Clinical features of dystonic tremor can resemble those of essential tremor, complicating diagnosis. Using task fMRI, DeSimone et al. reveal widespread network connectivity differences between patients with dystonic tremor versus patients with essential tremor and healthy controls, across cortical, subcortical and cerebellar regions.
Keywords: cerebellar function, dystonia, motor control, motor cortex, tremor
Abstract
Dystonia is a movement disorder characterized by involuntary muscle co-contractions that give rise to disabling movements and postures. A recent expert consensus labelled the incidence of tremor as a core feature of dystonia that can affect body regions both symptomatic and asymptomatic to dystonic features. We are only beginning to understand the neural network-level signatures that relate to clinical features of dystonic tremor. At the same time, clinical features of dystonic tremor can resemble that of essential tremor and present a diagnostic confound for clinicians. Here, we examined network-level functional activation and connectivity in patients with dystonic tremor and essential tremor. The dystonic tremor group included primarily cervical dystonia patients with dystonic head tremor and the majority had additional upper-limb tremor. The experimental paradigm included a precision grip-force task wherein online visual feedback related to force was manipulated across high and low spatial feedback levels. Prior work using this paradigm in essential tremor patients produced exacerbation of grip-force tremor and associated changes in functional activation. As such, we directly compared the effect of visual feedback on grip-force tremor and associated functional network-level activation and connectivity between dystonic tremor and essential tremor patient cohorts to better understand disease-specific mechanisms. Increased visual feedback similarly exacerbated force tremor during the grip-force task in dystonic tremor and essential tremor cohorts. Patients with dystonic tremor and essential tremor were characterized by distinct functional activation abnormalities in cortical regions but not in the cerebellum. We examined seed-based functional connectivity from the sensorimotor cortex, globus pallidus internus, ventral intermediate thalamic nucleus, and dentate nucleus, and observed abnormal functional connectivity networks in dystonic tremor and essential tremor groups relative to controls. However, the effects were far more widespread in the dystonic tremor group as changes in functional connectivity were revealed across cortical, subcortical, and cerebellar regions independent of the seed location. A unique pattern for dystonic tremor included widespread reductions in functional connectivity compared to essential tremor within higher-level cortical, basal ganglia, and cerebellar regions. Importantly, a receiver operating characteristic determined that functional connectivity z-scores were able to classify dystonic tremor and essential tremor with 89% area under the curve, whereas combining functional connectivity with force tremor yielded 94%. These findings point to network-level connectivity as an important feature that differs substantially between dystonic tremor and essential tremor and should be further explored in implementing appropriate diagnostic and therapeutic strategies.
Introduction
Recently, international experts in dystonia proposed rhythmic tremulous movement as an important feature for characterizing dystonia (Albanese et al., 2013). Clinical studies have reported moderate-to-high incidence of tremor affecting a dystonic body part (dystonic tremor) (Deuschl et al., 1998; Bhatia et al., 2018), especially in cervical dystonia patients who exhibit head and upper-limb tremors that may resemble essential tremor (Deuschl et al., 1997; Pal et al., 2000; Shaikh et al., 2008; Defazio et al., 2013, 2015; Erro et al., 2014). Behavioural studies have attempted to better understand the physiological mechanisms that distinguish dystonic tremor from essential tremor, such as regularity and frequency of tremor oscillations, electromyography, and brainstem excitability (Britton et al., 1994; Münchau et al., 2001; Shaikh et al., 2008, 2015; Nisticò et al., 2012a, b). What is less clear, and equally critical to the diagnostic and treatment framework, is understanding the brain function deficits associated with dystonic tremor and essential tremor.
Previous functional MRI studies performed mostly in focal dystonia have pointed to abnormal activation and functional connectivity within the sensorimotor cortex, basal ganglia, and cerebellum (Simonyan and Ludlow, 2010, 2012; Delnooz et al., 2013, 2015; Battistella et al., 2016, 2017; Burciu et al., 2017; Filip et al., 2017; Li et al., 2017). These studies have provided no consideration to the specific influence of dystonic tremor on brain function, especially in contrast with essential tremor patients that do not have dystonia. However, a recent study examined brain activation during a speech task in patients with isolated spasmodic dysphonia and spasmodic dysphonia with dystonic voice tremor (SD/DTV) (Kirke et al., 2017). Spasmodic dysphonia and SD/DTV were associated with activation abnormalities in cortical areas, putamen, and thalamus, whereas middle frontal gyrus and cerebellar activation was reduced in SD/DTV relative to spasmodic dysphonia (Kirke et al., 2017). This was interpreted to reflect that dystonia and dystonic tremor have distinct neural signatures, and that dystonic tremor is possibly positioned between dystonia and essential tremor based on symptomatology and brain abnormalities common to both conditions. Developing a functional perspective of brain signatures that characterize phenotypically variant forms of dystonic tremor and comparing these signatures to a cohort of essential tremor patients is critical to furthering this assertion.
The primary focus of the current study was to examine changes in blood oxygen level-dependent (BOLD) signal amplitude and functional connectivity that relate to changes in visual feedback during the completion of a grip-force task in patients with dystonic tremor. The dystonic tremor cohort included cervical dystonia patients with dystonic head tremor and the majority had additional upper-limb tremor, as well as one spasmodic dysphonia patient with upper-limb tremor. The rationale in using the force task stems from prior work demonstrating visual feedback-induced exacerbation of grip-force tremor that related to changes in BOLD amplitude in essential tremor (Archer et al., 2018). If changes in visual feedback are similarly robust in exacerbating grip-force tremor in dystonic tremor, this affords an opportunity to test the hypothesis of distinct functional abnormalities that relate to dystonic tremor and essential tremor. First, we used a voxel-wise BOLD amplitude analysis to test the hypothesis that dystonic tremor and essential tremor groups reveal differences in force-related functional activation. Second, we used systematically placed regions of interest in the cortex, basal ganglia, thalamus, and cerebellum to examine functional connectivity based on evidence that the pathways connecting these regions are affected in dystonia (Argyelan et al., 2009; Vo et al., 2015) and essential tremor (Pinto et al., 2003; Raethjen and Deuschl, 2012). Lastly, we performed binary logistic regression with receiver operating characteristic (ROC) analysis and leave-one-out cross-validation (LOOCV) to determine the effectiveness of tremor and functional connectivity in distinguishing between dystonic tremor and essential tremor.
Materials and methods
Participants
This study included 20 patients with dystonic tremor, 18 patients with essential tremor, and 18 healthy individuals who were asymptomatic to tremor. Patients were diagnosed by a movement disorders neurologist using established criteria (Deuschl et al., 1998; Albanese et al., 2013; Bhatia et al., 2018). The dystonic tremor group included 19 cervical dystonia patients with dystonic head tremor and 13 of these had additional upper-limb tremor. There was one spasmodic dysphonia patient who had unilateral dominant upper-limb dystonic tremor. The patients with essential tremor included were part of a previous study (Archer et al., 2018), and we excluded one patient from the essential tremor cohort here because the patient developed dystonia following the original publication. Clinical and demographic characteristics for healthy controls and patient groups are presented in Table 1. All experimental procedures were approved and monitored by the University of Florida Institutional Review Board and conducted in ethical accordance with the Declaration of Helsinki. Participants signed consent forms and were in full understanding of the experimental objectives.
Table 1.
Demographic and clinical characteristics
| Measure | Group | ||
|---|---|---|---|
| Control | DT | ET | |
| Sample size | 18 | 20 | 18 |
| Age, years | 63.7 ± 7.6 | 64.7 ± 7.9 | 65.1 ± 11.8 |
| Sex, male, female | 8, 10 | 5, 15 | 7, 11 |
| Handedness, right, left | 15, 3 | 17, 3 | 17, 1 |
| MVC, N | 58.9 ± 15.3 | 51.4 ± 17.4 | 50.5 ± 16.7 |
| MoCA, total | 27.8 ± 1.4 | 26.3 ± 2.9 | 27.6 ± 2.8 |
| FTM-TRS, total | 0.6 ± 0.9 | 21.9 ± 12.3 | 38.2 ± 18.8 |
| FTM-TRS, right upper-limb | 0 ± 0 | 2.3 ± 2.1 | 4.1 ± 1.6 |
| Disorder duration, years | - | 6.5 ± 6.3 | 23.0 ± 21.0 |
Count or data represented group means ± standard deviation for demographic and clinical variables for controls, dystonic tremor (DT), and essential tremor (ET).
FTM-TRS = Fahn-Talosa-Marin Tremor Rating Scale; MoCA = Montreal Cognitive Assessment.
MRI set-up and acquisition
MRI data were acquired following overnight withdrawal from tremor medications. In patients with cervical dystonia receiving botulinum toxin, MRI acquisition sessions were scheduled at least 3 months after the last injection. MRI sequences were acquired at 3 T (Philips Achieva) using a 32-channel radiofrequency head coil. Participants were supine in the scanner and performed each task with their right hand resting comfortably to their side. A visual display was projected using a head-coil mounted visor mirror positioned within the field of view at a distance of 35 cm. Cushions reduced head motion.
T1-weighted anatomical images were acquired using a 3D magnetization-prepared rapid gradient echo (MP-RAGE) sequence with the following parameters: repetition time = 8.2 ms, echo time = 3.7 ms, flip angle = 8°, field of view = 240 mm3, resolution = 1 mm isotropic, 170 continuous axial slices, 0 mm gap. Functional MRI data were acquired using a single-shot gradient echo-planar imaging (EPI) sequence with the following parameters: repetition time = 3000 ms, echo time = 30 ms, flip angle = 90°, field of view = 240 mm3, resolution = 3 mm isotropic, 55 continuous axial slices, 0 mm gap.
