Abstract
Regulation of the parasympathetic nervous system, indexed through high frequency heart rate variability (HF-HRV), is indicative of physical and psychological health. However, little is known about the trainability of this capacity. We investigated the effects of a 9-month mental training program (the ReSource Project; n = 298) on voluntary HF-HRV upregulation, assessed with a novel biofeedback procedure. The program consisted of attentional, interoceptive, socio-affective and socio-cognitive training elements, all of which potentially influence parasympathetic regulation. Based on known links between oxytocin and parasympathetic activity, we also explored the relationship of HF-HRV upregulation to the oxytocin receptor system. We found that HF-HRV during the biofeedback session increased after 3 months of training, concomitant with prolonged respiration cycles. Breathing-controlled changes in HF-HRV upregulation, indicative of improved parasympathetic control, were significantly increased after 6 months of training. Homozygous risk allele carriers (AA) of the oxytocin receptor gene polymorphism rs53576 showed initially lower parasympathetic control, but fully compensated for their initial deficits through the training. No changes were found for HF-HRV at rest. Our data demonstrate that a mental training intervention extending over several months can increase the capacity for voluntary regulation of HF-HRV, with important implications for improving individual and societal health.
Subject terms: Behavioural genetics, Autonomic nervous system, Human behaviour
Introduction
Many of the brain-body interactions underlying socio-affective processes implicate the body’s largest parasympathetic nerve: the vagus1–5. Activity of the vagus nerve can be measured non-invasively by quantifying the power of high frequency heart rate variability6 (HF-HRV). High frequency oscillations in heart rate are largely caused by the waxing and waning of the vagus’ slowing influence on the sinoatrial node. Specifically, the slowing influence of the vagus on the heart increases during exhalation and decreases during inhalation7. Thus, the distance of peaks and troughs in the oscillation of the heart rate indicates vagal activity.
Incidental upregulation of parasympathetic (vagal) activity, indexed by increases in HF-HRV, has been linked to the regulation of attention8 and emotion9, positive social engagement10, pro-social emotions, such as compassion11–13, and altruistic behaviour14,15. Recently, we have demonstrated that the ability to voluntarily increase HF-HRV predicts altruistic behaviour across a wide range of tasks16. This finding is in line with the role of the vagus in the mammalian care-system17,18, that is, the biological system underlying care-giving behaviour toward offspring and beyond. This system has been proposed as a biological basis of human altruism19,20. The neuropeptide oxytocin is central to this system. The vagus is densely populated with oxytocin receptors in rodents19,21–26 and intranasally administered and naturally secreted oxytocin increases HF-HRV in humans27–30. This further substantiates the role of the vagus in the care-system. Learning to voluntarily increase HF-HRV through biofeedback also has beneficial effects for diverse medical and psychopathological conditions such as asthma31,32, cardiovascular disease33, depression34, and PTSD35. To summarise, the increased parasympathetic tone associated with upregulated HF-HRV is marked by physiological and psychological quiescence which is conducive to regeneration of the organism and also facilitates positive social engagement and care-giving behaviours20,36–42.
Despite the importance of voluntary HF-HRV control for individual and societal health, little is known about its malleability. Biofeedback that explicitly targets voluntary HF-HRV control has been found to be effective31–35. It is, however, also conceivable that interventions using other self-regulatory methods to foster physiological and psychological well-being will also impact the ability to regulate HF-HRV. Secular meditation-based training programs (also called contemplative mental training) such as the 8-week MBSR43 (Mindfulness Based Stress Reduction) or MBCT44 (Mindfulness Based Cognitive Therapy) have gained attention recently because of their apparent health-benefits from reducing stress45 to increasing well-being46 and emotion regulation capacities47. As with physical exercises, however, there are many different types of mental training. While some programs focus on present-moment attention or mindfulness, others focus on emotional capacities, and yet others on meta-cognitive awareness of thoughts or the self48–50. To investigate (a) if contemplative mental training can boost our ability to regulate parasympathetic activity and (b) if yes, which type of practice is most efficient in doing so, we engaged 335 people in a 9-month training study, the ReSource project49. The intervention was comprised of secularised classical meditation exercises and “Contemplative Dyads”, which are 10-minute mental practices done with a partner51. The training was split into 3 modules of 3 months each (for details of the training, see Methods section). Each module had a different focus. In the first module, called the “Presence” module, the two core practices assigned for daily practice were a body scan and a breathing meditation. In the body scan participants feel their way through their body by successively focussing on the sensations of their feet, legs, pelvis, and so forth up to their head. In the breathing meditation, participants pay attention to the sensations of their breath, returning to these sensations whenever the mind has strayed. The two other 3-month modules of the ReSource training were called “Affect” and “Perspective” and also targeted the cultivation of intersubjective skills with classical meditation and a Contemplative Dyad as daily core practices. The Affect module focused on increasing socio-emotional capacities such as gratitude, compassion, loving-kindness, and acceptance of difficult emotions via “loving-kindness meditation” and an affective-based dyadic exercise. The Perspective module focused on socio-cognitive abilities such as meta-cognitive awareness, understanding the self, and taking the perspectives of other people with a thought observation meditation and a Contemplative Dyad (for details, see Methods section).
Two cohorts (n = 81 and n = 80) were initially trained over nine months (see Fig. 1, panel A). One cohort received the training in the order of Presence-Affect-Perspective, the other cohort in the order of Presence-Perspective-Affect. This counterbalanced design allows the use of the training groups as active control groups for each other, to detect effects which are specific to the module, rather than to mental training in general. A third cohort (n = 81) received only the Affect training (to control for the specific effect of the Presence module), and a retest control cohort (n = 90) did not receive any training but completed the same measurements at the same temporal distances. The intervention was delivered by a team of experienced meditation teachers and psychotherapists. Each module started with a 3-day residential retreat. While the participants were in silence, the teachers introduced them to the core practices and main topics of the respective modules. The retreat was followed by 13 weeks of module-specific training featuring weekly two hour sessions with teachers, and 30 minutes of daily home practice. Home practice was supported by a smartphone app and internet platform.
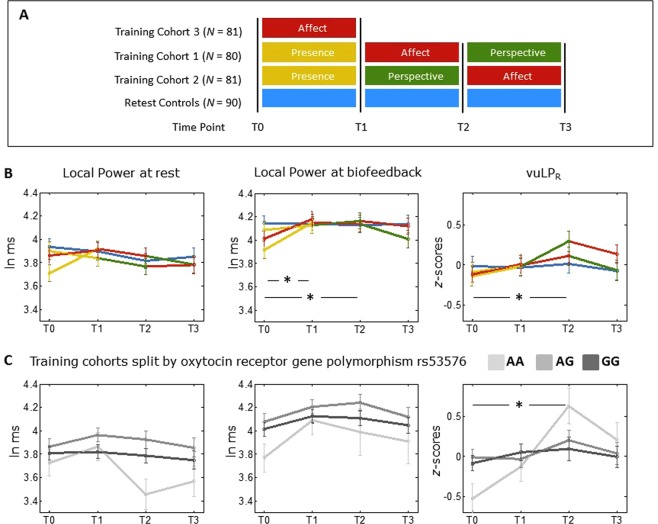
Figure 1.
Changes in the three HRV parameters across the training. (A) Design of the ReSource project (reproduced with permission from49): Three training cohorts undergo different types of mental training. Measurements are taken at every time point (x-axis). Δt between the time points = 3 months, total training duration = 9 months. A retest control cohort completes all measurements but does not undergo any training. (B) Variation in the three HRV parameters across the training period. ln ms = logarithm of milliseconds. vuLPR = voluntary upregulation of Local Power, controlling for changes in respiratory period. (C) Variation in the three HRV parameters in the trained cohorts across the training period, split by the oxytocin receptor gene rs53576 polymorphism. *p < 0.05, for the interaction between time and training from T0 to Tn, tested within a linear mixed model (see Methods). Error bars indicate standard error of the mean. Number of participants per timepoint are given in Table 1 (for Panel B) and Supplementary Table S2 (for Panel C). Note that the T0 data of panel B have been previously reported in16. Data of the graphs can also be found as tables in the Supplementary Material, Section F.
At four time points before and after each 3-month training module (i.e., at the beginning and after 3, 6, and 9 months), we investigated voluntary HF-HRV regulation using a newly developed biofeedback task16. In that task, participants were seated in front of a computer screen and asked to remain physically still. For five minutes we recorded HF-HRV at rest using an electrocardiogram. Subsequently, a spinning, three-dimensional ball was displayed on the screen (see Fig. 2). The altitude of this ball was determined by Local Power (LP), a measure of HF-HRV with high temporal resolution (for details, see16, and Methods section). Participants were asked to make the ball rise (which happened when Local Power increased), while remaining physically still. Participants were informed that the ball reflected some aspect of their “mental-bodily state”. However, no further information about this state was given, nor did we instruct any strategies to influence it (for the exact instructions see Supplementary Material, Section D).
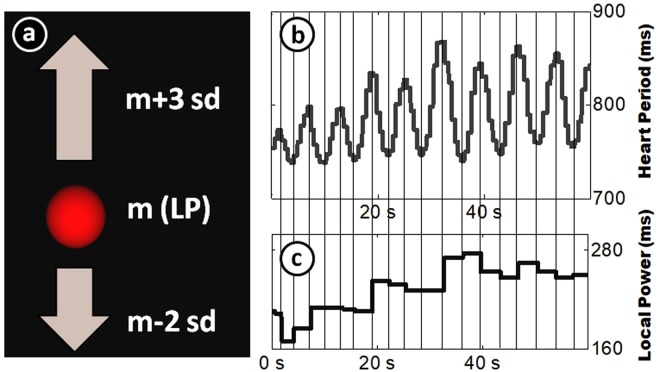
Figure 2.
