Summary
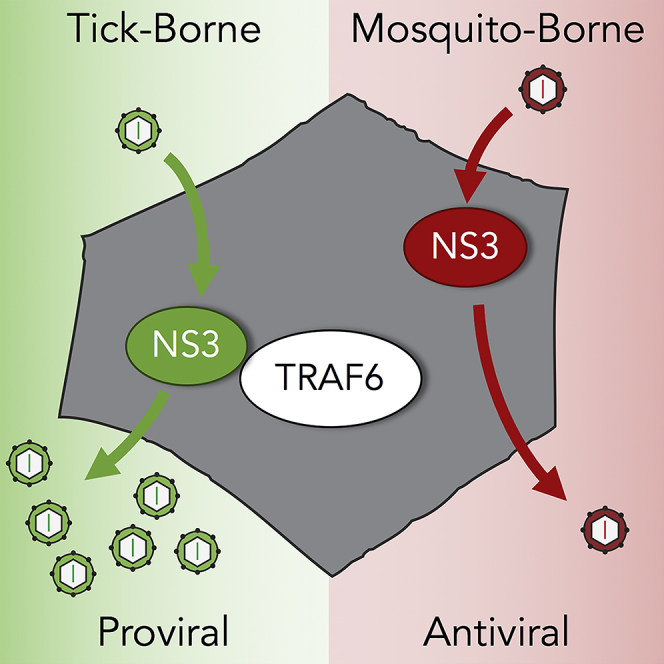
Tick-borne flaviviruses (TBFVs) can cause life-threatening encephalitis and hemorrhagic fever. To identify virus-host interactions that may be exploited as therapeutic targets, we analyzed the TBFV polyprotein in silico for antiviral protein-binding motifs. We obtained two putative tumor necrosis factor receptor-associated factor 6 (TRAF6)-binding motifs (TBMs) within the protease domain of the viral nonstructural 3 (NS3) protein. Here, we show that TBFV NS3 interacted with TRAF6 during infection and that TRAF6 supports TBFV replication. The proviral role of TRAF6 was not seen with mosquito-borne flaviviruses, consistent with the lack of conserved TBMs. Mutation of the second TBM within NS3 disrupted TRAF6 binding, coincident with reduced abundance of mature, autocatalytically derived form of the NS3 protease and significant virus attenuation in vitro. Our studies reveal insights into how flaviviruses exploit innate immunity for the purpose of viral replication and identify a potential target for therapeutic design.
Subject Areas: Molecular Interaction, Microbiology, Viral Microbiology
Graphical Abstract
Highlights
-
•
Langat virus (LGTV) NS3 protease interacts with TRAF6 during infection
-
•
Tick-borne, unlike mosquito-borne, flaviviruses use TRAF6 for optimal replication
-
•
E117A mutation of LGTV NS3 reduces TRAF6 binding and mature protease abundance
-
•
LGTV with a mutated TRAF6-binding motif is attenuated in vitro
Molecular Interaction; Microbiology; Viral Microbiology
Introduction
Viruses within the Flavivirus genus represent an overwhelming disease burden to humans, in worst cases causing either encephalitis or hemorrhagic fever with mortality rates up to 50% (Leyssen et al., 2000). The success of flaviviruses is in large part associated with transmission by arthropod vectors, either mosquito borne, represented by dengue virus (DENV), West Nile virus (WNV), and Zika virus, or tick borne, including tick-borne encephalitis virus (TBEV), Omsk hemorrhagic fever virus, Kyasanur Forest disease virus (KFDV), and Powassan virus (POWV). No specific antiviral therapy is available for the millions of individuals infected with flaviviruses each year. We rationalize that to develop desperately needed drugs and vaccines to treat and prevent flavivirus infections, we need to better understand how flaviviruses infect cells and cause disease.
The flavivirus chymotrypsin-like serine protease, nonstructural 3 (NS3), is an ideal target for broad-spectrum antiviral development (Luo et al., 2015). NS3 is relatively conserved between the flaviviruses and is essential for virus replication, functioning to cleave the viral polyprotein into mature individual proteins through a well-characterized enzymatic function (Li et al., 2014, Palanisamy et al., 2018). Mutant flaviviruses with an inactive NS3 protease are unable to generate infectious virions (Chambers et al., 2005). Despite substantial effort and resources directed toward the design of NS3 protease inhibitors, there has only been limited success and no compound has reached the clinical trial stage (Brecher et al., 2017). Innovative discovery approaches seek to expand potential drug targets by identifying protein-protein interactions necessary for viral protein function during infection (Geiss et al., 2009). We reasoned here that the identification of host proteins that interact with NS3 may direct us toward methods to inhibit NS3, either by modeling antiviral drugs from host antiviral interactions with NS3, or by identifying host proteins that NS3 uses during infection.
Bioinformatic analysis of the TBEV polyprotein revealed a potential interaction with the host protein tumor necrosis factor receptor-associated factor 6 (TRAF6). TRAF6 is an important antiviral intracellular molecule for the interleukin-1(IL-1)/Toll-like receptor (TLR) and retinoic acid-inducible gene I (RIG-I) signaling pathways (Inoue et al., 2007, Wu and Arron, 2003, Yoshida et al., 2008). It functions by linking innate immune receptor engagement to activation of protein kinase complexes, culminating in activation of transcription factors including nuclear factor-κB (NF-κB) and activator protein-1 (Walsh et al., 2015). TRAF6 is an E3 ubiquitin ligase and binds substrate proteins through a conserved TRAF6 binding motif (TBM). A canonical TBM is formed from the amino acid sequence P-X-E-X-X-Ac/Ar (X representing any amino acid, Ac/Ar representing either an acidic or aromatic residue) (Chung et al., 2007). TRAF6 has also been shown to bind to non-canonical TBMs that have alternative amino acids sequences (Gentry et al., 2004, Meads et al., 2010, Noels et al., 2007, Stack et al., 2013).
In this study, we discovered and experimentally validated a TBM in NS3 of tick-borne flaviviruses (TBFVs), which is not present in mosquito-borne flaviviruses (MBFVs). The protein interaction between TRAF6 and NS3 is disrupted by site-directed mutagenesis, and, in the context of the full TBFV genome, the mutation results in virus attenuation. We found that TBM mutation within NS3 impairs the expression of the mature, active form of the viral protease (NS3pro). NS3pro is only produced during infection by autoproteolytic cleavage and is essential for flavivirus replication. Our data suggest that TRAF6 actively participates in the TBFV replication cycle to maintain NS3pro expression, possibly through protein stabilization or protease activation. Thus TRAF6's structural and functional contributions to NS3pro maturation, in addition to the TRAF6-NS3 complex, serve as potential therapeutic targets that should be further explored for treatment of the TBFVs.
Results
TBFV, but Not MBFV, NS3pro Interacts with TRAF6
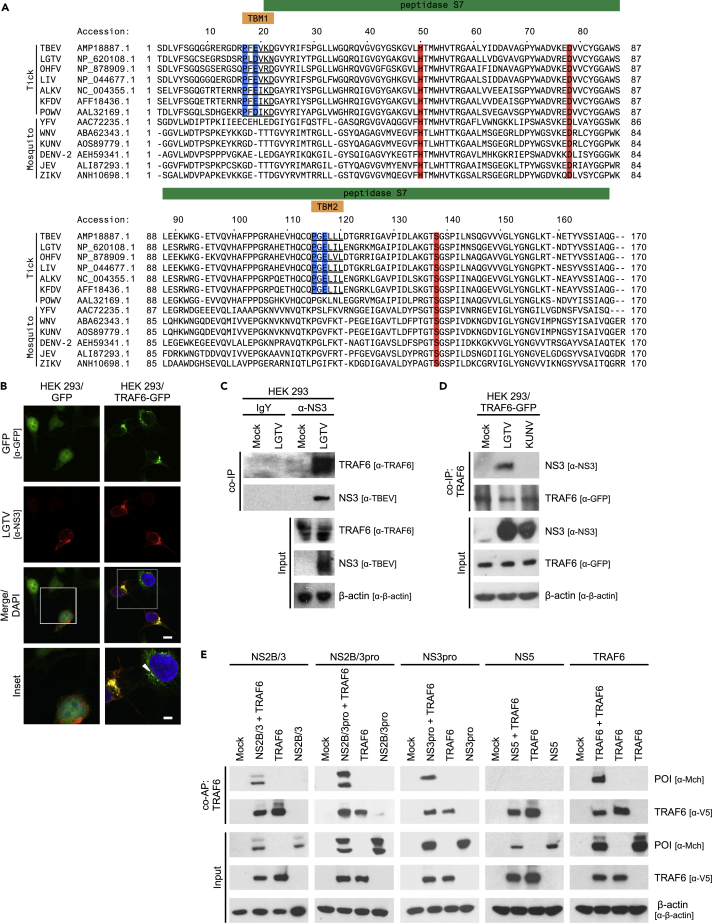
To investigate the cellular factors regulating TBFV replication, we performed a bioinformatic analysis of the TBEV polyprotein using the Eukaryotic Linear Motif Resource for Functional Sites in Proteins (Dinkel et al., 2013). Our primary screen revealed a solitary putative canonical TBM, P-F-E-V-K-D (TBM1), located within the protease domain of NS3 (NS3pro) between residues 17 and 22 (Figure 1A). Further analysis of NS3pro identified a non-canonical putative TBM, P-G-E-L-L-L (TBM2), at residues 115–120, in which the terminal amino acid of the consensus sequence is neither acidic nor aromatic (Figure 1A) (Gentry et al., 2004). Sequence alignment of NS3pro from other flaviviruses showed that the two putative TBMs are highly conserved among TBFVs (Figures 1A and S1A–S1C), with the exception of the evolutionarily isolated POWV (Hermance and Thangamani, 2017). TBM2 was not present in NS3 of POWV. Furthermore, we did not identify TBM1 or TBM2 in any MBFV (Figure 1A), suggesting a possible unique and specific interaction between TBFVs and TRAF6.
