Abstract
Background: Kawasaki disease (KD) may be associated with infection of unknown pathogen(s). For predicting of the etiology of KD, we evaluated epidemiological characteristics in KD, common infectious diseases and immune-mediated diseases in childhood.
Methods: We respectively, reviewed the data of patients with KD, influenza, aseptic meningitis, exanthem subitum (ES), Mycoplasma pneumoniae (MP) pneumonia, acute pyelonephritis (APN), Henoch-Schönlein purpura (HSP), acute poststreptococcal glomerulonephritis (APSGN), and childhood asthma. We compared and interpreted epidemiological data across the groups.
Results: In age distribution, KD, APN, and ES showed a similar pattern in that majority of patients were infants or young children, and other diseases showed a relatively even age-distribution which had a peak age, mainly 5–6 years, with bell-shape patterns. In annual-case pattern, there were epidemic years in aseptic meningitis and MP pneumonia, and the fluctuated annual cases were seen in other diseases. The trends of decreasing cases were seen in APSGN, HSP, and childhood asthma in recent years. In seasonal frequency, influenza or aseptic meningitis occurred in mainly winter or summer season, respectively. HSP and APSGN cases had less in summer, and KD, APN, and ES showed relatively even occurrence throughout a year without significant seasonal variations.
Conclusions: Our results suggest that KD agents may be associated with normal flora that are influenced by environmental changes, since pathogens of APN and ES could be regarded as normal flora that originate from the host itself or ubiquitously existing human reservoirs.
Keywords: Kawasaki disease, etiology, epidemiology, acute pyelonephritis, exanthem subitum, microbiota
Introduction
Kawasaki disease (KD) disease was a novel disease that appeared in East Asian countries in the order of Japan, South Korea, Taiwan, and China with a 5–10 year time-gap (1–4). After the first case report in the 1960s in Japan, KD has been reported in over 60 countries around the world (5). However, the incidence of KD is different across various populations; currently, the incidence in Western countries is lower by one tenth to one twentieth compared to East Asian countries, and has plateaued during recent decades. Whereas, the number of KD patients in East Asian countries has slowly and steadily increased after its first emergence (6). In these countries, KD occurs mostly in children between 6 months and 4 years of age, and has become a nationwide endemic disease within 2 decades after its first appearance. These findings suggest that KD spreads slowly to other regions within a nation and neighboring countries, which is contrasting to common infectious disease epidemics. KD pathogen(s) should satisfy with the epidemiological characteristics of KD.
The clinical manifestations of Kawasaki disease share similarities in some aspects of viral diseases, bacterial diseases, or infection-related immune mediated diseases such as systemic juvenile idiopathic rheumatoid arthritis or acute rheumatic fever (ARF). However, extensive studies searching for the etiologic agent(s) have turned up as failures until the present time (7). Common childhood infectious diseases and infection-related immune-mediated diseases, including KD, childhood idiopathic thrombocytopenia, and Henoch-Schönlein purpura (HSP) have low prevalence in older children and adults. Thus, these childhood diseases have been hypothesized to be associated with infections caused by unknown pathogens (8, 9). Since KD is an acute self-limiting systemic inflammation that involves multiple organs, it has been proposed that there are etiologic substances that induce systemic inflammation and target cell injuries, including coronary artery lesions (CALs), during or after the infection with KD pathogen (s) (10). Also, it has been proposed that KD pathogens may be a species of the host's normal flora based on clinical and epidemiological characteristics of KD (11). The epidemiological characteristics of a disease may aid in predicting the etiologic agent(s) of the disease. The identification of KD etiology is essential for understanding the disease, and the development of diagnostic tools and adequate treatment modalities.
In this study, to predict the etiology of KD, we evaluated and compared epidemiological characteristics between KD and common infectious diseases or immune-mediated diseases in children. After evaluating epidemiological parameters focused on age distribution, annual-case pattern and monthly-case pattern, we found that the epidemiological profiles in KD were similar to those in acute pyelonephritis (APN) or exanthema subitum (ES). We discussed the implications of these findings in KD epidemiology in Korea.
Materials and Methods
The subjects of this study were collected from the patients (0–15 years of age) who were admitted at The Catholic University of Korea Daejeon St. Mary's Hospital from January 1987 to December 2016. We evaluated the epidemiological characteristics of patients diagnosed with Mycoplasma pneumoniae (MP) pneumonia, influenza, aseptic meningitis, APN, ES, acute poststreptococcal glomerulonephritis (APSGN), HSP, childhood asthma (or recurrent wheezing episode), and KD. As an exception, the subjects diagnosed with influenza in this study were selected from outpatients who were positive for influenza in rapid diagnostic testing during the winter of influenza seasons. The diagnosis or selection criteria in each disease were referenced from other publications (12–20). Although the study period and the number of patients in each disease were not identical, we reevaluated the data that were used for previously published papers or collected new data for some diseases such as ES and HSP. Age, sex, age distribution pattern, annual-case pattern and monthly-case pattern were analyzed in each disease. For mean age, <12 months of age was regarded as 0 years of age for statistical analyses, except ES. In age distribution pattern, we focused on age predilection in infancy and younger children. In annual-case pattern, we searched whether the pattern shown cyclic epidemics during the study periods. As for seasonal variation, the disease was regarded as having seasonality when the number of seasonal cases showed over 10 or 15% difference in the number of cases between the highest season and the lowest season.
Ethics Statement
The written informed consents were obtained from the parents/caregivers of all children for the medical records to be used in this study at time of admission. The study was approved by the Institutional Review Board of The Catholic University of Korea Daejeon St. Mary's Hospital (DC18RESI0100).
