Abstract
The present study discusses the isolation of ursolic acid from the chloroform extract of Paulownia tomentosa (Thunb) Steud fruits and its cytotoxic effect has been assessed in-vitro was performed in different cells lines (A-549, MCF-7, HepG2) and in-vivo using N-diethylnitrosamine. The obtained results revealed that ursolic acid showed significant cytotoxic activity on MCF-7 and HepG2 cell lines in comparison to Doxorubicin as a reference drug. Moreover, we have assessed the inhibitory effects of Paulownia tomentosa fruit chloroform extract and the isolated ursolic acid on hepatocarcinogenesis was carried out for the first time using N-diethylnitrosamine, where the group treated with ursolic acid given orally after 8 weeks of cancer induction showed the most significant results in comparison to the chloroform extract. The effect of ursolic acid on intoxicated rats caused significant restoration of most of the normal hepatocytes architecture with regular dark nuclei and the group treated with Paulownia tomentosa fruits showed remarkable results with improvement in biochemical analysis.
Keywords: Biochemistry, Natural product chemistry, Pharmaceutical chemistry
1. Introduction
The liver is one of the most vital organs in the human body dealing with the metabolism and detoxification of external and internal chemical substances. Many toxins target the liver and cause hepatotoxic effects that can be observed through some biochemical parameters. As it is the main site of N-diethylnitrosamine (NDEA) metabolism, the production of ROS in liver may be responsible for its carcinogenic effects [1].
Many herbs have been proven to be effective as hepatoprotective agents; these hepatoprotective plants have the phytoconstituents such as iridoidglycosides, phenyl compounds, coumarins, essential oils, monoterpenoids, diterpenoids, triterpenoids, steroids, alkaloids and other nitrogenous compounds [2].
The chemical hepatocarcinogen NDEA is known to induce over expression of TGF-α (the growth factor transforming growth factor-α) which is closely involved in hepatocarcinogenesis and transformation in humans and animals. Furthermore, elevated expression of TGF-α in hepatic cirrhosis as TGF-α expression is higher in liver tissue of patients with chronic hepatic diseases compared with normal healthy subject [3].This phenomenon is thought to play an important role in the process of NDEA -induced hepatocarcinogenesis which is evidenced by changes in histopathological architecture and increased liver marker enzymes [4, 5].
Paulownia tomentosa (Thunb.) Steud. belongs to the family Scrophulariaceae, and it is commonly known as princess tree. It is a perennial tree native to China, Japan and the Far East but it has been adapted to the southern United States and it is even invasive now [6]. It has been used as an oriental medicine for the treatment of gonorrhea, bruise and deodorant. A decoction of the leaves was used to wash foul ulcers and to promote the growth of hair and prevent greying [7].
Ursolic acid is a naturally occurring pentacyclic triterpene, found in many plants and has a chemo protective activity in human. It possesses many important biological activities, such as anti-inflammatory, hepatoprotective, antiulcer, hypolipidemic and anti-atherosclerotic [8]. Numerous reports discussed ursolic acid in-vitro activities against tumor cell lines [9]. Several suggest possible mechanisms of action for tumor inhibition; i.e the proliferation of MCF-7 breast tumor cells by exerting an early cytostatic effect on the cell cycle at G1.The cytotoxic and cytostatic effects are likely to involve apoptosis (cell self-destruction). Also ursolic acid induced apoptosis in HepG2human hepatoblastoma cells in a dose-dependent manner, with DNA fragmentation, enhanced release of cytochrome c, and activation of caspase-3 (mediator for apoptosis). Expression of p21WAF1 (tumor suppressor) was increased, indicating possible involvement in mediating cell-cycle arrest. Also ursolic acid was found to inhibit tumor invasion by inhibited expression of both MMP-2 and MMP-9 at micromole concentrations, a finding that is consistent with the observed ability of ursolic acid to inhibit MMP expression in fibrosarcoma cells, and also inhibits proliferation and colony formation of tumor and limit the ability of tumor to invade and metastasize [10]. Ursolic acid reduces the proliferation of many tumor cell lines and many possible mechanisms of action have been addressed. Studies in MCF-7 breast carcinoma cells showed an early G1 cytostatic effect for ursolic acid [11, 12]. Enhancement of intracellular Ca2+ signaling is thought to play a role in reducing proliferation [9]. Ursolic acid has been previously isolated from Paulownia tomentosa Leaves [13, 14]. The Essential oil of the flowers of Paulownia tomentosa. Growing in Egypt play a roleas antimicrobial activity [15].The methanolic extract was reported as antioxidant Hypoglycemic [16].
