To the Editor
Specific immunotherapy is a highly effective treatment of respiratory allergy that can affect the course of disease and prevent the onset of allergic asthma.1 However, the treatment can cause anaphylactic reactions; therefore, most allergy vaccines must be given in small and increasing doses, requiring multiple administrations in short intervals.2 Furthermore, late-phase reactions mediated by T cells may occur, which have been observed even on the administration of allergen derivatives lacking IgE epitopes as long as they contain allergen-derived T-cell epitopes.
Detailed knowledge of allergen structures has allowed the design of new treatment forms for allergy, which hold promise to improve safety and efficacy and to simplify treatment schedules.3 Approaches that have been proposed for this purpose and that are currently undergoing clinical investigation either selectively target allergen-specific T cells4 or use allergen variants with preserved T-cell epitopes that aim at inducing allergen-specific–blocking IgG antibodies.5,6 A third approach aims at reducing both IgE-mediated adverse effects and T-cell–mediated adverse effects by using recombinant fusion proteins consisting of a carrier protein and allergen-derived peptides that induce allergen-specific protective IgG antibodies with carrier-specific T-cell help.7 We report the first clinical safety evaluation of such a vaccine, termed BM32. BM32 consists of 4 recombinant fusion proteins, BM321, BM322, BM325, and BM326 (see Fig E1 in this article’s Online Repository at www.jacionline.org). Each of these fusion proteins contains the PreS protein from hepatitis B virus, which humans encounter only when exposed to the virus or receiving PreS-containing hepatitis B vaccines, and nonallergenic peptides derived from the IgE binding sites of the 4 major grass pollen allergens Phl p 1, Phl p 2, Phl p 5, and Phl p 6.7 The PreS fusion proteins can be reproducibly obtained under good manufacturing practice conditions in gram amounts by recombinant expression in Escherichia coli as pure, soluble, unfolded proteins that do not aggregate.7 To clinically evaluate the potential of this vaccine to cause adverse effects, we studied immediate-type and late-phase reactions by skin prick test (SPT) as well as atopy patch test (APT) experiments in 60 grass pollen–allergic patients (see the Methods section and Table E1 in this article’s Online Repository at www.jacionline.org). The study was approved by the Ethics Committee of the Medical University of Vienna, and all patients gave written informed consent.
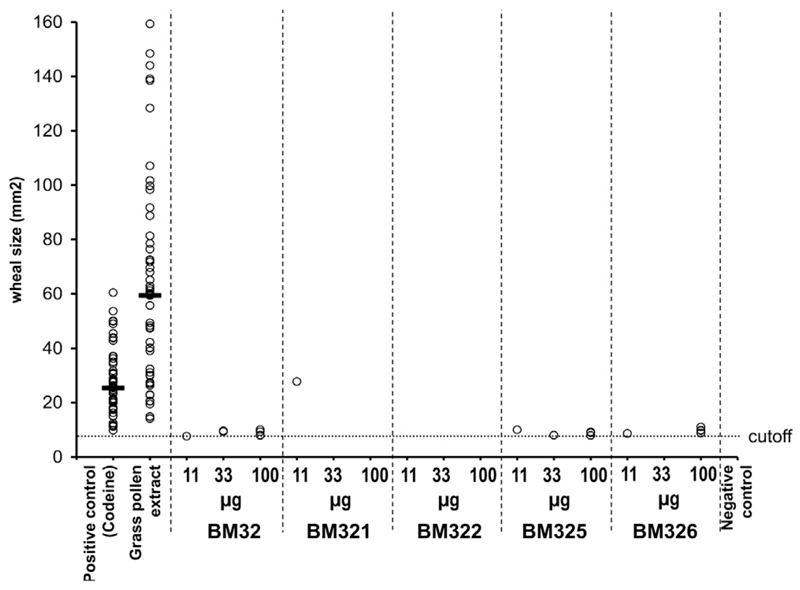
On study day 1, all participants underwent skin prick testing with 2 different commercial grass pollen extracts to ensure their sensitivity to grass pollen. BM32 was tested on study day 2, whereas separate SPTs with the 4 vaccine components (BM321, BM322, BM325, and BM326) were performed on study day 3. All tests with BM32 and the components were done using 3 concentrations (11, 33, 100 μg/mL) and in doublets. To ensure maximum allergic sensitivity of the study participants, all SPTs were done during the peak grass pollen season and all subjects suffered from allergic symptoms when the study was performed (see Fig E2 in this article’s Online Repository at www.jacionline.org). Results from SPTs, displayed in Fig 1, revealed an almost completely abolished ability of BM32 and its components to induce immediate-type allergic reactions, and positive SPT results to BM32 were exceptions, even at the highest dose of 100 μg of BM32. The individual BM32 components induced reactions in only 6 grass pollen–allergic individuals, of which all but one were close to the cutoff. All subjects showed SPT reactions to grass pollen extract (median wheal area, 59.8 ± 46.2 mm2; Fig 1). Our results thus show that BM32 and its components have a greatly diminished ability to induce such immediate-type allergic reactions and hence it is possible that a BM32-based vaccine may allow performing specific immunotherapy by administering high doses of the vaccine.