Force data acquisition
Force data were acquired using an MRI-compatible fibre-optic force transducer (Neuroimaging Solutions) with 0.025 N resolution housed in a precision grip apparatus. The force data were transmitted via fibre-optic cable to an SM130 Optical Sensing Interrogator (Micron Optics). The interrogator digitized force at 62.5 Hz, which was then converted to Newtons using LabVIEW (National Instruments).
Experimental design
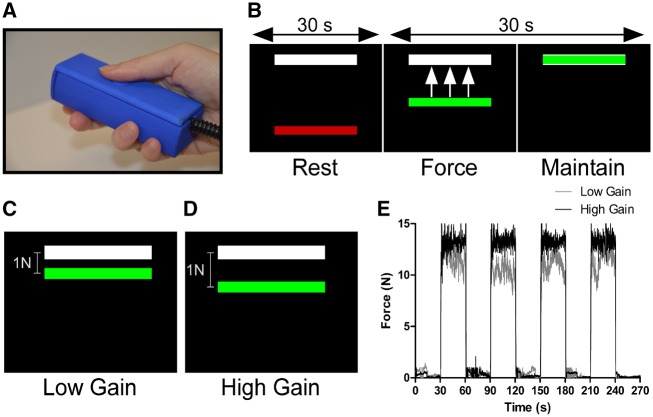
Maximum voluntary contraction (MVC), in Newtons, was obtained prior to imaging. Participants grasped the force transducer with their right hand between the thumb and fingers (Fig. 1A) and were instructed to sustain a maximum force for 3 s. MVC was obtained by computing the mean maximum force amplitude produced across three separate trials interleaved with 60 s rest. Participants were then accustomed to the visual display and force task during a training session outside the MRI environment.
Figure 1.
Experimental paradigm. (A) The force transducer was held with the right hand between the thumb and fingers and rested comfortably along the side of the body inside the scanner. (B) During the 30 s rest block, the visual display consisted of a high contrast black background with white target bar corresponding to 15% MVC and a stationary red colour bar. The transition of the colour bar to green cued participants to produce and maintain 15% MVC for 30 s and provided online visual feedback. (C) A 1-N difference between the colour and target bar was represented by a small spatial displacement and visual angle in the low feedback task and (D) a large spatial displacement and visual angle in the high feedback task. (E) An exemplar force trace for a single control participant during the high (black) and low feedback (grey) tasks showing force fluctuations around 15% MVC.
Task functional MRI was acquired across two separate tasks: low and high visual feedback. Each task was performed in a blocked design consisting of alternating rest and force blocks. The duration of each task was 270 s. Each block lasted 30 s, beginning and ending with a rest block. In each task, the visual display consisted of a high contrast black background and two horizontal bars—one fixed white bar representing a target force of 15% MVC, and one dynamic coloured bar that provided online visual feedback of the amount of force produced by the participant. During the rest block, the red bar remained stationary and participants were instructed to passively view the visual display. Transition of the coloured bar from red to green served as a cue for the participant to produce force corresponding to the target bar for 30 s while minimizing force fluctuations (Fig. 1B).
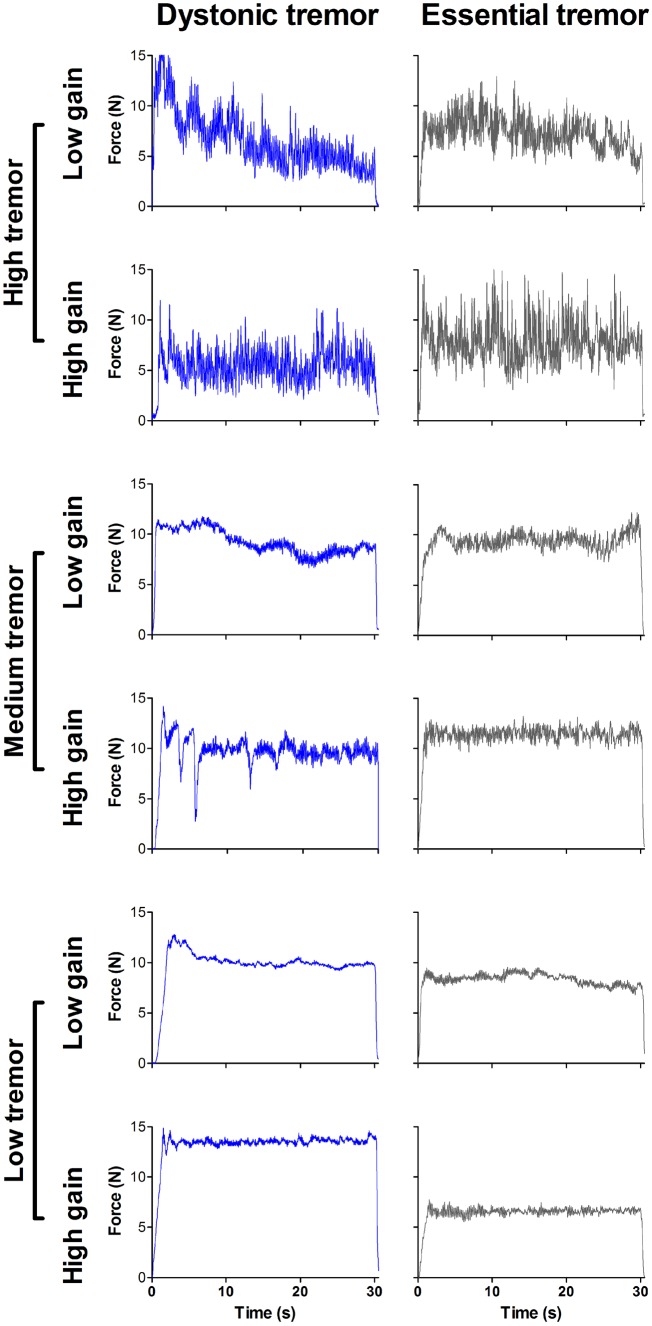
The goal to sustain a steady-state force of 15% MVC remained the same across feedback tasks. Visual feedback gain was altered across tasks (Vaillancourt et al., 2006; Coombes et al., 2010; Archer et al., 2018). We manipulated the optical height of the force amplitude fluctuations provided on the visual display such that in the low visual feedback task, a 1 N difference between colour and target bars was represented by a small spatial dissociation (Fig. 1C). Reducing the visual gain permitted small fluctuations in spatial amplitude of the colour bar, rendering a perceived attenuation of error feedback. In the high visual feedback task, the visual gain was larger such that a 1 N difference between target and colour bars was represented by a larger spatial displacement (Fig. 1D). Thus, the degree of error feedback became augmented by large spatial fluctuations of the colour bar. Exemplar force traces for a single control participant during the high and low feedback tasks are presented in Fig. 1E and representative traces allowing for visual comparison of high, medium, and low levels of force tremor in the dystonic tremor and essential tremor groups are presented in Fig. 2.
Figure 2.
Representative force tremor traces. Force traces for individual dystonic tremor (blue) and essential tremor (grey) patients depicting high (top), medium (middle), and low (bottom) degrees of force tremor for a single 30 s force block in both high and low visual feedback tasks.
Force data statistical analysis
Force data variables for high and low visual feedback including mean force (% MVC), force error [root mean square error: RMSE (N)], spectral power of force between 0–13 Hz (N2), and force tremor [sum of power of force (N2) between 4–12 Hz] were computed using MATLAB (Version R2017b). For each participant, the mean of each variable obtained from the middle 25 s of each force block was analysed. Values for mean force, force error, spectral power and force tremor in the low visual feedback task were subtracted from the high visual feedback task, producing high minus low difference scores (mean forceΔ, force errorΔ, spectral powerΔ, force tremorΔ). We also computed the peak force power (N2) and peak force frequency (Hz) across each of the force blocks in the low, high, and high minus low (Δ) feedback conditions, and the mean and standard deviation values were computed across the four blocks. All variables were examined for normality (Shapiro-Wilk) and homogeneity of variance (Levene’s) and submitted to either a one-way ANOVA or Kruskal-Wallis test. Significant F-statistics were decomposed using either Tukey’s honest significant difference test or Dunn’s test using rank sums and adjusted for multiple comparisons using the Benjamini-Hochberg false discovery rate (FDR) correction (Benjamini and Hochberg, 1995).
The next goal was to determine whether force tremor could classify dystonic tremor and essential tremor. A binary logistic regression model with forward selection determined the best predictive independent variables and accuracy of the model was tested using an ROC approach and LOOCV. The variables of interest were spectral powerΔ data within 0–3 and 4–12 Hz and standard deviation of peak force frequencyΔ. The 4–12 Hz bands represent the typical range for entrained motor unit activity producing upper-limb tremor in dystonic tremor and essential tremor (Elble, 1986; Elble et al., 1994; Deuschl and Elble, 2000; Shaikh et al., 2008; Neely et al., 2015) and the 0–3 Hz band can also be elevated in essential tremor (Neely et al., 2015). Standard deviation of peak frequency was selected because tremor frequency can be more irregular across time in dystonic tremor relative to essential tremor (Shaikh et al., 2008; Bove et al., 2018).
