Abstract
Angiogenesis is a highly complex and coordinated process in the brain. Under normal conditions, it is a vital process in growth and development, but under adverse conditions such as diabetes mellitus, it can lead to severe pathology. Astrocytes are a key constituent of the neurovascular unit and contribute to cerebral function, not only bridging the gap between metabolic supplies from blood vessels to neurons, but also regulating angiogenesis. Astrocytes affect angiogenesis by secreting angiogenic factors such as vascular endothelial growth factor (VEGF) into its microenvironment and regulating mitogenic activity in cerebral microvessel endothelial cells (CMEC). We hypothesized that astrocytes conditioned in high glucose media would produce and secrete decreased VEGF which would lead to impaired proliferation, migration, and tube formation of CMEC in vitro. Using neonatal rat astrocytes, we used normal glucose (NG, 5.5mM) vs. high glucose (HG, 25mM) feeding media and measured VEGF message and protein levels as well as secreted VEGF. We co-cultured conditioned astrocytes with isolated rat CMEC and measured mitogenic activity of endothelial cells using BrdU assay, scratch recovery assay, and tube formation assay. HG astrocytes produced and secreted decreased VEGF protein and resulted in impaired mitogenic activity when co-cultured with CMEC as demonstrated by decreased BrdU uptake, decreased scratch recovery, and slower tube formation. Our study provides insight into gliovascular adaptations to increased glucose levels resulting in impaired cellular cross-talk between astrocytes and CMEC which could be one explanation for cerebral microangiopathy seen in diabetic conditions.
Keywords: Cell biology, Neuroscience
1. Introduction
Angiogenesis is a highly complex and coordinated process requiring multiple angiogenic and regulatory factors, receptors, and intracellular signaling pathways [1, 2, 3]. Under normal conditions, it is a vital process in growth and development, but under adverse conditions such as diabetes mellitus, it can lead to severe pathology. Chronic hyperglycemia leads to changes in both small blood vessels (microangiopathy) and large blood vessels (macroangiopathy) [4]. Excessive angiogenesis plays a role in diabetic retinopathy and nephropathy, while insufficient angiogenesis contributes to impaired wound healing and embryonic vasculopathy in pregnancies complicated by maternal diabetes [4, 5, 6, 7, 8]. Understanding how high glucose conditions alters angiogenesis in the brain could provide insights into the cerebrovascular sequelae of diabetes mellitus.
The brain maintains its optimal continuous supply of oxygen and nutrients by physiologic and angiogenic adaptational changes [3]. Astrocytes are now viewed as key mechanistic elements of both neurovascular coupling and angiogenesis [9]. In the brain, the vascular network is predominantly formed by angiogenesis [9]. Astrocytes promote proper cortical blood vessel development in the brain as suggested by the observation that perinatal inhibition of astrocytogenesis resulted in a drastic reduction in the density and branching of cortical blood vessels [10]. Studies from our lab and others have established that astrocytes influence angiogenesis through their secreted products that comprise the “secretome” [11, 12, 13, 14]. Vascular endothelial growth factor (VEGF) is one of the most important angiogenic mitogens that is secreted by astrocytes into the brain microenvironment. The binding of VEGF to its receptors on the surface of endothelial cells activates intracellular tyrosine kinases, triggering multiple downstream signals that promote angiogenesis. Although there are multiple variants of the VEGF ligand and its receptor, the angiogenetic effects of this pathway are primarily mediated through the interaction of VEGF-A with its receptor. VEGF-A isoforms regulate the morphology and connectivity of capillary networks in the brain [15]. VEGF-A and its regulatory transcription factors are expressed by reactive astrocytes [16, 17]. Changes in astrocytic-derived VEGF-A could evoke changes in endothelial cell properties including migration, proliferation and angiogenesis [18]. We chose to focus our investigations on VEGF-A, the most common isoform of VEGF, which will be referred to as VEGF within this text.
The regulation of VEGF production by glucose has not been as extensively investigated as its regulation by oxygen, but elegant studies of VEGF mRNA expression in spheroids in vitro clearly demonstrated a similar regulation by glucose levels as oxygen levels [19, 20]. In rat kidney cells, high glucose media (25 mM) blunted the hypoxia-induced VEGF response at both the mRNA and protein level [21]. In vascular smooth muscle, glucose increases via an osmotic mechanism of VEGF synthesis and secretion but this effect is attenuated in the presence of insulin resistance [22]. Furthermore, human umbilical vein endothelial cells (HUVEC) exposed to high glucose conditions resulted in downregulation of VEGF which was associated with increased apoptosis [23]. These studies lead us to speculate that high glucose conditions would have similar effect upon the cerebral astrocyte.
The objective of this study is to investigate the effects of high glucose conditions on 1) neonatal astrocytic VEGF production and secretion; and 2) neonatal astrocytic angiogenic effect upon cerebral microvessel endothelial cell (CMEC) in co-culture. We hypothesized that high glucose conditions will decrease astrocytic expression and secretion of VEGF and this would result in decreased mitogenic activity in endothelial cells co-cultured with high glucose conditioned astrocytes.
2. Material and methods
2.1. Animal care
The animal protocols used in this study were approved by the Medical College of Wisconsin Institutional Animal Care and Use Committee. All rats had free access to a standard rat chow diet and drinking water. Rats were housed in a temperature (24 ± 2 °C), humidity (60 ± 10%), and 12-hour light cycle (lights on: 0600–1800) controlled environment.
