Abstract
Purpose
Lung adenocarcinoma (LA) is one of the major types of lung cancer. MicroRNAs (miRNAs) play an essential role in regulating responses of natural killer (NK) cells to cancer malignancy. However, the mechanism of miR-218-5p involved in the killing effect of NK cells to LA cells remains poorly understood.
Materials and Methods
The expression of miR-218-5p was examined by quantitative real-time polymerase chain reaction (qRT-PCR). Serine hydroxymethyl transferase 1 (SHMT1) level was detected by qRT-PCR or western blots. Cytokines production of interferon-γ (IFN-γ) and tumor necrosis factor-α (TNF-α) were detected by ELISA. The killing effect of NK cells to LA cells was investigated using lactate dehydrogenase cytotoxicity assay kit. The interaction of miR-218-5p and SHMT1 was probed by luciferase activity assay. Xenograft model was established to investigate the killing effect of NK cells in vivo.
Results
miR-218-5p was enhanced and SHMT1 was inhibited in NK cells of LA patients, whereas stimulation of interleukin-2 (IL-2) reversed their abundances. Addition of miR-218-5p reduced IL-2-induced cytokines expression and cytotoxicity in NK-92 against LA cells. Moreover, SHMT1 was negatively regulated by miR-218-5p and attenuated miR-218-5p-mediated effect on cytotoxicity, IFN-γ and TNF-α secretion in IL-2-activated NK cells. In addition, miR-218-5p exhaustion inhibited tumor growth by promoting killing effect of NK cells.
Conclusion
miR-218-5p suppresses the killing effect of NK cells to LA cells by targeting SHMT1, providing a potential target for LA treatment by ameliorating NK cells function.
Keywords: Lung adenocarcinoma, natural killer cells, miR-218-5p, SHMT1
INTRODUCTION
Lung cancer is a common tumor malignancy with high mortality worldwide.1 And lung adenocarcinoma (LA) is a major subtype that accounts for 40% of lung cancer with poor prognosis.2 With the development of chemo-therapy and immunological therapy, more strategies have been implicated in treatment of LA, whereas the survival rate is still low.3 Emerging research have reported that immune system is responsible for immunotherapy of lung cancer.4 Natural killer (NK) cell is one of the key immune cells and its cytolytic potential is limited in lung cancer, whereas increasing NK cells function can result in tumor regression.5 Potentiation of cytotoxicity, mediated by NK cells to LA, plays a vital role in overcoming tumor cell escape from immune system.6 NK cells control tumor by regulating pro-inflammatory cytokines production, such as interferon-γ (IFN-γ) and tumor necrosis factor-α (TNF-α), which can induce cytolysis of tumor cells.7 Therefore, it is promising to explore a novel driver that modulates the killing effect of NK cells to LA cells.
MicroRNAs (miRNAs) hold great promise in development of LA, and are associated with prognosis of LA.8 Previous research have reported that miRNAs may regulate the function of NK cells by enhancing or inhibiting the cytotoxic potential of NK cells.9 Moreover, miRNAs have been suggested to participate in NK cells activity impairment.10 For example, miR-183 is associated with NK cells function in lung cancer.11 The available evidence indicates that miR-218-5p plays an essential role in LA progression by regulating target gene expression.12 Notably, miR-218-5p is expressed in human NK cells, and may be involved in the development and function of NK cells.13 Therefore, we speculate miR-218-5p may be implicated in regulating NK cells function in LA.
A number of researchers have reported that serine hydroxymethyl transferase 1 (SHMT1) is required for tumor growth and disease progression.14,15 Previous work investigated the regulation of SHMT1 on lung cancer risk, and suggested SHMT1 may affect etiology of lung cancer.16 In LA, SHMT1 has an important impact on cell proliferation and apoptosis.17 However, there is no direct evidence in support of SHMT1 required for NK cells function. Intriguingly, bioinformatics analysis predicates the putative binding sites of miR-218-5p and SHMT1. Therefore, we assumed that SHMT1 is required for miR-218-5p-mediated function on the killing effect of NK cells to LA cells. In the present study, we detected the expressions of miR-218-5p and SHMT1 in NK cells of LA patients, and explored the potential mechanism that underlies miR-218-5p regulating the killing effect of NK cells to LA cells.
MATERIALS AND METHODS
Isolation of primary NK cells
Peripheral blood mononuclear cells were collected from 20 patients with LA and healthy volunteers, and were used to isolate human NK cells using NK cell enrichment kit (Thermo Fisher, Wilmington, DE, USA) according to the manufacturer's instructions. Highly pure NK cells were obtained by negative selection with magnetic labeling. Informed consent was signed by all patients, and the study was accepted by the Institutional Research Ethics Committee of The Affiliated Renhe Hospital of China Three Gorges University.
Cell culture and treatment
Human NK cell line NK-92, mouse NK cell line LNK, human LA cell line A549, and 293T cells were obtained from American Tissue Culture Collection (ATCC, Manassas, VA, USA). In this study, all cells were grown in RPMI-1640 (Gibco, Carlsbad, CA, USA) containing 10% fetal bovine serum (Gibco), 1% penicillin, and streptomycin (Invitrogen, Carlsbad, CA, USA) at 37℃ in an incubator with 5% CO2.