Task functional MRI data preprocessing
Preprocessing for functional MRI was carried out using a whole-brain analysis in Analysis of Functional NeuroImages (AFNI: Version 16.0.19) in combination with a cerebellum-optimized pipeline [spatially unbiased infra-tentorial template (SUIT): Diedrichsen, 2006; Diedrichsen et al., 2009] in statistical parametric mapping (SPM8). A combined 1.0-mm motion exclusion criterion in the x-, y-, and z-planes was used. The following preprocessing steps were performed: removal of the first three functional MRI volumes to account for scanner magnetization equilibrium, slice-timing correction, rigid-body volume registration, co-registration of the functional MRI scan to the T1-weighted anatomical scan, non-linear spatial warping of the functional MRI scan to the MNIavg152 template for the analysis of cerebral data, and cerebellar fine-tuning and normalization to the SUIT template for analysis of cerebellum data. Signal-to-noise ratio was improved by spatial smoothing using a full-width at half-maximum Gaussian kernel of 8 mm and 4 mm for normalized whole-brain and SUIT functional MRI data, respectively.
Task functional MRI statistical analysis
BOLD amplitude data were analysed in the framework of the general linear model. For high and low visual feedback separately, the instantaneous BOLD signal at each voxel and each time-point was scaled by the mean of the BOLD signal across its respective time series. The BOLD signals obtained from the rest and force blocks were modelled using a boxcar regressor convolved with a canonical haemodynamic response function. Non-specific sources of variance including censored head-motion parameters, white matter, and CSF calculated during preprocessing were included in the model as no-interest regressors. Using 3dDeconvolve, the regression was performed across the rest and force blocks to estimate the β-coefficient and its associated t-statistic for the BOLD contrast, separately for low and high feedback tasks. BOLDΔ statistical maps were generated for each participant by subtracting BOLD statistical maps in the low feedback task from the high feedback task. Between-group comparisons were examined using voxel-wise independent-samples t-tests on the BOLDΔ β-coefficients. Consistent with Archer et al. (2018), age and BOLD amplitude in the low visual feedback task were included as covariates. Significance level for contrasts was determined using 3dClustSim and set to P < 0.005 [P < 0.05 familywise error rate (FWER)-corrected] with a minimum cluster size of 243 mm3 [autocorrelation function (ACF): 0.56, 4.12, 6.88] for whole-brain and 189 mm3 (ACF: 0.62, 4.19, 4.51) for SUIT.
Task functional connectivity was analysed across the entire functional MRI run including rest and force blocks. The ANATICOR function in AFNI was incorporated to regress non-specific noise sources from the BOLD data including motion parameters, white matter, and CSF. The residual time series data (ANATICOR) for low visual feedback condition at each voxel was subtracted from its high visual feedback counterpart (ANATICORΔ). After obtaining the residual time series, 3DTcorr1D function was used to compute Pearson’s correlation coefficients between the time series of each region of interest and all other brain voxels, separately for each task. Seed regions of interest were placed within key nuclei of the cerebello-thalamo-basal ganglia-cortical circuit including the left and right dentate nucleus, ventral intermediate thalamic nucleus (VIM), globus pallidus internus (GPi), and sensorimotor cortex. The GPi and VIM are primary targets for deep brain stimulation (DBS) in dystonia and essential tremor, respectively (Koller et al., 2001; Vidailhet et al., 2005; Hung et al., 2007; Papavassiliou et al., 2008). The VIM has also emerged as a potential candidate for DBS in dystonic tremor (Morishita et al., 2010; Hedera et al., 2013; Cury et al., 2017). Region of interest labelling of GPi and VIM were derived from the Basal Ganglia Human Area Template (Prodoehl et al., 2008) and population-based stereotactic coordinates for VIM DBS (Papavassiliou et al., 2008), respectively. For the cortex, we used a functional seed location derived from previous work (Archer et al., 2018) in the sensorimotor cortex/inferior parietal lobule (SMC/IPL). We additionally used its mirror-symmetrical location to be consistent with left and right hemispheric placements of other seeds. Correlation coefficients were converted to z-scores using Fisher r-to-z transformations. High minus low functional connectivity (FCΔ) maps were computed using independent-samples t-tests on the high minus low z-scores for each region of interest, while covarying for age and z-score related to that specific region of interest in the low visual feedback task. T-tests were limited to voxels activated in the task using a mask file generated by combining the within-group BOLD activation maps for the high visual feedback task across all three groups. The significance threshold was determined using 3dClustSim and set to P < 0.005 (P < 0.05 FWER-corrected) with a minimum cluster size of 675 mm3 (ACF: 0.65, 4.71, 10.78). The larger cluster size for FCΔ data compared to the BOLDΔ was due to the inherent smoothness of the FCΔ data.
We sought to determine whether functional connectivity was effective in classifying dystonic tremor and essential tremor groups. First, we generated a functional connectivity mask by combining the FCΔ statistical maps for the dystonic versus essential tremor comparisons for all eight regions of interest (thresholded at P < 0.05 FWER-corrected). Next, we obtained the average z-scores from the ANATICORΔ time series for each dystonic tremor and essential tremor patient and across all voxels contained within the mask, separately for each region of interest. The average z-score for each patient and each region of interest was then entered into a binary logistic regression model with forward selection to determine the best set of independent variables that predicted group. Model accuracy was tested using ROC and LOOCV approaches.
Data availability
De-identified data are available from the corresponding author upon reasonable request.
Results
Change in force and tremor related to visual feedback
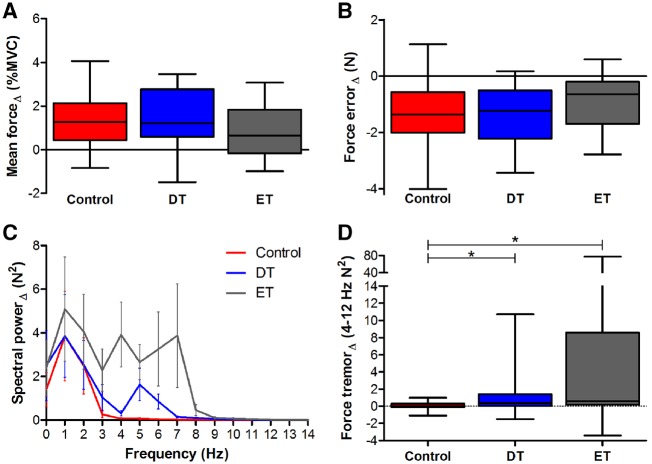
Within-group effects for mean forceΔ and RMSEΔ were examined using one-sample t-tests. All groups had significantly positive mean forceΔ [t(≥17) > 2.67, P < 0.02] (Fig. 3A). All groups had significantly negative RMSEΔ [t(≥17) < −3.78, P < 0.002], indicating error improved with visual feedback (Fig. 3B). One-way ANOVA models were not significant for mean forceΔ [F(2,53) = 1.37, P = 0.2] and RMSEΔ [F(2,53) = 1.59, P = 0.2]. Within-group effects for force tremorΔ were examined using one-sample Wilcoxon signed-rank tests. As expected, the control group did not demonstrate a significant force tremorΔ score (W = 31, P = 0.2). In contrast, dystonic tremor (W = 166, P < 0.002) and essential tremor (W = 112, P < 0.01) groups demonstrated significantly positive force tremorΔ scores, indicating increased tremor with increased visual feedback. A Kruskal-Wallis test on force tremorΔ data yielded a significant main effect of group [χ2(2) = 9.17, P = 0.01]. Dunn’s test revealed increased force tremorΔ for dystonic tremor (z = 2.35, P < 0.01) and essential tremor (z = 2.85, P < 0.01) compared to controls, but dystonic tremor and essential tremor did not differ (z = 0.57, P = 0.28) (Fig. 3D).
Figure 3.
Statistical analyses for force variables. Box and whisker plot summaries for (A) mean forceΔ, (B) force errorΔ, (C) spectral powerΔ (0–14 Hz), and (D) force tremorΔ (4–12 Hz) are shown for controls (red), dystonic tremor (DT, blue), and essential tremor (ET, grey). Asterisks represent a significant increase in force tremorΔ for the dystonic tremor and essential tremor groups compared to controls (P < 0.05 FDR-corrected).
Between-group effects of mean and standard deviation of peak force power and frequency were examined using Kruskal-Wallis tests and significant chi-squared values were decomposed using Dunn’s test. Mean and standard deviation peak power did not differ between groups in the low, high, or high minus low feedback tasks [χ2(2) > 6.1, P-values > 0.051]. Group effects were uncovered for mean peak frequency in the low and high feedback tasks [χ2(2) > 16.3, P-values < 0.0005]. Essential tremor had higher mean peak frequency values compared to controls and dystonic tremor (z > 13.13, P-values < 0.05 FDR-corrected). A main effect was also uncovered for standard devaition peak frequency in the high feedback task [χ2(2) = 20.16, P = 0.00004], where values were higher in essential tremor compared to controls and dystonic tremor (z > 3.62, P-values < 0.05 FDR-corrected) (Supplementary Fig. 1).
