Abstract
Recently, fibroblast growth factors are identified to play a vital role in the development and progression of human pancreatic cancer. FGF pathway is critical involved in numerous cellular processes through regulation of its downstream targets, including proliferation, apoptosis, migration, invasion, angiogenesis and metastasis. In this review article, we describe recent advances of FGFR signalling pathway in pancreatic carcinogenesis and progression. Moreover, we highlight the available chemical inhibitors of FGFR pathway for potential treatment of pancreatic cancer. Furthermore, we discuss whether targeting FGFR pathway is a novel therapeutic strategy for pancreatic cancer clinical management.
Keywords: FGF, FGFR, pancreatic cancer, target, therapy
1. INTRODUCTION
Pancreatic cancer is one of the common malignancies in human worldwide. In fact, 56 770 new cases of pancreatic cancer and 45 750 deaths have been expected this year in the United States.1 More than 400 000 deaths annually due to pancreatic cancer are observed in the worldwide.2 Pancreatic cancer is the third leading cause of cancer death behind lung cancer and colon cancer in the United States in 2018. However, deaths from pancreatic cancer are predicted to be the second leading cause of mortality in the United States by 2030.3 The causes of pancreatic cancer are still unclear, although accumulating evidence has suggested that pancreatic cancer occurrence is associated with several factors such as smoking, drinking, coffee consumption, high fat and high protein diet, and genetic background. In addition, the patients with diabetes and chronic pancreatitis have high risk for developing pancreatic cancer.4, 5 In contrast to the increase in survival for most cancer types, the 5‐year relative survival rate for pancreatic cancer is about 8% in the United States. One of the reasons is that pancreatic cancer is often diagnosed at a distant stage, which has 3% for the 5‐year survival rate.6 Because the early symptoms of pancreatic cancer are same as gastric disease such as upper abdominal discomfort and loss of appetite, most of the patients with pancreatic cancer often exhibit locally invasion or metastatic tumour when they are diagnosed.7 About 95% of pancreatic cancer cases are adenocarcinoma, known as PDAC (pancreatic ductal adenocarcinoma), which arises from the epithelium of a duct.8, 9, 10
In recent years, emerging evidence has demonstrated that vital genes and signalling pathways are critically involved in the tumorigenesis and progression of pancreatic cancer, such as K‐ras related proteins,11 Notch,12 Hedeghog,13 Wnt,14 F‐box proteins,15 PI3K (phosphatidylinositol 3‐Kinase)/Akt16 and mTOR (mammalian target of rapamycin).17 Several lines of evidence has revealed that various growth factor signalling pathways are participated in pancreatic tumorigenesis and progression, including TGF (transforming growth factor),18 EGF (epidermal growth factor),19 HGF (hepatocyte growth factor),20 IGF (insulin‐like growth factor),21 PDGF (platelet‐derived growth factor)22 and FGF (fibroblast growth factor).23, 24 Recently, FGF has been paid attention to pancreatic cancer development and progression. In fact, these pathways could have interplays. For example, Notch signalling activation increases FGF1‐mediated invasion in oral squamous cell carcinoma.25 FGF activates Ras‐MAPK pathway, leading to skin tumour induced by Pten deficient.26 Similarly, FGFR1 promotes activation of MAPK and mTOR pathway in palbociclib resistant non‐small‐cell lung cancer.27 Another study identified that FGF2 exerts tumour lymphangiogenesis via activating the Akt/mTOR/p70S6K.28 FGF signalling activates the expression of the sonic hedgehog receptor and Ptch2.29 In this review article, we will describe recent advances of FGF signalling pathway in pancreatic cancer. Moreover, we will dissect the available chemical inhibitors of FGF pathway for potential treatment of pancreatic cancer. Furthermore, we will discuss whether targeting FGF pathway is a novel therapeutic strategy for pancreatic cancer clinical management.
2. FGF/FGFR SIGNALLING PATHWAY
FGF, a kind of peptide molecule, has been identified to bind to its specific receptors of cell membrane and to govern cell growth. FGF is named due to its promotion of fibroblast proliferation and is located in various tissues. FGF is also called heparin conjugate growth factor because of its high affinity for heparin. At present, more than 20 members of the FGF family are identified, which are encoded by various genes.30 The structure of FGF protein contains heparin sulphate binding domain and FGFR binding domains.30 FGF1 (aFGF, acidic FGF) and FGF2 (bFGF, basic FGF) were originally thought to be potent mitogens for some cell types. FGF2 has two isoforms; the extracellular LMW isoform and predominantly nuclear HMW isoforms.31 Five types of FGFRs (FGFR1, 2, 3, 4, 5) have been reported and isoforms of FGFR‐1, ‐2, ‐3 have FGFR1b, FGFR1c, FGFR2b, FGFR2c, FGFR3a, FGFR3b, FGFR3c, FGFR4 and FGFR5.32, 33 Each isoform could have different location: FGFR3b is restricted to epithelial cell types, while FGFR3c is located in mesenchymal cell types. FGFR can bind to two FGF2 isoforms: LMW and HMW isoforms.31 FGFR proteins contain the cytoplasmic tyrosine kinase domain, a single‐pass transmembrane domain and extracellular immunoglobulin‐like domain.34 Interestingly, FGFR5 (also named as FGFRL1) lacks tyrosine kinase domain, which is different from the other four types.35
Clearly, FGFs as ligands bind to FGFRs and activate tyrosine kinase domain of FGFRs, leading to activation of FGF/FGFR signalling pathway. Interestingly, FGF1 also binds to heparin sulphate proteoglycans (HSPG), suggesting that HSPG could be a co‐receptor of FGF1. In addition, FGF1 co‐localizes with both proteoglycans CD44 and CSPG4 at the cell surface, indicating that these receptors could be storage molecular to create a reservoir of FGF1.36 Heparin and heparin sulphate glycosaminoglycans (HSGAGs) can stabilize FGFs against degradation.37 The activation of FGF/FGFR pathway regulates several downstream targets such as PI3K/Akt, MAPK (mitogen‐activated protein kinase) or PLCγ.38 FGF signalling pathway plays a role in a myriad of cellular biological and physiological processes such as proliferation, differentiation, survival, migration, invasion, metastasis, wound repair and angiogenesis.30, 39 FGF signalling pathway has been identified in tumorigenesis and progression in a variety of human cancers including pancreatic cancer. In the following sections, we will decipher the role of FGF/FGFR signalling pathway in pancreatic carcinogenesis.
3. THE ROLE OF FGF/FGFR IN PANCREATIC CANCER
3.1. FGF in pancreatic cancer
FGF‐1 and FGF‐2 are overexpressed in pancreatic carcinoma cells, which are associated with advanced tumour stage and shorter survival.40 In line with this finding, one study has demonstrated that the expression of FGF‐1, FGF‐2 and their receptors were highly increased in pancreatic adenocarcinomas compared with normal pancreatic tissue.41 Moreover, increased FGF and FGFR were associated with upregulation of iNOS (inducible nitric oxide synthase) and protein tyrosine nitration in pancreatic cancer tissues, predicting the potential involvement of oxidant stress in FGF pathway‐mediated pancreatic cancer development.41 Subsequently, this group identified that FGF‐1 signalling inhibited peroxynitrite‐induced cell death in pancreatic cancer, suggesting that FGF‐1 plays a vital role in pancreatic adenocarcinoma.42 Another study reported that FGF‐1 and FGF‐2 treatment led to induction of phosphorylation of E‐cadherin and beta‐catenin on tyrosine residues, resulting in an increase in cell adhesion, tubular differentiation and reduction of invasion in pancreatic cancer cells.43, 44
Twenty‐eight years ago, one study has shown that FGF‐2 at picomolar concentrations promoted cell proliferation via regulation of ornithine decarboxylase in AR4‐2J rat pancreatic cancer cell line.45 Moroever, more evidence has emerged to validate the role of FGF‐2 in pancreatic cancer. For exmaple, high expression of FGF‐2 was observed in PDAC, and patients with high level of FGF‐2 and VEGF (vascular endothelial growth factor) had shorter survival times.46 Consistently, tumour cell proliferative indices were significantly higher in pancreatic cancer cells with FGF‐2‐positive, indicating that the expression of FGF‐2 is associated with cell proliferation in pancreatic cancer.47 Similarly, a specific neutralizing antibody against FGF‐2 led to a 50% inhibition in cell proliferation in pancreatic cancer cells.48 Further, the high gradient of FGF‐2 enhanced cell invasiveness in pancreatic cancer cells, whereas inhibition of FGF pathway by anti‐FGF receptor antibody retarded cell invasion, demonstrating that FGF‐2 is involved in cell invasiveness in pancreatic cancer.49 Additionally, Pim‐3, a proto‐oncogene with serine/threonine kinase activity, promoted tumour neovascularization and tumour growth via upregulation of FGF‐2 in pancreatic cancer.50 Klotho, a transmembrane protein, suppressed cell growth in vitro and in vivo through inactivation of FGF‐2 pathway in pancreatic cancer.51 Interestingly, secretory FGF‐2 upregulation was exhibited to have the potential to inhibit spreading of pancreatic cancer cells.52