Biofeedback display and computation of Local Power. (Panel a) shows the display of the biofeedback task. A spinning ball is displayed on the screen. Its height is determined by Local Power (LP). (Panels b,c) illustrate the computation of LP: Shown is the heart period (HP) of a single participant over the course of 1 minute (Panel b). Every time the direction of the HP curve shifts (vertical lines), a new LP value (Panel c) is assigned, computed as the difference in HP to the previous shifting point. An average of the last two LP values is used for feedback. Figure reproduced with permission from16.
We investigated three interrelated parameters of autonomic regulation: Local Power at rest (LP_Rest), Local Power at biofeedback (LP_BF), and voluntary upregulation of Local Power, controlled for breathing (vuLPR; for details, see Methods section). LP_Rest characterises the parasympathetic influence on the heart without any self-regulatory efforts. LP_BF characterises the parasympathetic influence on the heart during biofeedback. And vuLPR characterises the ability to induce changes in parasympathetic activity, controlling for changes in the breathing pattern from rest to biofeedback, thus indexing the ability to regulate HF-HRV using other mechanisms than breathing control (such as upregulation of central vagal outflow).
Earlier studies have failed to find any effects of contemplative mental training on HF-HRV at rest52–54. Two studies, however, found that meditation (in the form of self-compassion training52 and a program focussing on emotion regulation53) leads to more situation-adequate adaptations of HF-HRV, that is, lower HF-HRV in stressful situations (vagal withdrawal) and higher HF-HRV during recovery from a stressor (vagal augmentation). Accordingly, we expected to find effects of the training not during LP_Rest, but during the two parameters indexing regulatory capacity (LP_BF and vuLPR). We assumed two principal mechanisms to facilitate these changes: Improvements in interoception (inner body sensing) and training of socio-emotional and socio-cognitive capacities. Interoceptive information partially travels from the body to the brain via the vagus nerve3. Improved interoception may thus affect vagal regulation. Additionally, continuous monitoring of bodily activity may make participants more aware of ways to use breathing to influence other physiological processes (e.g., change cardiac activity through breathing). The focus on interoception is particularly strong in the Presence module. However, the Affect and the Perspective modules also require participants to ground awareness in body sensations to focus on the present moment. Earlier studies have found that all three modules improve interoception55. Accordingly, we hypothesize that all modules will improve voluntary vagal regulation.
Previous research has established that vagal regulation underpins social engagement2,20,56, particularly, caregiving and altruistic behaviours14–16. As the Affect and the Perspective modules aimed at training these propensities, we hypothesized that they will also improve vagal regulation in the biofeedback task. As the biofeedback task itself does not involve social cues, though, this hypothesis rests on the assumption that the internal regulation schemes acquired during social training transfer to non-social situations. For instance, participants might be more benevolent with themselves during the task, promoting a more relaxed and thus parasympathetic state. Generally viable routes to vagal activation, such as voluntary relaxation57 and slower breathing58, might also be more effective in participants who have implicitly learned to activate the vagus through socio-affective training. This hypothesis would receive support from the data if the Affect and the Perspective module (which focus on social skills and incidentally also train interoception)55 prove to be more effective than the Presence module (which focuses primarily on interoception) in enhancing biofeedback performance. If they are less or only equally effective, we would not be able to disentangle the relative contributions of interoceptive and social training on changes in vagal regulation.
Based on the findings relating the oxytocin system to HF-HRV, we also explored how genetic differences in the oxytocin receptor system influence voluntary parasympathetic control. Specifically, we analysed the influence of the oxytocin receptor gene polymorphism rs53576. Homozygous carriers of the minor allele (A; hitherto referred to as ‘risk allele’) of rs53576 have been shown to exhibit lower interpersonal sensitivity59–62. For instance, risk allele carriers were shown to be less responsive to their toddler’s distress59 and had less pronounced cardiac reactions to infant’s crying sounds60. They show lower amygdala activation to social emotional stimuli, such as faces61, and show lower stress-buffering effects from the presence of a friend during a social stress-tests63. Assuming that this lowered interpersonal sensitivity is a function of diminished parasympathetic regulation, we explored whether AA-carriers perform worse in the biofeedback task. Because the training, especially the Affect and the Perspective module, is aimed at improving interpersonal skills, we furthermore assumed that it could diminish these initial differences between participants of different genotypes. Although motivated by previous theoretical reasoning and empirical data, these analyses had not been a primary goal of the ReSource project, as we did not know a priori which distribution of genotypes we would find in our sample and the ReSource project is lacking the sample size needed for rigorous genetic analyses. These additional analyses should thus be interpreted as purely explorative and in need of replication.
Results
Testing the specificity of the training modules
To determine whether the training modules had differential effects on HF-HRV and HF-HRV regulation we first compared two linear mixed models (for details see Methods). The first model included the four different conditions (Presence, Affect, Perspective, and no training) while a simpler nested model contained only training and no training (thus collapsing data across all training conditions). There were no differences in fit between these two models for any parameter (p > 0.30 for all). This indicates that the data were modelled equally well when only distinguishing between trained and untrained participants (regardless of the training type). We thus used this more parsimonious model for all subsequent analyses.
Changes in HRV regulation
As mentioned in the introduction, we analysed two ECG-derived parameters to characterise regulation of heart rate variability. Raw Local Power during the biofeedback (LP_BF) quantifies the power of high frequency oscillations in heart rate. It is strongly related to other heart rate variability parameters (r = 0.85 to 0.98)16, such as spectral high frequency power6 and peak-to-trough respiratory sinus arrhythmia64. It is influenced both by breathing patterns and vagal activity7,65. In order to isolate vagal activity, we additionally analysed voluntary upregulation of Local Power, controlling for respiration (vuLPR), as done in previous research16,52,66. This parameter indexes regulation of cardiac vagal activity beyond breathing effects, suggesting regulation of central vagal outflow (for more information on these parameters, see Methods section).
After 3 months of training (from T0 to T1), LP_BF had increased in the Training Cohorts (TCs), but not in the retest control cohort (Fig. 1B), as indicated by a time * training interaction, t(738.5) = 2.48, p = 0.007, Cohen’s d = 0.240 (this and all subsequent ts refer to the interaction term within the linear mixed model). Trained participants had also increased in respiratory period (measured by a strain gauge), as indicated by a time * training interaction, t(736.1) = 2.34, p = 0.010, d = 0.295. Increases in respiratory period (i.e., breathing more slowly) correlated with concomitant increases in LP_BF, r = 0.296, p < 0.001. No statistically significant changes occurred in the parameter of voluntary parasympathetic control, vuLPR, t(767.0) = 0.89, p = 0.188. Together these results indicate that the first three months of the training increase the capacity to regulate heart rate variability and that this effect is largely achieved through slower breathing.
The increase in LP_BF in the training as compared to the control cohort remained significant after six months of training, t(746.3) = 2.29, p = 0.011, d = 0.254. Importantly, at this time point there was also a significant time * training interaction in respiration-controlled voluntary upregulation, vuLPR, t(780.8) = 1.77, p = 0.039, d = 0.288 (Fig. 1B). This indicates that participants achieved control of heart rate variability partially through non-respiratory mechanisms, such as upregulation of central vagal outflow. This increased parasympathetic control emerges after participants have completed the initial training phases as well as one of the interpersonal training modules.
There was a slight drop in biofeedback performance between month 6 and month 9 of the study, rendering the effects from T0 to T3 no longer significant for LP_BF, t(747.6) = 1.31, p = 0.09, or vuLPR, t(784.6) = 1.31, p = 0.13. We also investigated HF-HRV during the initial five-minute resting period. No changes over the training were found in Local Power or Local Power controlled for breathing, p > 0.10 for all tests. This indicates that the training specifically affects regulatory capacities, but not autonomous activity at rest.
Descriptively, the control cohort had higher initial values on LP_BF as compared to the training cohort. Although this difference was not statistically significant, t(297) = 1.66, p = 0.10, it still may have influenced the results. Ceiling effects may have impeded control cohort increases in LP_BF. Further, the changes in the training cohorts might be partially attributed to regression towards the mean67. To address this, we included into the model (a) the baseline (T0) value of LP_BF, (b) the interaction of time * baseline, and (c) the interaction of baseline * time * training. All these terms were significant, p < 0.001 for all tests, with a positive beta for the baseline value and negative betas for both interaction terms. This indicates that (a) participants with higher values at baseline also had higher values in later training phases, that (b) participants with higher baseline values showed less change, and that (c) baseline values affected the training-dependent changes over time. Within this model the time * training interaction for changes in LP_BF from T0 to T1 was still significant, t(721.6) = 2.362, p = 0.009. However, the interaction became marginal from T0 to T2, t(733.2) = 1.33, p = 0.092. This indicates that regression towards the mean and ceiling effects may have slightly inflated the observed training-dependent changes in LP_BF. When doing the same control analyses for LP_Rest and vuLPR the pattern of significance remained unaffected.
