Abstract
Objective
The coarse fibres of areca nut and the continuous friction from occluding teeth are major causes of mechanical stress to the oral mucosa in conditions like oral submucous fibrosis and frictional keratosis. The continuous micro trauma provided in areca nut chewers, creates an environment where the keratinocytes exhibit alteration. Loricrin, is expressed abundantly in keratinizing epithelium in response to mechanical stress. Their expression or absence could play a role in malignant transformation. This study attempts to assess the potential of Loricrin as an early diagnostic marker in patients with chewing habit.
Methods
73 archival samples of formalin fixed, paraffin embedded tissue specimens histopathologically confirmed, were segregated as normal mucosa 11, hyperkeratotic 32 and oral submucous fibrosis 30 and stained with antibodies to Loricrin and graded as negative, mild, moderate and intense based on the staining intensity. Pearson's chi square test was done for statistical analysis.
Results
Loricrin expression was observed in all groups with staining in the stratum granulosum showing a significant association to habits (P = 0.000).
Conclusion
This prominent staining indicates a compensatory cytoskeletal rearrangement of surface epithelium during cell division in early oral submucous fibrosis showing potential as an early marker of the condition.
Keywords: Loricrin, Oral submucous fibrosis, Areca nut, Hyperkeratosis
1. Introduction
Epithelium is the primary protective barrier to the body's internal tissues from environmental stresses, chemical damage and bacterial infection.1 Keratinizing epithelia such as the epidermis and oral gingiva that are subjected to physical and chemical forces produce a protective toughened cornified cell envelope. Chronic trauma has been implicated as one of the causative factor in the progression of potentially malignant disorder to cancer.2
The incidence of an Oral Potentially Malignant Disorder (OPMD) undergoing malignant transformation into oral squamous cell carcinoma (OSCC) is high. A transformation rate of 3–19% and 5–15% has been seen in OPMDs like Oral submucous fibrosis (OSF) and Oral leukoplakia respectively.3, 4, 5 OSCC, which is an aggressive malignancy proceeds through a stepwise accumulation of genetic damage6 Genetic mutations often produce early phenotypic changes that may present as clinically apparent lesions. The normal integrity and morphology of epithelium is maintained by its cytoskeleton network components. Genetic and environmental factors can alter the normal proliferation and differentiation.7,8 For example, the continuous micro trauma provided by areca nut and other ingredients in chewing, plus friction from occluding teeth creates an environment where the keratinocytes of the epithelium tend to alter the Cornified epithelial Envelope (CE), a critical structure for barrier function at the outermost layer of the skin epidermis.9, 10, 11 Proteins of CE like Loricrin, are late differentiation markers of terminally differentiated keratinocytes. Their abundant presence in keratinizing epithelium, subjected to mechanical stress has led to the assumption that it might be responsible for barrier function. This study aims to correlate expression of loricrin in non-keratinizing epithelia of oral mucosa to the habit of chewing in OSF and hyperkeratosis conditions.2
2. Materials and methods
2.1. Study setting and sample size
The retrospective study was carried out in the department of oral and maxillofacial pathology of Ragas dental college and hospital in Chennai, India, using IHC on 73 formalin fixed paraffin embedded archival blocks. The study sample were segregated into 3 groups of normal mucosa (Group I; n = 11), hyperkeratosis (Group II; n = 32) and oral submucous fibrosis (Group III; n = 30) specimens. Group I sample site was chosen keeping in mind the 2006 Indian Council of Medical Research (ICMR) bioethical guidelines which advises against causing substantial damage to non-lesional areas, for the exclusive purpose of conducting a study, the normal mucosa that is trimmed/excised for approximation after extraction of III molars was taken after voluntary consent. Group II samples included cases of both frictional keratosis/physically induced (n = 21) and epithelial dysplastic lesions/substance abuse associated (n = 11). Data on gender, habit history, age, site duration were extracted from the archives. The study for evaluating the expression of Loricrin in these samples was done after approval by the Institutional Review Board.
2.2. Methodology
Sections of four micron thickness were used for both Haematoxylin and Eosin (H & E) staining and immunohistochemical (IHC) staining, with all reagents being analytical grade and procured from MERCK™. We used concentrated Loricrin polyclonal antibody along with Expose Mouse and Rabbit specific HRP/DAB detection IHC kit: Ab80436 (15 ml) as the primary and secondary antibody from Abcam Allied scientific Cambridge, MA, USA,.