2. Material and methods
2.1. Plant material
Fresh mature fruits of Paulownia tomentosa were collected in September 2007 from the Agricultural Museum, Giza, Egypt. Identified by Mrs. Trease Labib consultant of plant taxonomy at the Ministry of Agriculture and former director of El-Orman botanical garden. A voucher specimen (No.06-06-03-16) was kept at the Herbarium of El-Orman Botanical Garden. The collected fruits were air dried, separately powdered and kept in tightly-closed containers.
2.1.1. Extraction and isolation
Successive extraction for P. tomentosa fruits (80g) using Soxhlet apparatus starting with petroleum ether till complete defatting followed by chloroform till exhaustion. The chloroform extract (11g) was purified using charcoal powder, filtered and the filtrate was collected, evaporated under vacuum till dryness and weighted, then kept to be used for isolation of the main active constituents and evaluation the in vitro and in-vivo anticancer effects. The chloroform extracts of P. tomentosa fruits, chromatographed using TLC alongside with ursolic acid for comparison, using solvent systems benzene-ethyl acetate (86:14 v/v) and benzene-methanol (9:1 v/v) as development solvent systems and the plate was sprayed with vanillin sulphuric reagent then heated at 110 °C for 10 min and the results showed the presence of ursolic acid the major compound in the chloroform extract of Paulownia tomentosa fruits which was isolated by preparative TLC then purified and crystallized from methanol.
2.1.2. Ursolic acid compound
White powder, m.p. = 283 - 285 °C, freely soluble in CHCl3 and ether, partially soluble in alcohol.
H−1NMR of Ursolic acid compound (Figs. 1 and 2):(CDCI3, 400 MHz): δH 5.24 (1H, brs, H-12), 3.19 (1H, brs, H-3), 2.17 (1H, brs, H 18), 1.24 (3H, s, Me-23), 0.98 (3H, s, Me-24), 0.77 (3H, Me-25), 1.07 (3H, s, Me-26), 1.13 (3H, s, Me-27), 0.94 (3H, brs, Me-29), 0.92 (3H, brs, Me-30).
Fig. 1.
Chemical structure of ursolic acid.
Fig. 2.
Mass spectra of ursolic acid compound.
EI/MS, m/z (rel.int): 456 (M+), 439.10 (23), 457.68 (18), 304.21 (15), 315.49 (13), 395.07 (12), 411.92 (12), 393.25 (100) [8].
2.1.3. Material for in-vitro cytotoxicity testing
The in vitro antitumor screening was performed adopting previously reported procedures. Cells were suspended in RPMI 1640 medium for HepG2, MCF7 and DMEM for A549, 1% antibiotic-antimycotic mixture (10,000 u/ml potassium penicillin, 10,000 mg/ml streptomycin sulfate and 25 mg/ml amphotericin B) and 1% L-glutamine at 37°C, fewer than 5% CO2 and 95% humidity. Cells were seeded at concentration of 1 × 104 cells/well in fresh complete growth medium in 96-well microliter plates for 24 h. Media was aspirated, fresh medium (without serum) was added and cells were incubated with different concentrations of sample to give a final concentration of (100, 50, 25, 12.5, 6.25, 3.125, 0.78 and 1.56 Ug/ml). 0.5% DMSO was used as negative control and 100 mg/ml of doxorubicin was used as positive control. MTT assay was used for assessment of cytotoxicity. After 48 h of incubation, medium was aspirated, 40 ml MTT salt (2.5 mg/ml) were added to each well and incubated for further 4 h. To stop the reaction and dissolving the formed crystals, 200 ml of 10% sodium dodecyl sulfate (SDS) in deionized water were added to each well and incubated overnight at 37 °C. The absorbance was then measured at 595 nm and a reference wave length of 620 nm. A statistical significance was tested between samples and negative control (cells with vehicle) using independent t-test by SPSS 11 program. The % cytotoxicity was calculated according to the formula: [1-(OD compound/OD negative control)] x100]. A probit analysis was carried for LC50 determination using SPSS 11 program.