Results
A total of 7,832 patients diagnosed with 9 diseases, including KD, 5 infectious diseases, and 3 immune-mediated diseases were evaluated. The study period in the majority of diseases was over 10 years; for infectious diseases, MP pneumonia (2003–2012, n = 779) (12), influenza (2010–2017, n = 2,163) [(13, 14), unpublished observation], aseptic meningitis (1987–2003, n = 2,201) (15), APN (2005–2015, n = 320) (16) and ES (2005–2016, n = 429), and as for infection-related immune diseases, APSGN (1987–2013, n = 99) (17), HSP (1987–2015, n = 515) (unpublished observation) and childhood asthma (or recurrent wheezing episode) (2003–2014, n = 384) (18), and KD (1987–2016, n = 942) (19, 20) (Table 1).
Table 1.
Demographic findings in KD and other diseases (0–15 years of age).
| Diseases | Study period | No. of subjects | Mean cases/y | M:F ratio | Mean age (y) | Peak ages (y) |
|---|---|---|---|---|---|---|
| Kawasaki disease | 1987–2016 | 942 | 31 | 1.7:1 | 2.2 ± 1.6 | 1 |
| Mycoplasma pneumonia | 2003–2012 | 779 | 78 | 1.1:1 | 5.0 ± 3.2 | 4 |
| Influenza | 2010–2018 | 2,163* | 270 | 1.1:1 | 5.4 ± 3.8 | 3–5 |
| Aseptic meningitis | 1987–1998 | 2,201 | 115 | 2:1 | 6.0 ± 3.9 | 4–6 |
| Acute pyelonephritis | 2005–2015 | 320 | 29 | 1.4:1 | 1.5 ± 3.4 | 0 |
| Exanthem subitum | 2005–2016 | 429 | 36 | 1:1 | 1.0 ± 0.5 | 0 |
| APSGN | 1987–2014 | 99 | 4 | 2.3:1 | 8.3 ± 2.7 | 7–9 |
| Henoch-Shönlein purpura | 1987–2015 | 515 | 18 | 1.2:1 | 6.5 ± 3.0 | 5 |
| Childhood asthma | 2003–2014 | 384 | 32 | 1.2:1 | 5.6 ± 3.5 | 2–4 |
Outpatients; APSGN, acute poststreptoccocal glomerulonephritis.
Age, Sex, and Age Distribution in KD and Other Diseases
The male-to-female (M:F) ratio, mean age, and peak ages are shown in Table 1.
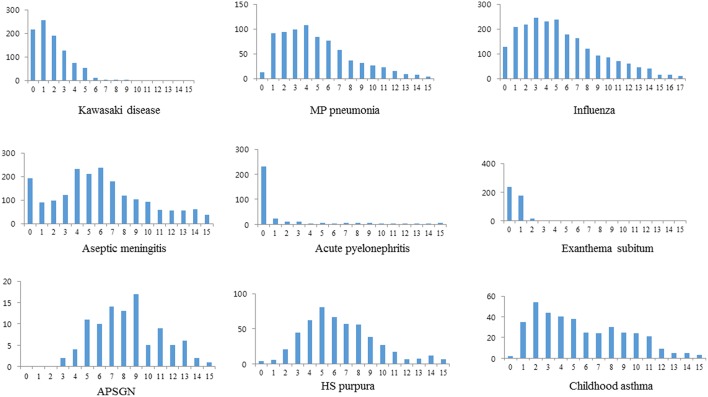
Male predominance was observed in all the diseases except ES (0.95:1), but the M:F ratios were somewhat different across the diseases. The highest M:F ratio was seen in APSGN (2.3:1), and the lower M:F ratios were seen in ES, MP pneumonia and influenza (1.1:1, respectively), and KD showed 1.7:1 ratio in this series. The lowest mean age was noted in order of ES (mean 1 year of age), APN (1.5 years), and KD (2.2 years) and the higher mean age was noted in APSGN (8.3 years) and HSP (6.5 years). In age distribution, KD, APN, and ES showed a similar pattern in that the majority of patients were infants and young children (0–4 years), and after this age period the prevalence decreased dramatically. There were only a few patients >3 years of age in ES and a few patients >5 years in KD, however there was relatively an even distribution with female predominance after >2 years in APN. A bell-shape distribution pattern with a peak prevalence, mainly in the 4–6 years age range was observed in other diseases, however peak ages were slightly different across the diseases (Figure 1). There was a trend where the peak ages were changed in the epidemic diseases such as MP pneumonia and influenza over time [(12), unpublished observation].
Figure 1.
Age distribution in Kawasaki disease and other infectious or infection-related immune-mediated diseases.