Sample standardisation: Foreskin specimen procured after circumcision for phymosis was fixed, processed, embedded, sectioned and stained in the same manner as the study samples and used as positive control. One positive control tissue slide was included for each batch of staining. The antibody was standardized to a dilution concentration of 1:500 with an incubation period of 1 hr. at room temperature.
2.3. Evaluation criteria for loricrin (LOR) staining
Loricrin localization within tissues and cells along with the degree of positivity were taken as parameters to evaluate Loricrin (LOR) staining. Tissue staining of Loricrin is normally localized to stratum granulosum and the corneal strata of stratified squamous epithelium in keratinized epithelia, while it is absent in non-keratinizing epithelium of the oral mucosa. Its cellular staining is localized to both cytoplasm and nucleoplasm of cells. Each case was graded as (-) nil or absence of stain, (+) mild, (++) moderate and (+++) intensively stained based on the intensity of staining taken up by the tissue as observed by three blinded observers independently.
2.4. Habit association among three groups
Assessment of the presence of habit history namely chewing, smoking and drinking were analysed through evaluation of archival records.
Statistical analysis: All data were entered and analysed using Statistical Package for Social Service version 17.0 (SPSS ™ Inc., IBM, IL, USA). Pearson's Chi-square test was done to compare intensity of staining between the groups and a P value < 0.05 was considered statistically significant. Kappa analysis was done to compare the intensity of Loricrin staining as observed between three observers.
3. Results
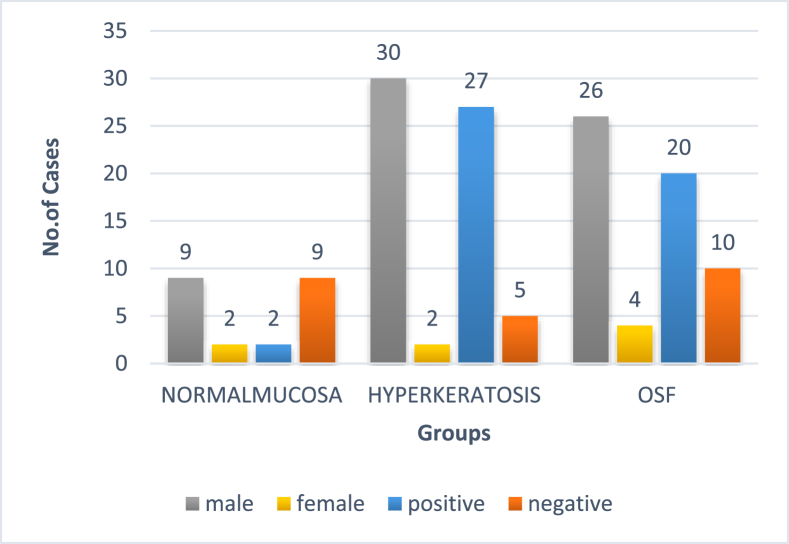
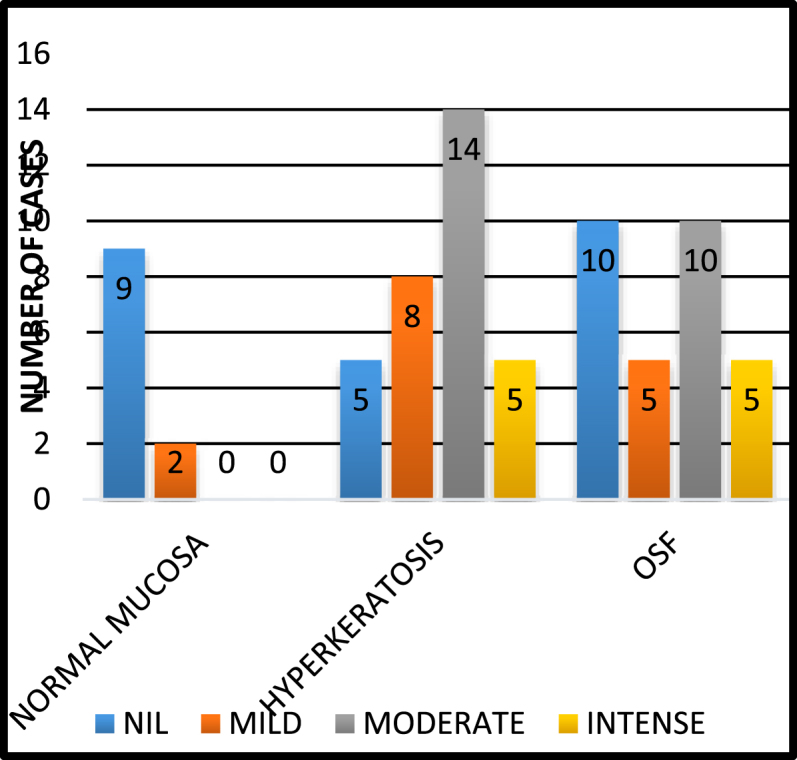
Gender distribution and stain positivity: Analysis revealed the samples to be predominantly from male patients with no significant relation between gender and study groups (P value 0.475). Histopathological evaluation of anti-bodies directed against Loricrin revealed a statistically significant relation between the positive expression of Loricrin and all three study groups (P = 0.000). A predominantly negative staining (81.8%) was observed in group I (normal mucosal specimens). While the group II and III samples under hyperkeratosis and OSF showed a predominantly positive staining of 84.4% and 66.7% respectively. Fig. 1, Fig. 2, Fig. 5.1.
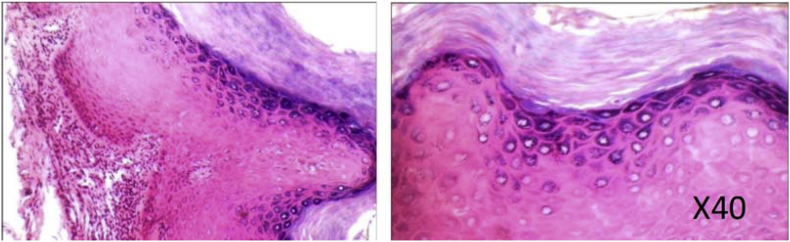
Fig. 1.
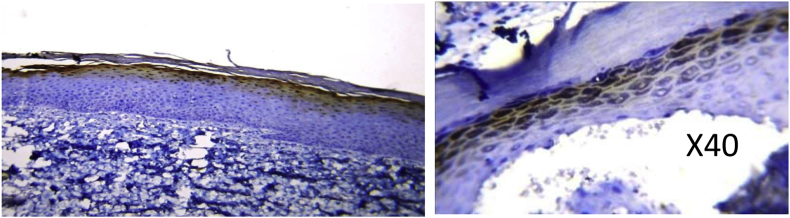
Chewing Habit induced HYPERKERATOSIS specimen (H & E) showing prominent granular cell layer and increased keratinization.
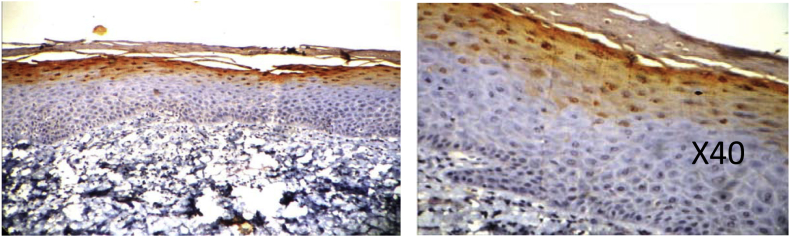
Fig. 2.
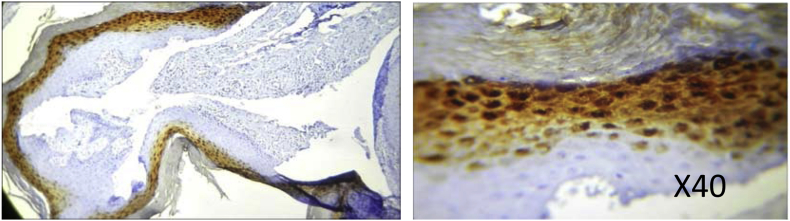
ORAL SUBMUCOUS FIBROSIS specimen from patient with areca nut use showing positive loricrin staining in the granular layer and few cells of stratum spinosum.
Fig. 5.1.
Gender and expression positivity.
