Abstract
Background
Being common, mild anemia is sometimes considered a mere consequence of aging; however, aging alone is unlikely to lead to anemia. Therefore, this study aimed to investigate the association between mild anemia and total mortality and cause-specific mortality in apparently healthy elderly subjects.
Methods
A retrospective cohort study was conducted on 10,114 apparently healthy elderly individuals who underwent cancer screening and routine medical check-ups at one Health Promotion Center between May 1995 and December 2007. We defined mild anemia as a hemoglobin concentration between 10.0 g/dL and 11.9 g/dL in women and between 10.0 g/dL and 12.9 g/dL in men. We assessed the relationship between the overall, cardiovascular (CV), and cancer mortality and mild anemia using Cox proportional hazard models.
Results
Mild anemia was present in 143 men (3.1%) and 246 women (6.1%). During an average follow-up of 7.6 years, 495 deaths occurred, including 121 CV and 225 cancer deaths. After adjustments, mild anemia was associated with a 128% increase in the risk of all-cause mortality hazard ratio (HR, 2.28; 95% confidence interval [CI], 1.54– 3.37) in men and cancer-related mortality (HR, 2.25; 95% CI, 1.22–4.13), particularly lung cancer (HR, 2.70; 95% CI, 1.03–7.08) in men, but not in women. In the subgroup analyses based on smoking status, obesity, and age, the associations were more prominent in never or former smoker groups and the older group.
Conclusion
The present study shows that overall and cancer-related mortality was associated with mild anemia in elderly men. Future prospective studies are needed to consolidate our findings.
Keywords: Anemia, Mortality, Aged, Cause of Death
INTRODUCTION
Globally, anemia affects 1.62 billion people, which corresponds to 24.8% of the world’s population [1]. It occurs at all stages of the life cycle but tends to increase with advancing age (the Health and Anemia Study) [2].
Anemia, especially mild (hemoglobin [Hgb] levels 10–12 g/dL for women and 10–13 g/dL for men) [3], is commonly considered to be a consequence of the aging process, not a significant condition. However, aging alone does not lead to anemia. Healthy individuals older than 60 years have shown stable Hgb levels in some cross-sectional studies [4].
In the past decades, several studies have shown that anemia was associated with a number of negative outcomes, especially in elderly people. In addition to its association with physical performance, disability in daily living, cognitive impairment, and decreased quality of life [5], anemia has also been considered as a strong predictor of hospitalization and mortality [6]. However, the subjects in most of these studies were hospitalized patients or those with cancer or cardiovascular disease (CVD) [7], with the results focusing on moderate to severe anemia (Hgb <10 mg/dL) in addition to mild anemia [6,7]. Recently, anemia, mostly mild, was reported to be related to cancer mortality from gastrointestinal tract, prostate, and genitourinary tract in communitydwelling men aged >40 years [8]. However, whether mild anemia is a survival predictor in the general population remains unclear.
Therefore, this study aimed to investigate the association between mild anemia and all-cause, cardiovascular, and cancer mortality in apparently healthy elderly subjects. We also assessed the relationship of site-specific cancer mortality with mild anemia and with vulnerable subgroups of smoking, obesity, and age groups who are high risk of death due to mild anemia.
METHODS
1. Study Population
A total of 10,114 Korean adults aged ≥60 years completed medical check-ups and cancer screenings at the Health Promotion Center at Seoul National University Hospital between May 1995 and December 2007. Among them, 823 without complete information on smoking and 242 without information on diabetes or hypertension were excluded. After further sequential exclusion of 20 subjects with severe renal dysfunction (glomerular filtration rate [GFR] <30 mL/min) [9], 109 with a significantly elevated inflammatory marker (C-reactive protein [CRP] of ≥2 mg/dL), and 44 with Hgb of <10 g/dL, 8,876 subjects were finally included. Further, 102 subjects diagnosed with cancer before medical check-ups were excluded, as were four subjects with known coronary artery disease (CAD) and 67 subjects who died during the first 2 years of follow-up to exclude patients with serious comorbidities. Finally, 8,703 subjects were included in our analysis. Adults with anemia related to chronic diseases were excluded, and the study population was defined as apparently healthy elderly individuals with the purpose of cancer screening and health check-up. Our protocol was approved by the Institution Review Board at the Seoul National University Hospital (IRB approval no., 1205-047-409). All the data acquired for the study were coded and filed separately in order to protect the subjects’ personal information. These files were only available for the authors, and written informed consent was obtained prior to participation in the study.
2. Baseline Measurements
Demographic information, past medical history, and social history were collected through a structured self-reported questionnaire, supervised by a trained nurse. Examinations and blood samplings were performed after an overnight fast. Systolic and diastolic blood pressures were measured in sitting position after a controlled 20-minute rest. Body mass index (BMI) was calculated as weight (kg)/height (m)2.
