Abstract
This article presents fibrosing mesenteric tuberculosis in a 19-year-old Arab boy who presented with weight loss, fever, abdominal pain, and distension. Abdominal contrast enhanced computed tomography (CECT) was performed which showed large infiltrative ill-defined mesenteric-based enhancing soft tissue phlegmonous mass with surrounding desmoplastic reaction causing retraction-kinking of small bowel loops associated with central necrotic mesenteric lymph nodes, multifocal small bowel wall thickening, and ascites. Abdominal tuberculosis is a diagnostic challenge particularly if pulmonary tuberculosis is absent as in this case. CT appears to be the modality of choice if clinical and epidemiological suspicion is high in order to ensure early treatment for a favorable outcome.
Keywords: Mesenteric tuberculosis, Computed tomography
Introduction
Tuberculosis (TB) is a prevalent communicable disease worldwide with a prevalence rate of 9 million. Southeast Asia and the western Pacific region represent 56% of the global TB burden. Extrapulmonary TB is seen in 15% of tubercular cases among which 11% due to abdominal tuberculosis. Abdomen tuberculosis is uncommon in developed western countries however it remains prominent in developing countries. Abdominal tuberculosis is the most common form of extrapulmonary TB and accounts for 11%. Coexisting pulmonary tuberculosis is seen in only 15%. Risk factors for abdominal tuberculosis are immune compromised status, HIV, diabetes mellitus, peritoneal dialysis, and immigrants from high prevalence areas of tuberculosis. Abdominal TB is more common between 25- and 45-year-old age groups and rare in the pediatric population and under the age of 20 as less as 1% [1], [2], [3], [4], [5], [6], [7], [8], [9]. Abdominal TB specifically peritoneal TB is often masquerading as inflammatory bowel disease, malignancy, or other infective conditions. In pediatric and adolescent patients, it is hard to diagnose at an early period due to the nonspecific clinical and radiological findings [8], [9], [10], [11], [12], [13], [14], [15], [16]. For this reason, it is necessary to be suspicious of TB based on an epidemiological location of the patient. In patients having no primary pulmonary focus, a delay can occur in the diagnosis of abdominal tuberculosis. The chest radiographs may be normal in 50%-60% of the patients with abdominal TB.
Case presentation
A 19-year-old Arab boy without a known medical condition admitted for evaluation of significant weight loss and low-grade chronic fever of 4 months’ duration. His other symptoms are fatigue, anorexia, and abdominal pain. A week prior to hospital admission, the patient had developed increasing abdomen pain and mild abdomen distention. Patient has no complaints of a chronic cough, cervical swelling, diarrhea, or constipation. On admission, the patient was febrile and cachexia. On physical examination, he had abdomen tenderness and rigidity. Laboratory findings on admission was hemoglobin 9 gm/L, platelet count 3 lac/mL, ESR 70, and CRP 20mg/L. Blood and urine culture were sterile. HIV antibodies were negative. Monteux test Tuberculin skin test was performed which turned out to be negative. Urine and blood show no serotonin or other abnormal metabolites. Patient underwent plain X-ray (Fig. 1), abdominal ultrasound (Fig. 2), contrast CT scan (Fig. 3), and abdominal MR imaging (Fig. 4). Patient had exploratory laparotomy which showed infiltrative mesentery mass, multifocal bowel wall thickening, bowel loops clumping – kinking, small bowel fistula, and extensive adhesion formation with multiple mesenteric lymph nodes. The patient underwent extensive adhesiolysis, mesenteric mass removal, and segmental bowel resection-anastomosis followed by antitubercular treatment for 6-month duration. Postoperative surgical specimen was sent for histopathology which showed caseating granulomas, lymphocyte infiltrates. PCR of the specimen was positive for mycobacterium tuberculosis.
Fig. 1.
An abdominal radiograph showing paucity of normal bowel gas pattern and ill-defined soft tissue opacity occupying quadrants.
Fig. 2.
USG abdomen showing clumped and matted small bowel loops forming a pseudo-mass (curved arrow).