Staining Intensity: Positive normal mucosal specimens showed mild staining, while majority of the hyperkeratosis samples (43.8%) had taken up staining with moderate intensity. Among OSF samples, equal cases (33.3%) showed moderate and negative staining. Similarly mild and intense staining were equally appreciable in 16.7% of the cases. A significant P-Value of 0.006 was appreciated on comparing the distribution of staining intensity of Loricrin among the groups (Fig. 5.2 Staining Intensity).
Fig. 5.2.
Staining intensity.
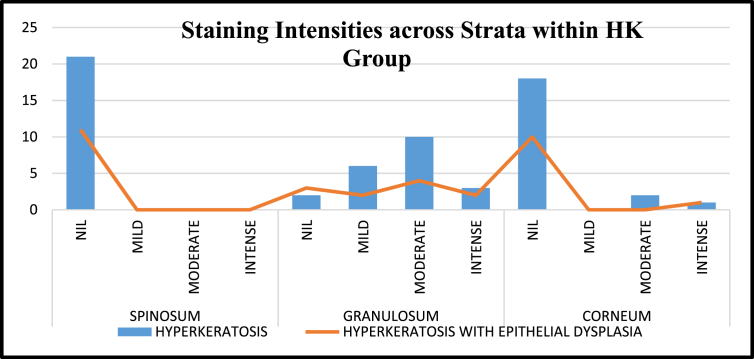
Staining index distribution in different epithelial strata (spinosum, granulosum and corneum): The spinosal layer of normal mucosa and hyperkeratosis samples did not show any staining. However the stratum spinosum (SS) in OSF samples showed different grades of staining intensities with 83.3% cases taking up no stain, while mild and intense staining (16.7%) was observed in equal number of cases. 33.3% of the cases showed moderate staining. The results were not statistically significant (P = 0.261)
The stratum granulosum (SG) of normal mucosa was positive for loricrin taking up mild staining (9.1%). Hyperkeratotic specimens however, showed the most staining in this layer, predominantly with moderate intensity (43.8%), though few with mild and intense staining were also observed. OSF on the other hand had an increase in the number of cases exhibiting an intense staining reaction (16.7%) followed by moderate (30.0%) and mild (13.3%) staining. The difference was statistically significant (P = 0.002)
The overall positivity of stratum corneum (SC), when compared to stratum granulosum was less in both hyperkeratosis 6.3% and OSF 13.3% specimens with no staining observed in the case of normal mucosa. The difference was not statistically significant (P = 0.786) (Fig. 6)
Fig. 6.
Loricrin Positivity and Staining intensity withinHR Grooup.
Distribution of habits among the three groups: Assessment of the presence of habit history (chewing, smoking and drinking) revealed that, out of the total 73 cases, 55 had habits and 18 had no habits. Among the individual groups, OSF had the most cases (n = 28) with positive habit history (50.9%), followed by hyperkeratosis (n = 26) (47.3%) and normal mucosa (n = 1) (1.8%). The difference among the groups were statistically significant. (p = 0.000) (Table 1)
Table-1.
Habits Pattern with respect to the three groups.
| Groups | (N) | Habits Present | Percentage % | Chewing Habit (n) | Smoking Habit(n) | Drinking Habit(n) |
|---|---|---|---|---|---|---|
| Total cases | 73 | 55 | 75.34% | |||
| Group I- Normal Mucosa | 11 | 1 | 1.8% | 1 | 1 | 0 |
| Group II- Hyperkeratosis | 32 | 26 | 47.27% | 25 | 18 | 17 |
| i)Frictional | 21 | 16 | 29.09% | 15/16 | 11/16 | 10/16 |
| ii)Dysplastic | 11 | 10 | 18.18% | 10/10 | 7/10 | 7/10 |
| Group III- OSF | 30 | 28 | 50.3% | 28 | 12 | 18 |
Chewing habit distribution and comparison among groups: Analysing the distribution of only the chewing habit, we found that the only positive case in the normal mucosa group had the habit of chewing. However this habit was predominantly evident in the OSF group followed by hyperkeratosis group. The difference in the habits was statistically significant (P = 0.000). Chewing habit compared within groups was also statistically significant (p = 0.000). (Table-1)
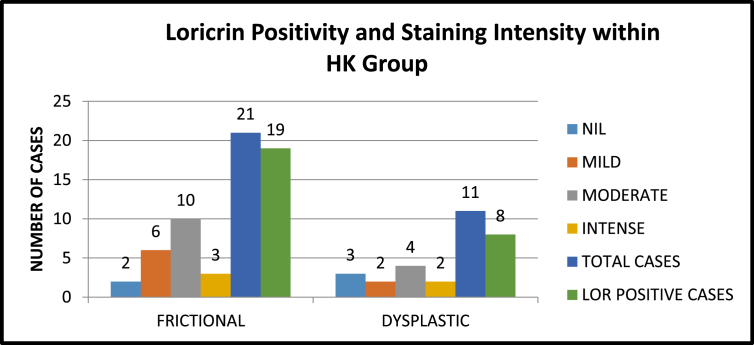
3.1. Comparing positivity and staining intensity of loricrin expression within hyperkeratosis
On comparing the positivity of Loricrin expression within the hyperkeratosis group, the physically induced hyperkeratosis group showed a greater positivity (90.5%) than the dysplastic group. (72.7%) and this difference in expression was not statistically significant. (P = 0.209) (Table-1)When the different intensities exhibited within this group was analysed, the physically induced cases showed mild intensity (18.2%). Moderate and intense staining was not observed in any case. The dysplastic specimens however showed mild, moderate and intense staining in 25 .0%, 43.8% and 15.6% of the cases respectively. {Statistically not significant. (P = 0.563)) (Kappa 0.4)}Fig. 3, Fig. 4 and Fig. 7.
Fig. 3.
