Abstract
Spinal metastasis is a rare presentation of paraganglioma and an effective therapy for nonresectable spinal metastatic paraganglioma (MPG) has not yet been established. We report the case of a 42-year-old woman with metastatic spinal cord compression caused by a relapsed spinal MPG after decompressive surgery. We performed transcatheter arterial embolization (TAE) in addition to systemic chemotherapy. After TAE, the neurologic symptoms improved, and the back pain was reduced. After 3 sessions of TAE, MRI revealed that the tumor at the level of the seventh thoracic vertebra had shrunk and the pressure on spinal cord had decreased. TAE might be a feasible treatment option for spinal MPG, even after surgery or irradiation.
Keywords: Metastatic paraganglioma, Transcatheter arterial embolization, Spinal metastasis, Spinal cord compression
Introduction
Paraganglioma (PG) is a rare neuroendocrine tumor with an annual incidence of only 1/100,000 [1], [2]. Approximately from 10% to 20% of patients will develop malignant PGs [3]; reports of spinal metastatic PGs (MPG) are quite rare. Surgical total resection is the mainstay of the treatment of spinal MPGs and radiation should be an alternative therapy when total resection is not advisable [4], [5]. An effective therapy for relapsed spinal MPGs has not yet been established. Here, we report the case of a 42-year-old woman with metastatic spinal cord compression (MSCC) caused by relapsed spinal MPG after decompressive surgery. We performed transcatheter arterial embolization (TAE) in addition to systemic chemotherapy, which included cyclophosphamide, vincristine, and dacarbazine (CVD). The patient's symptoms improved, and the tumor shrank.
Case report
A 42-year-old woman with a history of hypertension underwent surgical resection of a retroperitoneal PG in 2005. Postoperative blood pressure returned to normal range with no evidence of distant metastasis during a 4-year follow-up. However, the patient experienced severe back pain in 2009. Magnetic resonance imaging (MRI) revealed a spinal mass at the level of the seventh thoracic vertebra (Th7). The patient's blood pressure remained normal, but 123I-metaiodobenzylguanidine (123I-MIBG) showed strong uptake at the site of the mass, and the patient was diagnosed as having a spinal MPG. The mass widely invaded the chest wall and was unamenable to surgical resection. Therefore, CVD chemotherapy was administered for 2 cycles, and the patient was treated with 131I-MIBG at 5.55 MBq (150 mCi) for 3 sessions, and external beam radiotherapy (25 Gy in 5 fractions). After this therapy, the back pain was relieved.
However, after a 4-year follow-up, paralysis, paresthesia, and numbness of the lower limbs appeared in 2016. MRI revealed regrowth of the Th7 tumor, and the lesion protruded into the spinal canal. In addition, a new mass appeared on the right side of the fifth thoracic vertebra (Th5). Because the patient presented with bladder and bowel dysfunction, she was diagnosed as having MSCC caused by the relapsed spinal MPG. Urgent surgery (a sixth-eighth thoracic spinal laminectomy and decompressive surgery) was performed (Fig. 1). A pathologic examination confirmed the diagnosis of spinal MPG (Fig. 2). The patient underwent postoperative rehabilitation and CVD chemotherapy was administered for 7 cycles.
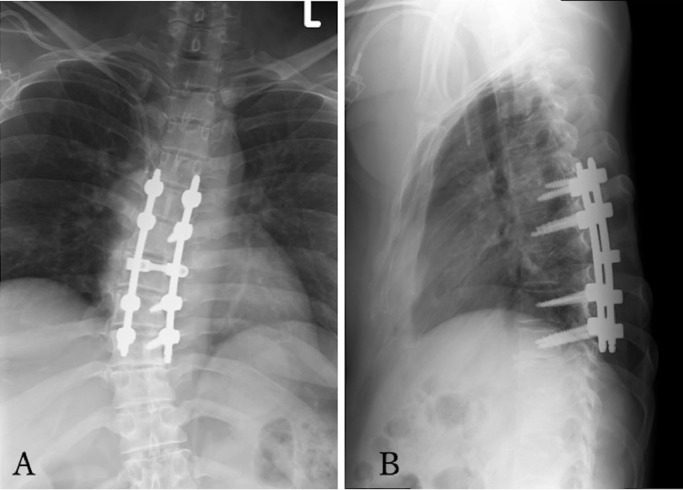
Fig. 1.
(A) Radiograph in anteroposterior view after arthrodesis of T5-T9. (B) Radiograph in lateral view after arthrodesis of T5-T9.