Figure 1.
LGTV NS3pro Interacts with TRAF6
(A) Sequence alignment of tick- or mosquito-borne flavivirus NS3pro. Putative TBMs are underlined, and anchor amino acids are highlighted in blue. Conserved residues of the catalytic triad are highlighted in red.
(B) Representative images of colocalization of LGTV NS3 and TRAF6. HEK293 cells expressing GFP or TRAF6-GFP were infected with LGTV at an MOI of 1. Cells were processed at 24 hpi, stained with anti-NS3 (red) and anti-GFP (green) antibodies, and visualized by confocal microscopy. Nuclei were stained with DAPI (blue); scale bar (white solid line), 10 μM. Yellow represents co-localized proteins. White box indicates area of enlargement in inset panel; inset scale bar (white solid line), 5 μM. White arrowhead indicates a distinct TRAF6 punctum observed in uninfected cells.
(C) CoIP of LGTV NS3 and endogenous TRAF6 during infection. HEK293 cells were infected with LGTV at an MOI of 1. Cell lysates were prepared at 48 hpi, incubated with either anti-NS3 or control IgY antisera, and precipitated with PrecipHen beads. Samples were analyzed by immunoblot with the indicated antibodies. Results representative of two independent experiments.
(D) CoIP of LGTV NS3 and TRAF6. HEK293 cells expressing TRAF6-GFP were infected with LGTV or KUNV at an MOI of 10. Cell lysates were immunoprecipitated with anti-GFP antibody and protein-A-conjugated agarose beads at 48 hpi. Samples were analyzed by immunoblot with the indicated antibodies. Results representative of three independent experiments.
(E) CoAP of recombinant LGTV NS3 constructs with TRAF6. HEK293 cells were transfected with 2 μg of each indicated plasmid. Cell lysates were pulled down using streptavidin-conjugated beads at 24 h post-transfection, and eluted proteins were analyzed by immunoblot with indicated antibodies. Results representative of three or more independent experiments. POI, protein of interest.
See also Figure S1.
Based on our bioinformatics results, we first examined a potential interaction between TBFV NS3 and TRAF6 using immunofluorescent microscopy. For this purpose, human embryonic kidney (HEK) 293 cells expressing either GFP or a GFP-tagged TRAF6 (TRAF6-GFP) were infected with Langat virus (LGTV), a prototypical member of the TBEV serogroup. Confocal analyses revealed strong co-localization of LGTV NS3 and TRAF6 proteins at 24 h post-infection (hpi) (Figure 1B). We also observed a change in the cellular distribution of TRAF6-GFP, from discrete puncta in uninfected cells to a large perinuclear aggregate during infection (Figure 1B). To validate the confocal results, we took a biochemical approach and preformed a co-immunoprecipitation (coIP) of endogenous TRAF6 with LGTV NS3. HEK293 cells were infected with LGTV, and at 48 hpi, anti-NS3 antibody was used to precipitate LGTV NS3. We were able to pull down endogenous TRAF6 with LGTV NS3 (Figure 1C). In addition, we tested the specificity of TBFV NS3 to interact with TRAF6 by preforming a coIP with HEK293 cells expressing TRAF6-GFP. Cells were either infected with LGTV or Kunjin virus (KUNV), an MBFV that lacks TBM1 and TBM2 (Figure 1A), and at 48 hpi, anti-GFP antibody was used to precipitate TRAF6-GFP. We were only able to pull down LGTV NS3 with TRAF6-GFP (Figure 1D). These results suggest that LGTV NS3 and TRAF6 proteins interact during TBFV infection.
To identify the structural and functional requirements of the LGTV NS3pro-TRAF6 interaction, we engineered epitope-tagged recombinant NS3 constructs and compared their interaction with wild-type (WT) TRAF6 using a co-affinity purification (coAP) technique based on biotin-streptavidin binding (Taylor et al., 2011). NS3 protease activity is dependent on a non-covalent interaction with NS2B (Erbel et al., 2006, Falgout et al., 1991). To express NS3 with a functional protease domain, we fused NS2B and NS3 together in a single open reading frame, as occurs in the polyprotein (NS2B/3). Once translated within the cell, the NS2B/3 polyprotein undergoes autocatalytic cleavage, freeing NS2B from NS3 (Bera et al., 2007, Liu et al., 2017). This cleavage event is detected on immunoblot as a doublet, with NS2B/3 having a larger molecular weight than NS3 alone. As a confirmation of the interaction detected during virus infection, TRAF6 precipitated both full-length LGTV NS2B/3 and truncated LGTV NS2B/3pro when expressed in HEK293 cells (Figure 1E). Next, we engineered the following two constructs to directly test the requirement of NS3 protease activity for NS3pro binding to TRAF6: (1) an LGTV NS3pro construct that lacks NS2B and thus has no enzymatic activity and (2) a mutated LGTV NS3 serine 138 to alanine, which disrupts the catalytic triad needed for protease activity (Pastorino et al., 2006). We found in both instances that NS3 was able to interact with TRAF6 regardless of a functional NS3 protease (Figures 1E, S1D, and S1E). Finally, LGTV NS5, the major interferon (IFN) antagonist of TBFVs (Best et al., 2005), was not precipitated with TRAF6 and served as a negative control, whereas TRAF6 forms a strong homomeric complex with alternatively tagged TRAF6 as previously reported (Megas et al., 2011) (Figure 1E). These results show that TRAF6 specifically interacts with the protease domain of LGTV NS3 and that the protease activity of NS3 is not required for this interaction.
TBFVs Use TRAF6 for Optimal Replication
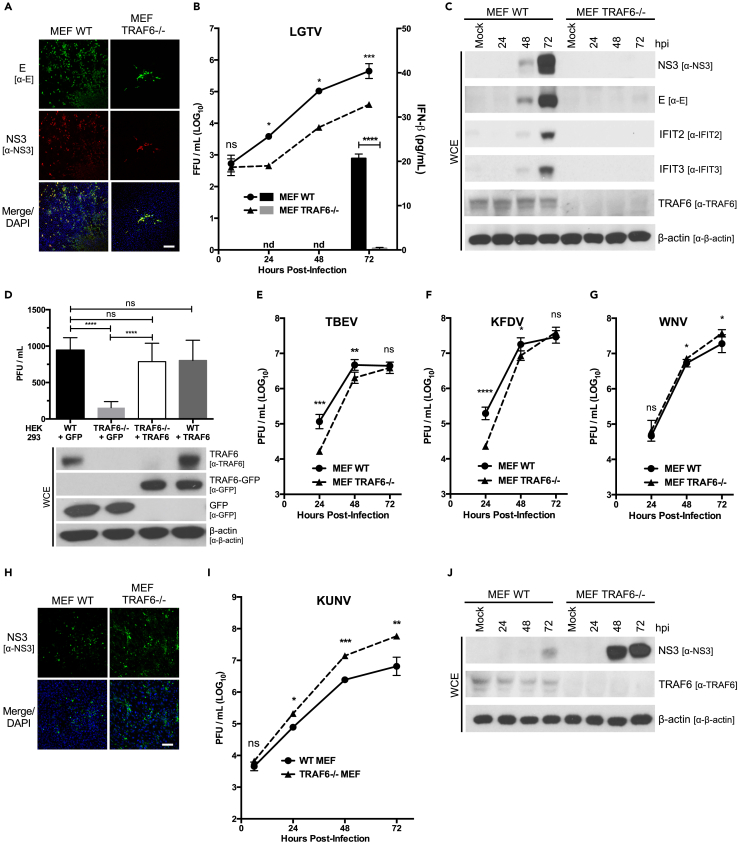
To assess the role of TRAF6 in TBFV replication, we infected WT and TRAF6−/− murine embryonic fibroblasts (MEFs) with LGTV. In contrast to what we predicted, we found that TRAF6 is needed for optimal LGTV replication (Figures 2A–2C and S2A). There was up to a 14.2-fold reduction in the release of infectious virions from TRAF6-deficient cells compared with cells expressing TRAF6 (Figure 2B). Moreover, NS3 and E proteins were readily detectable in LGTV-infected WT MEFs compared with TRAF6−/− MEFs (Figures 2C and S2A). We observed a similar reduction in LGTV replication when we infected CRISPR/Cas9-generated TRAF6−/− HEK293 cells (Figure S2B). To demonstrate that the reduction in LGTV titers was due to a lack of TRAF6, we complemented TRAF6−/− HEK293 cells with human TRAF6-GFP and found that LGTV titers were restored to WT levels (Figure 2D). In addition, we functionally depleted HEK293 cells of TRAF6 using a commercially available TRAF6 peptide inhibitor (Hou et al., 2014) and by overexpressing a TRAF6 construct with a mutation in the ligase catalytic site (Ning et al., 2008). In both cases, LGTV replication was significantly diminished to levels similar to TRAF6−/− cells (Figures S2C–S2E). Conversely, LGTV replication was similar between TRAF6-GFP overexpressing cells and control cells, suggesting that endogenous TRAF6 is sufficient for the observed proviral effect during LGTV infection (Figures 2D, S2D, and S2E). Furthermore, we infected TRAF6−/− cells with the highly virulent TBEV and KFDV and observed similar trends as with LGTV (Figures 2E, 2F, S2F, and S2G). There was a 9.9-fold reduction in TBEV and 8.6-fold reduction in KFDV titers in TRAF6−/− MEFs at 24 hpi (Figures 2E and 2F), suggesting that TRAF6 promotes the replication of TBFVs regardless of their virulence.
Figure 2.
TRAF6 Is a Proviral Factor for TBFVs
(A) Representative images of confocal microscopy of LGTV-infected WT or TRAF6−/− MEFs. MEFs were infected at an MOI of 1 and were processed at 24 hpi. Cells were immunostained with anti-E (green) and anti-NS3 (red) antibodies. Nuclei were stained with DAPI (blue); scale bar (white solid line), 200 μM.