HYPERKERATOSIS WITHOUT EPITHELIAL DYSPLASIA showing positive loricrin staining in the stratum granulosum in patient with Gutka (Pan) Usage.
Fig. 4.
HYPERKERATOSIS WITH EPITHELIAL DYSPLASIA showing positive loricrin staining in the stratum granulosum associated with chewing habit.
Fig. 7.
Staining and intensity aceoss Within HK group.
4. Discussion
The oral mucosa is exposed to a lot of stress, by both physical and chemical agents. To establish its integral role as an effective barrier with properties of flexibility and selective permeability, the mucosa of the oral cavity has been bestowed with specialized components like proteins, intermediate filaments and other cytoskeleton components. Loricrin is one such abundant protein that could play a role in the formation of a protective barrier. It is a late envelope proteins of the CE genes clustered in the “epidermal differentiation complex” on human chromosome1q21.1,12,13 Epithelia subjected to stress show an increase in the relative amount of these proteins, Loricrin is normally not expressed in non-keratinizing mucosa and other internal epithelia14, 15, 16 and its expression in certain conditions could be a means of providing protection against traumatic stimuli. Non-keratinizing epithelia show keratinization in conditions like OSF or hyperkeratosis. The differentiation of keratinocytes here could have been incited by stimuli both chemical and mechanical, like from chewing commercial and homemade “paan” which has areca nut and calcium-rich slaked lime as a primary component. Loricrin expression here, is seen as a compensatory mechanism. In our study, Loricrin has had a significant expression in all the three groups, [Normal mucosa (18.2%), hyperkeratosis (84.4%) and OSF (66.7%)] similar to a study by Li N et al.17 Analysis of the two positive normal mucosal cases in group I, revealed staining of mild intensity confined to the stratum granulosum (SG). The Positivity here could be attributed to the site of biopsy (retro molar region, exposed to friction from teeth and mastication) and chewing habit association respectively.18 The positivity in this region also emphasizes that while normal masticatory friction is tolerated, the environment significantly changes with external (habit) influences. This is a kind of adaptation by the keratinocytes to the changing environment of the oral cavity and can be seen by the altered presence of loricrin in areas that normally do not express it.17,19,20 The hyperkeratosis samples (Group II) showed a direct association between Loricrin expression and chewing habit with staining in both SG and SC ranging from moderate to intense respectively. Further emphasis was seen when hyperkeratosis with epithelial dysplasia (chemical stress) had greater association (90.9%) when compared to hyperkeratosis without epithelial dysplasia (physical stress) (76.2%). In OSF, (Group-III) a strong causal association (93.3%) of areca chewing habit was appreciable with all three staining intensities evident. The positivity of stratum spinosum indicates that the increased loricrin present here is not CE incorporated.20
This finding compounds the fact that continuous irritation or micro trauma alters the normal signalling mechanisms in the epidermis making them sensitive to altered/acute barrier disruption and varied external humidities. The barrier recovery process is further inhibited by factors like calcium concentration. Areca chewing, usually has slaked lime (calcium hydroxide) as one of its ingredient and its presence at both intra and inter-cellular level has been confirmed in the mucosa of betel nut chewers.21 Barrier disruption shows a decline in calcium levels at SG with a simultaneous increase in the lamellar body secretion and increased influx at SC interstices.22 Serine proteases and protease inhibitors at this level contain TGase (Transglutaminase substrate domains) cross linked to increased loricrin residues in CE. as a result of barrier disruption13,19 However, CE proteins like involucrin take over loricrin function in cases of increased trauma.23 This compensatory rearrangement of cytoskeleton during cell division paves way for other molecular events that occur during the formation of dysplasia and its transformation into malignancy.13,24 These are seen as decreased cell density and Retinoic Acid Receptors (RARs) of atrophic epithelium. Retinoic acid affects loricrin either through gene expression regulation or differentiation process and suppress the expression of loricrin in vitro.24