Hgb level and mean corpuscular volume (MCV) as well as serum total cholesterol, creatinine, and plasma glucose levels were measured using an automated analysis system (Toshiba, Tokyo, Japan; 2000, 2002, 2006). We defined mild anemia as Hgb between 10.0 g/dL and 11.9 g/dL in women and between 10.0 g/dL and 12.9 g/dL in men [3]. In addition, we defined microcytosis and macrocytosis as MCV of <83 fL and MCV >100 fL, respectively, according to the central laboratory’s reference limits. We estimated the GFR (eGFR) using the simplified Modification of Diet in Renal Disease formula [9]. The neutrophil/lymphocyte ratio (NLR) was calculated by dividing the circulating neutrophil count by the lymphocyte count, and the platelet lymphocyte ratio (PLR) was calculated by dividing the platelet count by the lymphocyte count. The prognostic nutritional index (PNI) was calculated using the albumin level and total lymphocyte count [10].
3. Outcomes
We merged the baseline data with the mortality data from the national death certificate files since December 31, 2008. Follow-up for death was completed in more than 98% of the cases [11], and the follow-up duration for each person was calculated as the difference between the baseline examination date and the censored mortality date for the deceased or since December 31, 2008 for survivors. Outcome was defined as all-cause, cardiovascular (International Classification of Diseases, 10th revision [ICD-10], codes I10–I15, I20, I21, I25, I44–I51, and I60–I74), and cancer mortality (ICD-10, codes C00–C97) [12].
4. Statistical Analysis
Data were presented as means with standard deviations or numbers with percentages using the Student t-test or the chi-square test. The Kaplan-Meier (KM) survival curves were obtained for mildly anemic and non-anemic populations, and the differences in survival between the groups were assessed using the log-rank test.
We then conducted the Cox proportional hazard regression models after adjusting for potential confounding factors, such as age, BMI, smoking (current smoker, ex-smoker, or non-smoker), hypertension medications, diabetes history, monthly income (<2, 2 to 4, 4 to 6, >6 (million Korean won per month), regular exercise (regular exercise or not), plasma fasting glucose, serum total cholesterol, eGFR, and CRP to identify the hazard ratios of all-cause mortality, as well as cardiovascular and cancer deaths in the mildly anemic group relative to the non-anemic group.
We also assessed the relationship between all-cause mortality and mild anemia in subgroups of men as follows: never/former smoker versus current smoker, lean (BMI of <25.0 kg/m2) versus overweight/obese (BMI of ≥25.0 kg/m2) [13], and younger (<64 years, median age of this study population) versus older (≥64 years). Two-tailed P<0.05 values were considered statistically significant, and all data management and analysis were conducted using the STATA ver. 10.0 (Stata Corp., College Station, TX, USA).
RESULTS
With 7.6 years of median follow-up (65,760 person-years of follow-up), 495 patients died. The baseline characteristics of the study population according to anemia status are shown in Table 1. More women (n=246, 6.1%) were mildly anemic than men (n=143, 3.1%).
Table 1.
Basic characteristics of the study population (n=8,703)
| Characteristic | Men (n=4,634) |
Women (n=4,069) |
||||
|---|---|---|---|---|---|---|
| Mild anemia (n=143) | Normal (n=4,491) | P-value | Mild anemia (n=246) | Normal (n=3,823) | P-value | |
| Age group (quartile) | ||||||
| 1st | 26 (1.7) | 1,535 (98.3) | <0.01 | 79 (5.5) | 1,355 (94.5) | 0.02 |
| 2nd | 28 (2.8) | 985 (97.2) | 50 (5.1) | 926 (94.9) | ||
| 3rd | 29 (3.0) | 924 (97.0) | 47 (5.7) | 772 (94.3) | ||