(B) Titration of infectious particles in the supernatant from WT or TRAF6−/− MEFs infected with LGTV (MOI of 0.1) for indicated times (h). Viral titers were determined by immunofocus assay (left y axis). IFN-β protein in supernatants from infected cells was quantitated by ELISA and is represented by a column graph (right y axis). Results are representative of three or more independent experiments performed in triplicate and plotted as mean ± SEM. ns, not significant; *p < 0.05, ***p < 0.001.
(C) Immunoblot analysis of whole-cell extracts from WT or TRAF6−/− MEFs infected with LGTV (MOI 0.1) for indicated times (h). Blots were probed with antibodies to LGTV NS3, LGTV E, IFIT2, IFIT3, TRAF6, and β-actin. Results representative of three or more independent experiments. See Figure S2A for longer exposures of LGTV NS3 and LGTV E.
(D) Titration of infectious particles in the supernatant from WT or TRAF6−/− HEK293 cells expressing either GFP or TRAF6-GFP infected with LGTV (MOI of 0.1) at 24 hpi. Viral titers were determined by plaque assay. Whole-cell extracts from the infected cells were analyzed by immunoblot using GFP, TRAF6, and β-actin antibodies. Data are average of three independent experiments performed in triplicate and plotted as mean ± SEM. ns, not significant; ****p < 0.0001.
(E–G) Titration of infectious particles in the supernatant from WT or TRAF6−/− MEFs infected with TBEV (E), KFDV (F), or WNV (G) (MOI of 0.1) for indicated times (h). Viral titers were determined by plaque assay. Data are average of three independent experiments performed in triplicate and plotted as mean ± SEM. ns, not significant; *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001.
(H) Representative images of confocal microscopy of KUNV-infected WT or TRAF6−/− MEFs. MEFs were infected at an MOI of 1 and were processed at 24 hpi. Cells were immunostained with anti-NS3 (green) antibodies. Nuclei were stained with DAPI (blue); scale bar (white solid line), 100 μM.
(I) Titration of infectious particles in the supernatant from WT or TRAF6−/− MEFs infected with KUNV (MOI of 0.1) for indicated times (h). Viral titers were determined by plaque assay. Data are representative of three or more independent experiments performed in triplicate and plotted as mean ± SEM. ns, not significant; *p < 0.05; **p < 0.01; ***p < 0.001.
(J) Immunoblot analysis of whole-cell extracts from WT or TRAF6−/− MEFs infected with KUNV (MOI 0.1) for indicated times (h). Blots were probed with antibodies to WNV NS3, TRAF6, and β-actin. Results representative of three or more independent experiments.
See also Figure S2.
To assess the interplay between TBFV replication, TRAF6, and IFN production, we quantified IFN-β in the supernatant of LGTV-infected TRAF6−/− MEFs. We were only able to detect IFN-β (20.6 pg/mL) at 72 hpi in the supernatant from WT MEFs. IFN-β remained undetectable in the supernatants from TRAF6−/− MEFs throughout the course of infection (Figure 2B). Moreover, the levels of IFN-β directly correlated with the expression of the IFN-stimulated genes, IFIT2 and IFIT3, as detected by immunoblot of the cell lysates (Figure 2C). These data indicate that the attenuation of LGTV replication in TRAF6−/− MEFs is not due to increased IFN production.
To compare the impact of TRAF6 on TBFVs to MBFVs, we infected TRAF6−/− MEFs with WNV and KUNV. We observed a striking difference between the MBFVs and TBFVs (Figures 2G–2J). The replication of WNV and KUNV was enhanced in the absence of TRAF6, both reaching higher viral titers in MEFs deficient in TRAF6 compared with WT (Figures 2G and 2I). Infection of TRAF6−/− MEFs resulted in up to 8.9-fold increase in KUNV titers and an increase in NS3 production compared with WT MEFs (Figures 2H–2J). Taken together, these results show that TRAF6 specifically supports TBFV replication, which is distinctly different from its antiviral role in MBFV infection.
Mutation of TBM2 Prevents TRAF6-NS3pro Interaction and Reduces Accumulation of the Mature Protease
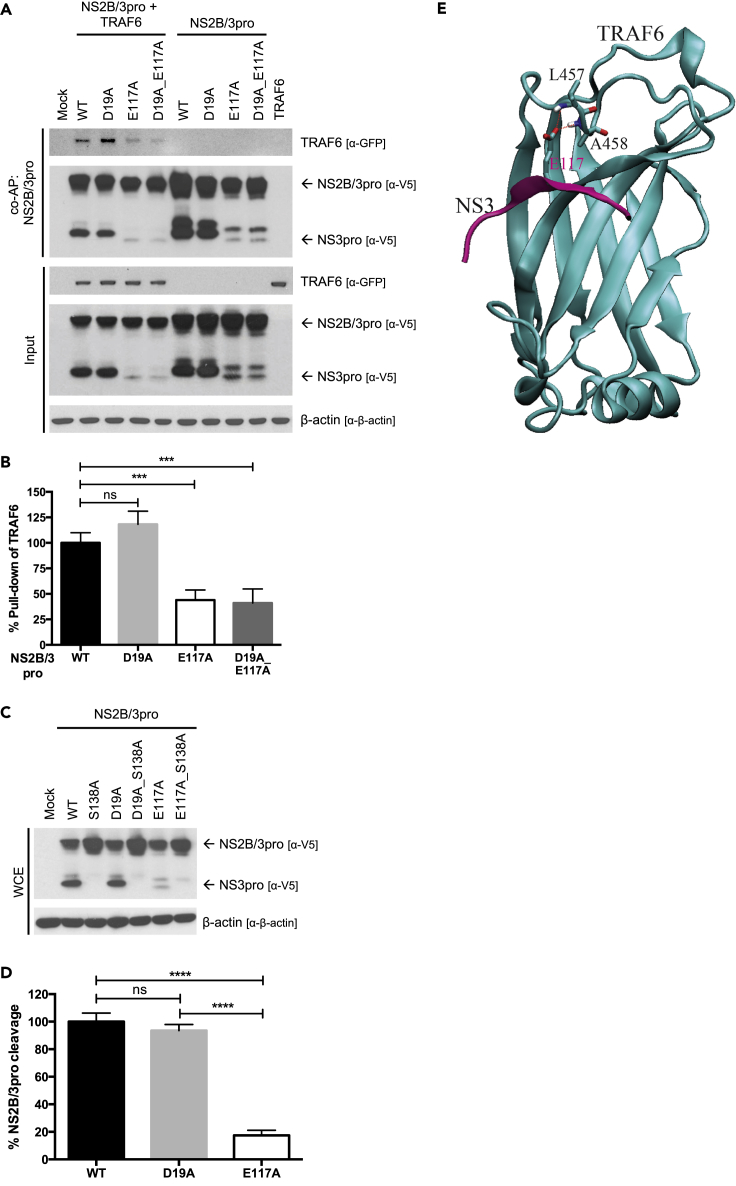
Previous studies have shown that mutation of the anchor glutamic acid residue in a TBM to an alanine disrupts TRAF6 binding (Verstak et al., 2014, Yu et al., 2016). To determine which TBM(s) in LGTV NS3pro interacts with TRAF6, we mutated the acidic amino acid in each TBM of LGTV NS2B/3pro to alanine. We tested the ability of each mutant to bind TRAF6 by coAP assay. We found that a D19A mutation in TBM1 had no effect on binding, whereas an E117A mutation in TBM2 resulted in a significant decrease in binding to TRAF6 (Figure 3A). E117A mutation also diminished the TRAF6 interaction to NS2B/3pro_D19A mutant (Figure 3A). Quantification of immunoblots using densitometry showed a 56.1% and a 58.6% decrease in pull down of TRAF6 by LGTV NS2B/3pro_E117A and LGTV NS2B/3pro_D19A_E117A, respectively (Figure 3B).
Figure 3.
An E117A Mutation in LGTV NS3pro Disrupts TRAF6 Binding and Mature Protease Accumulation
(A) CoAP of mutant LGTV NS2B/3pro constructs with TRAF6. HEK293 cells were transfected with 2 μg of each indicated plasmid. Cell lysates were pulled down using streptavidin-conjugated beads at 24 h post-transfection, and eluted proteins were analyzed by immunoblot with indicated antibodies. Results representative of three independent experiments.
(B) Quantification of TRAF6 pull-down by mutant LGTV NS2B/3pro constructs in (A). Precipitated TRAF6-GFP from three independent experiments were quantitated using ImageJ after normalization to β-actin. Data are presented as percent pull-down of TRAF6-GFP relative to NS2B/3pro WT ± SD. ns, not significant; ***p < 0.001.
(C) Immunoblot analysis of whole-cell extracts from HEK293 cells expressing mutant LGTV NS2B/3pro constructs. HEK293 cells were transfected with 2 μg of each indicated plasmid and processed 24 h post-transfection. Blots were probed with indicated antibodies. Results representative of three independent experiments.
(D) Quantification of cleaved NS3pro of mutant LGTV NS2B/3pro constructs in (C). Cleaved NS3pro from three independent experiments were quantitated using ImageJ after normalization to β-actin. Data are presented as percent cleavage relative to NS2B/3pro WT ± SD. ns, not significant; ****p < 0.0001.
(E) The modeled structure of the complex of the LGTV NS3pro TBM2 (colored in magenta) with the C-terminal MATH domain of TRAF6 (colored in cyan). Dashed lines indicate dual hydrogen bonds between the omega oxygen atom of E117 of NS3 and the backbone amide N-H groups of L457 and A458 of TRAF6.
See also Figure S3.