Fig 1.
Comparison of immediate SPT reactions to grass pollen extract, BM32, and individual BM32 components. Mean wheal areas (mm2, y-axis) induced with codein phosphate, grass pollen extract, and 3 concentrations (11, 33, 100 μg/mL) of BM32, BM321, BM322, BM325, and BM326 (x-axis) in the 60 study participants are displayed. Horizontal bars denote medians. The dotted line indicates the cutoff. Calculation: (1.5 mm2 × π) = 7.07 mm2.
To determine the ability of BM32 to induce delayed-type skin reactions, APTs were performed with either a mix of 160 or 80 μg of each of the 4 components, or a commercial grass pollen extract (Stallergenes, Antony, France) in Vaseline petroleum jelly (Unilever, London, United Kingdom) as a vehicle or the vehicle alone (negative control). APT reactions were evaluated according to the scoring of the European Task Force on Atopic Dermatitis after 48 hours and are presented in Table I. Ten percent (n = 6) of the subjects had a positive reaction (“+” reaction, n = 2; “++” reaction, n = 4) to grass pollen extract. None of the subjects had positive reactions with either concentration of BM32 or the vehicle (ie, Vaseline). Questionable reactions (ie, only erythema) were observed in 3 study participants. In 2 subjects, erythema was observed also for Vaseline (negative control). One subject developed erythema without papules to grass pollen extract. The results of atopy patch testing thus suggested that a reduction in allergen-specific T-cell epitopes in BM32 was achieved because BM32 did not induce positive APT reactions whereas 10% of the study participants had positive APT reactions to natural grass pollen allergens. These results were also corroborated by the finding that none of our patients reported late-phase reactions after skin prick testing with BM32.
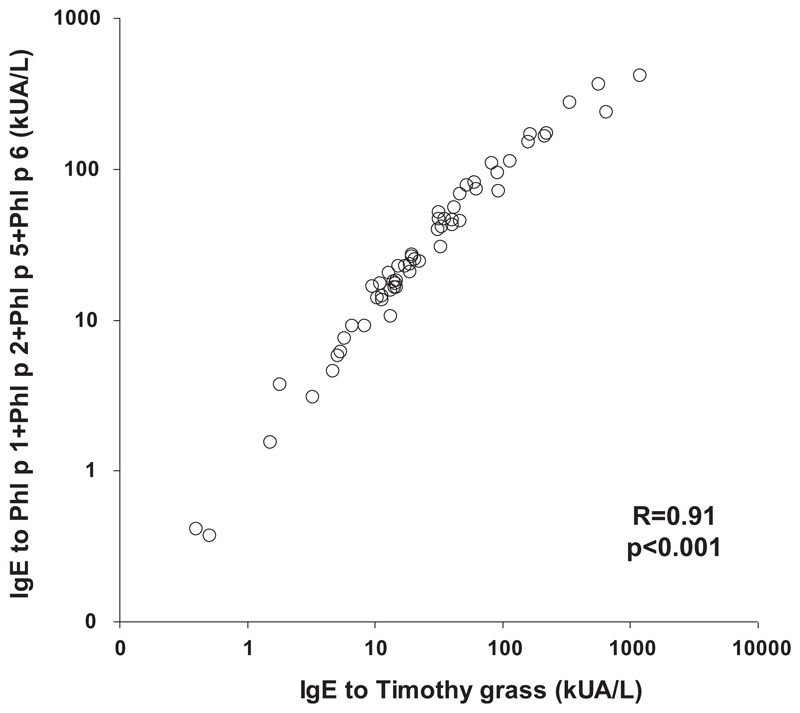
Table I.