2.2. Material for in-vivo hepatoprotective studies
2.2.1. Chemicals and biochemical kits
All chemicals used in the present study were of high analytical grade, products of Sigma (USA), Merck (Germany), BDH (England), diethyl nitrosamine (Sigma Co.) was used for induction of hepatic carcinogens in rats. Kits used for the quantitative determination of different parameters were purchased from Bio-diagnostic (Egypt).
2.2.2. Animals
Male albino rats weighting 130–150g supplied from the animal house of National Research Centre (Dokki, Giza, Egypt) and were used for experimental investigation. Animals were kept for two weeks to accommodate on laboratory conditions; they were kept under constant environmental and nutritional conditions, given food and water all over the period of the experiment. Appropriate anesthetic and sacrifice procedures were followed ensuring that animals didn't suffer at any stage of the experiments. Anesthetic procedures complied and were approved according to the ethical committee of the National Research Centre in Egypt with registration No.13–016.
2.2.3. Experimental design
2.2.3.1. Induction of liver damage
Liver damage in rats was induced by intraperitoneal injection of (200 mg/kg body weight) with N-diethyl-nitrosamine once weekly for eight weeks. Forty male Wister albino rats, weighing 130–150 g will be used in this study. Animals will be divided into 5 groups each of eight rats.
-
-
Group 1: Normal healthy control rats.
-
-
Group 2: Orally administered with chloroform extract of fruits to determine if there were any side effects.
-
-
Group 3: Given NDEA (200 mg/kg body weight) for 8 weeks.
-
-
Group 4: Given NDEA (200 mg/kg body weight) along with chloroform extract of fruits that will be orally administered (500 mg/kg).
-
-
Group 5 orally administered with isolated ursolic acid (500 mg/kg) after 8 weeks given NDEA.
After each experimental period of different groups NDEA, the sub-tongual vein was punctured in each animal in all groups, and blood samples were collected to be used for biochemical analysis. After blood collection, animals were sacrificed after decapitation, and then liver was rapidly removed, cut small sections then put in 10% formalin solution for histological and histopathological analysis, while the other sections were either used immediately or frozen at –80 °C for other biochemical analyses. Histological and histopathological analyses were conducted according to Hirsch et al. [17] to evaluate structural alterations of the hepatic parenchymal cells.
2.2.4. Parameters in case of NDEA induction
2.2.4.1. Liver functions enzymes in serum
The Albumin level according to Dumas et al. [18], Alanine Aminotransferase Activity (ALT) and Aspartate Aminotransferase Activity (AST) according to Rietman and Frankle [19],Alkaline Phosphatase Activity (ALP)according to Babson et al. [20], and γ Glutamyl Transferase Activity (GGT) according to Szasz, [21].
3. Results
3.1. Determination of in-vitro cytotoxic activity
The isolated ursolic acid compound was tested for its in-vitro cytotoxic activity against (A-549), (MCF-7) and (HepG2) at concentration 100 μg/ml using MTT assay [22]. The cytotoxic activity of the isolated compound gave 100% activity at 100 μg/ml concentration; this led to secondary screening to calculate its IC50 value as in Table 1.
Table 1.
IC50 of cytotoxic active compound isolated from P. tomentosa (Thunb.) Steud. fruits extract against different cell lines.
| Sample Name |
aIC50 (μg/1 × 104) |
||
|---|---|---|---|
| HEPG2 | A-549 | MCF-7 | |
| Compound 1 | 14.5 | 68.4 | 3.5 |
| Doxorubicin | 21.4 | 28.3 | 26.1 |
IC50: compound concentration required to inhibit the cell viability by 50%.
3.2. Determination of in-vivo cytotoxic activity
Experimental animals exposed to N-diethylnitrosamine NDEA, showed that transaminase activities (AST, ALT & ALP) were increased in plasma by the release of these enzymes from hepatic parenchyma cells, indicating a considerable hepatocellular injury by 165.57%, 55.81% and 78.77% respectively as compared to the control group. Therapeutic treatment with P. tomentosa, chloroform extract and the isolated ursolic acid modulated the hepatic liver enzymes with 65.60%, 3.81% and 9.44% for chloform extract treated group and with 7.53%, 112.62% and 0.32% for ursolic acid treated group respectively, with respect to control group. On the other hand control groups treated with either fruits extract or ursolic acid showing no significant change of the enzymes AST, ALT and ALP as revealed in Table 2, Fig. 3.
Table 2.