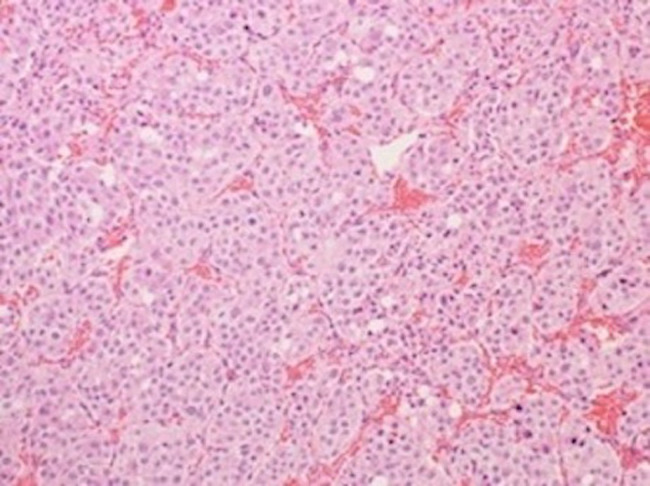
Fig. 2.
Pathohistology of the spinal metastasis (hematoxylin–eosin staining, × 200). The tumor is composed of heteromorphic cells with small and large circular nuclei with prominent nucleoli. The nests of tumor cells are separated by vascular septa. This is a so called Zellballen structure, which is characteristic of paraganglioma.
However, 8 months after surgery, remarkable back pain and numbness of the lower limbs recurred. Computed tomography showed regrowth of the tumor at Th7. Surgical resection was not considered practical and reirradiation unfeasible because of dose limitation to the spinal cord, therefore we performed TAE for local control and to relieve the symptoms, in addition to CVD chemotherapy.
First, TAE was performed 1 week after CVD chemotherapy administration. A 4.0 Fr catheter (Cobra; Terumo, Tokyo, Japan) was inserted from the right femoral artery using the Seldinger technique and an angiogram was obtained. The feeders of the tumor were the right sixth, the right seventh, the left eighth, and the right ninth intercostal arteries. These were superselectively catheterized using a 2.0 Fr microcatheter (Estream; Toray, Tokyo, Japan). Each angiogram demonstrated a tumor blush that corresponded to the spinal metastasis. Embolization was performed using 300-500 μm Embosphere microspheres (Merit Medical, South Jordan, UT) until tumor blush had disappeared (Fig. 3). However, as the right seventh intercostal arterial angiogram showed a posterior spinal artery besides tumor blush, embolization was not performed. The metastatic lesion of the Th5 spine had no symptoms, and TAE was not performed. During TAE, an anesthesiologist monitored the patient's blood pressure, but there was no obvious change in her circulation. On the same day, but after TAE, numbness of the lower limbs improved, and her back pain was relieved from a numerical rating scale of 10 to 1. Subsequently, we repeated TAE of the right sixth, the left eighth, and the right ninth intercostal arteries approximately every 4 weeks, in addition to CVD chemotherapy. After 3 sessions of TAE and CVD chemotherapy, MRI revealed that the Th7 spinal tumor had shrunk and the pressure on spinal cord had decreased (Fig. 4A and C). By contrast, the size of the metastatic lesion at the Th5 spine, for which we did not perform TAE, had increased slightly (Fig. 4B and D). The treatment course was good and there was no complication associated with this combined therapy. Currently, the patient is under treatment as an outpatient at our hospital.
Fig. 3.
(A) Angiogram of the right sixth intercostal artery demonstrates the tumor blush that corresponds to the spinal metastasis (arrow). (B) After TAE, the tumor blush disappears.
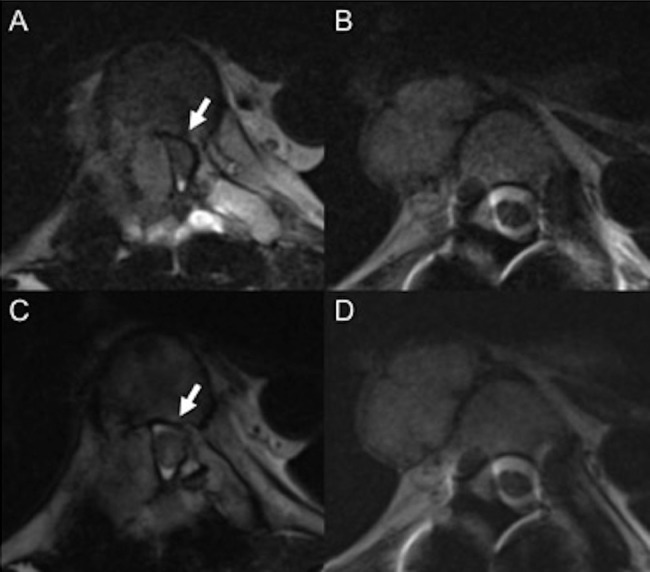
Fig. 4.