Modulation of HRV by oxytocin receptor gene polymorphism
Based on the links between the vagus nerve and the oxytocin system, we explored the potential influence of the oxytocin receptor gene polymorphism rs53576 on regulatory ability at baseline (T0). Genotype frequencies for rs53576 were N = 38 (13.24%) AA carriers, N = 120 (41.81%) AG carriers, and N = 129 (44.95%) GG carriers. This distribution is in Hardy-Weinberg equilibrium, χ2 = 0.494, p = 0.52. We found a significant relationship between rs53576 alleles and voluntary parasympathetic upregulation, vuLPR, F(2,284) = 3.198, p = 0.042 (see Supplementary Fig. S3). Post-hoc independent sample t-tests revealed that vuLPR was significantly lower in homozygous carriers of the risk allele (AA) than in AG carriers, p = 0.027, d = 0.411, or GG carriers, p = 0.014, d = 0.454. The difference between AG and GG carriers was not significant, t(247) = 0.335, p = 0.738, d = 0.043. No relationship was found between genotype and Local Power at rest, or LP_BF. The genetic influence thus seems to be specific to vagal regulation, but not resting vagal activity.
Modulation of training effects by oxytocin receptor gene polymorphism
We explored whether homozygous risk allele carriers would profit more strongly from the mental training, particularly the socio-affective and socio-cognitive training components, which might help them to overcome their initial deficits in interpersonal skills. Indeed, among participants who received the training, homozygous carriers of the risk allele (AA) of rs53576 showed significantly stronger vuLPR training effects than G-carriers (AG/GG), as indicated by a significant time * genotype interaction, from T0 to T2, t(566.1) = −2.30, p = 0.022, see Fig. 1, Panel C. By the end of the intervention (at T3), the three genotypes no longer differed significantly in vuLPR, F(2,140) = 0.234, p = 0.792. This indicates that homozygous risk allele carriers profit most strongly from the training and fully compensate their initial deficits in vuLPR. No gene-dependent trajectories were found for LP_BF or Local Power during the resting baseline, p > 0.10 for all tests, again suggesting that the influence of the rs53576 polymorphism is specific to voluntary vagal control.
There appears to be a slight drop in LP_Rest from T0 to T2 in the group of AA carriers. This drop was not significant, neither when tested as a time * genotype interaction from T0 to T2, t(534.1) = 0.691, p = 0.49, nor when tested as the effect of time within the AA group alone, t(222.57) = 0.98, p = 0.33, both tests two-sided. It is however noteworthy that this drop will have increased the effect size of gene-dependent change in vuLPR at T2 reported above, given the mathematical dependence of LP_Rest and vuLPR (see Methods).
Discussion
The goals of this study were to investigate whether voluntary upregulation of HF-HRV can be improved by contemplative mental training and if so, to determine which types of mental practices can bring about such increases in autonomic control. We used a novel biofeedback task to measure voluntary upregulation of HF-HRV, a cardiac marker of parasympathetic (vagal) control. We investigated this in the context of the ReSource project, a 9-month long mental training study consisting of three different 3-month training modules49. We showed that after 3 months of Presence training, a module focusing on present moment attention and interoception, participants indeed showed increased HF-HRV during the biofeedback task (measured by raw Local Power, a short-term measure of high frequency fluctuations in heart rate). No such changes were found for a retest control cohort. Increased regulation after these first 3 months of training, which included practices such as “breathing meditation” and “body scan”, was largely achieved by adopting slower breathing rates that are known to increase the influence of the vagus nerve on the heart7,65,68. We therefore suggest that the first months of training, which have a strong emphasis on body and breath observation, make participants more aware of ways to influence their bodily state through breath control. The ability to increase HF-HRV through slower breathing has been linked to symptom reduction in a variety of bodily and mental disorders, such as cardiovascular33 and pulmonary disease31,32, as well as depression34 and PTSD35, making these findings clinically relevant. It is noteworthy that participants in the control cohort showed descriptively slightly better biofeedback performance to begin with. These differences were, however, not significant and statistical control for baseline-dependency of change did not change the observed changes from T0 to T1, speaking for robust training-related increases. Still, future studies with less variance in the starting conditions may be helpful in replicating and validating our findings.
Interestingly, when using a breathing-controlled parameter of HF-HRV regulation (i.e., increases in Local Power from a resting baseline to the biofeedback, controlling for simultaneous increases in respiration cycle length), participants showed a significant increase after 6 months, that is, after completing either the Affect module or the Perspective module. These modules focused on socio-affective (compassion, loving-kindness) and socio-cognitive skills (perspective taking on self and others). In addition to classical meditation, these modules also included 10 minute contemplative dyadic exercises51. These findings suggest that the participants’ ability to increase the vagal influence of the heart has grown beyond breathing control. This could be attributed to the social nature of the training modules participants complete between month 3 and month 6. As the vagus is strongly implicated in social interaction9,42,69, intersubjective mental training may have had additional beneficial effects on its deliberate regulation. The vagus also plays an important role in the mammalian system underlying care-giving behaviour18–20,70. As the Affect module explicitly aims at fostering care-giving towards one-self (self-compassion)71 and others (pro-social behaviour), improvements in vagal upregulation might also be attributed to stronger activation of the care-system. Interestingly, however, our analyses revealed that there were no module-specific effects, but that it was rather training time which determined the observed increases on the two autonomic markers used here. Thus, the present data do not allow us to disentangle training-general from module-specific effects. We know from an earlier study55 that all of the investigated modules enhance the ability to feel the body (interoception, as measured by a heartbeat perception task). Thus, rather than the social nature of the Affect and Perspective training modules, it could also be an improved awareness of their own bodies which causes the observed enhancement in parasympathetic control. Future studies are warranted to disentangle the contributions of social and interoceptive training components on parasympathetic control. They could also strive to disentangle the effects of different training components such as silent retreats and weekly group sessions, as well as single meditation and dyadic practices on HF-HRV regulation.
All training effects were in a range of Cohen’s d = 0.240 and d = 0.295, which are conventionally labelled small effects72. However, this effect may play out in a lot of different physiological and psychological domains, given that HRV has been implicated in a wide range of outcomes from cardiac health, and emotion regulation to altruistic behaviour9,10,73.
The increases in HF-HRV control demonstrated after 6 months were no longer significant when tested 3 months later (at the end of the study). This unexpected finding may be due to the nature of the employed task. Whereas participants generally reacted with great interest to the biofeedback task in the initial measurement sessions, this interest may have declined through repetition, yielding changes in strategies or a lower involvement in the task. This may explain the slight drop of performance observable in the last measurement period. These fluctuations in interest in the task may be a limitation of the biofeedback task in repeated-measure designs. Apart from that, the data suggest that the task is sensitive to training-induced changes, rendering the novel biofeedback task a feasible tool for future research on voluntary autonomic regulation. An alternative explanation for the unexpected drop in HF-HRV control at T3 is that the training effects are volatile or that they characterize only a transient improvement. Investigation of participants’ performance at later time points (several months after the end of the training) may clarify this issue.
Finally, after having established that mental training can indeed have beneficial influences on voluntary parasympathetic regulation, we explored how this ability is modulated by the oxytocin system. The vagus nerve is densely populated by oxytocin receptors19,21–26 and intranasal administration of oxytocin has been found to increase HF-HRV in humans27–30. We thus explored whether individual differences on the oxytocin receptor gene polymorphism rs53576 modulated respiration-controlled HF-HRV regulation. We found that homozygous carriers of the risk allele (AA) of rs53576 showed a lower capacity for voluntary parasympathetic control at baseline. Previous studies have shown that AA-carriers exhibit lower interpersonal sensitivity59–62. Because relating to others is partially based on vagal regulation2,9,15,69,74,75 these two findings may be linked: The reduced social sensitivity of AA-carriers may also be partially rooted in a relative inability to regulate vagal activity. Furthermore, we found that the trajectories of training-related increases in autonomic control differ by genotype, with AA-carriers profiting most from the training. In fact, the increase in AA-carriers seems to drive the overall increases in the training cohorts. Possibly, their initial deficits may make the social exercises most fruitful for them, leading to stronger improvements than in G-allele carriers. Conversely, a ceiling effect may prevent G-carriers from increasing as much as homozygous A-carriers. Together, our explorative analyses and preliminary findings suggest a genetic basis for individual differences in parasympathetic control as well as its malleability through training. However, due to the relatively small sample sizes of the groups with an N of only 38 for AA carriers (and Ns of 120 and 129 for AG and GG carriers, respectively) these genetic analyses should be treated with great caution and future replication studies in bigger samples are clearly needed. It should also be noted that this genetic effect would not survive correction for multiple comparisons.
No effects of the training were found for Local Power at rest. This is in line with earlier studies investigating the effects of contemplative mental training on HF-HRV52–54. The only exception is a study by Tang et al.76. Notice, though, that this is the only study using normalised HF units instead of raw HF power, and that it uses a non-standard frequency band, 0.16–0.45, to determine the HF range. It thus seems that the training does not affect autonomic balance at rest, but rather the ability to induce changes therein. Both heart and breathing rhythm as well as the vagus nerve are under the control of evolutionarily old brainstem structures such as the pons and medulla oblongata77,78. Due to their vital role in preserving basic functions of the organism and their involvement in a large host of brain processes79, their basic activity may be slow to change. This may explain why it is hard to alter resting HF-HRV. The activity of those areas is, however, modulated by cortical activity, particularly by the medial prefrontal cortex80 (mPFC). This is probably how HF-HRV is affected through conscious effort81, such as the regulatory activity during biofeedback. We speculate that participants in our study have improved in the ability to exert a phasic influence of the mPFC on the brainstem, thus affecting HF-HRV. Such modulation is not tonic however, leaving resting HF-HRV unaltered. Future research with long-term practitioners of contemplative mental training (e.g., 15–40 years82) could clarify whether the modulatory effects become tonic, leading to permanently elevated levels of HF-HRV.
