Abstract
Branched chain amino acids (BCAAs) are building blocks for all life-forms. We review here the fundamentals of BCAA metabolism in mammalian physiology. Decades of studies have elicited a deep understanding of biochemical reactions involved in BCAA catabolism. In addition, BCAAs and various catabolic products act as signaling molecules, activating programs ranging from protein synthesis to insulin secretion. How these processes are integrated at an organismal level is less clear. Inborn errors of metabolism highlight the importance of organismal regulation of BCAA physiology. More recently, subtle alterations of BCAA metabolism have been suggested to contribute to numerous prevalent diseases, including diabetes, cancer, and heart failure. Understanding the mechanisms underlying altered BCAA metabolism and how they contribute to disease pathophysiology will keep researchers busy for the foreseeable future.
Keywords: branched chain amino acids, catabolism, diabetes, cancer, heart disease
THE BIOCHEMISTRY OF BRANCHED CHAIN AMINO ACIDS
Branched chain amino acids (BCAAs) cannot be synthesized by metazoans. Despite this, they are abundant components of animals, constituting approximately 35% of essential amino acids in most mammals (1–3). The functional R groups of all three BCAAs are branched (hence their name), small, and hydrophobic, rendering them critical components of most protein (4, 5). Together, BCAAs account for about 18% of amino acids and 63% of hydrophobic amino acids in protein across many life-forms (1–3, 6). The molar relative abundance of BCAAs to each other is nearly always approximately 1.6:2.2:1.0 Val:Leu:Ile, reflecting the linked nature of their synthesis and oxidation (see below). The three BCAAs are thus almost always eaten and combusted together (7, 8), and as such are also almost always studied as one entity, despite significant differences in their biological effects as outlined below. This tendency has often led to erroneous assumptions.
Synthesis
BCAAs are synthesized in bacteria, plants, and fungi, but not in animals. The synthesis of valine and isoleucine is carried out by the same enzymes, and leucine is created from α-ketoisovalerate, a transamination precursor of valine (Figure 1) (9). The carbons in valine (and leucine) are derived from the readily available and abundant pyruvate, but isoleucine carbons are derived from the relatively rare threonine, again reflecting the conserved ratio of abundance in protein. Because it does not occur in animals, BCAA synthesis has been successfully targeted for antimicrobials, herbicides, and antifungal agents (10).
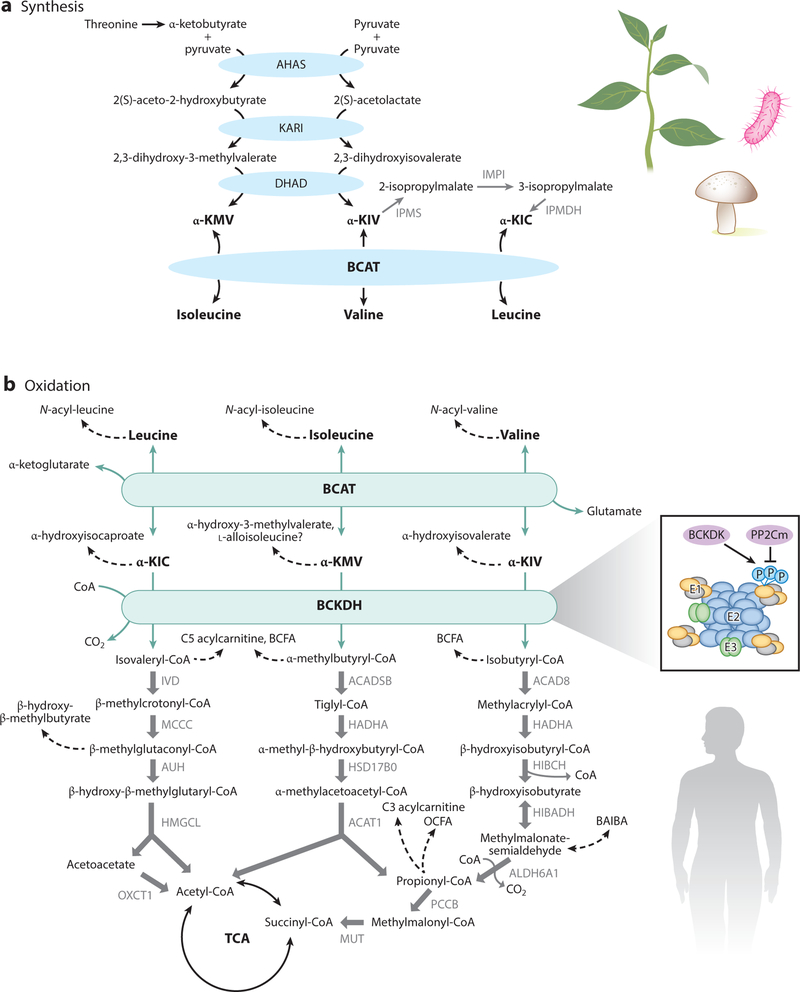
Figure 1.
BCAA synthesis and catabolism. Synthesis (a) occurs in plants, bacteria, and fungi. Oxidation (b) occurs in plants, bacteria, fungi, and animals. All three BCAAs share the BCAT and BCKDH steps, after which catabolism of each BCAA is unique. The BCKDH complex is composed of a core of 24 E2 subunits, which are docked by E1 heterotetamers and E3 dimers. BCKDK inhibits E1 via phosphorylation, which is reversed by PP2Cm. Abbreviations: ACAD8, acyl-CoA dehydrogenase family member 8; ACADSB, short/branched chain acyl-CoA dehydrogenase; ACAT1, acetyl-CoA acetyltransferase 1; AHAS, acetohydroxyacid synthase; α-KIC, α-ketoisocaproic acid; α-KIV, α-ketoisovaleric acid; α-KMV, α-ketomethylvaleric acid; ALDH6A1, aldehyde dehydrogenase 6 family member A1; AUH, AU RNA-binding protein/enoyl-coenzyme A hydratase; BAIBA, beta-amino-isobutyric acid; BCAA, branched chain amino acid; BCAT, branched chain amino transferase; BCFA, branched chain fatty acid; BCKDH, branched chain amino acid dehydrogenase; BCKDK, BCKDH kinase; CoA, coenzyme A; DHAD, dihydroxyacid dehydratase; HADHA, hydroxyacyl-CoA dehydrogenase subunit alpha; HIBADH, 3-hydroxyisobutyrate dehydrogenase; HIBCH, 3-hydroxyisobutyryl-CoA hydrolase; HMGCL, 3-hydroxymethyl-3-methylglutaryl-CoA lyase; HSD17B0, 2-methyl-3-hydroxybutyryl-CoA dehydrogenase; IPMDH, isopropylmalate dehydrogenase; IPMI, isopropylmalate isomerase; IPMS, isopropylmalate synthase; IVD, isovaleryl-CoA dehydrogenase; MCCC, methylcrotonoyl-CoA carboxylase; MUT, methylmalonyl-CoA mutase; OCFA, odd-chain fatty acid; OXCT1, 3-oxoacid CoA transferase; P, phosphorylation; PCCB, propionyl-CoA carboxylase subunit beta.
Catabolism
All life-forms catabolize BCAAs in a similarly linked process (Figure 1). In mammals, all three BCAAs are initially transaminated by branched chain amino transferases (BCATs) to form branched chain α-ketoacids (BCKAs) (11). The most common nitrogen acceptor is α-ketoglutarate (αKG), yielding glutamate (12). This reaction is rapid with a low free energy change and is thus likely in equilibrium in most cases. There is, however, a preference for the reverse reaction because (a) the Km for BCKAs is in the 100-μM range, while that for BCAAs is in the 1-mM range (somewhat higher for valine) (12, 13); and (b) the concentration of intracellular glutamate is typically relatively high (14). Two genes encode BCATs: BCAT1 (or cBCAT) encodes a cytoplasmic protein and is primarily expressed in the brain, whereas BCAT2 (or mBCAT) encodes a mitochondrial protein and is ubiquitously expressed (12, 15).
Irreversible initiation of BCKA oxidation occurs in the branched chain amino acid dehydrogenase (BCKDH) complex. The BCKDH complex is found on the inner surface of the inner membrane of mitochondria and shares many attributes with the pyruvate dehydrogenase (PDH) complex (16, 17). Like the PDH complex, BCKDH catalyzes an oxidative decarboxylation, releasing CO2 and covalently adding a coenzyme A (CoA) group to the oxidized BCKA product (18). CoA, a bulky and hydrophilic prosthetic moiety, traps all subsequent intermediates inside the mitochondria with one exemption: 3-hydroxyisobutyrate (3-HIB) in the valine catabolic pathway. The BCKDH complex has three components (19, 20): a thiamin-dependent decarboxylase, existing as an alpha2/beta2 heterotetramer (21), encoded by the BCKDHA and BCKDHB genes, respectively; a lipoate-dependent dihydrolipoyl transacylase that transfers the acyl groups to CoA, encoded by the DBT gene; and a FAD-dependent dihydrolipoyl dehydrogenase that transfers the released electrons to NAD+ and is encoded by the DLD gene (18). DLD also participates in other complexes, including the PDH complex, and may have moonlighting proteolytic functions (22). The rate of oxidation by the BCKDH complex is thought to be largely proportional to intracellular concentrations of BCKAs, as these are typically well below their Km for the BCKDH complex (~15–30 μM). Also similar to PDH, the BCKDH complex is tightly regulated by phosphory lation/dephosphorylation (16, 19). BCKDH kinase (BCKDK) adds phosphate on three residues of BCKDHA, thereby suppressing BCKDH activity (23–27). The complementary activating dephosphorylation is carried out by the recently identified phosphatase, PP2Cm (28, 29). BCKDK is allosterically suppressed by BCKAs [its greatest affinity is for α-ketoisocaproic acid (α-KIC)], thus allowing elevations in BCKAs to promote their own oxidation (30–33). Efficient product inhibition of BCKDH also occurs by nicotinamide adenine dinucleotide hydrate (NADH) and acyl-CoAs.
Like many sequential metabolic enzymes, BCAT2 and the BCKDH complex can be found physically associated in organized supramolecular complexes, often termed metabolons, allowing for substrate channeling from one enzyme to another. The association of BCKDHA with BCAT2 and the phosphorylation by BCKDK compete for the same loop on BCKDHA, such that BCAT2 binding increases BCKDH activity, while conversely, phosphorylation of BCKDHA destabilizes the interaction with BCAT2 (34).
After BCKDH decarboxylation, subsequent catabolism of BCAAs resembles fatty acid oxidation, and indeed, these two processes share a number of enzymatic subunits. Each set of reactions is mostly unique to each BCAA, and all occur inside the mitochondrial matrix. Ultimately, BCAA carbons are either lost as CO2 or enter the tricarboxylic acid (TCA) cycle. Specifically, valine (5C) loses 2 carbons to CO2 and contributes 3 carbons to the TCA as succinyl-CoA; leucine (6C) loses 1 carbon to CO2 and contributes 5 to the TCA in acetyl-CoAs; and isoleucine (6C) loses 1 carbon to CO2 and contributes 5 to the TCA as acetyl-CoA and succinyl-CoA (see 35 for a carbon tracing diagram). As a consequence, valine is considered glucogenic (i.e., succinyl-CoA is anaplerotic), whereas leucine is considered ketogenic, and isoleucine is mixed.
Catabolic Intermediates and By-Products
As noted above, all of these reactions occur in the mitochondrial matrix, and essentially all intermediate metabolites are trapped within the matrix by the CoA adduct. The main exception is 3-HIB, an intermediate in the valine catabolic pathway. The CoA adduct is removed in the preceding reaction and re-added after the generation of methylmalonic semialdehyde. As a result, 3-HIB can be secreted and is detected in plasma at 10–40-μM concentrations. Another possible exception is the last step of leucine oxidation, which yields acetyl-CoA plus acetoacetate, the latter of which could escape the matrix prior to ketone oxidation. In addition, some alternative metabolites can emanate from BCAA catabolism, although they are poorly studied and likely represent a small fraction of overall BCAA catabolic flux. Prior to oxidation by BCKDH, α-ketoacids can be reduced at the α-carbon to form branched chain α-hydroxy ketoacids (36, 37). These are present in healthy adult urine at very low levels (often below detection) and are directly degraded by α-hydroxyacid oxidases, probably in the liver (38). Additionally, a cytosolic dioxygenase converts a small percentage of α-KIC to beta-hydroxy-beta-methylbutyrate (HMB), which is present in human serum at approximately 2 μM (39). After oxidation by BCKDH, and prior to entry into propionyl-CoA or HMG-CoA, species that are normally bound to CoA can be released as 3-hydroxy acids (3-hydroxy-2-methylbutyric acid, 3-hydroxy-2-ethylpropionic acid, and 3-hydroxyisovaleric acid) (36). In healthy adults, keto-genesis is associated with urinary excretion of these 3-hydroxy acids, although in small amounts. Several intermediates of BCAA oxidation may also contribute to acylation of mitochondrial enzymes, but the biological significance of these side products of BCAA catabolism remains unclear (37).
