Abstract
We performed a retrospective analysis of 93 myelodysplastic syndromes (MDS) patients with intermediate 2 or high‐risk IPSS score to study the impact of Azacitidine (AZA) relative dose intensity (RDI) <80% on the overall survival (OS). There were 51.6% of patients who had full dose and 48.4% had dose reduction or delayed with a RDI <80%. Nineteen patients (20.4%) had RDI <80% before getting objective response. Overall and progression‐free survivals (OS, PFS) probabilities for the whole population were 58% (95% CI: 48‐69) and 47% (95% CI: 38‐58) at 1 year; 35% (95% CI: 26‐47) and 31% (95% CI: 23‐43) at two years, respectively. When analyzing the outcomes according to the response to AZA, median OS was 32 months (range: 26‐55) for responders and 8 months (range: 7‐12) for nonresponders, with a respective 1‐year and 2‐year OS probabilities of 91% vs 28% and 66% vs 6%, respectively (P < 0.001). Interestingly, there was no impact of dose reduction on OS nor on PFS, however, when analyzing the timing of dose reduction as time‐dependent variable, we found that patients who had dose reduction before achieving the objective response, had significantly lower OS (P = 0.02) and PFS (P = 0.01) compared to patients who had dose reduction after achieving the objective response. In multivariate analysis, acute myeloid leukemia with 21%‐30% blasts in BM and poor and very poor karyotype significantly impacted OS, (HR = 2.09, 95% CI: 1.27‐3.44, P = 0.004, and HR = 2.73, 95% CI: 1.6‐4.6, P < 0.001 respectively), as well as PFS (HR = 1.84, 95% CI: 1.07‐3.17, P = 0.028, and HR = 3.03, 95% CI: 1.7‐5.39, P < 0.001, respectively).
Keywords: Azacitidine, dose intensity, myelodisplastic syndromes
1. INTRODUCTION
Myelodysplastic syndromes (MDS) are a group of bone marrow stem cell disorders characterized by ineffective hematopoiesis leading to blood cytopenias and a high incidence of progression to acute myeloid leukemia (AML) occurring in one‐third of cases.1, 2, 3, 4 Irregular DNA hypermethylation is a hallmark of MDS5, 6, 7 and among hypomethylating agents, azacitidine (AZA) at a standard daily dose of 75 mg/m2 for 7 days spaced every 4 weeks, significantly reduces transfusion dependence, decreases the risk of transformation to AML, and improves quality of life (QOL) in high‐risk MDS (ie intermediate‐2 or high IPSS risk MDS).8, 9, 10
In routine clinical use, the dosage regimen of AZA could be adapted to the care environment of each treating center: 7 consecutively days or 5 days of treatment, 2 days off, and 2 days of treatment (5‐2‐2 regimen) are the most used. However, dose reduced regimen as a 5 days schedule or delayed treatment is also used in certain circumstances related mainly to the tolerance of the drug or a myelosuppression as a result of MDS.
In a phase III AZA‐001 trial11, 21% of cycles were longer than 35 days, and in the Spanish registry,12 57.5% of patients received reduced dose regimen with a total number of days less than 7 days.
Few studies have assessed the efficacy and safety of alternatives dosing schedule of AZA like a 5‐day schedule of subcutaneous or intravenous form or 5‐day dose‐intensified regimen and results from using these schedules are still not homogenous. Furthermore, the population analyzed was generally heterogeneous with a significant portion of lower‐risk MDS patients.13, 14, 15, 16, 17
The GFM group reported a large series (n = 282) of patients treated in the compassionate, patient‐named program of AZA in IPSS intermediate‐2 or high‐risk MDS and AML with ≤30% marrow blasts and showed that reduction of AZA schedule in 28% of patients did not significantly influence overall survival (OS) patients (median, 10.3 vs 14.3 months; P = 0.10).18
On the other hand, no prospective study has compared the standard AZA regimen to another dose schedule in responding patients or the analysis of a maintenance treatment with attenuated doses in responders comparatively to the standard regimen.
The primary endpoint of the present study is to assess the impact of the dose reduction and/or dose delay on the outcome of patients with MDS treated with AZA at our institution. The secondary endpoints were the evaluation of the efficacy of AZA in patients with high‐risk MDS and AML with 21%‐30% BM blasts according to MDS‐WHO criteria, safety, OS, and factors impacting on OS.