To summarise, we have shown that a mental training intervention increases the ability to voluntarily increase HF-HRV. This has implications for the use of such interventions in healthy and impaired populations. Mental training may prove particularly fruitful for people who exhibit altered or reduced vagal flexibility, as found in children at risk of psychopathology83 and people suffering from depression84 or social phobia85. Such programs may also be helpful for people suffering from psychotic symptoms, who have been found to benefit from increased HF-HRV control in a biofeedback study86. In addition, increased vagal control may have benefits for healthy individuals, as increases in vagal activity promote relaxation and restoration of the organism, thus increasing general well-being and resilience87–90. Furthermore, the ability to self-induce states of higher vagal activity may serve as a resource that allows individuals to more successfully reach out to other people in need, as suggested by previous studies14–16. Our results thus indicate that the investigated types of contemplative mental training may help build a healthier and more caring society.
Methods
Participants
Participants of the study were part of the ReSource Project, a large scale study on contemplative mental practice. Details about the recruitment procedure and randomised cohort assignment can be found in49, chapter 7. Briefly, all participants were mentally and physically healthy, had no regular meditation practice, and had not participated in a meditation retreat in the last 2 years. For the present study, physiological data of N = 298 participants (n = 251 in the training cohorts and n = 81 in a retest control cohort; 173 female, M age: 40.4; n = 287 with complete gene data) were available. Reasons for missing data are listed in the Supplementary Material, Section B. Participant characteristics for all study cohorts are listed in Table 1. We used data of all participants who had at least one time point in the study, as common for linear mixed models91. Note that the T0 data of this sample are identical to those used in Bornemann, et al.16. The present study also includes data from three subsequent time points (T1, T2, and T3) allowing the assessment of heart rate variability regulation through contemplative mental training over the course of the 9-month ReSource training. In addition, we explore how the oxytocin receptor gene polymorphism rs53576 affects parasympathetic regulation at baseline and as a result of training.
Table 1.
Participant characteristics across the four time points.
| T0 | T1 | T2 | T3 | |
|---|---|---|---|---|
|
Cohort 1 Presence - Affect - Perspective |
n = 64 (33f), M age: 40.6 (9.0) |
n = 76 (44f), M age: 41.5 (9.0) |
n = 76 (42f), M age: 41.6 (8.6) |
N = 73 (44f), M age: 41.4 (9.0) |
|
Cohort 2 Presence - Perspective - Affect |
n = 76 (45f) M age: 41.2 (9.8) |
n = 70 (43f), M age: 41.1 (9.7) |
n = 73 (46f), M age: 41.3 (9.7) |
n = 74 (44f), M age: 40.2 (9.7) |
|
Cohort 3 Affect |
n = 77 (47f) M age: 40.3 (8.8) |
n = 70 (41f), M age: 40.7 (9.0) |
||
|
Retest Control Group No Training |
n = 81 (48f), M age: 39.6 (9.3) |
n = 76 (47f), M age: 40.7 (9.0) |
n = 82 (48f), M age: 39.7 (9.4) |
n = 74 (47f), M age: 39.9 (9.5) |
| All samples combined |
n = 298 (173f), M age: 40.4 (9.2) |
n = 292 (175f), M age: 40.7 (9.2) |
n = 231 (137f), M age: 40.8 (9.2) |
n = 221 (131f), M age: 40.8 (9.4) |
f = females; m age = mean age (standard deviation in parenthesis). The T0 data have been reported on in16.
Ethics
The study was approved by the Research Ethics Committee of the University of Leipzig, number 376/12-ff and the Research Ethics Committee of the Humboldt University in Berlin, numbers 2013-02, 2013-29, and 2014-10. The study was registered with the Protocol Registration System of ClinicalTrials.gov under the title “Plasticity of the Compassionate Brain” with the ClinicalTrials.gov Identifier: NCT01833104. All methods were carried out following the relevant guidelines and regulations. All participants gave informed consent prior to the study.
Gene assessment
Genomic DNA was extracted from blood, drawn after the T0 measurement, using the Quick Gene DNA whole blood Kit (Kurabo, Japan). Genotyping of the rs53576 was performed using the TaqMan SNP Genotyping assay (Applied Biosystems; C___3290335_10). The TaqMan genotyping reaction was amplified on an Applied Biosystem 2720 Thermal Cycler 95 °C for 15 min, and 95 °C for 15 sec, and 60 °C for 1 min, for 40 cycles) and fluorescence was detected by using the Applied Biosystem 7500 Real-Time PCR systemon (Thermo Fisher Scientific Inc.). To assess genotyping reproducibility, a random ~5% selection of the sample were re-genotyped for all SNPs; all genotypes matched initially designated genotypes.
ReSource training
The overarching goal of the ReSource Project is to improve subjective well-being, mental and physical health, as well as compassion and intersubjective skills. To achieve these goals, the ReSource training aims to cultivate several attentional, socio-cognitive, and socio-affective processes taught in three separate modules called Presence, Affect, and Perspective. Each module was delivered over a period of three months, beginning with a 3-day silent retreat (silent except for the teacher instructions and explanations) and including 13 weekly group sessions of 2 hours and 30 minutes of daily practice. The training was facilitated by a team of experienced meditation teachers and psychotherapists and supported by an online-platform and a smartphone app. The three modules are described in detail in49, chapters 2 and 3.
Each module of the program has two core exercises, which participants were asked to practice 5 times a week, in addition to the weekly group sessions. Briefly, in the Presence module, participants learned to direct their attention to their present moment experience. The two core practices were Breathing Meditation and Body Scan. In the Breathing Meditation92, participants focused their attention on the sensations of their breathing, returning to these sensations whenever the mind had strayed. In the Body Scan43,93, participants attuned to bodily sensations, systematically and slowly moving the focus of attention from feet to head or head to feet, allowing sensations from all regions of the body to arise in the mind. In the Affect Module, participants practiced generating an attitude of kindness, care, and compassion for themselves and others. They learned how to approach difficult emotions with acceptance and to nurture prosocial motivations. The core practices in this module were a Loving-Kindness Meditation and a contemplative dyadic exercise referred to as the Affect Dyad. The Loving-Kindness Meditation94 involved connecting to an attitude of benevolence and care towards one self and others by using internally repeated phrases expressing these intentions and related imagery. The dyadic exercise can be conceptualized as a verbalized meditation. Participants sit facing each other. One of the participants shares a recent situation that evoked difficult emotions95–97 as well as a situation that evoked gratitude in them98–100. Focus is paid to how these situations affected their body. The other listens mindfully before they switch roles. In the Perspective Module, participants engaged in meta-cognition and in cognitive perspective-taking on one’s self and others. The core exercises in this module were an Observing-Thoughts Meditation and a dyadic exercise called the Perspective Dyad. In the Observing-Thoughts meditation43,101,102, participants practiced the observation of thoughts as mental events. They were taught to observe thoughts as natural events, comparable to sounds or bodily sensations. In the Perspective dyad, one partner chooses a recent situation and then randomly draws a previously identified personal “inner part” (a personality aspect in the framework of the Internal Family System)103,104. The participant then describes the chosen situation from the perspective of that inner part, thereby practicing perspective taking on one’s self. The partner listens mindfully and tries to identify the inner part whose perspective the other is speaking from, thereby training perspective-taking on others or Theory of Mind105.
Participants were required to log in on the online platform via computer or smartphone for each practice, allowing us to assess practice frequency and duration.
Study design
The ReSource study was designed to test effects of different types of mental training on a large array of behavioural, subjective, physiological and brain measures (see49, chapter 8). An overview of the design is given in Fig. 1A. Two cohorts underwent all three training modules in counterbalanced orders, that is, Presence-Affect-Perspective and Presence-Perspective-Affect. These serve as active control cohorts to each other, allowing us to compare the effects of the Affect module to those of the Perspective module (by comparing test scores at T2). It also makes it possible to compare the efficacy of different training orders (when comparing test scores after the entire training). An additional cohort underwent only the Affect training, allowing us to ascertain specific effects of a module with strong interoceptive focus (Presence) in comparison to a socio-affective training module (Affect). A retest control cohort underwent no training but completed the same measurements as the training cohorts. This allowed us to test for training general effects, not differentiating for training type. In the particular study reported here, we did not have any specific hypotheses about order effects, nor about differential effects of Perspective and Affect training. Rather, we were interested in whether the training has effects at all, and if so, if any module is more effective than the other. We thus adopted the modelling approach described below (see Model selection) that first tests for module specificity and then collapses all modules in case no specificity is revealed.
Physiological recordings
Heart rate and respiratory period were assessed throughout the resting measurement and the biofeedback task. For heart rate measurement, a standard Lead-II electrocardiogram was acquired by placing disposable Ag/AgCl Electrodes on the right mid-clavicle, left ribcage, and left outer clavicle. To measure respiration, a strain gauge was attached around the torso of participants at the level of the lower ribs, capturing both chest and abdominal breathing. Signals were recorded at 1000 Hz using a Biopac® (BIOPAC Systems Inc., Santa Barbara, CA) MP150 data acquisition system and the software AcqKnowledge 4.3. Data were processed and cleaned for artefacts (see Supplementary Material, Section C) with ARTiiFACT106 and custom MATLAB scripts (available on request).