To activate NK-92 or LNK cells, 20 ng/mL interleukin-2 (IL-2, Gibco) was introduced into cells for 24 h. SHMT1 overexpression vectors (SHMT1) cloned into pcDNA, miR-218-5p mimics (miR-218-5p), miR-218-5p inhibitor (anti-miR-218-5p), and negative control (miR-NC or anti-NC) were obtained from GenePharma (Shanghai, China). Transfection was performed in NK-92 or LNK cells using Lipofectamine 2000 (Invitrogen) according to the manufacturer's protocol.
Quantitative real-time polymerase chain reaction
Total RNA was isolated using Trizol reagent (Invitrogen) according to the manufacturer's instructions. Then cDNA was synthesized using TaqMan miRNA reverse transcription kit (Applied Biosystems, Foster City, CA, USA), followed by quantitative real-time polymerase chain reaction (qRT-PCR) with SYBR Green Master Mix (Applied Biosystems). All primers were listed as follows: miR-218-5p (Forward, 5′-TTGCGGATG GTTCCGTCAAGCA-3′; Reverse, 5′-ATCCAGTGCAGGGTCC GAGG-3′), U6 (Forward, 5′-CTCGCTTCG GCAGCACA-3′; Reverse, 5′-AACGCTTCACGAATTTGCGT-3′), SHMT1 (Forward, 5′-TTGCCTCGGAGAATTTCGCC-3′; Reverse, 5′-GTCCCGC CATAGT ATCTCTGG-3′), and β-actin (Forward, 5′-CAGCCTT CCTTCTTGGGTAT-3′; Reverse, 5′-TGGCATAGAGGTCTT TACGG-3′). The expression of miR-218-5p or SHMT1 was analyzed using 2−ΔΔCt method, and normalized to U6 or β-actin, respectively.
Western blots
Total protein was prepared in cell lysis buffer containing 1% protease inhibitor (Thermo Fisher). After quantification by BCA assay kit (Sigma, St. Louis, MO, USA), denatured proteins were separated by SDS-PAGE gel, transferred to polyvinylidene difluoride membranes (Millipore, Billerica, MA, USA), and then blocked with blocking reagent (Thermo Fisher) for 1 h at room temperature. Subsequently, the membranes were incubated with primary antibodies against SHMT1 or β-actin (Cell Signaling Technology, Danvers, MA, USA) overnight at 4℃. After being rinsed with TBST, membranes were hatched with secondary antibodies (Cell Signaling Technology) for 2 h at room temperature. The protein blots were visualized using enhanced chemiluminescence chromogenic substrate (Thermo Fisher), and investigated by Image Lab software (Bio-Rad, Hercules, CA, USA). β-actin was used as a standard for band intensities.
Enzyme linked immunosorbent assay
After treatment, cell supernatants were collected to detect levels of IFN-γ and TNF-α using commercial human ELISA Kit (Invitrogen) according to the manufacturer's instructions. Intensity of color was assayed at 450 nm with reference wave length at 620 nm using a microplate reader (Bio-Rad).
Cytotoxicity assay
The cytotoxicity of NK-92 to A549 cells was investigated by a lactate dehydrogenase (LDH) assay according to the manufacturer's instructions. Briefly, A549 cells were seeded into 96-well plates at a density of 1×104 cells per well, and then IL-2-treated NK-92 cells transfected with miR-218-5p, miR-NC, anti-miR-218-5p, anti-NC, pcDNA, or SHMT1 were introduced into each well, and co-cultured with A549 cells at effector/target cell ratio of 5:1 for 4 h. Following incubation, supernatants were collected, and the cytotoxicity of NK-92 cells was detected by LDH cytotoxicity assay kit (Thermo Fisher).
Luciferase activity assay
TargetScan analysis predicted putative binding sites of miR-218-5p and 3′ untranslated regions (3′-UTR) sequences of SHMT1. Luciferase report vectors with wild-type plasmid (SHMT1-wt) or mutant-type plasmid (SHMT1-mut) were generated using pGL3 vector (Promega, Madison, WI, USA), respectively. Luciferase reporter vectors, miR-218-5p, anti-miR-218-5p, or their negative control were co-transfected in 293T cells for 48 h using Lipofectamine 2000 according to the manufacturer's protocols. Luciferase activity was evaluated by luciferase assay kit (GeneCopoeia, Rockville, MD, USA).
Animals and xenograft model of LA
Every protocol was approved by the Animal Research committee of The Affiliated Renhe Hospital of China Three Gorges University. SPF BALB/c nude mice (male, 6-week-old) were purchased from Vital River Laboratory Animal Technology (Beijing, China). A549 cells (6×106 cells) were introduced into nude mice by subcutaneous injection (n=8 per group). At 2 h after LA model construction, IL-2-treated LNK cells (3×106 cells), with stable transfection of anti-miR-218-5p or anti-NC, were subcutaneously injected into the same sites. Tumors were examined every three days, and tumor volume was calculated with slide calipers by (0.5×length×width×height). Mice were sacrificed at 15 days after cell implantation, and tumor specimens were collected for molecular analyses.
Statistical analysis
Data were presented as the mean±SEM. Student's t test or one way ANOVA was used to evaluate the differences using SPSS 18.0 software (SPSS, Inc., Chicago, IL, USA). Statistically significant was considered as p values less than 0.05.
RESULTS
MiR-218-5p was enhanced while SHMT1 expression was impaired in NK cells of LA patients
To test whether miR-218-5p and SHMT1 participate in functioning of NK cells, their expressions were detected by qRT-PCR and western blots in NK cells from healthy control and LA patients (n=20). As a result, miR-218-5p expression was significantly increased in NK cells of LA patients compared to control group (Fig. 1A). However, a strong reduction of SHMT1 mRNA level was observed in NK cells in LA group compared to control group (Fig. 1B). Moreover, SHMT1 protein abundance showed similar trend in NK cells (Fig. 1C and D). These finding indicated that miR-218-5p and SHMT1 might play essential roles in NK cells in LA.