2. MATERIAL AND METHODS
2.1. Patients
We retrospectively analyzed the efficacy of AZA in patients older than 18 years of age with intermediate‐2 or high‐risk MDS (as defined by the WHO‐ 2008 criteria),19 according to the IPSS,8 nonproliferative chronic myelomonocytic leukemia with 10%‐19% marrow blasts (CMML‐2 with WBC<13G/L) or acute myelogenous leukemia (AML) with 20%‐30% blasts and multi‐lineage dysplasia, treated in the front‐line setting in the Hematology Department of Le Mans Hospital center between November 2008 and February 2018. This retrospective study was approved by the local ethics committee.
2.2. Treatment, dose relative intensity, and assessment of response
Azacitidine was administered subcutaneously according to the 5 days regimen of treatment, 2 days off and 2 days on treatment (5‐2‐2). Dose reduction of AZA including a 5 days regimen or delays to every 5 weeks were considered before hematological toxicity or nonhematologic‐related complications, and could be done for some patients depending on tolerance, compliance, and psychological tolerance after the achievement of best response objective. The dose intensity is the amount of AZA administered per unit time. The relative dose intensity (RDI) is the percentage of the dose received by the patient on the dose that theoretically should have administered.
Thus, a patient receiving a 5 days regimen of AZA every 4 weeks received RDI of 5/7 (71%) of AZA, and the one who receives 7 days every 5 weeks received in 6 months 83% of the RDI. We calculated the RDI of AZA for all patients and compared a group of patients who received RDI ≥80% of AZA with a group of patients who received RDI <80% of AZA.
Cytogenetic abnormalities were classified according to International System for Human Cytogenetic Nomenclature criteria,20, 21 IPSS8 criteria, and IPSS‐R criteria.22 Responses to treatment and disease status were evaluated according to IWG‐MDS‐ 200623 response criteria. The first evaluation of response was carried out after the fourth cycle of AZA. Progression defining events were death due to any reason, disease progression, disease relapse after response, and/or new cytogenetic aberration/clonal evolution.
2.3. Statistical analysis
All time‐to‐event analyses were performed from AZA initiation date using the Kaplan‐Meier method and the log‐rank test. OS was calculated from the start of AZA therapy until death from any cause, progression‐free survival (PFS) was defined as the time from AZA initiation to the time of first event (progression of disease or death). Clinical parameters that were found to have significant effect on survival in univariate analysis (those covariates with P values < 0.1) were selected through a stepwise algorithm and then reevaluated in a multivariate model with the addition of variables with clinical relevance (such as RDI <80 used as time‐dependent variable). Statistical analysis was performed using R statistical software (v3.5.1).
3. RESULTS
3.1. Patient characteristics
This retrospective analysis included 93 patients who received AZA in the front‐line setting at the Hematology Department of Le Mans Hospital center between November 2008 and February 2018. The median age was 77 years (range: 56‐89), the majority of the patients (59%) had more than 75 years, with a male predominance (sex ratio: 1.44). Sixty‐one patients (66%) had 2 or 3 cytopenias and forty‐five (48%) had transfusion dependence represented by the transfusion of ≥4 RBC/unit/8 weeks, or platelets, before the start of AZA regimen. Twenty‐two patients (24%) had AML with 20%‐30% blasts and multi‐lineage dysplasia. Cytogenetic analysis according to IPSS score was favorable, intermediate, or poor risk in 49%, 15%, and 29% of patients, respectively. The IPSS risk score was intermediate risk 2 in 57 patients (61%), and high risk in 31 patients (33%). Patient characteristics are shown in Table 1.
Table 1.