| 4th | 60 (5.4) | 1,047 (94.6) | 70 (8.3) | 770 (91.7) | ||
| Body mass index (kg/m2) | 21.7±2.7 | 23.9±2.7 | <0.01 | 23.4±2.9 | 24.6±3.0 | <0.01 |
| Systolic blood pressure (mm Hg) | 133.4±23.8 | 138.8±20.7 | <0.01 | 139.7±20.6 | 141.9±21.5 | 0.12 |
| Hypertension medication (yes) | 35 (24.5) | 1,039 (23.1) | 0.71 | 42 (17.1) | 1,008 (26.4) | <0.01 |
| Diabetes history (yes) | 20 (14.0) | 470 (10.5) | 0.18 | 20 (8.1) | 277 (7.3) | 0.61 |
| Smoking status | ||||||
| Never | 28 (19.6) | 1,061 (23.6) | 0.49 | 227 (92.3) | 3,588 (93.9) | 0.39 |
| Former | 68 (47.6) | 2,091 (46.6) | 11 (4.5) | 112 (2.9) | ||
| Current | 47 (32.9) | 1,339 (29.8) | 8 (3.3) | 123 (3.2) | ||
| Regular exercise (yes) | 66 (46.2) | 2,380 (53.0) | 0.11 | 80 (32.5) | 1,413 (37.0) | 0.16 |
| Monthly income (million Korean won) | ||||||
| <2 | 36 (25.9) | 660 (14.9) | <0.01 | 55 (23.2) | 889 (24.2) | 0.44 |
| 2–4 | 44 (31.7) | 1,197 (27.1) | 77 (32.5) | 1,065 (29.0) | ||
| 4–6 | 25 (18.0) | 1,325 (30.0) | 56 (23.6) | 815 (22.2) | ||
| >6 | 34 (24.5) | 1,237 (28.0) | 49 (20.7) | 907 (24.7) | ||
| Blood test findings | ||||||
| MCV (fL) | 93.1±8.2 | 94.1±4.1 | <0.01 | 91.9±5.5 | 92.4±3.8 | 0.10 |
| Microcytosis (MCV ≤82 fL) | 11 (7.7) | 12 (0.3) | <0.01 | 12 (4.9) | 19 (0.5) | <0.01 |
| Macrocytosis (MCV ≥101 fL) | 18 (12.6) | 242 (5.4) | <0.01 | 9 (3.7) | 65 (1.7) | 0.03 |
| Fasting blood glucose (mg/dL) | 103.1±25.8 | 105.9±29.9 | 0.26 | 98.0±17.1 | 102.3±26.6 | 0.01 |
| Total cholesterol (mg/dL) | 185.7±39.1 | 201.5±34.1 | <0.01 | 205.8±35.8 | 217.9±38.2 | <0.01 |
| Albumin (g/dL) | 4.08±0.30 | 4.31±0.26 | <0.01 | 4.16±0.27 | 4.30±0.26 | <0.01 |
| Renal function | ||||||
| Serum creatinine (mg/dL) | 1.08±0.26 | 1.04±0.15 | <0.01 | 0.83±0.16 | 0.82±0.12 | 0.28 |
| Modification of Diet in Renal Disease-GFR (mL/min/1.73 m2) | 77.0±17.4 | 78.2±13.2 | 0.30 | 77.3±19.1 | 76.8±13.6 | 0.61 |
| GFR <60 mL/min | 20 (14.0) | 346 (7.7) | <0.01 | 39 (15.9) | 384 (10.0) | <0.01 |
| C-reactive protein (mg/dL) (n=6,567) | 0.35±0.40 | 0.20±0.24 | <0.01 | 0.23±0.27 | 0.19±0.22 | 0.01 |
| Neutrophil/lymphocyte ratio* | 2.02±1.25 | 1.76±0.97 | <0.01 | 1.68±0.90 | 1.56±0.90 | 0.05 |
| Platelet/lymphocyte ratio† | 141.6±72.0 | 112.5±80.3 | <0.01 | 134.3±49.3 | 125.3±170.1 | 0.41 |
| Prognostic nutrition index‡ | 50.24±4.13 | 53.83±4.18 | <0.01 | 51.32±4.05 | 54.09±4.20 | <0.01 |
Values are presented as number (%) or mean±standard deviation.
MCV, mean corpuscular volume; GFR, glomerular filtration rate.
Calculated as neutrophil count/lymphocyte count.
Calculated as platelet count/lymphocyte count.
Calculated as albumin (g/L)+0.005×total lymphocyte count.
The mean age of men and women at baseline examinations were 64.2 years and 63.8 years, respectively, and those with mild anemia were more likely to be older for both genders. The mildly anemic group had significantly higher CRP levels and lower BMIs compared with the non-anemic group. NLR, PLR, and PNI were significantly associated with mild anemia, especially in men.
No significant differences in the smoking status were observed between the mild anemia and non-anemia groups, but the former group showed higher prevalence of microcytosis, macrocytosis, and chronic kidney disease (GFR of <60 mL/min) [9].
As shown in the KM curve of unadjusted survival, those with normal Hgb levels at baseline examination had better overall survival during the 13 years of follow-up than those with initial mild anemia. The relationship between mild anemia and overall survival was more pronounced in men than in women (P-values of log-rank test were <0.001 in men and 0.03 in women; P-value for interaction was <0.001) (Figure 1).
Figure. 1.