FGF‐5 has been reported to be involved in various biological processes including development, tissue growth, repair and morphogenesis.53 FGF‐5 was initially identified to be an oncogene in human cancers.54 FGF‐5 mRNA was detected in pancreatic cancer cells and secreted FGF‐5 protein was observed in conditioned medium of pancreatic cancer cells. Overexpression of FGF‐5 promoted the cell growth and increased MAPK activity in pancreatic cancer.55 Expression of FGFR‐1 IIIc variant mediated FGF‐5‐induced mitogenic responsiveness through the MAPK pathway in pancreatic ductal cells, indicating that FGF‐5 in conjunction with FGFR‐1 IIIc could contribute to pancreatic cancer pathobiology.56, 57
FGF‐7, also called as keratinocyte growth factor (KGF), is originally observed in mesenchymal cells, demonstrated that FGF‐7 might be involved in mesenchymal stimulation of epithelial cell proliferation.58 FGF‐7/KGF is frequently overexpressed in pancreatic cancer.59 KGF/FGF‐7 activated NF‐κB (nuclear factor kappa B) and subsequently induced the expression of VEGF, MMP‐9 and urokinase‐type plasminogen activator, leading to enhancement of migration and invasion in pancreatic ductal epithelial cells. This finding identify that KGF/FGF‐7 could be a malignancy‐contributing factor from tumour stroma.59 FGF10, a FGF‐7 subfamily member, exerted its biological responses via activation of FGFR2b. One study reported that FGF‐10 can participate in transmitting mesenchyme signalling to the epithelium and involved in pancreas development.60 Stimulation of pancreatic cancer cells with FGF‐1, FGF‐2, FGF‐7 and FGF‐10 resulted in changes in the expression of key genes such as SOX‐9 (SRY‐related HMG‐box gene 9), HNF3β (hepatocyte nuclear factor 3‐beta), GATA‐4, GATA‐6 and HES1 (hairy and enhancer of split‐1).61 This study suggests that these growth factors might be involved in pancreatic cancer development. FGF‐10 was observed in stromal cells surrounding the cancer cells in pancreatic cancer tissues. FGF‐10 induced cell migration and invasion through interaction with FGFR2 IIIb and increased expression level of MT1‐MMP (membrane type 1‐matrix metalloproteinase) and TGF‐β1 in pancreatic cancer.62 Consistently, FGF10 was significantly overexpressed in pancreatic cancer patients compared with healthy controls. FGF‐10 had differentially expressed in response to gemcitabine and erlotinib, suggesting that FGF‐10 could be a predictive biomarker for chemotherapeutic treatment response in pancreatic cancer patients.63
FGF‐13 was found to be significantly associated with the shorter survival and occurrence of liver metastasis in pancreatic cancer.64 This investigation identifies FGF‐13 as a novel prognostic biomarker in pancreatic cancer. Overexpression of FGF‐19 did not affect the cell proliferation, but inhibited cell migration, invasion and attachment via stimulation of FGFR4 in pancreatic cancer cells.65 Several knockout mouse phenotypes have demonstrated the role of FGFs in tumorigenesis. Ffg15 (human homolog, FGF19) deficiency impairs liver regeneration in mice.66 Moreover, fibrosis‐induced hepatocellular carcinoma development is retarded in Fgf15 knockout mice.67 Inducible Fgf13 ablation in cardiomyocytes enhances caveolae‐induced cardioprotection during cardiac pressure overload.68 Loss of Fgf21 leads to insulin resistance, pancreatic islet hyperplasia and dysfunction in mice. Fgf23 knockout mice impair the auditory system and the metabolism of phosphate and active vitamin D in the kidney.69, 70 Fgf19 transgenic mice developed hepatocellular carcinomas.71 Transgenic expression of FGF8 and FGF10 results in the development of hepatocytes and exocrine cells from pancreatic islet cells transdifferentiation.72 Prostate‐targeted Fgf8b transgenic mice have stromal activation and prostate cancer development.73 Fgf‐2 transgenic mice have glandular epithelial hyperplasia in the murine prostatic dorsal lobe.74 Without a doubt, the engineering mouse model is an ideal vehicle for studying the role of FGF in human cancers including pancreatic cancer.
3.2. FGF‐binding proteins in pancreatic cancer
FGF‐binding proteins (FGF‐BP) release FGFs from the extracellular matrix storage, leading to increased FGF activity. Therefore, FGF‐BP plays a critical role as an extracellular chaperone in FGF‐mediated signalling pathway and mitogenesis.75, 76, 77 Moreover, FGF‐BP expression is remarkably increased in a variety of human cancer tissues.78 FGF‐BP1 expression is highly elevated in pancreatic adenocarcinoma compared with normal pancreas, suggesting that FGF‐BP1 might a biomarker for high‐risk premalignant lesions.79 In consistent, FGF‐BP1 was found to be induced early during the pancreatic cancer initiation.80 These reports clearly indicate that FGF‐BP could become an indicator of early diagnosis for pancreatic cancer. The results from Fgfbp3 knockout mice showed that FGF‐BP3 impacts carbohydrate and lipid metabolism.81 To further investigate the role of FGF‐BP in tumorigenesis, Fgf‐bp engineering mice are required.
3.3. FGFR in pancreatic cancer
Twenty‐five years ago, aberrant expression of FGFR1 was observed in pancreatic cancer.82 Moreover, the 2‐immunoglobulin‐like form of FGFR1 was reported to involve in aberrant autocrine and paracrine pathways in pancreatic cancer.82 One study showed that inhibition of FGFR‐1 decreased cell growth in vitro and retarded tumour‐forming potential in vivo in pancreatic cancer. Moreover, FGF/FGFR‐1 exerted its function via regulation of receptor tyrosine phosphorylation and MAPK activation in pancreatic cancer.83 Overexpression of FGFR‐1α increased cell death via activation of caspase 3 and inhibition of Bcl‐xL (B‐cell lymphoma‐extra large)/BAX in pancreatic cancer cells. Moreover, FGFR‐1α overexpression suppressed cell growth and restored cytotoxic responses to chemotherapy.84 However, overexpression of FGFR‐1β led to formation of tumour xenograft and exhibited resistance to chemotherapy.84 Liu et al found that FGFR1 IIIb suppressed the formation and growth of tumours in mice, which have a reduced Ki‐67 and a lower level of tumour necrosis in tumours. This study showed that FGFR1 IIIb blocks the transformation phenotype of pancreatic cancer cells.85 Another study revealed that FGFR1 IIIb overexpression promoted the expression of SPARC (secreted protein acidic and rich in cysteine), which is a protein‐modulating cell–cell and cell–matrix interactions. FGFR1 IIIc overexpression decreased SPARC level in pancreatic cancer cells.86 This suggests that FGFR1‐III isoforms exert their function partly via modulation of SPARC expression in pancreatic cancer.
The FGFR1 IIIb induced cell proliferation after FGF‐1, FGF‐2 and FGF‐4 stimulations via production of a glycosylated 110kd protein in pancreatic cancer cells. The FGF‐1, FGF‐2 and FGF10 induced activation of MAPK and c‐Jun N‐terminal kinase and led to cell proliferation enhancement. Moreover, the FGFR1 IIIb increased single‐cell movement and plating efficacy. Thus, the FGFR1 IIIb could govern cell proliferation, adhesion and movement in pancreas.87 Blockade of FGF‐2‐induced proliferation of pancreatic cancer cells by an adenoviral vector encoding a truncated FGFR‐1 (AdtrFGFR‐1) led to decreased MAPK activation, implying that AdtrFGFR‐1 could be useful as a therapeutic agent in pancreatic cancer.88 Similarly, a recombinant adenovirus expressing soluble FGF receptor (AdsFGFR) suppressed tumour angiogenesis and tumour growth in vitro and in vivo, indicating that FGFR plays a key role in tumour angiogenesis.89 Clinically, high expression of FGFR was associated with the extent of malignancy and post‐operative survival in human PDAC.90
FGFR2 expression was observed in pancreatic cancer cells. Patients with high level of FGFR2 exhibited a shorter survival time in pancreatic cancer.62 Downregulation of FGFR‐2 by its shRNA infection targeting the IIIb and IIIc isoforms inhibited cell proliferation, migration and invasion in PDAC cells. Additionally, downregulation of FGFR‐2 led to decreased phosphorylation of ERK (extracellular signal‐regulated kinases) and VEGF‐A in PDAC cells after FGF‐2 stimulation. Moreover, inhibition of FGFR‐2 resulted in smaller tumours in nude mice, suggesting that FGFR‐2 could be a potential target for pancreatic cancer.91 Similarly, inhibition of FGFR signalling using shRNA led to cell kill in pancreatic cancer cells. Dovitinib treatment in combination with FGFR shRNA transfection achieved significant anti‐tumour effects in pancreatic cancer, especially in FGFR2 IIIb overexpressing pancreatic cancer cells.92 Furthermore, FGFR2 IIIc was highly expressed in PDAC tissues, which is associated with liver metastasis in PDAC patients. In line with the role of FGFR2 in PDAC, overexpression of FGFR2 IIIc promoted cell proliferation in vitro and enhanced tumour growth and live metastases in vivo via upregulation of p‐ERK (phosphorylated extracellular signal‐regulated kinase) in PDAC.93 One study showed that targeting the CYP2B1/cyclophosphamide suicide system to FGFRs led to tumour suppressive response and an increased survival rate in pancreatic cancer.94
FGFR4 was expressed in a majority of pancreatic cancer patients, and its expression was related to longer overall survival. FGFR4 stimulation led to increased cell adhesion to laminin and fibronection, and inhibited cell migration, suggesting that FGFR4 could contribute to tumour suppressive function via enhanced cell adhesion to extracellular matrix.65 Consistently, dominant‐negative FGFR‐4 and inhibitors of FGFR signalling inhibited matrix adhesion induced by N‐CAM (neural cell‐adhesion molecule) in pancreatic cancer. Moreover, N‐CAM promoted β1‐integrin‐involved cell–matrix adhesion via activation of FGFR signalling pathway.95 Additionally, FGFR4 knockout mice bred with FGF19 transgenic mice fail to develop liver tumours.71 The engineering mice are necessary to explore the function of FGFR in tumorigenesis.