Between-group effects in BOLDΔ
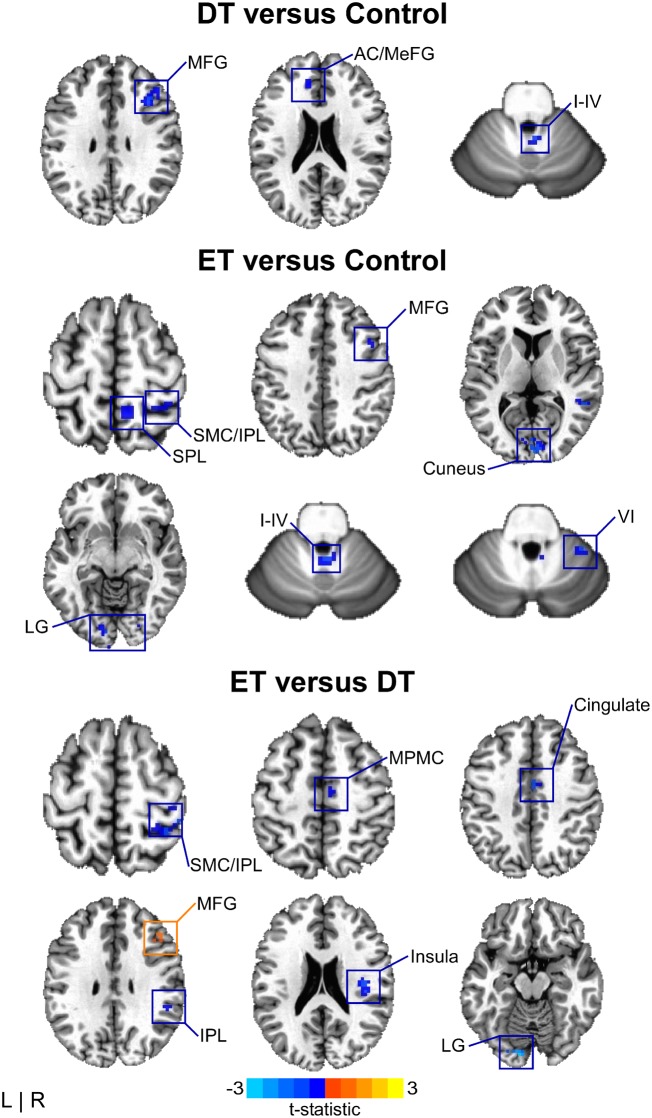
BOLDΔ amplitude for dystonic tremor was reduced relative to controls in frontal cortex, cingulate, and cerebellum (I–IV). Compared to controls, BOLDΔ amplitude in essential tremor was reduced in sensory cortex, parietal cortex, middle frontal gyrus, middle temporal gyrus, inferior occipital gyrus, visual cortex, and cerebellum (I–IV, VI, VIIIb). BOLDΔ in essential tremor was reduced relative to dystonic tremor in the sensorimotor cortex, inferior parietal lobule, cingulate, mesial premotor cortex, and visual cortex. In turn, BOLDΔ in dystonic tremor was reduced compared to essential tremor in the middle frontal gyrus (Fig. 4 and Supplementary Table 1).
Figure 4.
BOLDΔ between-group effects. Spatial colour coded t-statistical maps representing the mean difference in BOLDΔ amplitude for dystonic tremor (DT) versus controls (top), essential tremor (ET) versus controls (middle), and essential tremor versus dystonic tremor (bottom). Results are thresholded at P < 0.05 FWER-corrected. Positive (yellow-orange-red) values, denote a significantly positive difference for the contrast of interest, whereas negative (blue) values represent a significantly negative difference. I–IV, VI = cerebellar lobules I–IV and VI, respectively; AC = anterior cingulate; IPL = inferior parietal lobule; LG = lingual gyrus; MeFG = medial frontal gyrus; MFG = middle frontal gyrus; MPMC = mesial premotor cortex; SMC = sensorimotor cortex; SPL = superior parietal lobule.
Between-group effects in FCΔ
Left and right SMC/IPL regions of interest revealed abnormal FCΔ in dystonic tremor and essential tremor compared to controls within clusters comprising the sensorimotor cortex, premotor cortex, frontal cortex, parietal cortex, visual cortex, putamen and globus pallidus. The degree to which these areas were affected was greater in terms of cluster volume in dystonic tremor. Additionally, both SMC/IPL regions of interest in the dystonic tremor group showed reduced FCΔ with multiple cerebellar clusters (I–IV, V, VI, VIIb, VIIIa, VIIIb; crus I, vermis, and deep cerebellar nuclei), whereas cerebellar FCΔ was not affected in essential tremor (Fig. 5 and Supplementary Table 2).
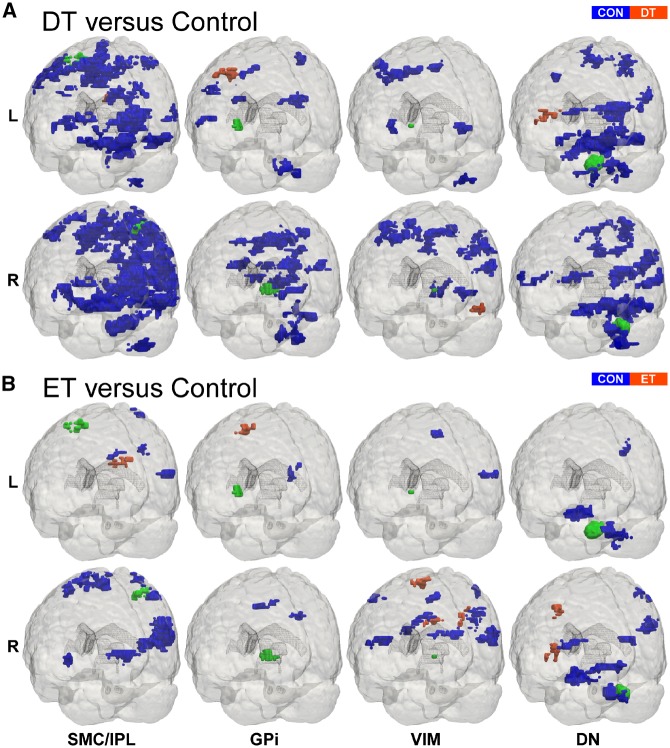
Figure 5.
Patient versus control FCΔ effects. 3D rendering (Madan, 2015) of whole-brain t-statistical maps comparing FCΔ for (A) dystonic tremor (DT) versus controls (CON) and (B) essential tremor (ET) versus controls (bottom). The x-axis denotes the seed region of interest in the SMC/IPL, GPi, VIM, and dentate nucleus (DN). The y-axis in each panel denotes the location of the seed region of interest in the left (L) and the right (R) hemispheres. In each panel, positive (red-orange) clusters, denote significantly increased FCΔ with the seed region of interest in the patient group (i.e. dystonic tremor or essential tremor) relative to controls, whereas negative (blue) clusters, denote significantly reduced FCΔ with the seed region of interest in patient groups relative to controls (P < 0.05 FWER-corrected).
Dystonic tremor revealed extensive patterns of deficient FCΔ for both SMC/IPL regions of interest compared to essential tremor (Fig. 6A). Left SMC/IPL FCΔ was reduced in dystonic tremor within an ipsilateral cluster comprising the inferior parietal lobule; and bilateral clusters in sensorimotor cortex, premotor cortex, superior parietal lobule, temporal cortex, insula, visual cortex, middle occipital gyrus, cingulate, putamen, and cerebellum (VI, crus I and II, vermis). Right SMC/IPL FCΔ was reduced in dystonic tremor within contralateral hemisphere clusters comprising parietal cortex, temporal cortex, and insula; ipsilateral clusters in sensorimotor cortex, premotor cortex, middle frontal gyrus, middle occipital gyrus, and middle temporal gyrus; and bilateral clusters comprising the cingulate, visual cortex, and cerebellum (I–IV, V, VI, VIIIa, vermis). Left SMC/IPL FCΔ was reduced in essential tremor compared to dystonic tremor within contralateral clusters comprising the middle frontal gyrus and insula, whereas right SMC/IPL FCΔ was reduced within contralateral clusters of the medial frontal gyrus and insula; and a bilateral cluster comprising paracentral lobule and cingulate.
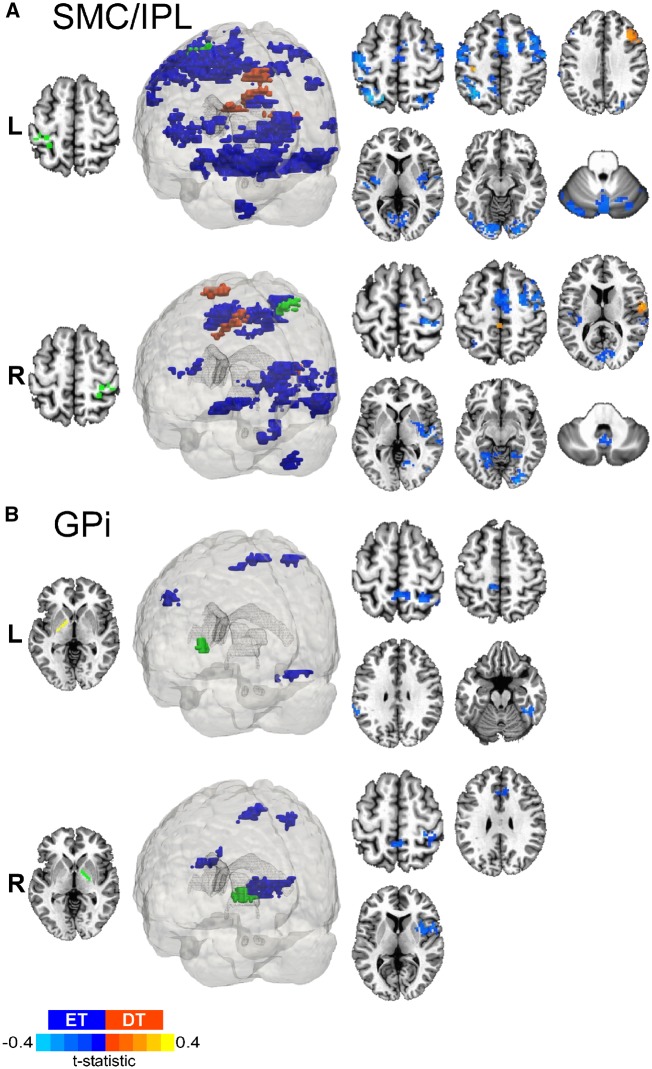
Figure 6.