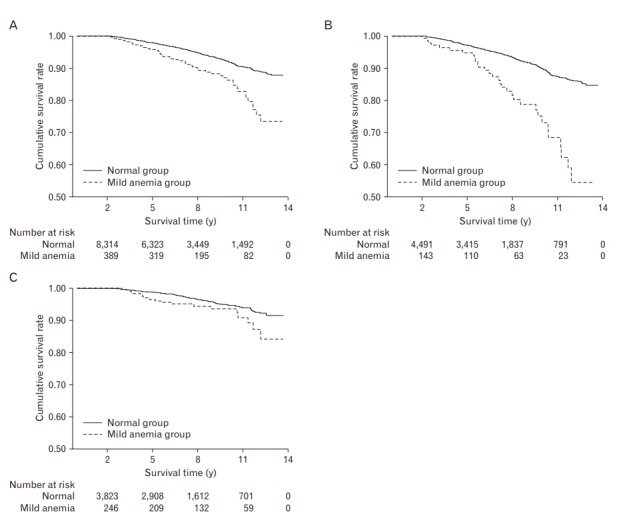
Kaplan-Meier curve on the effects of mild anemia on all-cause mortality. (A) Total study population: log-rank P<0.001. (B) Men: log-rank P<0.001. (C) Women: log-rank P=0.03.
After adjusting for potential confounders as shown in Table 1, mild anemia at baseline was associated with a 128% increase in the risk for all-cause mortality (hazard radio [HR], 2.28; 95% confidence interval [CI], 1.54–3.37) in men with statistical significance and a 56% increase in women although not statistically significant (Table 2). Regarding cancer mortality, mild anemia was significantly associated with mortality from cancer in men (HR, 2.25; 95% CI, 1.22–4.13], but not in women (HR, 1.56; 95% CI, 0.93–2.63). Cardiovascular mortality in mildly anemic subjects showed no significant difference compared with non-anemic subjects in both the genders. Further analyses for the associations between specific cancer deaths and mild anemia were conducted, and according to these results, only lung cancer deaths were significantly higher in the mild anemia group in men (HR, 2.70; 95% CI, 1.03–7.08) (Table 3). Table 4 shows the HRs of all-cause mortality of the mildly anemic group compared to the non-anemic group stratified by age, smoking, and obesity as follows: younger versus older (<64 years versus ≥64 years; men median age, 64 years), never/former smoker versus current smoker, and lean versus overweight/obese (BMI of <25.0 kg/m2 versus ≥25.0 kg/m2) in men. Due to the small number of current smokers and mortality rate, as well as the lack of association between mild anemia and all-cause mortality in women (Table 2), we only presented the stratified analyses in men. The risk of total mortality was significantly higher in the mild anemia group in never/former smokers only and not in current smokers (HR, 2.73; 95% CI, 1.62–4.61 in never/former smokers versus HR, 1.38; 95% CI, 0.72–2.67 in current smokers; P-value for interaction <0.001). In the stratified analysis according to the obesity status, total mortality was positively associated with mild anemia in both groups. The HR of mortality in overweight or obese subjects appeared to be higher than that in lean subjects; however, the interaction did not reach the statistical significance (HR, 1.88; 95% CI, 1.17–3.00 in the lean group versus HR, 3.97; 95% CI, 1.52–10.35 in overweight or obese group; P-value for interaction=0.59). In addition, total mortality was significantly associated with mild anemia in older subjects only (HR, 2.26; 95% CI, 1.41–3.61 older group versus HR, 1.62; 95% CI, 0.68–3.85 younger group; P-value for interaction <0.001).
Table 2.
HRs (95% CI) of mild anemia for all-cause, cardiovascular disease, and cancer deaths
| Variable | Men |
Women |
||||
|---|---|---|---|---|---|---|
| Case/person-years | ID | aHR (95% CI) | Case/person-years | ID | aHR (95% CI) | |
| All-cause mortality | ||||||
| Normal group | 310/33,589.2 | 922.9 | 1.00 | 136/29,020.8 | 468.6 | 1.00 |
| Mild anemia group | 31/1,077.8 | 2876.3 | 2.28 (1.54–3.37) | 18/2,071.8 | 868.8 | 1.56 (0.93–2.63) |
| Cardiovascular mortality | ||||||
| Normal group | 69/33,589.2 | 205.4 | 1.00 | 40/29,020.8 | 137.8 | 1.00 |
| Mild anemia group | 6/1,077.8 | 556.7 | 2.24 (0.95–5.30) | 6/2,071.8 | 289.6 | 1.83 (0.71–4.72) |
| Cancer mortality | ||||||
| Normal group | 147/33,589.2 | 437.6 | 1.00 | 58/29,020.8 | 199.9 | 1.00 |
| Mild anemia group | 12/1,077.8 | 1113.4 | 2.25 (1.22–4.13) | 8/2,071.8 | 386.1 | 1.78 (0.82–3.85) |
Adjusted for age, body mass index, smoking, hypertension medication, diabetes history, monthly income, regular exercise (>30 min at least 3 times a week), fasting glucose, total cholesterol, Modification of Diet in Renal Disease-glomerular filtration rate, and C-reactive protein.
aHR, adjusted hazard ratio; CI, confidence interval; ID, incidence density (per 100,000 person-years).