Numbers and grades of positive APT reactions to grass pollen extract, BM32, and the vehicle according to the grading system of the European Task Force on Atopic Dermatitis in the study population
| Positive reactions | Questionable reactions | ||
|---|---|---|---|
| + | ++ | ||
| Grass pollen extract | 2 (3.3) | 4 (6.7) | 2 (3.3) |
| BM32, 80 μg/mL | 0 (0) | 2 (3.3) | |
| BM32, 160 μg/mL | 0 (0) | 1 (1.7) | |
| Vaseline | 0 (0) | 2 (3.3) | |
Values are n (%).
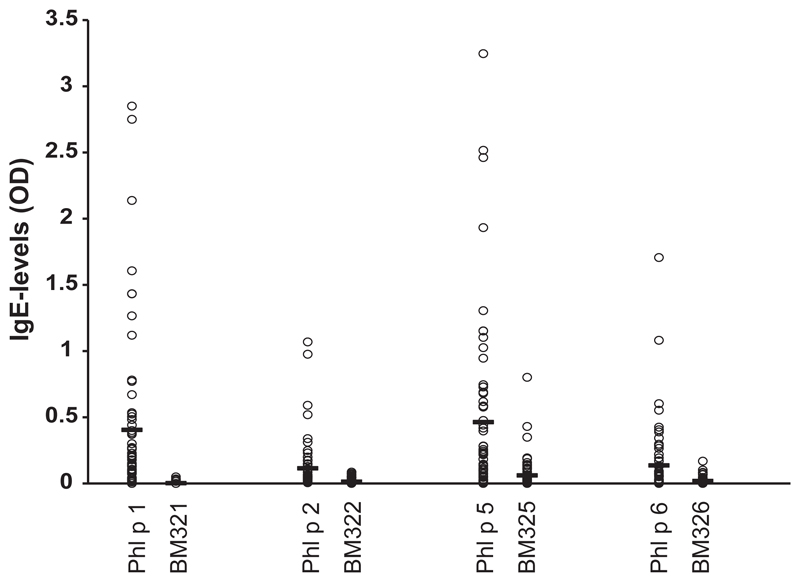
Blood samples were obtained from all study participants, and specific IgE levels to grass pollen extract and to individual grass pollen allergens were measured with the Phadia CAP system (Thermofisher, Uppsala, Sweden), while specific IgE to the 4 wild-type allergens and BM32 components was measured by ELISA. Fig E3 (in this article’s Online Repository at www.jacionline.org) shows that the BM32 components showed almost no IgE reactivity when compared with the wild-type allergens. The percentages of patients showing IgE reactivity to the recombinant allergens were high (Phl p 1, 97%; Phl p 2, 68%; Phl p 5, 82%; Phl p 6, 83%) (see Fig E3). There was a close correlation of timothy grass pollen specific IgE levels with the sum of IgE levels directed to the 4 recombinant allergens (rPhl p 1, rPhl p 2, rPhl p 5, and rPhl p 6), indicating that the 4 allergens contained most of the relevant IgE epitopes (see Fig E4 in this article’s Online Repository at www.jacionline.org). Furthermore, lymphoproliferation assays and cytokine measurements were performed in blood samples from 51 of the study participants. Lymphocyte proliferation was significantly lower to BM32 than to grass pollen extract, and BM32 also induced significantly lower secretion of proinflammatory cytokines (eg, IL-4, IL-5, IL-13, and IFN-γ) as well as of the tolerogenic cytokine (ie, IL-10) in PBMC cultures (see Table E2 in this article’s Online Repository at www.jacionline.org). It will thus be interesting to see in immunotherapy trials with BM32 (www.clinicaltrials.gov; NCT01445002, NCT01538979) whether the reduced production of TH2 cytokines is an advantage of BM32 or whether the reduced production of the TH1 cytokine IFN-γ and of the tolerogenic cytokine IL-10 is a disadvantage.
In summary, our study is the first that investigated the immediate as well as late skin reactivity of allergic patients to a novel type of allergy vaccines based on carrier-bound allergen peptides as exemplified for the BM32 proteins that are the active components of a hypoallergenic vaccine used for the treatment of grass pollen allergy. Our results indicate that BM32 may be used to formulate a vaccine that minimizes adverse effects caused by IgE-as well as T-cell–mediated reactions and hence may be used to treat grass pollen–allergic patients safely and effectively with high doses.