Dystonic tremor versus essential tremor SMC/IPL and GPi FCΔ. 3D rendering and axial slice representation of whole-brain t-statistical maps comparing FCΔ of (A) SMC/IPL and (B) GPi for the dystonic tremor (DT) versus essential tremor (ET) comparison. Seed regions of interest are shown in green and the top and bottom panels depict the between-group FCΔ effects originating from the left (L) and right (R) seeds, respectively. In each panel, positive (red-orange) clusters denote significantly increased FCΔ with the seed region of interest in the dystonic tremor group compared to essential tremor group, whereas negative (blue) clusters denote significantly reduced FCΔ with the seed region of interest in the dystonic tremor group compared to essential tremor group (P < 0.05 FWER-corrected).
Dystonic tremor and essential tremor revealed abnormal left and right GPi FCΔ compared to controls within the premotor cortex, parietal cortex, and supplementary motor area. The degree to which these areas were affected was greater in terms of cluster volume in the dystonic tremor group. Dystonic tremor revealed additional abnormalities in FCΔ compared to controls in sensorimotor cortex, frontal cortex, visual cortex, cingulate, insula, putamen, and thalamus, and extensively throughout the cerebellum (I–IV, VI, V, VIIb, VIIIa, VIIIb, IX, vermis) (Fig. 5 and Supplementary Table 3).
GPi FCΔ for both regions of interest was impaired in dystonic tremor compared to essential tremor (Fig. 6B). Left GPi FCΔ was reduced in dystonic tremor within contralateral clusters in sensorimotor cortex, superior parietal lobule, and fusiform gyrus; and bilateral clusters comprising paracentral lobule and inferior parietal lobule. Right GPi FCΔ was reduced in dystonic tremor within ipsilateral clusters of sensorimotor cortex, inferior parietal lobule, insula, and putamen; and bilateral paracentral lobule and cingulate.
VIM seeds in the dystonic tremor group revealed FCΔ abnormalities compared to controls in sensorimotor cortex, frontal cortex, parietal cortex, cingulate, precuneus, visual cortex, putamen, globus pallidus, and cerebellum (VIIIa, VIIIb, VIIb). VIM FCΔ in the essential tremor group was reduced compared to controls in the sensorimotor cortex, frontal cortex, parietal cortex, temporal cortex, and cingulate (Fig. 5 and Supplementary Table 4).
Left VIM FCΔ was reduced in dystonic tremor compared to essential tremor within bilateral clusters in the cuneus and insula; ipsilateral clusters in the sensorimotor cortex, inferior parietal lobule, and putamen; and contralateral clusters in the medial frontal gyrus, visual cortex, superior temporal gyrus, and cerebellum (V, VI, crus I). Left VIM FCΔ was reduced in essential tremor compared to dystonic tremor in a single contralateral cluster comprising the temporal cortex. Right VIM FCΔ was reduced in dystonic tremor compared to essential tremor in bilateral clusters in sensorimotor cortex and medial frontal gyrus; ipsilateral visual cortex, putamen, superior temporal gyrus, and insula; and contralateral inferior parietal lobule (Fig. 7A).
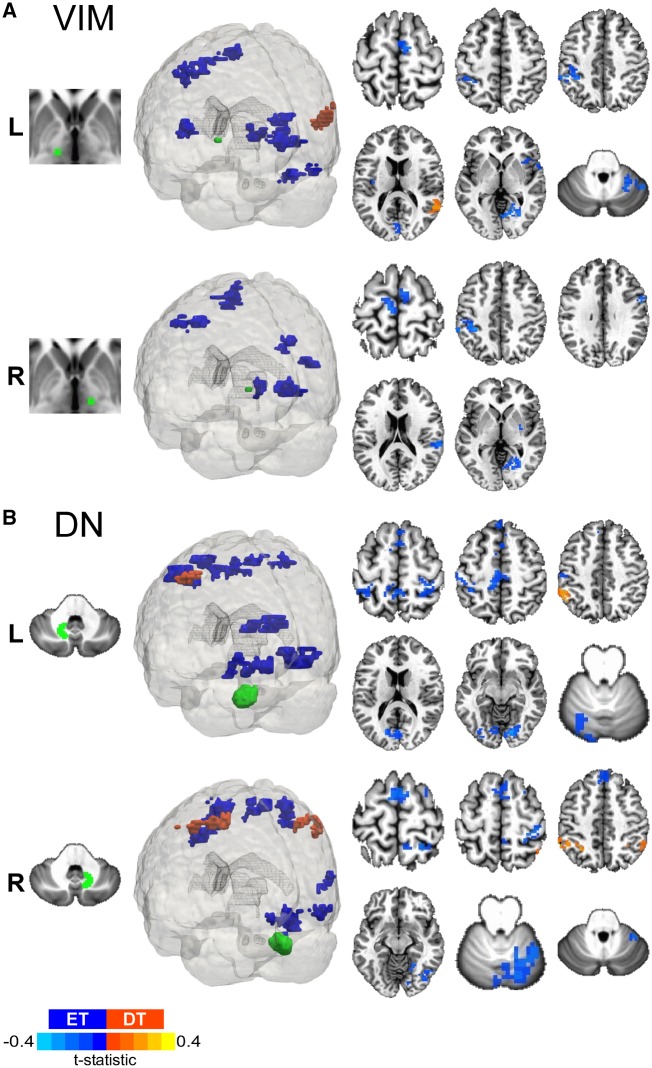
Figure 7.
Dystonic tremor versus essential tremor VIM and dentate nucleus FCΔ. 3D rendering and axial slice representation of whole-brain t-statistical maps comparing FCΔ of (A) VIM thalamus and (B) dentate nucleus (DN) for the dystonic tremor (DT) versus essential tremor (ET) comparison. Seed regions of interest are shown in green and the top and bottom panels depict the between-group FCΔ effects originating from the left (L) and right (R) seeds, respectively. In each panel, positive (red-orange) clusters denote significantly increased FCΔ with the seed region of interest in the dystonic tremor group compared to essential tremor group, whereas negative (blue) clusters denote significantly reduced FCΔ with the seed region of interest in the dystonic tremor group compared to essential tremor group (P < 0.05 FWER-corrected).
Left and right dentate nucleus regions of interest in dystonic tremor and essential tremor revealed abnormal FCΔ compared to controls within clusters of the sensorimotor cortex, premotor cortex, frontal cortex, parietal cortex, visual cortex, temporal cortex, insula, and cerebellum (I–IV, V, VI, crus II, vermis). The degree to which these regions were affected was greater in terms of cluster volume in the dystonic tremor group. Moreover, the dystonic tremor group revealed additional FCΔ impairments relative to controls in the cerebellum (VIIb, VIIIa, VIIIb, crus I) (Fig. 5 and Supplementary Table 5).
Left dentate nucleus FCΔ was reduced in dystonic tremor compared to essential tremor within ipsilateral inferior parietal lobule, occipital cortex, and cerebellum (VI); and bilateral clusters in sensorimotor cortex, mesial premotor cortex, frontal cortex, superior parietal lobule, paracentral lobule, and visual cortex. Right dentate nucleus FCΔ was reduced in dystonic tremor compared to essential tremor within the ipsilateral sensorimotor cortex, middle frontal gyrus, superior parietal lobule, paracentral lobule, visual cortex, temporal cortex, and cerebellum (V, VI, vermis); and a bilateral cluster in frontal cortex. Left dentate nucleus FCΔ was reduced in essential tremor compared to dystonic tremor in a single ipsilateral cluster in inferior parietal lobule, whereas right dentate nucleus FCΔ was reduced in essential tremor in inferior parietal lobule bilaterally (Fig. 7B).
Patient group classification
Spectral powerΔ data for the 0–3 and 4–12 Hz ranges (shown in Fig. 3C) combined with standard deviation of peak force frequencyΔ yielded moderately effective group classification in the ROC analysis (AUC = 0.77; 83% sensitivity, 65% specificity) and the LOOCV analysis [mean squared error (MSE) = 0.4]. The combined functional connectivity z-scores across all eight regions of interest in the high minus low feedback task provided an advantage in classifying patient groups compared to clinical tremor data alone (ROC: AUC = 0.89; 78% sensitivity, 90% specificity; LOOCV: MSE = 0.29). ROC classification was also evident when combining force (0–3 and 4–12 Hz spectral powerΔ and standard deviation peak force frequencyΔ) and FCΔ z-scores (ROC: AUC = 0.94; 78% sensitivity, 100% specificity; LOOCV: MSE = 0.34). The results of the logistic regression determined that left SMC/IPL FCΔ (F = 8.46, P = 0.004) and standard deviation peak force frequencyΔ (F = 4.83, P = 0.028) were significant predictors of patient cohort for connectivity and force data, respectively. Forward selection determined that left SMC/IPL FCΔ z-scores best predicted patient cohort (F = 8.46, P = 0.004). Left SMC/IPL FCΔ z-scores yielded an LOOCV MSE value of 0.19, suggesting high patient group classification accuracy (Supplementary Table 6).
Effect of head motion of FCΔ
To test whether cervical dystonia and dystonic head tremor in dystonic tremor contributed to MRI signal distortion artefacts and loss of connectivity strength, we first obtained the average motion (mm) for censored repetition times across low and high visual feedback tasks for each participant and submitted values to a one-way ANOVA with Welch correction. Average head motion across control (0.12 ± 0.03 mm), dystonic tremor (0.18 ± 0.09 mm), and essential tremor (0.14 ± 0.05 mm) groups revealed a significant main effect [F(2,30) = 4.76, P < 0.02]. Independent-samples t-tests revealed increased motion in dystonic tremor compared to controls [t(22) = 2.94, P < 0.02] but not essential tremor [t(28) = 1.95, P = 0.18]. Further examination of the data revealed that this effect was driven by two dystonic tremor patients in particular with head motion averages at 0.38 and 0.35 mm, and the average of the remaining subjects was 0.16 mm. Removal of these two subjects from the one-way ANOVA model failed to yield a significant main effect for motion. Next, we examined the between-group FCΔ effects for the dystonic tremor-essential tremor comparisons in all regions of interest with and without the inclusion of the two dystonic tremor patients. Results showed that FCΔ effects for all regions of interest did not differ when outlier motion dystonic tremor patients were excluded from the analysis (Supplementary Fig. 2). Finally, to determine if motion was related to FCΔ, we performed Pearson’s correlations between average head motion across low and high visual feedback tasks and average z-scores from the connectivity analysis. This analysis revealed non-significant correlations between motion and z-scores for any of the regions of interest in the dystonic tremor, essential tremor, and control groups (R2 < 0.15, P-values > 0.05), further indicating that head motion is not driving these results.