The evaluation of OSF by histological grading into early intermediate and advanced stages was done (data not included) with comparison of all three stages. While an increased expression was observed in the early and intermediate stages it was decreased in the advanced stages. This implies the limited protective capacity of loricrin when faced against persistent stresses over a prolonged period.20 However the presence in early stages itself can be in favour of determining the presence of the disease at an earlier stage. The decreased staining in SC could be attributed to continuous changes taking place in the environment around the epitopes leading to (a) their non-recognition (b) Loss during proteolysis and c) Masking by other cellular components.15
The property of keratinocytes to be altered in completely different conditions while preserving its original phenotype is seen when areca nut and other ingredients in chewing creates an environment similar to that of the dry regions of epidermis leading to expression of loricrin in a wet and humid oral cavity.19 In our study (data not included) the area of greatest concentration of the staining (nucleus or cytoplasm) was also observed. Greater concentration of the stain in the nucleus was observed in 14 OSF, 6 physically induced hyperkeratosis and 2 chemically induced hyperkeratosis samples. Intra nuclear accumulation of loricrin in SG has been seen as an introduction of potential nuclear targeting motifs in mutant c-terminal peptides leading to altered loricrin crosslinking and barrier function. The ability of environmental stimuli to produce inflammatory cytokines and chemo tactic factors in an antigen independent fashion, point to the fact that there is a fair chance of habit inducing mechanical stress altering the barrier function.26 Normally expression of loricrin is limited to the stratum granulosum of the keratinizing epithelia of the oral cavity and the epidermis. It is absent or not expressed in the non-keratinizing epithelia or the lining mucosa of the oral cavity. In the present study, the presence of staining being taken up by the stratum spinosum of oral submucous fibrosis specimens alone could be related to three factors.
-
•
Decreased cell density-as seen by the atrophic epithelium.
-
•
Micro trauma related to areca nut chewing and
-
•
Decrease in the number of (RAR) Retinoic acid receptors.
Increased expression of loricrin, seen in the early and intermediate grades of OSF than in the advanced stage points to its expression being directly related to the compensatory mechanism kicking in as an early response to stress at the site. Expression also evident in the hyperkeratosis group further establishes the fact that loricrin expression facilitates a modest level of protection to the changing environment. Chewing habit seen in almost all the patients clearly establishes the role of areca nut chewing as a key etiological factor in the pathogenesis of OSF by-inducing stress (both mechanical and chemical). Expression of Loricrin thus could be an early indicator of the various changes that could take place as a result of the continuous physical stimuli and could be a prognostic marker in oral potentially malignant disorders like OSF and leukoplakia.
5. Conclusion
This study, assessing Loricrin expression with an association to habits has been attempted for the first time in the Indian population and the present findings with regard to its expression support the view on the changing adaptability of the epithelia to new stimuli and indicates its use as an important prognostic marker for an earlier diagnosis of a malignant transformation. Further studies in this aspect could give us more valuable insights on oral mucosa and Loricrin interaction.
Ethical clearance
The study for evaluating the expression of Loricrin in these samples was done after approval and ethical clearance from the Institutional Review Board.
Support
No Source of support claimed.
Conflicts of interest
None.