2.2. Cell culture
Astrocytes- Sprague Dawley rat pups of 2–3 days of age were anesthetized with isoflurane, decapitated and the brain removed for preparation of astrocyte cultures as previously described [24]. Briefly, the brain was dissected free of meninges, and the hippocampus was isolated and cut into small pieces and transferred to a sterile dish containing 20 U/ml papain (Worthington Biochemical Corp) dissolved in DMEM/F12 media supplemented with 1% Penicillin-streptomycin, 0.1% Gentamicin, and 0.5% Fungizone. The tissue was incubated at 37 °C for 40 min with gentle agitation. The tissue was then centrifuged at 400 g for 4 minutes to remove the papain solution and resuspended in DMEM/F12 media supplemented with 10% FBS, 1% Penicillin-Streptomycin, and 0.1% Gentamicin. The tissues were triturated in the same media with a 1 ml pipette and the resulting cell suspension was filtered using a 40 μm cell strainer. The cell suspension was centrifuged at 400 g for 4 minutes and resuspended in astrocyte growth media: DMEM normal glucose (5.5 mM) or high glucose (25 mM) media supplemented with 10% FBS and 1% Penicillin-Streptomycin. Isolated cells were plated at a density of approximately 3000 cells per mm2. Many classical cell culture media are supplemented with approximately 5.5 mM D-glucose which approximates normal blood glucose levels in vivo. In contrast, glucose levels approaching 10 mM are close to pre-diabetic levels whereas 25 mM is considered diabetic-like conditions. Cell cultures were incubated at 37 °C in an atmosphere of 5% CO2 in air. Confluent monolayers of neonatal astrocytes formed within 7–10 days after the initial plating. The respective medium (normal glucose or high glucose) was changed 3 times a week. The primary cultures were maintained by passaging the cells when they were greater than 80% confluent. Confluent monolayers of brain astrocytes were used in studies after the 3rd passage.
Endothelial Cells- Sprague-Dawley rats (3–4 weeks of age) were anesthetized with isoflurane, decapitated and the brain was dissected free of meninges and the cerebral cortex was isolated, removing all large surface vessels. The tissue was minced and placed into papain solution (20 U/ml papain (Worthington Biochemical Corp) dissolved in DMEM/F12 media supplemented with 1% Penicillin-Streptomycin, 0.1% Gentamicin, and 0.5% Fungizone. The tissue was incubated at 37 °C for 40 min with gentle agitation. The tissue was then centrifuged at 400 g for 4 minutes to remove the papain solution. Centrifugation results in a pellet with 2 distinct layers, the top layer consisting mostly of microvessels and the lower layer containing mostly single cells. The top layer was carefully removed and resuspended in dissociation media (DMEM/F12 media supplemented with 10% FBS, 1% Penicillin-Streptomycin, and 0.1% Gentamicin), triturated using a 1 ml pipette, and filtered through a 70μm cell strainer where microvessels remain on the filter and single cells and debris pass-through and are discarded. Microvessels are collected off the strainer and placed in collagenase solution (1 mg/ml collagenase Type IV dissolved in DMEM/F12 media supplemented with 10% FBS, 1% Penicillin-Streptomycin, and 0.1% Gentamicin), incubated for 45 minutes at 37 °C, and then centrifuged at 400g for 4 minutes. The resuspended pellet is then layered on top of a 20% sucrose gradient and centrifuged at 600g for 5 minutes to further isolate the microvessel fraction and remove cellular debris. Microvessels were then plated using complete endothelial cell media (Cell Biologics Inc, IL) in culture dishes pre-treated for at least 4 hours with attachment factor solution (Cell Biologics Inc, IL) and incubated at 37 °C in an atmosphere of 5% CO2 in air. The endothelial cell culture medium was changed 24 hours after initial plating to remove any unattached cell debris. Confluent monolayers of cerebral microvessel endothelial cells formed within 10–12 days after the initial plating. Cells were maintained by changing media twice a week and passaging cells when they reached ∼80% confluence.
2.3. Protein isolation and immunoblot
Cultured astrocytes were lysed in RIPA lysis buffer (#20–188, EMD Millipore Corp., Billerica, MA) by running 5 minutes with Pink Eppendorf Bullet Blender Bead Lysis Kits (Next Advance) on Bullet Blender (Model: BBY24M, Next Advance), followed by 15 minutes incubation at 4 °C and centrifuged for 15 min at 15,000 g at 4 °C. Protein concentrations were determined with a bicinchoninic acid (BCA) protein assay kit (#23225, Thermo Scientific, Rockford, IL) according to the manufacturer's instructions, detected by Cytation 5 multi-Mode Reader (BIO-TEK Instruments, Inc.) with software version Gen 5 2.07.17. Equal amounts of each protein sample (total protein extract, 10μg) were separated by electrophoresis in 4–15% SDS-polyacrylamide gels (#456–8086, Mini-PROTEAN TGX precast Stain-free gels, Bio-Rad, Hercules, CA, USA). Total protein loading was determined by ChemiDoc MP Imaging System (Model No: Universal Hood III, BIO-RAD, Hercules, CA) and analyzed with Image Lab software (Version 4.0.1, Bia-Rad, Hercules, CA). Proteins were then transferred to polyvinylidene fluoride membranes with Trans-Blot Turbo Transfer Park (#1704156, Bio-Rad, Hercules, CA) on Trans-Blot Turbo Transfer System (Bio-Rad, Hercules, CA). After blocking with 5% Non-fat dried milk-TBST (Tris Buffer Saline-0.1% Tween 20), membranes were incubated overnight at 4 °C with primary antibody, rabbit polyclonal IgG against VEGF (sc-152, Santa Cruz Biotechnology, Dallas, TX, USA) with 1:200 dilution in TBST-1% BSA. After 5 minutes X 4 rinse with TBST, the membranes were then incubated with Goat anti-rabbit IgG (H + L) – HRP Conjugated secondary antibody (#170–6515, Bio-Rad, Hercules, CA) with 1:5000 dilution in TBST-1% BSA for 1 hour at room temperature, followed by 5 minutes X 4 rinse with TBST. The blots were developed in dark with SuperSignal West Pico chemiluminescent Substrate (#34080, Thermo Scientific, Rockford, IL) and the signals were captured with ChemiDoc MP Imaging System (Model No: Universal Hood III, BIO-RAD, Hercules, CA) and were then quantified with Image Lab software (Version 4.0.1, Bia-Rad, Hercules, CA).