Resting measurement
For assessment of resting HF-HRV, participants were asked to just sit still and “not think of anything in particular” for 5 minutes, as is common for resting HF-HRV assessment6. The resting measurement was always done before the biofeedback task.
Biofeedback task
The biofeedback task used to derive a measure of voluntary HF-HRV upregulation is described in more detail in16. Briefly, a red, spinning, three-dimensional ball was presented on the screen (see Fig. 2). Its vertical position was determined by a short-term estimate of HF-HRV (Local Power, see below). Participants were asked to make the ball go up by bringing themselves into a mental-bodily state that would be mirrored in the altitude of the ball. They were not told how to achieve that state but to experiment for themselves to find a state that is conducive to making the ball go up. Exact instructions can be found in Supplementary Material, Section D.
HRV Parameters
In the last decades, several methods have been developed to estimate vagal activity from an electrocardiogram6. All of them aim to quantify the variability of the heart period (the time from one heart beat to another), which is caused by the activity of the vagus nerve. The vagus has a tonic slowing influence on the heart. This influence increases during exhalation leading to longer heart periods, whereas it decreases during inhalation, leading to shorter heart periods7. These increments and decrements in heart period, which occur along with respiration, are the main source of variability in heart period (or heart rate, which is the inverse of heart period). The difference between heart period during exhalation and inhalation is indicative of vagal activity6. A widely used measure to quantify this is spectral high-frequency HRV. To obtain this measure, a series of heart periods (typically from recordings of at least 1 min in length) is subjected to a Fourier transformation to derive oscillations in different frequency bands. The frequency band which corresponds to adult human respiration (0.15–40 Hz) is dubbed ‘high frequency heart rate variability’, because it contains higher frequencies than those typically caused by other (e.g., sympathetic) influences on the heart. The power of this frequency band (its amplitude) is taken to indicate vagal activity. Another common measure is peak-to-trough Respiratory Sinus Arrhythmia (pt-RSA)64. Respiratory Sinus Arrhythmia generally denotes the phenomenon described above, that is, the close coupling of fluctuations in heart period with the respiration cycle. In pt-RSA a respiratory recording is used along with the heart period data. Pt-RSA is computed as the maximum heart period during exhalation minus the minimum heart period during inhalation. As the distribution of HRV measures is highly skewed, they are typically transformed by using their (natural) logarithm.
In this study, we used an HRV measure called Local Power (explained in Fig. 2; and also see16). Local Power (LP) was specifically developed for biofeedback purposes, as it can be computed online with high temporal resolution. It quantifies oscillations in heart period data by computing the difference of a trough to a successive peak in the heart period series or vice versa. Heart period data are continuously fed to the biofeedback program. Whenever there is a turning point in the data (a change from acceleration in heart period to deceleration or vice versa) the heart period at this point is saved. When the next turning point appears, the difference to the previous turning point is computed and fed back as the current estimate of vagal influence on the heart. Depending on participant characteristics (particularly breathing rate), LP can be fed back to participants with an average temporal resolution of 2.46 s (SD = 0.64 s). A previous study16 has shown that LP is highly correlated to both pt-RSA (r = 0.98) and spectral HF-HRV (r = 0.85). These measures would have had several disadvantages compared to LP when used for biofeedback. Pt-RSA has only about half the temporal resolution, meaning feedback would have been slower. Furthermore, it relies on respiratory data, the online assessment of which had proven to be error prone in pilot experiments. Spectral HF-HRV requires about 1 min of heart period data to be accurate6. Furthermore, it makes assumptions about the breathing frequency to be between 0.15 and 0.40. This range may be easily violated if participants adopt particularly low or fast breathing rhythms to influence their physiological state during biofeedback. We used LP in both the resting and the biofeedback condition to assure comparability.
It has been found that slower breathing enhances the vagal influence on the heart, as the effect of respiratory gating on the sinus node is more pronounced during slower breathing7,107,108. Thus, HRV is increased by slower breathing. Although slower breathing may eventually enhance parasympathetic activity109, initial changes in HRV are often only due to the gating effect of respiration on vagal transmission to the heart, and not to changes in central vagal outflow65. Thus, it has been recommended to control for respiratory effects when making inferences on changes in vagal activity from one condition (e.g., rest) to another (e.g., biofeedback)65.
Three HRV parameters are analysed in the current manuscript:
Local Power at Rest (LP_Rest). This parameter indexes short term fluctuations in heart rate, which are mostly due to central vagal outflow as well as the breathing pattern.
Local Power at biofeedback (LP_BF). This is the same parameter as (1), but during the biofeedback task. It thus indexes participants’ ability to induce oscillations in heart rate by both changes in central vagal outflow and breathing pattern.
Voluntary upregulation of Local Power, controlled for respiration (vuLPR), was computed (as in16) as the standardised residual of the linear regression of [RSP_BF–RSP_BF] on [LP_BF–LP_Rest], where RSP denotes mean respiratory period. This parameter indexes changes in heart rate oscillations from rest to biofeedback, which cannot be attributed to changes in respiration cycle length. It is thus a purer measure of the ability to increase central vagal outflow (voluntary parasympathetic control).
Model selection
To investigate changes in HF-HRV and HF-HRV regulation over time, we used linear mixed models, fit using R, version 3.1.2, and the modelling package lme4110. The model that was used to investigate differential change between the training cohorts and the retest control cohort included time, training, and their interaction as fixed effects and subject-specific intercepts as a random effect. Models including random slopes for training effects in addition to random intercepts were unidentifiable due to large eigenvalue ratios or did not converge due to non-positive Hessian matrices. Training type was first included as a variable with four categories coding for the four different conditions (Presence, Affect, Perspective, and no training). This model was tested against the simpler nested model that only tests for overall training effects (no training vs. training).
There were no significant differences in model fit between the two models for any of the HRV parameters as revealed by log likelihood chi-square tests, p > 0.30 for all tests. This indicates that the training types did not differ in their effect on resting or regulated HRV and that the data are modelled equally well by a simpler model that only distinguishes between trained and untrained participants. We thus used this more parsimonious model for all analyses. The main test of differential changes between the TCs and the RCC was the time*training interaction. We report contrasts, which probe the significance of changes in the DV from the initial value in the DV (at T0) to each of the three time points during the training (T1, T2, T3). This allowed us to test for changes in the DV that are not uniform over the entire training period (e.g., a DV that peaks at one time point and then stagnates or deteriorates). Within each model, contrasts were not corrected for multiple comparisons, which is justified because in the multilevel framework used here, estimates are “shrunk” toward a common mean using partial pooling, thus correcting for the increased risk of false positives typically incurred by multiple comparisons without compromising power111. The test statistic of the contrasts follows a Student’s t-distribution. One-tailed t-tests were used for contrasts for LP_BF and vuLPR because we specifically hypothesised increases in these parameters through the training rather than decreases.
Control variables
We repeated all analyses using age, sex, and BMI as control variables, because they potentially influence HF-HRV112,113. The pattern of significance remained the same in all analyses except for the effect of the rs53576 polymorphism on parasympathetic regulation at baseline (T0). The F-Test became marginal, F(2,280) = 0.08. The individual contrasts between the AA-carriers and the AG- and GG- carriers, however, remained significant, p = 0.049 and p = 0.027, respectively.
It has previously been reported that nicotine consumption reduces parasympathetic activity. We thus investigated the influence of smoking on the HRV parameters. There was a fairly low amount of smokers in our sample (11.3%) with an average cigarette consumption of 15.1 cigarettes/week (among the smokers only; SD = 13.18). We detected no influence of smoking on any of the HRV parameters, p > 0.4 for all tests, nor did the amount of cigarettes per week correlate to any of the parameters, p > 0.05 for all tests. There were also no interaction effects of smoking with the training effects, p > 0.15 for all tests.
Supplementary information
Acknowledgements
The ReSource Project in which this study was conducted is a collaborative effort by the Department of Social Neuroscience at the Max Planck Institute of Human Cognitive and Brain Sciences. We are thankful to all members of the department as well as countless outside collaborators who have supported us. We are particularly thankful to all ReSource teachers that taught the intervention program, to Astrid Ackermann, Christina Bochow, Matthias Bolz, and Sandra Zurborg for managing the study, to Hannes Niederhausen, Henrik Grunert, and Thorsten Kästner for their technical support, and to Sylvia Tydecks, Elisabeth Murzik, and Kerstin Träger for their help with recruitment and data collection. T.S., as principal investigator, received funding for the ReSource Project from (a) the European Research Council under the European Community’s Seventh Framework Program (FP7/2007–2013/ERC Grant Agreement Number 205557) and (b) from the Max Planck Society. IFB Adiposity Diseases is supported by the Federal Ministry of Education and Research (BMBF), Germany, FKZ: 01EO1501 (AD2-06E95 to Peter Kovacs).
Author Contributions
B.B. designed and conducted the experiments, assessed and analysed the data, and wrote the manuscript. P.K. performed the genotyping. T.S. designed and supervised the ReSource study, provided continuous feedback during data analysis and revised the final manuscript.
Data Availability
All data are available upon reasonable request from the Department of Social Neuroscience of the Max Planck Institute for Human Cognitive and Brain Sciences.
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary information accompanies this paper at 10.1038/s41598-019-44201-7.