Effect of the chloroform extract of Paulownia tomentosa (Thunb.) Steud. fruits & isolated ursolic acid on serum ALT, AST and ALP of rats.
| Parameters | Control | Control Treated fruits | Diethyl nitrosamine | Diethyl nitrosamine Treatment fruits | Treated Ursolic acid | ANOVA P ≤ |
|---|---|---|---|---|---|---|
| ALP | 65.82 ± 0.85c | 65.37 ± 0.98c | 174.80 ± 4.26a | 109 ± 1.88b | 70.78 ± 1.27c | 0.0001 |
| AST | 51.49 ± 0.83b | 33.22 ± 0.54d | 80.23 ± 1.16a | 49.53 ± 0.98bc | 44.99 ± 0.67bc | 0.0001 |
| ALT | 28.49 ± 0.32cd | 21.75 ± 0.39e | 50.99 ± 1.2a | 31.18 ± 0.59bc | 28.58 ± 0.34cd | 0.0001 |
⁃ Data are means ± SE of eight rats in each group.
⁃ Data are expressed as U/ml for ALT, AST and ALP.
⁃ Unshared letters between groups are significance value at p ≤ 0.05.
⁃ Statistical analysis is carried out using one way analysis of variance (ANOVA) by Co Stat Computer Program.
Fig. 3.
The hepatoprotective activity of the chloroform fruits extract of Paulownia tomentosa (Thunb.) Steud. & isolated ursolic acid on serum ALT, AST and ALP of rats.
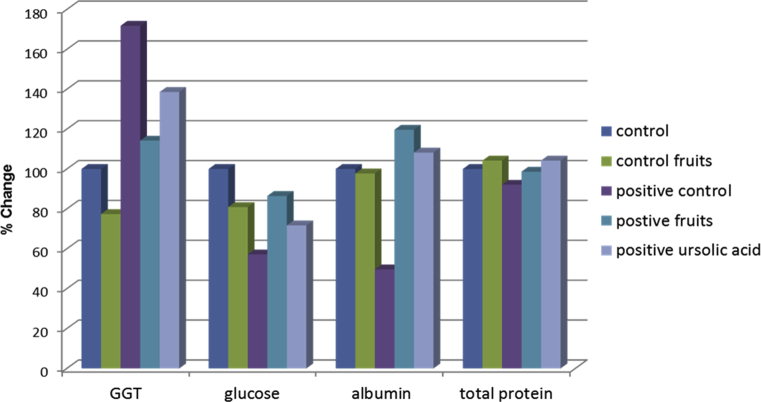
In case of albumin and total protein a significant reduction was recorded with NDEA given with different percentages 50.37% and 7.90% respectively, as compared to control group. After treated with fruits extract and ursolic acid, ameliorated these disturbances with different percentage of improvement, albumin record an elevation in the albumin level by 6.39% and 5.78% in groups treated with fruits and isolated ursolic acid respectively with comparison to control group, total protein results were close to the control group, although there was a slight elevation in case of ursolic acid treated group, according to GGT there was a significant increase in GGT liver enzymes by 71.8% as a result of NDEA induction with respect to control which ameliorated these disturbance by 20.377% and 38.61% respectively, as compared to the control group, no significant change in GGT was recorded in fruit extract normal group as compared to control. The significant decrease in GGT activity observed in rats treated with fruits extract and ursolic acid suggests a possible curative effect of P. tomentosa fruits extract in liver disturbance condition, which showed highly significant results near to control. For, hepatic glucose level, there was decrease by 42.83% in case of group given (NDEA),the treatment with fruits extract and ursolic acid ameliorated these decreases by 13.55% and 28.28% respectively compared to the control group as shown in Table 3, Fig. 4.
Table 3.
Effect of the chloroform extract of Paulownia tomentosa (Thunb.) Steud. Fruits & isolated ursolic acid on serum albumin, total protein, GGT & hepatic glucose of rats.
| Parameters | Control | Control Treated fruits | Diethyl nitrosamine | Diethyl nitrosamine Treatment fruits | Treated Ursolic acid | ANOVA P ≤ |
|---|---|---|---|---|---|---|
| GGT | 55.11 ± 0.97d | 42.66 ± 0.52e | 94.69 ± 1.77a | 62.95 ± 1.15cd | 76.39 ± 1.14b | 0.0001 |
| Glucose | 66.05 ± 0.62a | 53.42 ± 0.69c | 37.76 ± 0.69e | 57.10 ± o.69bc | 47.37 ± 0.60d | 0.0001 |
| T. Protein | 17.7 ± 0.18ab | 18.44 ± 0.13a | 16.3 ± 0.1c | 17.46 ± 0.12ab | 18.45 ± 0.15a | 0.002 |
| Albumin | 5.34 ± 0.08c | 5.22 ± 0.08c | 2.65 ± 0.09d | 6.39 ± 0.09ab | 5.78 ± 0.1bc | 0.0001 |
⁃ Data are means ± SE of eight rats in each group.