Fig. 1. MiR-218-5p and SHMT1 expressions were ectopic in NK cells of LA patients (n=20). (A) Expression of miR-218-5p was detected by qRT-PCR in NK cells of control or LA patients. (B) SHMT1 mRNA level was examined in NK cells in control and LA groups. (C and D) Abundance of SHMT1 protein was investigated by western blots. *p<0.05, LA vs. control group. LA, lung adenocarcinoma; SHMT1, serine hydroxymethyl transferase 1; qRT-PCR, quantitative real-time polymerase chain reaction; NK, natural killer.
Activated NK-92 cells promoted cytokines production, inhibited miR-218-5p, and induced SHMT1 protein expression
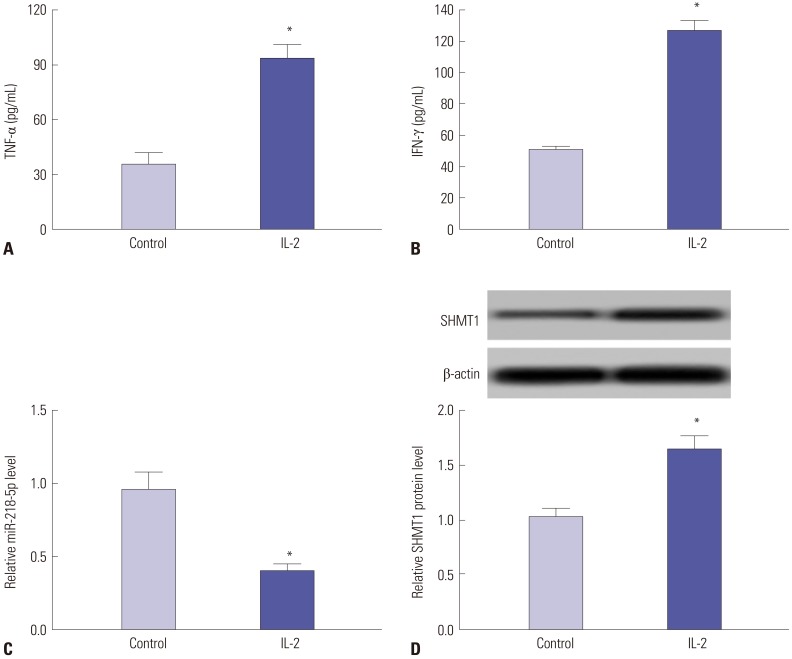
IL-2 was used to induce activation of NK cells in vitro. NK-92 cells showed enhanced TNF-α production after IL-2 stimulation (Fig. 2A). Meanwhile, IFN-γ expression was also elevated in NK-92 cells in response to IL-2 (Fig. 2B). These suggested that NK cells were activated by IL-2 treatment, indicating triggering of killing effect of NK cells. Subsequently, the expressions of miR-218-5p and SHMT1 were detected in activated NK-92 cells. The expression of miR-218-5p was impaired in activated NK cells compared to those without IL-2 treatment (Fig. 2C). Besides, IL-2 insult resulted in a great increase of SHMT1 protein level in NK cells compared to that before IL-2 stimulation (Fig. 2D). These results further showed that miR-218-5p and SHMT1 might be required for the functioning of NK cells.
Fig. 2. IL-2 induced activation of NK cells and dysregulation of miR-218-5p and SHMT1 expression. NK-92 cells were treated by IL-2 (20 ng/mL) for 24 h. (A and B) TNF-α and IFN-γ production levels were determined by ELISA in NK-92 cells with or without IL-2 stimulation. (C) Effect of IL-2 on miR-218-5p expression was investigated in NK-92 cells. (D) SHMT1 protein expression was detected in NK-92 cells after or before IL-2 treatment. *p<0.05, IL-2-treated group vs. control group. IL-2, interleukin-2; NK, natural killer; SHMT1, serine hydroxymethyl transferase 1; TNF-α, tumor necrosis factor-α; IFN-γ, interferon-γ.
Addition of miR-218-5p blocked the killing effect of NK cells to LA cells
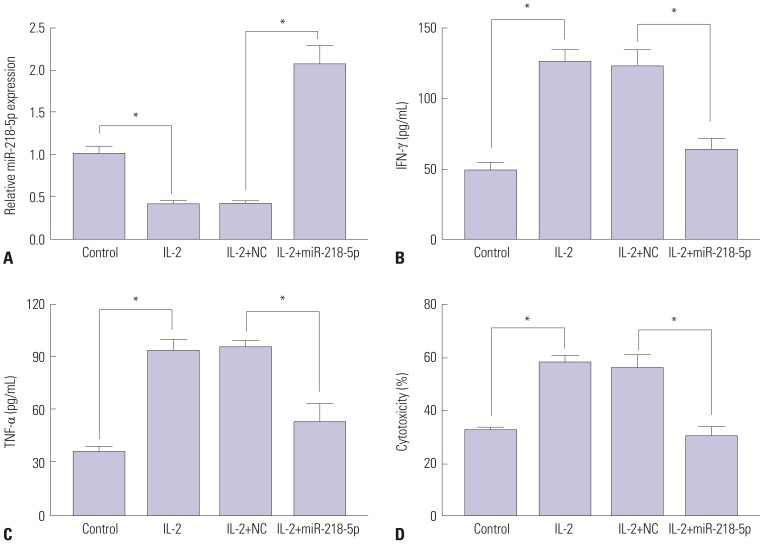
To evaluate the effect of miR-218-5p on killing effect of NK cells to LA cells, miR-218-5p mimics were introduced into NK-92 cells and co-cultured with A549 cells after IL-2 stimulation. MiR-218-5p expression was downregulated in NK-92 cells after IL-2 treatment, whereas addition of miR-218-5p reversed the level of miR-218-5p (Fig. 3A). Moreover, abundant presence of miR-218-5p inhibited IFN-γ and TNF-α secretion in NK-92 cells with treatment of IL-2, which activated cells and promoted cytokines production (Fig. 3B and C). In addition, the cytotoxicity of activated NK cells to A549 cells was investigated by co-culturing of NK-92 and A549 cells. Results showed that IL-2 stimulation improved the killing effect of NK-92 cells, while introduction of miR-218-5p displayed opposite effect (Fig. 3D). These results suggested that miR-218-5p overexpression can protect against the killing effect of NK cells to LA cells.