After performing coAP assays with LGTV NS2B/3pro mutants, we noticed that constructs with an E117A mutation had reduced levels of the cleaved, mature form of NS2B/3pro (Figure 3A). The disappearance of NS3pro could be due to a structural change in protein stability, or a functional change in protease activity leading to decreased autoproteolytic cleavage. To confirm that the lower-molecular-weight protein was produced by autocatalytic cleavage, we inactivated the protease by an additional S138A mutation, which resulted in a total loss of the lower molecular weight band (Figure 3C). Quantification of immunoblots by densitometry showed that the E117A mutation reduced mature NS3pro accumulation by 82.5% compared with the WT or D19A proteins (Figure 3D).
To further assess the TRAF6-NS3pro interaction at TBM2, we modeled the interaction of LGTV NS3pro and TRAF6 in silico. The crystal structures of TRAF6 and substrate ligands have been solved for several complexes and demonstrate a specific pattern of hydrogen bonding between the MATH domain of TRAF6 and the TBM of an interacting protein (Shi et al., 2015, Ye et al., 2002). Indeed, it was from these structures and through mutational analysis that the canonical TBM was determined. We set out to use these data to evaluate the structural interaction of LGTV NS3pro and TRAF6 through TBM2. Unfortunately, no crystal structure exists for any TBFV NS3. So, we began by first generating an LGTV NS3pro model using homology modeling with Murray Valley encephalitis virus NS3 crystal structure as a template (Assenberg et al., 2009) (Figure S3A). TBM2 of LGTV NS3pro was found to form a beta strand that is situated on the surface of the protein (Figure S3B). Next, we modeled the structure of the complex of the TBM2 of LGTV NS3pro with the MATH domain of TRAF6 (Figure 3E). The negatively charged omega oxygen atom of E117 of LGTV NS3 formed dual hydrogen bonds with the backbone amide N-H groups of L457 and A458 of TRAF6 (Figure 3E). The intermolecular interaction energies between E117 of LGTV NS3pro and their interacting protein partners in TRAF6 were calculated at the MP2/6-311++G** level using the supermolecular approach with the Gaussian 09 package (Frisch et al., 2009) as described previously (Mao et al., 2003). The resulting interaction energies in both the gas phase (ΔEgas) and the solution phase (ΔEsolution) are listed in Table 1. For comparison, the calculated intermolecular interaction energies between the mutated A117 residue of LGTV NS3 and their interacting protein partners are also listed. In the NS3pro_E117A mutant, the dual hydrogen bonds are lost, which results in a binding energy loss of 3.44 kcal/mol in the solution phase. Overall, both in silico and biological data support the conclusion that an E117A mutation in NS3pro interferes with TRAF6 binding. The unexpected result of the E117A mutation on the abundance of mature NS3pro warrants further studies into the potential effect(s) of TRAF6 on NS3pro structure and protease function.
Table 1.
Intermolecular Interaction Energies between E117 or A117 of LGTV NS3pro and the Backbone Amide N-H Groups of L457 and A458 of TRAF6
| Intermolecular Pair | ΔEgas (kcal/mol) | ΔEsolution (kcal/mol) |
|---|---|---|
| E117 of NS3 --- L457, A458 of TRAF6 | −19.23 | −3.60 |
| A117 of NS3 --- L457, A458 of TRAF6 | −0.09 | −0.16 |
ΔEgas and ΔEsolution represent intermolecular interaction energy in vacuum and in aqueous solution, respectively. Intermolecular interaction energies were calculated with the supermolecular approach at the MP2/6-311++G** level.
See also Figure S3.
An LGTV Mutant Virus Containing the E117A Mutation Exhibits a Small Plaque Phenotype and Is Attenuated In Vitro
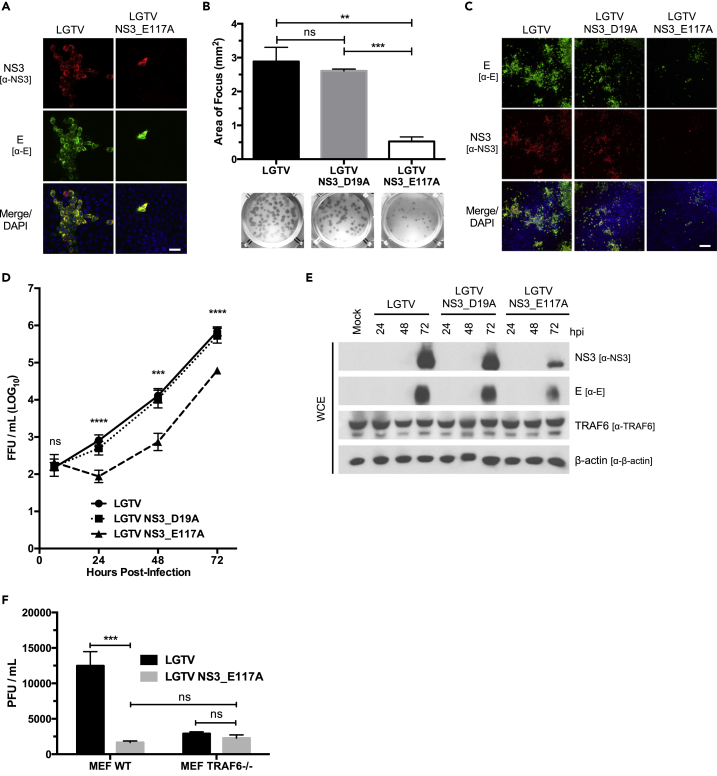
To test the impact of an E117A mutation on the ability of LGTV to replicate, we created the mutation in the LGTV infectious clone. The plaque-forming ability of the LGTV NS3_E117A mutant was noticeably reduced compared with that of the LGTV following transfection of equal amounts of in vitro transcribed mRNA (Figure 4A). Also, recovered LGTV NS3_E117A foci were reduced by 81.8% compared with recovered WT and NS3_D19A LGTV (Figure 4B). Consistently, we observed reduced replication of LGTV NS3_E117A in HEK293 cells compared with LGTV and LGTV NS3_D19A by immunofluorescent microscopy, immunofocus assay, and immunoblotting (Figures 4C–4E). There was up to a 17.4-fold reduction in the release of infectious virions from LGTV NS3_E117A-infected cells compared with cells infected with LGTV or LGTV NS3_D19A (Figure 3D). To test if LGTV NS3_E117A attenuation is due to an impaired ability to co-opt TRAF6 during replication, we infected WT and TRAF6−/− MEFs with LGTV NS3_E117A and LGTV. Similar to observations made with HEK293 cells, replication of LGTV NS3_E117A was reduced by 7.8-fold compared with LGTV in WT MEFs (Figure 4F). Alternatively, the attenuation of LGTV NS3_E117A was lost in TRAF6−/− MEFs; there was no statistical difference between titers from LGTV NS3_E117A and LGTV-infected TRAF6−/− MEFs (Figure 4F). Taken together, these data suggest that an E117A mutation in NS3 impairs LGTV replication and that this attenuation is dependent on TRAF6.
Figure 4.
LGTV NS3 E117A Mutant Virus Is Attenuated In Vitro
(A) Representative images of confocal microscopy of HEK293 cells transfected with either LGTV or LGTV_E117A in vitro-transcribed genomic RNA. Cells were processed at 72 h post-transfection and immunostained with anti-NS3 (red) and anti-E (green) antibodies. Nuclei were stained with DAPI (blue); scale bar (white solid line), 50 μM.
(B) Measurement of viral foci in Vero cells infected with recovered LGTV, LGTV NS3_D19A, or LGTV NS3_E117A. The area (mm2) of individual foci formed by each virus was calculated using ImageJ. Representative foci of each virus are presented below the column graph. Data are average of three independent experiments performed in triplicate and plotted as mean ± SEM. ns, not significant; **p < 0.01; ***p < 0.001.
(C) Representative images of confocal microscopy of HEK293 cells infected with LGTV, LGTV NS3_D19A, or LGTV NS3_E117A. HEK293 cells were infected at an MOI of 0.1 and were processed at 24 hpi. Cells were immunostained with anti-E (green) and anti-NS3 (red) antibodies. Nuclei were stained with DAPI (blue); scale bar (white solid line), 200 μM.
(D) Titration of infectious particles in the supernatant from HEK293 cells infected with LGTV, LGTV NS3_D19A, or LGTV NS3_E117A (MOI of 0.1) for indicated times (h). Viral titers were determined by immunofocus assay. Data are representative of three or more independent experiments performed in triplicate and plotted as mean ± SEM. ns, not significant; ***p < 0.001; ****p < 0.0001.
(E) Immunoblot analysis of whole-cell extracts from HEK293 cells infected with LGTV, LGTV NS3_D19A, or LGTV NS3_E117A (MOI 0.1) for indicated times (h). Blots were probed with antibodies to LGTV NS3, LGTV E, TRAF6, and β-actin. Results representative of three or more independent experiments.
(F) Titration of infectious particles in the supernatant from WT or TRAF6−/− MEFs infected with LGTV or LGTV NS3_E117A (MOI of 0.1) at 24 hpi. Viral titers were determined by plaque assay. Data are representative of three independent experiments performed in triplicate and plotted as mean ± SEM. ns, not significant; ***p < 0.001.
Discussion
In this study, we provide evidence that TRAF6 plays an unexpected proviral role in TBFV replication. We found that TRAF6 interacts with TBFV NS3pro in a sequence-dependent manner and that targeted inhibition of the complex formation leads to virus attenuation.
We have identified a non-canonical TBM that appears to be conserved among closely related TBFVs and is not present in MBFVs (Figure 1A). A physical interaction between flavivirus NS3 and TRAF6 has not been previously reported. It has been shown that NS3 from classical swine fever virus (CSFV), a pestivirus, binds and degrades TRAF6 to prevent activation of NF-κB signaling pathways during infection (Lv et al., 2017). Although CSFV is a member of the Flaviviridae, it differs from flaviviruses in several ways. (1) The genome of CSFV is composed of a different number and set of genes than flaviviruses (Tautz et al., 2015); (2) CSFV NS3 requires NS4A, not NS2B, as an essential cofactor of protease activity (Tautz et al., 2000); (3) CSFV is not transmitted by arthropod vectors; and (4) CSFV does not cause disease in humans (Blome et al., 2017).