Demographic and baseline characteristics of AZA‐treated patients
| Characteristics | N (%) |
|---|---|
| Age | |
| Median (range) | 77 (56‐89) |
| >75 years | 55 (59%) |
| ≤75 years | 38 (41%) |
| Gender (male) | 55 (59%) |
| PS ECOG | |
| 0‐1 | 61 (66%) |
| 2‐3 | 26 (28%) |
| Missing | 6 (6%) |
| Albumin (g/L) | |
| ≤35 | 34 (37%) |
| >35 | 47 (50%) |
| Missing | 12 (13%) |
| LDH level (UI/L) | |
| ≤250 | 40 (43%) |
| >250 | 42 (45%) |
| Missing | 11 (12%) |
| Ferritin level (ng/mL) | |
| ≥1000 | 19 (21%) |
| <1000 | 44 (47%) |
| Missing | 30 (32%) |
| Blasts | |
| Median (range) | 13% (4‐30) |
| Cytopenias | |
| 0‐1 | 32 (34%) |
| 2‐3 | 61 (66%) |
| WHO diagnosis | |
| CRMD | 2 (2%) |
| AREB1 | 10 (11%) |
| AREB2 | 44 (47%) |
| LMMC2 | 15 (16%) |
| AML | 22 (24%) |
| Transfusion dependence | |
| Yes | 45 (48%) |
| Cytogenetic risk (IPSS) | |
| Favorable | 46 (49%) |
| Intermediate | 14 (15%) |
| Poor | 27 (29%) |
| Missing | 6 (7%) |
| Cytogenetic risk (IPSS‐R) | |
| Very good | 6 (6%) |
| Good | 46 (50%) |
| Intermediate | 10 (11%) |
| Poor | 5 (5%) |
| Very poor | 20 (22%) |
| Missing | 6 (6%) |
| IPSS risk score | |
| Intermediate risk 2 (1.5‐2) | 57 (61.3%) |
| High risk (2.5‐3) | 31 (33.3%) |
| Missing | 5 (5.4%) |
| IPSS‐R risk score | |
| Intermediate > 3‐4.5 | 22 (24%) |
| High > 4.5‐6 | 35 (38%) |
| Very high > 6 | 30 (32%) |
| Missing | 6 (6%) |
AML, acute myeloid leukemia; AZA, Azacitidine.
3.2. Relative dose intensity
Forty‐eight patients (52%) had a full dose and 45 (48%) had dose reduction or delayed with a RDI <80%. Twenty‐eight patients (30%) received 5 days regimen, 22 (24%) patients were treated with cycles of more than 35 days, and five (5%) patients had received the two procedures. Patients with cycle delays or dose reductions received a median of 15 cycles (2‐68) while those without delays or dose reductions received a median of 6 cycles (1‐39). Twenty‐six patients (28%) had dose reduction and/or dose delayed with RDI <80% after achieving best objective response and 19 (20%) had a RDI <80% before reaching the best objective response. Twenty‐nine (31%) patients had a RDI <80% less before reaching cycle 12 of AZA and 16 (17%) patients had their dose reduced at cycle 12 or later (Table 2).
Table 2.
Relative dose Intensity of AZA < 80%
| Patients with AZA‐RDI < 80% | N | % |
|---|---|---|
| Total | 45 | 48 |
| 5 days regimen (A) | 28 | 30 |
| 5‐2‐2 regimen every 5 weeks or >5 weeks | 22 | 24 |
| Two procedures (A) + (B) | 5 | 5 |
| Patients achieved BOR | 26 | 28 |
| Patients did not achieve BOR | 19 | 20 |
| Patients who received less than 12 cycles of AZA | 29 | 31 |
| Patients who received 12 cycles of AZA or plus | 16 | 17 |
AZA, Azacitidine; BOR, best objectives response; RDI, relative dose intensity.
3.3. Response to treatment
Median time from first diagnosis to treatment start with AZA was 1.1 months (range: 0.2‐8.9). The median time from the start of AZA to treatment response was 4 months (range: 0.3‐8.97). The median number of AZA cycles was 9 (1‐68). Best hematological response according to IWG 2006 criteria was achieved in 53 patients (57%) after a median time of 4 months. According to IWG –MDS response criteria 2006, Overall response defined as CR, marrow CR, PR, and HI, was documented in 57% of patients (CR: 17.2%, marrow CR: 20.4%, PR: 9.7%, stable disease (SD) with hematological improvement: 9.7%) (Table 3). Forty‐two percent of patients were nonresponders including 25% of patients who achieved SD without hematologic improvement and 17% of patients who experienced treatment failure after AZA.
Table 3.
Response to treatment according to IWG‐MDS response criteria 2006
| Median (range) | N | (%) | |
|---|---|---|---|
| Complete response | 16 | 17.2 | |
| Partial response | 9 | 9.7 | |
| Bone marrow remission | 19 | 20.4 | |
| Stable disease | 32 | 34.4 | |
| with HI | 9 | 9.7 | |
| without HI | 23 | 24.7 | |
| Progression | 16 | 17.2 | |
| missing | 1 | 1.1 | |
| Hematological improvement | 35 | 32.5 | |
| Platelet (HI‐P) | 25/60 | 41.6 | |
| Erythroid (HI‐E) | 20/59 | 33.9 | |
| Neutrophil (HI‐N) | 14/56 | 25 | |
| Transfusion independence after AZA | |||
| Yes | 14/45 | 31.1 | |
| No | 31/45 | 68.9 | |
| Duration of AZA (No. of cycles) | 9 (1‐68) | ||
AZA, Azacitidine; HI: hematological improvement, MDS, myelodysplastic syndromes.