References
- 1.Presland R.B., Jurevic R.J. Making sense of the epithelial barrier: what molecular biology and genetics tell us about the functions of oral mucosal and epidermal tissues. J Dent Educ. 2002;66:564–574. [Pubmed- 12014572] [PubMed] [Google Scholar]
- 2.Saraswathi T.R., Ranganathan K., Shanmugam S., Sowmya R., Narasimhan P.D., Gunaseelan R. Prevalence of oral lesions in relation to habits: cross-sectional study in South India. Indian J Dent Res. 2006;17:121–125. doi: 10.4103/0970-9290.29877. [Pubmed-17176828] [DOI] [PubMed] [Google Scholar]
- 3.Axéll T., Pindborg J.J., Smith C.J., Van der Waal I. Oral white lesions with special reference to precancerous and tobacco-related lesions: conclusions of an international symposium held in Uppsala, Sweden, May18-211994.International Collaborative Group on Oral White Lesions. J Oral Pathol Med. 1996;25:49–54. doi: 10.1111/j.1600-0714.1996.tb00191.x. [ Pubmed-8667255] [DOI] [PubMed] [Google Scholar]
- 4.Murti P.R., Bhonsle R.B., Pindborg J.J., Daftary D.K., Gupta P.C., Mehta F.S. Malignant transformation rate in oral submucous fibrosis over a 17-year period. Community Dent Oral Epidemiol. 1985;13:340–341. doi: 10.1111/j.1600-0528.1985.tb00468.x. [ Pubmed-3866655] [DOI] [PubMed] [Google Scholar]
- 5.Al-dhohrah T., Mashrah M., Yao Z., Huang J. Aberrant DKK3 expression in the oral leukoplakia and oral submucous fibrosis: a comparative immunohisto chemical study. Eur J Histochem. 2016;60:2629. doi: 10.4081/ejh.2016.2629. [ Pubmed-27349317] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Choi S., Myers J.N. Molecular pathogenesis of oral squamous cell carcinoma implications for therapy critical reviews in oral biology and medicine. J Dent Res. 2008;87:14–32. doi: 10.1177/154405910808700104. [Pubmed-18096889] [DOI] [PubMed] [Google Scholar]
- 7.Wakamatsu K., Ogita H., Okabe N. Up-regulation of Loricrin expression by cell adhesion molecule nectin-1 through rap1-erk signalling in keratinocytes. J Biol Chem. 2007;282:18173–18181. doi: 10.1074/jbc.M611159200. [ Pubmed-17472964] [DOI] [PubMed] [Google Scholar]
- 8.Odani T., Ito D., Li M.H. Gene expression profiles of oral leukoplakia and carcinoma: genome-wide comparison analysis using oligonucleotide microarray technology. Int J Oncol. 2006;28:619–624. [ Pubmed-16465365] [PubMed] [Google Scholar]
- 9.Nithya S., Radhika T., Jeddy N. Loricrin – an overview. J Oral Maxillofac Pathol. 2015;19:64–68. doi: 10.4103/0973-029X.157204. [ Pubmed-26097310] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Candi E., Melino G., Mei G. Biochemical, structural, and transglutaminase substrate properties of human loricrin, the major epidermal cornified cell envelope Protein. J Biol Chem. 1995;270:26382–26390. doi: 10.1074/jbc.270.44.26382. [ Pubmed-7592892] [DOI] [PubMed] [Google Scholar]
- 11.Yanjia H., Xinchun J. The role of epithelial–mesenchymal transition in oral squamous cell carcinoma and oral submucous fibrosis. Clin Chim Acta. 2007;383:51–56. doi: 10.1016/j.cca.2007.04.014. [ Pubmed-17537414] [DOI] [PubMed] [Google Scholar]
- 12.Koch P.J., de Viragh P.A., Scharer E. Lessons from loricrin-deficient mice: compensatory mechanisms maintaining skin barrier function in the absence of a major cornified envelope protein. J Cell Biol. 2000;151:389–400. doi: 10.1083/jcb.151.2.389. [ Pubmed:11038185] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hitomi K. Transglutaminases in skin epidermis. Eur J Dermatol. 2005;15:313–319. Eur J Dermatol.2005; 15(5):313-19.[ Pubmed:16172037] [PubMed] [Google Scholar]