BCAAs also contribute to the synthesis of several unique lipid species, broadly categorized as N-acyl amino acids, branched chain fatty acids, and odd-chain fatty acids. Mammals appear to be capable of synthesizing all three types. In mammals, at least some N-acyl amino acids are synthesized by the secreted enzyme PM20D1, which covalently couples fatty acids to amino acids by an amide bond (40). Adipocytes and perhaps other cell types can synthesize odd-chain fatty acids by combining propionyl-CoA (with carbons derived from valine or isoleucine) and malonyl-CoA, followed by fatty acid chain extension via fatty acid synthase (35, 41). Fatty acid synthase can also elongate isobutyryl-CoA, isovaleryl-CoA, or 2-methylbutyryl-CoA to form branched chain fatty acids (42). These unique fatty acids are mostly synthesized in brown adipocytes, but their role remains unclear. All of these particular lipid species are present in normal serum but at low concentrations (43). Strikingly, branched chain fatty acids are found at very high levels in vernix caseosa, the white waxy substance found on newborn human skin, constituting 30% of fatty acid content (44).
SIGNALING BY BRANCHED CHAIN AMINO ACIDS AND THEIR CATABOLITES
mTOR
In addition to their structural and metabolic roles, BCAAs and many of their metabolites also have important allosteric regulatory and signaling effects. The best studied of these is the regulation of the mechanistic target of rapamycin (mTOR) pathway by leucine. Numerous cellular processes, including most notably protein synthesis and cellular growth, are controlled by the ubiquitous multiunit mTORC1 complex, and leucine is a potent activator of mTORC1 activity. The role of leucine as a growth-regulatory signal was first established in early experiments demonstrating that leucine stimulates muscle protein synthesis in vitro (45–47) and in perfused skeletal muscle preparations (47). Leucine (but not valine, isoleucine, or the BCKAs) promotes mTORC1 activation by directly binding Sestrin2 a negative regulator of mTORC1 activity (48) (Figure 2a). In the absence of leucine, Sestrin2 binds and inhibits GATOR2, a positive regulator of mTORC1 activity. When leucine is available at physiologically relevant concentrations, Sestrin2 releases GATOR2, promoting full mTORC1 activation (49). Upon activation, mTORC1 promotes protein synthesis and inhibits autophagy by phosphorylating several targets, including S6K, 4E-BP1, Ulk1, and TFEB/3. Leucine likely activates mTORC1 via other mechanisms as well, including via loaded leucyl tRNA synthetase (50–52). For a full discussion of mTOR signaling, we refer the reader to a number of excellent reviews (53–56).
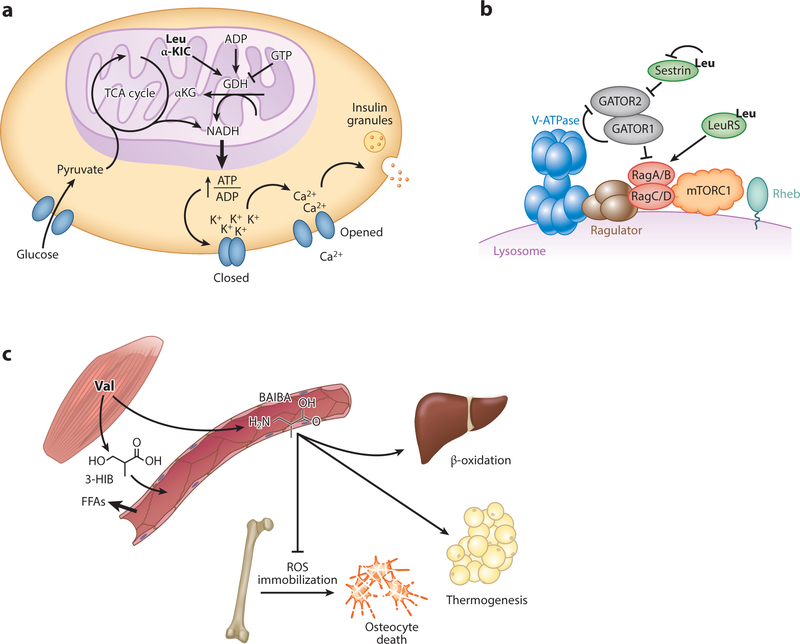
Figure 2.
(a) Leucine and α-KIC promote insulin release from pancreatic B cells via activation of glutamate dehydrogenase. (b) Leucine promotes mTORC1 activity by relieving Sestrin2-mediated inhibition and promoting LeuRS-mediated pathway activation. (c) Skeletal muscle secretes valine catabolites BAIBA and 3-HIB. BAIBA promotes hepatic B oxidation, adipocyte thermogenesis, and osteocyte survival; 3-HIB induces fatty acid transport across the endothelium and into skeletal muscle. Abbreviations: 3-HIB, 3-hydroxyisobutyrate; ADP, adenosine 5 -diphosphate; αKG, α-ketoglutarate; ATP, adenosine 5 -triphosphate; BAIBA, beta-amino-isobutyric acid; FFA, free fatty acid; GATOR1, GAP activity toward the Rag GTPases 1; GATOR2, GAP activity toward the Rag GTPases 2; GDH, glutamate dehydrogenase; GTP, guanosine triphosphate; Leu, leucine; LeuRS, leucyl tRNA synthetase; mTORC1, mechanistic target of rapamycin complex 1; NADH, nicotinamide adenine dinucleotide; TCA, tricarboxylic acid; ROS, reactive oxygen species; Val, valine; v-ATPase, vacuolar H+-adenosine triphosphatase ATPase.
Glutamate Dehydrogenase
Leucine also directly regulates protein-mediated insulin secretion in pancreatic islet beta cells. Leucine and α-KIC are strong insulin secretagogues in low-glucose states. The secretogenic activity is direct and not dependent on leucine oxidation because nonhydrolyzable analogs of leucine are equally secretogenic (57, 58). Instead, leucine promotes insulin release via activation of gluta-mate dehydrogenase (GDH), which catalyzes the oxidative deamination of glutamate to αKG (59). Under high-glucose states, GDH is inactive and suppressed by high GTP levels. When glucose drops below 5 mM, adenosine 5 -diphosphate (ADP) levels rise and can activate GDH, thereby providing reducing equivalents and promoting αKG entry into the TCA cycle. Both of these promote adenosine 5 -triphosphate (ATP) production, subsequent inhibition of KATP channels, depolarization of plasma membrane, and vesicular release of insulin (59–61). Leucine allosterically activates GDH under these conditions by increasing its affinity for ADP, thus further increasing the ATP energy charge, and consequently, insulin secretion. How leucine allosterically activates GDH is not known. Leucine can be a low-affinity substrate for GDH, so the catalytic may also be a site of allosteric activation (GDH functions as a homohexamer). Patients with mutations in GDH that cause hyperactivation in response to leucine (via loss of inhibition by GTP) lead to protein meal–induced hypoglycemia and hyperinsulinemia-hyperammonia syndrome (62). αKIC is also a strong insulin secretagogue, in part via its transamination, which yields both leucine to activate GDH and αKG to enter the TCA cycle (63). In addition, αKIC may directly inhibit KATP channels (64).
Valine Catabolites
Metazoans likely evolved to use leucine as a sensor for activation of mTOR signaling and insulin secretion because leucine is the most abundant essential amino acid; it thus serves well as an indicator of access to protein-derived amino acids. Nevertheless, other BCAA metabolites can also serve as signaling molecules. Two salient examples are 3-HIB and beta-amino-isobutyric acid (BAIBA), both related to valine catabolism (Figure 2c). As noted above, 3-HIB is the only intermediate metabolite of BCAAs that is separated from its covalent attachment to CoA; consequently, it is the only such metabolite that can easily leave the mitochondrial matrix. 3-HIB is thus in a position to report on rates of mitochondrial BCAA catabolic flux. 3-HIB is secreted by muscle and likely other tissues and is present in plasma in 30–50-μM concentrations (65). In muscle, secreted 3-HIB acts, in a paracrine fashion, on surrounding microvascular endothelial cells, where it promotes the transport of fatty acids out of the circulation, across the endothelial capillary wall, and to the myofibers. The pathway thus provides an important cross-regulatory link between BCAA and fatty acid consumptions, which represent two dominant fuel sources. Much remains to be learned about this pathway, including the receptor (if any) for 3-HIB and the mechanisms of transendothelial fatty acid transport.
BAIBA (technically also a BCAA) is not a direct intermediate of valine catabolism, but rather a potential side product, derived from methylmalonic semialdehyde, itself in rapid equilibrium with 3-HIB. Importantly, BAIBA can also be derived from thymine breakdown, and it is not always clear which source of BAIBA, thymine, or valine predominates under various studied conditions. Secretion of BAIBA, probably from muscle, has both paracrine and endocrine effects on muscle adipocytes and distal fat tissues, respectively (66). In these cells, BAIBA induces expression of the uncoupling protein UCP1, likely in part via activation of the nuclear receptor PPARα. BAIBA has also been reported to promote osteocyte survival, prevent bone loss (67), suppress renal fibroblast proliferation, prevent endoplasmic reticulum stress in hepatocytes (68), and suppress inflammation in adipocytes (69). Similar to 3-HIB, how BAIBA signals to its target cells remains ill defined, although in the case of osteocytes, the Mas-related G protein–coupled receptor type D appears to be directly targeted by BAIBA, preventing apoptosis in osteocytes (67). Both the 3-HIB and BAIBA pathways thus uncover novel extracellular metabolites with paracrine or endocrine signaling functions. They also underscore the important concept that the three BCAAs should not always be freely interchanged, conceptually or experimentally. Only valine oxidation can yield 3-HIB and BAIBA, whereas only leucine powerfully activates mTOR and GDH, for example.
ORGANISMAL PHYSIOLOGY
As noted, BCAAs are essential amino acids and cannot be synthesized by animals. Therefore, under homeostatic conditions, animals must maintain a precise balance between intake and loss of BCAAs. The diet is likely the only significant source of BCAAs; synthesis of BCAAs by gut microbiota has also been proposed, but it likely contributes a minor component. In terms of losses, oxidative catabolism of BCAAs dominates, as no appreciable amounts of BCAAs are lost in the urine. Circulating levels of BCAAs (approximately 200 μM of valine, 100 μM of leucine, and 60 μM of isoleucine) are maintained in the fasted state and return to these levels within hours after feeding; thus, the balance of BCAA intake/loss is under homeostatic control (70–72). Broadly, whole-animal BCAA physiology can be divided into a circulating pool and a tissue pool (Figure 3). BCAAs derived from the diet or released from protein breakdown appear in circulation. BCAAs are then disposed from circulation into tissues where they can be oxidized or incorporated into newly synthesized protein.
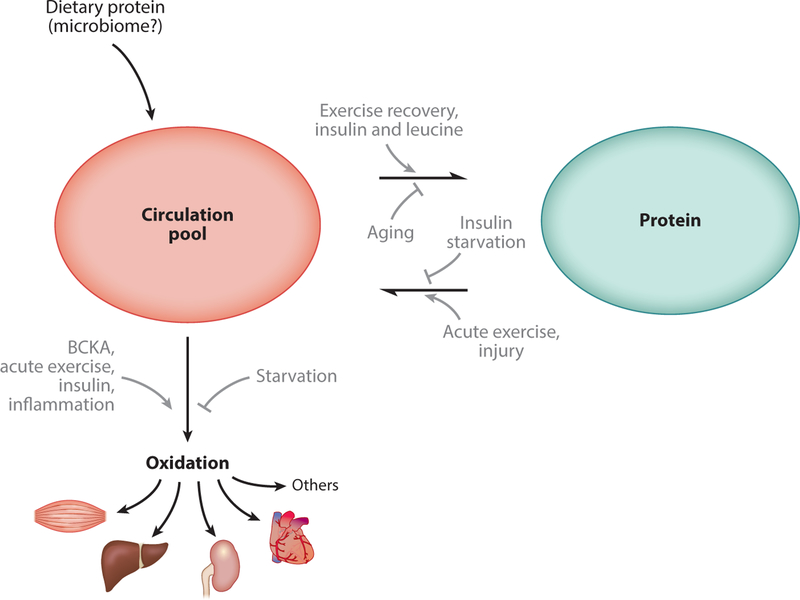
Figure 3.
Two-compartment model of whole-organism branched chain amino acid (BCAA) physiology. BCAAs appear in circulation when released from protein, from either the diet or tissues. BCAAs can leave the circulation to be deposited into new protein. All BCAAs are ultimately cleared from the system when oxidized in tissues. Many factors promote or inhibit each of these processes (grey arrows). The tissue-specific regulation of protein turnover and oxidation is poorly understood.
Dietary Intake
Dietary BCAA uptake is generally very efficient. Ingested BCAAs are usually derived from protein and are absorbed in the gut predominantly by short peptide carriers rather than by single amino acid carriers (73). After a protein-rich meal, circulating BCAA levels rise about 2–3 fold and decline back to baseline within 3 h, and the uptake kinetics differ depending on the protein source (74,75). The classic recommended protein intake to maintain minimal muscle mass is 0.8 g/kg/day, but modern recommendations for a healthy diet are higher (76). The range of protein intake in the United States varies widely from 0.9 g/kg/day in the fifth percentile to 2.2 g/kg/day in the ninety-fifth percentile for young adult males (77), perhaps reflecting the variety of popular diets. The average protein intake in males of 1.7 g/kg/day translates to approximately 88, 145, and 66 mg/kg/day of valine, leucine, and isoleucine. Protein intake varies by age and sex: It is on average higher in males than females and declines with age but comprises close to 15% of calories in all groups (77). Notably, typical laboratory rodent diets used for research contain 30% protein by calories, although the typical Western diet contains about 20%. BCAAs account for only 2–5% of dietary energy sources.