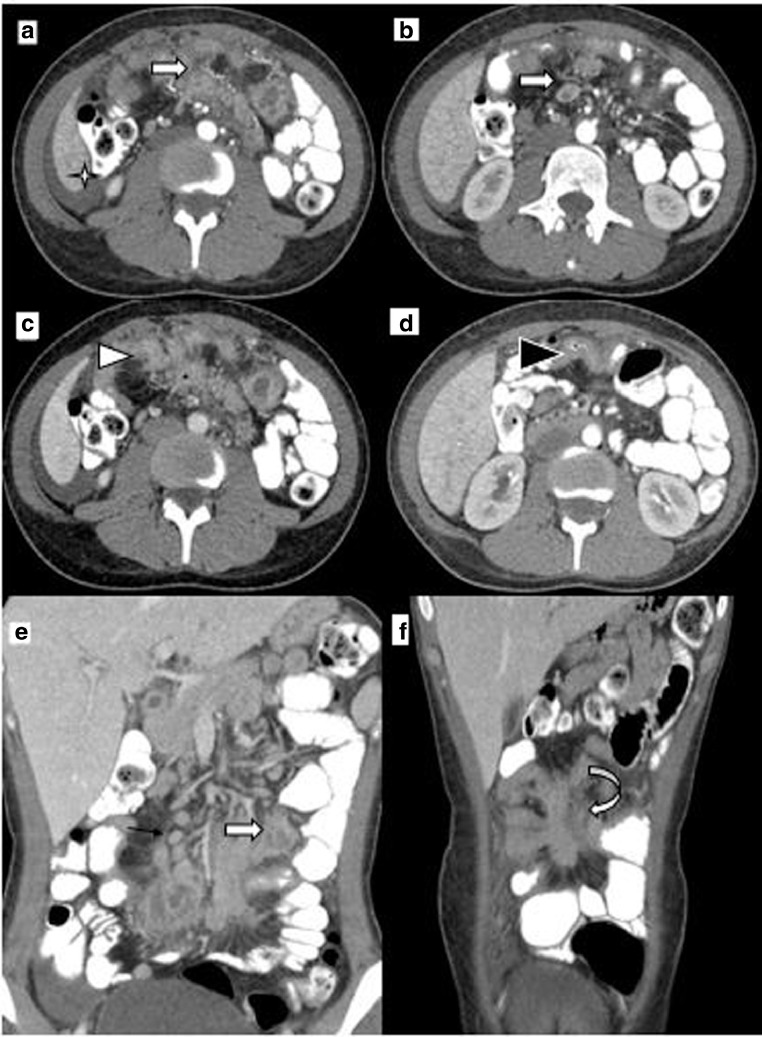
Fig. 3.
CT abdomen: (a) late arterial phase showing multiple discrete and conglomerate mesenteric lymph nodal masses (block white arrow) and perihepatic free fluid (white star). (b) Portal phase showing central low attenuating discrete lymph node (block white arrow). (c) Showing conglomerate mesenteric lymph node mass causing tethering, retraction, kinking, and matting of small intestinal loops (white arrowhead). (d) Showing jejunal loop wall thickening (black arrowhead). (e) Coronal reformation showing multiple central low attenuating discrete (thin black arrow) and conglomerate mesenteric lymph nodal mass (block white arrow). (f) Coronal reformation showing mesenteric based lymph nodal mass with surrounding desmoplastic reaction causing tethering, inking, and retraction of small bowel loops (curved white arrow).
Fig. 4.
MRI abdomen: (a) T2 fat-suppressed image showing ill-defined heterogeneous iso to hyperintense mesenteric based soft tissue mass with surrounding desmoplastic reaction causing tethering, retraction, and kinking of small bowel loops (block white arrow). (b and c) DWI image shows hyperintense signal (black arrowhead) with corresponding apparent diffusion coefficent (ADC) map showing hypointensity (white arrowhead) suggesting diffusion restriction. (d) Postcontrast T1WI showing multiple discrete and conglomerate central hypointense and peripheral enhancing mesenteric lymph nodes (curved white arrow). (e) Postcontrasts T1WI showing smoothly thickened enhancing peritoneum (block white arrow) and nodular mesentery (thin black arrow). (f) Coronal postcontrasts T1WI image showing multiple discrete and conglomerate mesenteric lymph nodes (black arrowhead) and clumping of small bowel loops forming a pseudo-mass (curved black arrow).