⁃ Data are expressed as g/dl for serum protein and albumin as g/l
⁃ Data are expressed as g/l hepatic glucose and U/mg protein for GGT
⁃ Unshared letters between groups are significance value at p≤0.05.
⁃ Statistical analysis is carried out using one way analysis of variance (ANOVA) by Co Stat Computer Program.
Fig. 4.
The hepatoprotective activity of the chloroform fruits extract of Paulownia tomentosa (Thunb.) Steud. & isolated ursolic acid on GGT, glucose, albumin and total protein of rats.
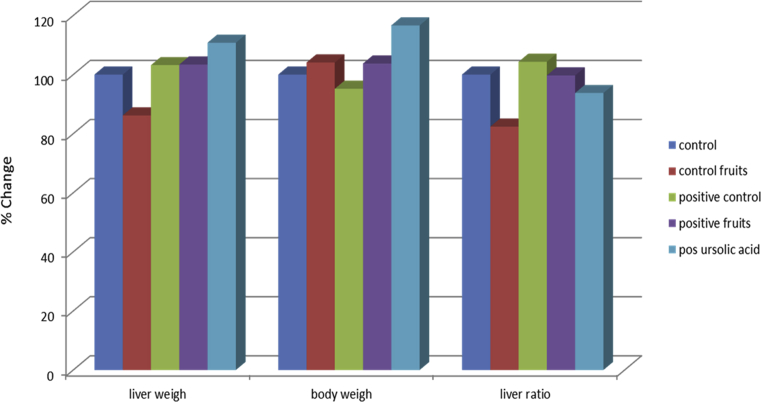
Table 4& Fig. 5 showed the changes in rats liver weight, body weight and relative liver weight due to NDEA induction wheretherewasincreaseinliverweightby3.2%ascomparedto control and decrease in the body weight by -4.79%, relative liver weight were increased slightly by 4.33% in NDEA group compared to control. Therapeutic treatment with fruits record 3.4%, 3.748% and 0.27% also according to ursolic acid record 10.8%, 16.65% and 6.233% with respect to control.
Table 4.
Effect of the chloroform extract of Paulownia tomentosa (Thunb.) Steud. fruits & isolated ursolic acid on liver weigh, body weight and liver ratio.
| Parameters | Control | Control treated Fruits | NDEA | NDEA Treated fruits | Treated Ursolic acid | ANOVA P ≤ |
|---|---|---|---|---|---|---|
| Liver weigh | 5.00 ± 0.06ab | 4.31 ± 0.02c | 5.16 ± 0.11ab | 5.17 ± 0.05ab | 5.54 ± 0.14a | 0.021 |
| Body weigh | 136.33 ± 1.5bc | 141.9 ± 0.69b | 129.8 ± 0.94cd | 141.4 ± 1.10bc | 159.0 ± 1.87a | 0.0001 |
| Liver ratio | 3.69 ± 0.04ab | 3.04 ± 0.012e | 3.85 ± 0.12ab | 3.68 ± 0.058ab | 3.46 ± 0.057bc | 0.004 |
• Data are means ± SE of eight rats in each group.
• Data are expressed as grams.
• Unshared letters between groups are significance value at p ≤ 0.05.
• Statistical analysis is carried out using one way analysis of variance (ANOVA) by Co Stat Computer Program.
Fig. 5.
The hepatoprotective activity of the chloroform fruits extract of Paulownia tomentosa (Thunb.) Steud. & isolated ursolic acid on liver weigh, body weight and liver ratio.