4. FGFR INHIBITORS FOR PANCREATIC CANCER TREATMENT
Several FGFR inhibitors have been discovered for potential treatment of human cancers including pancreatic cancer96 (Table 1). For example, SSR128129E is an orally effective allosteric FGFR inhibitor, which has no effect on other related RTKs. Chemical SSR128129E (SSR) inhibits responses mediated by FGFR1‐4. SSR was reported to inhibit the proliferation and migration of pancreatic tumour cell line in response to FGF‐7.97 Dovitinib, formerly known as TKI258, a tyrosine kinase inhibitor to FGFRs, PDGFRβ (platelet‐derived growth factor receptor beta) and VEGFR2, inhibited activation of signalling intermediates in pancreatic cancer cells upon FGF‐1 and FGF‐2 treatment. TKI258 repressed surviving level, enhanced activity of gemcitabine and reduced motility of pancreatic cancer cells. Moreover, TKI258 inhibited tumour growth and lymph node metastases in mouse model, suggesting that TKI258 could be an effective agent for human pancreatic cancer.98 Dovitinib treatment exhibited pro‐apoptotic effect in pancreatic cancer cells with heightened FGFR signalling activation via regulation of Akt/Mcl‐1 axis.92 Recently, a phase 1b study showed that dovitinib with gemcitabine and capecitabine achieved efficacy signals in advanced pancreatic cancer.99
Table 1.
FGFR inhibitors in cancer treatment
| Inhibitors | Targets | Function | Adverse events | Ref. |
|---|---|---|---|---|
| BGJ398 | FGFR1‐3 | Inhibits cell proliferation; exerts anti‐tumour activity in several tumour types including lung cancer, bladder, urothelial cancers, cholangiocarcinoma | Hyperphosphatemia, constipation, decreased appetite, diarrhoea, fatigue, alopecia, nausea in patients | 109, 124, 125, 126, 127 |
| SSR128129E | FGFR1‐4 | Inhibits proliferation, angiogenesis and metastasis in pancreatic, breast and colon cancer cells | A therapeutic dose minimally elevated plasma levels of the prothrombotic PAI‐1, a minor anaemia in mice | 97 |
| Dovitinib (TKI258) | FGFR, PDGFRβ, VEGFR2 | Inhibits tumour growth, motility and metastasis; enhances the therapeutic effect of gemcitabine and capecitabine | Fatigue, neutropenia, thrombocytopenia, anaemia, nausea, palmar‐plantar erythrodysesthesia syndrome in patients | 98, 99 |
| Lenvatinib | FGFR1‐4, KIT, RET, VEGFR1‐3, PDGFRα | Inhibits tumour growth, angiogenesis in pancreatic cancer, hepatocellular cancer and melanoma | Hypertension, palmar‐plantar erythrodysesthesia syndrome, decrease appetite, proteinuria, fatigue, nausea | 100, 128, 129 |
| Masitinib | c‐Kit, FGFR and PDGFR | Inhibits inflammation, combined with gemcitabine exhibited synergy on proliferation inhibition | Back pain, constipation, pulmonary embolism, vomiting, nausea, rash, thrombocytopenia, thrombosis, hypokalemia, pyrexia, neutropenia and anaemia | 101, 102, 103, 104 |
| PD173074 | FGFR1, VEGFR2 | Blocks the proliferation and induces apoptosis. Inhibits stem cell proliferation and self‐renewal | No body weight loss and appearance change in mice | 105, 108, 130 |
| Nintedanib | VEGFR1/2/3, FGFR1/2/3, PDGFRα/β | Inhibits cell proliferation, induces apoptosis, enhances gemcitabine, or afatinib, or docetaxel, or cisplatin inhibitory effect | Diarrhoea, asthenia, nausea, vomiting, anaemia, anorexia, hepatic enzyme elevation, hypertension, hypothyroidism, hand‐foot syndrome, cardiac disorder, haematological abnormalities. Nintedanib plus docetaxel leads to sepsis, pneumonia, respiratory failure and pulmonary embolism | 110, 111, 112, 113, 131, 132, 133, 134, 135, 136, 137, 138, 139, 140 |
| Ponatinib | FGFRs, Bcr‐Abl, Src, PDGFRa, VEGFR2 | Anti‐tumour activity in leukaemia. Combines an MEK inhibitor to inhibit pancreatic cancer cell growth | Hypertension, myelosuppression, cerebrovascular, vaso‐occlusive disease, lipase and rash | 115, 141, 142, 143, 144, 145 |
Lenvatinib, an oral inhibitor of multiple RTKs targeting FGFR1‐4, VEGFR1‐3, PDGFRα, RET and KIT. One study has shown that lenvatinib suppressed in vivo angiogenesis induced by overexpressed FGF in pancreatic cancer. Notably, lenvatinib also inhibited tumour growth in tumour xenograft models. This report indicates that lenvatinib inhibited FGF‐ and VEGF‐driven angiogenesis in pancreatic cancer.100 Masitinib, a tyrosine kinase inhibitor of several targets, inhibits c‐Kit, FGFR and PDGFR. Masitinib could decrease inflammation in pancreatic cancer patients with increased pain scores.101 Masitinib and gemcitabine combination exhibited synergy in vitro on proliferation of pancreatic cancer cells.102 The efficacy and safety of masitinib/gemcitabine have been evaluated and shown to extend survival and median time‐to‐progression in pancreatic cancer.103, 104 PD173074, an effective inhibitor of FGFR1, inhibited neoangiogenesis and mitogenesis, induced apoptosis, leading to inhibition of orthotopic tumour growth in pancreatic cancer mouse model.105 In addition, PD173074 inhibited cell proliferation and self‐renewal of pancreatic cancer stem cells via suppression of Oct4, Sox‐2, Nanog, c‐Myc, XIAP (X‐linked inhibitor of apoptosis protein), Bcl‐2 and survivin. However, it has no direct evidence to show the role of FGF/FGFR in pancreatic cancer stem cells. Two papers suggest that FGF signalling and FGF10 were involved in enhancing differentiation of pluripotent stem cells into pancreatic progenitors.106, 107 Moreover, PD173074 induced cell apoptosis via upregulation of caspase‐3 and cleaved PARP (poly‐ADP ribose polymerase) in pancreatic cancer cells. PD173074 also inhibited the activation of c‐Met, Src, ERK1/2 and NF‐κB in pancreatic cancer cells.108 BGJ398 is an effective, bioactive FGFR1/2/3 inhibitor with low inhibitory effect on FGFR4, which inhibited cell proliferation of pancreatic cancer.109
Nintedanib (BIBF 1120), a triple tyrosine kinase inhibitor that targets VEGFR1/2/3, FGFR1/2/3 and PDGFRα/β signalling, inhibited tumour growth, enhanced the activity of gemcitabine and decreased metastatic burden in orthotopic pancreatic xenografts, suggesting that nintedanib could be a potent anti‐angiogenesis agent for pancreatic cancer.110 Moreover, nintedanib inhibited cell proliferation, induced apoptosis via blocking PI3K/MAPK activity and enhanced gemcitabine inhibitory effects in pancreatic cancer.111 Furthermore, nintedanib was identified as a highly effective therapeutic for neuroendocrine carcinoma of the pancreas using transgenic mouse model.112 Notably, nintedanib plus afatinib exhibit anti‐tumour activity with a manageable safety in pancreatic cancer.113 Ponatinib (AP24534) is an effective multitargeted inhibitor that act on FGFRs, Bcr‐Abl, Src kinase, PDGFRα, VEGFR2, Akt, ERK1/2 and other kinases.114 Ponatinib plus an MEK inhibitor were effective in inhibition of pancreatic cancer cell growth.115 BGJ398 is an effective, bioactive FGFR1/2/3 inhibitor with low inhibitory effect on FGFR4, which inhibited cell proliferation of pancreatic cancer.109 We believe that more FGFR inhibitors will be discovered for the treatment of pancreatic cancer. It is noteworthy that using these FGFR inhibitors could cause side effects on cancer patients. For instance, TKIs could lead to adverse effects on viral organs, including the cardiovascular system and liver.116 Hypertension is associated with the treatment of nintedanib, lenvatinib, ponatinib, cabozantinib and trametinib.116 Moreover, ponatinib treatment for chronic myeloid leukaemia results in cardiovascular adverse effects, such as vascular occlusive event.117 Due to inhibition of VEGFR by these TKIs, these inhibitors’ application could lead to bleeding and thrombosis.118 Hence, it is required to reduce adverse effects of FGFR inhibitors.