Annual-Case Patterns in KD and Other Diseases
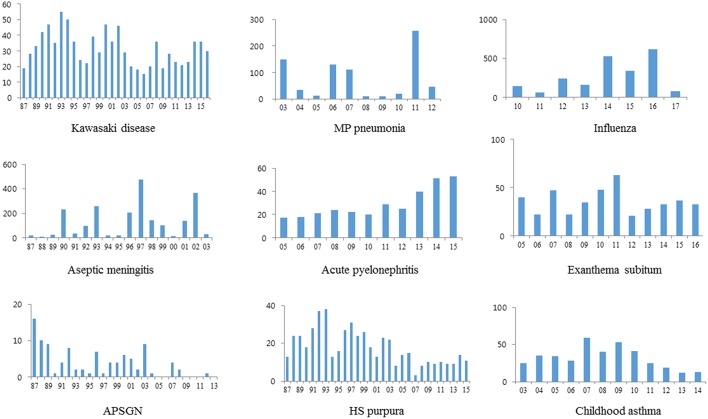
Cyclic epidemics caused by possibly new strains of pathogens, such as macrolide-resistant strains were noted in MP pneumonia and in aseptic meningitis caused by mainly enteroviruses; all had similar patterns with nationwide epidemics or outbreaks in Korea (12, 15). Influenza occurred every winter and early spring, but the number of cases and peak epidemic months were different in 2009 pandemic and seasonal influenza, across seasonal influenza after 2009 pandemic [(14), unpublished observation]. Relatively an even, but fluctuating annual cases were noted in KD, APN, ES, HSP, and childhood asthma, except APSGN (Figure 2). The mean annual cases in each disease are shown Table 1, and the results may be helpful to estimate the incidence of each disease in our city, since nationwide KD epidemiological studies in Korea have been performed every 3 years (21, 22). There was a trend of decreasing number of cases in the recent years or decades in APSGN, HSP and childhood asthma, whereas an increasing trend was noted in APN, compared to the past years or decades (Figure 2).
Figure 2.
Annual cases in KD and other diseases.
Monthly or Seasonal-Case Pattern in KD and Other Diseases
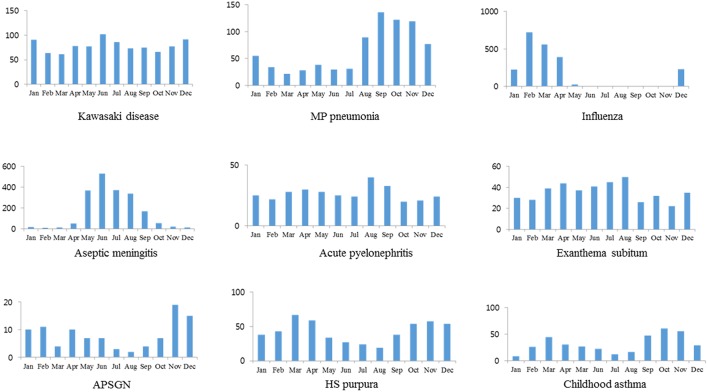
A higher number of cases were seen in the fall and winter in MP pneumonia. Aseptic meningitis was noted mainly during the summer whereas influenza mainly during winter and early spring seasons. There was a trend showing a lower number of cases in the summer in HSP, and a higher number of cases in the winter for APSGN. A higher number of cases were seen during the fall and spring for childhood asthma. Although KD affected more patients during summer than any other season in this series, KD had no difference in seasonal variation according to the definition of the seasonality. Also, no seasonality was seen in APN and ES (Table 2 and Figure 3).
Table 2.
Seasonality in KD and other diseases.
| Diseases | Spring (%) | Summer (%) | Fall (%) | Winter (%) | Seasonality |
|---|---|---|---|---|---|
| Kawasaki disease | 22.9 | 27.7 | 23.1 | 26.2 | No seasonality |
| Mycoplasma pneumonia | 11.2 | 19.1 | 48.4 | 21.3 | Fall predominance |
| Influenza | 45.3 | 0.2 | 0.1 | 54.4 | Spring, winter |
| Aseptic meningitis | 22.2 | 63.5 | 12.3 | 1.9 | Summer predominance |
| Acute pyelonephritis | 26.9 | 27.8 | 23.1 | 22.2 | No seasonality |
| Exanthem subitum | 28.4 | 31.7 | 18.6 | 21.8 | No seasonality |
| APSGN | 21.2 | 12.1 | 30.3 | 36.4 | Winter, fall |
| Henoch-Shönlein purpura | 31.1 | 13.6 | 29.1 | 26.2 | Low in summer |
| Childhood asthma | 26.8 | 13.5 | 43 | 16.7 | Fall, Spring |
APSGN, acute poststreptococcal glomerulonephritis.
Figure 3.
Monthly or seasonal distribution of cases in KD and other diseases.
Discussion
It is hypothesized that KD may have an etiologic agent(s), although many studies searching for the etiology of KD have failed. Etiologic agents in infectious diseases originate from an external source and invade into the host. However, at present time, they could be classified as exogenous pathogens and the endogenous pathogens; the former is generally accepted as agents that are newly introduced to humans from other animal species or other places, whereas the latter originates from the host in the forms of normal flora (or commensals) or those becoming a normal flora from initially being an exogenous pathogen. For examples, measles in immune-innocent populations in the past and acquired immunodeficiency syndrome (AIDS) were caused by new external pathogens. On the other hand, the pathogens causing APN or acute otitis media, such as E. coli or Streptococcus pneumoniae, could be categorized as endogenous pathogens. It is well-known that a newly introduced disease by an exogenous pathogen has affected all aged persons in a population and spread to other populations in relatively a short-time period of time after its first emergence. Over time, infected persons and groups have immunity to the pathogens, and young children and infants would remain susceptible, especially during cyclic epidemic viral infections. Accordingly, epidemiologic characteristics of infectious diseases or infection-related diseases in the populations at a given time may differ.
In the present study, we found that epidemiological characteristics in KD were most similar to those in APN or ES among the 8 evaluated diseases. In age distribution, these 3 diseases had an age predilection in infancy and young children; 79.7% of patients in APN, 95.8% in ES, and 50.1% in KD were 0–1 years of age, and 87.5% of patients in APN, near 100% in ES, and 91.7% in KD were 0–4 years of age. In the view of epidemiological and clinical aspects, an infected infant with pathogens from these diseases are difficult to disperse the pathogens to other infants. Other diseases showed a relatively even age-distribution throughout childhood, though peak ages were slightly different across the diseases. This suggests that younger children may have less chance on exposure to the pathogens and older children and young adult groups may have immunity or tolerability to the pathogens causing the diseases.