3.3. Histopathological findings
Histology examination of liver sections Fig. 6 of control animals (A) showed normal hepatocyte cell. H&E stain. (A*) normal hepatocyte cell Masson’ trichome stains record normal collagen distribution. (B) NDEA group showed appearance of hepatocellular carcinoma with enlarged hyperchromatic nuclei and scattered mitosis after 8 weeks of NDEA administration H&E stain. (B*) cirrhosis cells and damage cell wall of hepatic cells shown through collagen increase distribution stained with Masson' trichome. (C) Revealed the effect of ursolic acid which orally given after 8 weeks of diethyl nitrosamine administration, restoration of most of the normal hepatocytes architecture with regular dark nuclei. (D) H&E stain showed significant regression of hepatic fibrosis in the cirrhotic rats treated with fruits extracts after 8 weeks of induction of cirrhosis and (D*) Masson’ trichome stain showing initial improvement and initial regression of the fibrosis. The biochemical findings are supported by the histopathological observations, which revealed induction of hepatocellular damage and the enhancement of liver cells, through hepatic enzyme function Fig. 7.
Fig. 6.
Liver sections of control animals [H&E] stain (A), (A*) normal hepatocyte cell Masson’ trichome stains. (B) DEN group [H&E] stain. (B*) collagen increase distribution stained with Masson trichome. (C) Revealed the effect of ursolic acid [H&E] stain (C)*the same section stained with. Masson's trichome. (D) H&E stain cirrhotic rats treated with fruits extracts (D*) Masson’ trichome stain showing initial improvement.
Fig. 7.
A quantitative analysis of Fig. 6.
3.4. Statistical analysis
Data presented as mean ± standard error (SE), Statistical analysis is carried out using one way analysis of variance (ANOVA) Computer program by Co Stat Computer Program significant at p ≤ 0.05.
4. Discussion
N-nitrosodiethylamine is one of the most important environmental carcinogen of this class, has been suggested to cause the generation of reactive oxygen species (ROS) resulting in oxidative stress and cellular injury [4]. Ursolic acid is a hydroxy penta cyclic triterpene, which has a chemoprotective activity in human and found in many plant, it is the major component of some traditional medicinal herbs, It possesses many important biological activities, such as hepatoprotective, anti-inflammatory, antiulcer, hypolipidemic and ant atherosclerotic [8].
On the basis of physical, chromatographic and spectral data and also by comparison with published data [8],compound (1) was established and assigned as ursolic acid, which was identified in leaves, fruits and flowers using different chromatographic and spectroscopic techniques.
From the Table 1 and Fig. 2. The isolated ursolic acid showed the most promising and has the highest selectivity on MCF-7 with IC50 3.5 μg/ml even more than the reference drug 'Doxorubicin' with IC50 26.1 μg/ml. Moreover, ursolic acid showed strong cytotoxic activity against HepG2 cell line with IC50 21.4 μg/ml.
Elevated serum concentrations of this protein can be achieved in the adult by exposure to hepatocarcinogen agents. It has the high specificity for hepatocarcinoma. Its serum concentration confirms hepatocarcinoma and for the diagnosis of tumor response to therapy [5].
The significant observed improvement in all liver parameters, albumin, glucose and total protein in the treated rats with fruits ext. or ursolic acid, suggests a possible protective effect of ursolic acid in liver disturbance condition which showed highly significant results as compared to control. Lower level of total protein showing the reduction in biosynthesis of protein due to destructions and dissociation of polyribosome's on endoplasmic reticulum caused by toxicity of NDEA [23].
Liver mass recovery accelerates by using ursolic acid, it stimulates hepatocyte proliferation after partial hepatectomy, and this may be associated with the stimulation of C/EBPβ expression [24]. NDEA has been shown to be metabolized to its active ethyl radical, which can interact with DNA causing mutation and subsequent oncogenesis [25].Ursolic acid has been shown to inhibit DNA replication [26].
The present study has assessed the inhibitory effects of Paulownia tomentosa (Thunb.) Steud. Flowers, fruits and isolated ursolic acid on hepatocarcinogenesis for the first time using diethyl nitrosamine induced rats' hepatocarcinogenesis model. Transaminase activities AST, ALT, ALP, GGT, were increased remarkably in plasma by the release of these enzymes from hepatic parenchymal cells, which were indicating a considerable hepatocellular injury while albumin, total protein showing reduction against NDEA treatment in agreement [4].