Fig. 3. Enrichment of miR-218-5p blocked the killing effect of NK cells to LA cells. (A) Altered abundance of miR-218-5p was detected in IL-2-avtivated NK-92 cells with miR-218-5p or NC transfection. (B and C) IFN-γ and TNF-α secretion were examined in NK-92 cells after IL-2 and miR-218-5p treatment. (D) Killing effect of NK-92 to LA cells was evaluated by LDH cytotoxicity assay kit, and the effect of miR-218-5p on killing effect was detected in NK-92 cells transfected with miR-218-5p mimics. *p<0.05, IL-2-treated group vs. control group, and IL-2+miR-218-5p group vs. IL-2+NC group. NK, natural killer; LA, lung adenocarcinoma; IL-2, interleukin-2; NC, negative control; IFN-γ, interferon-γ; TNF-α, tumor necrosis factor-α; LDH, lactate dehydrogenase.
SHMT1 was a target of miR-218-5p
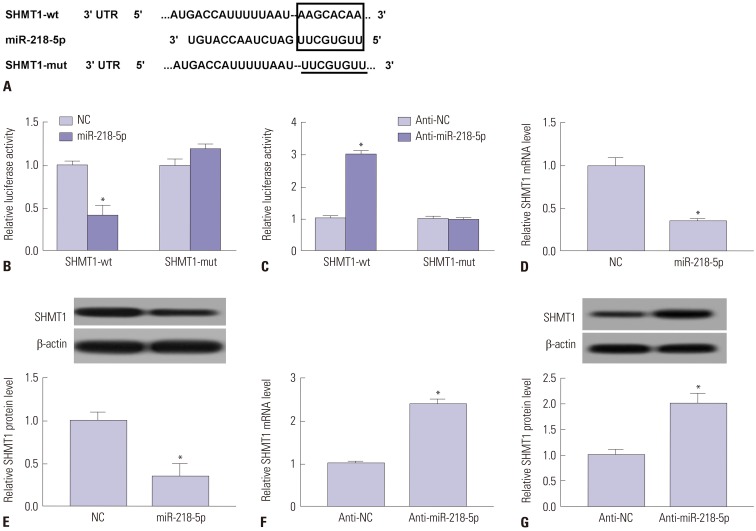
Since miR-218-5p and SHMT1 were involved in dysfunction of NK cells, we next probed the potential link of miR-218-5p and SHMT1 in NK-92 cells. Bioinformatics assay described the putative binding sites of miR-218-5p and SHMT1 by TargetScan, indicating SHMT1 might be a direct target of miR-218-5p (Fig. 4A). Next, we investigated the interaction in 293T cells by luciferase activity assay. SHMT1-wt and miR-218-5p were co-transfected into 293T cells, and luciferase activity was obviously inhibited in miR-218-5p group compared to NC group, whereas there was not significant difference of the activity in response to SHMT1-mut (Fig. 4B). Conversely, luciferase activity of cells with SHMT1-wt showed a higher level in anti-miR-218-5p group compared to that in anti-NC group. However, miR-218-5p deletion failed to show efficacy in affecting the activity of cells transfected with SHMT1-mut (Fig. 4C). These uncovered the interaction between miR-218-5p and SHMT1. Accordingly, the effect of miR-218-5p on SHMT1 expression was investigated in NK-92 cells. Addition of miR-218-5p led to a great loss of SHMT1 mRNA and protein level compared to NC group (Fig. 4D and E). Nevertheless, the absence of miR-218-5p showed an opposite effect, revealed by elevated SHMT1 abundance at mRNA and protein level (Fig. 4F and G), indicating miR-218-5p was negatively correlated with SHMT1 in NK cells.
Fig. 4. SHMT1 was a target of miR-218-5p. (A) Bioinformatics assay indicated putative binding sites of miR-218-5p and 3′-UTR of SHMT1 by TargetScan. (B and C) Wt or mut of SHMT1-transfected 293T cells were used to investigate luciferase activity by co-transfection of miR-218-5p or anti-miR-218-5p, respectively. (D and E) Effect of miR-218-5p on SHMT1 expression was detected in NK-92 cells at mRNA and protein level. (F and G) Abundances of SHMT1 mRNA and protein were altered in NK-92 cells after anti-miR-218-5p transfection. *p<0.05, miR-218-5p group vs. NC group, and anti-miR-218-5p group vs. anti-NC group. SHMT1, serine hydroxymethyl transferase 1; NK, natural killer.