Hematological improvement was obtained in 35 patients (32.5%), with a better response in platelet lineage (42.6%) and erythroid lineage (33.9%). Neutrophil improvement was shown in 25% of patients. Among the 45 patients who had transfusion dependence before the start of AZA, 14 patients (31.1%) had transfusion independence at least 8 weeks according to IWG2006‐MDS response criteria. Response details are summarized in Table 3.
3.4. Causes of AZA discontinuation and death
At the last follow‐up, 82 patients (88.2%) discontinued AZA. The causes of discontinuation were a lack of significant improvement or disease progression which involved in 16 patients (17.2%), relapse in 28 patients (30.1%), and alteration of general conditions in 10 patients (10.7%). Infections occurred in 17 patients (18.3%), hemorrhage in four patients (4.3%), heart failure in three patients (3.2%), and second cancer occurred in one patient (1.1%).Three patients (3.2%) discontinued treatment due to personal choice.
At the time of analysis, 17 patients (18.3%) were still alive, among them 11 (11.8%) were still treated with AZA. Seventy‐six patients (81.7%) have died, among them fifty‐two (55.9%) after discontinuation of AZA, all of complications related to disease progression.
3.5. Survival outcomes
After a median follow‐up of 12 months (1‐71), a total of 76 events occurred, 39 relapse and 37 deaths without relapse, the median OS and PFS for the whole population were 15 months (range: 1‐71) and 12 months (range: 1‐70), respectively. OS and PFS probabilities for the whole population were 58% (95% CI: 48‐69) and 47% (95% CI: 38‐58) at one year; 35% (95% CI: 26‐47) and 31% (95% CI: 23‐43) at 2 years, respectively (Figure 1A,C). When analyzing the outcomes according to response to AZA, median OS was 32 months (range: 26‐55) for responders (defined as CR/marrow CR/PR/HI) and 8 months (range: 1‐12) for nonresponders, with a respective 1‐year and 2‐year OS probabilities of 91% vs 28% and 66% vs 6%, respectively (P < 0.001) (Figure 1B). A total of 40 patients had transformation to AML after a median time of 11 months (range: 1‐68). The median response duration was 3.9 months.
Figure 1.
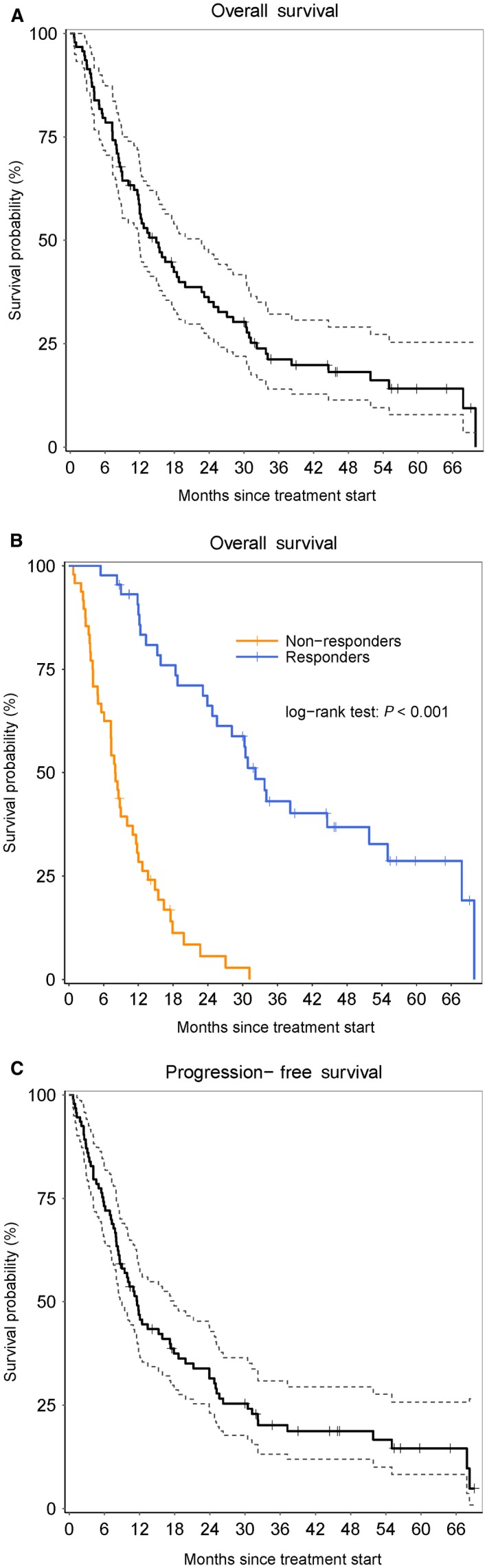
A, Overall survival for the whole population. B, Overall survival according to response after azacitidine (AZA). C, Progression‐free survival for the whole population