Table 3.
HRs (95% CI) of mild anemia for deaths from four site-specific cancer
| Site-specific cancer | Men |
Women |
||||
|---|---|---|---|---|---|---|
| Case/person-years | ID | aHR (95% CI) | Case/person-years | ID | aHR (95% CI) | |
| Upper gastrointestinal cancer | ||||||
| Normal group | 19/33,589.2 | 56.6 | 1.00 | 9/29,020.8 | 31.0 | 1.00 |
| Mild anemia group | 2/1,077.8 | 185.6 | 2.62 (0.57–12.15) | 1/2,071.8 | 48.3 | 2.21 (0.25–19.41) |
| Lower gastrointestinal cancer | ||||||
| Normal group | 13/33,589.2 | 38.7 | 1.00 | 6/29,020.8 | 20.7 | 1.00 |
| Mild anemia group | 2/1,077.8 | 185.6 | 3.72 (0.78–17.78) | 1/2,071.8 | 48.3 | 3.61 (0.38–34.16) |
| Hepatobiliary cancer | ||||||
| Normal group | 47/33,589.2 | 139.9 | 1.00 | 19/29,020.8 | 65.5 | 1.00 |
| Mild anemia group | 2/1,077.8 | 185.6 | 1.45 (0.34–6.18) | 3/2,071.8 | 144.8 | 1.61 (0.45–5.73) |
| Lung cancer | ||||||
| Normal group | 43/33,589.2 | 128.0 | 1.00 | 10/29,020.8 | 34.5 | 1.00 |
| Mild anemia group | 5/1,077.8 | 463.9 | 2.70 (1.03–7.08) | 1/2,071.8 | 48.3 | 0.89 (0.11–7.57) |
Adjusted for age, body mass index, smoking, hypertension medication, diabetes history, income, regular exercise (>30 min at least 3 times a week), fasting glucose, total cholesterol, Modification of Diet in Renal Disease-glomerular filtration rate, and C-reactive protein.
aHR, adjusted hazard ratio, CI, confidence interval; ID, incidence density (per 100,000 person-years).
Table 4.
HRs (95% CI) of mild anemia group for total death stratified by current smoking, obesity, and age in men
| Variable | Category | Case/person-years | ID | aHR (95% CI) |
|---|---|---|---|---|
| Current smoking* | ||||
| Never or former smoker (n=3,248) | Normal group | 173/23,099.1 | 748.9 | 1.00 (reference) |
| Mild anemia group | 20/718.2 | 2,784.7 | 2.73 (1.62–4.61) | |
| Current smoker (n=1,386) | Normal group | 137/10,490.1 | 1,306.0 | 1.00 (reference) |
| Mild anemia group | 11/359.6 | 3,059.4 | 1.38 (0.72–2.67) | |
| Obesity | ||||
| Lean (BMI <25.0 kg/m2, n=3,109) | Normal group | 222/22,370.0 | 992.4 | 1.00 (reference) |
| Mild anemia group | 25/954.1 | 2,620.3 | 1.88 (1.17–3.00) | |
| Overweight or Obese (BMI ≥25.0 kg/m2, n=1,525) | Normal group | 88/11,219.3 | 784.4 | 1.00 (reference) |
| Mild anemia group | 6/123.7 | 4,851.5 | 3.97 (1.52–10.35) | |
| Age* | ||||
| Younger (≤63 y, n=2,574) | Normal group | 120/19,207.5 | 624.8 | 1.00 (reference) |
| Mild anemia group | 6/445.7 | 1,346.1 | 1.62 (0.68–3.85) | |
| Older (≥64 y, n=2,060) | Normal group | 190/14,381.7 | 1,321.1 | 1.00 (reference) |
| Mild anemia group | 25/632.0 | 3,955.5 | 2.26 (1.41–3.61) |
Adjusted for age, body mass index, smoking, hypertension medication, diabetes history, income, regular exercise (>30 min at least three times a weeks), fasting glucose, total cholesterol, Modification of Diet in Renal Disease-glomerular filtration rate, and C-reactive protein.
aHR, adjusted hazard ratio, CI, confidence interval; ID, incidence density (per 100,000 person-years); BMI, body mass index.
P-value for interaction <0.05.
DISCUSSION
This study presented the associations between mild anemia and total mortality, as well as cardiovascular- and cancer-related mortalities in apparently healthy Korean elderly individuals aged ≥60 years. We specifically investigated mild anemia, which does not generally exhibit any specific symptoms, in relatively healthy subjects without known serious illnesses such as cancer, CAD, and renal failure at baseline health check-ups.