Protein Breakdown
Isotope tracing studies in the fasted state have consistently demonstrated that BCAAs and other essential amino acids appear in circulation at rates proportional to their concentration in protein (78, 79), consistent with protein breakdown being the primary source of BCAA in the circulation. Typically, the combined rate of appearance of BCAAs from normal protein breakdown is approximately 0.76 g/kg/day in overnight fasted adults, which is more than double the average intake of 0.35 g/kg/day, reflecting significant cycling in and out of the protein pool. Most of the BCAAs that appear in circulation are reincorporated into newly synthesized protein, typically accounting for 70–90% of disposal in the fasted state (80–82). Which tissues serve as the source of BCAAs is difficult to measure directly, and it likely differs under different conditions. However, current estimates suggest that skeletal muscle, the liver, and the gut account for most protein breakdown, reflecting both the large mass of skeletal muscle protein (about 38% of whole-body protein) and the fast turnover in the liver and gut (83).
Protein Synthesis
In the absence of significant secretion or absorption of protein, rates of protein synthesis and breakdown in each tissue must be equal under homeostatic conditions. Most studies aimed at measuring protein synthesis in vivo have focused on skeletal muscle. A summary of these collective findings establishes that protein synthesis requires both an anabolic signal and the amino acid building blocks to make new protein. Importantly, BCAAs, and specifically leucine, contribute to the anabolic signal. Perfusion of isolated skeletal muscle with BCAAs stimulates protein synthesis as efficiently as a complete amino acid mixture, and conversely, perfusion of muscle with an amino acid mixture lacking BCAAs fails to promote synthesis (84). Leucine exerts the most potent growth-promoting effect as evidenced by the fact that oral gavage with leucine, but not isoleucine or valine, stimulates protein synthesis in skeletal muscle (85). Incubation of skeletal muscle with leucine, but not isoleucine or valine, stimulates protein synthesis nearly as well as a complete BCAA mixture (85). Importantly, in vivo, other anabolic signals such as insulin must accompany leucine in order to promote protein synthesis. For example, oral administration of leucine leads to increased plasma insulin and stimulates protein synthesis, but when plasma insulin levels are maintained at fasting levels by somatostatin infusion, protein synthesis is inhibited (86). Many of these effects are likely mediated by mTOR, whose maximal activation requires both hormonal signals (e.g., insulin, or insulin-like growth factor) and amino acid signals (e.g., leucine via Sestrin2) (55, 56, 87). Interestingly, the relative importance of insulin versus amino acid is likely different in the splanchnic bed or viscera, where insulin has little effect while amino acids powerfully inhibit breakdown and activate synthesis (88). More detail can be found in the extensive literature on protein synthesis (83, 89, 90). The potent anabolic effects of BCAAs have led to growing interest in their use as a supplement to exercise (Figure 4), typically in the form of a whey protein shake consumed immediately after exercise. Resistance exercise is a powerful anabolic signal, which synergizes with protein or BCAA intake; combining exercise and protein intake thus leads to maximum protein synthesis (91). The literature on this topic is vast, and we refer the reader to detailed discussions (92, 93).
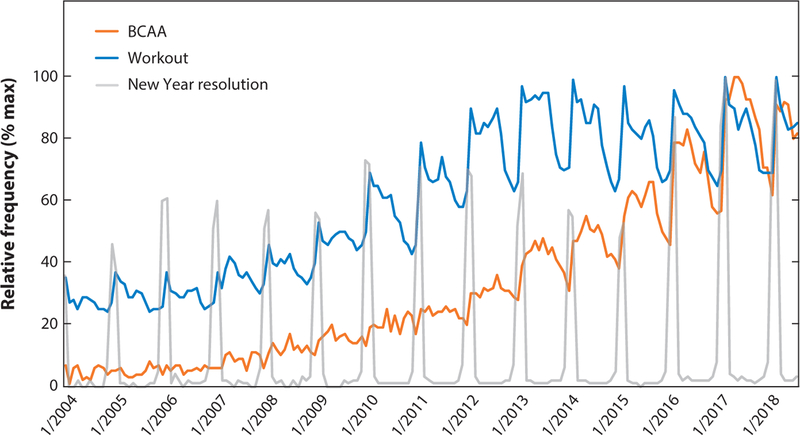
Figure 4.
The graph illustrates increasing public interest in branched chain amino acids (BCAAs) since 2004. The relative frequency of Google searches for the indicated keyword is shown, revealing the rising cyclical interest in “BCAA” in close correlation with the term “workout” and rising each year in January, coincident with the term “New Year resolution.”
BCAA Oxidation
BCAAs that are not reincorporated in the protein pool are instead oxidized to maintain homeostasis. Oxidation must occur at appreciable amounts, because at a steady state of protein maintenance where there is no net gain or loss of BCAAs, disposal must match intake.
Because BCKAs can activate their own oxidation, oxidation increases after feeding (74, 75). Conversely, briefly restricting food reduces BCAA oxidation causing plasma BCAA levels to rise (interestingly, more so than other amino acids). If fasting continues into starvation, however, BCAA oxidation once again increases, likely in large part to provide gluconeogenic precursors to the liver (94). In severe starvation, BCAA oxidation rates fall again, presumably to conserve essential amino acids. Various factors in addition to BCAA availability modulate BCAA oxidation rates. For example, insulin increases whole-body BCAA oxidation when amino acid concentration is maintained (95). Inflammatory cytokines can double whole-body BCAA oxidation in rats (96). Moreover, thyroid hormone increases BCAA oxidation before it changes energy expenditure, glucose metabolism, or fat metabolism (97, 98), although interestingly, it appears to inhibit oxidation in the liver (99).
Exercise also strongly affects BCAA oxidation. The flux of BCAA oxidation increases during a bout of acute endurance exercise in proportion to (submaximal) intensity (81, 100–103). It is unclear if the relative preference for BCAA oxidation versus other substrates is also increased. This increase in BCAA oxidation is not accompanied by increases in oxidation of other essential amino acids. Females oxidize less leucine than males during exercise (101, 104), and this is at least in part mediated by estrogen (105). In animals, endurance training clearly drives an adaptation for increased BCAA oxidation in skeletal muscle (106), probably through induction of the transcriptional activator PGC-1α (106–110). However, results from human studies testing the hypothesis that endurance training actually increases BCAA oxidation during exercise are controversial (104).
In all of these cases, the molecular mechanisms driving changes in BCAA oxidation—and in which tissues oxidation is being modulated—are unclear. In fact, there is no good consensus on the relative distribution of BCAA oxidation between different tissues. The oxidation of BCAAs occurs after transamination to BCKAs, and these two processes can occur in different tissues. In fact, some have argued that liver lacks BCAT activity and that BCAAs are largely transaminated in the muscle and then shuttled to the liver for oxidation (111), although it should be noted that nonhepatocyte cells in the liver do express BCAT enzymes (112). Regardless, the relative distribution of BCKA oxidation between different tissues has been challenging to address. Enzymes of BCAA catabolism are expressed throughout the body, in contrast to those of all other essential amino acids, which are largely confined to the liver (111). To estimate BCAA oxidation flux in various tissues, many groups have used ex vivo assays of BCAT and BCKDH activities in extracts or slices from different tissues (7). In general, such studies indicate that BCAT enzyme activity is highest in heart, kidney, stomach, and pancreas, and is lowest in liver; BCKDH enzyme activity is highest in liver, less in heart and kidney, and lowest in muscle, adipose tissue, and brain. For BCKDH, these measured activities typically reflect the phosphorylation status of BCKDH and, interestingly, correlate poorly with mRNA or protein expression of BCKDH enzymes.
However, these studies with cell extracts or purified enzymes fail to account for the numerous in vivo regulatory factors (e.g., availability of BCAAs, product inhibition, redox state, subcellular compartmentalization). Direct measurements of BCAA oxidation can be made in vivo using isotopic tracer contributions to each tissue, but such studies are not practical in humans. In mouse studies, such steady-state heavy isotope infusion studies in vivo have recently demonstrated that specific rates of BCAA oxidation in fact actively occur in all tissues examined (110). Interestingly, in the pancreas, BCAAs appear to be a dominant source of oxidative fuel, accounting for >20% of carbons incorporated into the TCA cycle. Overall, skeletal muscle oxidizes more BCAAs than any other tissue. Strikingly, oxidative flux correlates poorly with extent of phosphorylation of BCKDH, again reflecting the likely numerous other factors that dictate BCAA catabolic flux in vivo.
Finally, it should be noted that an implicit assumption in most whole-body studies to date has been that BCAAs and BCKAs are transported easily and quickly in and out of cells. This area of BCAA physiology, however, remains poorly understood. There are many amino acid transporters that are often capable of transporting a suite of amino acids, with significant redundancy (reviewed in 113). The most prominent transporter of BCAAs into cells is the large neutral amino acid (LNAA) transporter, a heterodimer composed of LAT1 and its molecular chaperone CD98 (SLC7A5 and SLC3A2, respectively) (114–116). Genetic and pharmacologic inhibition studies indicate that, at least for some cell types in cell culture, LNAA mediates most BCAA uptake (117). To what extent these transporters are rate limiting under physiological conditions is not clear. The LNAA also transports aromatic amino acids (AAAs), and the frequent correlation between plasma levels of BCAAs and AAAs (e.g., in prediabetes) has been ascribed to competition for these transporters (118). The transporter is highly expressed in the blood-brain barrier, where it has been estimated to be 96% saturated with LNAAs, mostly leucine and phenylalanine (119). Based on this observation, treatment with BCAAs has been proposed to competitively prevent uptake of AAAs into the brain in, for example, hepatic encephalopathy, with variable results (120). If so, then LNAA transport does likely contribute a rate-limiting step in systemic BCAA homeostasis, although the LNAA transporter may have lower affinity for BCAAs in tissues other than the brain. BCKAs may be transported by nonspecific monocarboxylate transporters (121).
BCAAs are also important for interorgan nitrogen exchange, most often studied between muscle and liver (122). BCAT enzymes operate near equilibrium in most tissues, and rates of transamination generally far outstrip rates of BCKA oxidation, thus allowing BCAA amino groups to contribute significantly to the transamination pool. Alanine is a major circulating metabolite (with higher turnover than glutamine, glycerol, or pyruvate) (79), which is in large part secreted by skeletal muscle; it is also an important source for gluconeogenesis in the liver (123). Muscle secretion of alanine requires amination of pyruvate, and strikingly, leucine alone accounts for 20% of this nitrogen (124). Valine and isoleucine likely contribute proportionally. BCAAs thus participate as nitrogen donors both to move nitrogen to the liver for urea synthesis and to facilitate moving carbons to the liver for gluconeogenesis. Of note, net transfer of nitrogen from BCAAs requires the concomitant removal of BCKAs to prevent the reverse reaction, which is achieved via either BCKA secretion or oxidation (125). BCAA nitrogen can also be transferred to glutamine, a substrate for gluconeogenesis in the kidney (126) and in the brain; to the synthesis of both excitatory glutamate and inhibitory GABA neurotransmitters; and to the neuroprotective astrocyte/neuron glutamine/glutamate shuttle (127).
In summary, whole-body BCAA metabolism reflects a balance between protein ingestion, cycling of protein synthesis and breakdown, and BCAA oxidation. Large gaps still exist in our understanding of how these processes are regulated and how they differ between tissues.
BRANCHED CHAIN AMINO ACIDS IN DISEASE
Inborn Errors of BCAA Metabolism
Inborn errors of BCAA metabolism have demonstrated the importance of evolutionarily honed BCAA homeostatic mechanisms to prevent excess of BCAAs or their derivatives. Maple syrup urine disease (MSUD), first described in the 1950s (128–130), is an autosomal recessive disease caused by mutations in the first two subunits of BCKDH (the BCKDHA/BCKDHB heterotetramer or DBT) and occurs in approximately 1:200,000 births. Mutations in the third subunit of BCKDH, DLT, lead to more severe and distinct disease because DLT is shared with PDH and αGDH. Plasma BCAAs, α-ketoacids, and hydroxy-BCAAs are high, and elevations in lalloisoleucine are pathognomonic (131). Accumulation of a rare catabolic product, sotolone, gives the urine its characteristic odor (132, 133). The disease presentations are variable, in part depending on which subunit of BCKDH is affected (134). Untreated, MSUD leads to encephalopathy, cerebral edema, and death. The mechanism of encephalopathy remains unclear, but it likely involves disturbed neurotransmission. BCAAs, especially leucine, donate via BCAT transamination one-third or more of the amino groups in brain glutamate, the major excitatory neuro-transmitter, and are critical to maintain nitrogen homeostasis in the astrocyte/neuron glutamate/glutamine cycle (127, 135, 136); elevations in α-KIC may thus contribute to glutamate depletion. In addition, as noted above, BCAAs (especially leucine) compete with AAAs for transport across the blood-brain barrier, thus potentially limiting important neurotransmitter precursors. Numerous other mechanisms have been proposed (137). Upon diagnosis, patients are treated by aggressive protein withdrawal, and then amino acid–defined diets are slowly reintroduced to maintain BCAA levels as close to normal as possible (138). Liver transplantation is curative, which demonstrates that providing ~10% of total body BCKDH activity is sufficient to restore BCAA homeostasis (139). Conversely, MSUD patients can serve as liver donors (140), indicating that BCKDH activity outside the liver is also sufficient to maintain BCAA homeostasis. Gene therapy to deliver functional BCKDH or edit the endogenous mutations may provide viable alternatives to liver transplant.