2.4. RNA isolation and real time PCR
Real-time PCR was used to measure the expression of VEGF mRNA in primary cultures of rat brain astrocytes. Analysis of relative gene expression data using Samples' total RNAs were isolated by RNeasy Mini Kit (#74104, Qiagen Inc., Valencia, CA) and RNA concentrations were determined by Cytation 5 multi-Mode Reader (BIO-TEK Instruments, Inc.). cDNA was synthesized on IQ5 Multicolor Real-Time PCR Detection System (Bio-Rad, Hercules, CA) using High Capacity cDNA Reverse Transcription Kit (#4368814, Applied Biosystems, Foster City, CA) according to the manufacturer's instructions. PCR was carried out on CFX384 Real Time System (Model No: CFX384 Optics Module, Bio-Rad, Hercules, CA). Reaction systems were 3μl Bulls eye TaqProbe 2X qPCR MasterMix-Low Rox (#BEQPCR-PL, Midwest scientific, Valley Park, MO), 2 μl Primer-Probe (diluted from 20X, see following for detail), 1 μl cDNA (1:20 dilution), loading to MicroAmp Optical 384-well reaction plate (#4309849, Applied Biosystems, Foster City, CA). Applied primers were GAPDH (Ref Seq #NM_017008 [1], Assy ID: Rn.PT.58.35727291, Integrated DNA Technologies, Inc. (IDT), Skokie, IL), VEGF (exon 1b-4) (Ref Seq #NM_001110333 [3], Assay ID: Rn.PT.58.34097147, IDT, Skokie, IL). PCR runs: hot start 10 minutes at 95 °C followed by 40 cycles of 10 sections at 95 °C and 60 sections at 60 °C, terminated by cooling to 4 °C. PCR results were obtained by CFX Manager Software (Version 3.1, Bio-Rad, Hercules, CA) and analyzed with the 2−Δ ΔCT method [25].
2.5. Quantification of secreted VEGF in culture
Secreted astrocytic VEGF from confluent cell culture supernatant was collected after 24 hours serum starve and measured in duplicate using a colorimetric solid phase sandwich ELISA (Rat VEGF Immunoassay, R&D Systems, Minneapolis, MN, USA) after concentration (Amicon Ultra-4, Sigma-Aldrich, St.Louis, MO). The assay was carried out according to manufacturer's instructions. Measurements were performed using a microplate reader (FLUOstar Omega, BMG Labtech, Cary, NC).
2.6. Monolayer scratch recovery assay
Cocultures of astrocytes and brain capillary endothelial cells were performed using protocols previously described with slight modifications [26]. Briefly, confluent endothelial cells were trypsinized and resuspended in endothelial cell media with additives. Endothelial cells were plated at approximately 10,000 cells per centimeter square on attachment factor solution from Cell Applications, Inc. treated transwells. Endothelial cells were grown to confluence over 7 days prior to starting coculture, with normal glucose endothelial cell media being changed every 2–3 days. Approximately 5 days to starting coculture, astrocytes were plated at approximately 15,000 cells per centimeter square in transwell inserts and maintained in either normal glucose or high glucose astrocytic media. On the day of coculture, confluent endothelial cells in transwells were washed with PBS twice and transwell inserts with confluent astrocyte monolayers were placed in each endothelial cell containing well with corresponding astrocytic media (normal glucose vs high glucose). Endothelial cells and astrocytes were co-cultured for 2 days without changing media. On the third day, scratch migration assay was performed using methods previously described [27]. Briefly, endothelial cell monolayer was scraped using a p200 pipette tip. Debris was not washed away to preserve conditioned astrocytic media. To enable imaging the area with scratch injury, a mark made perpendicularly to the scratch on the underside of the plate using an ultra-fine tip marker. The cells were returned to the CO2 incubator between imaging, which occurred hourly after scratch. Endothelial cells alone were subject to scratch assay respective astrocyte feeding media (high glucose or normal glucose) for negative control. Using bright field microscopy, scratch injury site was located using reference point and images were acquired from at least 3 random areas. Distance between injury was measured and averaged for each timepoint and condition.
2.7. Immunofluorescence microscopy for 5-bromo-2′-Deoxyuridine (BrdU)
Proliferating endothelial cells in culture were labeled by incubation with BrdU as described previously [28, 29] with minor modifications. Cocultures of astrocytes and brain capillary endothelial cells were performed using the same techniques as described in the scratch recovery assay (as above) using transwell plates over 3 days. For this experiment, endothelial cells were plated at approximately 1 × 104 cells per square centimeters on polyester 0.4μm pore transwell inserts pretreated with attachment factor while the astrocytes were plated at 1.5 × 104 cells per square centimeter in a 6 well plate. The endothelial cells on transwell insert were labeled with BrdU solution for 8 hours, washed twice with PBS, and fixed with 4% paraformaldehyde-4% sucrose in 0.1M PBS. After incubation with BrdU, the transwell insert was cut from the plate using a sharp blade and mounted on a slide. Antigen retrieval was performed, cells were blocked with 1% BSA-TBST for 1 hour at room temperature, and incubated with primary antibody (Mouse anti-BrdU, MCA2483GA, Bio-Rad, Hercules, CA) diluted to 1:100 in 0.1M TBST-3%BSA overnight at 4 °C. After washing, cells were incubated with secondary antibody (Goat anti-Mouse IGG, Cross-absorbed, Alexa Fluor 546, A11003, Invitrogen, Carlsbad, CA) diluted to 1:200 in 0.1M TBST-1%BSA for 1 hour at room temperature. Cells were then washed and counterstained with 300 nM DAPI (D9542, Sigma, St. Louis, MO) in 0.1 M PBS (pH 7.4) for 1 minute. After wash, cells were coverslipped with anti-fade mounting medium and scanned with Olympus VS100 Fluorescent Slide Scanner. Total cell number and total BrdU-IR cell number were obtained using ImageJ software.