References
- 1.Thayer JF, Lane RD. Claude Bernard and the heart–brain connection: Further elaboration of a model of neurovisceral integration. Neuroscience & Biobehavioral Reviews. 2009;33:81–88. doi: 10.1016/j.neubiorev.2008.08.004. [DOI] [PubMed] [Google Scholar]
- 2.Porges SW. The Polyvagal Theory: phylogenetic contributions to social behavior. Physiology & Behavior. 2003;79:503–513. doi: 10.1016/s0031-9384(03)00156-2. [DOI] [PubMed] [Google Scholar]
- 3.Craig B. How do you feel? Interoception: the sense of the physiological condition of the body. Nature reviews. Neuroscience. 2002;3:655–666. doi: 10.1038/nrn894. [DOI] [PubMed] [Google Scholar]
- 4.Craig B. Human feelings: why are some more aware than others? Trends in Cognitive Sciences. 2004;8:239–241. doi: 10.1016/j.tics.2004.04.004. [DOI] [PubMed] [Google Scholar]
- 5.Critchley HD. Neural mechanisms of autonomic, affective, and cognitive integration. J Comp Neurol. 2005;493:154–166. doi: 10.1002/cne.20749. [DOI] [PubMed] [Google Scholar]
- 6.Task-Force Heart Rate Variability - Standards of measurement, physiological interpretation, and clinical use. Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Circulation. 1996;93:1043–1065. doi: 10.1161/01.CIR.93.5.1043. [DOI] [PubMed] [Google Scholar]
- 7.Eckberg DL. The human respiratory gate. The Journal of Physiology. 2003;548:339–352. doi: 10.1113/jphysiol.2003.037192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Duschek S, Muckenthaler M, Werner N, del Paso GA. Relationships between features of autonomic cardiovascular control and cognitive performance. Biol Psychol. 2009;81:110–117. doi: 10.1016/j.biopsycho.2009.03.003. [DOI] [PubMed] [Google Scholar]
- 9.Butler EA, Wilhelm FH, Gross JJ. Respiratory sinus arrhythmia, emotion, and emotion regulation during social interaction. Psychophysiology. 2006;43:612–622. doi: 10.1111/j.1469-8986.2006.00467.x. [DOI] [PubMed] [Google Scholar]
- 10.Hastings, P. D. & Miller, J. G. In Prosocial Development - A Multidimensional Approach (eds Padilla-Walker, L. M. & Carlo, G.) 112–127 (Oxford University Press, 2014).
- 11.Stellar JE, Cohen AB, Oveis C, Keltner D. Affective and Physiological Responses to the Suffering of Others: Compassion and Vagal Activity. Journal of Personality and Social Psychology. 2015;108:572–585. doi: 10.1037/pspi0000010. [DOI] [PubMed] [Google Scholar]
- 12.Gill KL, Calkins SD. Do aggressive/destructive toddlers lack concern for others? Behavioral and physiological indicators of empathic responding in 2-year-old children. Development and Psychopathology. 2003;15:55–71. doi: 10.1017/S095457940300004X. [DOI] [PubMed] [Google Scholar]
- 13.Rockliff H, Gilbert P, McEwan K, Lightman S, Glover D. A pilot exploration of heart rate variability and salivary cortisol responses to compassion-focused imagery. Clinical Neuropsychiatry. 2008;5:132–139. [Google Scholar]
- 14.Miller JG, Kahle S, Hastings PD. Roots and Benefits of Costly Giving: Children Who Are More Altruistic Have Greater Autonomic Flexibility and Less Family Wealth. Psychol Sci. 2015;26:1038–1045. doi: 10.1177/0956797615578476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Barraza JA, Alexander V, Beavin LE, Terris ET, Zak PJ. The heart of the story: Peripheral physiology during narrative exposure predicts charitable giving. Biol Psychol. 2015;105:138–143. doi: 10.1016/j.biopsycho.2015.01.008. [DOI] [PubMed] [Google Scholar]
- 16.Bornemann B, Kok BE, Böckler A, Singer T. Helping from the heart: Voluntary upregulation of heart rate variability predicts altruistic behavior. Biological Psychology. 2016;119:54–63. doi: 10.1016/j.biopsycho.2016.07.004. [DOI] [PubMed] [Google Scholar]
- 17.Panksepp, J. In About a Body: working with the embodied mind in psychotherapy (eds Corrigall, J., Payne, H. & Wilkinson, H.) (Hove, UK & NYC, 2006).
- 18.Depue RA, Morrone-Strupinsky JV. A neurobehavioral model of affiliative bonding: Implications for conceptualizing a human trait of affiliation. Behavioral and Brain Sciences. 2005;28:313–395. doi: 10.1017/s0140525x05000063. [DOI] [PubMed] [Google Scholar]
- 19.Carter CS. Oxytocin pathways and the evolution of human behavior. Annual review of psychology. 2014;65:17–39. doi: 10.1146/annurev-psych-010213-115110. [DOI] [PubMed] [Google Scholar]
- 20.Preston SD. The origins of altruism in offspring care. Psychological Bulletin. 2013;139:1305–1341. doi: 10.1037/a0031755. [DOI] [PubMed] [Google Scholar]
- 21.Tribollet E, Charpak S, Schmidt A, Dubois-Dauphin M, Dreifuss J. Appearance and transient expression of oxytocin receptors in fetal, infant, and peripubertal rat brain studied by autoradiography and electrophysiology. The Journal of Neuroscience. 1989;9:1764–1773. doi: 10.1523/JNEUROSCI.09-05-01764.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Charpak S, Armstrong W, Mühlethaler M, Dreifuss J-J. Stimulatory action of oxytocin on neurones of the dorsal motor nucleus of the vagus nerve. Brain research. 1984;300:83–89. doi: 10.1016/0006-8993(84)91342-8. [DOI] [PubMed] [Google Scholar]
- 23.Higa KT, Mori E, Viana FF, Morris M, Michelini LC. Baroreflex control of heart rate by oxytocin in the solitary-vagal complex. American Journal of Physiology-Regulatory, Integrative and Comparative Physiology. 2002;282:R537–R545. doi: 10.1152/ajpregu.00806.2000. [DOI] [PubMed] [Google Scholar]
- 24.Boccia M, Petrusz P, Suzuki K, Marson L, Pedersen CA. Immunohistochemical localization of oxytocin receptors in human brain. Neuroscience. 2013;253:155–164. doi: 10.1016/j.neuroscience.2013.08.048. [DOI] [PubMed] [Google Scholar]
- 25.Loup F, Tribollet E, Dubois-Dauphin M, Pizzolato G, Dreifuss J. Localization of oxytocin binding sites in the human brainstem and upper spinal cord: an autoradiographic study. Brain research. 1989;500:223–230. doi: 10.1016/0006-8993(89)90317-X. [DOI] [PubMed] [Google Scholar]
- 26.Sofroniew M, Weindl A, Schrell U, Wetzstein R. Immunohistochemistry of vasopressin, oxytocin and neurophysin in the hypothalamus and extrahypothalamic regions of the human and primate brain. Acta histochemica. Supplementband. 1980;24:79–95. [PubMed] [Google Scholar]
- 27.Engert V, Koester AM, Riepenhausen A, Singer T. Boosting recovery rather than buffering reactivity: Higher stress-induced oxytocin secretion is associated with increased cortisol reactivity and faster vagal recovery after acute psychosocial stress. Psychoneuroendocrinology. 2016;74:111–120. doi: 10.1016/j.psyneuen.2016.08.029. [DOI] [PubMed] [Google Scholar]
- 28.Kubzansky LD, Mendes WB, Appleton AA, Block J, Adler GK. A heartfelt response: Oxytocin effects on response to social stress in men and women. Biological Psychology. 2012;90:1–9. doi: 10.1016/j.biopsycho.2012.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kemp AH, et al. Oxytocin increases heart rate variability in humans at rest: implications for social approach-related motivation and capacity for social engagement. PLoS One. 2012;7:e44014. doi: 10.1371/journal.pone.0044014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Norman GJ, et al. Oxytocin increases autonomic cardiac control: moderation by loneliness. Biol Psychol. 2011;86:174–180. doi: 10.1016/j.biopsycho.2010.11.006. [DOI] [PubMed] [Google Scholar]
- 31.Lehrer PM, et al. Biofeedback treatment for asthma. Chest Journal. 2004;126:352–361. doi: 10.1378/chest.126.2.352. [DOI] [PubMed] [Google Scholar]
- 32.Lehrer PM, et al. Heart rate variability biofeedback: effects of age on heart rate variability, baroreflex gain, and asthma. Chest Journal. 2006;129:278–284. doi: 10.1378/chest.129.2.278. [DOI] [PubMed] [Google Scholar]
- 33.Del Pozo JM, Gevirtz RN, Scher B, Guarneri E. Biofeedback treatment increases heart rate variability in patients with known coronary artery disease. American heart journal. 2004;147:545. doi: 10.1016/j.ahj.2003.08.013. [DOI] [PubMed] [Google Scholar]
- 34.Karavidas MK, et al. Preliminary results of an open label study of heart rate variability biofeedback for the treatment of major depression. Appl Psychophysiol Biofeedback. 2007;32:19–30. doi: 10.1007/s10484-006-9029-z. [DOI] [PubMed] [Google Scholar]
- 35.Zucker TL, Samuelson KW, Muench F, Greenberg MA, Gevirtz RN. The effects of respiratory sinus arrhythmia biofeedback on heart rate variability and posttraumatic stress disorder symptoms: A pilot study. Appl Psychophysiol Biofeedback. 2009;34:135–143. doi: 10.1007/s10484-009-9085-2. [DOI] [PubMed] [Google Scholar]
- 36.Metz CN, Tracey KJ. It takes nerve to dampen inflammation. Nat Immunol. 2005;6:756–757. doi: 10.1038/ni0805-756. [DOI] [PubMed] [Google Scholar]
- 37.Hamill, R. W., Shapiro, R. E. & Vizzard, M. A. In Primer on the Autonomic Nervous System (Third Edition) (eds Biaggioni, I., Burnstock, G., Low, P. A. & Paton, J. F. R.) 17–26 (Academic Press, 2012).