TRAF6 is conventionally associated with host innate immune protection because of its pivotal role in IL-1/TLR and RIG-I signaling (Inoue et al., 2007, Yoshida et al., 2008). It was previously shown that prototypical RNA viruses replicate better and are more cytopathic in TRAF6-deficient cells because of a mitigated IFN response (Konno et al., 2009, Yoshida et al., 2008). Studies examining the role of TRAF6 during flavivirus infection have been limited to MBFVs. DENV and Japanese encephalitis virus (JEV) have been found to up-regulate cellular miR-146a to decrease expression and signaling of TRAF6; suppression of TRAF6 leads to decreased NF-κB activation, Jak-STAT signaling, and IFN production, and in the case of DENV, also inhibited virus-induced autophagy (Pu et al., 2017, Sharma and Verma, 2015, Wu et al., 2013). The effect of TBFV NS3 binding to TRAF6 is an intriguing and unexplored question for follow-up studies, especially if TBFV NS3 binding to TRAF6 inhibits the canonical antiviral signaling role for TRAF6, while using TRAF6 to benefit virus replication.
It was unexpected when we observed better replication of TBFVs in WT MEFs compared with TRAF6−/− MEFs (Figures 2A–2C, 2E, and 2F). Indeed, because our findings differ from precedent, we took multiple approaches to validate the proviral effects of TRAF6 in TBFV infection. (1) We used two independently generated TRAF6 knockout cell lines: embryonically derived TRAF6−/− MEFs and CRISPR/Cas9 generated TRAF6−/− HEK293 cells (Figures 2A–2C and S2B). (2) We functionally depleted WT cells of TRAF6 by overexpressing an enzymatically inactive TRAF6 mutant (Figures S2D and S2E). (3) We treated WT cells with a TRAF6 inhibitor peptide (Figure S2C). (4) We mutated TBM2 within a TBFV genome (Figure 4). Under all these conditions, we consistently saw TBFV attenuation. Furthermore, the phenotype could be rescued by the expression of TRAF6 (Figure 2D).
When we assessed MBFV replication in our TRAF6−/− MEFs, using WNV and KUNV, we observed a reciprocal phenotype than what was seen with TBFVs. MBFV replication was enhanced in the absence of TRAF6, similar to previous DENV and JEV studies (Figures 2G–2J). As predicted from our sequence analysis (Figure 1A), KUNV NS3 did not interact with TRAF6 during infection (Figure 1D). Furthermore, we saw better LGTV replication in WT MEFs compared with TRAF6−/− MEFs despite higher IFN-β production (Figure 2B). Taken all together, our findings demonstrate that the proviral function of TRAF6 is specific to TBFVs.
An E117A mutation in TBM2 of NS3pro revealed that interaction with TRAF6 is needed for the accumulation of the mature processed form of the protease. Note that TRAF6-NS3pro binding is not essential for protein function. This allowed us to design and recover mutant LGTV NS3_E117A, which was attenuated to similar levels seen with WT LGTV in TRAF6-deficient cells (Figures 4D and S2B). We failed to precipitate TRAF6 from LGTV NS3_E117A-infected cells (data not shown); these experiments were confounded by reduced viral protein levels associated with the LGTV NS3_E117A (Figure 4E). LGTV NS3_E117A attenuation is most likely a result of decreased interaction with TRAF6 because the difference between mutant and WT virus is lost in TRAF6−/− MEFs (Figure 4F). In contrast, overexpression of TRAF6 did not enhance virus production, suggesting that the endogenous level of TRAF6 is sufficient for optimal virus replication (Figures 2D and S2D), albeit viral protein levels were modestly elevated (Figure S2E).
We are currently evaluating how TRAF6 benefits TBFV replication, looking specifically for structural changes to NS3pro when complexed with TRAF6 and changes to overall protease activity. TRAF6 is an E3 ubiquitin ligase and performs its normal physiological function by ubiquitinating itself and substrate proteins (Walsh et al., 2015). TBFV NS3pro may be modified by TRAF6-mediated non-degradative ubiquitination; this hypothesis is supported by our observation of LGTV attenuation in cells overexpressing an enzymatically inactive TRAF6 (Figures S2D and S2E). It has previously been shown that K27-linked ubiquitination of DENV NS3 facilitates NS2B recruitment and NS2B/3pro complex formation; the ligase has yet to be identified (Liu et al., 2017).
The flavivirus NS3 is an attractive therapeutic target because it is essential for virus replication and has well-characterized enzymatic functions (Leyssen et al., 2000). Substantial effort and resources have been put into the design of inhibitors of NS3pro activity, but there has only been limited success and no compound has reached the clinical trial stage. It has been challenging to develop NS3pro inhibitors because the active site is shallow and highly charged (Luo et al., 2015). We propose that the TRAF6-NS3pro interaction may serve as an antiviral target. Specific disruption of the TRAF6-NS3pro complex, as demonstrated by our use of a TRAF6 inhibitor peptide in this study (Figure S2C), could indirectly inhibit NS3 function and be accomplished without having to overcome the multiple obstacles that are associated with development of compounds that directly inactivate NS3. Likewise, we speculate that the attenuation and small plaque phenotype of the E117A mutant virus in vitro may result in impaired in vivo pathogenesis as reported for other mutant flaviviruses, including LGTV (Blaney et al., 2002, Eastman and Blair, 1985, Hanley et al., 2002, Rumyantsev et al., 2006).
Overall, our study demonstrates that specific flavivirus therapeutic targets may be identified by first understanding how viruses and host cell proteins interact. By identifying a specific interaction between TRAF6 and TBFV NS3pro, we revealed a mechanism used by viruses to co-opt host cell antiviral molecules for viral gain.
Limitations of the Study
There are still many issues to be investigated regarding the role of TRAF6 in TBEV infection, including (1) the exact mechanism as to how a TRAF6-NS3pro interaction affects mature NS3pro accumulation and ultimately virus replication; TRAF6, a E3 ubiquitin ligase, may ubiquitinate NS3 directly or act as a scaffold protein bring together other host or viral proteins; and (2) the importance of TRAF6 in TBFV pathogenesis in primary cells, including dendritic cells and neurons, and within in vivo models. (3) Our investigations only examine the proviral role of TRAF6 in TBFV infection; however, TBFV antagonism of the antiviral activity of TRAF6 likely contributes to the overall success of TBFVs to cause disease.
Methods
All methods can be found in the accompanying Transparent Methods supplemental file.
Acknowledgments
We thank Drs. Malathi Krishnamurthy, R. Mark Wooten, and Stanislaw Stepkowski for their guidance and intellectual contributions in helping shape the overarching direction of research. We also thank Dr. Jyl S. Matson for the use of her plate reader and Dr. Alexander G. Pletnev for providing the LGTV infectious clone. We are pleased to acknowledge the Ohio Supercomputer Center for a generous allocation of supercomputer time. This work was supported in part by the Intramural Research Program of the National Institutes of Health and National Institute of Allergy and Infectious Diseases: ZIA-AI001125 to S.M.B. and 1K22-AI099020 to R.T.T.
Author Contributions
Conceptualization, B.H.Y., S.M.B., and R.T.T.; Methodology, B.H.Y., S.A., X. H., and R.T.T.; Formal Analysis, B.H.Y., S.A., X. H., and R.T.T.; Investigation, B.H.Y., T.G.B., K.L.M., A.O.I., K.J.L., J.B.P., S.A., X.H., and R.T.T.; Writing – Original Draft, B.H.Y. and R.T.T.; Writing – Review & Editing, B.H.Y., S.M.B., S.C., X.H., and R.T.T.; Funding Acquisition, S.M.B. and R.T.T.; Resources, S.C., S.M.B., X.H., and R.T.T.; Visualization, B.H.Y. and R.T.T.; Supervision, R.T.T.
Declaration of Interests
The authors declare no competing interests.
Published: May 31, 2019
Footnotes
Supplemental Information can be found online at https://doi.org/10.1016/j.isci.2019.05.010.