Several previous studies involving the community-dwelling elderly population confirmed an independent association between anemia and the risk of mortality [6]. We also found that mild anemia in our elderly subjects increased the risks of all-cause and cancer-related mortalities in men, but not in women. Cardiovascular-related mortality was not related to mild anemia in both gender.
These associations can be explained by several potential hypotheses. Anemia can occur in various conditions, and in the elderly population, approximately one third of anemic persons had nutritional deficiency, and another one third had chronic inflammation, chronic kidney disease, or both. The remaining one third had unexplained anemia [14].
Based on these data, the first hypothesis for our results is that anemia is accompanied with chronic diseases including chronic kidney disease, infectious or inflammatory diseases, and severe trauma and hidden neoplastic diseases [14,15], which could be confounders in the association between anemia and mortality. Although subjects diagnosed with cancer were excluded from the study population and the presence of chronic diseases was considered by adjusting for hypertension and/or diabetes, renal function, and an inflammatory marker before the analysis, all of the information on chronic disease history possibly accompanied by anemia would not have been fully considered in the analysis.
Second, one of the important causes of death in elderly people is trauma. Anemia has been known as a significant independent risk factor for accidental falls and fractures in elderly people, an even stronger risk factor for serious injuries such as hip and head injuries [15].
Third, the association between anemia and cancer-related deaths is consistent with previous investigations showing a relationship between mild anemia with cancer and all-cause mortality in both healthy subjects and cancer patients [3,8,16]. Anemia may be one of the most common signs secondary to not only tumor based on mechanisms, such as blood loss and bone marrow invasion by malignant cells, but also cancer treatments. A previous study reported that the Hgb level is a strong independent prognostic factor to control cancer using radiation therapy [17], and a predictor of local recurrence in patients with breast cancer [18]. Recently, anemia, mostly mild, was reported to be a survival predictor for gastrointestinal tract, prostate, and genitourinary tract cancers in community-dwelling men aged >40 years, but not women from Australia [8]. In our study, in line with previous results, mild anemia was significantly associated with cancer-related mortality in apparently cancer-free men aged ≥60 years, particularly lung cancer. Recently, we reported that nutritional insufficiency, potentially related to mild anemia in elderly people and mediated by chronic inflammation, is a predictor of cancer survival, especially lung cancer [19]. This finding is consistent with those in other studies [20]. In this study, total cholesterol, albumin level, and the proportion of better PNI were also significantly lower both in men and women with mild anemia than their counterparts. Moreover, inflammatory markers, represented by highs-sensitivity-CRP, NLR, and PLR, were higher especially in men with mild anemia. Nutritional imbalance and chronic inflammation may be the possible explanation for the association between mild anemia and cancer mortality in Korean men. However, this finding is subject to further investigation as there were relatively few site-specific cancer deaths in this study.
Finally, a national cancer screening program has been established in Korea starting at age 40 years for cervical, colorectal, stomach, and breast cancers for all women, and additional liver cancer screening for high-risk groups including hepatitis B or C carriers as previously described in detail [21]. Therefore, the relationship between mild anemia and mortality from lung cancer, which has no proven screening program and is relatively common in men, appeared prominent in this study.
We further performed a subgroup analysis to investigate the effects of the major risk factors for cancer (smoking, obesity, and age) on the risk of total deaths in men. According to the subgroup analyses, mild anemia was associated with total mortality in never or former smokers, but not in current smokers. Mild anemia can be categorized into two subgroups: true anemia group and laboratory error group. However, the subgroup stratified analysis according to MCV status was limited due to the small number of individuals in the mild anemic groups. Additionally, in the subgroup analysis according to age, significant associations were observed in the older group only. Although the associations were significant regardless of obesity, the HRs seemed to be higher in the overweight or obese group than in the lean group.
Perhaps, various factors were involved in these outcomes. First, carbon monoxide (CO) in cigarette smoke binds more avidly to Hgb than oxygen (carboxyhemoglobin [HbCO]), this induce a hypoxic state and decrease the amount of oxygen available in the myocardium [22]. Moreover, elevated HbCO level stimulates the body to produce more red blood cells, which elevate the Hgb levels. Therefore, they may be objectively measured as smokers without anemia even though severely hypoxic. These individuals would still be vulnerable to hypoxia-related diseases such as CVD, with patients congestive heart disease suffering particularly from serious outcomes [23]. In short, even smokers with normal Hgb levels can be more vulnerable to hypoxia-related mortality. In addition, this might lead to the cancellation of any significant difference in the total mortality between smokers without and with anemia.
Next, obesity is known to be associated with several physiologic conditions, including obstructive sleep apnea, respiratory muscle impairment, and depressed central ventilator drive [24], which can lead to hypoxic conditions. In addition, frailty in the obese group exhibited a significantly higher risk of death than in their normal-weight counterparts in Korean elderly people [25].