- 38.Stock S, Uvnäs-Moberg K. Increased plasma levels of oxytocin in response to afferent electrical stimulation of the sciatic and vagal nerves and in response to touch and pinch in anaesthetized rats. Acta Physiologica Scandinavica. 1988;132:29–34. doi: 10.1111/j.1748-1716.1988.tb08294.x. [DOI] [PubMed] [Google Scholar]
- 39.Uvnäs-Moberg K. Role of efferent and afferent vagal nerve activity during reproduction: integrating function of oxytocin on metabolism and behaviour. Psychoneuroendocrinology. 1994;19:687–695. doi: 10.1016/0306-4530(94)90050-7. [DOI] [PubMed] [Google Scholar]
- 40.McCall C, Singer T. The animal and human neuroendocrinology of social cognition, motivation and behavior. Nat Neurosci. 2012;15:681–688. doi: 10.1038/nn.3084. [DOI] [PubMed] [Google Scholar]
- 41.McCorry LK. Physiology of the Autonomic Nervous System. American Journal of Pharmaceutical Education. 2007;71:78. doi: 10.5688/aj710478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Porges SW. Social engagement and attachment. Annals of the New York Academy of Sciences. 2003;1008:31–47. doi: 10.1196/annals.1301.004. [DOI] [PubMed] [Google Scholar]
- 43.Kabat-Zinn, J. Full catastrophe living: Using the wisdom of your body and mind to face stress, pain, and illness. (Delta Trade Paperbacks, 1990).
- 44.Williams, M., Teasdale, J., Segal, Z. & Kabat-Zinn, J. The Mindful Way Through Depression - Freeing Yourself from Chronic Unhappiness (2007).
- 45.Chiesa A, Serretti A. Mindfulness-based stress reduction for stress management in healthy people: a review and meta-analysis. Journal of alternative and complementary medicine (New York, N.Y.) 2009;15:593–600. doi: 10.1089/acm.2008.0495. [DOI] [PubMed] [Google Scholar]
- 46.Carmody J, Baer RA. Relationships between mindfulness practice and levels of mindfulness, medical and psychological symptoms and well-being in a mindfulness-based stress reduction program. Journal of behavioral medicine. 2008;31:23–33. doi: 10.1007/s10865-007-9130-7. [DOI] [PubMed] [Google Scholar]
- 47.Lutz A, Brefczynski-Lewis J, Johnstone T, Davidson RJ. Regulation of the Neural Circuitry of Emotion by Compassion Meditation: Effects of Meditative Expertise. PloS One. 2008;3:e1897. doi: 10.1371/journal.pone.0001897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dahl CJ, Lutz A, Davidson RJ. Reconstructing and deconstructing the self: cognitive mechanisms in meditation practice. Trends in cognitive sciences. 2015;19:515–523. doi: 10.1016/j.tics.2015.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Singer, T. et al. The ReSource Project: Background, Design, Samples, and Measurements (2nd ed.). (Max Planck Institute for Human Cognitive and Brain Sciences, 2016).
- 50.Engen HG, Singer T. Affect and motivation are critical in constructive meditation. Trends in cognitive sciences. 2016;20:159–160. doi: 10.1016/j.tics.2015.11.004. [DOI] [PubMed] [Google Scholar]
- 51.Kok BE, Singer T. Effects of contemplative dyads on engagement and perceived social connectedness over 9 months of mental training: A randomized clinical trial. Jama psychiatry. 2017;74:126–134. doi: 10.1001/jamapsychiatry.2016.3360. [DOI] [PubMed] [Google Scholar]
- 52.Arch JJ, et al. Self-compassion training modulates alpha-amylase, heart rate variability, and subjective responses to social evaluative threat in women. Psychoneuroendocrinology. 2014;42:49–58. doi: 10.1016/j.psyneuen.2013.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kemeny ME, et al. Contemplative/emotion training reduces negative emotional behavior and promotes prosocial responses. Emotion. 2012;12:338–350. doi: 10.1037/a0026118. [DOI] [PubMed] [Google Scholar]
- 54.Grossman, P., Deuring, G., Walach, H., Schwarzer, B. & Schmidt, S. Mindfulness-based Intervention does not Influence Cardiac Autonomic Control or Pattern of Physical Activity in Fibromyalgia During Daily Life: An Ambulatory, Multi-measure Randomized Controlled Trial*. The Clinical Journal of Pain (2016). [DOI] [PubMed]
- 55.Bornemann B, Singer T. Taking time to feel our body: Steady increases in heartbeat perception accuracy and decreases in alexithymia over 9 months of contemplative mental training. Psychophysiology. 2017;54:469–482. doi: 10.1111/psyp.12790. [DOI] [PubMed] [Google Scholar]
- 56.Patriquin MA, Scarpa A, Friedman BH, Porges SW. Respiratory sinus arrhythmia: A marker for positive social functioning and receptive language skills in children with autism spectrum disorders. Developmental psychobiology. 2013;55:101–112. doi: 10.1002/dev.21002. [DOI] [PubMed] [Google Scholar]
- 57.Sarang P, Telles S. Effects of two yoga based relaxation techniques on heart rate variability (HRV) International Journal of Stress Management. 2006;13:460–475. doi: 10.1037/1072-5245.13.4.460. [DOI] [Google Scholar]
- 58.Peng CK, et al. Heart rate dynamics during three forms of meditation. International journal of cardiology. 2004;95:19–27. doi: 10.1016/j.ijcard.2003.02.006. [DOI] [PubMed] [Google Scholar]
- 59.Bakermans-Kranenburg MJ, van Ijzendoorn MH. Oxytocin receptor (OXTR) and serotonin transporter (5-HTT) genes associated with observed parenting. Social cognitive and affective neuroscience. 2008;3:128–134. doi: 10.1093/scan/nsn004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Riem MM, Pieper S, Out D, Bakermans-Kranenburg MJ, van IJzendoorn MH. Oxytocin receptor gene and depressive symptoms associated with physiological reactivity to infant crying. Social cognitive and affective neuroscience. 2011;6:294–300. doi: 10.1093/scan/nsq035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tost H, et al. A common allele in the oxytocin receptor gene (OXTR) impacts prosocial temperament and human hypothalamic-limbic structure and function. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:13936–13941. doi: 10.1073/pnas.1003296107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.McQuaid, R. J., McInnis, O. A., Matheson, K. & Anisman, H. Distress of ostracism: oxytocin receptor gene polymorphism confers sensitivity to social exclusion. Social cognitive and affective neuroscience, nsu166 (2015). [DOI] [PMC free article] [PubMed]
- 63.Chen FS, et al. Common oxytocin receptor gene (OXTR) polymorphism and social support interact to reduce stress in humans. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:19937–19942. doi: 10.1073/pnas.1113079108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Grossman P, Beek JV, Wientjes C. A comparison of three quantification methods for estimation of respiratory sinus arrhythmia. Psychophysiology. 1990;27:702–714. doi: 10.1111/j.1469-8986.1990.tb03198.x. [DOI] [PubMed] [Google Scholar]
- 65.Grossman P, Karemaker J, Wieling W. Prediction of tonic parasympathetic cardiac control using respiratory sinus arrhythmia: the need for respiratory control. Psychophysiology. 1991;28:201–216. doi: 10.1111/j.1469-8986.1991.tb00412.x. [DOI] [PubMed] [Google Scholar]
- 66.Cribbet MR, Williams PG, Gunn HE, Rau HK. Effects of tonic and phasic respiratory sinus arrhythmia on affective stress responses. Emotion. 2011;11:188–193. doi: 10.1037/a0021789. [DOI] [PubMed] [Google Scholar]
- 67.Bland JM, Altman DG. Regression towards the mean. BMJ: British Medical Journal. 1994;308:1499. doi: 10.1136/bmj.308.6942.1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Berntson GG, Cacioppo JT, Quigley KS. Respiratory sinus arrhythmia: autonomic origins, physiological mechanisms, and psychophysiological implications. Psychophysiology. 1993;30:183–196. doi: 10.1111/j.1469-8986.1993.tb01731.x. [DOI] [PubMed] [Google Scholar]
- 69.Geisler FC, Kubiak T, Siewert K, Weber H. Cardiac vagal tone is associated with social engagement and self-regulation. Biol Psychol. 2013;93:279–286. doi: 10.1016/j.biopsycho.2013.02.013. [DOI] [PubMed] [Google Scholar]
- 70.Panksepp J. Affective consciousness: Core emotional feelings in animals and humans. Consciousness and cognition. 2005;14:30–80. doi: 10.1016/j.concog.2004.10.004. [DOI] [PubMed] [Google Scholar]
- 71.Germer, C. K. The Mindful Path to Self-Compassion. (Guilford Press, 2009).
- 72.Ellis, P. D. The essential guide to effect sizes: Statistical power, meta-analysis, and the interpretation of research results. (Cambridge University Press, 2010).