Methods
Patients and study design
Sixty grass pollen–allergic patients (men, 32; women, 28) with a mean age of 29 years were included in the study (Table E1). These patients had a positive case history indicative of grass pollen allergy and a positive skin reaction to timothy grass pollen extract (Stallergenes) and to a 5-grass pollen mix (Stallergenes). Codein phosphate (Stallergenes) was used as a positive control. Only subjects with a positive reaction to grass pollen (ie, a mean weal diameter of >3 mm, corresponding to wheal area of >7.1 mm2) were included in the study. Patients were excluded if they suffered from autoimmune diseases or were under long-term treatment with systemic corticosteroids, immunosuppressive drugs, or psychoactive drugs. Before the study, patients were not allowed to use oral antihistamines for 3 days and local (in the skin test area) and systemic corticosteroids for 14 days. Patients were allowed to use topical nasal or ocular antihistamines or topical nasal corticosteroids before and during the study, but were prohibited from using oral antihistamines or corticosteroids during the study.
The study was approved by the Ethics Committee of the Medical University of Vienna, and all patients gave written informed consent. The study was carried out in accordance with the Code of Ethics of the World Medical Association (Declaration of Helsinki). The study protocol was deposited at www.clinicaltrials.gov before the first patient was included in the trial (NCT01538979).
Skin prick testing
SPTs were performed on lower forearms of patients. Skin test solutions were applied at a distance of 3 cm or more and were pricked with commercial prick lancets (Allergopharma, Reinbek, Germany). After 20 minutes, the wheal reactions were surrounded with a felt pen, photo-documented, and transferred to paper using adhesive tape. Three different SPTs were performed during the study. On study day 1, a screening SPT was performed using 2 different commercial SPT solutions (timothy grass pollen extract and a mix of pollen extracts from 5 different grasses) as well as codein phosphate (positive control) and negative control solution. All commercial skin test solutions were obtained from Stallergenes. Only eligible subjects who had positive skin reactions with grass pollen extracts (n = 60) underwent skin prick testing with BM32 and its components and atopy patch testing. Blood samples for measurements of total IgE, allergen-specific and BM32-specific IgE, and PBMC proliferations and cytokine measurements were obtained on study day 1 from eligible subjects. On study day 2, SPTs with 3 different concentrations (11, 33, and 100 μg/mL) of BM32 were performed in doublets on the other lower forearm which had not been used for SPT 1. On study day 3, an SPTwith 3 concentrations (11, 33, and 100 μg/mL) of BM321, BM322, BM325, and BM326 was performed in doublets, using both lower forearms for testing. On days 2 and 3, codein phosphate (Stallergenes), timothy grass pollen extract (Stallergenes), negative control solution (Stallergenes), and diluent (ie, 0.9% sodium chloride solution) were used as positive and negative controls, respectively. Wheals were measured by planimetry using the program ImageJ (http://imagej.nih.gov/ij/).
Atopy patch testing
APTs were performed using 12-mm aluminum cups (Finn Chambers on Scanpor, Large, Epitest Ltd Oy, Tuusula, Finland) containing either 160 μg or 80 μg each of BM321, BM322, BM325, and BM326, or a commercial grass pollen extract for atopy patch testing (Stallergenes) in Vaseline petroleum jelly as a vehicle or the vehicle alone (negative control). APTs were performed on the patients’ upper back on day 2. Before administration, the skin area was stripped using an adhesive tape. After 48 hours, the aluminum cups were removed, the test area was immediately marked, and photo documentation was done. Reactions were graded according to European Task Force on Atopic Dermatitis scoring.E1