More recent work shows that mutations in BCKDK, leading to excess, rather than restricted BCAA oxidation may lead to autism spectrum disorder with epilepsy (141, 142). In addition, homozygous mutations in SLC7A5, a component of the LNAA transporter (see above), have been found in patients with autistic traits. Deletion of Slc7A5 in endothelial cells of mice leads to low brain BCAAs and severe neurological symptoms, and intracerebroventricular administration of BCAAs partially reverses these abnormalities (143). Thus, excess or insufficient BCAAs/BCKAs in the brain contributes to neurologic diseases, underscoring the importance of BCAA homeostasis for normal brain function, although in both cases the mechanisms remain unclear.
Diabetes
Unlike the clear neurotoxic effects of large excesses in BCAAs that are seen in MSUD patients, the possible pathogenic consequences of milder elevations in BCAAs are only slowly coming to light. Elevations of BCAA levels in blood of patients with obesity and insulin resistance were first noted in the 1960s (144, 145). Recent work has revitalized these observations and supports the notion that elevations in BCAAs in fact contribute causally to insulin resistance, as supported by the following observations: (a) Unbiased metabolomic studies with plasma from normal subjects with normal insulin sensitivity identified elevations in plasma BCAAs as the strongest predictor for developing diabetes in the subsequent decade or more, indicating that changes in BCAA metabolism precede detectable insulin resistance (146–149); (b) Mendelian genetics studies revealed that polymorphisms near the PPM2 gene (encoding for the BCKDH phosphatase) that affect BCAA levels also increase the risk of insulin resistance (150); and (c) BCAAs infused into the circulation of healthy adults are sufficient to impair glucose disposal (151). Moreover, adding BCAAs to a high-fat diet worsens the development of glucose intolerance in rodents (147), whereas limiting BCAAs improves glucose tolerance and insulin sensitivity (152). Together, these data support the notion that BCAAs contribute to insulin resistance, likely via a mechanism dependent on excess lipid availability. Conversely, insulin resistance itself likely can cause elevations in BCAAs, establishing a feed-forward loop (153, 154).
Identifying the mechanisms that lead to BCAA elevations may present novel targets for interventions early in the progression to insulin resistance. Multiple mechanisms are likely at play, involving multiple organs (Figure 5). To date, nearly all mechanistic studies are largely based on rodent studies. In adipose tissue of insulin resistant people and animals, gene expression of nearly every enzyme required for BCAA oxidation is suppressed (155–160). Cell culture studies suggest that hypoxia, endoplasmic reticulum stress, and inflammation contribute to this suppression (161, 162), and thiazolidinediones rescue expression in vivo (158, 163). In the liver, BCKDH phosphorylation is increased, which is likely driven by high BCKDK expression (159, 164–166). Suppression of BCAA oxidation in liver and adipose tissue promotes elevations in plasma BCAAs, likely shunting BCAA oxidation to permissive organs (118). Consistent with these observations, recent studies in whole animals with steady-state heavy isotope infusions revealed in db/db mice blunted BCAA oxidation in fat and liver, with consequent significant shunting of oxidation to skeletal muscle (110). Recent work also showed that fructose ingestion induces the hepatic transcription factor ChREBP-β, which in turn activates BCKDK transcription, thus linking fructose and BCAA metabolism. This suggests a mechanism by which the modern dietary choices of high fructose and protein consumption synergistically conspire to elevate plasma BCAAs (166). Reversing the effects of BCKDK by liver-targeted overexpression of the PPCM phosphatase PPM1K, or by systemic pharmacological inhibition of BCKDK, improved glucose tolerance in Zucker fatty rats (166), strongly supporting the causal role of reduced liver BCAA oxidation in the development of insulin resistance.
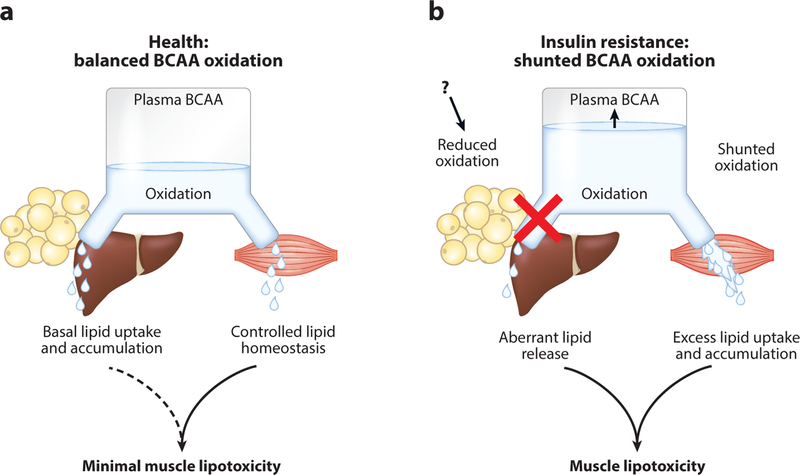
Figure 5.
Proposed model of casual relationship between altered tissue branched chain amino acid (BCAA) oxidation and elevated circulating BCAA levels in insulin resistance. In healthy conditions (a), BCAA oxidation is balanced among different organs. Genetic and environmental factors suppress BCAA oxidation in the liver and adipose tissues (b), causing increased circulating BCAA levels and overflow of BCAAs into skeletal muscle, which results in lipotoxicity and insulin resistance. Adapted with permission from Reference 110.
How elevations in BCAAs cause insulin resistance remains unclear. In fact, it remains uncertain if elevations in BCAAs per se promote insulin resistance; alternative explanations include the consequences of decreased oxidation in some tissues (e.g., adipose) or shunted increased oxidation in others (e.g., muscle). Few mechanisms have been proposed to connect impaired BCAA oxidation in the liver with direct effects within the liver. Interestingly, BCKDK in the liver appears to also phosphorylate and inhibit ATP citrate lyase, a rate-limiting step for de novo lipogenesis, possibly providing an alternative explanation for insulin resistance in the face of elevated BCKDK (166). The near complete loss of BCAA oxidation in adipose tissue may also have important cell-autonomous consequences. In cultured adipocytes, leucine and isoleucine contribute 30% of lipogenic acetyl-CoA, and BCAA oxidation is required for differentiation (35). These observations predict that loss of BCAA oxidation could impair lipid storage in adipocytes, thus contributing to ectopic lipid deposition and insulin resistance. Adipocytes also use BCAAs to synthesize odd-chain fatty acids (35, 41), but the role for these unique lipids is unknown.
Skeletal muscle is the predominant site of glucose disposal after a carbohydrate load (167), and thus it represents a critical site of insulin resistance. Several mechanisms have been proposed to connect elevated BCAAs or increased BCAA oxidation to insulin resistance in skeletal muscle. mTOR activation by leucine has been investigated, but mTOR does not appear to be responsible for the effects of BCAAs on insulin resistance, because treating rats with rapamycin does not abrogate the effects of BCAAs (147). In fact, in general, diets supplemented with leucine only, rather than all three BCAAs, tend to improve insulin resistance rather than worsen it (168). Competition of elevated BCAA oxidation with oxidation of other substrates, notably glucose, has also been proposed, but this mechanism is unlikely because the relative contribution of BCAAs to total muscle fuel oxidation is small (110). In one proposed mechanism that connects BCAAs and ec-topic lipid accumulation, high BCAA oxidation in skeletal muscle depletes the intracellular pool of glycine, thereby impairing lipid export of acyl-glycine adducts, resulting in accumulation of acyl-CoA species (165). Glycine levels frequently correlate inversely with BCAAs (145–147), and a low-BCAA diet raised glycine back to normal levels (165). Finally, elevated oxidation of va-line likely increases production of 3-HIB, increasing fatty acid uptake via paracrine promotion of transendothelial fatty acid transport (65). Plasma concentrations of 3-HIB are associated with the future development of diabetes, even after adjusting for body mass index and plasma BCAAs (169). Despite this multitude of potential mechanisms, no studies have definitively demonstrated that any of these changes in BCAA oxidation in specific tissues are sufficient to cause insulin resistance.
Cancer
Because BCAAs are essential amino acids, a growing tumor must obtain them from either the circulation or surrounding tissue. Alterations in circulating BCAA levels in patients diagnosed with cancer have long been noted (170–173). Recent retrospective metabolomic studies demonstrated that elevated plasma BCAA levels are associated with a greater than twofold increased risk in pancreatic cancer and precede clinical presentation by many years. The observation was recapitulated in mice genetically engineered to develop pancreatic ductal adenocarcinoma and is likely caused by subclinical systemic protein breakdown during early tumorigenesis, which is presumed to service the BCAA needs of the growing tumor (171). Interestingly, the same appears not to be true of other tumors, even when driven by the same mutations in KRAS and p53 (174). Whether these alterations in systemic BCAA metabolism contribute to tumor growth or metastasis remains unclear. Regardless, opportunities for biomarker development are an area of intense investigation (175, 176).
A recent surge of studies on tumor BCAA metabolism has focused largely on BCAT1, the expression of which is altered in numerous cancers, and in many cases correlates with poor outcome (177–179). Notably, in glioblastomas containing wild-type isocitrate dehydrogenase (IDH), half express high levels of BCAT1, whereas IDHmut tumors suppress BCAT1 expression (180). The latter suppression may be mediated by the oncometabolite 2-hydroxyglutarate (2-HG) that is generated by mutant IDH. 2-HG potently inhibits dioxygenases, including histone demethylases (181), leading to widespread suppressive hypermethylation of promoters, including that of BCAT1 (180). Conversely, several mechanisms to explain elevated BCAT1 expression in various cancers have been proposed, including increased binding of BCAT1 mRNA transcript to RNA-binding protein MSI2 (182) chromatin hyperacetylation of the BCAT1 gene by the MLL1 fusion protein (183), and transcriptional activation by the myc oncogene (184, 185). It remains unclear precisely how BCAT1 expression promotes tumor growth, but it likely differs between tumors. In IDHwt acute myeloid leukemia, BCAT1 expression correlates with shorter survival and has been proposed to mimic IDHmut acute myeloid leukemia by virtue of depleting αKG, leading to inactivation of αKG-dependent dioxygenases, which is analogous to inhibition of the same dioxygenases by 2-HG in IDHmut cells. Suppression of dioxygenases ultimately promotes growth via HIF-1α stabilization and by altering the epigenomic landscape (186). Conversely, 2-HG produced in IDHmut glioma cells inhibits BCAT1, thus limiting the supply of glutamate and opening a vulnerability to treatment with inhibitors of glutaminase, the other main source of glutamate (187). In chronic myeloid leukemia, BCAT1 is proposed to promote blast crisis by aminating BCKAs to produce BCAAs, leading to progrowth mTOR activation; however, it is unclear how BCAT1 enzymatic activity should be limited to only one direction (182). BCAT1 overexpression also promotes mTORC1 activity in breast cancer through unclear mechanisms (188). In summary, data strongly point to an important role for BCAT1 in multiple cancer types, likely via multiple different mechanisms unique to each cancer.
Heart Failure
BCAA metabolism has also garnered much attention in the context of cardiovascular disease and heart failure. Elevations in plasma BCAAs and their metabolites are independently associated with cardiovascular disease risk (189–191), a topic reviewed elsewhere (192). Additionally, circulating and cardiac BCAAs and BCKAs rise in response to ischemic and hemodynamic murine models of heart failure (193–195). In these studies, the increase in cardiac and plasma BCAAs coincides with diminished expression of multiple components of the BCAA catabolic pathway. However, the heart consumes far fewer BCAAs than other organs (196–198), making it unlikely that diminished cardiac BCAA catabolism alone accounts for increased plasma BCAAs. Suppression of whole-body BCAA catabolism via deletion of PP2Cm elevates circulating and cardiac BCAA levels and worsens cardiac response to aortic constriction and ischemia/reperfusion injury (193, 194). Additionally, dietary BCAA supplementation worsens contractility and increases infarct size following myocardial infarction (195). Conversely, pharmacological promotion of systemic BCAA catabolism lowers circulating and cardiac BCAA levels and improves cardiac function in both hemodynamic and ischemic challenges (193–195). These data strongly support the notion that alterations of BCAA metabolism contribute to heart failure in numerous contexts.