Discussion
Extrapulmonary TB is seen in 15% of tubercular patients among which 11% due to abdominal tuberculosis. Gastrointestinal tuberculosis is a most common form of extrapulmonary and accounts for 11%. Coexisting pulmonary tuberculosis is seen in only 15%. Gastrointestinal tuberculosis occurs via the following route: aspiration of infected sputum, ingestion of infected milk with mycobacterium tuberculosis or mycobacterium bovis, hematogenous spread, local spread from infected abdominal or pelvic organs [4], [14].
Gastrointestinal tuberculosis is categorized into following 4 parts.
Intestinal tuberculosis
Ileocecal junction is the most common site affected followed by ileum and jejunum. Most commonly thickening of the terminal ileum and medial cecal wall is commonly associated with thickening and gapping of Ilio-cecal valve. In chronic case terminal ileal stenosis, shrunken, and pulled up cecum seen. Isolated multifocal Ileal and jejunal loops involvement can be seen but rare and most commonly seen in association with TB peritonitis [4], [6], [7]. In our case, multifocal wall thickening of jejunum and ileal loops noted with no proximal small bowel dilatation.
Peritoneal tuberculosis
Peritoneal tuberculosis is classified into 3 types; (a) wet type: gross ascites with or without smoothly thickened peritoneum, (b) dry type: thickening of peritoneum with dense adhesions and micronodule formation seen, and (c) fibrosing/fixed type: a rare form of peritonitis where necrotic matted lymph node mass in mesentery can cause surrounding desmoplastic reaction giving stellate mesentery appearance resulting in clumping, tethering of bowel loops, bowel wall thickening, bowel obstruction, fistula formation, cold abscess, and loculated ascites [4], [6], [11]. In our case, CT and MRI showed mesenteric based soft tissue mass causing multifocal jejunal and ileal wall thickening, tethering, retraction, and kinking of small bowel loops associated with central necrotic mesenteric lymph nodes and mild ascites. Exploratory laparotomy confirmed CT and MRI findings.
Tubercular lymphadenopathy
Most commonly enlarged lymph nodes are seen in the mesentery and the retroperitoneum. Most of the lymph nodes show central liquefactive necrosis. Lymph nodes can be discrete or matted forming a mass within the mesentery or in the retroperitoneum. In a chronic case, lymph nodes can show calcification [1], [11], [17]. In our case, there were both central causative necrotic and noncaseating lymph nodes.
Tubercular ascites
Tubercular ascites is most commonly seen in association with TB peritonitis. Ascites can be free, localized, or loculated. CT shows low to high attenuation fluid. High attenuation can be due to high protein and cellular contents. Combination of the fat fluid level in ascites along with necrotic lymph node is pathognomic of tubercular ascites. Ascites can show delayed enhancement on contrast enhancement MRI [6], [17]. Tuberculous ascites show lower apparent diffusion coefficient than other fluid due to presence of inflammatory cells with restricted diffusion [18]. In our case, there was mild low attenuating free fluid in the pelvic cavity.
Role of CT and MRI in abdominal tuberculosis
MRI helps in early detection of peritoneal thickening, well delineation of central necrosis in lymph nodes based on T1/T2 signal intensity. MR imaging can detect caseation or liquefactive necrosis of the lymph nodes [4], [14]. In our case, there was T1 hypointense, T2 hypointense signal intensity of the mesenteric lymph node suggesting caseation necrosis than liquefactive. Diffusion-weighted MR imaging can used for evaluation of gastrointestinal and peritoneal tuberculosis. Diffusion-weighted MR imaging shows restricted diffusion with low apparent diffusion coefficient of tuberous lesion due to excess fibrous tissue, presence of calcification, and associated granulation tissues that decrease intracellular space [19], [20], [21], [22], [23], [24]. MR enterography has an advantage in the characterization of bowel wall thickening, dynamic assessment of bowel function, and for follow-up with no worry of radiation, unlike CT. Thus, in patients with contraindication for intravenous contrast, noncontrast MRI is far better than plain CT. Also, CT gives no information regarding the functional assessment of the bowel loops which can be assessed by dynamic cine MRI. Both CT and MRI can diagnose abdominal tuberculosis with high sensitivity and specificity. CT has an advantage over MRI in being a faster, less expensive investigation with better spatial resolution, lesser artifacts and can simultaneously evaluate chest, abdomen, and pelvis in a single examination. Virtual CT endoscopy may have a role in detection of changes of the intestinal wall in tuberculous lesion [25].