Recombinant allergens, BM32
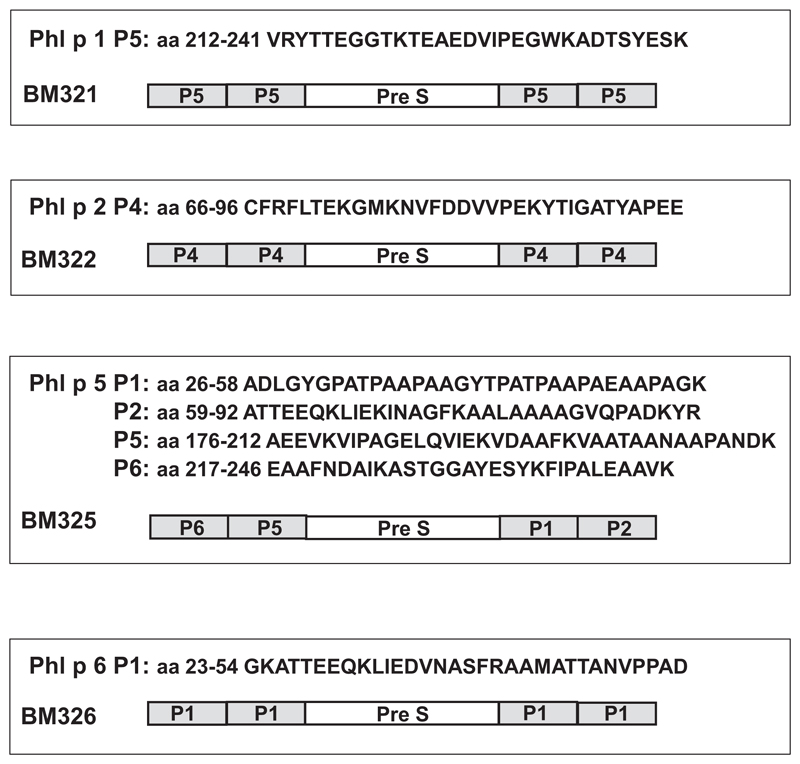
Recombinant Phl p 1, rPhl p 2, rPhl p 5, and rPhl p 6 were obtained from Biomay AG, Vienna, Austria (http://www.biomay.com/). The design and in vitro immunologic characterization of the 4 recombinant hypoallergenic grass pollen allergen derivatives (BM32 components; ie, BM321, BM322, BM325, and BM326) has been described earlier.E2 The BM32 components are recombinant fusion proteins consisting of the PreS protein from hepatitis B virus as carrier and nonallergenic peptides from the major timothy grass pollen allergens Phl p 1, Phl p 2, Phl p 5, and Phl p 6 (Fig E1). They were expressed in Escherichia coli and purified according to good manufacturing practice standards by Biomay AG. An equimolar mix of BM321, BM322, BM325, and BM326 has been designated “BM32.”
Determination of total and allergen-specific IgE, lymphoproliferation assays, and cytokine measurements
Blood samples for the preparation of serum and PBMCs were drawn from the antecubital vein before skin testing. Total IgE and IgE specific levels for timothy grass pollen extract, rPhl p 1, rPhl p 2, rPhl p 5, and rPhl p 6 were measured using the Phadia CAP system. To compare IgE reactivities to BM32 components and the corresponding allergens, IgE levels specific for the recombinant allergens and the BM32 components were also measured by ELISA. ELISA plates (Nunc Maxisorp, Roskilde, Denmark) were coated with 3 μg/mL of recombinant Phl p 1, Phl p 2, Phl p 5, or Phl p 6, or BM321, BM322, BM325, or BM326, and diluted in 100 mM sodium carbonate-bicarbonate buffer, pH = 9.6, for 5 hours at room temperature. Comparable coating of recombinant allergens and BM32 components had been verified in a pilot experiment, in which the 4 recombinant allergens and the corresponding 4 BM32 components were coated on the same plate as described above and detected with titrated rabbit sera raised against each of the recombinant allergens and BM32 components in dilution steps of 1:2, starting at a dilution of 1:1000. ELISA experiments were performed as described previously.E3 Bound IgE antibodies were detected using a peroxidase-linked goat anti-human IgE antibody (KPL, Gaitherburg, Md) diluted 1:2500 in PBST/0.5% BSA. ABTS (2,2’-Azino-bis[3-ethylbenzothiazoline-6-sulfonic acid] diammonium salt, A1888, Sigma-Aldrich, St Louis, Mo) was used for the enzymatic color reaction. Plates were read at 405nm.
For lymphoproliferation assays, PBMCs were isolated from heparinised blood samples by Ficoll (Amersham Pharmacia Biotech, Little Chalfont, United Kingdom) density gradient centrifugation. PBMCs (2 × 105/well) were cultured in triplicates in 96-well plates (Nunclone; Nalge Nunc International, Roskilde, Denmark) in 100 μL serum-free Ultra Culture medium (BioWhittaker, Rockland, Me) supplemented with 2 mM l-glutamin (Sigma-Aldrich), 50 μM β-mercaptoethanol (Sigma-Aldrich), and 0.1 mg gentamicin per milliliter (Sigma-Aldrich) at 37°C and 5% CO2 in a humidified atmosphere. Cells were stimulated with BM32 (0.5 μg each of BM321, BM322, BM325, and BM326 per well) and, for comparison, with 50 μg of timothy grass pollen extract per well (Stallergenes), corresponding to approximately 0.5 μg of the single Phl p 5 allergen,E4 which was established in titration experiments to be the optimal concentration for stimulation, or, for control purposes with medium alone. After 6 days of culture, 0.5 μCi [3H]thymidine (Amersham Pharmacia Biotech) was added per well and 16 hours thereafter incorporated radioactivity was measured by liquid scintillation counting using a microbeta scintillation counter (Wallac ADL, Freiburg, Germany). Mean cpm (counts per minute) values were calculated from the triplicates, and stimulation indices were calculated as the quotient of the cpm obtained for cultures with antigen and medium alone.