There was significant decrease of AST, ALT, GGT and ALP activities observed in rats treated with fruits and ursolic acid suggested a possible protective effect of P. tomentosa especially with ursolic acid in liver disturbance condition also Treatment with fruits extract ameliorated the disturbances in all total protein, albumin, glucose with different percentage of improvement. These biochemical findings were further confirmed by histopathological studies. On histopathological sections of the liver cell, fruits and ursolic acid treatment revealed that it had antitumor activity, evidenced from the absence of cellular necrosis and inflammatory infiltrates in the liver section of rats. Ursolic acid is able to restore the biochemical and histological changes caused by the development of hepatocellular carcinoma.
5. Conclusion
The present study has assessed the inhibitory effects of Paulownia tomentosa, fruits and the isolated ursolic acid on hepatocarcinogenesis is for the first time using a NDEA induced rats hepatocarcinogenesis model. Ursolic acid plays a role in accelerating liver proliferation, recovering liver function, and protecting the integrity of hepatocytes against liver damage.
Declarations
Author contribution statement
Sanaa Ali, Nabaweya Ibrahim, Magdy Mohammed, Seham El-Hawary, Esraa Refaat: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Funding statement
This work was supported by the National Research Centre, Giza, Egypt.
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
References
- 1.Bansal A.K., Bansal M., Soni G., Bhavnagar D. Protective role of Vitamin E pre-treatment on N-nitrosodiethylamine induced oxidative stress in rat liver. Chem. Biol. Interact. 2005;156:3101–3111. doi: 10.1016/j.cbi.2005.08.001. [DOI] [PubMed] [Google Scholar]
- 2.Valan M.F., John de britto A., Venkataraman R. Phytoconstituents with hepatoprotective activity. Int. J. Chem. Sci. 2010;8(3):1421–1432. [Google Scholar]
- 3.Kagawa M., Sano T., Ishibashi N., Hashimoto M., Okuno M., Moriwaki H. An acyclic retinoid, NIK-333, inhibits N-diethylnitrosamine-induced rat hepatocarcinogenesis through suppression of TGF-α expression and cell proliferation. Carcinogenesis. 2004;25(6):979–985. doi: 10.1093/carcin/bgh093. [DOI] [PubMed] [Google Scholar]
- 4.Hamed M.A., Aly H.F., Ali S.A., Metwalley N.S., Hassan S.A., Ahmed S.A. In vitro and in vivo assessment of some functional foods against initiation of hepatocellular carcinoma. J. Basic. Appl. Sci. Res. 2012;2(1):471–483. [Google Scholar]
- 5.Ali S.A., Ahmed S.A., Farrag A.H. Protective and prophylactic role of brassicaceae vegetables extracts on N-nitrosodiethylamine induced initiation of hepatocellular carcinoma in rat liver. Res. Rev.: Journal of Pharmacology and Toxicological Studies. 2015;1:1–19. [Google Scholar]
- 6.Vol. III. Sanghai Science and Technology press; 1985. p. 1925. (Chinese Drugs Encyclopedia). [Google Scholar]
- 7.Duke J.A., Ayensu E.S. Reference Publ., Inc; 1985. Medicinal Plants of China. [Google Scholar]
- 8.Novotny L., Abdel-Hamid M.E., Hamza H., Masterova I., Grancai D. Development of LC-MS method for determination of ursolic acid: application to the analysis of ursolic acid in Staphylea holocarpa Hemsl. J. Pharm. Biomed. Anal. 2003;31:961–968. doi: 10.1016/s0731-7085(02)00706-9. [DOI] [PubMed] [Google Scholar]
- 9.Novotny L., Vachalkova A., Biggs D. Ursolic acid: an anti-tumorigenic and chemopreventive activity mini review. Neoplasma. 2002;48:241–246. [PubMed] [Google Scholar]
- 10.Neto C.C. Cranberry and its phytochemicals: a review of in-vitro anticancer studies. J. Nutr. 2007;137:186S–193S. doi: 10.1093/jn/137.1.186S. [DOI] [PubMed] [Google Scholar]