In the annual-case pattern, cyclic epidemics were noted in MP pneumonia and aseptic meningitis, and marked fluctuated cases with a different peak week were noted in influenza. The annual-case pattern in KD was relatively even, but fluctuations in annual cases were noted as well as in APSGN, HSP and childhood asthma, and APN and ES in infectious diseases. APN may be the most common systemic bacterial infection in early childhood in developed countries (23). A majority of uropathogens causing APN in infants are E. coli or other uropathogens such as Klebsiella spp., Proteus spp., and Enterococcus spp., and they may originate from the intestinal commensals in the host. It is possible that after colonization of a strain of E. coli or other uropathoegns transferred from other persons, the pathogens invade into the host on occasional events and elicit immune reactions (24). Although KD and APN affect mainly infants and young children, older children and adults are also affected (25). Moreover, recurrent cases are not uncommon in the both diseases. These findings suggest that immature immune function in early childhood may be associated with both diseases, and pathogens may be multiple in KD (11). ES is an acute systemic viral infection, and is characterized by sudden appearances of generalized maculopapular or morbilliform rashes just after defervescence. The etiologic agent is a species belonging to the human Herpes virus group, herpes virus type 6 or rarely type 7. Herpes viruses have a characteristic of latent infections after initial infection. ES may have no differences in age predilection, possibly sero-prevalence rate, and clinical manifestations across populations around the world and have occurred throughout every year with no seasonal variations (26, 27). Herpes virus groups become latent in the host after the initial infection as shown in herpes zoster, herpes labialis, and reactivation of cytomegalovirus and Epstein-Barr virus (EBV) in depressed immune state of the host (28). It was reported that EBV infection or herpes virus 6 infection was related to certain clinical manifestations in KD such as otorrhea or BCG inoculation site inflammation (29, 30). It is a reasonable presumption that etiologic viruses in ES may have been introduced to humans a long-time ago, and may be coexisting within healthy human reservoirs including family members of infected infants with ES, and can be activated at any time. On occasion, the latent viruses in healthy carriers who may have a transient immune disturbance, can be reactivated and be spread to infants who have no immunity to the viruses.
In the present study, monthly case or seasonal case patterns in KD were also the most similar to those in APN or ES. Similar to this study, Nagao et al. (31) reported that the super-annual periodicity of KD was the most similar to ES among seven childhood infectious diseases including ES, herpangina, hand-foot-mouth disease, chicken pox, pharyngoconjunctival fever, erythema infectiosum, and GAS infection. They suggested that the KD pathogen is transmitted through close contact and persists asymptomatically in most hosts, likely ES (31), and this suggestion is accordance with our presumed characteristics of KD pathogens. Although the rate of KD has slowly increased with slight seasonal predominance in nationwide studies in Japan and Korea (22, 32), KD has occurred with annual fluctuations with seasonal variation each year in a given location as shown in this study. These data suggest that KD appears as local outbreaks rather than as a simultaneous nationwide epidemic as shown in viral or MP infections in Korea.
We have proposed that KD pathogens may be a species of normal flora of the host (10, 11). The disturbance of microbiota of the host, i.e., dysbiosis, has been reported in a variety of diseases, including obesity, autism spectrum disorders, allergic diseases, cancers, and autoimmune diseases such as inflammatory bowel disease, JIA and KD (33–38). It has been known that microbiota differ across ethnic groups along with different cultural environments such as diets, antibiotic use, and possibly genetic factors (39–41). Kinumaki et al. reported that Streptococcus spp. in intestinal microbiota markedly increased during the acute phase of KD, and suggested that KD-related streptococci may be involved in the pathogenesis of KD (38). Recently, Esposito et al. reviewed the possible role of the microbiota-host partnership on etiology and pathogenesis of KD (42). Because normal flora spread via colonization in individuals in populations, a new disease that is associated with normal flora may take a long period of time during which to spread, and would show different incidences across populations at given times as shown in KD epidemiology in Asian countries. Since pathogens exist in healthy human reservoirs as normal flora, the diseases may occur mainly in immunologically or genetically susceptible groups such as young children group that have developing immune system and microbiota (43, 44). Also, the epidemiology in these diseases can change over time along with an increasing number of people who obtain pathogens as normal flora in the populations, since normal flora that have adapted to the host may be less virulent compared to initial external pathogens. For example, scarlet fever caused by Group A beta-hemolytic streptococcus (GAS) was a severe disease in the past, with two notorious complications: acute rheumatic fever (ARF) and APSGN. Now, scarlet fever, ARF and APSGN have become rare diseases with a milder clinical phenotype in the developed countries (17, 45). However, pharyngitis caused by GAS without complications is not uncommon, and a relatively high proportion of GAS carriers among healthy children have been reported in the developed countries (46, 47). In addition, GAS strains have been susceptible to penicillin throughout the antibiotic era, with few genetic variations (48). Given that the evolutional purpose of external pathogens may be to become a species of normal flora in the host, these findings suggest that GAS strains may be changing to be part of normal flora in humans, though ARF still occurs in small populations (49). Thus, it is natural that initially severe diseases take on milder phenotypes over time, as shown in scarlet fever, pandemic influenza, and AIDS (13, 45, 50). It was also reported that recently diagnosed KD patients showed a less severe clinical phenotype, manifesting a higher incidence of incomplete KD and a lower incidence of CALs and less activated laboratory values such as C-reactive protein and albumin compared to patients who developed the disease in earlier periods (51).