Discussion
This study examined the effects of visual feedback on force tremor and associated changes in BOLD activation and connectivity during the completion of a grip-force task in dystonic tremor patients. The findings here are convincing that like dystonia, dystonic tremor is a network disorder involving large-scale BOLD amplitude and connectivity changes across cortical, basal ganglia, and cerebellar regions. A secondary goal of the current study was to develop a perspective for network-level BOLD activation and connectivity that distinguishes dystonic tremor from essential tremor, which yielded several important findings. Increased visual feedback exacerbated force tremor during the grip-force task in both patient groups. Dystonic tremor and essential tremor were characterized by distinct BOLDΔ amplitude abnormalities in higher-level cortical and visual regions but not in the cerebellum. Furthermore, dystonic tremor was characterized by widespread reductions in FCΔ compared to essential tremor including cortical, visual, basal ganglia, and cerebellar regions, whereas essential tremor demonstrated only minor cortical FCΔ reductions compared to dystonic tremor. Importantly, FCΔ z-scores were able to separate dystonic tremor and essential tremor patient cohorts with very good sensitivity and specificity. These findings provide support that dystonic tremor involves distinct brain networks from essential tremor, and points to large-scale network connectivity as a key feature distinguishing these disorders.
Visual feedback increases force tremor in dystonic tremor
Increasing visual feedback produced positive mean force tremorΔ in dystonic tremor and essential tremor compared to controls. Exacerbated tremor within the 4–12 Hz power spectral range replicates our prior work in essential tremor (Archer et al., 2018) and supports the hypothesis that increasing the availability of visual information worsens tremor (Feys et al., 2003; Keogh et al., 2004; Gironell et al., 2012). Previous studies have classified dystonic tremor and essential tremor based on physiological mechanisms of tremor (Münchau et al., 2001; Shaikh et al., 2008). Some studies, which have used accelerometer recordings and filter the low frequency rhythm, have reported tremor that is more irregular in dystonic tremor compared to essential tremor (Shaikh et al., 2008; Bove et al., 2018). This was not apparent in the current study, as essential tremor had increased standard deviation of peak force frequency compared to controls and dystonic tremor. We believe this is likely related to the isometric force task. In isometric force for healthy adults and those with Parkinson’s disease, peak power is around 1 Hz with residual tremor power between 4–8 Hz (Vaillancourt et al., 2001). The same can be seen for healthy older and young adults (Vaillancourt et al., 2003). If a patient has considerable tremor, then the power in the 4–8 Hz band increases to be greater than the power in the 0–2 Hz band. Thus, in essential tremor patients who have more tremor than the dystonic tremor patients at the hand producing force, the peak frequency may occur at either low or tremor frequencies thereby increasing variability.
Distinct cortical and visual BOLD difference impairments in dystonic tremor and essential tremor
BOLDΔ amplitude in dystonic tremor was reduced in the frontal cortex and cerebellum (I–IV) compared to controls. Notably, BOLDΔ amplitude in the primary visual cortex was not affected. Although our behavioural results reinforce the assertion that tremor severity is influenced by availability of visual information (Feys et al., 2003; Keogh et al., 2004; Gironell et al., 2012), the BOLDΔ results suggest visual feedback-induced tremor exacerbation in dystonic tremor need not be coupled to associated dysfunction of visual regions akin to present and prior evidence in essential tremor (Neely et al., 2015; Archer et al., 2018). Functional abnormalities of prefrontal and cerebellar regions in patients with cervical dystonia have been previously reported, although it was unclear whether these patients also had tremor (Galardi et al., 1996; Burciu et al., 2017; Filip et al., 2017). Similar to our findings, Kirke et al. (2017) reported deficient activation of the middle frontal gyrus and cerebellum (lobule VII) in SD/DTV relative to spasmodic dysphonia. Discrepant cerebellar abnormalities in our study (lobule I–IV) compared to their work may be attributable to task modality or phenotypic differences across the two cohorts. Our study shows impaired adaptation of BOLD signal in response to visual feedback similar to essential tremor, and further implicates the role of cerebello-frontal cortex network in the pathogenesis of dystonic tremor.
Dystonic tremor and essential tremor groups demonstrated functionally distinct higher-level cortical abnormalities. BOLDΔ amplitude of the middle frontal gyrus was reduced in dystonic tremor relative to both control and essential tremor groups. In turn, activation of the sensorimotor cortex and inferior parietal lobule were reduced in essential tremor relative to both control and dystonic tremor groups. Dysfunction of the parieto-motor network in essential tremor has been interpreted to possibly reflect a cortical domain of increased kinetic tremor oscillations. For example, increased force tremorΔ in essential tremor was found to correlate with sensorimotor and parietal BOLDΔ signal (Archer et al., 2018). Moreover, high-density EEG in essential tremor patients revealed reduced sensorimotor beta-band desynchronization and bi-directional connectivity between the motor and parietal cortices that was related to feedback induced exacerbation of force tremor of the arm (Roy et al., 2018). These differences in findings for dystonic tremor and essential tremor suggest functionally disparate higher-level cortical mechanisms that will need further exploration.
Functional abnormalities of the cerebellum have been a consistent finding in cervical dystonia (Magyar-Lehmann et al., 1997; Burciu et al., 2017; Filip et al., 2017) and essential tremor (Bucher et al., 1997; Buijink et al., 2015a, b; Neely et al., 2015; Broersma et al., 2016; Archer et al., 2018). Similar to these studies, we uncovered reduced cerebellar BOLDΔ amplitude in dystonic tremor (lobule I–IV) and essential tremor (lobules I–IV, VI, and VIIIb) compared to controls, although no difference between patient groups was uncovered. Kirke et al. (2017) recently interpreted common and uncommon functional substrates between patients with spasmodic dysphonia and SD/DTV to reflect that dystonia and dystonic tremor represent heterogeneous disorders, and that dystonic tremor is likely positioned between dystonia and essential tremor based on symptomology and brain abnormalities common to both dystonia and essential tremor. Our BOLDΔ findings may further support this interpretation given that dystonic tremor and essential tremor revealed common cerebellar and distinct higher-level (i.e. sensorimotor cortex, inferior parietal lobule, visual cortex, middle frontal gyrus) neural signatures.
Distinct network-level functional connectivity changes in dystonic tremor and essential tremor
The notion that a distributed motor network is involved in the pathogeneses of dystonia and essential tremor incites motivation to examine network-level connectivity dynamics among cortical, basal ganglia, and cerebellar systems. Relative to controls and independent of the region of interest, increased visual feedback in dystonic tremor caused changes in inter- and intra-regional FCΔ interactions among a widely distributed network within and beyond the cerebello-basal ganglia-cortical pathway. SMC/IPL and dentate nucleus seeds revealed bi-directional cortico-cerebellar changes in FCΔ. The SMC/IPL also showed abnormal FCΔ with basal ganglia regions and the GPi showed abnormal FCΔ with cortical and cerebellar regions. Interestingly, whereas BOLDΔ amplitude of visual regions was not affected in dystonic tremor, all regions of interest revealed abnormal FCΔ with visual cortex, suggesting that visual feedback-induced tremor exacerbation in dystonic tremor may relate to dysfunctional connectivity and not activation. Functional connectivity in cervical dystonia has received little attention and to our knowledge has not been examined in dystonic tremor. Delnooz et al. (2013) reported abnormal resting state connectivity of sensorimotor, visual, and executive control (prefrontal, cingulate, parietal) networks. Li et al. (2017) recently reported higher connectivity in cervical dystonia in the cortex, basal ganglia, thalamus, and cerebellum originating from a bilateral region of interest in the precentral gyrus implicated in isometric head movement. Here, we show that cortical (prefrontal, premotor, parietal, temporal, visual), basal ganglia, and cerebellar FCΔ originating from a sensorimotor seed location is abnormal in dystonic tremor and the majority of clusters showed reduced FCΔ. The extent of FCΔ abnormalities in dystonic tremor may be primary to dystonia itself, but we cannot confirm this in the current study. That is, changes in FCΔ of the cortex, basal ganglia, thalamus, and cerebellum support the notion that cervical dystonia is a disorder affecting widely distributed functional brain networks (Neychev et al., 2011; Prudente et al., 2014; Jinnah et al., 2017). Future studies including a subset of dystonia patients without tremor is important to better understanding the pathophysiological distinction between dystonia and dystonic tremor, and how these functional substrates relate to essential tremor.