We tested in univariate analysis, the variables that could impact outcome after AZA were as follows: age, gender, ferritin level, LDH level, albumin level, number of cytopenias, transfusion dependence, AML, poor karyotype (IPSS score), poor and very poor karyotype (IPSS‐R score), high‐risk IPSS score, high and very high‐risk IPSS‐R score, RDI <80% before and after getting BOR. This univariate analysis showed a significant negative impact of number cytopenia, transfusion dependence, AML, poor karyotype (IPSS score), poor and very poor karyotype (IPSS‐R score), and high IPSS score on both OS and PFS (Table 4). Interestingly, there was no impact of dose reduction on OS nor on PFS, however, when analyzing the timing of dose reduction as time‐dependent variable, we found that patients who had dose reduction before achieving the objective response, had significantly lower OS (P = 0.02) and PFS (P = 0.01) compared to patients who had dose reduction after achieving the objective response. Significant factors in univariate analysis were studied in a multivariate model and the only two factors that were found to impact OS were AML with 21%‐30% blasts in BM (HR = 2.09, 95% CI: 1.27‐3.44, P = 0.004) and poor and very poor karyotype according to IPSS‐R (HR = 2.73, 95% CI: 1.6‐4.6, P < 0.001), as well as the same variables for PFS, with HR = 1.84, 95% CI: 1.07‐3.17, P = 0.028, and HR = 3.03, 95% CI: 1.7‐5.39, P < 0.001 respectively (Table 5).
Table 4.
Univariate analysis on OS and PFS
| Variable | OS | PFS | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | Cox P‐value | HR | 95% CI | Cox P‐value | |
| Cytopenias: 2‐3 | 1.66 | [1.04‐2.63] | 0.032 | 1.64 | [1.03‐2.60] | 0.036 |
| Transfusion dependence | 1.78 | [1.12‐2.82] | 0.014 | 1.68 | [1.06‐2.65] | 0.027 |
| AML with 21%‐30% blasts in the bone marrow | 2.01 | [1.22‐3.31] | 0.006 | 1.83 | [1.11‐3.00] | 0.017 |
| Poor karyotype (IPSS score) | 2.73 | [1.49‐4.99] | 0.001 | 2.92 | [1.62‐5.26] | <0.001 |
| Poor and very poor karyotype (IPSS‐R score) | 2.86 | [1.60‐5.09] | <0.001 | 2.87 | [1.66‐4.94] | <0.001 |
| High IPSS score | 2.62 | [1.83‐3.76] | <0.001 | 2.47 | [1.71‐3.58] | <0.001 |
| <80% RDI (time‐dependent) | 0.732 | [0.44‐1.16] | 0.182 | 1.02 | [0.69‐1.51] | 0.911 |
AML, acute myeloid leukemia; OS, Overall survivals; PFS, progression‐free survivals; RDI, relative dose intensity.
Table 5.
Multivariate analysis on OS and PFS
| Variable | OS | PFS | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | Cox P‐value | HR | 95% CI | Cox P‐value | |
| AML with 21%‐30% blasts in the bone marrow | 2.09 | [1.27‐3.44] | 0.004 | 1.84 | [1.07‐3.17] | 0.028 |
| Poor and very poor karyotype (IPSS‐R score) | 2.73 | [1.60‐4.65] | <0.001 | 3.03 | [1.70‐5.39] | <0.001 |
| <80% RDI (time‐dependent) | 0.77 | [0.48‐1.24] | 0.282 | 0.87 | [0.54‐1.40] | 0.558 |
AML, acute myeloid leukemia; OS, Overall survivals; PFS, progression‐free survivals; RDI, relative dose intensity.