Supplemental Information
References
- Assenberg R., Mastrangelo E., Walter T.S., Verma A., Milani M., Owens R.J., Stuart D.I., Grimes J.M., Mancini E.J. Crystal structure of a novel conformational state of the flavivirus NS3 protein: implications for polyprotein processing and viral replication. J. Virol. 2009;83:12895–12906. doi: 10.1128/JVI.00942-09. [DOI] [PMC free article] [PubMed] [Google Scholar]; Assenberg, R., Mastrangelo, E., Walter, T.S., Verma, A., Milani, M., Owens, R.J., Stuart, D.I., Grimes, J.M., Mancini, E.J. (2009). Crystal structure of a novel conformational state of the flavivirus NS3 protein: implications for polyprotein processing and viral replication. J. Virol. 83, 12895-12906. [DOI] [PMC free article] [PubMed]
- Bera A.K., Kuhn R.J., Smith J.L. Functional characterization of cis and transActivity of the flavivirus NS2B-NS3 protease. J. Biol. Chem. 2007;282:12883–12892. doi: 10.1074/jbc.M611318200. [DOI] [PubMed] [Google Scholar]; Bera, A.K., Kuhn, R.J., Smith, J.L. (2007). Functional characterization of cis and transActivity of the flavivirus NS2B-NS3 protease. J. Biol. Chem. 282, 12883-12892. [DOI] [PubMed]
- Best S.M., Morris K.L., Shannon J.G., Robertson S.J., Mitzel D.N., Park G.S., Boer E., Wolfinbarger J.B., Bloom M.E. Inhibition of interferon-stimulated JAK-STAT signaling by a tick-borne flavivirus and identification of NS5 as an interferon antagonist. J. Virol. 2005;79:12828–12839. doi: 10.1128/JVI.79.20.12828-12839.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]; Best, S.M., Morris, K.L., Shannon, J.G., Robertson, S.J., Mitzel, D.N., Park, G.S., Boer, E., Wolfinbarger, J.B., Bloom, M.E. (2005). Inhibition of interferon-stimulated JAK-STAT signaling by a tick-borne flavivirus and identification of NS5 as an interferon antagonist. J. Virol. 79, 12828-12839. [DOI] [PMC free article] [PubMed]
- Blaney J.E., Jr., Johnson D.H., Manipon G.G., Firestone C.-Y., Hanson C.T., Murphy B.R., Whitehead S.S. Genetic basis of attenuation of dengue virus type 4 small plaque mutants with restricted replication in suckling mice and in SCID mice transplanted with human liver cells. Virology. 2002;300:125–139. doi: 10.1006/viro.2002.1528. [DOI] [PubMed] [Google Scholar]; Blaney, J.E., Jr., Johnson, D.H., Manipon, G.G., Firestone, C.-Y., Hanson, C.T., Murphy, B.R., Whitehead, S.S. (2002). Genetic basis of attenuation of dengue virus type 4 small plaque mutants with restricted replication in suckling mice and in SCID mice transplanted with human liver cells. Virology 300, 125-139. [DOI] [PubMed]
- Blome S., Staubach C., Henke J., Carlson J., Beer M. Classical swine fever—an updated review. Viruses. 2017;9:86. doi: 10.3390/v9040086. [DOI] [PMC free article] [PubMed] [Google Scholar]; Blome, S., Staubach, C., Henke, J., Carlson, J., Beer, M. (2017). Classical swine fever-an updated review. Viruses 9, 86-25. [DOI] [PMC free article] [PubMed]
- Brecher M., Li Z., Liu B., Zhang J., Koetzner C.A., Alifarag A., Jones S.A., Lin Q., Kramer L.D., Li H. A conformational switch high-throughput screening assay and allosteric inhibition of the flavivirus NS2B-NS3 protease. PLoS Pathog. 2017;13:e1006411. doi: 10.1371/journal.ppat.1006411. [DOI] [PMC free article] [PubMed] [Google Scholar]; Brecher, M., Li, Z., Liu, B., Zhang, J., Koetzner, C.A., Alifarag, A., Jones, S.A., Lin, Q., Kramer, L.D., Li, H. (2017). A conformational switch high-throughput screening assay and allosteric inhibition of the flavivirus NS2B-NS3 protease. PLoS Pathog. 13, e1006411. [DOI] [PMC free article] [PubMed]
- Chambers T.J., Droll D.A., Tang Y., Liang Y., Ganesh V.K., Murthy K., Nickells M. Yellow fever virus NS2B-NS3 protease: characterization of charged-to-alanine mutant and revertant viruses and analysis of polyprotein-cleavage activities. J. Gen. Virol. 2005;86:1403–1413. doi: 10.1099/vir.0.80427-0. [DOI] [PubMed] [Google Scholar]; Chambers, T.J., Droll, D.A., Tang, Y., Liang, Y., Ganesh, V.K., Murthy, K., Nickells, M.., (2005). Yellow fever virus NS2B-NS3 protease: characterization of charged-to-alanine mutant and revertant viruses and analysis of polyprotein-cleavage activities. J. Gen. Virol. 86, 1403-1413. [DOI] [PubMed]
- Chung J.Y., Lu M., Yin Q., Lin S.-C., Wu H. Molecular basis for the unique specificity of TRAF6. Adv. Exp. Med. Biol. 2007;597:122–130. doi: 10.1007/978-0-387-70630-6_10. [DOI] [PubMed] [Google Scholar]; Chung, J.Y., Lu, M., Yin, Q., Lin, S.-C., Wu, H. (2007). Molecular basis for the unique specificity of TRAF6. Adv. Exp. Med. Biol. 597, 122-130. [DOI] [PubMed]
- Dinkel H., Van Roey K., Michael S., Davey N.E., Weatheritt R.J., Born D., Speck T., Kruger D., Grebnev G., Kuban M. The eukaryotic linear motif resource ELM: 10 years and counting. Nucleic Acids Res. 2013;42:D259–D266. doi: 10.1093/nar/gkt1047. [DOI] [PMC free article] [PubMed] [Google Scholar]; Dinkel, H., Van Roey, K., Michael, S., Davey, N.E., Weatheritt, R.J., Born, D., Speck, T., Kruger, D., Grebnev, G., Kuban, M., et al. (2013). The eukaryotic linear motif resource ELM: 10 years and counting. Nucleic Acids Res. 42, D259-D266. [DOI] [PMC free article] [PubMed]
- Eastman P.S., Blair C.D. Temperature-sensitive mutants of Japanese encephalitis virus. J. Virol. 1985;55:611–616. doi: 10.1128/jvi.55.3.611-616.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]; Eastman, P.S., Blair, C.D. (1985). Temperature-sensitive mutants of Japanese encephalitis virus. J. Virol. 55, 611-616. [DOI] [PMC free article] [PubMed]
- Erbel P., Schiering N., D'Arcy A., Renatus M., Kroemer M., Lim S.P., Yin Z., Keller T.H., Vasudevan S.G., Hommel U. Structural basis for the activation of flaviviral NS3 proteases from dengue and West Nile virus. Nat. Struct. Mol. Biol. 2006;13:372–373. doi: 10.1038/nsmb1073. [DOI] [PubMed] [Google Scholar]; Erbel, P., Schiering, N., D'Arcy, A., Renatus, M., Kroemer, M., Lim, S.P., Yin, Z., Keller, T.H., Vasudevan, S.G., Hommel, U. (2006). Structural basis for the activation of flaviviral NS3 proteases from dengue and West Nile virus. Nat. Struct. Mol. Biol. 13, 372-373. [DOI] [PubMed]
- Falgout B., Pethel M., Zhang Y., Lai C.-J. Both nonstructural proteins NS2B and NS3 are required for the proteolytic processing of dengue virus nonstructural proteins. J. Virol. 1991;65:2467–2475. doi: 10.1128/jvi.65.5.2467-2475.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]; Falgout, B., Pethel, M., Zhang, Y., Lai, C.-J. (1991). Both nonstructural proteins NS2B and NS3 are required for the proteolytic processing of dengue virus nonstructural proteins. J. Virol. 65, 2467-2475. [DOI] [PMC free article] [PubMed]
- Frisch, M.J., Trucks, G., Schlegel, H., Scuseria, G.E., Robb, M.A., Cheeseman, J.R., Scalmani, G., Barone, G.A., Petersson, G.A., Nakatsuji, H., et al., Gaussian 09, Revision A.02, Gaussian Inc., 2009.
- Geiss B.J., Stahla H., Hannah A.M., Gari H.H., Keenan S.M. Focus on flaviviruses: current and future drug targets. Future Med. Chem. 2009;1:327–344. doi: 10.4155/fmc.09.27. [DOI] [PMC free article] [PubMed] [Google Scholar]; Geiss, B.J., Stahla, H., Hannah, A.M., Gari, H.H., Keenan, S.M. (2009). Focus on flaviviruses: current and future drug targets. Future Med. Chem. 1, 327-344. [DOI] [PMC free article] [PubMed]
- Gentry J.J., Rutkoski N.J., Burke T.L., Carter B.D. A functional interaction between the p75 neurotrophin receptor interacting factors, TRAF6 and NRIF. J. Biol. Chem. 2004;279:16646–16656. doi: 10.1074/jbc.M309209200. [DOI] [PubMed] [Google Scholar]; Gentry, J.J., Rutkoski, N.J., Burke, T.L., Carter, B.D. (2004). A functional interaction between the p75 neurotrophin receptor interacting factors, TRAF6 and NRIF. J. Biol. Chem. 279, 16646-16656. [DOI] [PubMed]
- Hanley K.A., Lee J.J., Blaney J.E., Murphy B.R., Whitehead S.S. Paired charge-to-alanine mutagenesis of dengue virus type 4 NS5 generates mutants with temperature-sensitive, host range, and mouse attenuation phenotypes. J. Virol. 2002;76:525–531. doi: 10.1128/JVI.76.2.525-531.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]; Hanley, K.A., Lee, J.J., Blaney, J.E., Murphy, B.R., Whitehead, S.S. (2002). Paired charge-to-alanine mutagenesis of dengue virus type 4 NS5 generates mutants with temperature-sensitive, host range, and mouse attenuation phenotypes. J. Virol. 76, 525-531. [DOI] [PMC free article] [PubMed]