5. CONCLUSION AND PERSPECTIVE
In summary, FGF plays an important role in the development and progression of human pancreatic cancer because FGF pathway is critical involved in numerous cellular processes including proliferation, apoptosis, migration, invasion, angiogenesis and metastasis (Figure 1). FGF/FGFR has been revealed to participate in its regulatory functions through regulation of its downstream targets (Table 2). Therefore, targeting FGF/FGFR pathway might be an effective strategy for treating pancreatic cancer. However, several questions should be addressed regarding role of FGF/FGFR in pancreatic cancer. Since the upstream and downstream components involved in FGF/FGFR pathway are largely unknown, it is required to identify these components that could be helpful for discovery of new inhibitor of FGFR for pancreatic cancer treatment. Because FGF/FGFR could have different roles in various organisms, it is better to find an approach for discovery of FGF/FGFR inhibitors in the specific organism with minimal effect on other organisms. Because available FGFR inhibitors target multiple molecules, which could lead to side effect function, it is better to develop the specific inhibitor for one molecule. Blocking a single FGFR with a monoclonal antibody could be helpful for cancer patients with amplification or constitutive activation of a special subtype of FGFR. Due to that most cancers with upregulation of FGFs and FGFR subtypes, targeting one FGFR by its antibody or siRNA might not acquire the treatment benefit. Recently, several microRNAs (miRNAs) have been identified to target FGF/FGFR pathway in human cancer.119, 120, 121 For example, miR‐214 inhibits the expression of FGFR‐1, leading to suppression of hepatocellular carcinoma metastasis.119 One study showed that miR‐99a targets FGFR3 in epithelial ovarian cancer cells.120 Another study validated the miRNA panel, including let‐7c, miR‐155 and miR‐218, could be useful for prediction of response to ponatinib in lung cancer cells.121 FGF2 was a direct target of miR‐186‐5p in glioblastoma multiforme.122 Moreover, FGF‐2 regulates cell proliferation, migration and angiogenesis via governing NDY1/KDM2B‐miR‐101‐EZH2 pathway in bladder cancer.123 However, studies for role of miRNAs regulating FGF/FGFR in pancreatic cancer progression are not available. How to use FGFR inhibitors in combination with chemotherapeutic drugs to maximize the treatment benefit in cancer patients? Taken together, uncovering the molecular mechanism regarding how FGF pathway is involved in pancreatic tumorigenesis would shed light onto the discovery of new effective inhibitors of FGFR.
Figure 1.
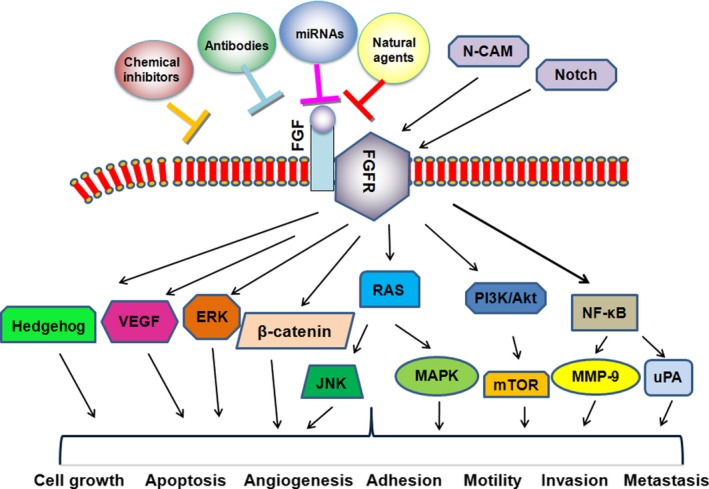
Role of the FGF/FGFR signalling pathway in the development and progression of pancreatic cancer. Fibroblast growth factor (FGF) signalling pathway regulates numerous cellular processes such as cell proliferation, apoptosis, angiogenesis, migration, invasion and metastasis. FGF/FGFR could be regulated by Notch, N‐CAM and miRNAs. FGF/FGFR exhibits its physiological functions via regulation of its downstream targets. The chemical inhibitors of FGF/FGFR, antibodies and natural agents could block FGF signalling pathway. Thus, targeting FGF/FGFR could be an effective approach for the treatment of pancreatic cancer patients
Table 2.
Role of FGF/FGFR in pancreatic cancer
| FGF/FGFR | Targets | Function | Reference |
|---|---|---|---|
| FGF‐1 | Induction of phosphorylation of E‐cadherin and β‐catenin, regulation of SOX‐9, HNF3β, HES1 | Overexpression; associates with advanced tumour stage and shorter survival | 40, 41, 43, 44, 61 |
| FGF‐2 | Induction of phosphorylation of E‐cadherin and β‐catenin, regulation of SOX‐9, HNF3β, HES1, ornithine decarboxylase | Overexpression; associates with advanced tumour stage and shorter survival; promotes cell growth and invasion | 40, 41, 43, 44, 47, 49, 61 |
| FGF‐5 | Induction of MAPK activity | Overexpression; promotes the cell growth | 55 |
| FGF‐7 | Activates NF‐κB, VEGF, MMP‐9 and uPA, regulation of SOX‐9, HNF3β, HES1 | Overexpression; promotes migration and invasion | 59, 61 |
| FGF‐10 | Increases MT1‐MMP and TGF‐β1, regulation of SOX‐9, HNF3β, HES1 | Induces cell migration and invasion. Overexpressed; a biomarker for chemotherapeutic treatment response | 61, 62, 63 |
| FGF‐13 | Not identified | Associates with the shorter survival and occurrence of liver metastasis in pancreatic cancer | 64 |
| FGF‐19 | Stimulation of FGFR4 | Inhibits cell migration, invasion and attachment | 65 |
| FGF‐BP1 | Not identified | Overexpression; Induces early during the pancreatic cancer initiation | 79, 80 |
| FGFR‐1 | Activation of MAPK, caspase 3, inhibition of Bcl‐xL/Bax and SPARC | Controls cell growth, cell death, adhesion, movement and tumour angiogenesis | 83, 84, 85, 86, 87, 89 |
| FGFR‐2 | ERK, VEGF‐A | Overexpression; associates with a shorter survival rate; inhibits cell proliferation, migration and invasion | 62, 91, 93 |
| FGFR‐4 | PLC‐γ, PI3K, MAPK | Associates with longer overall survival; increases cell adhesion, inhibits cell migration | 65, 95 |
CONFLICT OF INTEREST
The authors declare that they have no conflict of interest.
AUTHOR CONTRIBUTIONS
All authors are involved in writing this manuscript and approved this article.
ACKNOWLEDGEMENT
This work was supported by grant from National Natural Science Foundation of China (NSFC No. 81572936, 81773186) and Research Fund for Lin He's Academician Workstation of New Medicine and Clinical Translation.
Kang X, Lin Z, Xu M, Pan J, Wang Z‐W. Deciphering role of FGFR signalling pathway in pancreatic cancer. Cell Prolif. 2019;52:e12605 10.1111/cpr.12605
Contributor Information
Jun Pan, Email: panjun@wmu.edu.cn.
Zhi‐wei Wang, Email: zwang6@bidmc.harvard.edu.