Cytokine levels were determined in supernatants from identically prepared PBMC cultures. The concentrations of the cytokines IL-1b, IL-2, IL-4, IL-5, IL-6, IL-7, IL-10, IL-12, IL-13, IL-17, G-CSF, GM-CSF, IFN-γ, and TNF-α were measured using the xMAP Luminex fluorescent bead-based technology (R&D Systems, Minneapolis, Minn) according to the manufacturer’s instructions using a Luminex 100 System (Luminex Corp, Austin, Tex).
Statistical methods
For both groups of subjects (subjects with negative APT reactions, n = 46, and subjects with positive APT reactions, n = 5, respectively), cytokine levels measured after in vitro stimulation of T cells using grass pollen extract were described by median and 25th and 75th percentiles and compared with the respective levels induced by stimulation with BM32, using Wilcoxon-Mann-Whitney U test. Exact (for n < 10 subjects) or asymptotic (for n ≥ 10 subjects) P values were calculated by 2-sided tests, and values of 0.05 or less were regarded statistically significant. According to the exploratory character of the study, no adjustment for multiple testing was done. Statistical calculations were done using IBM SPSS Statistics, Version 20.0 (IBM Corp, Armonk, NY).
Extended Data
Fig E1.
Schematic representation of BM32 components BM321, BM322, BM325, and BM326 showing the sequences of the allergen-derived peptides and their position in the PreS fusion proteins.
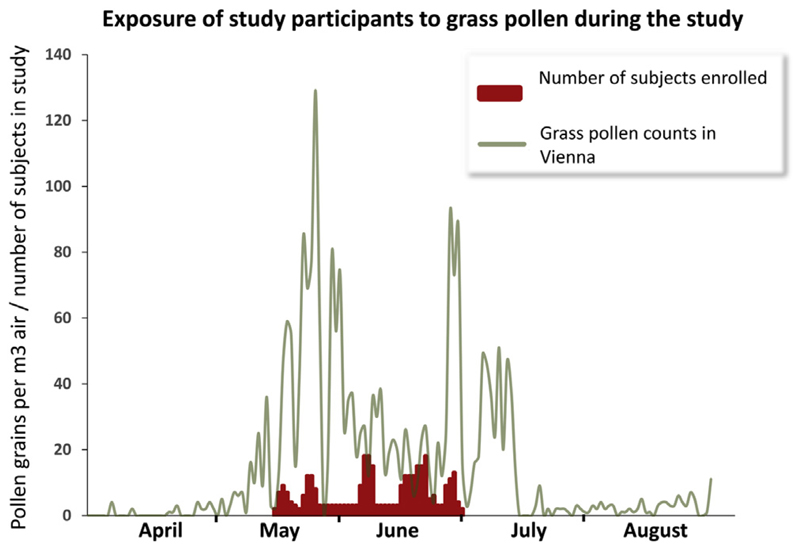
Fig E2.
Exposure of study participants to grass pollen during the study. The x-axis displays the time of grass pollen exposure in Vienna from April 1 to August 31. The y-axis shows pollen counts (gray line, grains per m3) as well as the number of subjects enrolled at the respective time points (red columns).
Fig E3.
Comparison of IgE reactivities to recombinant allergens and BM32 components in the study population. Shown are IgE levels (y-axis, OD values) specific for each of the allergens and the corresponding BM32 components (x-axis). Mean IgE levels are indicated by horizontal bars.
Fig E4.
Correlation of timothy grass pollen allergen-specific IgE with recombinant allergen-specific IgE in the study population. Shown are IgE levels to timothy grass pollen extract (x-axis) and the sums of IgE levels to Phl p 1, Phl p 2, Phl p 5, and Phl p 6 (y-axis) in kUA/L.