2.8. Endothelial cell tube formation assay
Cocultures of astrocytes and brain capillary endothelial cells were prepared as described previously [11]. Briefly, confluent astrocytes were trypsinized and resuspended in Dulbecco's modified Eagle's medium containing 10% FBS and antibiotics. Cells were plated at 6,000 cells per square centimeter on fibronectin-coated coverslips. After 24 hours of incubation, the medium was removed, and endothelial cells were plated at approximately 20,000 cells per coverslip in complete endothelial cell media (Cell Biologics Inc, IL). Control coverslips lacking either the astrocytes or endothelial cells were incubated in the same medium as used for co-cultures. Medium was changed every 3 days until visualized.
2.9. Statistics
All data presented are expressed as mean ± SD where applicable. Student's t-test determined statistical significance as demonstrated for a p value < 0.05. For scratch assay, statistical difference between normal glucose and high glucose and timepoints was evaluated using a two-way ANOVA. Differences between groups at specific timepoints was measured using Student's t-test as significance was demonstrated for a p value < 0.05.
3. Results
3.1. High glucose media decreases VEGF message and protein levels in astrocytes
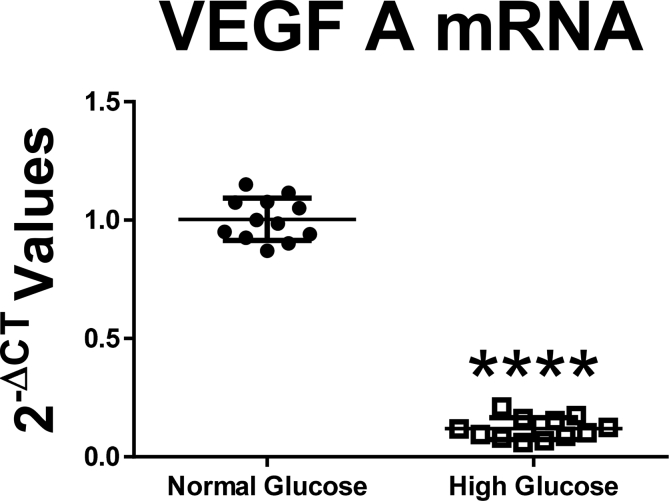
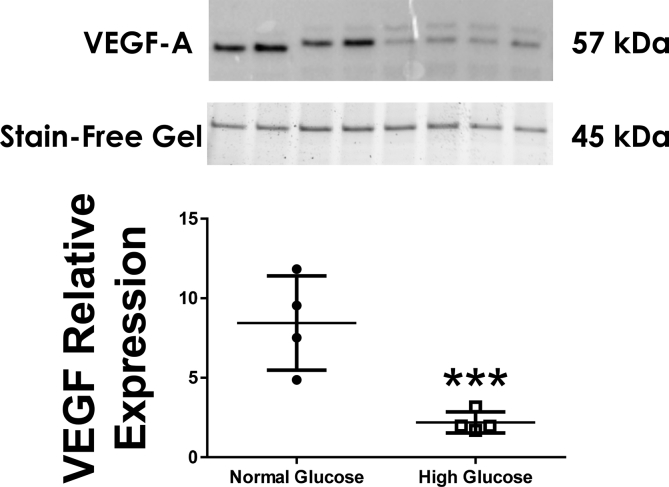
To determine the ability of the neonatal astrocyte to produce VEGF in response to altered glucose concentrations, we measured levels of both VEGF message and protein levels in astrocytic lysates. Astrocytes cultured in high glucose media had significantly decreased VEGF mRNA levels compared to normal glucose media (Fig. 1: difference between the means -88.42 ± 2.91%, n = 12, p < 0.0001). Astrocytes cultured in high glucose media had significantly decreased VEGF protein level compared to normal glucose media (Fig. 2: n = 4, p < 0.01).
Fig. 1.
Astrocytes cultured in high glucose media had significantly decreased VEGF mRNA levels compared to normal glucose media (Difference between the means -88.42 ± 2.91%, n = 12, p < 0.0001).
Fig. 2.
Astrocytes cultured in high glucose media had significantly decreased VEGF protein level compared to normal glucose media (n = 4, p < 0.01).
3.2. High glucose media decreases VEGF protein section from astrocytes
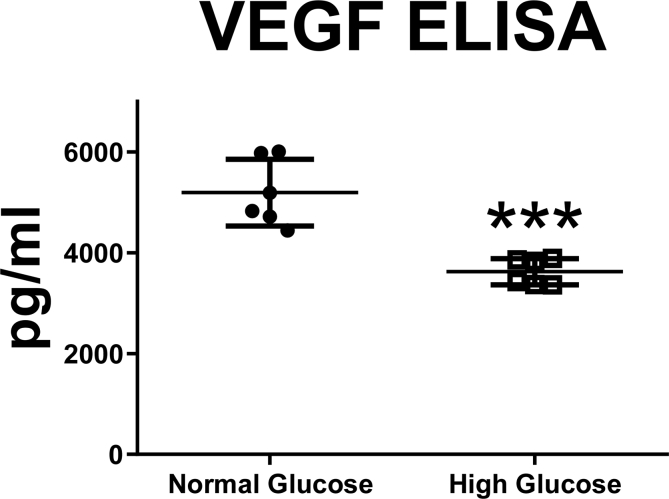
To determine the ability of the neonatal astrocyte to secrete VEGF we measured VEGF levels in the media after cellular stimulation. Astrocytes cultured in high glucose media secreted significantly decreased levels of VEGF into media compared to normal glucose media (Fig. 3: n = 6, *p < 0.001).
Fig. 3.
Astrocytes cultured in high glucose media secreted significantly decreased levels of VEGF into media compared to normal glucose media (n = 6, *p < 0.001).