REFERENCES
- 1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019;69(1):7‐34. [DOI] [PubMed] [Google Scholar]
- 2. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394‐424. [DOI] [PubMed] [Google Scholar]
- 3. Rahib L, Smith BD, Aizenberg R, Rosenzweig AB, Fleshman JM, Matrisian LM. Projecting cancer incidence and deaths to 2030: the unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res. 2014;74(11):2913‐2921. [DOI] [PubMed] [Google Scholar]
- 4. Kirkegard J, Mortensen FV, Cronin‐Fenton D. Chronic pancreatitis and pancreatic cancer risk: a systematic review and meta‐analysis. Am J Gastroenterol. 2017;112(9):1366‐1372. [DOI] [PubMed] [Google Scholar]
- 5. Zhang JJ, Jia JP, Shao Q, Wang YK. Diabetes mellitus and risk of pancreatic cancer in China: a meta‐analysis based on 26 case‐control studies. Prim Care Diabetes. 2018;S1751-9918(18)30091-3 10.1016/j.pcd.2018.11.015. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 6. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68(1):7‐30. [DOI] [PubMed] [Google Scholar]
- 7. Zhang Q, Zeng L, Chen Y, et al. Pancreatic cancer epidemiology, detection, and management. Gastroenterol Res Pract. 2016;2016:8962321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Arcidiacono PG, Bhutani MS, Giovannini M. EURO‐EUS 2003: Pancreatic tumor – impact of endoscopic ultrasonography on diagnosis, staging and treatment. Cancer Biol Ther. 2004;3(5):477‐481. [DOI] [PubMed] [Google Scholar]
- 9. McGough N, Cummings JH. Coeliac disease: a diverse clinical syndrome caused by intolerance of wheat, barley and rye. Proc Nutr Soc. 2005;64(4):434‐450. [DOI] [PubMed] [Google Scholar]
- 10. Garcea G, Dennison AR, Pattenden CJ, Neal CP, Sutton CD, Berry DP. Survival following curative resection for pancreatic ductal adenocarcinoma. A systematic review of the literature. JOP. 2008;9(2):99‐132. [PubMed] [Google Scholar]
- 11. Mann KM, Ying H, Juan J, Jenkins NA, Copeland NG. KRAS‐related proteins in pancreatic cancer. Pharmacol Ther. 2016;168:29‐42. [DOI] [PubMed] [Google Scholar]
- 12. Gao J, Long B, Wang Z. Role of Notch signaling pathway in pancreatic cancer. Am J Cancer Res. 2017;7(2):173‐186. [PMC free article] [PubMed] [Google Scholar]
- 13. Gu D, Schlotman KE, Xie J. Deciphering the role of hedgehog signaling in pancreatic cancer. J Biomed Res. 2016;30(5):353‐360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Javadinia SA, Shahidsales S, Fanipakdel A, et al. Therapeutic potential of targeting the Wnt/beta‐catenin pathway in the treatment of pancreatic cancer. J Cell Biochem. 2018. [DOI] [PubMed] [Google Scholar]
- 15. Wang H, Maitra A, Wang H. The emerging roles of F‐box proteins in pancreatic tumorigenesis. Semin Cancer Biol. 2016;36:88‐94. [DOI] [PubMed] [Google Scholar]
- 16. Ebrahimi S, Hosseini M, Shahidsales S, et al. Targeting the Akt/PI3K signaling pathway as a potential therapeutic strategy for the treatment of pancreatic cancer. Curr Med Chem. 2017;24(13):1321‐1331. [DOI] [PubMed] [Google Scholar]
- 17. Rozengurt E. Mechanistic target of rapamycin (mTOR): a point of convergence in the action of insulin/IGF‐1 and G protein‐coupled receptor agonists in pancreatic cancer cells. Front Physiol. 2014;5:357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Shen W, Tao GQ, Zhang Y, Cai B, Sun J, Tian ZQ. TGF‐beta in pancreatic cancer initiation and progression: two sides of the same coin. Cell Biosci. 2017;7:39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hao C, Li Z, Zhang X, et al. Expression and clinical significance of EGF and TGF‐alpha in chronic pancreatitis and pancreatic cancer. Minerva Endocrinol. 2018;43(3):253‐258. [DOI] [PubMed] [Google Scholar]
- 20. Modica C, Tortarolo D, Comoglio PM, Basilico C, Vigna E. MET/HGF co‐targeting in pancreatic cancer: a tool to provide insight into the tumor/stroma crosstalk. Int J Mol Sci. 2018;19(12):E3920 10.3390/ijms19123920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Vaccaro V, Melisi D, Bria E, et al. Emerging pathways and future targets for the molecular therapy of pancreatic cancer. Expert Opin Ther Targets. 2011;15(10):1183‐1196. [DOI] [PubMed] [Google Scholar]
- 22. Lee J, Lee J, Yun JH, et al. Autocrine DUSP28 signaling mediates pancreatic cancer malignancy via regulation of PDGF‐A. Sci Rep. 2017;7(1):12760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ndlovu R, Deng LC, Wu J, Li XK, Zhang JS. Fibroblast growth factor 10 in pancreas development and pancreatic cancer. Front Genet. 2018;9:482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Coleman SJ, Bruce C, Chioni AM, Kocher HM, Grose RP. The ins and outs of fibroblast growth factor receptor signalling. Clin Sci (Lond). 2014;127(4):217‐231. [DOI] [PubMed] [Google Scholar]
- 25. Weaver AN, Burch MB, Cooper TS, et al. Notch signaling activation is associated with patient mortality and increased FGF1‐mediated invasion in squamous cell carcinoma of the oral cavity. Mol Cancer Res. 2016;14(9):883‐891. [DOI] [PubMed] [Google Scholar]
- 26. Mathew G, Hannan A, Hertzler‐Schaefer K, et al. Targeting of Ras‐mediated FGF signaling suppresses Pten‐deficient skin tumor. Proc Natl Acad Sci USA. 2016;113(46):13156‐13161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Haines E, Chen T, Kommajosyula N, et al. Palbociclib resistance confers dependence on an FGFR‐MAP kinase‐mTOR‐driven pathway in KRAS‐mutant non‐small cell lung cancer. Oncotarget. 2018;9(60):31572‐31589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Matsuo M, Yamada S, Koizumi K, Sakurai H, Saiki I. Tumour‐derived fibroblast growth factor‐2 exerts lymphangiogenic effects through Akt/mTOR/p70S6kinase pathway in rat lymphatic endothelial cells. Eur J Cancer. 2007;43(11):1748‐1754. [DOI] [PubMed] [Google Scholar]
- 29. Morales AV, Espeso‐Gil S, Ocaña I, et al. FGF signaling enhances a sonic hedgehog negative feedback loop at the initiation of spinal cord ventral patterning. Dev Neurobiol. 2016;76(9):956‐971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Turner N, Grose R. Fibroblast growth factor signalling: from development to cancer. Nat Rev Cancer. 2010;10(2):116‐129. [DOI] [PubMed] [Google Scholar]
- 31. Coleman SJ, Chioni A‐M, Ghallab M, et al. Nuclear translocation of FGFR1 and FGF2 in pancreatic stellate cells facilitates pancreatic cancer cell invasion. EMBO Mol Med. 2014;6(4):467‐481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ornitz DM, Marie PJ. Fibroblast growth factor signaling in skeletal development and disease. Genes Dev. 2015;29(14):1463‐1486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Babina IS, Turner NC. Advances and challenges in targeting FGFR signalling in cancer. Nat Rev Cancer. 2017;17(5):318‐332. [DOI] [PubMed] [Google Scholar]
- 34. Alabed SJ, Khanfar M, Taha MO. Computer‐aided discovery of new FGFR‐1 inhibitors followed by in vitro validation. Future Med Chem. 2016;8(15):1841‐1869. [DOI] [PubMed] [Google Scholar]
- 35. Wiedemann M, Trueb B. Characterization of a novel protein (FGFRL1) from human cartilage related to FGF receptors. Genomics. 2000;69(2):275‐279. [DOI] [PubMed] [Google Scholar]
- 36. Zhen Y, Haugsten EM, Singh SK, Wesche J. Proximity labeling by a recombinant APEX2‐FGF1 fusion protein reveals interaction of FGF1 with the proteoglycans CD44 and CSPG4. Biochemistry. 2018;57(26):3807‐3816. [DOI] [PubMed] [Google Scholar]
- 37. Hacker U, Nybakken K, Perrimon N. Heparan sulphate proteoglycans: the sweet side of development. Nat Rev Mol Cell Bio. 2005;6(7):530‐541. [DOI] [PubMed] [Google Scholar]
- 38. Beenken A, Mohammadi M. The FGF family: biology, pathophysiology and therapy. Nat Rev Drug Discov. 2009;8(3):235‐253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Korc M, Friesel RE. The role of fibroblast growth factors in tumor growth. Curr Cancer Drug Targets. 2009;9(5):639‐651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Yamanaka Y, Friess H, Buchler M, et al. Overexpression of acidic and basic fibroblast growth factors in human pancreatic cancer correlates with advanced tumor stage. Cancer Res. 1993;53(21):5289‐5296. [PubMed] [Google Scholar]
- 41. Vickers SM, MacMillan‐Crow LA, Green M, Ellis C, Thompson JA. Association of increased immunostaining for inducible nitric oxide synthase and nitrotyrosine with fibroblast growth factor transformation in pancreatic cancer. Arch Surg. 1999;134(3):245‐251. [DOI] [PubMed] [Google Scholar]