Differentials
Fibrosing mesenteric tuberculosis can often masquerade as inflammatory bowel disease and malignancy. Conditions which can mimic are mesenteric carcinoid, sclerosing mesenteritis, peritoneal carcinomatosis, and atypical desmoid tumor. However, in our case the presence of mesenteric mass, necrotic lymph nodes, smoothly thickened enhancing peritoneum, multifocal small intestinal wall thickening, and ascites were highly suggestive of tubercular etiology than neoplastic or other inflammatory cause.
Conclusion
Abdominal tuberculosis with predominant mesenteric involvement is rare with fatal complication if undiagnosed and left untreated. Peritoneal TB often masquerades as inflammatory bowel disease, malignancy, and other infective conditions. CT appears to be the modality of choice if clinical and epidemiological suspicion is high in order to ensure early treatment for a favorable outcome.
Teaching point
Presence of mesenteric mass, multisegment small bowel wall thickening, necrotic lymph nodes, and ascites in appropriate clinical settings and epidemiology, highly suggest mesenteric tuberculosis. CT abdomen remains investigation of choice for mesenteric tuberculosis as it can evaluate chest, abdomen, and pelvis simultaneously.
Footnotes
Acknowledgments: Dr. Shafeek Abubacker – Language help, writing assistance, and proofreading. Dr. Uma Mahesh matapathi – Proofreading and writing assistance. Dr. Selvakumar Subbaraman – Proofreading and writing assistance.
Competing Interests: The authors have declared that no competing interests exist.
References
- 1.Joshi A.R., Basantani A.S., Patel R.C. Role of CT & MRI in abdominal tuberculosis. Curr Radiol Rep. 2014;2:66. [Google Scholar]
- 2.Usta M., Urganci N., Dalgic N., Kızılkan N., Kurtaraner T., Karadag A. Clinical presentation in a series of eight children with abdominal tuberculosis. Experience of a single-center in Turkey. Iran J Pediatr. 2017;27:9766. [Google Scholar]
- 3.Sial M., Wieland M.L. Peritoneal tuberculosis is an uncommon site of extrapulmonary infection caused by Mycobacterium tuberculosis. Current concepts in the management of tuberculosis. Mayo Clin Proc. 2011;86:348–361. doi: 10.4065/mcp.2010.0820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Karaman A., Erden A., Karaman H., Ates I., Yılmaz N. A case with peritoneal tuberculosis. Ankara Med J. 2012;12:103–105. [Google Scholar]
- 5.Vanhoenacker F.M., De Backer A.I., Maes M., Op De beeck B., Van Altena R., Van Beckevoort D. Imaging of gastrointestinal and abdominal tuberculosis. Eur Radiol. 2004;14:E103–E115. doi: 10.1007/s00330-003-2047-9. [DOI] [PubMed] [Google Scholar]
- 6.Sanai F.M., Bzeizi K.I. Systematic review: tuberculous peritonitis – presenting features, diagnostic strategies, and treatment. Aliment Pharmacol Ther. 2005;22:685–700. doi: 10.1111/j.1365-2036.2005.02645.x. [DOI] [PubMed] [Google Scholar]
- 7.Akhan O., Pringot J. Imaging of abdominal tuberculosis. Euro Radiol. 2002;12:312–323. doi: 10.1007/s003300100994. [DOI] [PubMed] [Google Scholar]
- 8.Rathi P., Gambhir P. Abdominal tuberculosis. Assoc Phys India. 2016;64:38–47. [PubMed] [Google Scholar]
- 9.Sanai F.M., Bzeizi K.I. Systematic review: tuberculous peritonitis – presenting features, diagnostic strategies, and treatment. Aliment Pharmacol Ther. 2005;22:685–700. doi: 10.1111/j.1365-2036.2005.02645.x. [DOI] [PubMed] [Google Scholar]