SHMT1 was required for miR-218-5p-mediated killing effect of NK cells to LA cells
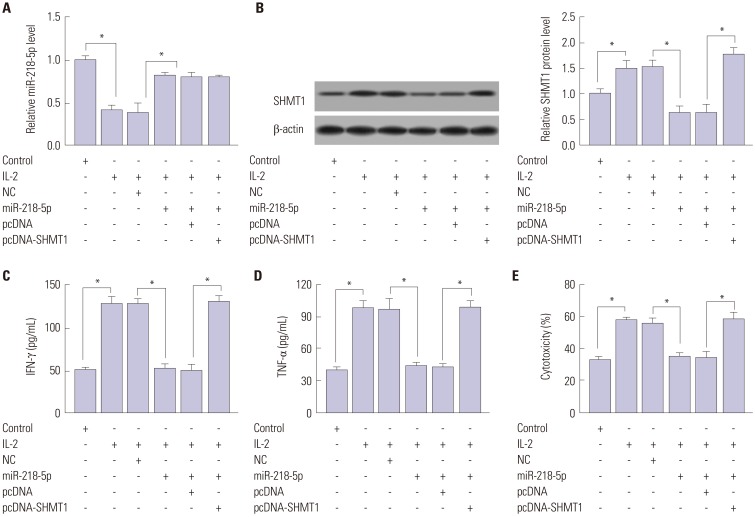
To probe whether SHMT1 was associated with miR-218-5p-mediated limited capacity for killing effect of NK cells, miR-218-5p or (and) SHMT1 were transfected into IL-2-treated NK-92 cells. MiR-218-5p abundance was markedly impaired after IL-2 insult and miR-218-5p mimics protected the expression, while SHMT1 overexpression could not afford miRNA expression (Fig. 5A). Besides, SHMT1 expression was promoted in NK-92 cells by IL-2 stimulation and miR-218-5p addition inhibited SHMT1 abundance, whereas SHMT1 overexpression effectively increased protein levels (Fig. 5B). IL-2 facilitated IFN-γ production and miR-218-5p blocked cytokine secretion, while SHMT1 reversed the expression of IFN-γ in NK-92 cells (Fig. 5C). Moreover, similar trend was uncovered in terms of TNF-α level in NK cells (Fig. 5D). Likewise, SHMT1 ablated the effect of miR-218-5p on cytotoxicity of NK cells to A549 cells, which enhanced the killing effect (Fig. 5E). These data indicated that miR-218-5p overexpression repressed the killing effect of NK cells to LA cells by regulating SHMT1 expression.
Fig. 5. Introduction of SHMT1 reversed miR-218-5p-mediated inhibitory role in the killing effect of NK-92 to LA cells. (A) Expression of miR-218-5p was examined in NK-92 cells after treatment. (B) Ectopic SHMT1 protein level was detected in miR-218-5p or SHMT1-transfected NK-92 cells after IL-2 insult. (C and D) Effect of SHMT1 on IFN-γ and TNF-α expression in IL-2-activated NK-92 cells was investigated. (E) Cytotoxicity of NK-92 with SHMT1 transfection to A549 cells was evaluated. *p<0.05, IL-2-treated group vs. control group, IL-2+miR-218-5p group vs. IL-2+NC group, and IL-2+miR-218-5p+SHMT1 group vs. IL-2+miR-218-5p+pcDNA group. SHMT1, serine hydroxymethyl transferase 1; NK, natural killer; IFN-γ, interferon-γ; TNF-α, tumor necrosis factor-α; IL-2, interleukin-2.
MiR-218-5p depletion limited LA tumor growth in vivo
To further explore the effect of miR-218-5p on cytotoxicity of NK cells to LA cells in vivo, activated LNK cells were transfected with anti-miR-218-5p or anti-NC, and then introduced into mice model of LA (n=8 per group). Tumor volume was progressively increased, and absence of miR-218-5p decreased tumor growth compared to anti-NC treatment group (Fig. 6A). Moreover, the expression of SHMT1 was examined in tumor tissues. An evident increase in protein level was observed in miR-218-5p deficiency group compared to NC group (Fig. 6B and C). These findings suggested that miR-218-5p exhaustion weakened tumor growth in vivo by enhancing the killing effect of NK cells to LA cells.
Fig. 6. Abrogation of miR-218-5p enhanced the killing effect of NK to LA cells in vivo. IL-2-treated LNK cells were transfected with anti-miR-218-5p and introduced into LA tumor in nude mice (n=8 per group). (A) Tumor volume was examined every three days. (B and C) Expression of SHMT1 protein was detected in the tumor tissues. *p<0.05, anti-miR-218-5p group vs. anti-NC group. NK, natural killer; LA, lung adenocarcinoma; IL-2, interleukin-2; LNK, mouse NK cell line; SHMT1, serine hydroxymethyl transferase 1.