The mechanisms by which BCAAs affect cardiac function, however, remain poorly understood. As in the case of insulin resistance, multiple mechanisms likely contribute. Diminished cardiac BCAA catabolism per se is not likely to compromise ATP production in heart failure because BCAA oxidation contributes to a negligible amount (<5%) of ATP production even in the healthy heart (110, 199; reviewed in 200, 201). It is thus more likely that altered concentrations of BCAAs or BCKAs in the heart affect function. High levels of intracellular cardiac leucine may activate mTOR, thus promoting cardiac insulin resistance and hypertrophy. Indeed, mTOR inhibition mitigates cardiac dysfunction in multiple heart failure models (195, 202, 203; reviewed in 204). Conversely, even transient exposure of isolated rodent hearts to high concentrations of BCAAs impairs contractility. This may occur in part via inhibition of mitochondrial ATP production, as high levels of BCAAs inhibit both pyruvate and αKG dehydrogenases (193, 205). Overall, however, how alterations of systemic BCAA metabolism alter heart failure remains unclear.
CONCLUSION
BCAAs have been the subject of often intense study since their discovery in the mid-nineteenth century. More than 50,000 studies are reported in PubMed. Space constraints invariably precluded us from covering all that is to be said about these fascinating molecules. We have succinctly reviewed here the basic biochemistry and physiology of mammalian BCAA metabolism, much of which was elucidated in the latter part of the twentieth century. This is followed by examples of more recent investigations pointing to a potentially important role for BCAAs in the development of numerous prevalent diseases that increasingly afflict the modern world. Armed with improved understanding of BCAA physiology and pathophysiology, we anticipate that interventions appropriately targeting BCAA metabolism will help improve treatment of these modern ailments.
ACKNOWLEDGMENTS
We apologize for omitting a number of additional important topics and studies due to space constraints. The authors were supported by grants from the US National Institutes of Health: T32 GM-07229 (M.N.), F30HL142186–01 (D.M.), and R01 DK107667, DK114103 (Z.A.).
Footnotes
DISCLOSURE STATEMENT
The authors are not aware of any affiliations, memberships, funding, or financial holdings that might be perceived as affecting the objectivity of this review.
LITERATURE CITED
- 1.Davis TA, Fiorotto ML, Reeds PJ. 1993. Amino acid compositions of body and milk protein change during the suckling period in rats. J. Nutr 123(5):947–56 [DOI] [PubMed] [Google Scholar]
- 2.Harper A, Block K, Cree T. 1983. Branched-chain amino acids: nutritional and metabolic interrelationships In Proceedings of the Fourth Symposium on Protein Metabolism and Nutrition, ed. Pion R, Arnal M, Bonin D, pp. 159–81. Paris: INRA [Google Scholar]
- 3.Moura A, Savageau MA, Alves R. 2013. Relative amino acid composition signatures of organisms and environments. PLOS ONE 8(10):e77319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chou PY, Fasman GD. 1973. Structural and functional role of leucine residues in proteins. J. Mol. Biol 74(3):263–81 [DOI] [PubMed] [Google Scholar]
- 5.Dill KA. 1990. Dominant forces in protein folding. Biochemistry 29(31):7133–55 [DOI] [PubMed] [Google Scholar]
- 6.Schweigert BS, Bennett BA, Guthneck BT. 1954. Amino acid composition of organ meats. J. Food Sci 19(1–6):219–23 [Google Scholar]
- 7.Brosnan JT, Brosnan ME. 2006. Branched-chain amino acids: enzyme and substrate regulation. J. Nutr 136(1):207S–11S [DOI] [PubMed] [Google Scholar]
- 8.Shimomura Y, Obayashi M, Murakami T, Harris RA. 2001. Regulation of branched-chain amino acid catabolism: nutritional and hormonal regulation of activity and expression of the branched-chain α-keto acid dehydrogenase kinase. Curr. Opin. Clin. Nutr. Metab. Care 4(5):419–23 [DOI] [PubMed] [Google Scholar]
- 9.McCourt JA, Duggleby RG. 2006. Acetohydroxyacid synthase and its role in the biosynthetic pathway for branched-chain amino acids. Amino Acids 31(2):173–210 [DOI] [PubMed] [Google Scholar]
- 10.Amorim Franco TM, Blanchard JS. 2017. Bacterial branched-chain amino acid biosynthesis: structures, mechanisms, and drugability. Biochemistry 56(44):5849–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ichihara A, Koyama E. 1966. Transaminase of branched chain amino acids. J. Biochem 59(2):160–69 [DOI] [PubMed] [Google Scholar]
- 12.Ichihara A, Yamasaki Y, Masuji H, Sato J. 1975. Isozyme patterns of branched chain amino acid transaminase during cellular differentiation and carcinogenesis In Isozymes, Vol. 3, ed. Markert CL, pp. 875–89. Amsterdam: Elsevier [Google Scholar]
- 13.Kadowaki H, Knox WE. 1982. Cytoslic and mitochondrial isoenzymes of branched-chain amino acid aminotransferase during development of the rat. Biochem. J 202:777–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Krebs HA, Lund P. 1977. Aspects of the regulation of the metabolism of branched-chain amino acids. Adv. Enzyme Regul 15:375–94 [DOI] [PubMed] [Google Scholar]
- 15.Goto M, Shinno H, Ichihara A. 1977. Isozyme patterns of branched-chain amino acid transaminase in human tissues and tumors. GANN Jpn. J. Cancer Res 68(5):663–67 [PubMed] [Google Scholar]
- 16.Patel MS, Nemeria NS, Furey W, Jordan F. 2014. The pyruvate dehydrogenase complexes: structure-based function and regulation. J. Biol. Chem 289(24):16615–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wieland OH. 1983. The mammalian pyruvate dehydrogenase complex: structure and regulation In Reviews of Physiology, Biochemistry and Pharmacology, Vol. 96, ed. Adrian RH, zur Hausen H, Helmreich E, Holzer H, Jung R, et al. , pp. 123–70. Berlin: Springer; [DOI] [PubMed] [Google Scholar]
- 18.Johnson WA, Connelly JL, Glynn MT. 1972. Cellular localization and characterization of bovine liver branched-chain α-keto acid dehydrogenases. Biochemistry 11(10):1967–73 [DOI] [PubMed] [Google Scholar]
- 19.Paxton R, Harris RA. 1982. Isolation of rabbit liver branched chain α-ketoacid dehydrogenase and regulation by phosphorylation. J. Biol. Chem 257(3):14433–39 [PubMed] [Google Scholar]
- 20.Pettit FH, Yeaman SJ, Reed LJ. 1978. Purification and characterization of branched chain α-keto acid dehydrogenase complex of bovine kidney. PNAS 75(10):4881–85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Danner DJ, Lemmon SK, Elsas LJ II. 1978. Substrate specificity and stabilization by thiamine pyrophosphate of rat liver branched chain α-ketoacid dehydrogenase. Biochem. Med 19(1):27–38 [DOI] [PubMed] [Google Scholar]
- 22.Babady NE, Pang Y-P, Elpeleg O, Isaya G. 2007. Cryptic proteolytic activity of dihydrolipoamide dehydrogenase. PNAS 104(15):6158–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Harris RA, Popov KM, Shimomura Y, Zhao Y, Jaskiewicz J, et al. 1992. Purification, characterization, regulation and molecular cloning of mitochondrial protein kinases. Adv. Enzyme Regul 32:267–84 [DOI] [PubMed] [Google Scholar]
- 24.Popov KM, Shimomura Y, Harris RA. 1991. Purification and comparative study of the kinases specific for branched chain α-ketoacid dehydrogenase and pyruvate dehydrogenase. Protein Expr. Purif 2(4):278–86 [DOI] [PubMed] [Google Scholar]
- 25.Popov KM, Zhao Y, Shimomura Y, Kuntz MJ, Harris RA. 1992. Branched-chain α-ketoacid dehydrogenase kinase. Molecular cloning, expression, and sequence similarity with histidine protein kinases. J. Biol. Chem 267(19):13127–30 [PubMed] [Google Scholar]
- 26.Shimomura Y, Nanaumi N, Suzuki M, Popov KM, Harris RA. 1990. Purification and partial characterization of branched-chain α-ketoacid dehydrogenase kinase from rat liver and rat heart. Arch. Biochem. Biophys 283(2):293–99 [DOI] [PubMed] [Google Scholar]
- 27.Tuor U, Simone C, Bascaramurty S. 1992. Local blood-brain barrier in the newborn rabbit: postnatal changes in α-aminoisobutyric acid transfer within medulla, cortex, and selected brain areas. J. Neurochem 59(3):999–1007 [DOI] [PubMed] [Google Scholar]
- 28.Damuni Z, Reed LJ. 1987. Purification and properties of the catalytic subunit of the branched-chain α-keto acid dehydrogenase phosphatase from bovine kidney mitochondria. J. Biol. Chem 262(11):5129–32 [PubMed] [Google Scholar]
- 29.Damuni Z, Merryfield ML, Humphreys JS, Reed LJ. 1984. Purification and properties of branched-chain α-keto acid dehydrogenase phosphatase from bovine kidney. PNAS 81(14):4335–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Crowell PL, Block KP, Repa JJ, Torres N, Nawabi MD, et al. 1990. High branched-chain α-keto acid intake, branched chain α-keto acid dehydrogenase activity, and plasma and brain amino acid and plasma keto acid concentrations in rats. Am. J. Clin. Nutr 52(2):313–19 [DOI] [PubMed] [Google Scholar]
- 31.Frick G, Tai L, Blinder L, Goodman H. 1981. l-leucine activates branched chain α-keto acid dehydrogenase in rat adipose tissue. J. Biol. Chem 256(6):2618–20 [PubMed] [Google Scholar]
- 32.Lau KS, Fatania HR, Randle PJ. 1982. Regulation of the branched chain 2-oxoacid dehydrogenase kinase reaction. FEBS Lett 144(1):57–62 [DOI] [PubMed] [Google Scholar]
- 33.Waymack P, DeBuysere M, Olson M. 1980. Studies on the activation and inactivation of the branched chain α-keto acid dehydrogenase in the perfused rat heart. J. Biol. Chem 255(20):9773–81 [PubMed] [Google Scholar]
- 34.Islam MM, Nautiyal M, Wynn RM, Mobley JA, Chuang DT, Hutson SM. 2010. Branched-chain amino acid metabolon, interaction of glutamate dehydrogenase with the mitochondrial branched-chain amino-transferase (BCATm). J. Biol. Chem 285(1):265–76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Green CR, Wallace M, Divakaruni AS, Phillips SA, Murphy AN, et al. 2016. Branched-chain amino acid catabolism fuels adipocyte differentiation and lipogenesis. Nat. Chem. Biol 12(1):15–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liebich HM, Först C. 1984. Hydroxycarboxylic and oxocarboxylic acids in urine: products from branched-chain amino acid degradation and from ketogenesis. J. Chromatogr. B Biomed. Sci. Appl 309:225–42 [DOI] [PubMed] [Google Scholar]
- 37.Anderson KA, Huynh FK, Fisher-Wellman K, Stuart JD, Peterson BS, et al. 2017. SIRT4 is a lysine deacylase that controls leucine metabolism and insulin secretion. Cell Metab 25(4):838–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jones JM, Morrell JC, Gould SJ. 2000. Identification and characterization of HAOX1, HAOX2, and HAOX3, three human peroxisomal 2-hydroxy acid oxidases. J. Biol. Chem 275(17):12590–97 [DOI] [PubMed] [Google Scholar]
- 39.Van Koevering M, Nissen S. 1992. Oxidation of leucine and alpha-ketoisocaproate to beta-hydroxy-beta-methylbutyrate in vivo. Am. J. Physiol. Endocrinol. Metab 262(1):E27–31 [DOI] [PubMed] [Google Scholar]
- 40.Long JZ, Svensson KJ, Bateman LA, Lin H, Kamenecka T, et al. 2016. The secreted enzyme PM20D1 regulates lipidated amino acid uncouplers of mitochondria. Cell 166(2):424–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Crown SB, Marze N, Antoniewicz MR. 2015. Catabolism of branched chain amino acids contributes significantly to synthesis of odd-chain and even-chain fatty acids in 3T3-L1 adipocytes. PLOS ONE 10(12):e0145850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wallace M, Green CR, Roberts LS, Lee YM, McCarville JL, et al. 2018. Enzyme promiscuity drives branched-chain fatty acid synthesis in adipose tissues. Nat. Chem. Biol 14:1021–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mika A, Stepnowski P, Kaska L, Proczko M, Wisniewski P, et al. 2016. A comprehensive study of serum odd- and branched-chain fatty acids in patients with excess weight: odd- and branched-chain fatty acids in obesity. Obesity 24(8):1669–76 [DOI] [PubMed] [Google Scholar]