In the subgroup analysis according to age, frailty can be considered as an important cause of these results. Frailty increases with age, although old age itself does not induce frailty. These elderly people are vulnerable to stressors, such as trauma and acute illness, which can contribute to the increased risk for disability and death [26]. Frailty, complicated by mild anemia and malnourishment, may lead to serious health outcomes in this population. Moreover, systemic inflammation due to undernutrition might be correlated with an increase in resting energy expenditure, loss of lean body mass, and decreased performance and survival with aging [27].
Our study has several strengths, including a large, generally healthy elderly population with long-term follow-up. In addition, because we excluded individuals who died during the first 2 years of follow-up, our study population consisted of more healthy individuals. Another strength of our study is that the data were from the national registry on mortality with reliable causes of death and no loss to follow-up.
The limitations of this study should also be discussed. First, this study was not designed to establish causality. Second, the study population was restricted to individuals who had routine medical checkups, possibly making our subjects less representative of the general elderly population in Korea. Third, the national cancer screening program in Korea might attenuate the relationship between mild anemia and common cancer deaths including in the stomach, liver, and breast in Korea.
In conclusion, our study showed that all-cause and cancer-related mortalities were higher in mildly anemic elderly men. Physicians should consider finding the cause of anemia including common cancer screening, even in their apparently healthy, cancer-free elderly male patients and consider appropriate treatment. Further research is needed to elucidate the mechanism of the associations between mild anemia and mortality, as well as to confirm that treating the anemia reduces mortality.
Footnotes
No potential conflict of interest relevant to this article was reported.
REFERENCES
- 1.World Health Organization . Global database on anemia: worldwide prevalence of anemia 1993-2005. Geneva: World Health Organization; 2008. [Google Scholar]
- 2.Ania BJ, Suman VJ, Fairbanks VF, Rademacher DM, Melton LJ 3rd. Incidence of anemia in older people: an epidemiologic study in a well defined population. J Am Geriatr Soc. 1997;45:825–31. doi: 10.1111/j.1532-5415.1997.tb01509.x. [DOI] [PubMed] [Google Scholar]
- 3.Riva E, Tettamanti M, Mosconi P, Apolone G, Gandini F, Nobili A, et al. Association of mild anemia with hospitalization and mortality in the elderly: the Health and Anemia population-based study. Haematologica. 2009;94:22–8. doi: 10.3324/haematol.13449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zauber NP, Zauber AG. Hematologic data of healthy very old people. JAMA. 1987;257:2181–4. [PubMed] [Google Scholar]
- 5.Lipschitz D. Medical and functional consequences of anemia in the elderly. J Am Geriatr Soc. 2003;51(3 Suppl):S10–3. doi: 10.1046/j.1532-5415.51.3s.6.x. [DOI] [PubMed] [Google Scholar]
- 6.Culleton BF, Manns BJ, Zhang J, Tonelli M, Klarenbach S, Hemmelgarn BR. Impact of anemia on hospitalization and mortality in older adults. Blood. 2006;107:3841–6. doi: 10.1182/blood-2005-10-4308. [DOI] [PubMed] [Google Scholar]
- 7.Archbold RA, Balami D, Al-Hajiri A, Suliman A, Liew R, Cooper J, et al. Hemoglobin concentration is an independent determinant of heart failure in acute coronary syndromes: cohort analysis of 2310 patients. Am Heart J. 2006;152:1091–5. doi: 10.1016/j.ahj.2006.07.020. [DOI] [PubMed] [Google Scholar]
- 8.Chalmers KA, Knuiman MW, Divitini ML, Bruce DG, Olynyk JK, Milward EA. Long-term mortality risks associated with mild anaemia in older persons: the Busselton Health Study. Age Ageing. 2012;41:759–64. doi: 10.1093/ageing/afs150. [DOI] [PubMed] [Google Scholar]
- 9.Botev R, Mallie JP, Couchoud C, Schuck O, Fauvel JP, Wetzels JF, et al. Estimating glomerular filtration rate: Cockcroft-Gault and Modification of Diet in Renal Disease formulas compared to renal inulin clearance. Clin J Am Soc Nephrol. 2009;4:899–906. doi: 10.2215/CJN.05371008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Proctor MJ, Morrison DS, Talwar D, Balmer SM, Fletcher CD, O’Reilly DS, et al. A comparison of inflammation-based prognostic scores in patients with cancer: a Glasgow Inflammation Outcome Study. Eur J Cancer. 2011;47:2633–41. doi: 10.1016/j.ejca.2011.03.028. [DOI] [PubMed] [Google Scholar]