DISCUSSION
LA is a major subtype of lung cancer which threatens people's health all over the world.2 Over the past few years, immune-based therapy has gained more attention, and increasing number of investigators showed great promise in cytotoxicity of NK cells to tumor cells. NK cells played important roles in various types of cancer, including hepatocellular carcinoma and colorectal cancer.18,19 Likewise, immune T cell and NK cell were involved in LA progression by varying pathways.20 Besides, miRNAs have been suggested to be associated with control of immune response in diverse cancers by regulating functions of T or NK cells.21 For instance, miR-146a suppressed NK cells function via mediating signal transducer and activator of transcription 1 (STAT1).22 However, miR-30c could confer NK cells cytotoxicity by regulating natural-killer group 2 member D (NKG2D).23 A novel miRNA, miR-218-5p, has been reported as a potential biomarker in many cancers, such as colorectal cancer, osteoarthritis, and LA.24,25,26 However, the study on involvement of miR-218-5p in NK cells function remains poorly understood. In the present study, we obtained NK cells from LA patients or healthy controls, and detected the expressions of miR-218-5p and SHMT1 in NK cells, and showed that both miR-218-5p and SHMT1 were expressed in NK cells.
Tumor cells have been reported to be killed by immune cells, while tumor immune escape supported tumor development by triggering the resistance to cytotoxicity of immune cells, such as NK cells.5 Previous research revealed that transforming growth factor-β (TGF-β) and IL in the microenvironment might disturb the balance of limitation of NK cell function in cancers.27 Furthermore, IL-2 was widely used to induce activation of NK cells to inhibit tumor processes.28,29 Therefore, we also introduced IL-2 into NK cells and found that IL-2 stimulated IFN-γ and TNF-α secretion, and altered the abundances of miR-218-5p and SHMT1. This was consistent with another finding that suggested miR-218-5p had low expressions in activated NK cells.13 We hypothesized that miR-218-5p and SHMT1 might be required for activation of NK cells, which could provide a novel biomarker of regulating NK cells function. To test the hypothesis, we probed whether miR-218-5p might affect the killing effect of NC cells to LA cells. Our results showed that miR-218-5p overexpression inhibited inflammatory cytokines production and cytotoxicity against LA cells, indicating that miR-218-5p was negatively correlated with the killing effect of NK cells. Similarly, miR-889 compromised the killing effect of NK cells by increasing resistance to cytotoxicity of NK cells.18 Apart from miR-889, miR-615-5p also blocked NK cells cytotoxicity to tumor cells by regulating insulin-like growth factor-1R (IGF-1R) expression.30 These findings uncovered that miRNAs might play an essential role in regulating NK cells cytotoxicity.
miRNAs interacted with the development and function of NK cells by transcriptional and post-transcriptional regulation.31 MiR-218-5p was negatively correlated with poor prognosis of lung cancer by targeting IL-6 and STAT3.32 Besides, slug and zinc finger E-box binding homeobox 2 (ZEB2) were also targeted by miR-218-5p, and played essential roles in epithelial-mesenchymal transition and metastasis in lung cancer.33 SHMT1 was reported to be crucial for tumor progression in various cancer types, including lung cancer.14,16,34 In addition, SHMT1 might participate in cell proliferation and apoptosis by miR-198 targeting in LA.17 Previous study revealed that vitamin B6 might regulate inflammatory response by mediating SHMT-related pathway.35 Moreover, SHMT1 was suggested to stimulate the production of inflammatory cytokines in ovarian cancer.36 Besides, currently available evidence have indicated SHMT1 was required for B-lymphoma cell growth.34 These findings uncovered that SHMT1 might be regarded as a target for immunotherapy of tumors. However, the mechanism that SHMT1 requires for functioning of NK cells still remains unclear. Hence, we probed a link of miR-218-5p with SHMT1, and investigated the effect of SHMT1 on functioning of NK cells. Our results showed that addition of SHMT1 ameliorated NK cell function. Murine xenograft model in vivo was responsible for preclinical drug trials, and it has been widely used for NK cells function or LA progression investigation.29,37 In this study, we found that miR-218-5p also regulated the killing effect of NK cells in vivo, revealed by reduced tumor volume. MiR-218-5p and SHMT1 were involved in not only cytotoxicity of NK cells but also in LA progression, whereupon the mechanism by which miR-218-5p and SHMT1 affected LA processes should be investigated further in future studies.
In summary, miR-218-5p was enhanced in NK cells but inhibited by IL-2 treatment. Moreover, miR-218-5p overturned the killing effect of NK cells to LA cells by regulating SHMT1 expression in vitro and in vivo. This might indicate that miR-218-5p has a potential to regulate NK cell functions to address anti-tumor role in cancers, such as LA.
Footnotes
The authors have no potential conflicts of interest to disclose.
- Conceptualization: Quanjun Yang.
- Data curation: Jingjing Li.
- Formal analysis: Yili Hu.
- Funding acquisition: Xiaofei Tang.
- Investigation: Lili Yu.
- Methodology: Lihua Dong.
- Project administration: Diandian Chen.
- Resources: Quanjun Yang, Lihua Dong.
- Software: Diandian Chen.
- Supervision: Jingjing Li.
- Validation: Xiaofei Tang, Yili Hu.
- Visualization: Quanjun Yang, Jingjing Li.
- Writing—original draft: Quanjun Yang, Jingjing Li.
- Writing—review & editing: Jingjing Li, Quanjun Yang, Yili Hu.