- 44.Ran-Ressler RR, Devapatla S, Lawrence P, Brenna JT. 2008. Branched chain fatty acids are constituents of the normal healthy newborn gastrointestinal tract. Pediatr. Res 64(6):605–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Buse MG, Reid SS. 1975. Leucine. A possible regulator of protein turnover in muscle. J. Clin. Investig 56(5):1250–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chang Hong S-O, Layman DK. 1984. Effects of leucine on in vitro protein synthesis and degradation in rat skeletal muscles. J. Nutr 114(7):1204–12 [DOI] [PubMed] [Google Scholar]
- 47.Li JB, Jefferson LS. 1978. Influence of amino acid availability on protein turnover in perfused skeletal muscle. Biochim. Biophys. Acta 544(2):351–59 [DOI] [PubMed] [Google Scholar]
- 48.Wolfson RL, Chantranupong L, Saxton RA, Shen K, Scaria SM, et al. 2015. Sestrin2 is a leucine sensor for the mTORC1 pathway. Science 351:43–48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Saxton RA, Knockenhauer KE, Wolfson RL, Chantranupong L, Pacold ME, et al. 2016. Structural basis for leucine sensing by the Sestrin2-mTORC1 pathway. Science 351(6268):53–58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bonfils G, Jaquenoud M, Bontron S, Ostrowicz C, Ungermann C, De Virgilio C. 2012. Leucyl-tRNA synthetase controls TORC1 via the EGO complex. Mol. Cell 46(1):105–10 [DOI] [PubMed] [Google Scholar]
- 51.Han JM, Jeong SJ, Park MC, Kim G, Kwon NH, et al. 2012. Leucyl-tRNA synthetase is an intracellular leucine sensor for the mTORC1-signaling pathway. Cell 149(2):410–24 [DOI] [PubMed] [Google Scholar]
- 52.Yoon M-S, Son K, Arauz E, Han JM, Kim S, Chen J. 2016. Leucyl-tRNA synthetase activates Vps34 in amino acid-sensing mTORC1 signaling. Cell Rep 16(6):1510–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Efeyan A, Zoncu R, Sabatini DM. 2012. Amino acids and mTORC1: from lysosomes to disease. Trends Mol. Med 18(9):524–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jewell JL, Russell RC, Guan K-L. 2013. Amino acid signalling upstream of mTOR. Nat. Rev. Mol. Cell Biol 14(3):133–39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Laplante M, Sabatini DM. 2012. mTOR signaling in growth control and disease. Cell 149(2):274–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wolfson RL, Sabatini DM. 2017. The dawn of the age of amino acid sensors for the mTORC1 pathway. Cell Metab 26(2):301–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Christensen HN, Hellman B, Sehlin J, Tager HS, Täljedal I-B. 1971. In vitro stimulation of insulin release by non-metabolizable, transport-specific amino acids. Biochim. Biophys. Acta Biomembr 241(2):341–48 [DOI] [PubMed] [Google Scholar]
- 58.Fajans SS, Quibrera R, Pek S, Floyd J, Christensen HN, et al. 1971. Stimulation of insulin release in the dog by a nonmetabolizable amino acid. Comparison with leucine and arginine. J. Clin. Endocrinol. Metab 33(1):35–41 [DOI] [PubMed] [Google Scholar]
- 59.Sener A, Malaisse WJ. 1980. l-leucine and a nonmetabolized analogue activate pancreatic islet glutamate dehydrogenase. Nature 288(5787):187–89 [DOI] [PubMed] [Google Scholar]
- 60.Gao ZY, Li G, Najafi H, Wolf BA, Matschinsky FM. 1999. Glucose regulation of glutaminolysis and its role in insulin secretion. Diabetes 48(8):1535–42 [DOI] [PubMed] [Google Scholar]
- 61.Wilson DF, Cember ATJ, Matschinsky FM. 2018. Glutamate dehydrogenase: role in regulating metabolism and insulin release in pancreatic β-cells. J. Appl. Physiol 125(2):419–28 [DOI] [PubMed] [Google Scholar]
- 62.Stanley CA, Lieu YK, Hsu BYL, Burlina AB, Greenberg CR, et al. 1998. Hyperinsulinism and hyper-ammonemia in infants with regulatory mutations of the glutamate dehydrogenase gene. N. Engl. J. Med 338(19):1352–57 [DOI] [PubMed] [Google Scholar]
- 63.Gao Z, Young RA, Li G, Najafi H, Buettger C, et al. 2003. Distinguishing features of leucine and α-ketoisocaproate sensing in pancreatic β-cells. Endocrinology 144(5):1949–57 [DOI] [PubMed] [Google Scholar]
- 64.Bränström R, Efendic S, Berggren P-O, Larsson O. 1998. Direct inhibition of the pancreatic β-cell ATP-regulated potassium channel by α-ketoisocaproate. J. Biol. Chem 273(23):14113–18 [DOI] [PubMed] [Google Scholar]
- 65.Jang C, Oh SF, Wada S, Rowe GC, Liu L, et al. 2016. A branched-chain amino acid metabolite drives vascular fatty acid transport and causes insulin resistance. Nat. Med 22(4):421–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Roberts LD, Boström P, O’Sullivan JF, Schinzel RT, Lewis GD, et al. 2014. β-Aminoisobutyric acid induces browning of white fat and hepatic β-oxidation and is inversely correlated with cardiometabolic risk factors. Cell Metab 19(1):96–108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kitase Y, Vallejo JA, Gutheil W, Vemula H, Jähn K, et al. 2018. β-Aminoisobutyric acid, l -BAIBA, is a muscle-derived osteocyte survival factor. Cell Rep 22(6):1531–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Shi C-X, Zhao M-X, Shu X-D, Xiong X-Q, Wang J-J, et al. 2016. β-Aminoisobutyric acid attenuates hepatic endoplasmic reticulum stress and glucose/lipid metabolic disturbance in mice with type 2 diabetes. Sci. Rep 6:21924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Jung TW, Park HS, Choi GH, Kim D, Lee T. 2018. β-Aminoisobutyric acid attenuates LPS-induced inflammation and insulin resistance in adipocytes through AMPK-mediated pathway. J. Biomed. Sci 25(1):27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Everman S, Mandarino LJ, Carroll CC, Katsanos CS. 2015. Effects of acute exposure to increased plasma branched-chain amino acid concentrations on insulin-mediated plasma glucose turnover in healthy young subjects. PLOS ONE 10(3):e0120049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Louard RJ, Barrett EJ, Gelfand RA. 1990. Effect of infused branched-chain amino acids on muscle and whole-body amino acid metabolism in man. Clin. Sci 79(5):457–66 [DOI] [PubMed] [Google Scholar]
- 72.Wahren J, Felig P, Hagenfeldt L. 1976. Effect of protein ingestion on splanchnic and leg metabolism in normal man and in patients with diabetes mellitus. J. Clin. Investig 57(4):987–99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Grimble G 2000. Mechanisms of peptide and amino acid transport and their regulation In Proteins, Peptides and Amino Acids in Enteral Nutrition, ed. Fürst P, Young V, pp. 63–88. Basel: Karger; [DOI] [PubMed] [Google Scholar]
- 74.Boirie Y, Dangin M, Gachon P, Vasson M-P, Maubois J-L, Beaufrère B. 1997. Slow and fast dietary proteins differently modulate postprandial protein accretion. PNAS 94(26):14930–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Dangin M, Boirie Y, Garcia-Rodenas C, Gachon P, Fauquant J, et al. 2001. The digestion rate of protein is an independent regulating factor of postprandial protein retention. Am. J. Physiol. Endocrinol. Metab 280(2):E340–48 [DOI] [PubMed] [Google Scholar]
- 76.Wolfe RR, Cifelli AM, Kostas G, Kim I-Y. 2017. Optimizing protein intake in adults: interpretation and application of the recommended dietary allowance compared with the acceptable macronutrient distribution range. Adv. Nutr. Int. Rev. J 8(2):266–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Fulgoni VL. 2008. Current protein intake in America: analysis of the National Health and Nutrition Examination Survey, 2003–2004. Am. J. Clin. Nutr 87(5):1554S–57S [DOI] [PubMed] [Google Scholar]
- 78.Bier DM. 1989. Intrinsically difficult problems: the kinetics of body proteins and amino acids in man. Diabetes/Metab. Rev 5(2):111–32 [DOI] [PubMed] [Google Scholar]
- 79.Hui S, Ghergurovich JM, Morscher RJ, Jang C, Teng X, et al. 2017. Glucose feeds the TCA cycle via circulating lactate. Nature 551:115–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Matthews DE, Motil KJ, Rohrbaugh DK, Burke JF, Young VR, Bier DM. 1980. Measurement of leucine metabolism in man from a primed, continuous infusion of L-[1-13C] leucine. Am. J. Physiol. Endocrinol. Metab 238(5):E473–79 [DOI] [PubMed] [Google Scholar]
- 81.Wolfe RR, Goodenough RD, Wolfe MH, Royle GT, Nadel ER. 1982. Isotopic analysis of leucine and urea metabolism in exercising humans. J. Appl. Physiol 52(2):458–66 [DOI] [PubMed] [Google Scholar]
- 82.Short KR, Vittone JL, Bigelow ML, Proctor DN, Nair KS. 2004. Age and aerobic exercise training effects on whole body and muscle protein metabolism. Am. J. Physiol. Endocrinol. Metab 286(1):E92–101 [DOI] [PubMed] [Google Scholar]
- 83.Waterlow JC. 2006. Protein Turnover Wallingford, UK: CABI [Google Scholar]
- 84.May ME, Buse MG. 1989. Effects of branched-chain amino acids on protein turnover. Diabetes/Metab. Rev 5(3):227–45 [DOI] [PubMed] [Google Scholar]
- 85.Anthony JC, Yoshizawa F, Anthony TG, Vary TC, Jefferson LS, Kimball SR. 2000. Leucine stimulates translation initiation in skeletal muscle of postabsorptive rats via a rapamycin-sensitive pathway. J. Nutr 130(10):2413–19 [DOI] [PubMed] [Google Scholar]
- 86.Anthony JC, Lang CH, Crozier SJ, Anthony TG, MacLean DA, et al. 2002. Contribution of insulin to the translational control of protein synthesis in skeletal muscle by leucine. Am. J. Physiol. Endocrinol. Metab 282(5):E1092–101 [DOI] [PubMed] [Google Scholar]
- 87.Wang X, Proud CG. 2006. The mTOR pathway in the control of protein synthesis. Physiology 21(5):362–69 [DOI] [PubMed] [Google Scholar]
- 88.Nygren J, Nair KS. 2003. Differential regulation of protein dynamics in splanchnic and skeletal muscle beds by insulin and amino acids in healthy human subjects. Diabetes 52(6):1377–85 [DOI] [PubMed] [Google Scholar]
- 89.James HA, O’Neill BT, Nair KS. 2017. Insulin regulation of proteostasis and clinical implications. Cell Metab 26(2):310–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Rooyackers OE, Nair KS. 1997. Hormonal regulation of human muscle protein metabolism. Annu. Rev. Nutr 17(1):457–85 [DOI] [PubMed] [Google Scholar]
- 91.Biolo G, Tipton KD, Klein S, Wolfe RR. 1997. An abundant supply of amino acids enhances the metabolic effect of exercise on muscle protein. Am. J. Physiol. Endocrinol. Metab 273(1):E122–29 [DOI] [PubMed] [Google Scholar]
- 92.Shad BJ, Thompson JL, Breen L. 2016. Does the muscle protein synthetic response to exercise and amino acid-based nutrition diminish with advancing age? A systematic review. Am. J. Physiol. Endocrinol. Metab 311(5):E803–17 [DOI] [PubMed] [Google Scholar]
- 93.Stokes T, Hector A, Morton R, McGlory C, Phillips S. 2018. Recent perspectives regarding the role of dietary protein for the promotion of muscle hypertrophy with resistance exercise training. Nutrients 10(2):180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Fryburg DA, Barrett EJ, Louard RJ, Gelfand RA. 1990. Effect of starvation on human muscle protein metabolism and its response to insulin. Am. J. Physiol. Endocrinol. Metab 259(4):E477–82 [DOI] [PubMed] [Google Scholar]
- 95.Castellino P, Luzi L, Simonson DC, Haymond M, DeFronzo RA. 1987. Effect of insulin and plasma amino acid concentrations on leucine metabolism in man. Role of substrate availability on estimates of whole body protein synthesis. J. Clin. Investig 80(6):1784–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Holeček M, Šprongl L, Skopec F, Andrýs C, Pecka M. 1997. Leucine metabolism in TNF-α and endotoxin-treated rats: contribution of hepatic tissue. Am. J. Physiol. Endocrinol. Metab 273(6):E1052–58 [DOI] [PubMed] [Google Scholar]
- 97.Riis ALD, Jørgensen JOL, Gjedde S, Nørrelund H, Jurik AG, et al. 2005. Whole body and forearm substrate metabolism in hyperthyroidism: evidence of increased basal muscle protein breakdown. Am. J. Physiol. Endocrinol. Metab 288(6):E1067–73 [DOI] [PubMed] [Google Scholar]
- 98.Riis ALD, Jørgensen JOL, Ivarsen P, Frystyk J, Weeke J, Møller N. 2008. Increased protein turnover and proteolysis is an early and primary feature of short-term experimental hyperthyroidism in healthy women. J. Clin. Endocrinol. Metab 93(10):3999–4005 [DOI] [PubMed] [Google Scholar]
- 99.Kobayashi R, Shimomura Y, Otsuka M, Popov KM, Harris RA. 2000. Experimental hyperthyroidism causes inactivation of the branched-chain α-ketoacid dehydrogenase complex in rat liver. Arch. Biochem. Biophys 375(1):55–61 [DOI] [PubMed] [Google Scholar]