The essential tremor group revealed abnormal FCΔ relative to controls independent of the region of interest. The magnitude of the effects for each region of interest in terms of number of regional dysfunctional connections and cluster volume were typically less than that of the dystonic tremor versus control comparison. Future work involving weighted graph theory network analysis (Rubinov and Sporns, 2010) would be useful in determining whether the strength of functional connections is effective in distinguishing dystonic tremor and essential tremor. FCΔ between the SMC/IPL and sensorimotor, premotor, prefrontal, parietal, temporal, visual and basal ganglia regions was abnormal in essential tremor relative to controls. This finding is important because the sensorimotor cortex and inferior parietal lobule have been consistently shown to be hypoactive in essential tremor here and in prior work (Archer et al., 2018). Evidence that the cortico-cortico connectivity originating from this region is abnormal in essential tremor is in line with previous studies (Fang et al., 2013; Gallea et al., 2015). A recent study in essential tremor revealed changes in resting state functional connectivity within the VIM-motor cortex-cerebellar circuit (Fang et al., 2016). Here, essential tremor revealed abnormal FCΔ of the VIM thalamus within a distributed cortical network but not the cerebellum. Essential tremor demonstrated abnormal FCΔ originating from the dentate nucleus to cortical regions, but not originating from the SMC/IPL to cerebellar regions. Abnormal cerebello-cortico FCΔ fits well with prior evidence that functional coupling between the cerebellum and sensorimotor cortex is disturbed in essential tremor during the completion of a motor task (Buijink et al., 2015b; Neely et al., 2015). Distinct FCΔ abnormalities were revealed in the patient group comparisons. It was clear that FCΔ was impaired to a greater degree in dystonic tremor independent of the seed region. For seed locations in the SMC/IPL, VIM thalamus, and dentate nucleus, FCΔ for dystonic tremor was reduced compared to essential tremor across a widely distributed network involving cortical, subcortical, and cerebellar regions, whereas reductions in cortical FCΔ primarily characterized essential tremor relative to dystonic tremor. As well, GPi FCΔ was reduced in dystonic tremor compared to essential tremor across multiple cortical and basal ganglia regions. Most importantly, combining FCΔ z-scores across all regions of interest yielded effective classification of patient groups in the ROC analysis (AUC = 0.89; 78% sensitivity, 90% specificity) and LOOCV analysis (MSE = 0.29). Collectively, these findings support distinct network-level connectivity within and beyond the cerebello-basal ganglia-cortical network despite similar feedback-dependent task performance and tremor outcomes.
Conclusions
The current study provides novel evidence of distinct task-related BOLDΔ amplitude and FCΔ changes in dystonic tremor and essential tremor. Dystonic tremor and essential tremor are characterized by distinct functional activation signatures in higher-level cortical and visual regions, but common cerebellar impairments. A salient finding was that the magnitude of the FCΔ effects was more widespread in the dystonic tremor group, affecting cortical, subcortical, and cerebellar systems independent of the region of interest. Furthermore, these differences in FCΔ across cortical, subcortical, and cerebellar regions proved effective in classifying dystonic tremor and essential tremor groups with very good sensitivity and specificity. This study provides a fresh perspective that network-level activation and connectivity differ substantially between dystonic tremor and essential tremor and should be further explored in future studies for improved diagnostic and therapeutic strategies.
Supplementary Material
Acknowledgements
A portion of this work was performed in the McKnight Brain Institute at the National High Magnetic Field Laboratory’s AMRIS Facility, which is supported by National Science Foundation Cooperative Agreement No. DMR-1644779 and the State of Florida.
Glossary
Abbreviations
- BOLD
blood oxygen level-dependent
- FCΔ
high minus low functional connectivity
- GPi
globus pallidus internus
- LOOCV
leave-one-out cross-validation
- MVC
maximum voluntary contraction
- ROC
receiver operating characteristic
- SMC/IPL
combined voxels of sensorimotor cortex and inferior parietal lobule
- VIM
ventral intermediate nucleus
Funding
This work was supported by grants from the National Institutes of Health (R01 NS058487, K23 NS092957) and Tyler’s Hope Foundation.
Competing interests
D.B.A has received grant support from the Parkinson’s Foundation. D.E.V. has received grant support from NIH, NSF, and Tyler’s Hope Foundation. He is co-founder and manager of Neuroimaging Solutions, LLC. A.W.S. has received grant support from Benign Essential Blepharospasm Research Foundation, Dystonia Coalition, Dystonia Medical Research Foundation, and National Organization for Rare Disorders and grant support from NIH (KL2 and K23 NS092957–01A1).
References
- Albanese A, Bhatia K, Bressman SB, Delong MR, Fahn S, Fung VS et al. Phenomenology and classification of dystonia: a consensus update. Mov Disord 2013; 28: 863–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Archer DB, Coombes SA, Chu WT, Chung JW, Burciu RG, Okun MS et al. A widespread visually-sensitive functional network relates to symptoms in essential tremor. Brain 2018; 141: 472–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Argyelan M, Carbon M, Niethammer M, Uluğ AM, Voss HU, Bressman SB et al. Cerebellothalamocortical connectivity regulates penetrance in dystonia. J Neurosci 2009; 29: 9740–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Battistella G, Fuertinger S, Fleysher L, Ozelius LJ, Simonyan K. Cortical sensorimotor alterations classify clinical phenotype and putative genotype of spasmodic dysphonia. Eur J Neurol 2016; 23: 1517–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Battistella G, Termsarasab P, Ramdhani RA, Fuertinger S, Simonyan K. Isolated focal dystonia as a disorder of large-scale functional networks. Cereb Cortex 2017; 27: 1203–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc Ser B Stat Methodol 1995; 57: 289–300. [Google Scholar]
- Bhatia KP, Bain P, Bajaj N, Elble RJ, Hallett M, Louis ED et al. Consensus Statement on the classification of tremors. from the task force on tremor of the International Parkinson and Movement Disorder Society. Mov Disord 2018; 33: 75–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bove F, Di Lazzaro G, Mulas D, Cocciolillo F, Di Giuda D, Bentivoglio AR. A role for accelerometry in the differential diagnosis of tremor syndromes. Funct Neurol 2018; 33: 45–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Britton TC, Thompson PD, Day BL, Rothwell JC, Findley LJ, Marsden CD. Rapid wrist movements in patients with essential tremor. The critical role of the second agonist burst. Brain 1994; 117 (Pt 1): 39–47. [DOI] [PubMed] [Google Scholar]
- Broersma M, van der Stouwe AMM, Buijink AWG, de Jong BM, Groot PFC, Speelman JD et al. Bilateral cerebellar activation in unilaterally challenged essential tremor. Neuroimage Clin 2016; 11: 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bucher SF, Seelos KC, Dodel RC, Reiser M, Oertel WH. Activation mapping in essential tremor with functional magnetic resonance imaging. Ann Neurol 1997; 41: 32–40. [DOI] [PubMed] [Google Scholar]
- Buijink AW, Broersma M, van der Stouwe AM, van Wingen GA, Groot PF, Speelman JD et al. Rhythmic finger tapping reveals cerebellar dysfunction in essential tremor. Parkinsonism Relat Disord 2015a; 21: 383–8. [DOI] [PubMed] [Google Scholar]
- Buijink AW, van der Stouwe AM, Broersma M, Sharifi S, Groot PF, Speelman JD et al. Motor network disruption in essential tremor: a functional and effective connectivity study. Brain 2015b; 138: 2934–47. [DOI] [PubMed] [Google Scholar]
- Burciu RG, Hess CW, Coombes SA, Ofori E, Shukla P, Chung JW et al. Functional activity of the sensorimotor cortex and cerebellum relates to cervical dystonia symptoms. Hum Brain Mapp 2017; 38: 4563–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coombes SA, Corcos DM, Sprute L, Vaillancourt DE. Selective regions of the visuomotor system are related to gain-induced changes in force error. J Neurophysiol 2010; 103: 2114–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cury RG, Fraix V, Castrioto A, Pérez Fernández MA, Krack P, Chabardes S et al. Thalamic deep brain stimulation for tremor in Parkinson disease, essential tremor, and dystonia. Neurology 2017; 89: 1416–23. [DOI] [PubMed] [Google Scholar]
- Defazio G, Conte A, Gigante AF, Fabbrini G, Berardelli A. Is tremor in dystonia a phenotypic feature of dystonia? Neurology 2015; 84: 1053–9. [DOI] [PubMed] [Google Scholar]
- Defazio G, Gigante AF, Abbruzzese G, Bentivoglio AR, Colosimo C, Esposito M et al. Tremor in primary adult-onset dystonia: prevalence and associated clinical features. J Neurol Neurosurg Psychiatry 2013; 84: 404–8. [DOI] [PubMed] [Google Scholar]
- Delnooz CC, Pasman JW, Beckmann CF, van de Warrenburg BP. Task-free functional MRI in cervical dystonia reveals multi-network changes that partially normalize with botulinum toxin. PLoS One 2013; 8: e62877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delnooz CC, Pasman JW, Beckmann CF, van de Warrenburg BP. Altered striatal and pallidal connectivity in cervical dystonia. Brain Struct Funct 2015; 220: 513–23. [DOI] [PubMed] [Google Scholar]