- 11.Es-saady D., Simon A., Jayat-Vignoles C., Chulia A.J., Delage C. MCF-7 cell cycle arrested at G1 through ursolic acid and increased reduction of tetrazolium salts. Anticancer Res. 1996;16(1):481–486. [PubMed] [Google Scholar]
- 12.Es-saady D., Simon A., Ollier M., Maurizis J.C., Chulia A.J., Delage C. Inhibitory effect of ursolic acid on B16 proliferation through cell cycle arrest. Cancer Lett. 1996;106(2):193–197. doi: 10.1016/0304-3835(96)04312-1. [DOI] [PubMed] [Google Scholar]
- 13.Tanabe Y., Kondo S., Takahashi K. Constituents of medical plants III. Constituents of leaves of Paulownia tomentosa and Rhododendron kaemfperi. Kanazawa Daigaku Yakugakubu Kenkyu Nempo. 1962;12:7–14. [Google Scholar]
- 14.Jing H., Lai-Jiu S.A. Study on the new extraction process of ursolic acid from the Paulownia tomentosa (Thunb.) Steud leaves. J. Northwest Univ. 2003;33(3):304–306. [Google Scholar]
- 15.Ibrahim N.A., El-Hawary S.S., Mohammed M.M.D., Faraid M.A., Nayera A.M., Refaat E.S. Chemical composition, antimicrobial activity of the essential oil of the flowers of Paulownia tomentosa (Thunb.) Steud. Growing in Egypt. J. Appl. Sci. Res. 2013;9(4):3228–3232. [Google Scholar]
- 16.Ibrahim N.A., El-Hawary S.S., Ali S.A., Mohammed M.M.D., Refaat E.A. Chemical constituents of Paulownia tomentosa (Thunb.) Steud. Fam. Scrophulariaceae and its role against hyperglycemia. World J. Pharmaceut. Res. 2015;4(8):2445–2466. [Google Scholar]
- 17.Hirsch C., Zouain C.S., Alves J.B., Goes A.M. Induction of protective immunity and modulation of granulomatous hypersensitivity in mice using PIII, an anionic fraction of Schistosoma mansoni adult worm. Parasitology. 1997;115:21–28. doi: 10.1017/s0031182097001078. [DOI] [PubMed] [Google Scholar]
- 18.Dumas B.T., Watson W.A., Biggs H.G. Albumin standard and the measurement of serum albumin with Bromocresol green. Clin. Chem. Acta. 1971;3:87–96. doi: 10.1016/0009-8981(71)90365-2. [DOI] [PubMed] [Google Scholar]
- 19.Rietman S., Frankle S.A. Colorimetric method for determination of serum glutamic oxaloacetic and glutamic pyruvic transaminases. Am. J. Clin. Pathol. 1957;28:56–63. doi: 10.1093/ajcp/28.1.56. [DOI] [PubMed] [Google Scholar]
- 20.Babson A.L., Greely S.J., Coleman C.M., Philips G.E. Phenolphthalein monophosphate as a substrate for serum alkaline phosphatase. Clin. Chem. 1966;12:482–490. [PubMed] [Google Scholar]
- 21.Szasz G.A. Kinetic photometric method for serum gamma glutamyl transpeptidase. Clin. Chem. 1969;15:124–136. [PubMed] [Google Scholar]
- 22.Mosmann T. Rapid colorimetric assays for cellular growth and survival: application to proliferation and cytotoxicity assays. J. Immunol. Methods. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- 23.Kazmi I., Narooka A., Afzal M., Singh R., Al-Abbasi F.A., Ahmad A., Anwar F. Anticancer effect of ursolic acid stearoyl glucoside in chemically induced hepatocellular carcinoma. J. Physiol. Biochem. 2013;69:687–695. doi: 10.1007/s13105-013-0245-8. [DOI] [PubMed] [Google Scholar]
- 24.Yong-Ri J., Jing-ling J., Cheng-Hao L., Xi-Xu P., Nan-Ge J. Ursolic acid enhances mouse liver regeneration after partial hepatectomy. Pharmaceut. Biol. 2012;50(4):523–528. doi: 10.3109/13880209.2011.611143. [DOI] [PubMed] [Google Scholar]
- 25.Chakraborty T., Chatterjee A., Rana A., Dhachinamoorthi D., Kumar P.A., Chatterjee M. Carcinogen-induced early molecular events and its implication in the initiation of chemical hepatocarcinogenesis in rats: chemopreventive role of vanadium on this process. Biochim. Biophys. Acta. 2007;1772:48–59. doi: 10.1016/j.bbadis.2006.10.019. [DOI] [PubMed] [Google Scholar]
- 26.Kim D.K., Baek J.H., Kang C.M., Yoo M.A., Sung J.W., Chung H.Y.N.D., Kim Y.H., Choi Lee S.H., Kim K.W. Apoptotic activity of ursolic activity may correlate with the inhibition of initiation of DNA replication. Int. J. Cancer. 2000;87:629–636. [PubMed] [Google Scholar]