- 73.Acharya, U. R., Joseph, K. P., Kannathal, N., Min, L. C. & Suri, J. S. In Advances in cardiac signal processing 121–165 (Springer, 2007).
- 74.Miller JG, et al. Children’s dynamic RSA change during anger and its relations with parenting, temperament, and control of aggression. Biol Psychol. 2013;92:417–425. doi: 10.1016/j.biopsycho.2012.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Muhtadie Luma, Koslov Katrina, Akinola Modupe, Mendes Wendy Berry. Vagal flexibility: A physiological predictor of social sensitivity. Journal of Personality and Social Psychology. 2015;109(1):106–120. doi: 10.1037/pspp0000016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Tang YY, et al. Central and autonomic nervous system interaction is altered by short-term meditation. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:8865–8870. doi: 10.1073/pnas.0904031106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Leslie R. Neuroactive substances in the dorsal vagal complex of the medulla oblongata: nucleus of the tractus solitarius, area postrema, and dorsal motor nucleus of the vagus. Neurochemistry international. 1985;7:191–211. doi: 10.1016/0197-0186(85)90106-8. [DOI] [PubMed] [Google Scholar]
- 78.Pocock, G., Richards, C. D. & Richards, D. A. Human physiology. (Oxford university press, 2013).
- 79.Loewy AD, Wallach JH, McKellar S. Efferent connections of the ventral medulla oblongata in the rat. Brain Research Reviews. 1981;3:63–80. doi: 10.1016/0165-0173(81)90012-6. [DOI] [PubMed] [Google Scholar]
- 80.Lane, R. D., Reiman, E. M., Ahern, G. L. & Thayer, J. F. In Brain and Cognition. 97–100 (Academinc Press Inc 525 B ST, STE 1900, San Diego, CA 92101–4495, USA).
- 81.Thayer JF, Ahs F, Fredrikson M, Sollers JJ, III., Wager TD. A meta-analysis of heart rate variability and neuroimaging studies: implications for heart rate variability as a marker of stress and health. Neuroscience and biobehavioral reviews. 2012;36:747–756. doi: 10.1016/j.neubiorev.2011.11.009. [DOI] [PubMed] [Google Scholar]
- 82.Lutz A, Greischar LL, Rawlings NB, Ricard M, Davidson RJ. Long-term meditators self-induce high-amplitude gamma synchrony during mental practice. Proceedings of the national Academy of Sciences. 2004;101:16369–16373. doi: 10.1073/pnas.0407401101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Calkins, S. D. The emergence of self-regulation: Biological and behavioral control mechanisms supporting toddler competencies. Socioemotional development in the toddler years: Transitions and transformations, 261–284 (2007).
- 84.Rottenberg J, Wilhelm FH, Gross JJ, Gotlib IH. Vagal rebound during resolution of tearful crying among depressed and nondepressed individuals. Psychophysiology. 2003;40:1–6. doi: 10.1111/1469-8986.00001. [DOI] [PubMed] [Google Scholar]
- 85.Schmitz J, Krämer M, Tuschen‐Caffier B, Heinrichs N, Blechert J. Restricted autonomic flexibility in children with social phobia. Journal of Child Psychology and Psychiatry. 2011;52:1203–1211. doi: 10.1111/j.1469-7610.2011.02417.x. [DOI] [PubMed] [Google Scholar]
- 86.Clamor A, Koenig J, Thayer JF, Lincoln TM. A randomized-controlled trial of heart rate variability biofeedback for psychotic symptoms. Behaviour research and therapy. 2016;87:207–215. doi: 10.1016/j.brat.2016.10.003. [DOI] [PubMed] [Google Scholar]
- 87.Geisler FC, Vennewald N, Kubiak T, Weber H. The impact of heart rate variability on subjective well-being is mediated by emotion regulation. Personality and Individual Differences. 2010;49:723–728. doi: 10.1016/j.paid.2010.06.015. [DOI] [Google Scholar]
- 88.Boehm JK, Kubzansky LD. The heart’s content: the association between positive psychological well-being and cardiovascular health. Psychological bulletin. 2012;138:655. doi: 10.1037/a0027448. [DOI] [PubMed] [Google Scholar]
- 89.Sharpley CF. Heart rate reactivity and variability as psychophysiological links between stress, anxiety, depression, and cardiovascular disease: implications for health psychology interventions. Australian Psychologist. 2002;37:56–62. doi: 10.1080/00050060210001706686. [DOI] [Google Scholar]
- 90.Smeets T. Autonomic and hypothalamic–pituitary–adrenal stress resilience: Impact of cardiac vagal tone. Biological Psychology. 2010;84:290–295. doi: 10.1016/j.biopsycho.2010.02.015. [DOI] [PubMed] [Google Scholar]
- 91.Boisgontier MP, Cheval B. The anova to mixed model transition. Neuroscience & Biobehavioral Reviews. 2016;68:1004–1005. doi: 10.1016/j.neubiorev.2016.05.034. [DOI] [PubMed] [Google Scholar]
- 92.Wallace, B. A. The Attention Revolution - Unlocking the Power of the Focussed Mind. (Wisdom Publications, 2006).
- 93.Hart, W. The Art of Living: Vipassana-Meditation as Taught by S.N. Goenka. (Harper and Row, 1987).
- 94.Salzberg, S. Loving-Kindness - The Revolutionary Art of Happiness. (Shambala Publications, 1995).
- 95.Frattaroli J. Experimental disclosure and its moderators: A meta-analysis. Psychological Bulletin. 2006;132:823–865. doi: 10.1037/0033-2909.132.6.823. [DOI] [PubMed] [Google Scholar]
- 96.Pasupathi M. Emotion regulation during social remembering: Differences between emotions elicited during an event and emotions elicited when talking about it. Memory. 2003;11:151–163. doi: 10.1080/741938212. [DOI] [PubMed] [Google Scholar]
- 97.Rimé, B. In Handbook of emotion regulation (ed. Gross, J. J.) Ch. 466–485, (Guilford Press, 2007).
- 98.Steindl-Rast, D. Gratefulness, the heart of prayer - An Approach to Life in Fullness. (Paulist Press, 1984).
- 99.Emmons RA, McCullough ME. Counting blessings versus burdens: An experimental investigation of gratitude and subjective well-being in daily life. Journal of Personality and Social Psychology. 2003;84:377–389. doi: 10.1037/0022-3514.84.2.377. [DOI] [PubMed] [Google Scholar]
- 100.Bartlett MY, DeSteno D. Gratitude and Prosocial Behavior. Psychological Science. 2006;17:319–325. doi: 10.1111/j.1467-9280.2006.01705.x. [DOI] [PubMed] [Google Scholar]
- 101.Fronsdal, G. The Issue at Hand - Essays on Buddhist Mindfulness Practice (2001).
- 102.Krishnamurti, J. On mind and thought. (Harper, 1993).
- 103.Holmes, T. Parts Work: An Illustrated Guide to Your Inner Life. (Winged Heart Press, 2007).
- 104.Schwartz, R. C. Internal family systems therapy. (Guilford Press, 1997).
- 105.Frith C, Frith U. Theory of mind. Current Biology. 2005;15:R644–R645. doi: 10.1016/j.cub.2005.08.041. [DOI] [PubMed] [Google Scholar]
- 106.Kaufmann T, Sütterlin S, Schulz S, Vögele C. ARTiiFACT: a tool for heart rate artifact processing and heart rate variability analysis. Behav Res. 2011;43:1161–1170. doi: 10.3758/s13428-011-0107-7. [DOI] [PubMed] [Google Scholar]
- 107.Berntson GG, et al. Heart rate variability: origins methods and interpretative caveats. Psychophysiology. 1997;34:623–648. doi: 10.1111/j.1469-8986.1997.tb02140.x. [DOI] [PubMed] [Google Scholar]
- 108.Eckberg DL. Point: counterpoint: respiratory sinus arrhythmia is due to a central mechanism vs. respiratory sinus arrhythmia is due to the baroreflex mechanism. Journal of applied physiology. 2009;106:1740–1742. doi: 10.1152/japplphysiol.91107.2008. [DOI] [PubMed] [Google Scholar]
- 109.Pramanik T, et al. Immediate effect of slow pace bhastrika pranayama on blood pressure and heart rate. The Journal of Alternative and Complementary Medicine. 2009;15:293–295. doi: 10.1089/acm.2008.0440. [DOI] [PubMed] [Google Scholar]
- 110.Bates, D., Mächler, M., Bolker, B. & Walker, S. Fitting Linear Mixed-Effects Models Using lme4. Journal of Statistical Software; Vol 1, Issue 1 (2015).
- 111.Gelman A, Hill J, Yajima M. Why we (usually) don’t have to worry about multiple comparisons. Journal of Research on Educational Effectiveness. 2012;5:189–211. doi: 10.1080/19345747.2011.618213. [DOI] [Google Scholar]
- 112.Koenig J, et al. Body mass index is related to autonomic nervous system activity as measured by heart rate variability—a replication using short term measurements. The journal of nutrition, health & aging. 2014;18:300–302. doi: 10.1007/s12603-014-0022-6. [DOI] [PubMed] [Google Scholar]
- 113.Antelmi I, et al. Influence of age, gender, body mass index, and functional capacity on heart rate variability in a cohort of subjects without heart disease. The American journal of cardiology. 2004;93:381–385. doi: 10.1016/j.amjcard.2003.09.065. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data are available upon reasonable request from the Department of Social Neuroscience of the Max Planck Institute for Human Cognitive and Brain Sciences.