- 42. Vickers SM, MacMillan‐Crow L, Huang Z, Thompson JA. Acidic fibroblast growth factor (FGF‐1) signaling inhibits peroxynitrite‐induced cell death during pancreatic tumorigenesis. Free Radic Biol Med. 2001;30(9):957‐966. [DOI] [PubMed] [Google Scholar]
- 43. El‐Hariry I, Pignatelli M, Lemoine NR. FGF‐1 and FGF‐2 modulate the E‐cadherin/catenin system in pancreatic adenocarcinoma cell lines. Br J Cancer. 2001;84(12):1656‐1663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. El‐Hariry I, Pignatelli M, Lemoine NR. FGF‐1 and FGF‐2 regulate the expression of E‐cadherin and catenins in pancreatic adenocarcinoma. Int J Cancer. 2001;94(5):652‐661. [DOI] [PubMed] [Google Scholar]
- 45. De Vries L, Tahiri‐Jouti N, Bensaid M, et al. Regulation of proliferation by fibroblast growth factor in a pancreatic cancer cell line. Digestion. 1990;46(Suppl 2):162‐165. [DOI] [PubMed] [Google Scholar]
- 46. Kuwahara K, Sasaki T, Kuwada Y, Murakami M, Yamasaki S, Chayama K. Expressions of angiogenic factors in pancreatic ductal carcinoma: a correlative study with clinicopathologic parameters and patient survival. Pancreas. 2003;26(4):344‐349. [DOI] [PubMed] [Google Scholar]
- 47. Yamazaki K, Nagao T, Yamaguchi T, Saisho H, Kondo Y. Expression of basic fibroblast growth factor (FGF‐2)‐associated with tumour proliferation in human pancreatic carcinoma. Virchows Arch. 1997;431(2):95‐101. [DOI] [PubMed] [Google Scholar]
- 48. Leung HY, Gullick WJ, Lemoine NR. Expression and functional activity of fibroblast growth factors and their receptors in human pancreatic cancer. Int J Cancer. 1994;59(5):667‐675. [DOI] [PubMed] [Google Scholar]
- 49. Hasegawa Y, Takada M, Yamamoto M, Saitoh Y. The gradient of basic fibroblast growth factor concentration in human pancreatic cancer cell invasion. Biochem Biophys Res Commun. 1994;200(3):1435‐1439. [DOI] [PubMed] [Google Scholar]
- 50. Liu B, Wang Z, Li HY, Zhang B, Ping B, Li YY. Pim‐3 promotes human pancreatic cancer growth by regulating tumor vasculogenesis. Oncol Rep. 2014;31(6):2625‐2634. [DOI] [PubMed] [Google Scholar]
- 51. Abramovitz L, Rubinek T, Ligumsky H, et al. KL1 internal repeat mediates klotho tumor suppressor activities and inhibits bFGF and IGF‐I signaling in pancreatic cancer. Clin Cancer Res. 2011;17(13):4254‐4266. [DOI] [PubMed] [Google Scholar]
- 52. Escaffit F, Estival A, Bertrand C, Vaysse N, Hollande E, Clemente F. FGF‐2 isoforms of 18 and 22.5 kDa differentially modulate t‐PA and PAI‐1 expressions on the pancreatic carcinoma cells AR4‐ 2J: consequences on cell spreading and invasion. Int J Cancer. 2000;85(4):555‐562. [DOI] [PubMed] [Google Scholar]
- 53. Kehler JS, David VA, Schäffer AA, et al. Four independent mutations in the feline fibroblast growth factor 5 gene determine the long‐haired phenotype in domestic cats. J Hered. 2007;98(6):555‐566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Bates B, Hardin J, Zhan X, Drickamer K, Goldfarb M. Biosynthesis of human fibroblast growth factor‐5. Mol Cell Biol. 1991;11(4):1840‐1845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Kornmann M, Ishiwata T, Beger HG, Korc M. Fibroblast growth factor‐5 stimulates mitogenic signaling and is overexpressed in human pancreatic cancer: evidence for autocrine and paracrine actions. Oncogene. 1997;15(12):1417‐1424. [DOI] [PubMed] [Google Scholar]
- 56. Kornmann M, Lopez ME, Beger HG, Korc M. Expression of the IIIc variant of FGF receptor‐1 confers mitogenic responsiveness to heparin and FGF‐5 in TAKA‐1 pancreatic ductal cells. Int J Pancreatol. 2001;29(2):85‐92. [DOI] [PubMed] [Google Scholar]
- 57. Kornmann M, Lopez M, Beger H, Korc M. Expression of the IIIc variant of FGF receptor‐1 confers mitogenic responsiveness to heparin and FGF‐5 in TAKA‐1 pancreatic ductal cells. Int J Gastrointest Cancer. 2001;29(2):85‐92. [PubMed] [Google Scholar]
- 58. Rubin JS, Osada H, Finch PW, Taylor WG, Rudikoff S, Aaronson SA. Purification and characterization of a newly identified growth factor specific for epithelial cells. Proc Natl Acad Sci USA. 1989;86(3):802‐806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Niu J, Chang Z, Peng B, et al. Keratinocyte growth factor/fibroblast growth factor‐7‐regulated cell migration and invasion through activation of NF‐kappaB transcription factors. J Biol Chem. 2007;282(9):6001‐6011. [DOI] [PubMed] [Google Scholar]
- 60. Bellusci S, Grindley J, Emoto H, Itoh N, Hogan BL. Fibroblast growth factor 10 (FGF10) and branching morphogenesis in the embryonic mouse lung. Development. 1997;124(23):4867‐4878. [DOI] [PubMed] [Google Scholar]
- 61. Gnatenko DA, Kopantzev EP, Sverdlov ED. Variable effects of growth factors on developmental gene expression in pancreatic cancer cells. Dokl Biochem Biophys. 2018;481(1):217‐218. [DOI] [PubMed] [Google Scholar]
- 62. Nomura S, Yoshitomi H, Takano S, et al. FGF10/FGFR2 signal induces cell migration and invasion in pancreatic cancer. Br J Cancer. 2008;99(2):305‐313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Torres C, Perales S, Alejandre MJ, et al. Serum cytokine profile in patients with pancreatic cancer. Pancreas. 2014;43(7):1042‐1049. [DOI] [PubMed] [Google Scholar]
- 64. Missiaglia E, Dalai I, Barbi S, et al. Pancreatic endocrine tumors: expression profiling evidences a role for AKT‐mTOR pathway. J Clin Oncol. 2010;28(2):245‐255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Motoda N, Matsuda Y, Onda M, Ishiwata T, Uchida E, Naito Z. Overexpression of fibroblast growth factor receptor 4 in high‐grade pancreatic intraepithelial neoplasia and pancreatic ductal adenocarcinoma. Int J Oncol. 2011;38(1):133‐143. [PubMed] [Google Scholar]
- 66. Kong B, Huang J, Zhu Y, et al. Fibroblast growth factor 15 deficiency impairs liver regeneration in mice. Am J Physiol Gastrointest Liver Physiol. 2014;306(10):G893‐902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Uriarte I, Latasa MU, Carotti S, et al. Ileal FGF15 contributes to fibrosis‐associated hepatocellular carcinoma development. Int J Cancer. 2015;136(10):2469‐2475. [DOI] [PubMed] [Google Scholar]
- 68. Wei EQ, Sinden DS, Mao L, Zhang H, Wang C, Pitt GS. Inducible Fgf13 ablation enhances caveolae‐mediated cardioprotection during cardiac pressure overload. Proc Natl Acad Sci USA. 2017;114(20):E4010‐E4019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Lysaght AC, Yuan Q, Fan Y, et al. FGF23 deficiency leads to mixed hearing loss and middle ear malformation in mice. PLoS ONE. 2014;9(9):e107681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Shimada T, Kakitani M, Yamazaki Y, et al. Targeted ablation of Fgf23 demonstrates an essential physiological role of FGF23 in phosphate and vitamin D metabolism. J Clin Invest. 2004;113(4):561‐568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. French DM, Lin BC, Wang M, et al. Targeting FGFR4 inhibits hepatocellular carcinoma in preclinical mouse models. PLoS ONE. 2012;7(5):e36713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Yamaoka T, Yoshino K, Yamada T, et al. Transgenic expression of FGF8 and FGF10 induces transdifferentiation of pancreatic islet cells into hepatocytes and exocrine cells. Biochem Biophys Res Commun. 2002;292(1):138‐143. [DOI] [PubMed] [Google Scholar]
- 73. Elo TD, Valve EM, Seppanen JA, et al. Stromal activation associated with development of prostate cancer in prostate‐targeted fibroblast growth factor 8b transgenic mice. Neoplasia. 2010;12(11):915‐927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Konno‐Takahashi N, Takeuchi T, Nishimatsu H, et al. Engineered FGF‐2 expression induces glandular epithelial hyperplasia in the murine prostatic dorsal lobe. Eur Urol. 2004;46(1):126‐132. [DOI] [PubMed] [Google Scholar]
- 75. Mongiat M, Otto J, Oldershaw R, Ferrer F, Sato JD, Iozzo RV. Fibroblast growth factor‐binding protein is a novel partner for perlecan protein core. J Biol Chem. 2001;276(13):10263‐10271. [DOI] [PubMed] [Google Scholar]
- 76. Tassi E, Al‐Attar A, Aigner A, et al. Enhancement of fibroblast growth factor (FGF) activity by an FGF‐binding protein. J Biol Chem. 2001;276(43):40247‐40253. [DOI] [PubMed] [Google Scholar]
- 77. Ray PE, Tassi E, Liu XH, Wellstein A. Role of fibroblast growth factor‐binding protein in the pathogenesis of HIV‐associated hemolytic uremic syndrome. Am J Physiol Regul Integr Comp Physiol. 2006;290(1):R105‐113. [DOI] [PubMed] [Google Scholar]