4. DISCUSSION
The approved AZA dosing schedule is 75 mg/m2/day on days 1‐7 of each 28‐day treatment cycle (AZA 7). However, the consecutively 7 day regimen is most often not applied over the entire duration of treatment for multiple reasons.
In a prospective, longitudinal, multicenter US patients registry from community‐based hematology clinics (n = 331), it has been shown that only 17% of patients treated with AZA received the FDA‐approved continuous 7‐day dosing schedule; 51% received <7 days; 30% received 7 days with pauses; and 2% >7 days.24
In the Spanish compassionate use registry, 200 patients with either a confirmed diagnosis of WHO‐defined MDS, or de novo or secondary AML according to WHO criteria with 20%‐30% bone marrow blasts were treated with AZA: Among them 66 (33.0%) received AZA 5; 56 (28.0%) received AZA 5‐2‐2; and 78 (39.0%) received AZA 7 consecutively days regimen. ORRs appeared higher with the AZA 5‐2‐2 dosing schedule (67.9%) compared with AZA 7 (51.3%) and AZA 5 (39.4%), (overall, P = 0.0094), but there was no impact on survival. Otherwise, 58.5% of patients included in the study presented IPSS Low‐ or Int‐1‐risk MDS.16
Recently, an update of data registry of the GESMD between 2000 and 2013 included 251 patients with higher risk MDS patients (defined by an IPSS risk score of 1.5 or more) treated with AZA. Dosing schedule was available in 179 patients (71%). Seventy‐six patients received a standard 7‐day regimen (42.5%), and the remaining 103 (57.5%) were treated with less intensive dosing. There were no differences in survival between these two dosing schedules (P = 0.12).12
The MD Anderson group reported a retrospective pooled analysis of 2 decitabine, another HMA, clinical trials in 182 patients with de novo or secondary MDS, either of intermediate or high risk or of any French‐American‐British subtype and showed that patients who had dose modifications, cycle delays or dose reductions, had significantly higher ORRs compared with those who had none of these, but median OS values were similar to those of patients who had neither. Otherwise, patients with cycle delays or dose reductions received a median of six cycles of decitabine compared with those without cycle delays or dose reductions who received a median of two cycles.25
The MD Anderson group also studied the impact of RDI in patients with intermediate or high‐risk IPSS‐MDS or CMML treated with decitabine, in a monocentric retrospective series and showed OS rates significantly higher for patients who had dose reduction/or dose delayed after getting best objective response compared to those who had dose reduction/or dose delayed prior to best objective response or those with no dose reduction/or dose delayed.26
This could be explained by a longer exposure to treatment over time in patients with delayed/dose reduction compared to neither who received fewer cycles of AZA25 and the need for an initial intensity dose before obtaining a response to treatment and then maintaining of a certain degree of impregnation of HMA. In this line, it was shown that a low dose but high‐dose intensity schedule of decitabine at 20 mg/m2 intravenously daily over 1 hour for 5 consecutive days, every 4 week, optimizes hypo methylation induction, as well as clinical results based on IWG criteria, compared to the 10 mg/m2 intravenously over 1 hour daily for 10 days or 20 mg/m2 subcutaneously daily for 5 days regimens.27
The impact of the time of dose reduction on survival, or the maintenance therapy with reduced dose in patients who achieved best responses was not reported in patients treated with AZA. This is particularly important in MDS since patients are expected to receive long‐term treatment which can last several years, particularly in responders, but that can be a source of psychological intolerance, fatigue related to incessant trips to the hospital, alteration of QOL, and decision of discontinuation of AZA‐treatment, while it was demonstrated that additional cycles of AZA therapy have improved the quality of response in patients with higher‐risk MDS28 and a median of 9 cycles of AZA was previously showed to be involved with prolonged OS versus conventional care.11
The effect of dose relative intensity was largely unknown and to the best of our knowledge, this is the first report to address in depth the effect of dose relative intensity on patients' outcomes with high‐risk MDS treated with AZA. Nevertheless, this study has tried to provide answers to a question ever raised which is whether yes or no, responding patients beyond a certain time if they still need the same dose of AZA.
In this present study with the limitations related to its retrospective nature, we showed that 51.6% of patients had a full dose and 48.4% a dose reduction or delayed dose with a RDI <80%. Nineteen patients (20.4%) had RDI <80% before getting BOR, result of hematological toxicity or nonhematologic‐related complications that would lead the treating physician to reduce the dose(s) of AZA or delay subsequent doses. Twenty‐six patients (27.9%) expected dose reduction/or dose delayed after getting best objective response setting and 16 (17.2%) had their dose reduced at cycle 12 or after, for compliance or psychological tolerance.