- Hermance M.E., Thangamani S. Powassan virus: an emerging arbovirus of public health concern in North America. Vector-Borne Zoonotic Dis. 2017;17:453–462. doi: 10.1089/vbz.2017.2110. [DOI] [PMC free article] [PubMed] [Google Scholar]; Hermance, M.E., Thangamani, S. (2017). Powassan virus: an emerging arbovirus of public health concern in North America. Vector-Borne Zoonotic Dis. 17, 453-462. [DOI] [PMC free article] [PubMed]
- Hou W., Jin Y.-H., Kang H.S., Kim B.S. Interleukin-6 (IL-6) and IL-17 synergistically promote viral persistence by inhibiting cellular apoptosis and cytotoxic T cell function. J. Virol. 2014;88:8479–8489. doi: 10.1128/JVI.00724-14. [DOI] [PMC free article] [PubMed] [Google Scholar]; Hou, W., Jin, Y.-H., Kang, H.S., Kim, B.S. (2014). Interleukin-6 (IL-6) and IL-17 synergistically promote viral persistence by inhibiting cellular apoptosis and cytotoxic T cell function. J. Virol. 88, 8479-8489. [DOI] [PMC free article] [PubMed]
- Inoue J.-I., Gohda J., Akiyama T. Characteristics and biological functions of TRAF6. Adv. Exp. Med. Biol. 2007;597:72–79. doi: 10.1007/978-0-387-70630-6_6. [DOI] [PubMed] [Google Scholar]; Inoue, J.-I., Gohda, J., Akiyama, T. (2007). Characteristics and biological functions of TRAF6. Adv. Exp. Med. Biol. 597, 72-79. [DOI] [PubMed]
- Konno H., Yamamoto T., Yamazaki K., Gohda J., Akiyama T., Semba K., Goto H., Kato A., Yujiri T., Imai T. TRAF6 establishes innate immune responses by activating NF-kappaB and IRF7 upon sensing cytosolic viral RNA and DNA. PLoS One. 2009;4:e5674. doi: 10.1371/journal.pone.0005674. [DOI] [PMC free article] [PubMed] [Google Scholar]; Konno, H., Yamamoto, T., Yamazaki, K., Gohda, J., Akiyama, T., Semba, K., Goto, H., Kato, A., Yujiri, T., Imai, T., et al. (2009). TRAF6 establishes innate immune responses by activating NF-kappaB and IRF7 upon sensing cytosolic viral RNA and DNA. PLoS One 4, e5674. [DOI] [PMC free article] [PubMed]
- Leyssen P., Clercq E., Neyts J. Perspectives for the treatment of infections with Flaviviridae. Clin. Microbiol. Rev. 2000;13:67–82. doi: 10.1128/cmr.13.1.67-82.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]; Leyssen, P., Clercq, E., Neyts, J. (2000). Perspectives for the treatment of infections with Flaviviridae. Clin. Microbiol. Rev. 13, 67-82. [DOI] [PMC free article] [PubMed]
- Li K., Phoo W.W., Luo D. Functional interplay among the flavivirus NS3 protease, helicase, and cofactors. Virol. Sin. 2014;29:74–85. doi: 10.1007/s12250-014-3438-6. [DOI] [PMC free article] [PubMed] [Google Scholar]; Li, K., Phoo, W.W., Luo, D. (2014). Functional interplay among the flavivirus NS3 protease, helicase, and cofactors. Virol. Sin. 29, 74-85. [DOI] [PMC free article] [PubMed]
- Liu H., Zhang L., Sun J., Chen W., Li S., Wang Q., Yu H., Xia Z., Jin X., Wang C. Endoplasmic reticulum protein SCAP inhibits dengue virus NS2B3 protease by suppressing its K27-linked polyubiquitylation. J. Virol. 2017;91:1–17. doi: 10.1128/JVI.02234-16. [DOI] [PMC free article] [PubMed] [Google Scholar]; Liu, H., Zhang, L., Sun, J., Chen, W., Li, S., Wang, Q., Yu, H., Xia, Z., Jin, X., Wang, C. (2017). Endoplasmic reticulum protein SCAP inhibits dengue virus NS2B3 protease by suppressing its K27-linked polyubiquitylation. J. Virol. 91, 1-17. [DOI] [PMC free article] [PubMed]
- Luo D., Vasudevan S., Lescar J. The flavivirus NS2B–NS3 protease–helicase as a target for antiviral drug development. Antiviral Res. 2015;118:148–158. doi: 10.1016/j.antiviral.2015.03.014. [DOI] [PubMed] [Google Scholar]; Luo, D., Vasudevan, S., Lescar, J. (2015). The flavivirus NS2B-NS3 protease-helicase as a target for antiviral drug development. Antiviral Res. 118, 148-158. [DOI] [PubMed]
- Lv H., Dong W., Cao Z., Li X., Wang J., Qian G., Lv Q., Wang C., Guo K., Zhang Y. TRAF6 is a novel NS3-interacting protein that inhibits classical swine fever virus replication. Sci. Rep. 2017;7:6737. doi: 10.1038/s41598-017-06934-1. [DOI] [PMC free article] [PubMed] [Google Scholar]; Lv, H., Dong, W., Cao, Z., Li, X., Wang, J., Qian, G., Lv, Q., Wang, C., Guo, K., Zhang, Y. (2017). TRAF6 is a novel NS3-interacting protein that inhibits classical swine fever virus replication. Sci. Rep. 7, 6737. [DOI] [PMC free article] [PubMed]
- Mao L.S., Wang Y.L., Liu Y.M., Hu X.C. Multiple intermolecular interaction modes of positively charged residues with adenine in ATP-binding proteins. J. Am. Chem. Soc. 2003;125:14216–14217. doi: 10.1021/ja036096p. [DOI] [PubMed] [Google Scholar]; Mao, L.S., Wang, Y.L., Liu, Y.M., Hu, X.C. (2003). Multiple intermolecular interaction modes of positively charged residues with adenine in ATP-binding proteins. J. Am. Chem. Soc. 125, 14216-14217. [DOI] [PubMed]
- Meads M.B., Li Z.W., Dalton W.S. A novel TNF receptor-associated factor 6 binding domain mediates NF- B signaling by the common cytokine receptor subunit. J. Immonol. 2010;185:1606–1615. doi: 10.4049/jimmunol.0902026. [DOI] [PMC free article] [PubMed] [Google Scholar]; Meads, M.B., Li, Z.W., Dalton, W.S. (2010). A novel TNF receptor-associated factor 6 binding domain mediates NF- B signaling by the common cytokine receptor subunit J. Immonol. 185, 1606-1615. doi:10.4049/jimmunol.0902026. [DOI] [PMC free article] [PubMed]
- Megas C., Hatzivassiliou E.G., Yin Q., Marinopoulou E., Hadweh P., Vignali D.A.A., Mosialos G. Mutational analysis of TRAF6 reveals a conserved functional role of the RING dimerization interface and a potentially necessary but insufficient role of RING-dependent TRAF6 polyubiquitination towards NF-κB activation. Cell Signal. 2011;23:772–777. doi: 10.1016/j.cellsig.2010.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]; Megas, C., Hatzivassiliou, E.G., Yin, Q., Marinopoulou, E., Hadweh, P., Vignali, D.A.A., Mosialos, G. (2011). Mutational analysis of TRAF6 reveals a conserved functional role of the RING dimerization interface and a potentially necessary but insufficient role of RING-dependent TRAF6 polyubiquitination towards NF-κB activation. Cell Signal. 23, 772-777. [DOI] [PMC free article] [PubMed]
- Ning S., Campos A.D., Darnay B.G., Bentz G.L., Pagano J.S. TRAF6 and the three C-terminal lysine sites on IRF7 are required for its ubiquitination-mediated activation by the tumor necrosis factor receptor family member latent membrane protein 1. Mol. Cell. Biol. 2008;28:6536–6546. doi: 10.1128/MCB.00785-08. [DOI] [PMC free article] [PubMed] [Google Scholar]; Ning, S., Campos, A.D., Darnay, B.G., Bentz, G.L., Pagano, J.S. (2008). TRAF6 and the three C-terminal lysine sites on IRF7 are required for its ubiquitination-mediated activation by the tumor necrosis factor receptor family member latent membrane protein 1. Mol. Cell. Biol. 28, 6536-6546. [DOI] [PMC free article] [PubMed]
- Noels H., van Loo G., Hagens S., Broeckx V., Beyaert R., Marynen P., Baens M. A Novel TRAF6 binding site in MALT1 defines distinct mechanisms of NF-κB activation by API2·MALT1 fusions. J. Biol. Chem. 2007;282:10180–10189. doi: 10.1074/jbc.M611038200. [DOI] [PubMed] [Google Scholar]; Noels, H., van Loo, G., Hagens, S., Broeckx, V., Beyaert, R., Marynen, P., Baens, M. (2007). A Novel TRAF6 binding site in MALT1 defines distinct mechanisms of NF-κB activation by API2·MALT1 fusions. J. Biol. Chem. 282, 10180-10189. [DOI] [PubMed]
- Palanisamy N., Akaberi D., Lennerstrand J. Protein backbone flexibility pattern is evolutionarily conserved in the Flaviviridae family: a case of NS3 protease in Flavivirus and Hepacivirus. Mol. Phylogenet. Evol. 2018;118:58–63. doi: 10.1016/j.ympev.2017.09.015. [DOI] [PubMed] [Google Scholar]; Palanisamy, N., Akaberi, D., Lennerstrand, J. (2018). Protein backbone flexibility pattern is evolutionarily conserved in the Flaviviridae family: a case of NS3 protease in Flavivirus and Hepacivirus. Mol. Phylogenet. Evol. 118, 58-63. [DOI] [PubMed]
- Pastorino B.A.M., Peyrefitte C.N., Grandadam M., Thill M.C.E., Tolou H.J., Bessaud M. Mutagenesis analysis of the NS2B determinants of the Alkhurma virus NS2B-NS3 protease activation. J. Gen. Virol. 2006;87:3279–3283. doi: 10.1099/vir.0.82088-0. [DOI] [PubMed] [Google Scholar]; Pastorino, B.A.M., Peyrefitte, C.N., Grandadam, M., Thill, M.C.E., Tolou, H.J., Bessaud, M. (2006). Mutagenesis analysis of the NS2B determinants of the Alkhurma virus NS2B-NS3 protease activation. J. Gen. Virol. 87, 3279-3283. [DOI] [PubMed]