Pathogenesis of KD and APN as well as other infectious and immune-mediated diseases remains to be further studied. APN has a focus in renal cortical parenchyma, where replicated uropathogens, byproducts from APN agents such as toxins and pathogen-associated molecular patterns (PAMPs), substances from injured host cells, and those from activated immune cells are produced. When these diverse substances spread from the focus into systemic circulation, clinical manifestations such as fever, tissue cell injury and/or bacteremia begin to occur, and the host immune system may respond to the substances. The majority of patients infected with APN pathogens may be asymptomatic and their disease are self-limited if systemic spread did not occur and the substances in the focus were controlled as a localized inflammation (24). On the other hand, ARF or APSGN are classic representatives of infection-related immune-mediated disease. In ARF, after 1–4 weeks after initial GAS infection when symptoms and signs of the initial bacterial infection (pharyngitis) subside, acute onset of fever and other symptoms, such as carditis, arthritis, and rarely skin rash (erythema marginatum), develop (49). The majority of patients with ARF or APSGN show a self-limited clinical course, although severely affected patients have long-term complications such as severe rheumatic heart disease or chronic renal failure, similar to giant coronary artery aneurysms in KD. Only a small proportion of patients with GAS infection are affected with ARF or APSGN. Some patients with ARF or APSGN may have preceding asymptomatic GAS infections, and other group streptococci, other bacteria or viruses are associated with postinfectious glomerulonephritis or heart tissue inflammation, including myocarditis and possibly heart valve diseases, without evidence of GAS infection (52, 53). It is believed that there are etiologic substances that induce various clinical manifestations in KD as well as in ARF and APSGN after initial infection (10). These substances include not only those originating from the pathogens but also those from the injured host cells, including damage-associated molecular patterns (DAMPs) and those from immune cells activated by infectious insults. It have been proposed that these substances bind to specific target cells of organs such as heart and joints in ARF, kidneys in APSGN, or coronary arteries or other organs in KD. The host immune system may control these substances based on their size and biochemical properties (the protein-homeostasis-system hypothesis) (10, 54, 55).
Based on the data from epidemiological and clinical studies of KD, the pathogenesis of KD could be explained as follows. Along with economic growth and westernization in East Asian countries in order of Japan, South Korea, Taiwan, and China, a species in normal flora (microbiota) in people may be affected by environmental changes such as improved hygiene or increased consumption of westernized foods including red meats (10, 11). These species (KD agents) slowly spread via colonization as normal flora to other people, including the parents and guardians of KD patients, and are eventually colonized in infants and young children predisposed to KD. On occasion, colonized KD agents invade the host via unknown events; if they are intestinal commensals, a mechanism similar to APN (via hematogeneous route in young infants) may be activated (24). Invading KD pathogens make a focus (replication site) elsewhere within the host, possibly near the upper respiratory or intestinal tracts (portal of entry) or in the target organ via systemic or local circulation. The majority of patients infected by the KD agents may be asymptomatic and have self-limited disease. However, during or after recovery from this infection, similar to ARF or APSGN, KD develops when the substances from a focus spread abruptly into systemic circulation (10). There are various incubation periods in ARF and APSGN after initial GAS infection, and no intact pathogens or structural components of the pathogen have been detected in the pathologic lesions in ARF, APSGN, KD, and other infection-related immune-mediated diseases. Furthermore, GAS strains can reside in intracellular compartments in host cells such as tonsillar epithelial cells and macrophages (56, 57), and small extracellular bacteria such as mycoplasma species can proliferate within cells and spread to other organ cells (58). Therefore, it is possible that the etiologic substances may originate from injured host cells, including a kind of DAMPs, or from immune cells exposed to infectious insults, than from various pathogens. Immune/repair system of the host, including immunoglobulins (IgG, IgM, and IgM) and platelets, begin to control the substances and take part in tissue cell repair (59). Thus, the prognoses of KD and other immune-mediated diseases depend on the ability of the host immune system to control the diverse substances from target cells injured by initial insults. Early immune modulators (corticosteroids or intravenous immunoglobulin) may act on hyperactive immune reactions performed by the non-specific adaptive immune cells (T cells and B cells) in the early stage of each disease (10, 54, 60). However, despite early treatment, a few patients with KD experience severe or giant aneurysms, and these patients may have insufficient immune function to control the substances originating from injured target cells (61). Young children with immature immune function may be prone to invasion of KD pathogens as occurs in APN in infants, and the probability of invasion of KD agents in young children may be similar across different populations (20). Since microbiota may differ across populations, marked differences in racial incidence in KD or other immune-mediated diseases such as HSP, inflammatory bowel disease, and Bechet disease may be associated with the colonization state of the different etiologic agents in the populations (20).
This retrospective study has some limitations. Epidemiological data observed in a single hospital may not match the nationwide data. However, we found that outbreaks of infectious diseases such as MP pneumonia, influenza, and aseptic meningitis in our city had occurred concurrently with nationwide epidemics. Demographic and some epidemiologic characteristics of KD, APN, APSGN, HSP, childhood asthma, and possibly ES were similar to the data in published papers in Korea (data not shown).
In conclusion, epidemiological characteristics of KD were similar to APN or ES in age distribution, annual case pattern, and seasonal variations. The pathogens of APN and ES could be regarded as one species of normal flora, since they may have originated from the host itself or ubiquitously existing human reservoirs. KD may also be associated with a species in the normal flora that may be influenced by environmental changes.
Ethics Statement
The written informed consents were obtained from the parents/caregivers of all children for the medical records to be used in this study at time of admission. The study was approved by the Institutional Review Board of The Catholic University of Korea Daejeon St. Mary's Hospital (DC18RESI0100).