Table E1. Demographic, clinical, and serologic characteristics of study participants.
| Characteristic | n (%) | Median ± SE (kUA/L) |
|---|---|---|
| Demographic | ||
| Age (y), mean | 29 | |
| Min | 18 | |
| Max | 49 | |
| Sex | ||
| Male | 32 (53) | |
| Female | 28 (47) | |
| Clinical | ||
| Symptoms of grass pollen allergy | ||
| Rhinitis | 58 (97) | |
| Conjunctivitis | 56 (93) | |
| Lung symptoms | 25 (42) | |
| Dermatitis | 24 (40) | |
| Serologic | ||
| Specific IgE | ||
| Antigen* | ||
| Grass pollen | 60 (100) | 19.4 ± 24.6 |
| Phl p 1 | 58 (97) | 11.5 ± 4.6 |
| Phl p 2 | 41 (68) | 5.4 ± 2.9 |
| Phl p 5 | 49 (82) | 12.3 ± 3.7 |
| Phl p 6 | 50 (83) | 3.9 ± 2.6 |
| Total IgE (kU/L) | 168 ± 174 | |
SE, Standard error.
No. (%) of reactive patients.
Table E2. Lymphocyte proliferation and cytokine responses in PBMCs from analyzed subjects with positive and negative APT reactions.
| Positive APT reaction (n = 5) | Negative APT reaction (n = 46) | |||||
|---|---|---|---|---|---|---|
| BM32 | P value | Grass pollen extract | BM32 | P value | Grass pollen extract | |
| SI | 1.22 (0.92/2.21) | * | 5.79 (3.68/15.35) | 1.66 (0.59/7.30) | † | 9.65 (0.23/27.38) |
| IL-2 | 0.00 (0.00/0.00) | * | 0.00 (0.00/16.44) | 0.00 (0.00/80.57) | * | 0.00 (0.00/49.32) |
| IL-4 | 0.00 (0.00/0.10) | † | 0.99 (0.41/1.22) | 0.00 (0.00/0.57) | † | 1.00 (0.725/3.46) |
| IL-5 | 55.07 (0.00/292.3) | NS‡ | 146.5 (35.59/1,045) | 8.01 (0.00/308.71) | † | 73.26 (0.00/1,566) |
| IL-13 | 89.83 (0.00/154.61) | † | 504.5 (247.7/1,086) | 1.40 (0.00/403.58) | † | 399.1 (0.00/3,736) |
| IL-1b | 0.00 (0.00/3.55) | † | 242.5 (68.58/357.77) | 0.54 (0.00/173.99) | † | 157.8 (13.71/1,152) |
| IL-6 | 67.8 (0.00/104.31) | † | 16,763 (13,272/43,340) | 73.91 (0.00/3,273.94) | † | 25,159 (1,735/401,099) |
| IL-7 | 0.00 (0.00/0.00) | * | 0.29 (0.00/0.60) | 0.00 (0.00/0.89) | † | 0.51 (0.00/1.78) |
| IL-10 | 4.15 (0.00/8.97) | † | 36.74 (20.33/75.97) | 1.90 (0.00/24.88) | † | 43.02 (0.00/169.03) |
| IL-12 | 0.19 (0.00/0.55) | NS‡ | 0.32 (0.00/0.97) | 0.05 (0.00/2.08) | † | 0.80 (0.00/2.92) |
| IL-17 | 0.00 (0.00/6.17) | † | 84.09 (45.64/205.67) | 0.00 (0.00/101.9) | † | 83.49 (3.77/665.19) |
| G-CSF | 0.00 (0.00/3.14) | † | 1,337 (586/2,573) | 0.00 (0.00/151.45) | † | 1,061 (71.6/5,952) |
| GM-CSF | 0.00 (0.00/0.00) | † | 69.41 (25.07/106.49) | 0.00 (0.00/18.11) | † | 59.60 (5.04/523.53) |
| IFN-γ | 0.00 (0.00/28.25) | NS‡ | 1,202 (6.78/4,722) | 0.00 (0.00/2,574) | † | 950.0 (0.00/8118) |
| TNF-α | 0.00 (0.00/0.47) | * | 68.41 (0.00/84.47) | 0.00 (0.00/26.22) | † | 47.83 (0.00/343.61) |
NS, Not significant; SI, stimulation index.
Stimulation indices and cytokine levels (median: boldface, range) are shown after stimulation of PBMCs with BM32 or grass pollen extract in subjects with (n = 5) or without (n = 46) positive APT reaction to grass pollen allergens.
Statistically significant differences between BM32- and grass pollen extract–stimulated cultures (P < .05).
Statistically significant differences between BM32- and grass pollen extract–stimulated cultures (P < .01).
Statistically nonsignificant differences between BM32- and grass pollen extract–stimulated cultures.