3.3. High glucose conditioned astrocytes decreased CMEC mitogenic activity in co-culture
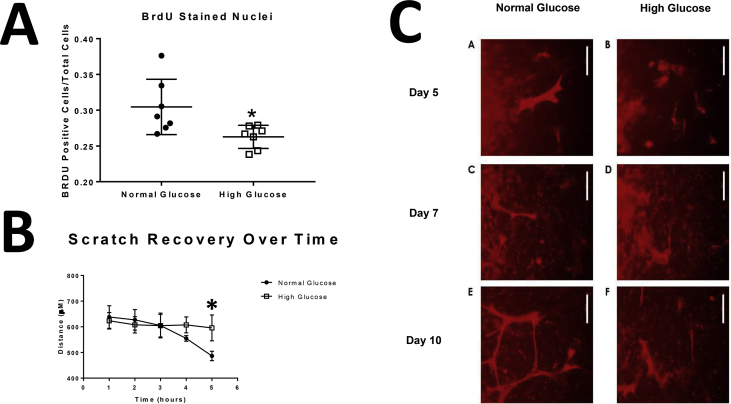
To determine the effect of glucose concentration on astrocytic cross-talk with endothelial cells, we focused on key mitogenic activities known to occur when astrocytes and endothelial cells are co-cultured [30]. Cell proliferation was estimated using BrdU incorporation in endothelial cells co-cultured with astrocytes. BrdU levels in CMEC co-cultured with astrocytes conditioned in high glucose media was decreased compared to CMEC that were co-cultured with astrocytes conditioned in normal glucose media (Fig. 4A: n = 7, *p < 0.05). To evaluate CMEC migration and proliferation, we used CMEC monolayer scratch recovery assay. When scratched, cell monolayers respond to disruption of the monolayer with an increase in migration and proliferation. CMEC co-cultured with astrocytes conditioned in high glucose resulted in decreased scratch recovery assay results compared to CMEC co-cultured with astrocytes conditioned in normal glucose (Fig. 4B: Time accounts for 66.13% of the total variance between the two curves at time point 5 hours, where F = 56.01, p value < 0.0001). Tube formation is an integrated angiogenic activity involving proliferation, migration, and invasion. Although tube formation is present in CMEC co-cultured in both high and normal glucose conditioned astrocytes, the time to tube formation is slower in CMEC co-cultured in high glucose conditioned astrocytes compared to normal glucose conditioned astrocytes. At 5 hour timepoint, there was a significant difference between normal glucose and high glucose scratch recovery (Fig. 4C: n = 5, *p < 0.05). Our observations suggest that glucose concentration significantly changes the astrocyte-CMEC cross-talk that affects proliferation rates of CMEC.
Fig. 4.
Astrocytes conditioned in high glucose media and co-cultured with cerebral microvessel endothelial cells resulted in decreased mitogenic activity. Astrocytes conditioned in high glucose media and co-cultured with cerebral microvessel endothelial cells resulted in decreased BrdU levels (Fig. 4A: n = 7, *p < 0.05), decreased scratch recovery assay results (Fig. 4B: Time accounts for 66.13% of the total variance between the two curves at time point 5 hours, where F = 56.01, p value < 0.0001), and slower tube formation compared to astrocytes conditioned to normal glucose media (Fig. 4C: n = 5).
4. Discussion
The spatial arrangement of astrocytes within the neurovascular unit (located between the vasculature and neurons) points to their important role in the uptake of glucose from the circulation, its metabolism, and transfer of energy to neurons. In view of the intricate metabolic interdependence of the cellular constituents of the neurovascular unit, dysregulation of astrocytic function under hyperglycemic conditions could lead to significant impairments in cellular functions [31, 32]. Several studies have focused upon the effects of high glucose conditions on astrocytes in relation to neurotoxicity, oxidative stress, and inflammation in the brain [33, 34, 35] but few have investigated the secreted products. Astrocytes are in constant reciprocal communication with the cerebral microenvironment predominantly by using secreted proteins, which control and regulate many biological and pathological processes in the nervous system. The novel findings in this study are two-fold: 1) high glucose conditions change the astrocytic capacity to produce and secrete VEGF, a key angiogenic factor; and 2) high glucose conditions change the ability of the astrocyte to affect mitogenic activity of CMEC in co-culture. These results suggest that exposure to increased glucose levels can alter the cellular cross-talk between key cellular constituents of the neurovascular unit and lead to altered angiogenesis.
The astrocyte is a highly responsive cellular constituent of the neurovascular unit and can significantly affect the function of the brain by altering its “secretome”. The astrocytic secretome is comprised of hundreds of proteins that influences neuronal and vascular development during all stages of life [36]. The astrocyte sheds extracellular vesicles of different sizes, from 150-500nm and can influence angiogenic responses upon other cells in co-culture [37]. Our findings presented in this study confirm that astrocytes produce VEGF in vitro and high glucose conditions (25 mM) not only decreases VEGF message and protein in culture but also decrease astrocytic VEGF secretion compared to normal glucose conditions (5.5 mM) (Figs. 1, 2, and 3). Our results are consistent with a report demonstrating that in an in vitro murine whole conceptus culture, high glucose (20 mM) insult results in reduced levels of VEGF-A in offspring, which in turn, leads to abnormal VEGF receptor signaling, resulting in embryopathy [38]. These results compliment the additional observations by the same group that addition of exogenous VEGF-A blunts the hyperglycemia-induced vasculopathy seen in offspring of diabetic mothers [38]. In contrast to our findings, Wang et al. demonstrated an increase in VEGF mRNA levels in response to high glucose conditions in retinal astrocytes [39]. This group's VEGF results were reported as part of a cytokine response to high glucose in the eye and underscore the diverse or even opposing roles of astrocytes in diabetic complications in the CNS. There is emerging evidence to support that there is considerable heterogeneity of astrocytes and what we once believed was a cell that provided passive support to neuronal activity may indeed be a central player in CNS function [40]. One is left to speculate that astrocytes may have different responses to metabolic substrate depending on their location and that cellular functions of the astrocyte may not be generalizable throughout the CNS.