References
- 1.Global Burden of Disease Cancer Collaboration. Fitzmaurice C, Allen C, Barber RM, Barregard L, Bhutta ZA, Brenner H, et al. Global, regional, and national cancer incidence, mortality, years of life lost, years lived with disability, and disability-adjusted life-years for 32 cancer groups, 1990 to 2015: a systematic analysis for the global burden of disease study. JAMA Oncol. 2017;3:524–548. doi: 10.1001/jamaoncol.2016.5688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Inamura K. Clinicopathological characteristics and mutations driving development of early lung adenocarcinoma: tumor initiation and progression. Int J Mol Sci. 2018;19:1259. doi: 10.3390/ijms19041259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Donington JS, Kim YT, Tong B, Moreira AL, Bessich J, Weiss KD, et al. Progress in the management of early-stage non-small cell lung cancer in 2017. J Thorac Oncol. 2018;13:767–778. doi: 10.1016/j.jtho.2018.04.002. [DOI] [PubMed] [Google Scholar]
- 4.Shroff GS, de Groot PM, Papadimitrakopoulou VA, Truong MT, Carter BW. Targeted therapy and immunotherapy in the treatment of non-small cell lung cancer. Radiol Clin North Am. 2018;56:485–495. doi: 10.1016/j.rcl.2018.01.012. [DOI] [PubMed] [Google Scholar]
- 5.Aktas¸ ON, Öztürk AB, Erman B, Erus S, Tanju S, Dilege Ş. Role of natural killer cells in lung cancer. J Cancer Res Clin Oncol. 2018;144:997–1003. doi: 10.1007/s00432-018-2635-3. [DOI] [PubMed] [Google Scholar]
- 6.Le Maux Chansac B, Missé D, Richon C, Vergnon I, Kubin M, Soria JC, et al. Potentiation of NK cell-mediated cytotoxicity in human lung adenocarcinoma: role of NKG2D-dependent pathway. Int Immunol. 2008;20:801–810. doi: 10.1093/intimm/dxn038. [DOI] [PubMed] [Google Scholar]
- 7.Wang R, Jaw JJ, Stutzman NC, Zou Z, Sun PD. Natural killer cell-produced IFN-γ and TNF-α induce target cell cytolysis through up-regulation of ICAM-1. J Leukoc Biol. 2012;91:299–309. doi: 10.1189/jlb.0611308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Maemura K, Watanabe K, Ando T, Hiyama N, Sakatani T, Amano Y, et al. Altered editing level of microRNAs is a potential biomarker in lung adenocarcinoma. Cancer Sci. 2018;109:3326–3335. doi: 10.1111/cas.13742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shirshev SV, Nekrasova IV, Gorbunova OL, Orlova EG, Maslennikova IL. MicroRNA in hormonal mechanisms of regulation of NK cell function. Dokl Biochem Biophys. 2017;474:168–172. doi: 10.1134/S160767291703005X. [DOI] [PubMed] [Google Scholar]
- 10.Rizzo R, Soffritti I, D'Accolti M, Bortolotti D, Di Luca D, Caselli E. HHV-6A/6B infection of NK cells modulates the expression of miRNAs and transcription factors potentially associated to impaired NK activity. Front Microbiol. 2017;8:2143. doi: 10.3389/fmicb.2017.02143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Donatelli SS, Zhou JM, Gilvary DL, Eksioglu EA, Chen X, Cress WD, et al. TGF-β-inducible microRNA-183 silences tumor-associated natural killer cells. Proc Natl Acad Sci U S A. 2014;111:4203–4208. doi: 10.1073/pnas.1319269111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li YJ, Zhang W, Xia H, Zhang BS, Chen P, Zhao YL, et al. miR-218 suppresses epithelial-to-mesenchymal transition by targeting Robo1 and Ecop in lung adenocarcinoma cells. Future Oncol. 2017;13:2571–2582. doi: 10.2217/fon-2017-0398. [DOI] [PubMed] [Google Scholar]
- 13.Victor AR, Weigel C, Scoville SD, Chan WK, Chatman K, Nemer MM, et al. Epigenetic and posttranscriptional regulation of CD16 expression during human NK cell development. J Immunol. 2018;200:565–572. doi: 10.4049/jimmunol.1701128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gupta R, Yang Q, Dogra SK, Wajapeyee N. Serine hydroxymethyl transferase 1 stimulates pro-oncogenic cytokine expression through sialic acid to promote ovarian cancer tumor growth and progression. Oncogene. 2017;36:4014–4024. doi: 10.1038/onc.2017.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bahari G, Hashemi M, Naderi M, Sadeghi-Bojd S, Taheri M. Association of SHMT1 gene polymorphisms with the risk of childhood acute lymphoblastic leukemia in a sample of Iranian population. Cell Mol Biol (Noisy-le-grand) 2016;62:45–51. [PubMed] [Google Scholar]
- 16.Wang L, Lu J, An J, Shi Q, Spitz MR, Wei Q. Polymorphisms of cytosolic serine hydroxymethyltransferase and risk of lung cancer: a case-control analysis. Lung Cancer. 2007;57:143–151. doi: 10.1016/j.lungcan.2007.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wu S, Zhang G, Li P, Chen S, Zhang F, Li J, et al. miR-198 targets SHMT1 to inhibit cell proliferation and enhance cell apoptosis in lung adenocarcinoma. Tumour Biol. 2016;37:5193–5202. doi: 10.1007/s13277-015-4369-z. [DOI] [PubMed] [Google Scholar]