T2-weighted MRI before TAE (A-B). T2-weighted MRI after three sessions of TAE (C-D). (A) A tumor in the right Th7 pedicle strongly compresses the dural sac from the right side (arrow). (B) A tumor (3.3 cm × 2.1 cm) on the right side of the Th5 vertebral body (arrow head). (C) The tumor in the Th7 right pedicle shrinks and compression of the dural sac is reduced. (D) The size of the tumor on the right side of Th5 vertebral body, for which we did not perform TAE, increases slightly (3.4 cm × 2.3 cm) compared with B.
Discussion
MSCC is a medical emergency that requires rapid treatment to reduce pain and to preserve neurologic functioning [6], [7]. Surgical resection is the mainstay of treatment for MSCC caused by spinal MPG and radiotherapy should be chosen as an alternative when total resection is unfeasible [3], [4]. Relapsed cases are often difficult to rescue. Here, we performed TAE as a rescue treatment for a relapsed case after surgical resection and radiotherapy. TAE was performed 1 week after administering CVD chemotherapy and we performed this combined therapy for 3 sessions. The lesion of Th7 for which we performed TAE shrank; however, the lesion of Th5 for which we did not perform TAE increased slightly. This result indicates that TAE might contribute to local control. As far as we know, there have been no reports of TAE performed for spinal MPGs. To our knowledge, this is the first report of TAE in addition to CVD chemotherapy for relapsed spinal MPG.
TAE suppresses tumor growth by interrupting its blood supply [8]. There are similar reports that transcatheter arterial chemoembolization provides a longer antitumor effect because of a partial response to local anticancer drugs [9]. In the present case, because systemic chemotherapy (CVD therapy) was continued, only TAE was performed. The efficiency of TAE for hypervascular tumors has been clarified and the vascularity of the tumor is important for TAE [8], [9], [10]. PGs are known as hypervascular tumors; therefore, TAE may have been effective in reducing PGs. In this case, the tumor shrank after TAE and good pain relief was obtained. Koike et al reported transcatheter arterial chemoembolization/TAE on 24 bone metastases and found good pain relief was obtained in 20 patients (response rate: 83.3%) [10].
While TAE is effective as a treatment for bone metastases, embolization in the nontarget region is a complication of TAE. Before embolization, it is necessary to check the feeders of the tumor and confirm that there is no blood flow in nontarget regions. When blood flow is observed in a nontarget region, we consider a blood flow alteration technique using coils. When the lesions are associated with spinal arteries, TAE should not be performed to avoid spinal cord infarction. In this case, as the right seventh intercostal arterial angiogram showed a posterior spinal artery, we did not perform TAE for this vessel.
We performed TAE for the Th7 spinal MPG in 3 sessions and the patient's symptoms improved and the tumor shrank. TAE can be performed even after surgery or radiotherapy and TAE with systemic chemotherapy is also applicable. By contrast with radiotherapy using dose limitation, TAE can be repeated when necessary [7]. In conclusion, we consider TAE is a feasible treatment option for spinal MPG, even after surgery or irradiation.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.radcr.2019.05.004.