- 100.el-Khoury AE, Forslund A, Olsson R, Branth S, Sjodin A, et al. 1997. Moderate exercise at energy balance does not affect 24-h leucine oxidation or nitrogen retention in healthy men. Am. J. Physiol. Endocrinol. Metab 273(2):E394–407 [DOI] [PubMed] [Google Scholar]
- 101.Phillips SM, Atkinson SA, Tarnopolsky MA, MacDougall JD. 1993. Gender differences in leucine kinetics and nitrogen balance in endurance athletes. J. Appl. Physiol 75(5):2134–41 [DOI] [PubMed] [Google Scholar]
- 102.Wagenmakers AJM, Brookes JH, Coakley JH, Reilly T, Edwards RHT. 1989. Exercise-induced activation of the branched-chain 2-oxo acid dehydrogenase in human muscle. Eur. J. Appl. Physiol. Occupat. Physiol 59(3):159–67 [DOI] [PubMed] [Google Scholar]
- 103.White TP, Brooks GA. 1981. [U-14C]-glucose, -alanine, and -leucine oxidation in rats at rest and two intensities of running. Am. J. Physiol. Endocrinol. Metab 240(2):E155–65 [DOI] [PubMed] [Google Scholar]
- 104.McKenzie S, Phillips SM, Carter SL, Lowther S, Gibala MJ, Tarnopolsky MA. 2000. Endurance exercise training attenuates leucine oxidation and BCOAD activation during exercise in humans. Am. J. Physiol. Endocrinol. Metab 278(4):E580–87 [DOI] [PubMed] [Google Scholar]
- 105.Hamadeh MJ, Devries MC, Tarnopolsky MA. 2005. Estrogen supplementation reduces whole body leucine and carbohydrate oxidation and increases lipid oxidation in men during endurance exercise. J. Clin. Endocrinol. Metab 90(6):3592–99 [DOI] [PubMed] [Google Scholar]
- 106.Overmyer K, Evans C, Qi N, Minogue C, Carson J, et al. 2015. Maximal oxidative capacity during exercise is associated with skeletal muscle fuel selection and dynamic changes in mitochondrial protein acetylation. Cell Metab 21(3):468–78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Dohm GL, Hecker AL, Brown WE, Klain GJ, Puente FR, et al. 1977. Adaptation of protein metabolism to endurance training. Biochem. J 164:705–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Hatazawa Y, Tadaishi M, Nagaike Y, Morita A, Ogawa Y, et al. 2014. PGC-1α-mediated branched-chain amino acid metabolism in the skeletal muscle. PLOS ONE 9(3):e91006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Hatazawa Y, Senoo N, Tadaishi M, Ogawa Y, Ezaki O, et al. 2015. Metabolomic analysis of the skeletal muscle of mice overexpressing PGC-1α. PLOS ONE 10(6):e0129084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Neinast MD, Jang C, Hui S, Murashige DS, Chu Q, et al. 2018. Quantitative analysis of the whole-body metabolic fate of branched-chain amino acids. Cell Metab In press [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Hutson S, Wallin R, Hall T. 1992. Identification of mitochondrial branched chain aminotransferase and its isoforms in rat tissues. J. Biol. Chem 267(22):15681–86 [PubMed] [Google Scholar]
- 112.Ding C, Li Y, Guo F, Jiang Y, Ying W, et al. 2016. A cell-type-resolved liver proteome. Mol. Cell. Proteom 15(10):3190–202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Palacín M, Estévez R, Bertran J, Zorzano A. 1998. Molecular biology of mammalian plasma membrane amino acid transporters. Physiol. Rev 78(4):969–1054 [DOI] [PubMed] [Google Scholar]
- 114.Kanai Y, Segawa H, Miyamoto K, Uchino H, Takeda E, Endou H. 1998. Expression cloning and characterization of a transporter for large neutral amino acids activated by the heavy chain of 4F2 antigen (CD98). J. Biol. Chem 273(37):23629–32 [DOI] [PubMed] [Google Scholar]
- 115.Mastroberardino L, Spindler B, Pfeiffer R, Skelly PJ, Loffing J, et al. 1998. Amino-acid transport by heterodimers of 4F2hc/CD98 and members of a permease family. Nature 395(6699):288–91 [DOI] [PubMed] [Google Scholar]
- 116.Oxender DL, Christensen HN. 1963. Distinct mediating systems for the transport of neutral amino acids by the Ehrlich cell. J. Biol. Chem 238(11):3686–99 [PubMed] [Google Scholar]
- 117.Verrey F 2003. System L: heteromeric exchangers of large, neutral amino acids involved in directional transport. Pflügers Arch 445(5):529–33 [DOI] [PubMed] [Google Scholar]
- 118.Newgard CB. 2012. Interplay between lipids and branched-chain amino acids in development of insulin resistance. Cell Metab 15(5):606–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Smith QR, Momma S, Aoyagi M, Rapoport SI. 1987. Kinetics of neutral amino acid transport across the blood-brain barrier. J. Neurochem 49(5):1651–58 [DOI] [PubMed] [Google Scholar]
- 120.Gluud LL, Dam G, Les I, Marchesini G, Borre M, et al. 2017. Branched-chain amino acids for people with hepatic encephalopathy. Cochrane Database Syst. Rev 9:1–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Silva LS, Poschet G, Nonnenmacher Y, Becker HM, Sapcariu S, et al. 2017. Branched-chain ketoacids secreted by glioblastoma cells via MCT1 modulate macrophage phenotype. EMBO Rep 2017:e201744154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Harper Alfred E 1989. Chairman’s remarks: thoughts on the role of branched-chain α-keto acid dehydrogenase complex in nitrogen metabolism. Ann. N.Y. Acad. Sci 573(1):267–73 [DOI] [PubMed] [Google Scholar]
- 123.Nurjhan N, Bucci A, Perriello G, Stumvoll M, Dailey G, et al. 1995. Glutamine: a major gluconeogenic precursor and vehicle for interorgan carbon transport in man. J. Clin. Investig 95(1):272–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Haymond MW, Miles JM. 1982. Branched chain amino acids as a major source of alanine nitrogen in man. Diabetes 31(1):86–89 [DOI] [PubMed] [Google Scholar]
- 125.Odessey R, Khairallah EA, Goldberg AL. 1974. Origin and possible significance of alanine production by skeletal muscle. J. Biol. Chem 249(23):7623–29 [PubMed] [Google Scholar]
- 126.Stumvoll M, Meyer C, Perriello G, Kreider M, Welle S, Gerich J. 1998. Human kidney and liver gluconeogenesis: evidence for organ substrate selectivity. Am. J. Physiol. Endocrinol. Metab 274(5):E817–26 [DOI] [PubMed] [Google Scholar]
- 127.Sperringer JE, Addington A, Hutson SM. 2017. Branched-chain amino acids and brain metabolism. Neurochem. Res 42(6):1697–709 [DOI] [PubMed] [Google Scholar]
- 128.Dancis J, Levitz M, Miller S, Westall RG. 1959. Maple syrup urine disease. Br. Med. J 1(5114):90–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Menkes JH. 1959. Maple syrup disease: isolation and identification of organic acids in the urine. Pediatrics 23(2):348–53 [PubMed] [Google Scholar]
- 130.Menkes JH, Hurst PL, Craig JM. 1954. A new syndrome: progressive familial infantile cerebral dysfunction associated with an unusual urinary substance. Pediatrics 14(5):462–67 [PubMed] [Google Scholar]
- 131.Schadewaldt P, Bodner-Leidecker A, Hammen H-W, Wendel U. 1999. Significance of l-alloisoleucine in plasma for diagnosis of maple syrup urine disease. Clin. Chem 45(10):1734–40 [PubMed] [Google Scholar]
- 132.Podebrad F, Heil M, Reichert S, Mosandl A, Sewell A, Böhles H. 1999. 4,5-Dimethyl-3-hydroxy-2[5H]-furanone (sotolone)—the odour of maple syrup urine disease. J. Inherit. Metab. Dis 22(2):107–14 [DOI] [PubMed] [Google Scholar]
- 133.Sewell AC, Mosandl A, Böhles H. 1999. False diagnosis of maple syrup urine disease owing to ingestion of herbal tea. N. Engl. J. Med 341(10):769. [DOI] [PubMed] [Google Scholar]
- 134.Adeva-Andany MM, López-Maside L, Donapetry-García C, Fernández-Fernández C, Sixto-Leal C.2017. Enzymes involved in branched-chain amino acid metabolism in humans. Amino Acids 49(6):1005–28 [DOI] [PubMed] [Google Scholar]
- 135.Yudkoff M 1997. Brain metabolism of branched-chain amino acids. Glia 21(1):92–98 [DOI] [PubMed] [Google Scholar]
- 136.Yudkoff M, Nissim I, Kim S, Pleasure D, Hummeler K, Segal S. 1983. [15N] leucine as a source of [15N] glutamate in organotypic cerebellar explants. Biochem. Biophys. Res. Commun 115(1):174–79 [DOI] [PubMed] [Google Scholar]
- 137.Burrage LC, Nagamani SCS, Campeau PM, Lee BH. 2014. Branched-chain amino acid metabolism: from rare Mendelian diseases to more common disorders. Hum. Mol. Genet 23(R1):R1–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Gambello MJ, Li H. 2018. Current strategies for the treatment of inborn errors of metabolism. J. Genet. Genom 45(2):61–70 [DOI] [PubMed] [Google Scholar]
- 139.Díaz VM, Camarena C, de la Vega Á, Martínez-Pardo M, Díaz C, et al. 2014. Liver transplantation for classical maple syrup urine disease: long-term follow-up. J. Pediatr. Gastroenterol. Nutr 59(5):636–39 [DOI] [PubMed] [Google Scholar]
- 140.Barshop BA, Khanna A. 2005. Domino hepatic transplantation in maple syrup urine disease. N. Engl. J. Med 353(22):2410–11 [DOI] [PubMed] [Google Scholar]
- 141.García-Cazorla A, Oyarzabal A, Fort J, Robles C, Castejón E, et al. 2014. Two novel mutations in the BCKDK (branched-chain keto-acid dehydrogenase kinase) gene are responsible for a neurobehavioral deficit in two pediatric unrelated patients. Hum. Mutat 35(4):470–77 [DOI] [PubMed] [Google Scholar]
- 142.Novarino G, El-Fishawy P, Kayserili H, Meguid NA, Scott EM, et al. 2012. Mutations in BCKD-kinase lead to a potentially treatable form of autism with epilepsy. Science 338(6105):394–97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Tčrlungeanu DC, Deliu E, Dotter CP, Kara M, Janiesch PC, et al. 2016. Impaired amino acid transport at the blood brain barrier is a cause of autism spectrum disorder. Cell 167(6):1481–94.e18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Adibi SA. 1968. Influence of dietary deprivations on plasma concentration of free amino acids of man. J. Appl. Physiol 25(1):52–57 [DOI] [PubMed] [Google Scholar]
- 145.Felig P, Marliss E, Cahill GF. 1969. Plasma amino acid levels and insulin secretion in obesity. N. Engl. J. Med 281(15):811–16 [DOI] [PubMed] [Google Scholar]
- 146.Wang TJ, Larson MG, Vasan RS, Cheng S, Rhee EP, et al. 2011. Metabolite profiles and the risk of developing diabetes. Nat. Med 17(4):448–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Newgard CB, An J, Bain JR, Muehlbauer MJ, Stevens RD, et al. 2009. A branched-chain amino acid-related metabolic signature that differentiates obese and lean humans and contributes to insulin resistance. Cell Metab 9(4):311–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Liu J, Semiz S, van der Lee SJ, van der Spek A, Verhoeven A, et al. 2017. Metabolomics based markers predict type 2 diabetes in a 14-year follow-up study. Metabolomics 13(9):104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Guasch-Ferré M, Hruby A, Toledo E, Clish CB, Martínez-González MA, et al. 2016. Metabolomics in prediabetes and diabetes: a systematic review and meta-analysis. Diabetes Care 39(5):833–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Lotta LA, Scott RA, Sharp SJ, Burgess S, Luan J, et al. 2016. Genetic predisposition to an impaired metabolism of the branched-chain amino acids and risk of type 2 diabetes: a Mendelian randomisation analysis. PLOS Med 13(11):e1002179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Harris L-ALS, Smith GI, Patterson BW, Ramaswamy RS, Okunade AL, et al. 2017. Alterations in 3-hydroxyisobutyrate and FGF21 metabolism are associated with protein ingestion-induced insulin resistance. Diabetes 66(7):1871–78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Cummings NE, Williams EM, Kasza I, Konon EN, Schaid MD, et al. 2017. Restoration of metabolic health by decreased consumption of branched-chain amino acids: a low BCAA diet restores metabolic health. J. Physiol 596(4):623–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Mahendran Y, Jonsson A, Have CT, Allin KH, Witte DR, et al. 2017. Genetic evidence of a causal effect of insulin resistance on branched-chain amino acid levels. Diabetologia 60(5):873–78 [DOI] [PubMed] [Google Scholar]
- 154.Wang Q, Holmes MV, Davey Smith G, Ala-Korpela M. 2017. Genetic support for a causal role of insulin resistance on circulating branched-chain amino acids and inflammation. Diabetes Care 40(12):1779–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Herman MA, She P, Peroni OD, Lynch CJ, Kahn BB. 2010. Adipose tissue branched chain amino acid (BCAA) metabolism modulates circulating BCAA levels. J. Biol. Chem 285(15):11348–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Lips MA, Van Klinken JB, van Harmelen V, Dharuri HK, ‘t Hoen PAC, et al. 2014. Roux-en-Y gastric bypass surgery, but not calorie restriction, reduces plasma branched-chain amino acids in obese women independent of weight loss or the presence of type 2 diabetes. Diabetes Care 37(12):3150–56 [DOI] [PubMed] [Google Scholar]