- 18.Xie H, Zhang Q, Zhou H, Zhou J, Zhang J, Jiang Y, et al. microRNA-889 is downregulated by histone deacetylase inhibitors and confers resistance to natural killer cytotoxicity in hepatocellular carcinoma cells. Cytotechnology. 2018;70:513–521. doi: 10.1007/s10616-017-0108-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhao H, Su W, Kang Q, Xing Z, Lin X, Wu Z. Natural killer cells inhibit oxaliplatin-resistant colorectal cancer by repressing WBSCR22 via upregulating microRNA-146b-5p. Am J Cancer Res. 2018;8:824–834. [PMC free article] [PubMed] [Google Scholar]
- 20.Lavin Y, Kobayashi S, Leader A, Amir ED, Elefant N, Bigenwald C, et al. Innate immune landscape in early lung adenocarcinoma by paired single-cell analyses. Cell. 2017;169:750–765. doi: 10.1016/j.cell.2017.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jasinski-Bergner S, Mandelboim O, Seliger B. The role of microRNAs in the control of innate immune response in cancer. J Natl Cancer Inst. 2014;106:dju257. doi: 10.1093/jnci/dju257. [DOI] [PubMed] [Google Scholar]
- 22.Xu D, Han Q, Hou Z, Zhang C, Zhang J. miR-146a negatively regulates NK cell functions via STAT1 signaling. Cell Mol Immunol. 2017;14:712–720. doi: 10.1038/cmi.2015.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ma Y, Gong J, Liu Y, Guo W, Jin B, Wang X, et al. MicroRNA-30c promotes natural killer cell cytotoxicity via up-regulating the expression level of NKG2D. Life Sci. 2016;151:174–181. doi: 10.1016/j.lfs.2016.03.012. [DOI] [PubMed] [Google Scholar]
- 24.Liu M, Yin K, Guo X, Feng H, Yuan M, Liu Y, et al. Diphthamide biosynthesis 1 is a novel oncogene in colorectal cancer cells and is regulated by MiR-218-5p. Cell Physiol Biochem. 2017;44:505–514. doi: 10.1159/000485087. [DOI] [PubMed] [Google Scholar]
- 25.Lu J, Ji ML, Zhang XJ, Shi PL, Wu H, Wang C, et al. MicroRNA-218-5p as a potential target for the treatment of human osteoarthritis. Mol Ther. 2017;25:2676–2688. doi: 10.1016/j.ymthe.2017.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tian F, Li R, Chen Z, Shen Y, Lu J, Xie X, et al. Differentially expressed miRNAs in tumor, adjacent, and normal tissues of lung adenocarcinoma. Biomed Res Int. 2016;2016:1428271. doi: 10.1155/2016/1428271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Marcais A, Viel S, Grau M, Henry T, Marvel J, Walzer T. Regulation of mouse NK cell development and function by cytokines. Front Immunol. 2013;4:450. doi: 10.3389/fimmu.2013.00450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pan C, Xiang L, Pan Z, Wang X, Li J, Zhuge L, et al. MiR-544 promotes immune escape through downregulation of NCR1/NKp46 via targeting RUNX3 in liver cancer. Cancer Cell Int. 2018;18:52. doi: 10.1186/s12935-018-0542-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang LL, Zhang LF, Shi YB. miR-24 inhibited the killing effect of natural killer cells to colorectal cancer cells by downregulating Paxillin. Biomed Pharmacother. 2018;101:257–263. doi: 10.1016/j.biopha.2018.02.024. [DOI] [PubMed] [Google Scholar]
- 30.Rahmoon MA, Youness RA, Gomaa AI, Hamza MT, Waked I, El Tayebi HM, et al. MiR-615-5p depresses natural killer cells cytotoxicity through repressing IGF-1R in hepatocellular carcinoma patients. Growth Factors. 2017;35:76–87. doi: 10.1080/08977194.2017.1354859. [DOI] [PubMed] [Google Scholar]
- 31.Leong JW, Wagner JA, Ireland AR, Fehniger TA. Transcriptional and post-transcriptional regulation of NK cell development and function. Clin Immunol. 2017;177:60–69. doi: 10.1016/j.clim.2016.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yang Y, Ding L, Hu Q, Xia J, Sun J, Wang X, et al. MicroRNA-218 functions as a tumor suppressor in lung cancer by targeting IL-6/STAT3 and negatively correlates with poor prognosis. Mol Cancer. 2017;16:141. doi: 10.1186/s12943-017-0710-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shi ZM, Wang L, Shen H, Jiang CF, Ge X, Li DM, et al. Downregulation of miR-218 contributes to epithelial-mesenchymal transition and tumor metastasis in lung cancer by targeting Slug/ZEB2 signaling. Oncogene. 2017;36:2577–2588. doi: 10.1038/onc.2016.414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ducker GS, Ghergurovich JM, Mainolfi N, Suri V, Jeong SK, Hsin-Jung Li S, et al. Human SHMT inhibitors reveal defective glycine import as a targetable metabolic vulnerability of diffuse large B-cell lymphoma. Proc Natl Acad Sci U S A. 2017;114:11404–11409. doi: 10.1073/pnas.1706617114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ueland PM, McCann A, Midttun Ø, Ulvik A. Inflammation, vitamin B6 and related pathways. Mol Aspects Med. 2017;53:10–27. doi: 10.1016/j.mam.2016.08.001. [DOI] [PubMed] [Google Scholar]
- 36.Gupta R, Yang Q, Dogra SK, Wajapeyee N. Serine hydroxymethyl transferase 1 stimulates pro-oncogenic cytokine expression through sialic acid to promote ovarian cancer tumor growth and progression. Oncogene. 2017;36:4014–4024. doi: 10.1038/onc.2017.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lv Q, Hu JX, Li YJ, Xie N, Song DD, Zhao W, et al. MiR-320a effectively suppresses lung adenocarcinoma cell proliferation and metastasis by regulating STAT3 signals. Cancer Biol Ther. 2017;18:142–151. doi: 10.1080/15384047.2017.1281497. [DOI] [PMC free article] [PubMed] [Google Scholar]