- 11.Korean Statistical Information Service . Daejeon: Statistics Korea; 2005. 2005 Annual report on the cause of death statistics: based on vital registration [Internet] [cited 2008 Dec 31]. Available from: http://kostat.go.kr/portal/korea/index.action. [Google Scholar]
- 12.Park MS, Chung SY, Chang Y, Kim K. Physical activity and physical fitness as predictors of all-cause mortality in Korean men. J Korean Med Sci. 2009;24:13–9. doi: 10.3346/jkms.2009.24.1.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.International Association for the Study of Obesity . The Asia-Pacific perspective: redefining obesity and its treatment. Sydney: World Health Organization Western Pacific Region; 2002. [Google Scholar]
- 14.Price EA, Schrier SL. Unexplained aspects of anemia of inflammation. Adv Hematol. 2010;2010:508739. doi: 10.1155/2010/508739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Duh MS, Mody SH, Lefebvre P, Woodman RC, Buteau S, Piech CT. Anaemia and the risk of injurious falls in a community-dwelling elderly population. Drugs Aging. 2008;25:325–34. doi: 10.2165/00002512-200825040-00005. [DOI] [PubMed] [Google Scholar]
- 16.Caro JJ, Salas M, Ward A, Goss G. Anemia as an independent prognostic factor for survival in patients with cancer: a systemic, quantitative review. Cancer. 2001;91:2214–21. [PubMed] [Google Scholar]
- 17.Lavey RS. Clinical trial experience using erythropoietin during radiation therapy. Strahlenther Onkol. 1998;174 Suppl 4:24–30. [PubMed] [Google Scholar]
- 18.Dubsky P, Sevelda P, Jakesz R, Hausmaninger H, Samonigg H, Seifert M, et al. Anemia is a significant prognostic factor in local relapse-free survival of premenopausal primary breast cancer patients receiving adjuvant cyclophosphamide/methotrexate/5-fluorouracil chemotherapy. Clin Cancer Res. 2008;14:2082–7. doi: 10.1158/1078-0432.CCR-07-2068. [DOI] [PubMed] [Google Scholar]
- 19.Ko YJ, Kwon YM, Kim KH, Choi HC, Chun SH, Yoon HJ, et al. Highsensitivity C-reactive protein levels and cancer mortality. Cancer Epidemiol Biomarkers Prev. 2012;21:2076–86. doi: 10.1158/1055-9965.EPI-12-0611. [DOI] [PubMed] [Google Scholar]
- 20.Sun K, Chen S, Xu J, Li G, He Y. The prognostic significance of the prognostic nutritional index in cancer: a systematic review and metaanalysis. J Cancer Res Clin Oncol. 2014;140:1537–49. doi: 10.1007/s00432-014-1714-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Korea Centers for Disease Control and Prevention . Cheongju: Korea Centers for Disease Control and Prevention; 2005. The third Korea National Health and Nutrition Examination Survey (KNHANES III) [Internet] [cited 2008 Dec 31]. Available from: http://knhanes.cdc.go.kr. [Google Scholar]
- 22.Aronow WS, Cassidy J, Vangrow JS, March H, Kern JC, Goldsmith JR, et al. Effect of cigarette smoking and breathing carbon monoxide on cardiovascular hemodynamics in anginal patients. Circulation. 1974;50:340–7. doi: 10.1161/01.cir.50.2.340. [DOI] [PubMed] [Google Scholar]
- 23.Allred EN, Bleecker ER, Chaitman BR, Dahms TE, Gottlieb SO, Hackney JD, et al. Short-term effects of carbon monoxide exposure on the exercise performance of subjects with coronary artery disease. N Engl J Med. 1989;321:1426–32. doi: 10.1056/NEJM198911233212102. [DOI] [PubMed] [Google Scholar]
- 24.Piper AJ, Grunstein RR. Current perspectives on the obesity hypoventilation syndrome. Curr Opin Pulm Med. 2007;13:490–6. doi: 10.1097/MCP.0b013e3282ef6894. [DOI] [PubMed] [Google Scholar]
- 25.Lee Y, Kim J, Han ES, Ryu M, Cho Y, Chae S. Frailty and body mass index as predictors of 3-year mortality in older adults living in the community. Gerontology. 2014;60:475–82. doi: 10.1159/000362330. [DOI] [PubMed] [Google Scholar]
- 26.Fried LP, Tangen CM, Walston J, Newman AB, Hirsch C, Gottdiener J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56:M146–56. doi: 10.1093/gerona/56.3.m146. [DOI] [PubMed] [Google Scholar]
- 27.Sanchez-Lara K, Turcott JG, Juarez E, Guevara P, Nunez-Valencia C, Onate-Ocana LF, et al. Association of nutrition parameters including bioelectrical impedance and systemic inflammatory response with quality of life and prognosis in patients with advanced non-small-cell lung cancer: a prospective study. Nutr Cancer. 2012;64:526–34. doi: 10.1080/01635581.2012.668744. [DOI] [PubMed] [Google Scholar]