Our group has previously demonstrated that astrocytes play an important role in regulating angiogenesis in the brain through its secretome [11]. In co-cultures of astrocytes and capillary endothelium, we observed morphological changes in both cell types resulting in astrocytes with “footlike” projections and intermittent gap junctions forming within the endothelial cells [11]. In vivo, the abluminal surface of the cerebral microvessels is almost completely covered by the foot processes of astrocytes [41]. This close relationship allows for factors secreted by the astrocyte to induce physiologic responses from the cerebral microvasculature. In our study, we investigated the mitogenic response of isolated CMEC co-cultured with astrocytes pre-conditioned in either normal glucose or high glucose medium. To demonstrate angiogenesis in vitro, cell assays are required to assess proliferation, migration, and tube formation. The quantitative capacity of these in vitro studies is particularly important, as it provides a confidence that is not always readily acquired with more complicated in vivo experiments. Using the BrdU assay, the proliferative capacity of endothelial cells co-cultured with high glucose conditioned astrocytes was significantly lower compared to those co-cultured with normal glucose conditioned astrocytes (Fig. 2A). Using the endothelial cell monolayer scratch recovery assay, the migration capacity of endothelial cells was decreased in co-culture with astrocytes conditioned in high glucose compared to normal glucose, but not with high glucose media alone (Fig. 2B). And using the tube formation assay, the ability to form tubes from endothelial cell monolayer was slower in co-culture with high glucose conditioned astrocytes compared to normal glucose (Fig. 2C). These findings taken together with our observations that astrocytes cultured in high glucose media produce decreased levels of VEGF suggest that astrocytes respond to changes in glucose by altering its secretome which could lead to impaired astrocytic cross-talk with other cells. Given that the astrocyte plays a critical role in regulating the function of the neurovascular unit, the results from our studies are important because to our knowledge, this is the first study to demonstrate a direct effect of glucose on astrocytic angiogenic capacity.
Vasculogenesis seems to be genetically programmed, but the process of angiogenesis is regulated by a complex interplay of inhibitory and stimulatory growth factors [42]. Early events in vessel formation and invasive capillary sprouting are triggered by VEGF, which predominantly act on endothelial cells [43]. VEGF-A, which has the highest angiogenic activity of the VEGF family induces and regulates the formation of new blood vessels during development and is a mitogen and survival-promoting factor for endothelial cells and controls endothelial cell migration and differentiation during vascular development [44]. For this reason, our study has specifically investigated VEGF-A. This could be viewed as a limitation given that there are several different angiogenic factors that the astrocyte produces including angiopoietin 1 and 2 [45], arachidonic acid metabolites [11], and Wnt family growth factors [46] to name a few. Furthermore, astrocytes require close interaction with endothelial cell to induce angiogenesis, therefore the endothelial cells were not maintained in normal glucose during co-culture and this exposure prevents us from saying the changes we see are from astrocytic secretome changes alone. The contribution of glucose conditions on the endothelial cell alone were outside the scope of this study. Nevertheless, the observations within this study give support to the hypothesis that high glucose conditions profoundly change the cross-talk between astrocytes and endothelial cell and merit further studies to delineate these effects.
In conclusion, in vitro responses of cultured neonatal astrocytes in high glucose conditions results in decreased production and secretion of VEGF and when co-cultured with CMEC results in impaired mitogenic activity. Responses of the astrocyte to environmental glucose changes provides insight into cellular adaptations that could explain cerebral microangiopathy seen in diabetic conditions. Further studies are required to elucidate the specific mechanisms that explain how high glucose alters the regulation of VEGF production and secretion in the astrocyte. Our study provides clear evidence that high glucose conditions alters astrocytic-endothelial cell cross-talk which lead to impaired angiogenesis.
Declarations
Author contribution statement
Susan Cohen: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Qiuli Liu, Matthew Wright: Performed the experiments.
Jodi Garvin: Contributed reagents, materials, analysis tools or data.
Kevin Rarick: Performed the experiments; Analyzed and interpreted the data; Wrote the paper.
David Harder: Conceived and designed the experiments; Wrote the paper.
Funding statement
This work was supported by NHLBI of the National Institutes of Health under award number HL033833-32 and Children's Hospital of Wisconsin Research Institute Pilot Award funding.
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
References
- 1.Dore-Duffy P., LaManna J.C. Physiologic angiodynamics in the brain. Antioxidants Redox Signal. 2007;9(9):1363–1371. doi: 10.1089/ars.2007.1713. [DOI] [PubMed] [Google Scholar]
- 2.LaManna J.C., Chavez J.C., Pichiule P. Structural and functional adaptation to hypoxia in the rat brain. J. Exp. Biol. 2004;207(Pt 18):3163–3169. doi: 10.1242/jeb.00976. [DOI] [PubMed] [Google Scholar]