Author Contributions
K-YL designed the study, collected data, contributed to interpretation of results, and drafted the manuscript. J-WR contributed to data collection and drafted the initial manuscript. HK and J-WH contributed to data collection and revised the manuscript. All authors have read and approved submission of the final version of the manuscript.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors would like to acknowledge our colleges on data collection during the study periods of each disease.
References
- 1.Kawasaki T. Acute febrile muco-cutaneous lymph node syndrome in young children with unique digital desquamation. Arerugi. (1967)16:178–222. [PubMed] [Google Scholar]
- 2.Park JS, Suh CJ, Cho SH, Lee DB. Mucocutaneous lymph node syndrome: five case report. J Korean Pediatr Soc. (1973) 16:61–7. [Google Scholar]
- 3.Lue HC, Philip S, Chen MR, Wang JK, Wu MH. Surveillance of Kawasaki disease in Taiwan and review of the literature. Acta Paediatr Taiwan. (2004) 45:8–14. [PubMed] [Google Scholar]
- 4.Zhang T, Yanagawa H, Nakamura Y. The profiles of Kawasaki disease in China. J Epidemiol. (2001) 11:103–8. 10.2188/jea.11.103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Uehara R, Belay ED. Epidemiology of Kawasaki disease in Asia, Europe, and the United States. J Epidemiol. (2012) 22:79–85. 10.2188/jea.JE20110131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Singh S, Vignesh P, Burgner D. The epidemiology of Kawasaki disease: a global update. Arch Dis Child. (2015) 100:1084–8. 10.1136/archdischild-2014-307536 [DOI] [PubMed] [Google Scholar]
- 7.McCrindle BW, Rowley AH, Newburger JW, Burns JC, Bolger AF, Gewitz M, et al. Diagnosis, treatment, and long-term management of kawasaki disease: a scientific statement for health professionals from the American Heart Association. Circulation. (2017) 135:e927–99. 10.1161/CIR.0000000000000484 [DOI] [PubMed] [Google Scholar]
- 8.Kühne T. Diagnosis and management of immune thrombocytopenia in childhood. Hamostaseologie. (2017) 37:36–44. 10.5482/HAMO-16-06-0017 [DOI] [PubMed] [Google Scholar]
- 9.Chen JY, Mao JH. Henoch-Schönlein purpura nephritis in children: incidence, pathogenesis and management. World J Pediatr. (2015) 11:29–34. 10.1007/s12519-014-0534-5 [DOI] [PubMed] [Google Scholar]
- 10.Lee KY, Rhim JW, Kang JH. Kawasaki disease: laboratory findings and an immunopathogenesis on the premise of a “protein homeostasis system”. Yonsei Med J. (2012) 53:262–75. 10.3349/ymj.2012.53.2.262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee KY, Han JW, Lee JS. Kawasaki disease may be a hyperimmune reaction of genetically susceptible children to variants of normal environmental flora. Med Hypotheses. (2007) 69:642–51. 10.1016/j.mehy.2006.12.051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim EK, Youn YS, Rhim JW, Shin MS, Kang JH, Lee KY. Epidemiological comparison of three Mycoplasma pneumoniae pneumonia epidemics in a single hospital over 10 years. Korean J Pediatr. (2015) 58:172–7. 10.3345/kjp.2015.58.5.172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rhim JW, Go EJ, Lee KY, Youn YS, Kim MS, Park SH, et al. Pandemic 2009 H1N1 virus infection in children and adults: A cohort study at a single hospital throughout the epidemic. Int Arch Med. (2012) 5:13. 10.1186/1755-7682-5-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rhim JW, Lee KY, Youn YS, Kang JH, Kim JC. Epidemiological and clinical characteristics of childhood pandemic 2009 H1N1 virus infection: an observational cohort study. BMC Infect Dis. (2011) 11:225. 10.1186/1471-2334-11-225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee KY, Burgner D, Lee HS, Hong JH, Lee MH, Kang JH, et al. The changing epidemiology of pediatric aseptic meningitis in Daejeon, Korea from 1987 to 2003. BMC Infect Dis. (2005) 5:97. 10.1186/1471-2334-5-97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Huh SM, Park BK, Kang HM, Rhim JW, Suh JS, Lee KY. Clinical implications of DMSA scan in childhood acute pyelonephritis. Child Kidney Dis. (2017) 21:107–13. 10.3339/jkspn.2017.21.2.107 [DOI] [Google Scholar]
- 17.Kuem SW, Hur SM, Youn YS, Rhim JW, Suh JS, Lee KY. Changes in acute poststreptococcal glomerulonephritis: an observation study at a single Korean hospital over two decades. Child Kidney Dis. (2015) 19:112–7. 10.3339/chikd.2015.19.2.112 [DOI] [Google Scholar]
- 18.Rhim JW, Kang HM, Yang EA, KY Lee. Epidemiological relationship between Mycoplasma pneumoniae pneumonia and recurrent wheezing episode in children: an observational study at a single hospital in Korea. BMJ Open. (2019) 9:e026461. 10.1136/bmjopen-2018-026461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee KY, Han JW, Lee HS, Hong JH, Hahn SH, Lee JS, et al. Epidemiologic study of Kawasaki disease at a single hospital in Daejeon, Korea (1987 through 2000). Pediatr Infect Dis J. (2004) 23:52–5. 10.1097/01.inf.0000105201.92839.ec [DOI] [PubMed] [Google Scholar]