- 157.Pietiläinen KH, Naukkarinen J, Rissanen A, Saharinen J, Ellonen P, et al. 2008. Global transcript profiles of fat in monozygotic twins discordant for BMI: pathways behind acquired obesity. PLOS Med 5(3):e51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Sears DD, Hsiao G, Hsiao A, Yu JG, Courtney CH, et al. 2009. Mechanisms of human insulin resistance and thiazolidinedione-mediated insulin sensitization. PNAS 106(44):18745–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.She P, Van Horn C, Reid T, Hutson SM, Cooney RN, Lynch CJ. 2007. Obesity-related elevations in plasma leucine are associated with alterations in enzymes involved in branched-chain amino acid metabolism. AJP Endocrinol. Metab 293(6):E1552–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Wiklund P, Zhang X, Pekkala S, Autio R, Kong L, et al. 2016. Insulin resistance is associated with altered amino acid metabolism and adipose tissue dysfunction in normoglycemic women. Sci. Rep 6:24540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Burrill JS, Long EK, Reilly B, Deng Y, Armitage IM, et al. 2015. Inflammation and ER stress regulate branched-chain amino acid uptake and metabolism in adipocytes. Mol. Endocrinol 29(3):411–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Lo KA, Labadorf A, Kennedy NJ, Han MS, Yap YS, et al. 2013. Analysis of in vitro insulin-resistance models and their physiological relevance to in vivo diet-induced adipose insulin resistance. Cell Rep 5(1):259–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Hsiao G, Chapman J, Ofrecio JM, Wilkes J, Resnik JL, et al. 2011. Multi-tissue, selective PPARγ modulation of insulin sensitivity and metabolic pathways in obese rats. Am. J. Physiol. Endocrinol. Metab 300(1):E164–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Lian K, Du C, Liu Y, Zhu D, Yan W, et al. 2015. Impaired adiponectin signaling contributes to disturbed catabolism of branched-chain amino acids in diabetic mice. Diabetes 64(1):49–59 [DOI] [PubMed] [Google Scholar]
- 165.White PJ, Lapworth AL, An J, Wang L, McGarrah RW, et al. 2016. Branched-chain amino acid restriction in Zucker-fatty rats improves muscle insulin sensitivity by enhancing efficiency of fatty acid oxidation and acyl-glycine export. Mol. Metab 5(7):538–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.White PJ, McGarrah RW, Grimsrud PA, Tso S-C, Yang W-H, et al. 2018. The BCKDH kinase and phosphatase integrate BCAA and lipid metabolism via regulation of ATP-citrate lyase. Cell Metab 27(6):1281–93.e7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.DeFronzo RA, Tripathy D. 2009. Skeletal muscle insulin resistance is the primary defect in type 2 diabetes. Diabetes Care 32(Suppl. 2):S157–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Pedroso J, Zampieri T, Donato J. 2015. Reviewing the effects of l-leucine supplementation in the regulation of food intake, energy balance, and glucose homeostasis. Nutrients 7(5):3914–37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Mardinoglu A, Gogg S, Lotta LA, Stancakova A, Nerstedt A, et al. 2018. Elevated plasma levels of 3-hydroxyisobutyric acid are associated with incident type 2 diabetes. EBioMedicine 27:151–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Budhathoki S, Iwasaki M, Yamaji T, Yamamoto H, Kato Y, Tsugane S. 2016. Association of plasma concentrations of branched-chain amino acids with risk of colorectal adenoma in a large Japanese population. Ann. Oncol 28(4):818–23 [DOI] [PubMed] [Google Scholar]
- 171.Mayers JR, Wu C, Clish CB, Kraft P, Torrence ME, et al. 2014. Elevation of circulating branched-chain amino acids is an early event in human pancreatic adenocarcinoma development. Nat. Med 20(10):1193–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Nezami Ranjbar MR, Luo Y, Di Poto C, Varghese RS, Ferrarini A, et al. 2015. GC-MS based plasma metabolomics for identification of candidate biomarkers for hepatocellular carcinoma in Egyptian cohort. PLOS ONE 10(6):e0127299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Watanabe A, Higashi T, Sakata T, Nagashima H. 1984. Serum amino acid levels in patients with hepatocellular carcinoma. Cancer 54(9):1875–82 [DOI] [PubMed] [Google Scholar]
- 174.Mayers JR, Torrence ME, Danai LV, Papagiannakopoulos T, Davidson SM, et al. 2016. Tissue of origin dictates branched-chain amino acid metabolism in mutant Kras-driven cancers. Science 353(6304):1161–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Bi X, Henry CJ. 2017. Plasma-free amino acid profiles are predictors of cancer and diabetes development. Nutr. Diabetes 7(3):e249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Wei J, Xie G, Zhou Z, Shi P, Qiu Y, et al. 2011. Salivary metabolite signatures of oral cancer and leukoplakia. Int. J. Cancer 129(9):2207–17 [DOI] [PubMed] [Google Scholar]
- 177.Chang IW, Wu WJ, Wang YH, Wu TF, Liang PI, et al. 2015. BCAT1 overexpression is an indicator of poor prognosis in patients with urothelial carcinomas of the upper urinary tract and urinary bladder. Histopathology 68(4):520–32 [DOI] [PubMed] [Google Scholar]
- 178.Xu Y, Yu W, Yang T, Zhang M, Liang C, et al. 2018. Overexpression of BCAT1 is a prognostic marker in gastric cancer. Hum. Pathol 75:41–46 [DOI] [PubMed] [Google Scholar]
- 179.Zheng YH, Hu WJ, Chen BC, Grahn THM, Zhao YR, et al. 2016. BCAT1, a key prognostic predictor of hepatocellular carcinoma, promotes cell proliferation and induces chemoresistance to cisplatin. Liver Int 36(12):1836–47 [DOI] [PubMed] [Google Scholar]
- 180.Tönjes M, Barbus S, Park YJ, Wang W, Schlotter M, et al. 2013. BCAT1 promotes cell proliferation through amino acid catabolism in gliomas carrying wild-type IDH1. Nat. Med 19(7):901–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Xu W, Yang H, Liu Y, Yang Y, Wang P, et al. 2011. Oncometabolite 2-hydroxyglutarate is a competitive inhibitor of α-ketoglutarate-dependent dioxygenases. Cancer Cell 19(1):17–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Hattori A, Tsunoda M, Konuma T, Kobayashi M, Nagy T, et al. 2017. Cancer progression by reprogrammed BCAA metabolism in myeloid leukaemia. Nature 545(7655):500–4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Oktyabri D, Ishimura A, Tange S, Terashima M, Suzuki T. 2016. DOT1L histone methyltransferase regulates the expression of BCAT1 and is involved in sphere formation and cell migration of breast cancer cell lines. Biochimie 123:20–31 [DOI] [PubMed] [Google Scholar]
- 184.Eden A, Simchen G, Benvenisty N. 1996. Two yeast homologs of ECA39, a target for c-Myc regulation, code for cytosolic and mitochondrial branched-chain amino acid aminotransferases. J. Biol. Chem 271(34):20242–45 [DOI] [PubMed] [Google Scholar]
- 185.Zhou W, Feng X, Ren C, Jiang X, Liu W, et al. 2013. Over-expression of BCAT1, a c-Myc target gene, induces cell proliferation, migration and invasion in nasopharyngeal carcinoma. Mol. Cancer 12(1):53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Raffel S, Falcone M, Kneisel N, Hansson J, Wang W, et al. 2017. BCAT1 restricts αKG levels in AML stem cells leading to IDHmut-like DNA hypermethylation. Nature 551:384–88 [DOI] [PubMed] [Google Scholar]
- 187.McBrayer SK, Mayers JR, DiNatale GJ, Shi DD, Khanal J, et al. 2018. Transaminase inhibition by 2-hydroxyglutarate impairs glutamate biosynthesis and redox homeostasis in glioma. Cell 175(1):101–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Zhang L, Han J. 2017. Branched-chain amino acid transaminase 1 (BCAT1) promotes the growth of breast cancer cells through improving mTOR-mediated mitochondrial biogenesis and function. Biochem. Biophys. Res. Commun 486(2):224–31 [DOI] [PubMed] [Google Scholar]
- 189.Bhattacharya S, Granger CB, Craig D, Haynes C, Bain J, et al. 2014. Validation of the association between a branched chain amino acid metabolite profile and extremes of coronary artery disease in patients referred for cardiac catheterization. Atherosclerosis 232(1):191–96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Shah SH, Bain JR, Muehlbauer MJ, Stevens RD, Crosslin DR, et al. 2010. Association of a peripheral blood metabolic profile with coronary artery disease and risk of subsequent cardiovascular events. Circ. Cardiovasc. Genet 3(2):207–14 [DOI] [PubMed] [Google Scholar]
- 191.Shah SH, Sun J-L, Stevens RD, Bain JR, Muehlbauer MJ, et al. 2012. Baseline metabolomic profiles predict cardiovascular events in patients at risk for coronary artery disease. Am. Heart J 163(5):844–50 [DOI] [PubMed] [Google Scholar]
- 192.McGarrah RW, Crown SB, Zhang G-F, Shah SH, Newgard CB. 2018. Cardiovascular metabolomics. Circ. Res 122(9):1238–58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Li T, Zhang Z, Kolwicz SC, Abell L, Roe ND, et al. 2017. Defective branched-chain amino acid catabolism disrupts glucose metabolism and sensitizes the heart to ischemia-reperfusion injury. Cell Metab 25(2):374–85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Sun H, Olson KC, Gao C, Prosdocimo DA, Zhou M, et al. 2016. Catabolic defect of branched-chain amino acids promotes heart failure. Circulation 133(21):2038–49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Wang W, Zhang F, Xia Y, Zhao S, Yan W, et al. 2016. Defective branched chain amino acid catabolism contributes to cardiac dysfunction and remodeling following myocardial infarction. Am. J. Physiol. Heart Circ. Physiol 311(5):H1160–69 [DOI] [PubMed] [Google Scholar]
- 196.McNulty PH, Jacob R, Deckelbaum LI, Young LH. 2000. Effect of hyperinsulinemia on myocardial amino acid uptake in patients with coronary artery disease. Metab. Clin. Exp 49(10):1365–69 [DOI] [PubMed] [Google Scholar]
- 197.Schwartz RG, Barrett EJ, Francis CK, Jacob R, Zaret BL. 1985. Regulation of myocardial amino acid balance in the conscious dog. J. Clin. Investig 75(4):1204–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Turer AT, Stevens RD, Bain JR, Muehlbauer MJ, van der Westhuizen J, et al. 2009. Metabolomic profiling reveals distinct patterns of myocardial substrate use in humans with coronary artery disease or left ventricular dysfunction during surgical ischemia/reperfusion. Circulation 119(13):1736–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Buse MG, Biggers JF, Friderici KH, Buse JF. 1972. Oxidation of branched chain amino acids by isolated hearts and diaphragms of the rat: the effect of fatty acids, glucose, and pyruvate respiration. J. Biol. Chem 247(24):8085–96 [PubMed] [Google Scholar]
- 200.De Jong KA, Lopaschuk GD. 2017. Complex energy metabolic changes in heart failure with preserved ejection fraction and heart failure with reduced ejection fraction. Can. J. Cardiol 33(7):860–71 [DOI] [PubMed] [Google Scholar]
- 201.Lopaschuk GD. 2017. Metabolic modulators in heart disease: past, present, and future. Can. J. Cardiol 33(7):838–49 [DOI] [PubMed] [Google Scholar]
- 202.McMullen JR. 2004. Inhibition of mTOR signaling with rapamycin regresses established cardiac hyper-trophy induced by pressure overload. Circulation 109(24):3050–55 [DOI] [PubMed] [Google Scholar]
- 203.Völkers M, Toko H, Doroudgar S, Din S, Quijada P, et al. 2013. Pathological hypertrophy amelioration by PRAS40-mediated inhibition of mTORC1. PNAS 110(31):12661–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Sciarretta S, Forte M, Frati G, Sadoshima J. 2018. New insights into the role of mTOR signaling in the cardiovascular system. Circ. Res 122(3):489–505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Jackson RH, Singer TP. 1983. Inactivation of the 2-ketoglutarate and pyruvate dehydrogenase complexes of beef heart by branched chain keto acids. J. Biol. Chem 258(3):1857–65 [PubMed] [Google Scholar]