Acknowledgments
This study was funded by Biomay AG, Vienna, Austria, the Christian Doppler Laboratory for Allergy Research, by the SFB Program F46 of the Austrian Science Fund (FWF, grants nos. F4605 and F4613), and the Austrian Research Promotion Agency (grant nos. 830432 and 835415).
Footnotes
Disclosure of potential conflict of interest: V. Niederberger has received consultancy fees from Biomay AG, and her institution has recently received funding for other studies from Biomay AG. K. Marth has received payment for delivering lectures from Thermo Fisher. J. Eckl-Dorna’s institution has received funding from the Austrian Science Fund (FWF). A. Neubauer, F. Stolz, and R. Henning are employees and shareholders of Biomay AG, which has received grants from the Austrian Research Promotion Agency (FFG; grant nos. 830432 and 835415) and receives royalties from Thermo Fisher. R. Valenta’s institution has received funding from the FWF, Biomay AG, and Thermo Fisher; he has received consultancy fees from Biomay AG and Thermo Fisher. The rest of the authors declare that they have no relevant conflicts of interest.
References
- 1.Jacobsen L, Niggemann B, Dreborg S, Ferdousi HA, Halken S, Host A, et al. Specific immunotherapy has long-term preventive effect of seasonal and perennial asthma: 10-year follow-up on the PAT study. Allergy. 2007;62:943–8. doi: 10.1111/j.1398-9995.2007.01451.x. [DOI] [PubMed] [Google Scholar]
- 2.Winther L, Malling HJ, Mosbech H. Allergen-specific immunotherapy in birch- and grass-pollen-allergic rhinitis, II: side-effects. Allergy. 2000;55:827–35. doi: 10.1034/j.1398-9995.2000.00368.x. [DOI] [PubMed] [Google Scholar]
- 3.Valenta R, Campana R, Marth K, van Hage M. Allergen-specific immunotherapy: from therapeutic vaccines to prophylactic approaches. J Int Med. 2012;272:144–57. doi: 10.1111/j.1365-2796.2012.02556.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Larche M. Update on the current status of peptide immunotherapy. J Allergy Clin Immunol. 2007;119:906–9. doi: 10.1016/j.jaci.2007.02.015. [DOI] [PubMed] [Google Scholar]
- 5.Niederberger V, Horak F, Vrtala S, Spitzauer S, Krauth MT, Valent P, et al. Vaccination with genetically engineered allergens prevents progression of allergic disease. Proc Natl Acad Sci U S A. 2004;101:14677–82. doi: 10.1073/pnas.0404735101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pellaton C, Perrin Y, Boudousquie C, Barbier N, Wassenberg J, Corradin G, et al. Novel birch pollen specific immunotherapy formulation based on contiguous overlapping peptides. Clin Transl Allergy. 2013;3:17. doi: 10.1186/2045-7022-3-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Focke-Tejkl M, Weber M, Niespodziana K, Neubauer A, Huber H, Henning R, et al. Development and characterization of a recombinant, hypoallergenic, peptide-based vaccine for grass pollen allergy. J Allergy Clin Immunol. 2015;135:1207–17. doi: 10.1016/j.jaci.2014.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E1.Turjanmaa K, Darsow U, Niggemann B, Rance F, Vanto T, Werfel T. EAACI/GA2LEN position paper: present status of the atopy patch test. Allergy. 2006;61:1377–84. doi: 10.1111/j.1398-9995.2006.01136.x. [DOI] [PubMed] [Google Scholar]
- E2.Focke-Tejkl M, Weber M, Niespodziana K, Neubauer A, Huber H, Henning R, et al. Development and characterization of a recombinant, hypoallergenic, peptide-based vaccine for grass pollen allergy. J Allergy Clin Immunol. 2015;135:1207–17. doi: 10.1016/j.jaci.2014.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E3.Pree I, Reisinger J, Focke M, Vrtala S, Pauli G, van Hage M, et al. Analysis of epitope-specific immune responses induced by vaccination with structurally folded and unfolded recombinant Bet v 1 allergen derivatives in man. J Immunol. 2007;179:5309–16. doi: 10.4049/jimmunol.179.8.5309. [DOI] [PubMed] [Google Scholar]
- E4.Focke M, Marth K, Flicker S, Valenta R. Heterogeneity of commercial timothy grass pollen extracts. Clin Exp Allergy. 2008;38:1400–8. doi: 10.1111/j.1365-2222.2008.03031.x. [DOI] [PubMed] [Google Scholar]