- 20.Rhim JW, Youn YS, Han JW, Lee SJ, Oh JH, Lee KY. Changes in Kawasaki disease during 2 decades at a single institution in Daejeon, Korea. Pediatr Infect Dis J. (2014) 33:372–5. 10.1097/INF.0000000000000123 [DOI] [PubMed] [Google Scholar]
- 21.Park YW, Park IS, Kim CH, Ma JS, Lee SB, Kim CH, et al. Epidemiologic study of Kawasaki disease in Korea, 1997-1999: comparison with previous studies during 1991-1996. J Korean Med Sci. (2002) 17:453–6. 10.3346/jkms.2002.17.4.453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim GB, Park S, Eun LY, Han JW, Lee SY, Yoon KL, et al. Epidemiology and clinical features of Kawasaki disease in South Korea, 2012-2014. Pediatr Infect Dis J. (2017) 36:482–5. 10.1097/INF.0000000000001474 [DOI] [PubMed] [Google Scholar]
- 23.Bhat RG, Katy TA, Place FC. Pediatric urinary tract infections. Emerg Med Clin North Am. (2011) 29:637–53. 10.1016/j.emc.2011.04.004 [DOI] [PubMed] [Google Scholar]
- 24.Lee KY. New insights for febrile urinary tract infection (acute pyelonephritis) in children. Child Kidney Dis. (2016) 20:37–44. 10.3339/jkspn.2016.20.2.37 [DOI] [Google Scholar]
- 25.Kontopoulou T, Kontopoulos DG, Vaidakis E, Mousoulis GP. Adult Kawasaki disease in a European patient: a case report and review of the literature. J Med Case Rep. (2015) 9:75. 10.1186/s13256-015-0516-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Enders G, Biber M, Meyer G, Helftenbein E. Prevalence of antibodies to human herpesvirus 6 in different age groups, in children with exanthema subitum, other acute exanthematous childhood diseases, Kawasaki syndrome, and acute infections with other herpesviruses and HIV. Infection. (1990) 18:12–5. 10.1007/BF01644173 [DOI] [PubMed] [Google Scholar]
- 27.Agut H, Bonnafous P, Gautheret-Dejean A. Update on infections with human herpesviruses 6A, 6B, and 7. Med Mal Infect. (2017) 47:83–91. 10.1016/j.medmal.2016.09.004 [DOI] [PubMed] [Google Scholar]
- 28.Collins-McMillen D, Goodrum FD. The loss of binary: Pushing the herpesvirus latency paradigm. Curr Clin Microbiol Rep. (2017) 4:124–31. 10.1007/s40588-017-0072-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pavone P, Cocuzza S, Passaniti E, Longo MR, Verrotti A, Serra A, et al. Otorrhea in Kawasaki disease diagnosis complicated by an EBV infection: coincidental disease or a true association. Eur Rev Med Pharmacol Sci. (2013) 17:989–93. [PubMed] [Google Scholar]
- 30.Kakisaka Y, Ohara T, Katayama S, Suzuki T, Sasai S, Hino-Fukuyo N, et al. Human herpes virus type 6 can cause skin lesions at the BCG inoculation site similar to Kawasaki disease. Tohoku J Exp Med. (2012) 228:351–3. 10.1620/tjem.228.351 [DOI] [PubMed] [Google Scholar]
- 31.Nagao Y, Urabe C, Nakamura H, Hatano N. Predicting the characteristics of the aetiological agent for Kawasaki disease from other paediatric infectious diseases in Japan. Epidemiol Infect. (2016) 144:478–92. 10.1017/S0950268815001223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Makino N, Nakamura Y, Yashiro M, Ae R, Tsuboi S, Aoyama Y, et al. Descriptive epidemiology of Kawasaki disease in Japan, 2011-2012: from the results of the 22nd nationwide survey. J Epidemiol. (2015) 25:239–45. 10.2188/jea.JE20140089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cani PD, Delzenne NM. Interplay between obesity and associated metabolic disorders: new insights into the gut microbiota. Curr Opin Pharmacol. (2009) 9:737–43. 10.1016/j.coph.2009.06.016 [DOI] [PubMed] [Google Scholar]
- 34.Doenyas C. Gut microbiota, inflammation, and probiotics on neural development in autism spectrum disorder. Neuroscience. (2018) 374:271–86. 10.1016/j.neuroscience.2018.01.060 [DOI] [PubMed] [Google Scholar]
- 35.Hörmannsperger G, Clave T, Haller D. Gut matters: microbe-host interactions in allergic diseases. J Allergy Clin Immunol. (2012) 129:1452–9. 10.1016/j.jaci.2011.12.993 [DOI] [PubMed] [Google Scholar]
- 36.Chen J, Domingue JC, Sears CL. Microbiota dysbiosis in select human cancers: evidence of association and causality. Semin Immunol. (2017) 32:25–34. 10.1016/j.smim.2017.08.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Arvonen M, Berntson L, Pokka T, Karttunen TJ, Vähäsalo P, Stoll ML. Gut microbiota-host interactions and juvenile idiopathic arthritis. Pediatr Rheumatol Online J. (2016) 14:44. 10.1186/s12969-016-0104-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kinumaki A, Sekizuka T, Hamada H, Kato K, Yamashita A, Kuroda M. Characterization of the gut microbiota of Kawasaki disease patients by metagenomic analysis. Front Microbiol. (2015) 6:824. 10.3389/fmicb.2015.00824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lin L, Zhang J. Role of intestinal microbiota and metabolites on gut homeostasis and human diseases. BMC Immunol. (2017) 18:2. 10.1186/s12865-016-0187-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Harusato A, Chassaing B. Insights on the impact of diet-mediated microbiota alterations on immunity and diseases. Am J Transplant. (2018)18:550–5. 10.1111/ajt.14477 [DOI] [PubMed] [Google Scholar]