Appendix. Supplementary materials
References
- 1.Simpson L.N., Hughes B.D., Karikari I.O., Mehta A.I., Hodges T.R., Cummings T.J. Catecholamine-secreting paraganglioma of the thoracic spinal column: report of an unusual case and review of the literature. Neurosurgery. 2012;70(4):E1049–E1052. doi: 10.1227/NEU.0b013e31822e5aae. (discussion E52) [DOI] [PubMed] [Google Scholar]; Simpson L.N., Hughes B.D., Karikari I.O., et al.AU: Please note that as per the journal style, if there are more than six authors, the first six author names are listed followed by ‘et al.’ Therefore, in Ref(s). 1,4,5,8,9,10.Please provide the names of all authors if the author group consists of six authors or fewer (2012) Catecholamine-secreting paraganglioma of the thoracic spinal column: report of an unusual case and review of the literature. Neurosurgery70(4): E1049–E1052(discussion E52) [DOI] [PubMed]
- 2.Lehmen J.A., Babbel D.M., Mikhitarian K., Choma T.J. Paraganglioma presenting as metastatic lesion in a cervical vertebra: a case report and review of the literature. Spine. 2010;35(5):E152–E154. doi: 10.1097/BRS.0b013e3181cf2c96. [DOI] [PubMed] [Google Scholar]; Lehmen J.A., Babbel D.M., Mikhitarian K., Choma T.J. (2010) Paraganglioma presenting as metastatic lesion in a cervical vertebra: a case report and review of the literature. Spine35(5): E152–E154 [DOI] [PubMed]
- 3.Lau D., La Marca F., Camelo-Piragua S., Park P. Metastatic paraganglioma of the spine: case report and review of the literature. Clin Neurol Neurosurg. 2013;115(9):1571–1574. doi: 10.1016/j.clineuro.2013.01.006. [DOI] [PubMed] [Google Scholar]; Lau D., La Marca F., Camelo-Piragua S., Park P. (2013) Metastatic paraganglioma of the spine: case report and review of the literature. Clin Neurol Neurosurg115(9):1571–1574 [DOI] [PubMed]
- 4.Jia Q., Yin H., Yang J., Wu Z., Yan W., Zhou W. Treatment and outcome of metastatic paraganglioma of the spine. Eur Spine J. 2017 doi: 10.1007/s00586-017-5140-5. [DOI] [PubMed] [Google Scholar]; Jia Q., Yin H., Yang J., et al. (2017) Treatment and outcome of metastatic paraganglioma of the spine. Eur Spine J. doi:10.1007/s00586-017-5140-5 [DOI] [PubMed]
- 5.Yin M., Huan Q., Sun Z., He S., Xia Y., Mo W. Clinical characteristics and surgical treatment of spinal paraganglioma: a case series of 18 patients. Clin Neurol Neurosurg. 2017;158:20–26. doi: 10.1016/j.clineuro.2017.03.019. [DOI] [PubMed] [Google Scholar]; Yin M., Huan Q., Sun Z., et al. (2017) Clinical characteristics and surgical treatment of spinal paraganglioma: a case series of 18 patients. Clin Neurol Neurosurg158:20–26 [DOI] [PubMed]
- 6.Prasad D., Schiff D. Malignant spinal cord compression. Lancet Oncol. 2005;6:15–24. doi: 10.1016/S1470-2045(04)01709-7. [DOI] [PubMed] [Google Scholar]; Prasad D., Schiff D. (2005) Malignant spinal cord compression. Lancet Oncol6:15–24 [DOI] [PubMed]
- 7.Rades D., Abrahm J.L. The role of radiotherapy for metastatic epidural spinal cord compression. Nat Rev Clin Oncol. 2010;7:590–598. doi: 10.1038/nrclinonc.2010.137. [DOI] [PubMed] [Google Scholar]; Rades D., Abrahm J.L. (2010) The role of radiotherapy for metastatic epidural spinal cord compression. Nat Rev Clin Oncol7:590–598. [DOI] [PubMed]
- 8.Chuang V.P., Wallace S., Swanson D., Zornoza J., Handel S.F., Schwarten D.A. Arterial occlusion in the management of pain from metastatic renal carcinoma. Radiology. 1979;133:611–614. doi: 10.1148/133.3.611. [DOI] [PubMed] [Google Scholar]; Chuang V.P., Wallace S., Swanson D., et al. (1979) Arterial occlusion in the management of pain from metastatic renal carcinoma. Radiology133:611–614 [DOI] [PubMed]
- 9.Chiras J., Adem C., Calee J.N., Spelle L., Cormier E., Rose M. Selective intra-arterial chemoembolization of pelvic and spine bone metastases. Eur Radiol. 2004;13:1774–1780. doi: 10.1007/s00330-004-2240-5. [DOI] [PubMed] [Google Scholar]; Chiras J., Adem C., Calee J.N., et al. (2004) Selective intra-arterial chemoembolization of pelvic and spine bone metastases. Eur Radiol13:1774–1780 [DOI] [PubMed]
- 10.Koike Y., Takizawa K., Ogawa Y., Muto A., Yoshimatsu M., Yagihashi K. Transcatheter arterial chemoembolization (TACE) or embolization (TAE) for symptomatic bone metastases as a palliative treatment. Cardiovasc Intervent Radiol. 2011;34:793–801. doi: 10.1007/s00270-010-0031-8. [DOI] [PubMed] [Google Scholar]; Yuya K., Kenji T., Yukihisa O., et al. (2011) Transcatheter arterial chemoembolization (TACE) or embolization (TAE) for symptomatic bone metastases as a palliative treatment. Cardiovasc Intervent Radiol34:793–801. doi: 10.1007/s00270-010-0031-8 [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.