- 3.Benderro G.F., Sun X., Kuang Y., Lamanna J.C. Decreased VEGF expression and microvascular density, but increased HIF-1 and 2alpha accumulation and EPO expression in chronic moderate hyperoxia in the mouse brain. Brain Res. 2012;1471:46–55. doi: 10.1016/j.brainres.2012.06.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kota S.K., Meher L.K., Jammula S., Kota S.K., Krishna S.V.S., Modi K.D. Aberrant angiogenesis: the gateway to diabetic complications. Indian J. Endocrinol. Metab. 2012;16(6):918–930. doi: 10.4103/2230-8210.102992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wilkinson-Berka J.L. Vasoactive factors and diabetic retinopathy: vascular endothelial growth factor, cycoloxygenase-2 and nitric oxide. Curr. Pharmaceut. Des. 2004;10(27):3331–3348. doi: 10.2174/1381612043383142. [DOI] [PubMed] [Google Scholar]
- 6.Osterby R., Nyberg G. New vessel formation in the renal corpuscles in advanced diabetic glomerulopathy. J. Diabet. Complicat. 1987;1(4):122–127. doi: 10.1016/s0891-6632(87)80069-7. [DOI] [PubMed] [Google Scholar]
- 7.Tahergorabi Z., Khazaei M. Imbalance of angiogenesis in diabetic complications: the mechanisms. Int. J. Prev. Med. 2012;3(12):827–838. doi: 10.4103/2008-7802.104853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Troncoso F., Acurio J., Herlitz K., Aguayo C., Bertoglia P., Guzman-Gutierrez E. Gestational diabetes mellitus is associated with increased pro-migratory activation of vascular endothelial growth factor receptor 2 and reduced expression of vascular endothelial growth factor receptor 1. PLoS One. 2017;12(8) doi: 10.1371/journal.pone.0182509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dallerac G., Rouach N. Astrocytes as new targets to improve cognitive functions. Prog. Neurobiol. 2016;144:48–67. doi: 10.1016/j.pneurobio.2016.01.003. [DOI] [PubMed] [Google Scholar]
- 10.Ma S., Kwon H.J., Huang Z. A functional requirement for astroglia in promoting blood vessel development in the early postnatal brain. PLoS One. 2012;7(10) doi: 10.1371/journal.pone.0048001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang C., Harder D.R. Cerebral capillary endothelial cell mitogenesis and morphogenesis induced by astrocytic epoxyeicosatrienoic acid. Stroke; J. Cereb. Circ. 2002;33(12):2957–2964. doi: 10.1161/01.str.0000037787.07479.9a. [DOI] [PubMed] [Google Scholar]
- 12.Munzenmaier D.H., Harder D.R. Cerebral microvascular endothelial cell tube formation: role of astrocytic epoxyeicosatrienoic acid release. Am. J. Physiol. Heart Circ. Physiol. 2000;278(4):H1163–H1167. doi: 10.1152/ajpheart.2000.278.4.H1163. [DOI] [PubMed] [Google Scholar]
- 13.Scott A., Powner M.B., Gandhi P., Clarkin C., Gutmann D.H., Johnson R.S. Astrocyte-derived vascular endothelial growth factor stabilizes vessels in the developing retinal vasculature. PLoS One. 2010;5(7) doi: 10.1371/journal.pone.0011863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Weidemann A., Krohne T.U., Aguilar E., Kurihara T., Takeda N., Dorrell M.I. Astrocyte hypoxic response is essential for pathological but not developmental angiogenesis of the retina. Glia. 2010;58(10):1177–1185. doi: 10.1002/glia.20997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ruhrberg C., Gerhardt H., Golding M., Watson R., Ioannidou S., Fujisawa H. Spatially restricted patterning cues provided by heparin-binding VEGF-A control blood vessel branching morphogenesis. Genes Dev. 2002;16(20):2684–2698. doi: 10.1101/gad.242002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Proescholdt M.A., Jacobson S., Tresser N., Oldfield E.H., Merrill M.J. Vascular endothelial growth factor is expressed in multiple sclerosis plaques and can induce inflammatory lesions in experimental allergic encephalomyelitis rats. J. Neuropathol. Exp. Neurol. 2002;61(10):914–925. doi: 10.1093/jnen/61.10.914. [DOI] [PubMed] [Google Scholar]
- 17.Argaw A.T., Gurfein B.T., Zhang Y., Zameer A., John G.R. VEGF-mediated disruption of endothelial CLN-5 promotes blood-brain barrier breakdown. Proc. Natl. Acad. Sci. U. S. A. 2009;106(6):1977–1982. doi: 10.1073/pnas.0808698106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chow J., Ogunshola O., Fan S.Y., Li Y., Ment L.R., Madri J.A. Astrocyte-derived VEGF mediates survival and tube stabilization of hypoxic brain microvascular endothelial cells in vitro. Brain Res. Dev. Brain Res. 2001;130(1):123–132. doi: 10.1016/s0165-3806(01)00220-6. [DOI] [PubMed] [Google Scholar]
- 19.Shweiki D., Neeman M., Itin A., Keshet E. Induction of vascular endothelial growth factor expression by hypoxia and by glucose deficiency in multicell spheroids: implications for tumor angiogenesis. Proc. Natl. Acad. Sci. U. S. A. 1995;92(3):768–772. doi: 10.1073/pnas.92.3.768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Benjamin L.E. Glucose, VEGF-A, and diabetic complications. Am. J. Pathol. 2001;158(4):1181–1184. doi: 10.1016/S0002-9440(10)64066-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Katavetin P., Miyata T., Inagi R., Tanaka T., Sassa R., Ingelfinger J.R. High glucose blunts vascular endothelial growth factor response to hypoxia via the oxidative stress-regulated hypoxia-inducible factor/hypoxia-responsible element pathway. J. Am. Soc. Nephrol. 2006;17(5):1405–1413. doi: 10.1681/ASN.2005090918. [DOI] [PubMed] [Google Scholar]
- 22.Doronzo G., Viretto M., Russo I., Mattiello L., Anfossi G., Trovati M. Effects of high glucose on vascular endothelial growth factor synthesis and secretion in aortic vascular smooth muscle cells from obese and lean zucker rats. Int. J. Mol. Sci. 2012;13(8):9478–9488. doi: 10.3390/ijms13089478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yu M., Lu G., Zhu X., Huang Z., Feng C., Fang R. Downregulation of VEGF and upregulation of TL1A expression induce HUVEC apoptosis in response to high glucose stimuli. Mol. Med. Rep. 2016;13(4):3265–3272. doi: 10.3892/mmr.2016.4924. [DOI] [PubMed] [Google Scholar]