- 78. Czubayko F, Smith RV, Chung HC, Wellstein A. Tumor growth and angiogenesis induced by a secreted binding protein for fibroblast growth factors. J Biol Chem. 1994;269(45):28243‐28248. [PubMed] [Google Scholar]
- 79. Tassi E, Henke RT, Bowden ET, et al. Expression of a fibroblast growth factor‐binding protein during the development of adenocarcinoma of the pancreas and colon. Cancer Res. 2006;66(2):1191‐1198. [DOI] [PubMed] [Google Scholar]
- 80. Tassi E, Wellstein A. The angiogenic switch molecule, secreted FGF‐binding protein, an indicator of early stages of pancreatic and colorectal adenocarcinoma. Semin Oncol. 2006;33(6 Suppl 11):S50‐56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Tassi E, Garman KA, Schmidt MO, et al. Fibroblast growth factor binding protein 3 (FGFBP3) impacts carbohydrate and lipid metabolism. Sci Rep. 2018;8(1):15973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Kobrin MS, Yamanaka Y, Friess H, Lopez ME, Korc M. Aberrant expression of type I fibroblast growth factor receptor in human pancreatic adenocarcinomas. Cancer Res. 1993;53(20):4741‐4744. [PubMed] [Google Scholar]
- 83. Wagner M, Lopez ME, Cahn M, Korc M. Suppression of fibroblast growth factor receptor signaling inhibits pancreatic cancer growth in vitro and in vivo. Gastroenterology. 1998;114(4):798‐807. [DOI] [PubMed] [Google Scholar]
- 84. Vickers SM, Huang ZQ, MacMillan‐Crow L, Greendorfer JS, Thompson JA. Ligand activation of alternatively spliced fibroblast growth factor receptor‐1 modulates pancreatic adenocarcinoma cell malignancy. J Gastrointest Surg. 2002;6(4):546‐553. [DOI] [PubMed] [Google Scholar]
- 85. Liu Z, Neiss N, Zhou S, et al. Identification of a fibroblast growth factor receptor 1 splice variant that inhibits pancreatic cancer cell growth. Cancer Res. 2007;67(6):2712‐2719. [DOI] [PubMed] [Google Scholar]
- 86. Chen G, Tian X, Liu Z, et al. Inhibition of endogenous SPARC enhances pancreatic cancer cell growth: modulation by FGFR1‐III isoform expression. Br J Cancer. 2010;102(1):188‐195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Liu Z, Ishiwata T, Zhou S, et al. Human fibroblast growth factor receptor 1‐IIIb is a functional fibroblast growth factor receptor expressed in the pancreas and involved in proliferation and movement of pancreatic ductal cells. Pancreas. 2007;35(2):147‐157. [DOI] [PubMed] [Google Scholar]
- 88. Kleeff J, Kothari NH, Friess H, Fan H, Korc M. Adenovirus‐mediated transfer of a truncated fibroblast growth factor (FGF) type I receptor blocks FGF‐2 signaling in multiple pancreatic cancer cell lines. Pancreas. 2004;28(1):25‐30. [DOI] [PubMed] [Google Scholar]
- 89. Compagni A, Wilgenbus P, Impagnatiello MA, Cotten M, Christofori G. Fibroblast growth factors are required for efficient tumor angiogenesis. Cancer Res. 2000;60(24):7163‐7169. [PubMed] [Google Scholar]
- 90. Ohta T, Yamamoto M, Numata M, et al. Expression of basic fibroblast growth factor and its receptor in human pancreatic carcinomas. Br J Cancer. 1995;72(4):824‐831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Matsuda Y, Yoshimura H, Suzuki T, Uchida E, Naito Z, Ishiwata T. Inhibition of fibroblast growth factor receptor 2 attenuates proliferation and invasion of pancreatic cancer. Cancer Sci. 2014;105(9):1212‐1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Zhang H, Hylander BL, LeVea C, et al. Enhanced FGFR signalling predisposes pancreatic cancer to the effect of a potent FGFR inhibitor in preclinical models. Br J Cancer. 2014;110(2):320‐329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Ishiwata T, Matsuda Y, Yamamoto T, Uchida E, Korc M, Naito Z. Enhanced expression of fibroblast growth factor receptor 2 IIIc promotes human pancreatic cancer cell proliferation. Am J Pathol. 2012;180(5):1928‐1941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Huch M, Abate‐Daga D, Roig JM, et al. Targeting the CYP2B 1/cyclophosphamide suicide system to fibroblast growth factor receptors results in a potent antitumoral response in pancreatic cancer models. Hum Gene Ther. 2006;17(12):1187‐1200. [DOI] [PubMed] [Google Scholar]
- 95. Cavallaro U, Niedermeyer J, Fuxa M, Christofori G. N‐CAM modulates tumour‐cell adhesion to matrix by inducing FGF‐receptor signalling. Nat Cell Biol. 2001;3(7):650‐657. [DOI] [PubMed] [Google Scholar]
- 96. O'Sullivan H, Kelleher FC, Lavelle M, et al. Therapeutic potential for FGFR inhibitors in SOX9‐FGFR2 coexpressing pancreatic cancer. Pancreas. 2017;46(8):e67‐e69. [DOI] [PubMed] [Google Scholar]
- 97. Bono F, DeSmet F, Herbert C, et al. Inhibition of tumor angiogenesis and growth by a small‐molecule multi‐FGF receptor blocker with allosteric properties. Cancer Cell. 2013;23(4):477‐488. [DOI] [PubMed] [Google Scholar]
- 98. Taeger J, Moser C, Hellerbrand C, et al. Targeting FGFR/PDGFR/VEGFR impairs tumor growth, angiogenesis, and metastasis by effects on tumor cells, endothelial cells, and pericytes in pancreatic cancer. Mol Cancer Ther. 2011;10(11):2157‐2167. [DOI] [PubMed] [Google Scholar]
- 99. Ma WW, Xie H, Fetterly G, et al. A phase Ib study of the FGFR/VEGFR inhibitor dovitinib with gemcitabine and capecitabine in advanced solid tumor and pancreatic cancer patients. Am J Clin Oncol. 2018; 42(2):184-189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Yamamoto Y, Matsui J, Matsushima T, et al. Lenvatinib, an angiogenesis inhibitor targeting VEGFR/FGFR, shows broad antitumor activity in human tumor xenograft models associated with microvessel density and pericyte coverage. Vasc Cell. 2014;6:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Waheed A, Purvey S, Saif MW. Masitinib in treatment of pancreatic cancer. Expert Opin Pharmacother. 2018;19(7):759‐764. [DOI] [PubMed] [Google Scholar]
- 102. Humbert M, Casteran N, Letard S, et al. Masitinib combined with standard gemcitabine chemotherapy: in vitro and in vivo studies in human pancreatic tumour cell lines and ectopic mouse model. PLoS ONE. 2010;5(3):e9430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Mitry E, Hammel P, Deplanque G, et al. Safety and activity of masitinib in combination with gemcitabine in patients with advanced pancreatic cancer. Cancer Chemother Pharmacol. 2010;66(2):395‐403. [DOI] [PubMed] [Google Scholar]
- 104. Deplanque G, Demarchi M, Hebbar M, et al. A randomized, placebo‐controlled phase III trial of masitinib plus gemcitabine in the treatment of advanced pancreatic cancer. Ann Oncol. 2015;26(6):1194‐1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Buchler P, Reber HA, Roth MM, Shiroishi M, Friess H, Hines OJ. Target therapy using a small molecule inhibitor against angiogenic receptors in pancreatic cancer. Neoplasia. 2007;9(2):119‐127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Memon B, Karam M, Al‐Khawaga S, Abdelalim EM. Enhanced differentiation of human pluripotent stem cells into pancreatic progenitors co‐expressing PDX1 and NKX6.1. Stem Cell Res Ther. 2018;9(1):15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Greggio C, De Franceschi F, Figueiredo‐Larsen M, et al. Artificial three‐dimensional niches deconstruct pancreas development in vitro. Development. 2013;140(21):4452‐4462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Lai SW, Bamodu OA, Tsai WC, et al. The therapeutic targeting of the FGFR1/Src/NF‐kappaB signaling axis inhibits pancreatic ductal adenocarcinoma stemness and oncogenicity. Clin Exp Metastasis. 2018;35:663‐677. [DOI] [PubMed] [Google Scholar]
- 109. Lehnen NC, von Massenhausen A, Kalthoff H, et al. Fibroblast growth factor receptor 1 gene amplification in pancreatic ductal adenocarcinoma. Histopathology. 2013;63(2):157‐166. [DOI] [PubMed] [Google Scholar]
- 110. Kutluk Cenik B, Ostapoff KT, Gerber DE, Brekken RA. BIBF 1120 (nintedanib), a triple angiokinase inhibitor, induces hypoxia but not EMT and blocks progression of preclinical models of lung and pancreatic cancer. Mol Cancer Ther. 2013;12(6):992‐1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Awasthi N, Hinz S, Brekken RA, Schwarz MA, Schwarz RE. Nintedanib, a triple angiokinase inhibitor, enhances cytotoxic therapy response in pancreatic cancer. Cancer Lett. 2015;358(1):59‐66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Bill R, Fagiani E, Zumsteg A, et al. Nintedanib is a highly effective therapeutic for neuroendocrine carcinoma of the pancreas (PNET) in the Rip1Tag2 transgenic mouse model. Clin Cancer Res. 2015;21(21):4856‐4867. [DOI] [PubMed] [Google Scholar]