An important finding in our study is that patients who had treatment dose reduced before achieving the objective response had significantly lower OS and PFS compared to those who had dose reduction after achieving response, this should be taken in high consideration when proceeding to dose reduction. Prospective evaluation of an approach conceiving a loading dose for induction of a best objective response followed by a maintenance schedule is to be considered for patients with MDS treated with AZA and HMA in general. In Japan, a prospective phase III trial (JALSG MDS212: UMIN000009633) that compares AZA: 75 mg/m2, 5 days regimen and AZA 75 mg/m2, 7 days regimen in high‐risk MDS patients, with OS as the end point, is ongoing. This study could provide valuable follow‐up information on the findings of our study.
Laribi K, Bolle D, Alani M, et al. Impact of the relative dose intensity on survival of patients with high‐risk myelodysplastic syndromes treated with Azacitidine. Cancer Med. 2019;8:2188–2195. 10.1002/cam4.2121
REFERENCES
- 1. Tefferi A, Vardiman JW. Myelodysplastic syndromes. N Engl J Med. 2009;361(19):1872‐1885. [DOI] [PubMed] [Google Scholar]
- 2. Bennett Jm, Catovsky D, Daniel Mt, et al. Proposals for the classification of the myelodysplastic syndromes. Br J Haematol. 1982;51:189‐199. [PubMed] [Google Scholar]
- 3. Nimer SD. Myelodysplastic syndromes. Blood. 2008;111:4841‐4851. [DOI] [PubMed] [Google Scholar]
- 4. Gidaro A, Deliliers GL, Gallipoli P, Arquati M, Wu MA, Castelli R. Laboratory and clinical risk assessment to treat myelodysplatic syndromes. Clin Chem Lab Med. 2016;54:1411‐1426. [DOI] [PubMed] [Google Scholar]
- 5. Santini V, Kantarjian HM, Issa JP. Changes in DNA methylation in neoplasia: pathophysiology and therapeutic implications. Ann Intern Med. 2001;134:573‐586. [DOI] [PubMed] [Google Scholar]
- 6. Leone G, Teofili L, Voso MT, Lubbert M. DNA methylation and demethylating drugs in myelodysplastic syndromes and secondary leukemias. Haematologica. 2002;87:1324‐1341. [PubMed] [Google Scholar]
- 7. Pleyer L, Greil R. Digging deep into ‘‘dirty'' drugs‐modulation of the methylation machinery. Drug Metab Rev. 2015;47(2):252‐279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Greenberg P, Cox C, LeBeau MM, et al. International scoring system for evaluating prognosis in myelodysplastic syndromes. Blood. 1997;89(6):2079‐2088. [PubMed] [Google Scholar]
- 9. Silverman LR, Demakos EP, Peterson BL, et al. Randomized controlled trial of azacitidine in patients with the myelodysplastic syndrome: a study of the cancer and leukemia group B. J Clin Oncol. 2002;20(10):2429‐2440. [DOI] [PubMed] [Google Scholar]
- 10. Silverman LR, McKenzie DR, Peterson BL, Holland JF, Backstrom JT, Beach Cl, et al.; Cancer and Leukemia Group B . Further analysis of trials with azacitidine in patients with myelodysplastic syndrome: studies 8421, 8921, and 9221 by the Cancer and Leukemia Group B. J Clin Oncol. 2006;24(24):3895‐3903. [DOI] [PubMed] [Google Scholar]
- 11. Fenaux P, Mufti GJ, Hellstrom‐Lindberg E, et al.; International Vidaza High‐Risk MDS Survival Study Group . Efficacy of azacitidine compared with that of conventional care regimens in the treatment of higher‐risk myelodysplastic syndromes: a randomised, open‐label, phase III study. Lancet Oncol. 2009;10(3):223‐232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bernal T, Martínez‐Camblor P, Sánchez‐García J, et al.; Group on Myelodysplastic Syndromes ; PETHEMA Foundation ; Spanish Society of Hematology . Effectiveness of azacitidine in unselected high‐risk myelodysplastic syndromes: results from the Spanish registry. Leukemia. 2015;29(9):1875‐1881. [DOI] [PubMed] [Google Scholar]
- 13. Martin MG, Walgren RA, Procknow E, et al. A phase II study of 5‐day intravenous azacitidine in patients with myelodysplastic syndromes. Am J Hematol. 2009;84(9):560‐564. [DOI] [PubMed] [Google Scholar]