- 41.Korpela K, de Vos WM. Antibiotic use in childhood alters the gut microbiota and predisposes to overweight. Microb Cell. (2016) 3:296–8. 10.15698/mic2016.07.514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Esposito S, Polinori I, Rigante D. The gut microbiota-host partnership as a potential driver of Kawasaki syndrome. Front Pediatr. (2019) 7:124. 10.3389/fped.2019.00124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wopereis H, Oozeer R, Knipping K, Belzer C, Knol J. The first thousand days - intestinal microbiology of early life: establishing a symbiosis. Pediatr Allergy Immunol. (2014) 25:428–38. 10.1111/pai.12232 [DOI] [PubMed] [Google Scholar]
- 44.Simon AK, Hollander GA, McMichael A. Evolution of the immune system in humans from infancy to old age. Proc Biol Sci. (2015) 282:20143085. 10.1098/rspb.2014.3085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Quinn RW. Comprehensive review of morbidity and mortality trends for rheumatic fever, streptococcal disease, and scarlet fever: the decline of rheumatic fever. Rev Infect Dis. (1989) 11:928–53. [DOI] [PubMed] [Google Scholar]
- 46.Kaplan EL, Gastanaduy AS, Huwe BB. The role of the carrier in treatment failures after antibiotic for group A streptococci in the upper respiratory tract. J Lab Clin Med. (1981) 98:326–35. [PubMed] [Google Scholar]
- 47.Roberts AL, Connolly KL, Kirse DJ, Evans AK, Poehling KA, Peters TR, et al. Detection of group A Streptococcus in tonsils from pediatric patients reveals high rate of asymptomatic streptococcal carriage. BMC Pediatr. (2012) 12:3. 10.1186/1471-2431-12-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Brook I. Treatment challenges of Group A beta-hemolytic streptococcal pharyngo-tonsillitis. Int Arch Otorhinolaryngol. (2017) 21:286–96. 10.1055/s-0036-1584294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gewitz MH, Baltimore RS, Tani LY, Sable CA, Shulman ST, Carapetis J, et al. Revision of the Jones Criteria for the diagnosis of acute rheumatic fever in the era of Doppler echocardiography: a scientific statement from the American Heart Association. Circulation. (2015) 131:1806–18. 10.1161/CIR.0000000000000205 [DOI] [PubMed] [Google Scholar]
- 50.Mirani G, Williams PL, Chernoff M, Abzug MJ, Levin MJ, Seage GR, III, et al. IMPAACT P1074 Study Team. Changing trends in complications and mortality rates among US youth and young adults with HIV Infection in the era of combination antiretroviral therapy. Clin Infect Dis. (2015) 61:1850–61. 10.1093/cid/civ687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kil HR, Yu JW, Lee SC, Rhim JW, Lee KY. Changes in clinical and laboratory features of Kawasaki disease noted over time in Daejeon, Korea. Pediatr Rheumatol. (2017) 15:60. 10.1186/s12969-017-0192-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kambham N. Postinfectious glomerulonephritis. Adv Anat Pathol. (2012) 19:338–47. 10.1097/PAP.0b013e31826663d9 [DOI] [PubMed] [Google Scholar]
- 53.Trachtenberg BH, Hare JM. Inflammatory cardiomyopathic syndromes. Circ Res. (2017) 121:803–18. 10.1161/CIRCRESAHA.117.310221 [DOI] [PubMed] [Google Scholar]
- 54.Lee KY. A common immunopathogenesis mechanism for infectious diseases: the protein-homeostasis-system hypothesis. Infect Chemother. (2015) 47:12–26. 10.3947/ic.2015.47.1.12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lee KY. A unified pathogenesis for kidney diseases, including genetic diseases and cancers, by the protein-homeostasis-system hypothesis. Kidney Res Clin Pract. (2017) 36:132–44. 10.23876/j.krcp.2017.36.2.132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Osterlund A, Engstrand L. An intracellular sanctuary for Streptococcus pyogenes. in human tonsillar epithelium: studies of asymptomatic carriers and in vitro cultured biopsies. Acta Otolaryngol. (1997) 117:883–8. [DOI] [PubMed] [Google Scholar]
- 57.O'Neill AM, Thurston TL, Holden DW. Cytosolic replication of Group A streptococcus. in human macrophages. MBio. (2016) 7:e00020–16. 10.1128/mBio.00020-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hegde S, Hegde S, Spergser J, Brunthaler R, Rosengarten R, Chopra-Dewasthaly R. In vitro and in vivo cell invasion and systemic spreading of Mycoplasma agalactiae in the sheep infection model. Int J Med Microbiol. (2014) 304:1024–31. 10.1016/j.ijmm.2014.07.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Han JW, Oh JH, Rhim JW, Lee KY. Correlation between elevated platelet count and immunoglobulin levels in the early convalescent stage of Kawasaki disease. Medicine (Baltimore). (2017) 96:e7583. 10.1097/MD.0000000000007583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lee KY. Pneumonia, acute respiratory distress syndrome, and early immune-modulator therapy. Int J Mol Sci. (2017) 18:E388. 10.3390/ijms18020388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Seo YM, Kang HM, Lee SC, Yu JW, Kil HR, Rhim JE, et al. Clinical implications in laboratory parameter values in acute Kawasaki disease for early diagnosis and proper treatment. Korean J Pediatr. (2018) 61:160–6. 10.3345/kjp.2018.61.5.160 [DOI] [PMC free article] [PubMed] [Google Scholar]