- Pu J., Wu S., Xie H., Li Y., Yang Z., Wu X., Huang X. miR-146a Inhibits dengue-virus-induced autophagy by targeting TRAF6. Arch. Virol. 2017;162:3645–3659. doi: 10.1007/s00705-017-3516-9. [DOI] [PMC free article] [PubMed] [Google Scholar]; Pu, J., Wu, S., Xie, H., Li, Y., Yang, Z., Wu, X., Huang, X. (2017). miR-146a Inhibits dengue-virus-induced autophagy by targeting TRAF6. Arch. Virol. 162, 3645-3659. [DOI] [PMC free article] [PubMed]
- Rumyantsev A.A., Murphy B.R., Pletnev A.G. A tick-borne langat virus mutant that is temperature sensitive and host range restricted in neuroblastoma cells and lacks neuroinvasiveness for immunodeficient mice. J. Virol. 2006;80:1427–1439. doi: 10.1128/JVI.80.3.1427-1439.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]; Rumyantsev, A.A., Murphy, B.R., Pletnev, A.G. (2006). A tick-borne langat virus mutant that is temperature sensitive and host range restricted in neuroblastoma cells and lacks neuroinvasiveness for immunodeficient mice. J. Virol. 80, 1427-1439. [DOI] [PMC free article] [PubMed]
- Sharma N., Verma R. miR-146a suppresses cellular immune response during Japanese encephalitis virus JaOArS982 strain infection in human microglial cells. J. Neuroinflammation. 2015;12:1–16. doi: 10.1186/s12974-015-0249-0. [DOI] [PMC free article] [PubMed] [Google Scholar]; Sharma, N., Verma, R. (2015). miR-146a suppresses cellular immune response during Japanese encephalitis virus JaOArS982 strain infection in human microglial cells. J. Neuroinflammation 12, 1-16. [DOI] [PMC free article] [PubMed]
- Shi Z., Zhang Z., Zhang Z., Wang Y., Li C., Wang X., He F., Sun L., Jiao S., Shi W., Zhou Z. Structural Insights into mitochondrial antiviral signaling protein (MAVS)-tumor necrosis factor receptor-associated factor 6 (TRAF6) signaling. J. Biol. Chem. 2015;290:26811–26820. doi: 10.1074/jbc.M115.666578. [DOI] [PMC free article] [PubMed] [Google Scholar]; Shi, Z., Zhang, Z., Zhang, Z., Wang, Y., Li, C., Wang, X., He, F., Sun, L., Jiao, S., Shi, W., Zhou, Z. (2015). Structural Insights into mitochondrial antiviral signaling protein (MAVS)-tumor necrosis factor receptor-associated factor 6 (TRAF6) signaling. J. Biol. Chem. 290, 26811-26820. [DOI] [PMC free article] [PubMed]
- Stack J., Hurst T.P., Flannery S.M., Brennan K., Rupp S., Oda S., Khan A.R., Bowie A.G. Poxviral protein A52 stimulates p38 mitogen-activated protein kinase (MAPK) activation by causing tumor necrosis factor receptor-associated factor 6 (TRAF6) self-association leading to transforming growth factor β-activated Kinase 1 (TAK1) Recruitment. J. Biol. Chem. 2013;288:33642–33653. doi: 10.1074/jbc.M113.485490. [DOI] [PMC free article] [PubMed] [Google Scholar]; Stack, J., Hurst, T.P., Flannery, S.M., Brennan, K., Rupp, S., Oda, S., Khan, A.R., Bowie, A.G. (2013). Poxviral protein A52 stimulates p38 mitogen-activated protein kinase (MAPK) activation by causing tumor necrosis factor receptor-associated factor 6 (TRAF6) self-association leading to transforming growth factor β-activated Kinase 1 (TAK1) Recruitment. J. Biol. Chem. 288, 33642-33653. [DOI] [PMC free article] [PubMed]
- Tautz N., Kaiser A., Thiel H.-J. NS3 serine protease of bovine viral diarrhea virus: characterization of active site residues, NS4A cofactor domain, and protease–cofactor interactions. Virology. 2000;273:351–363. doi: 10.1006/viro.2000.0425. [DOI] [PubMed] [Google Scholar]; Tautz, N., Kaiser, A., Thiel, H.-J. (2000). NS3 serine protease of bovine viral diarrhea virus: characterization of active site residues, NS4A cofactor domain, and protease-cofactor interactions. Virology 273, 351-363. [DOI] [PubMed]
- Tautz N., Tews B.A., Meyers G. The molecular biology of pestiviruses. Adv. Virus Res. 2015;93:47–160. doi: 10.1016/bs.aivir.2015.03.002. [DOI] [PubMed] [Google Scholar]; Tautz N., Tews B.A. and Meyers G., The molecular biology of pestiviruses, Adv. Virus Res. 93, 2015, 47–160. [DOI] [PubMed]
- Taylor R.T., Lubick K.J., Robertson S.J., Broughton J.P., Bloom M.E., Bresnahan W.A., Best S.M. TRIM79α, an interferon-stimulated gene product, restricts tick-borne encephalitis virus replication by degrading the viral RNA polymerase. Cell Host Microbe. 2011;10:185–196. doi: 10.1016/j.chom.2011.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]; Taylor, R.T., Lubick, K.J., Robertson, S.J., Broughton, J.P., Bloom, M.E., Bresnahan, W.A., Best, S.M. (2011). TRIM79α, an interferon-stimulated gene product, restricts tick-borne encephalitis virus replication by degrading the viral RNA polymerase. Cell Host Microbe 10, 185-196. [DOI] [PMC free article] [PubMed]
- Verstak B., Stack J., Ve T., Mangan M., Hjerrild K., Jeon J., Stahl R., Latz E., Gay N., Kobe B. The TLR signaling adaptor TRAM interacts with TRAF6 to mediate activation of the inflammatory response by TLR4. J. Leukoc. Biol. 2014;96:427–436. doi: 10.1189/jlb.2A0913-487R. [DOI] [PMC free article] [PubMed] [Google Scholar]; Verstak, B., Stack, J., Ve, T., Mangan, M., Hjerrild, K., Jeon, J., Stahl, R., Latz, E., Gay, N., Kobe, B., et al. (2014). The TLR signaling adaptor TRAM interacts with TRAF6 to mediate activation of the inflammatory response by TLR4. J. Leukoc. Biol. 96, 427-436. [DOI] [PMC free article] [PubMed]
- Walsh M.C., Lee J., Choi Y. Tumor necrosis factor receptor- associated factor 6 (TRAF6) regulation of development, function, and homeostasis of the immune system. Immunol. Rev. 2015;266:72–92. doi: 10.1111/imr.12302. [DOI] [PMC free article] [PubMed] [Google Scholar]; Walsh, M.C., Lee, J., Choi, Y. (2015). Tumor necrosis factor receptor- associated factor 6 (TRAF6) regulation of development, function, and homeostasis of the immune system. Immunol. Rev. 266, 72-92. [DOI] [PMC free article] [PubMed]
- Wu H., Arron J.R. TRAF6, a molecular bridge spanning adaptive immunity, innate immunity and osteoimmunology. BioEssays. 2003;25:1096–1105. doi: 10.1002/bies.10352. [DOI] [PubMed] [Google Scholar]; Wu, H., Arron, J.R. (2003). TRAF6, a molecular bridge spanning adaptive immunity, innate immunity and osteoimmunology. BioEssays 25, 1096-1105. [DOI] [PubMed]
- Wu S., He L., Li Y., Wang T., Feng L., Jiang L., Zhang P., Huang X. miR-146a facilitates replication of dengue virus by dampening interferon induction by targeting TRAF6. J. Infect. 2013;67:329–341. doi: 10.1016/j.jinf.2013.05.003. [DOI] [PubMed] [Google Scholar]; Wu, S., He, L., Li, Y., Wang, T., Feng, L., Jiang, L., Zhang, P., Huang, X. (2013). miR-146a facilitates replication of dengue virus by dampening interferon induction by targeting TRAF6 J. Infect. 67, 329-341. [DOI] [PubMed]
- Ye H., Arron J.R., Lamothe B., Cirilli M., Kobayashi T., Shevde N., Segal D., Dzivenu O., Vologodskaia M., Yim M. Distinct molecular mechanism for initiating TRAF6 signalling. Nature. 2002;418:443–446. doi: 10.1038/nature00888. [DOI] [PubMed] [Google Scholar]; Ye, H., Arron, J.R., Lamothe, B., Cirilli, M., Kobayashi, T., Shevde, N., Segal, D., Dzivenu, O., Vologodskaia, M., Yim, M., et al. (2002). Distinct molecular mechanism for initiating TRAF6 signalling. Nature 418, 443-446. [DOI] [PubMed]
- Yoshida R., Takaesu G., Yoshida H., Okamoto F., Yoshioka T., Choi Y., Akira S., Kawai T., Yoshimura A., Kobayashi T. TRAF6 and MEKK1 play a pivotal role in the RIG-I-like helicase antiviral pathway. J. Biol. Chem. 2008;283:36211–36220. doi: 10.1074/jbc.M806576200. [DOI] [PMC free article] [PubMed] [Google Scholar]; Yoshida, R., Takaesu, G., Yoshida, H., Okamoto, F., Yoshioka, T., Choi, Y., Akira, S., Kawai, T., Yoshimura, A., Kobayashi, T. (2008). TRAF6 and MEKK1 play a pivotal role in the RIG-I-like helicase antiviral pathway. J. Biol. Chem. 283, 36211-36220. [DOI] [PMC free article] [PubMed]
- Yu J., Yun H., Shin B., Kim Y., Park E.-S., Choi S., Yu J., Amarasekara D.S., Kim S., Inoue J.-I. Interaction of tumor necrosis factor receptor-associated factor 6 (TRAF6) and Vav3 in the receptor activator of nuclear factor κB (RANK) Signaling complex enhances osteoclastogenesis. J. Biol. Chem. 2016;291:20643–20660. doi: 10.1074/jbc.M116.728303. [DOI] [PMC free article] [PubMed] [Google Scholar]; Yu, J., Yun, H., Shin, B., Kim, Y., Park, E.-S., Choi, S., Yu, J., Amarasekara, D.S., Kim, S., Inoue, J.-I., et al. (2016). Interaction of tumor necrosis factor receptor-associated factor 6 (TRAF6) and Vav3 in the receptor activator of nuclear factor κB (RANK) Signaling complex enhances osteoclastogenesis. J. Biol. Chem. 291, 20643-20660. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.