- 14. Fujimaki K, Miyashita K, Kawasaki R, Tomita N. Efficacy and safety of a 5‐day regimen of azacitidine for patients with high‐risk myelodysplastic syndromes. Eur J Haematol. 2015;97:228‐231. [DOI] [PubMed] [Google Scholar]
- 15. Pierdomenico F, Esteves S, Almeida A. Efficacy and tolerability of 5‐day azacytidine dose‐intensified regimen in higher‐risk MDS. Ann Hematol. 2013;92(9):1201‐1206. [DOI] [PubMed] [Google Scholar]
- 16. García‐Delgado R, de Miguel D, Bailén A, et al. Effectiveness and safety of different azacitidine dosage regimens in patients with myelodysplastic syndromes or acute myeloid leukemia. Leuk Res. 2014;38(7):744‐750. [DOI] [PubMed] [Google Scholar]
- 17. Lyons RM, Cosgriff TM, Modi SS, et al. Hematologic response to three alternative dosing schedules of azacitidine in patients with myelodysplastic syndromes. J Clin Oncol. 2009;27(11):1850‐1856. [DOI] [PubMed] [Google Scholar]
- 18. Itzykson R, Thepot S, Quesnel B, et al.; Groupe Francophone des Myelodysplasies (GFM) . Prognostic factors for response and overall survival in 282 patients with higher‐risk myelodysplastic syndromes treated with azacitidine. Blood. 2011;117(2):403‐411. [DOI] [PubMed] [Google Scholar]
- 19. Swerdlow S, Campo E, Harris NL, et al. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues. Lyon, France, International Agency for Research of Cancer; 2008. [Google Scholar]
- 20. An international system for human cytogenetic nomenclature (1978) ISCN (1978). Report of the Standing Committee on Human Cytogenetic Nomenclature. Cytogenet Cell Genet. 1978;21(6):309‐409. [DOI] [PubMed] [Google Scholar]
- 21. Chun K, Hagemeijer A, Iqbal A, Slovak ML. Implementation of standardized international karyotype scoring practices is needed to provide uniform and systematic evaluation for patients with myelodysplastic syndrome using IPSS criteria: an International Working Group on MDS Cytogenetics Study. Leuk Res. 2010;34(2):160‐165. [DOI] [PubMed] [Google Scholar]
- 22. Greenberg Pl, Tuechler H, Schanz J, et al. Revised international prognostic scoring system for myelodysplastic syndromes. Blood. 2012;120(12):2454‐2465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Cheson BD, Greenberg PL, Bennett JM, et al. Clinical application and proposal for modification of the International Working Group (IWG) response criteria in myelodysplasia. Blood. 2006;108(2):419‐425. [DOI] [PubMed] [Google Scholar]
- 24. Sekeres MA, Jaroslaw MS, Maciejewski P, et al. A study comparing dosing regimens and efficacy of subcutaneous to intravenous azacitidine (AZA) for the treatment of myelodysplastic syndromes (MDS). Blood. 2009;114. abstract:3797. [Google Scholar]
- 25. Jabbour E, Garcia‐Manero G, Cornelison AM, et al. The effect of decitabine dose modification and myelosuppression on response and survival in patients with myelodysplastic syndromes. Leuk Lymphoma. 2015;56(2):390‐394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ghanem H, Cornelison AM, Garcia‐Manero G, et al. Decitabine can be safely reduced after achievement of best objective response in patients with myelodysplastic syndrome. Clin Lymphoma Myeloma Leuk. 2013;13(Suppl 2):S289‐S294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kantarjian H, Oki Y, Garcia‐Manero G, et al. Results of a randomized study of 3 schedules of low‐dose decitabine in higher‐risk myelodysplastic syndrome and chronic myelomonocytic leukemia. Blood. 2007;109(1):52‐57. [DOI] [PubMed] [Google Scholar]
- 28. Silverman LR, Fenaux P, Mufti GJ, et al. Continued azacitidine therapy beyond time of first response improvesquality of response in patients with higher‐risk myelodysplastic syndromes. Cancer. 2011;117:2697‐2702. [DOI] [PMC free article] [PubMed] [Google Scholar]