- 14.Elias P.M. Stratum corneum defensive functions –an integerated view. J Invest dermatol. 2005;125:183–200. doi: 10.1111/j.0022-202X.2005.23668.x. [ Pubmed-16098026] [DOI] [PubMed] [Google Scholar]
- 15.Hohl D., Mehrel T., Lichti U., Turner M.L., Roop D.R., Steinert P.M. Characterization of human loricrin; Structure and function of a new class of epidermal cell envelope proteins. J Biol Chem. 1991;266:6626–6636. [ Pubmed-2007607] [PubMed] [Google Scholar]
- 16.Steinert P.M., Marekov L.N. The proteins elafin, filaggrin, keratin intermediate filaments, loricrin, and small proline-rich proteins 1 and 2 are isodipeptide cross-linked components of the human epidermal cornified cell envelope. J Biol Chem. 1995;270:17702–17711. doi: 10.1074/jbc.270.30.17702. [ Pubmed-7543090] [DOI] [PubMed] [Google Scholar]
- 17.Li N., Jian X.C., Xu C.J. Expression of loricrin and cytochrome P450 3A5 in oral submucous fibrosis and their significance. Hua xi kou qiang yi xue za zhi. West China J Stomatol. 2009;27:29–33. [ Pubmed-19323390] [PubMed] [Google Scholar]
- 18.Bellato L., Martinelli-Kläy C.P., Martinelli C.R., Lombardi T. Alveolar ridge keratosis - a retrospective clinicopathological study. Head Face Med. 2013;9(12) doi: 10.1186/1746-160X-9-12. [ Pubmed-23587097] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Katou F., Shirai N., Kamakura S., Tagami H., Nagura H., Motegi K. Differential expression of cornified cell envelope precursors in normal skin, intra orally transplanted skin and normal oral mucosa. Br J Dermatol. 2003;148:898–905. doi: 10.1046/j.1365-2133.2003.05288.x. [Pubmed-12786819] [DOI] [PubMed] [Google Scholar]
- 20.Li N., JianX, Hu Y., Xu C., Yao Z., Zhong X. Discovery of novel biomarkers in oral submucous fibrosis by microarray analysis. Cancer Epidemiol Biomark Prev. 2008;17:2249–2258. doi: 10.1158/1055-9965.EPI-07-2908. [ Pubmed-18768491] [DOI] [PubMed] [Google Scholar]
- 21.Trivedy C.R., Craig G., Warnakulasuriya S. The oral health consequences of chewing areca nut. Addict Biol. 2002;7:115–125. doi: 10.1080/13556210120091482. [ Pubmed-11900631] [DOI] [PubMed] [Google Scholar]
- 22.Hohl D., Lichti U., Breitkreutz D., Steinert P.M. Roop DR Transcription of the human loricrin gene in vitro is induced by calcium and cell density and suppressed by Retinoic acid. J Investig Dermatol. 1991;96:414–418. doi: 10.1111/1523-1747.ep12469779. [ Pubmed-2007780] [DOI] [PubMed] [Google Scholar]
- 23.Ekanayake-Mudiyanselage S., Aschauer H., Schmook F.P., Jensen J.M., Meingassner J.G., Proksch E. Expression of epidermal keratins and the cornified envelope protein involucrin is influenced by permeability barrier disruption. J Investig Dermatol. 1998;111:517–523. doi: 10.1046/j.1523-1747.1998.00318.x. [ Pubmed-9740250] [DOI] [PubMed] [Google Scholar]
- 24.Fisher C., Blumenberg M., Canic M.T. Retinoid receptors and keratinocytes. Crit Rev Oral Biol Med. 1995;6:284–301. doi: 10.1177/10454411950060040201. [ PubMed-8664420] [DOI] [PubMed] [Google Scholar]
- 25.Dermot K.B., ArmstrongKevinEMckenna, Hughes Anne E. Novel insertional mutation in loricrin in. J Investig Dermatol. 1998;111:702–704. doi: 10.1046/j.1523-1747.1998.00343.x. [ PubMed-9764857] [DOI] [PubMed] [Google Scholar]
- 26.Barker J.N., Mitra R.S., Griffiths C.E., Dixit V.M., Nickoloff B.J. Keratinocytes as initiators of inflammation Lancet. 1991;337:211–214. doi: 10.1016/0140-6736(91)92168-2. [PubMed-1670850] [DOI] [PubMed] [Google Scholar]