- Deuschl G, Bain P, Brin M. Consensus statement of the Movement Disorder Society on tremor. Ad Hoc Scientific Committee. Mov Disord 1998; 13 (Suppl 3): 2–23. [DOI] [PubMed] [Google Scholar]
- Deuschl G, Elble RJ. The pathophysiology of essential tremor. Neurology 2000; 54: S14–20. [PubMed] [Google Scholar]
- Deuschl G, Heinen F, Guschlbauer B, Schneider S, Glocker FX, Lücking CH. Hand tremor in patients with spasmodic torticollis. Mov Disord 1997; 12: 547–52. [DOI] [PubMed] [Google Scholar]
- Diedrichsen J. A spatially unbiased atlas template of the human cerebellum. Neuroimage 2006; 33: 127–38. [DOI] [PubMed] [Google Scholar]
- Diedrichsen J, Balsters JH, Flavell J, Cussans E, Ramnani N. A probabilistic MR atlas of the human cerebellum. Neuroimage 2009; 46: 39–46. [DOI] [PubMed] [Google Scholar]
- Elble RJ. Physiologic and essential tremor. Neurology 1986; 36: 225–31. [DOI] [PubMed] [Google Scholar]
- Elble RJ, Higgins C, Leffler K, Hughes L. Factors influencing the amplitude and frequency of essential tremor. Mov Disord 1994; 9: 589–96. [DOI] [PubMed] [Google Scholar]
- Erro R, Rubio-Agusti I, Saifee TA, Cordivari C, Ganos C, Batla A et al. Rest and other types of tremor in adult-onset primary dystonia. J Neurol Neurosurg Psychiatry 2014; 85: 965–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang W, Chen H, Wang H, Zhang H, Puneet M, Liu M et al. Essential tremor is associated with disruption of functional connectivity in the ventral intermediate nucleus–motor cortex–cerebellum circuit. Hum Brain Mapp 2016; 37: 165–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang W, Lv F, Luo T, Cheng O, Liao W, Sheng K et al. Abnormal regional homogeneity in patients with essential tremor revealed by resting-state functional MRI. PLoS One 2013; 8: e69199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feys P, Helsen WF, Liu X, Lavrysen A, Loontjens V, Nuttin B et al. Effect of visual information on step-tracking movements in patients with intention tremor due to multiple sclerosis. Mult Scler 2003; 9: 492–502. [DOI] [PubMed] [Google Scholar]
- Filip P, Gallea C, Lehéricy S, Bertasi E, Popa T, Mareček R et al. Disruption in cerebellar and basal ganglia networks during a visuospatial task in cervical dystonia. Mov Disord 2017; 32: 757–68. [DOI] [PubMed] [Google Scholar]
- Galardi G, Perani D, Grassi F, Bressi S, Amadio S, Antoni M et al. Basal ganglia and thalamo-cortical hypermetabolism in patients with spasmodic torticollis. Acta Neurol Scand 1996; 94: 172–6. [DOI] [PubMed] [Google Scholar]
- Gallea C, Popa T, García-Lorenzo D, Valabregue R, Legrand AP, Marais L et al. Intrinsic signature of essential tremor in the cerebello-frontal network. Brain 2015; 138: 2920–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gironell A, Ribosa-Nogué R, Pagonabarraga J. Withdrawal of visual feedback in essential tremor. Parkinsonism Relat Disord 2012; 18: 402–3. [DOI] [PubMed] [Google Scholar]
- Hedera P, Phibbs FT, Dolhun R, Charles PD, Konrad PE, Niemat JS et al. Surgical targets for dystonic tremor: considerations between the globus pallidus and ventral intermediate thalamic nucleus. Parkinsonism Relat Disord 2013; 19: 684–6. [DOI] [PubMed] [Google Scholar]
- Hung SW, Hamani C, Lozano AM, Poon YY, Piboolnurak P, Miyasaki JM et al. Long-term outcome of bilateral pallidal deep brain stimulation for primary cervical dystonia. Neurology 2007; 68: 457–9. [DOI] [PubMed] [Google Scholar]
- Jinnah HA, Neychev V, Hess EJ. The anatomical basis for dystonia: the motor network model [Review]. Tremor Other Hyperkinet Mov (N Y) 2017; 7: 506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keogh J, Morrison S, Barrett R. Augmented visual feedback increases finger tremor during postural pointing. Exp Brain Res 2004; 159: 467–77. [DOI] [PubMed] [Google Scholar]
- Kirke DN, Battistella G, Kumar V, Rubien-Thomas E, Choy M, Rumbach A et al. Neural correlates of dystonic tremor: a multimodal study of voice tremor in spasmodic dysphonia. Brain Imaging Behav 2017; 11: 166–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koller WC, Lyons KE, Wilkinson SB, Troster AI, Pahwa R. Long-term safety and efficacy of unilateral deep brain stimulation of the thalamus in essential tremor. Mov Disord 2001; 16: 464–8. [DOI] [PubMed] [Google Scholar]
- Li Z, Prudente CN, Stilla R, Sathian K, Jinnah HA, Hu X. Alterations of resting-state fMRI measures in individuals with cervical dystonia. Hum Brain Mapp 2017; 38: 4098–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madan CR. Creating 3D visualizations of MRI data: A brief guide. F1000Res 2015; 4: 466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magyar-Lehmann S, Antonini A, Roelcke U, Maguire RP, Missimer J, Meyer M et al. Cerebral glucose metabolism in patients with spasmodic torticollis. Mov Disord 1997; 12: 704–8. [DOI] [PubMed] [Google Scholar]
- Morishita T, Foote KD, Haq IU, Zeilman P, Jacobson, Okun MS. Should we consider Vim thalamic deep brain stimulation for select cases of refractory dystonic tremor. Stereotact Funct Neurosurg 2010; 88: 98–104. [DOI] [PubMed] [Google Scholar]
- Münchau A, Schrag A, Chuang C, MacKinnon CD, Bhatia KP, Quinn NP et al. Arm tremor in cervical dystonia differs from essential tremor and can be classified by onset age and spread of symptoms. Brain 2001; 124: 1765–76. [DOI] [PubMed] [Google Scholar]
- Neely KA, Kurani AS, Shukla P, Planetta PJ, Wagle Shukla A, Goldman JG et al. Functional brain activity relates to 0–3 and 3–8 Hz force oscillations in essential tremor. Cereb Cortex 2015; 25: 4191–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neychev VK, Gross RE, Lehéricy S, Hess EJ, Jinnah HA. The functional neuroanatomy of dystonia [Review]. Neurobiol Dis 2011; 42: 185–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nisticò R, Pirritano D, Novellino F, Salsone M, Morelli M, Valentino P et al. Blink reflex recovery cycle in patients with essential tremor associated with resting tremor. Neurology 2012a; 79: 1490–5. [DOI] [PubMed] [Google Scholar]
- Nisticò R, Pirritano D, Salsone M, Valentino P, Novellino F, Condino F et al. Blink reflex recovery cycle in patients with dystonic tremor: a cross-sectional study. Neurology 2012b; 78: 1363–5. [DOI] [PubMed] [Google Scholar]
- Pal PK, Samii A, Schulzer M, Mak E, Tsui JK. Head tremor in cervical dystonia. Can J Neurol Sci 2000; 27: 137–42. [PubMed] [Google Scholar]
- Papavassiliou E, Rau G, Heath S, Abosch A, Barbaro NM, Larson PS et al. Thalamic deep brain stimulation for essential tremor: relation of lead location to outcome. Neurosurgery 2008; 62: 884–94. [DOI] [PubMed] [Google Scholar]
- Pinto AD, Lang AE, Chen R. The cerebellothalamocortical pathway in essential tremor. Neurology 2003; 60: 1985–7. [DOI] [PubMed] [Google Scholar]
- Prodoehl J, Yu H, Little DM, Abraham I, Vaillancourt DE. Region of interest template for the human basal ganglia: comparing EPI and standardized space approaches. Neuroimage 2008; 39: 956–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prudente CN, Hess EJ, Jinnah HA. Dystonia as a network disorder: what is the role of the cerebellum? [Review]. Neuroscience 2014; 260: 23–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raethjen J, Deuschl G. The oscillating central network of Essential tremor. Clin Neurophysiol 2012; 123: 61–4. [DOI] [PubMed] [Google Scholar]
- Roy A, Coombes SA, Chung JW, Archer DB, Okun MS, Hess CW et al. Cortical dynamics within and between parietal and motor cortex in essential tremor. Mov Disord 2018; 34: 95–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubinov M, Sporns O. Complex network measures of brain connectivity: uses and interpretations. Neuroimage 2010; 52: 1059–69. [DOI] [PubMed] [Google Scholar]
- Shaikh AG, Jinnah HA, Tripp RM, Optican LM, Ramat S, Lenz FA et al. Irregularity distinguishes limb tremor in cervical dystonia from essential tremor. J Neurol Neurosurg Psychiatry 2008; 79: 187–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaikh AG, Zee DS, Jinnah HA. Oscillatory head movements in cervical dystonia: Dystonia, tremor, or both? Mov Disord 2015; 30: 834–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonyan K, Ludlow CL. Abnormal activation of the primary somatosensory cortex in spasmodic dysphonia: an fMRI study. Cereb Cortex 2010; 20: 2749–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonyan K, Ludlow CL. Abnormal structure-function relationship in spasmodic dysphonia. Cereb Cortex 2012; 22: 417–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaillancourt DE, Haibach PS, Newell KM. Visual angle is the critical variable mediating gain-related effects in manual control. Exp Brain Res 2006; 173: 742–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaillancourt DE, Newell KM. Aging and the time and frequency structure of force output variability. J Appl Physiol 2003; 94: 903–12. [DOI] [PubMed] [Google Scholar]
- Vaillancourt DE, Slifkin AB, Newell KM. Intermittency in the visual control of force in Parkinson’s disease. Exp Brain Res 2001; 138: 118–27. [DOI] [PubMed] [Google Scholar]
- Vidailhet M, Vercueil L, Houeto JL, Krystkowiak P, Benabid AL, Cornu P et al. Bilateral deep-brain stimulation of the globus pallidus in primary generalized dystonia. N Engl J Med 2005; 352: 459–67. [DOI] [PubMed] [Google Scholar]
- Vo A, Sako W, Niethammer M, Carbon M, Bressman SB, Uluğ AM et al. Thalamocortical connectivity correlates with phenotypic variability in dystonia. Cereb Cortex 2015; 25: 3086–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
De-identified data are available from the corresponding author upon reasonable request.