- 10.Debi U., Ravisankar V., Prasad K.K., Sinha S.K., Sharma A.K. Abdominal tuberculosis of the gastrointestinal tract. World J Gastroenterol. 2014;20:14831–14840. doi: 10.3748/wjg.v20.i40.14831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zissin R., Gayer G., Chowers M., Shapiro-Feinberg M., Kots E., Hertz M. Computerized tomography findings of abdominal tuberculosis: Report of 19 cases. Isr Med Assoc. 2001;3:414–418. [PubMed] [Google Scholar]
- 12.Abdel Razek A.A., Abu Zeid M.M., Bilal M., Abdel Wahab N.M. Virtual CT colonoscopy versus conventional colonoscopy: a prospective study. Hepatogastroenterology. 2005;52:1698–1702. [PubMed] [Google Scholar]
- 13.Abdelaal A., Alfrey R., Abdelaziem S., Abunada M., Alfaky A., Ibrahim W.H. Role of laparoscopic peritoneal biopsy in the diagnosis of peritoneal tuberculosis. A seven-year experience. Chirurgia. 2014;109:330–334. [PubMed] [Google Scholar]
- 14.Abdel Razek A.A. Imaging of connective tissue diseases of the head and neck. Neuroradiol J. 2016;29:222–230. doi: 10.1177/1971400916639605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Razek A.A., Castillo M. Imaging appearance of granulomatous lesions of head and neck. Eur J Radiol. 2010;76:52–60. doi: 10.1016/j.ejrad.2009.05.021. [DOI] [PubMed] [Google Scholar]
- 16.Abdel Razek A., Suresh M. Imaging of sialadenitis. Neuroradiol J. 2017;30:205–215. doi: 10.1177/1971400916682752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.da Rocha E.L., Pedrosa B.C., Bormann R.L., Kierszenbaum M.L., Torres L.R., D’Ippolito G. Abdominal tuberculosis: a radiological review with emphasis on computed tomography and magnetic resonance imaging findings. Radiol Bras. 2015;48:181–191. doi: 10.1590/0100-3984.2013.1801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Razek A.A.K.A., Samir S. Differentiation malignant from benign pericardial effusion with diffusion-weighted MRI. Clin Radiol. 2019;74:325. doi: 10.1016/j.crad.2019.01.005. e19-325.e24. [DOI] [PubMed] [Google Scholar]
- 19.Abd-El Khalek Abd-ALRazek A., Fahmy D.M. Diagnostic value of diffusion-weighted imaging and apparent diffusion coefficient in assessment of the activity of crohn disease: 1.5 or 3 T. J Comput Assist Tomogr. 2018;42:688–696. doi: 10.1097/RCT.0000000000000754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Abdel Razek A.A., Soliman N., Elashery R. Apparent diffusion coefficient values of mediastinal masses in children. Eur J Radiol. 2012;81:1311–1314. doi: 10.1016/j.ejrad.2011.03.008. [DOI] [PubMed] [Google Scholar]
- 21.Abdel Razek A.A., Gaballa G., Elhawarey G., Megahed A.S., Hafez M., Nada N. Characterization of pediatric head and neck masses with diffusion-weighted MR imaging. Eur Radiol. 2009;19:201–208. doi: 10.1007/s00330-008-1123-6. [DOI] [PubMed] [Google Scholar]
- 22.Abdel Razek A.A., Nada N. Role of diffusion-weighted MRI in differentiation of masticator space malignancy from infection. Dentomaxillofac Radiol. 2013;42(4) doi: 10.1259/dmfr.20120183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Abdel Razek A., Samir S. Diagnostic performance of diffusion-weighted MR imaging in differentiation of diabetic osteoarthropathy and osteomyelitis in diabetic foot. Eur J Radiol. 2017;89:221–225. doi: 10.1016/j.ejrad.2017.02.015. [DOI] [PubMed] [Google Scholar]
- 24.Abdel Razek A.A.K. Routine and advanced diffusion imaging modules of the salivary glands. Neuroimaging Clin N Am. 2018;28:245–254. doi: 10.1016/j.nic.2018.01.010. [DOI] [PubMed] [Google Scholar]
- 25.Abdel Razek A.A., Abu Zeid M.M., Bilal M., Abdel Wahab N.M. Virtual CT colonoscopy versus conventional colonoscopy: a prospective study. Hepatogastroenterology. 2005;52:1698–1702. [PubMed] [Google Scholar]