- 24.Wang L., Cossette S.M., Rarick K.R., Gershan J., Dwinell M.B., Harder D.R. Astrocytes directly influence tumor cell invasion and metastasis in vivo. PLoS One. 2013;8(12) doi: 10.1371/journal.pone.0080933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Livak K.J., Schmittgen T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25(4):402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 26.Czupalla C.J., Liebner S., Devraj K. In vitro models of the blood-brain barrier. Methods Mol. Biol. 2014;1135:415–437. doi: 10.1007/978-1-4939-0320-7_34. [DOI] [PubMed] [Google Scholar]
- 27.Guo S., Lok J., Liu Y., Hayakawa K., Leung W., Xing C. Assays to examine endothelial cell migration, tube formation, and gene expression profiles. Methods Mol. Biol. 2014;1135:393–402. doi: 10.1007/978-1-4939-0320-7_32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sun X., Hu X., Wang D., Yuan Y., Qin S., Tan Z. Establishment and characterization of primary astrocyte culture from adult mouse brain. Brain Res. Bull. 2017;132:10–19. doi: 10.1016/j.brainresbull.2017.05.002. [DOI] [PubMed] [Google Scholar]
- 29.Teng R.J., Eis A., Bakhutashvili I., Arul N., Konduri G.G. Increased superoxide production contributes to the impaired angiogenesis of fetal pulmonary arteries with in utero pulmonary hypertension. Am. J. Physiol. Lung Cell Mol. Physiol. 2009;297(1):L184–L195. doi: 10.1152/ajplung.90455.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kirkpatrick C.J., Fuchs S., Unger R.E. Co-culture systems for vascularization--learning from nature. Adv. Drug Deliv. Rev. 2011;63(4-5):291–299. doi: 10.1016/j.addr.2011.01.009. [DOI] [PubMed] [Google Scholar]
- 31.Eddleston M., Mucke L. Molecular profile of reactive astrocytes--implications for their role in neurologic disease. Neuroscience. 1993;54(1):15–36. doi: 10.1016/0306-4522(93)90380-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rungger-Brandle E., Dosso A.A., Leuenberger P.M. Glial reactivity, an early feature of diabetic retinopathy. Investig. Ophthalmol. Vis. Sci. 2000;41(7):1971–1980. [PubMed] [Google Scholar]
- 33.Bahniwal M., Little J.P., Klegeris A. High glucose enhances neurotoxicity and inflammatory cytokine secretion by stimulated human astrocytes. Curr. Alzheimer Res. 2017;14(7):731–741. doi: 10.2174/1567205014666170117104053. [DOI] [PubMed] [Google Scholar]
- 34.Hsieh H.L., Lin C.C., Hsiao L.D., Yang C.M. High glucose induces reactive oxygen species-dependent matrix metalloproteinase-9 expression and cell migration in brain astrocytes. Mol. Neurobiol. 2013;48(3):601–614. doi: 10.1007/s12035-013-8442-6. [DOI] [PubMed] [Google Scholar]
- 35.Hsieh H.L., Chi P.L., Lin C.C., Yang C.C., Yang C.M. Up-regulation of ROS-dependent matrix metalloproteinase-9 from high-glucose-challenged astrocytes contributes to the neuronal apoptosis. Mol. Neurobiol. 2014;50(2):520–533. doi: 10.1007/s12035-013-8628-y. [DOI] [PubMed] [Google Scholar]
- 36.Jha M.K., Seo M., Kim J.H., Kim B.G., Cho J.Y., Suk K. The secretome signature of reactive glial cells and its pathological implications. Biochim. Biophys. Acta. 2013;1834(11):2418–2428. doi: 10.1016/j.bbapap.2012.12.006. [DOI] [PubMed] [Google Scholar]
- 37.Proia P., Schiera G., Mineo M., Ingrassia A.M., Santoro G., Savettieri G. Astrocytes shed extracellular vesicles that contain fibroblast growth factor-2 and vascular endothelial growth factor. Int. J. Mol. Med. 2008;21(1):63–67. [PubMed] [Google Scholar]
- 38.Pinter E., Haigh J., Nagy A., Madri J.A. Hyperglycemia-induced vasculopathy in the murine conceptus is mediated via reductions of VEGF-A expression and VEGF receptor activation. Am. J. Pathol. 2001;158(4):1199–1206. doi: 10.1016/S0002-9440(10)64069-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang J., Li G., Wang Z., Zhang X., Yao L., Wang F. High glucose-induced expression of inflammatory cytokines and reactive oxygen species in cultured astrocytes. Neuroscience. 2012;202:58–68. doi: 10.1016/j.neuroscience.2011.11.062. [DOI] [PubMed] [Google Scholar]
- 40.Schitine C., Nogaroli L., Costa M.R., Hedin-Pereira C. Astrocyte heterogeneity in the brain: from development to disease. Front. Cell. Neurosci. 2015;9(76) doi: 10.3389/fncel.2015.00076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Abbott N.J., Ronnback L., Hansson E. Astrocyte-endothelial interactions at the blood-brain barrier. Nat. Rev. Neurosci. 2006;7(1):41–53. doi: 10.1038/nrn1824. [DOI] [PubMed] [Google Scholar]
- 42.Acker T., Plate K.H. Role of hypoxia in tumor angiogenesis-molecular and cellular angiogenic crosstalk. Cell Tissue Res. 2003;314(1):145–155. doi: 10.1007/s00441-003-0763-8. [DOI] [PubMed] [Google Scholar]
- 43.Maury J.J., Choo A.B., Chan K.K. Technical advances to genetically engineering human embryonic stem cells. Integr. Biol. (Camb). 2011;3(7):717–723. doi: 10.1039/c1ib00019e. [DOI] [PubMed] [Google Scholar]
- 44.Ferrara N. Vascular endothelial growth factor: basic science and clinical progress. Endocr. Rev. 2004;25(4):581–611. doi: 10.1210/er.2003-0027. [DOI] [PubMed] [Google Scholar]
- 45.Alvarez J.I., Katayama T., Prat A. Glial influence on the blood brain barrier. Glia. 2013;61(12):1939–1958. doi: 10.1002/glia.22575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lee H.S., Han J., Bai H.J., Kim K.W. Brain angiogenesis in developmental and pathological processes: regulation, molecular and cellular communication at the neurovascular interface. FEBS J. 2009;276(17):4622–4635. doi: 10.1111/j.1742-4658.2009.07174.x. [DOI] [PubMed] [Google Scholar]