- 113. Bahleda R, Hollebecque A, Varga A, et al. Phase I study of afatinib combined with nintedanib in patients with advanced solid tumours. Br J Cancer. 2015;113(10):1413‐1420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Musumeci F, Greco C, Grossi G, Molinari A, Schenone S. Recent studies on ponatinib in cancers other than chronic myeloid leukemia. Cancers (Basel). 2018;10(11):E430 10.3390/cancers10110430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Sahu N, Chan E, Chu F, et al. Cotargeting of MEK and PDGFR/STAT3 pathways to treat pancreatic ductal adenocarcinoma. Mol Cancer Ther. 2017;16(9):1729‐1738. [DOI] [PubMed] [Google Scholar]
- 116. Shah RR, Morganroth J. Update on cardiovascular safety of tyrosine kinase inhibitors: with a special focus on QT interval, left ventricular dysfunction and overall risk/benefit. Drug Saf. 2015;38(8):693‐710. [DOI] [PubMed] [Google Scholar]
- 117. Douxfils J, Haguet H, Mullier F, Chatelain C, Graux C, Dogne JM. Association between BCR‐ABL tyrosine kinase inhibitors for chronic myeloid leukemia and cardiovascular events, major molecular response, and overall survival: a systematic review and meta‐analysis. JAMA Oncol. 2016. 10.1001/jamaoncol.2015.5932 [DOI] [PubMed] [Google Scholar]
- 118. Gacche RN, Meshram RJ. Angiogenic factors as potential drug target: efficacy and limitations of anti‐angiogenic therapy. Biochim Biophys Acta. 2014;1846(1):161‐179. [DOI] [PubMed] [Google Scholar]
- 119. Wang J, Li J, Wang X, Zheng C, Ma W. Downregulation of microRNA‐214 and overexpression of FGFR‐1 contribute to hepatocellular carcinoma metastasis. Biochem Biophys Res Commun. 2013;439(1):47‐53. [DOI] [PubMed] [Google Scholar]
- 120. Jiang H, Qu L, Wang Y, Cong J, Wang W, Yang X. miR‐99a promotes proliferation targeting FGFR3 in human epithelial ovarian cancer cells. Biomed Pharmacother. 2014;68(2):163‐169. [DOI] [PubMed] [Google Scholar]
- 121. Ren S, Rivard CJ, Yu H, et al. A miRNA panel predicts sensitivity of FGFR inhibitor in lung cancer cell lines. Clin Lung Cancer. 2018;19(5):450‐456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Wang F, Jiang H, Wang S, Chen B. Dual functional microRNA‐186‐5p targets both FGF2 and RelA to suppress tumorigenesis of glioblastoma multiforme. Cell Mol Neurobiol. 2017;37(8):1433‐1442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Kottakis F, Polytarchou C, Foltopoulou P, Sanidas I, Kampranis SC, Tsichlis PN. FGF‐2 regulates cell proliferation, migration, and angiogenesis through an NDY1/KDM2B‐miR‐101‐EZH2 pathway. Mol Cell. 2011;43(2):285‐298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Nogova L, Sequist LV, Perez Garcia JM, et al. Evaluation of BGJ398, a fibroblast growth factor receptor 1–3 kinase inhibitor, in patients with advanced solid tumors harboring genetic alterations in fibroblast growth factor receptors: results of a global phase I, dose‐escalation and dose‐expansion study. J Clin Oncol. 2017;35(2):157‐165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Pal SK, Rosenberg JE, Hoffman‐Censits JH, et al. Efficacy of BGJ398, a fibroblast growth factor receptor 1–3 inhibitor, in patients with previously treated advanced urothelial carcinoma with FGFR3 alterations. Cancer Discov. 2018;8(7):812‐821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Goyal L, Saha SK, Liu LY, et al. Polyclonal secondary FGFR2 mutations drive acquired resistance to FGFR inhibition in patients with FGFR2 fusion‐positive cholangiocarcinoma. Cancer Discov. 2017;7(3):252‐263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Javle M, Lowery M, Shroff RT, et al. Phase II study of BGJ398 in patients With FGFR‐altered advanced cholangiocarcinoma. J Clin Oncol. 2018;36(3):276‐282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Ikeda K, Kudo M, Kawazoe S, et al. Phase 2 study of lenvatinib in patients with advanced hepatocellular carcinoma. J Gastroenterol. 2017;52(4):512‐519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Hong DS, Kurzrock R, Wheler JJ, et al. Phase I dose‐escalation study of the multikinase inhibitor lenvatinib in patients with advanced solid tumors and in an expanded cohort of patients with melanoma. Clin Cancer Res. 2015;21(21):4801‐4810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Miyake M, Ishii M, Koyama N, et al. 1‐tert‐butyl‐3‐[6‐(3,5‐dimethoxy‐phenyl)‐2‐(4‐diethylamino‐butylamino)‐pyrido[2,3 ‐d]pyrimidin‐7‐yl]‐urea (PD173074), a selective tyrosine kinase inhibitor of fibroblast growth factor receptor‐3 (FGFR3), inhibits cell proliferation of bladder cancer carrying the FGFR3 gene mutation along with up‐regulation of p27/Kip1 and G1/G0 arrest. J Pharmacol Exp Ther. 2010;332(3):795‐802. [DOI] [PubMed] [Google Scholar]
- 131. Eisen T, Shparyk Y, Macleod N, et al. Effect of small angiokinase inhibitor nintedanib (BIBF 1120) on QT interval in patients with previously untreated, advanced renal cell cancer in an open‐label, phase II study. Invest New Drugs. 2013;31(5):1283‐1293. [DOI] [PubMed] [Google Scholar]
- 132. Reck M, Kaiser R, Mellemgaard A, et al. Docetaxel plus nintedanib versus docetaxel plus placebo in patients with previously treated non‐small‐cell lung cancer (LUME‐Lung 1): a phase 3, double‐blind, randomised controlled trial. Lancet Oncol. 2014;15(2):143‐155. [DOI] [PubMed] [Google Scholar]
- 133. Molife LR, Omlin A, Jones RJ, et al. Randomized phase II trial of nintedanib, afatinib and sequential combination in castration‐resistant prostate cancer. Future Oncol. 2014;10(2):219‐231. [DOI] [PubMed] [Google Scholar]
- 134. Droz JP, Medioni J, Chevreau C, et al. Randomized phase II study of nintedanib in metastatic castration‐resistant prostate cancer postdocetaxel. Anticancer Drugs. 2014;25(9):1081‐1088. [DOI] [PubMed] [Google Scholar]
- 135. Mross K, Büchert M, Frost A, et al. Vascular effects, efficacy and safety of nintedanib in patients with advanced, refractory colorectal cancer: a prospective phase I subanalysis. BMC Cancer. 2014;14:510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Dizon DS, Sill MW, Schilder JM, et al. A phase II evaluation of nintedanib (BIBF‐1120) in the treatment of recurrent or persistent endometrial cancer: an NRG Oncology/Gynecologic Oncology Group Study. Gynecol Oncol. 2014;135(3):441‐445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Han JY, Kim HY, Lim KY, Hwangbo B, Lee JS. A phase II study of nintedanib in patients with relapsed small cell lung cancer. Lung Cancer. 2016;96:108‐112. [DOI] [PubMed] [Google Scholar]
- 138. Eisen T, Loembé A‐b, Shparyk Y, et al. A randomised, phase II study of nintedanib or sunitinib in previously untreated patients with advanced renal cell cancer: 3‐year results. Br J Cancer. 2015;113(8):1140‐1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Reck M, Mellemgaard A, Novello S, et al. Change in non‐small‐cell lung cancer tumor size in patients treated with nintedanib plus docetaxel: analyses from the phase III LUME‐lung 1 study. Onco Targets Ther. 2018;11:4573‐4582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Forster M, Hackshaw A, DePas T, et al. A phase I study of nintedanib combined with cisplatin/gemcitabine as first‐line therapy for advanced squamous non‐small cell lung cancer (LUME‐Lung 3). Lung Cancer. 2018;120:27‐33. [DOI] [PubMed] [Google Scholar]
- 141. Jain P, Kantarjian H, Jabbour E, et al. Ponatinib as first‐line treatment for patients with chronic myeloid leukaemia in chronic phase: a phase 2 study. Lancet Haematol. 2015;2(9):e376‐383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Shacham‐Abulafia A, Raanani P, Lavie D, et al. Real‐life experience with ponatinib in chronic myeloid leukemia: a multicenter observational study. Clin Lymphoma Myeloma Leuk. 2018;18(7):e295‐e301. [DOI] [PubMed] [Google Scholar]
- 143. Breccia M, Abruzzese E, Castagnetti F, et al. Ponatinib as second‐line treatment in chronic phase chronic myeloid leukemia patients in real‐life practice. Ann Hematol. 2018;97(9):1577‐1580. [DOI] [PubMed] [Google Scholar]
- 144. Cortes JE, Kim DW, Pinilla‐Ibarz J, et al. A phase 2 trial of ponatinib in Philadelphia chromosome‐positive leukemias. N Engl J Med. 2013;369(19):1783‐1796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Lipton JH, Chuah C, Guerci‐Bresler A, et al. Ponatinib versus imatinib for newly diagnosed chronic myeloid leukaemia: an international, randomised, open‐label, phase 3 trial. Lancet Oncol. 2016;17(5):612‐621. [DOI] [PubMed] [Google Scholar]


