeking in rats. Psychopharmacology (Berl) 227: 403–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez V, Moore CF, Brunzell DH, Lynch WJ (2014) Sex differences in the effect of wheel running on subsequent nicotine-seeking in a rat adolescent-onset self-administration model. Psychopharmacology 231: 1753–1762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stowe M (1976) Quarterly section research report Reynolds RJ. [Google Scholar]
- Thompson MF, Poirier GL, Davila-Garcia MI, Huang W, Tam K, Robidoux M, Dubuke ML, Shaffer SA, Colon-Perez L, Febo M, DiFranza JR, King JA (2018) Menthol enhances nicotine-induced locomotor sensitization and in vivo functional connectivity in adolescence. J Psychopharmacol 32: 332–343. [DOI] [PubMed] [Google Scholar]
- Ton HT, Smart AE, Aguilar BL, Olson TT, Kellar KJ, Ahern GP (2015) Menthol enhances the desensitization of human alpha 3 beta 4 nicotinic acetylcholine receptors. Molecular Pharmacology 88: 256–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhl GR, Walther D, Behm FM, Rose JE (2011) Menthol preference among smokers: association with TRPA1 variants. Nicotine & Tobacco Research 13: 1311–1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valentine JD, Hokanson JS, Matta SG, Sharp BM (1997) Self-administration in rats allowed unlimited access to nicotine. Psychopharmacology 133: 300–304. [DOI] [PubMed] [Google Scholar]
- Villanti AC, Giovino GA, Barker DC, Mowery PD, Sevilimedu V, Abrams DB (2012) Menthol brand switching among adolescents and young adults in the National Youth Smoking Cessation Survey. American Journal of Public Health 102: 1310–1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villanti AC, Mowery PD, Delnevo CD, Niaura RS, Abrams DB, Giovino GA (2016a) Changes in the prevalence and correlates of menthol cigarette use in the USA, 2004–2014. Tob Control 25: ii14–ii20. [DOI] [PubMed] [Google Scholar]
- Villanti AC, Rath JM, Williams VF, Pearson JL, Richardson A, Abrams DB, Niaura RS, Vallone DM (2016b) Impact of exposure to electronic cigarette advertising on susceptibility and trial of electronic cigarettes and cigarettes in US young adults: a randomized controlled trial. Nicotine Tob Res 18: 1331–9. [DOI] [PubMed] [Google Scholar]
- Wackowski O, Delnevo CD (2007) Menthol cigarettes and indicators of tobacco dependence among adolescents. Addict Behav 32: 1964–9. [DOI] [PubMed] [Google Scholar]
- Wang T, Wang B, Chen H (2014) Menthol facilitates the intravenous self-administration of nicotine in rats. Front Behav Neurosci 8: 437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westman EC, Behm FM, Rose JE (1996) Dissociating the nicotine and airway sensory effects of smoking. Pharmacol Biochem Be 53: 309–315. [DOI] [PubMed] [Google Scholar]
- WHO (2016) Banning menthol in tobacco products
- Wickham RJ, Nunes EJ, Hughley S, Silva P, Walton SN, Park J, Addy NA (2018) Evaluating oral flavorant effects on nicotine self-administration behavior and phasic dopamine signaling. Neuropharmacology 128: 33–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willis DN, Liu B, Ha MA, Jordt SE, Morris JB (2011) Menthol attenuates respiratory irritation responses to multiple cigarette smoke irritants. FASEB J 25: 4434–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yingst JM, Veldheer S, Hammett E, Hrabovsky S, Foulds J (2017) Should electronic cigarette use be covered by clean indoor air laws? Tob Control 26: e16–e18. [DOI] [PMC free article] [PubMed] [Google Scholar]


